Abstract
Objective
Multiple aspects of religion have been linked with a variety of physical health outcomes; however, rarely have investigators attempted to empirically test the mechanisms through which religiosity impacts health. The links between religious participation, religious coping, and diurnal cortisol patterns over a 10-year period in a national sample of adults in the United States were investigated.
Method
Participants included 1,470 respondents from the Midlife in the United States (MIDUS) study who provided reports on religious participation, religious coping, and diurnal cortisol.
Results
Religious participation predicted steeper (“healthier”) cortisol slopes at the 10-year follow-up, controlling for potential confounds. Further, religious struggle (religious coping marked by tension and strain about religious and spiritual issues) mediated the prospective association between religious participation and cortisol slope, such that greater religious attendance predicted lower levels of religious struggle 10 years later, which in turn was linked with a steeper cortisol slope; this effect remained strong when controlling for general emotional coping and social support. Positive religious coping was unrelated to diurnal cortisol patterns.
Conclusion
These findings identify religious struggle as a mechanism through which religious participation impacts diurnal cortisol levels and suggest that diurnal cortisol is a plausible pathway through which aspects of religion influence long-term physical health.
Keywords: religion, coping, health, MIDUS
Investigations point to religion as important aspects of life, such that 90% of Americans believe in God or a higher spiritual power (Lee & Newberg, 2005), greater than 60% of Americans belong to a church (Iannaccone, 1998), and over 40% of Americans attend religious services weekly or almost weekly (Newport, 2010). Many findings highlight the link between religious participation and improved health, even when controlling for demographic factors, health behaviors, social support, and mood (Powell, Shahabi, & Thoresen, 2003). Moreover, a large body of research has shown that individuals use religious strategies to cope with stressful life events (Pargament, Smith, Koenig, & Perez, 1998). Overall, research points to a beneficial relationship between multiple aspects of religion, including service attendance and religious coping, and health such that these aspects of religion have been linked with a variety of desirable physical health outcomes, including lower blood pressure (Koenig, Pargament, & Nielsen, 1998), better lipid profiles (Friedlander, Kark, & Stein, 1987), and greater survival for individuals with a chronic illness (Ironson, Solomon, et al., 2002). Although the relationship between religious constructs and health is frequently investigated, rarely have researchers attempted to empirically test the mechanisms by which these constructs impact health outcomes.
Mechanisms proposed to explain the relationship between religious participation and health include social support, health behaviors, and coping strategies (Powell, et al., 2003). The current investigation focuses on religious coping as the mechanism by which religion influences health-related biology, specifically diurnal cortisol. Pargament (2001) asserted that stressful life events can be interpreted through religious terms via spiritual and religious coping appraisals, implying that religion and spirituality can offer individuals a unique strategy to cope with stress. Religious coping is often conceptualized as efforts to understand or deal with life stress in relation to the sacred, including notions of God, higher or divine powers, or divine-like qualities (Pargament, Feuille, & Burdzy, 2011). As opposed to religious participation that quantifies behavior, religious coping attempts to understand how an individual considers and utilizes aspects of religion in daily life when faced with a stressful event. Further, the literature has demonstrated that religious coping measures can be stronger predictors of health outcomes in times of stress when compared to religious participation (Pargament, 2001), but no studies to our knowledge have prospectively examined how religious coping may explain the relationship between religious participation and diurnal cortisol patterns.
Importantly, religious coping can be broken down into positive religious coping strategies and negative religious coping, more recently termed religious struggle. Positive religious coping includes a benevolent understanding and reinterpretation of a stressor in a positive, potentially beneficial light, including searching for a spiritual connection in times of stress, a collaborative relationship with the sacred, and/or seeking spiritual support (Pargament, et al., 2011). Negative religious coping is a general term used to describe maladaptive ways religion can be used to cope. Religious struggle, is a specific type of negative religious coping that is marked by tension and strain about religious and spiritual issues, the reinterpretation of stressors as punishment from the sacred, and/or spiritual discontent (Magyar-Russell et al., 2014). Religious struggle is a normative and expected process in dealing with life stress with outcomes dependent on how these struggles are managed, such that resolved religious struggles are associated with growth and transformation but unresolved religious struggle or strain is linked with hopelessness and poor psychological health (Magyar-Russell, et al., 2014; Pargament, Murray-Swank, Magyar, & Ano, 2005). Positive religious coping has been linked with lower levels of anxiety and better physical well-being, whereas religious struggle is associated with greater depressive symptoms, poor physical health, and even greater risk of mortality (Ai, Seymour, Tice, Kronfol, & Bolling, 2009; Sherman, Simonton, Latif, Spohn, & Tricot, 2005). Recent work with patients undergoing hemodialysis has highlighted the independent effects of religious struggle such that greater religious struggle was related to greater psychological distress and lower health-related quality of life whereas positive religious coping was related to only improved health-related quality of life (Ramirez et al., 2012). Furthermore, greater endorsement of religious struggle has also been linked to less frequent religious service attendance, lending support to idea that religious attendance may influence religious struggles overtime and contribute to beneficial health effects (Fitchett et al., 2004). Overall, there is a large body of research that investigates the relationship between religious coping and psychological well-being and physical health (Pargament, 1996).
Recent work has begun to investigate the biological mechanisms through which religious coping may influence health. For patients undergoing cardiac surgery, greater amounts of religious struggle were linked with increased levels of a circulating pro-inflammatory cytokine, interleukin (IL)-6 (Ai, et al., 2009). Furthermore, pre-operative anxiety was indirectly linked to post-operative depression via religious struggle and IL-6 in patients undergoing open heart surgery (Ai, Pargament, Appel, & Kronfol, 2010). Given preliminary work suggesting the importance of inflammatory pathways, the current work proposes an additional biological pathway through which religious participation and religious coping influence health is the hypothalamic-pituitary-adrenal (HPA) axis and its hormonal product, cortisol. Cortisol is the primary stress hormone secreted by the HPA axis following stressful experiences and is an important component in the regulation of the immune, cardiovascular, metabolic, and homeostatic systems (Sephton, Sapolsky, Kraemer, & Spiegel, 2000). Cortisol exhibits a diurnal pattern where approximately 30–45 minutes after morning waking there is a peak in daily cortisol production, which then declines over the course of the day to its nadir during bedtime (Gunnar, Wewerka, Frenn, Long, & Griggs, 2009). Dysregulation in cortisol patterns, represented by a less steep decline in cortisol over the day (“flatter” cortisol slope) is linked with depression (Gallagher-Thompson et al., 2006), increased cellular aging (Tomiyama et al., 2012), and mortality (Kumari, Shipley, Stafford, & Kivimaki, 2011).
Prior work supports the investigation of the relationship between religious practices and diurnal cortisol patterns. For example, in a sample of HIV-positive men, increased faith in a higher being was associated with lower cortisol levels collected over a 15-hour time period (Ironson, Balbin, et al., 2002). Additionally, in a sample of women with breast cancer, greater spirituality was associated with lower evening cortisol levels across a 3-day period (Sephton, Koopman, Schaal, Thoresen, & Spiegel, 2001). Despite the recent interest in understanding the effect of religion and spirituality on health and illness, no studies to date have examined psychological mechanisms (e.g., religious coping) through which religious participation may affect the HPA axis. Further, to the authors’ knowledge, all of the prior studies of religious participation and health-related biology (e.g., cortisol) have been cross-sectional in design.
The current work investigated the associations between religious participation, religious coping, and diurnal cortisol over a 10-year period in a large sample of adults in the United States. Based on prior work, we hypothesized that religious participation would prospectively predict steeper cortisol slopes, and would do so, at least in part, through lower religious struggle and higher positive religious coping assessed at follow-up. Further, to test the robustness of these associations, a number of potential confounds were controlled for, as well as plausible alternative mechanisms such emotion focused coping, and social support.
Method
The data for this study were taken from the Midlife in the United States Project (MIDUS), a two-wave survey of adults, aged 25 to 74. This study included salivary cortisol collection for a portion of the participants as part of the National Study of Daily Experiences (NSDE). Phone interviews and self-reported questionnaires were collected from 1995–1996 (Wave 1) and again in 2004–2006 (Wave 2). A subset of individuals who completed Wave 2 data collection then completed Wave 2 of NSDE (2004–2009) where cortisol samples were collected (See Almeida, McGonagle, & King, 2009, for a more detailed description). Religious participation data was collected at Wave 1 and used in the present analyses. Religious coping, emotion focused coping, and social support data collected at Wave 2 and cortisol samples collected at Wave 2 of NSDE were used in the present analyses.
Sample
For the current study, only those individuals who provided religious participation data at Wave 1 and religious coping, social support, emotion focused coping, and cortisol data at Wave 2 were included. The sample consisted of 1,470 adults (56% female, 92.6% White/Caucasian, 67.8% completed some college or more; age, M = 47.66 years, SD = 12.04 years).
Measures
Religious Participation
Religious participation at Wave 1 was assessed using two questions, asking participants how often they attended religious services (M = 2.82, SD =3.64) and how often they attended meetings of religious groups (M = 1.00, SD = 2.28) in a typical month. Items were highly correlated (r >.95) and thus were combined into a composite measure for religious participation by computing the mean of both religious service attendance and meetings of religious group attendance. This composite measure captures multiple forms of religious participation. Due to the high positive skew of this variable, the natural log of the variable was computed and used in analyses.
Religious Coping
The Brief Multidimensional Measure of Religion and Spirituality (BMMRS; Fetzer Institute, 1999) was used in the current study to assess religious and spiritual coping at Wave 2. Participants completed questions assessing the extent to which they employ religious coping strategies in daily life using a 4-point scale (1 = none; 4 = a great deal). To determine the two religious coping composites, a principle axis factor analysis was conducted using varimax rotation. Two factors were extracted based on eigenvalues greater than one; factor loadings ranged from .68–.92. Positive religious coping items included: I look to God for strength, support, and guidance; I work together with God as partners; I think about how my life is part of a larger spiritual force, and reverse-coded I try to make sense of the situation and decide what to do without relying on God (M = 2.79, SD = .91; α = .84). The mean value of the four items was used in the present analyses. Religious/spiritual struggle included: I wonder whether God has abandoned me; I feel God is punishing me for my sins or lack of spirituality (M = 1.26, SD = .53; α = .69). The mean value of the two items was used for the religious struggle composite in the present analyses. The positive and religious struggle composites were modestly inversely related (r = −.03, p = .04).
Previous research has often included “I try to make sense of the situation and decide what to do without relying on God” within the religious struggle/negative religious coping composite (Fetzer Institute, 1999; Pargament, et al., 1998); however, when this item was included in the religious struggle composite, the internal consistency was very low (α = .33). The results presented below do not change whether including this item in the religious struggle composite or reverse coded within the positive religious coping composite.
Emotion Focused Coping
Emotion focused coping was assessed at Wave 2 via 12-items from the COPE (Carver, Scheier, & Weintraub, 1989). The score is the sum of 12-items including coping styles focused on and venting of emotion, denial, and behavioral disengagement. Higher scores represent higher levels of emotion focused coping (M = 22.01, SD = 5.38; α =.83). Emotion focused coping was assessed to determine if an effect of religious coping on diurnal cortisol would remain significant when controlling for general emotion focused coping.
Salivary cortisol
Salivary cortisol was assessed using Salivettes (Sarstedt, Rommelsdorft, Germany). On average, saliva collection during NSDE II occurred 25.55 months (SD = 12.72) after the MIDUS II assessment. On days 2–5 of the 8-day NSDE II study period, participants self-collected saliva samples at four time points each day; immediately upon waking, 30 minutes later to assess cortisol awakening response (CAR), before lunch, and at bedtime. Cortisol concentrations were quantified with a commercially available luminescence immunoassay (IBL, Hamburg, Germany) with intra-assay and interassay coefficients of variability less than 5% (Polk, Cohen, Doyle, Skoner, & Kirschbaum, 2005). Saliva collection compliance was assessed using nightly telephone interviews and paper-and-pencil logs included in the collection kits. Cortisol values were base-10 log-transformed to correct for positive skew in the cortisol distribution (Adam & Kumari, 2009) and a constant of 4 was added after the log transformation so that all transformed scores were positive.
Social Support
Social support was assessed for the following relationships: family members, friends, and spouse/partner (Walen & Lachman, 2000). Items were answered on a 4-point scale where higher scores reflect greater amounts of support received. The mean values of support provided by family (M = 3.54, SD = .58, α =.84), friends (M = 3.30, SD = .66, α =.88), and spouse/partner (M = 3.63, SD = .53, α =.86) were then averaged to provide an overall measure of support received (M = 3.47, SD = .46; α =.85). Social support was included as an additional covariate to determine if an effect of religious constructs on diurnal cortisol remained significant when controlling for social support.
Diurnal Cortisol Covariates
Covariates included age, gender (male = 1, female = 2), education (0 = high school or less, 1 = some college or more), waist circumference (a measure of central adiposity), race/ethnicity (0 = white, 1 = nonwhite), and average wake time across the days of salivary cortisol sampling. These are standard covariates in diurnal cortisol studies (Adam & Kumari, 2009).
Data Analysis
Prior to conducting HLM analyses, bivariate correlations were conducted to determine the relationships among religion variables. Because of the strong diurnal rhythm of cortisol, Hierarchical Linear Modeling (HLM; Raudenbush, Bryk, & Congdon, 2013) was used for cortisol data analyses. HLM allows for the simultaneous estimation of multiple cortisol parameters (cortisol at wakeup, CAR, and slope) and the prediction of individual differences in diurnal cortisol profiles. Following prior diurnal cortisol research (Adam & Kumari, 2009), Time Since Waking, Time Since Waking-squared, and CAR (dummy coded 1 for the second cortisol sample of the day and 0 for all other samples) were modeled at Level-1 to provide estimates of each participant’s diurnal cortisol rhythm (Level 1: Cortisol = π0iIntercept + π1iTimeij + π2iCARij + π3iTime2ij + εij). The CAR coefficient reflects a latent estimate of the size of each individual’s CAR (for more information regarding this standard approach see Adam, 2006). Level-2 (person-level) effect of religious participation at Wave 1 was included as a predictor (Level 2: π0 to π2 = βi0 to βij × Religious Participation + rij). To control for potential confounding effects, age, gender, ethnicity, education, waist circumference, and wake time were included at Level-2 (Level 2: π0 to π2 = βi0 to βij × Control Variables + rij). Next, whether positive religious coping and religious struggle at Wave 2 mediated the effects of religious participation at Wave 1 on cortisol parameters was tested (Level 2: π0 to π2 = βi0 to βij × Religious Participation + βij × Positive Religious Coping + βij × Religious Struggle + rij). Monte Carlo confidence intervals were calculated to determine the indirect associations between religious participation, religious coping, and diurnal cortisol parameters (Selig & Preacher, 2009). Finally, emotion focused coping and social support at Wave 2 was controlled for as potential confounds/alternative mechanisms of the proposed mediating effect of religious coping. In line with prior studies (e.g., Adam, Hawkley, Kudielka, & Cacioppo, 2006), cortisol intercept, slope (effect of time), and CAR were all allowed to vary randomly at Level-2 (i.e., treated as random effects), while Time Since Waking-squared was treated as a fixed effect with no Level-2 predictors. Person-level variables were all grand-mean centered, with the exception of gender, ethnicity, and education. All significance tests were 2-tailed with robust standard errors.
Results
Religious participation at Wave 1 was significantly associated with greater positive religious coping (r = .53, p < .001) and lower religious struggle (r = −.08, p < .01) at Wave 2. Emotion focused coping was significantly positively associated with religious struggle (r = .31, p < .001) and with positive religious coping (r = .05, p = .001). As shown in Model 1 of Table 1, participants’ cortisol values showed the expected diurnal pattern across the day, with high values at wakeup (β00 = 5.179; SE = 0.025, p < .001), an increase in levels in the first 30 minutes after waking (CAR; β20 = 0.142; SE = 0.017, p < .001), and a decline in cortisol levels across the day (β30 = 0.001; SE = 0.0001, p < .001).
Table 1.
Multilevel Growth-Curve Models of Diurnal Cortisol Parameters
| Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|
| Fixed effect (independent variable) |
Estimate (SE) | P | Estimate (SE) | P | Estimate (SE) | P |
| Wake Up Cortisol, π0 | ||||||
| Average Wakeup Cortisol
(Intercept), β00 |
5.179 (0.025) | <.001 | 5.179 (0.025) | <.001 | 5.176 (0.026) | <.001 |
| Age, β01 | 0.002 (0.001) | .001 | 0.002 (0.001) | .001 | 0.002 (0.001) | .002 |
| Female, β02 | −0.063 (0.013) | <.001 | −0.062 (0.013) | <.001 | −0.059 (0.014) | <.001 |
| Waist Circumference, β03 | −0.001 (0.001) | .041 | −0.001 (0.001) | .045 | −0.001 (0.001) | .066 |
| Ethnicity, β04 | −0.104 (0.038) | .006 | −0.102 (0.038) | .007 | −0.098 (0.036) | .007 |
| Education, β05 | 0.052 (0.014) | <.001 | 0.050 (0.014) | .001 | 0.046 (0.014) | .001 |
| Average Waketime, β06 | −0.006 (0.007) | .372 | −0.006 (0.008) | .370 | −0.005 (0.007) | .433 |
| W1 Religious Participation, β07 | 0.014 (0.007) | .071 | 0.013 (0.008) | .099 | 0.012 (0.008) | .118 |
| W2 Religious Struggle, β08 | -- | -- | −0.020 (0.012) | .113 | −0.008 (0.014) | .556 |
| W2 Emotion Focused Coping, β09 | -- | -- | -- | -- | −0.002 (0.002) | .156 |
| W2 Social Support, β010 | -- | -- | -- | -- | 0.019 (0.018) | .285 |
| Time Since Waking, π1 | ||||||
| Average Linear Slope, β10 | −0.061 (0.003) | <.001 | −0.061 (0.003) | <.001 | −0.061 (0.003) | <.001 |
| Age, β11 | 0.000 (0.000) | <.001 | 0.000 (0.000) | <.001 | 0.000 (0.000) | <.001 |
| Female, β12 | 0.001 (0.001) | .474 | 0.001 (0.001) | .588 | 0.001 (0.001) | .627 |
| Waist Circumference, β13 | −0.001 (0.001) | .928 | −0.000 (0.000) | .850 | −0.000 (0.000) | .851 |
| Ethnicity, β14 | 0.015 (0.003) | <.001 | 0.014 (0.003) | <.001 | 0.014 (0.003) | <.001 |
| Education, β15 | −0.005 (0.001) | .001 | −0.004 (0.001) | .004 | −0.004 (0.001) | .004 |
| Average Waketime, β16 | −0.001 (0.001) | .169 | −0.001 (0.001) | .165 | −0.001 (0.001) | .167 |
| W1 Religious Participation, β17 | −0.002 (0.001) | .024 | −0.002 (0.001) | .055 | −0.002 (0.001) | .054 |
| W2 Religious Struggle, β18 | -- | -- | 0.005 (0.001) | <.001 | 0.005 (0.001) | <.001 |
| W2 Emotion Focused Coping, β19 | -- | -- | -- | -- | 0.000 (0.000) | .890 |
| W2 Social Support, β110 | -- | -- | -- | -- | 0.000 (0.002) | .945 |
| Cortisol Awakening Response, π2 | ||||||
| Average CAR, β20 | 0.142 (0.017) | <.001 | 0.142 (0.017) | <.001 | 0.142 (0.018) | <.001 |
| Age, β21 | 0.001 (0.000) | .019 | 0.001 (0.000) | .015 | 0.001 (0.000) | .021 |
| Female, β22 | 0.037 (0.010) | <.001 | 0.037 (0.010) | <.001 | 0.037 (0.010) | <.001 |
| Waist Circumference, β23 | −0.000 (0.000) | .536 | −0.000 (0.000) | .510 | −0.000 (0.000) | .544 |
| Ethnicity, β24 | 0.022 (0.023) | .339 | 0.020 (0.023) | .368 | 0.021 (0.023) | .346 |
| Education, β25 | −0.009 (0.010) | .370 | −0.008 (0.010) | .457 | −0.008 (0.010) | .434 |
| Average Waketime, β26 | −0.004 (0.004) | .326 | −0.004 (0.004) | .327 | −0.004 (0.004) | .346 |
| W1 Religious Participation, β27 | 0.006 (0.006) | .314 | 0.007 (0.006) | .264 | 0.006 (0.006) | .291 |
| W2 Religious Struggle, β28 | -- | -- | 0.012 (0.010) | .212 | 0.014 (0.010) | .141 |
| W2 Emotion Focused Coping, β29 | -- | -- | -- | -- | −0.000 (0.001) | .871 |
| W2 Social Support, β210 | -- | -- | -- | -- | 0.008 (0.011) | .439 |
| Time Since Waking Squared, π3 | ||||||
| Average Curvature, β30 | 0.001 (0.000) | <.001 | 0.001 (0.001) | <.001 | 0.001 (0.001) | <.001 |
Note. Intercepts indicate average log-transformed cortisol values at wakeup; average slopes of time since waking indicate change in log-transformed cortisol per 1-hour change in time; average slopes of time since waking-squared indicate change in log-transformed cortisol per 1-hour change in time-squared. CAR = Cortisol Awakening Response. For education, 0 = high school or less, 1 = some college or more, and for ethnicity, 0 = white, 1 = nonwhite. W1 = Data collected at Wave 1. W2 = Data collected at Wave 2.
Religious participation was prospectively associated with a steeper (healthier) cortisol slope (β17 = −0.002; SE = 0.001, p < .05) across the four days of saliva sampling at the 10-year follow up. As displayed in Model 1 of Table 1, when controlling for the effects of age, gender, ethnicity, education, average wake up time, and waist circumference, religious participation was a significant predictor of steeper cortisol slope. Religious participation was unrelated to either wakeup cortisol levels or CAR.
Mediation by Religious Coping
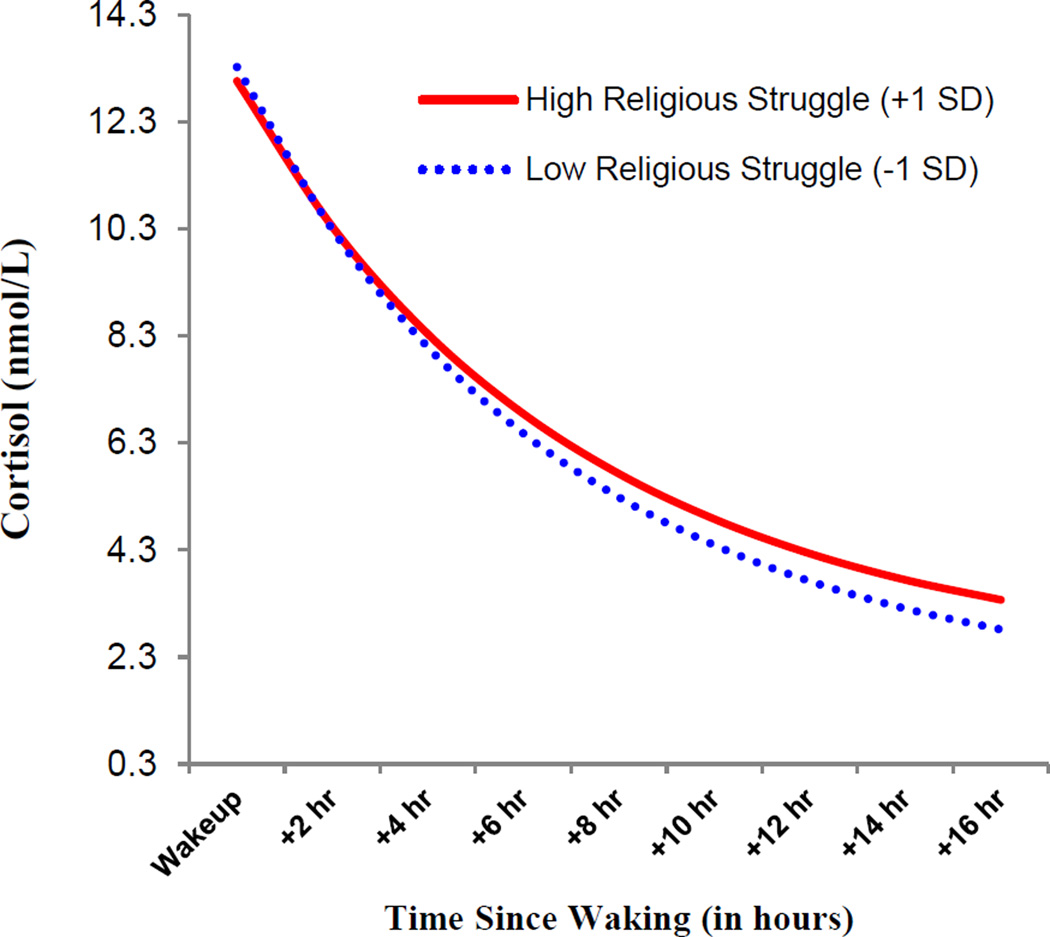
Next, whether the associations between Wave 1 religious participation and steeper diurnal cortisol slope could be explained by engagement in religious coping strategies at Wave 2 was examined. Using the Monte Carlo method for assessing 2-2-1 multilevel model mediation with 20,000 repetitions, confidence intervals (95%) for indirect effects were estimated with an online calculator (Selig & Preacher, 2009). Simple regression showed that religious participation significantly predicted lower levels of religious struggle at the 10-year follow-up (b = −0.05, SE = 0.12, p < .001). Religious participation, religious struggle, and demographic covariates were then included as predictors of diurnal cortisol patterns using HLM. As shown in Mode1 2 of Table 1, the effect of religious participation on diurnal cortisol was no longer significant (β17 = −0.002; SE = 0.001, p = .055). Religious struggle significantly predicted a flatter diurnal cortisol slope (β18 = 0.005; SE = 0.001, p < .001). Monte Carlo analyses showed significant indirect effects of religious participation on diurnal cortisol slope via religious struggle (95% CI: −0.000413, −0.000094). Further, as displayed in Model 3, when adding the emotion focused coping variable and social support to the model, the effects of religious struggle remained a significant predictor of flatter diurnal cortisol slope, and neither emotion focused coping nor social support significantly predicted cortisol slope1. The associations between religious struggle and diurnal cortisol are depicted in Figure 1.
Figure 1.
Associations between religious struggle and diurnal cortisol at the 10-year follow-up. High values for religious struggle are plotted at +1 standard deviation and low values are plotted at −1 standard deviation from the mean.
For positive religious coping, simple regression showed that religious participation significantly predicted positive religious coping (b = .62, SE = 0.02, p < .001). However, when religious participation, positive religious coping, religious struggle, and demographic covariates were entered as predictors of diurnal cortisol patterns using HLM, positive religious coping was not a significant predictor of any cortisol parameters, yet religious struggle remained a significant predictor of a flatter diurnal cortisol slope (β18 = 0.005, SE = 0.001, p < .001).
Discussion
The results of the current study found that prior religious participation predicted diurnal cortisol profiles at a 10-year follow up in a large national sample of adults in the United States. Specifically, prior religious participation, including time attending religious services and meetings regarding religious organizations, was prospectively associated with a steeper (“healthier”) cortisol slope. Importantly, this relationship remained strong when controlling for demographic factors. Notably, our findings also point to a psychological mechanism through which religious participation is prospectively associated with HPA function: Greater religious participation predicted lower levels of religious struggle 10 years later, which in turn was linked with a steeper cortisol slope. The effects of religious struggle held when controlling for emotion focused coping styles and social support, suggesting a unique effect of religious coping, namely religious struggle, on HPA function. Positive religious coping was not significantly related to diurnal cortisol profiles, nor was emotion focused coping style or social support.
To our knowledge, this is the first study to examine the prospective relationship between prior religious participation and diurnal cortisol parameters, highlighting a biological mechanism by which religious participation may benefit health outcomes. Prior work has pointed to a complex relationship between religion and health; however, few have attempted to understand how religion may influence health and health-related physiology from a psychological standpoint. Our findings also demonstrate a potentially meaningful deleterious health impact of religious struggle. Previous literature has linked religious coping styles marked by religious struggle with poor health outcomes, including poorer functional ability in patients undergoing medical rehabilitation (Fitchett, Rybarczyk, DeMarco, & Nicholas, 1999) and greater mortality in elderly patients who are hospitalized (Pargament, et al., 2001). A previous cross-sectional study found that higher religious attendance was linked with lower religious struggle (Fitchett, et al., 2004) and longitudinal work has linked greater religious struggle with greater mortality risk (Pargament, et al., 2001). This work is the first to longitudinally examine the relationships among prior religious participation, religious struggle, and biological processes.
The current study makes two important methodological and theoretical advances over prior work: 1) using a prospective design to test the links between prior religious participation and health-related biology over a 10-year time span, highlighting the cumulative effect of religious participation on health, and 2) identifying later religious struggle as a plausible mechanism through which religious participation impacts physical health. Interestingly, our results indicate that while religious participation is linked to greater positive religious coping over time, the observed benefit of religious participation on diurnal cortisol is not driven by greater positive religious coping, emotional focused coping style, or social support. Emotion focused coping and, especially, social support, have been considered as alternative explanations for the association between religious participation and physical health. However, the data from this study suggest that, at least with regard to the links between religious participation and diurnal cortisol, neither general coping nor social support mediate the links between religious participation and diurnal cortisol patterns. Moreover, this work suggests that prior religious participation may aid in the resolution of religious struggle—reducing negative religious coping—or perhaps avoid religious struggle, thus potentially benefiting health. The fact that those who more often participate in religious activities less often engage in religious struggle points to the possibility that religiously active individuals have more adaptive ways of managing stress—independent of positive religious coping—that may benefit cortisol functioning, and, potentially, long-term health and longevity. By engaging in lower levels of religious or spiritual conflict or viewing God or a higher divine power in a punitive role during times of stress, individuals may be employing more adaptive coping strategies that benefit health. It is possible that lower levels of religious struggles may allow individuals to feel more in control and able to manage stress when situations appear uncontrollable (Lutgendorf, Russell, Ullrich, Harris, & Wallace, 2004).
Although the effects presented are small, they are still meaningful and comparable to those reported in a meta-analysis investigating the effects of religious coping on psychological adjustment (Ano & Vasconcelles, 2005). Researchers have noted that the effect sizes of many popular associations between behavior and health are also small, including relationships between increased television viewing and healthy food choices with the risk for cardiovascular disease (Grøntved & Hu, 2011; He, Nowson, Lucas, & MacGregor, 2007; Robles, Slatcher, Trombello, & McGinn, 2014). While these effect sizes are small, they are still considered targets to improve health.
One potential explanation for the observed effects in the current study is culture. There are strong cultural and social ties to religious participation and religious coping (Taylor, 2001). Although a great strength of the current study is utilizing a national sample, it is still a predominately white and educated sample within the United States. It is possible that the current relationships found would not generalize to other ethnicities and cultures within the U.S., to countries where religion and spirituality are not as common (e.g., Western Europe), or in samples with greater proportions of Eastern religions where religious coping may take different forms. Further, by only having an assessment of religious coping at Wave 2, the data are unable to address how resolution of religious struggles overtime may influence diurnal cortisol patterns and health. It is possible that a portion of the effects observed in the current study is due to a decrease in religious struggle overtime.
Although numerous studies have pointed to religious participation as a protective factor against mortality (Pargament, et al., 2001), few have identified biological mechanisms underlying this relationship. The current study points to the possibility of significant and lasting beneficial effects of religious participation and lower religious struggle on HPA axis activity. Research has linked diurnal cortisol, specifically flatter diurnal cortisol slopes, with chronic illness and, importantly, mortality (Kumari, et al., 2011). Recent work has also identified IL-6, an immune factor important in many chronic illnesses and inflammation processes (e.g., diabetes, atherosclerosis), as a mediator of the relationship between religious participation and decreased mortality (Lutgendorf, et al., 2004). Converging reports also point to IL-6 as an important factor in the religion-mood relationship (Ai, et al., 2010; Ai, et al., 2009). Moving forward, the contribution of diurnal cortisol and various immune markers should be investigated as potential mediators of the relationship between religion and health to continue to clarify this complex relationship. Furthermore, research may benefit from understanding how psychological states influence the relationships observed in the current study and if they change the relationships between religious participation, coping, and health over time. Alternatively, research may also benefit by investigating whether better health over time leads to greater religious participation. The majority of work to date examines the effects of religion on health but few have determined the effects of health on religion.
The current work does have limitations worth noting. The variables used to operationalize religious participation were only assessed during Wave 1 data collection, which prevented examining the effects of changes across time in religious participation on diurnal cortisol parameters. Similarly, religious coping and diurnal cortisol were only at assessed at one time point. Finally, as the structure of religious activities for a given religion may affect how often an individual attends religious services or religious groups, the measure used to assess religious participation may not be appropriate for all religions. As researchers continue to investigate the complex relationships between religion and health, perhaps specific inventories or items that tap into the multiple aspects of religious participation across various religions is necessary.
Future work also should investigate other possible cognitive and affective mechanisms that may help to clarify the religion-health link. Researchers have hypothesized numerous mechanisms by which religion and spirituality may provide benefits to health outcomes, including positive affect, compassion, locus of control, and a sense of meaning in life (Powell, et al., 2003). For people who view religion and spirituality as an important part of life, it can make up a crucial part of their meaning system through which they interpret life experiences, and in turn influence goals, beliefs, and daily habits (e.g., physical activity, eating habits). A sense of meaning in life provides motivation to maintain and promote good health but also can provide a rationale for stressful life events when they arise (Dull & Skokan, 1995). It is likely that that the health benefits of religious participation are at least partially derived from a greater sense of life meaning.
Taken together, these findings provide strong evidence of a long-term association between religious participation and an important biological process linked to health and mortality. Although this work identified lower religious struggle as a psychological mechanism of the religion-health link, more research is needed to answer the key question of why religion appears to typically confer health benefits, as well as conditions under which religion may actually have detrimental health effects. This work also raises the question of how medical professionals or religious institutions can help individuals either avoid or resolve religious struggle that arises in times of stress to benefit health. Although scholars have studied the impact of religious practices on people and culture for several hundred years, only now are researchers beginning to understand the reasons behind their potentially powerful impact on health.
Acknowledgments
The MIDUS I study (Midlife in the U.S.) was supported by the John D. and Catherine T. MacArthur Foundation Research Network on Successful Midlife Development. The MIDUS II research was supported by a grant from the National Institute on Aging (P01-AG020166) to conduct a longitudinal follow-up of the MIDUS I investigation.
Footnotes
Religious support (Fetzer Institute, 1999) was also explored as a potential confound/mechanism to determine if the effects of coping and participation were robust to religious support; however, due to missing data, utilizing this variable substantially reduced the sample size (n = 897). Notably, the effects of religious struggle remained significant when utilizing religious support as a covariate with the smaller sample.
References
- Adam EK. Transactions among adolescent trait and state emotion and diurnal and momentary cortisol activity in naturalistic settings. Psychoneuroendocrinology. 2006;31(5):664–679. doi: 10.1016/j.psyneuen.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Adam EK, Hawkley LC, Kudielka BM, Cacioppo JT. Day-to-day dynamics of experience-cortisol associations in a population-based sample of older adults. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:17058–17063. doi: 10.1073/pnas.0605053103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam EK, Kumari M. Assessing salivary cortisol in large-scale, epidemiological research. Psychoneuroendocrinology. 2009;34(10):1423–1436. doi: 10.1016/j.psyneuen.2009.06.011. [DOI] [PubMed] [Google Scholar]
- Ai AL, Pargament KI, Appel HB, Kronfol Z. Depression following open - heart surgery: A path model involving interleukin - 6, spiritual struggle, and hope under preoperative distress. Journal of Clinical Psychology. 2010;66(10):1057–1075. doi: 10.1002/jclp.20716. [DOI] [PubMed] [Google Scholar]
- Ai AL, Seymour EM, Tice TN, Kronfol Z, Bolling SF. Spiritual struggle related to plasma interleukin-6 prior to cardiac surgery. Psychology of Religion and Spirituality. 2009;1:112–128. [Google Scholar]
- Almeida DM, McGonagle K, King H. Assessing daily stress processes in social surveys by combining stressor exposure and salivary cortisol. Biodemography and Social Biology. 2009;55(2):219–237. doi: 10.1080/19485560903382338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ano GG, Vasconcelles EB. Religious coping and psychological adjustment to stress: A meta-analysis. Journal of Clinical Psychology. 2005;61(4):461–480. doi: 10.1002/jclp.20049. [DOI] [PubMed] [Google Scholar]
- Carver CS, Scheier MF, Weintraub JK. Assessing coping strategies: a theoretically based approach. Journal of personality and social psychology. 1989;56(2):267. doi: 10.1037//0022-3514.56.2.267. [DOI] [PubMed] [Google Scholar]
- Dull VT, Skokan LA. A cognitive model of religion's influence on health. Journal of Social Issues. 1995;51(2):49–64. [Google Scholar]
- Fetzer Institute. Multidimensional measurement of religiousness/spirituality for use in health research. A report of the Fetzer Institute/National Institute on Aging Working Group. 1999 [Google Scholar]
- Fitchett G, Murphy PE, Kim J, Gibbons JL, Cameron JR, Davis JA. Religious Struggle: Prevalence, Correlates and Mental Health Risks in Diabetic, Congestive Heart Failure, and Oncology Patients. International Journal of Psychiatry in Medicine. 2004;34(2):179–196. doi: 10.2190/UCJ9-DP4M-9C0X-835M. [DOI] [PubMed] [Google Scholar]
- Fitchett G, Rybarczyk BD, DeMarco GA, Nicholas JJ. The role of religion in medical rehabilitation outcomes: A longitudinal study. Rehabilitation Psychology. 1999;44(4):333. [Google Scholar]
- Friedlander Y, Kark JD, Stein Y. Religious observance and plasma lipids and lipoproteins among 17-year-old Jewish residents of Jerusalem. Preventive medicine. 1987;16(1):70–79. doi: 10.1016/0091-7435(87)90007-7. [DOI] [PubMed] [Google Scholar]
- Gallagher-Thompson D, Shurgot GR, Rider K, Gray HL, McKibbin CL, Kraemer HC, Thompson LW. Ethnicity, stress, and cortisol function in Hispanic and non-Hispanic white women: A preliminary study of family dementia caregivers and noncaregivers. The American journal of geriatric psychiatry. 2006;14(4):334–342. doi: 10.1097/01.JGP.0000206485.73618.87. [DOI] [PubMed] [Google Scholar]
- Grøntved A, Hu FB. Television viewing and risk of type 2 diabetes, cardiovascular disease, and all-cause mortality: a meta-analysis. Journal of Amercian Medicine. 2011;305(23):2448–2455. doi: 10.1001/jama.2011.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar MR, Wewerka S, Frenn K, Long JD, Griggs C. Developmental changes in hypothalamus-pituitary-adrenal activity over the transition to adolescence: Normative changes and associations with puberty. Development and Psychopathology. 2009;21(1):69–85. doi: 10.1017/S0954579409000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He F, Nowson C, Lucas M, MacGregor G. Increased consumption of fruit and vegetables is related to a reduced risk of coronary heart disease: meta-analysis of cohort studies. Journal of human hypertension. 2007;21(9):717–728. doi: 10.1038/sj.jhh.1002212. [DOI] [PubMed] [Google Scholar]
- Iannaccone LR. Introduction to the Economics of Religion. Journal of economic literature. 1998;36(3):1465–1495. [Google Scholar]
- Ironson G, Balbin E, Schneiderman N, Boll TJ, Johnson SB, Perry NW, Jr, Rozensky RH. Handbook of clinical health psychology: Volume 1. Medical disorders and behavioral applications. Washington, DC, US: American Psychological Association; 2002. Health psychology and infectious diseases; pp. 5–36. [Google Scholar]
- Ironson G, Solomon GF, Balbin EG, O’Cleirigh C, George A, Kumar M, Woods TE. The Ironson-Woods Spirituality/Religiousness Index is associated with long survival, health behaviors, less distress, and low cortisol in people with HIV/AIDS. Annals of Behavioral Medicine. 2002;24(1):34–48. doi: 10.1207/S15324796ABM2401_05. [DOI] [PubMed] [Google Scholar]
- Koenig HG, Pargament KI, Nielsen J. Religious coping and health status in medically ill hospitalized older adults. The Journal of Nervous and Mental Disease. 1998;186(9):513–521. doi: 10.1097/00005053-199809000-00001. [DOI] [PubMed] [Google Scholar]
- Kumari M, Shipley M, Stafford M, Kivimaki M. Association of diurnal patterns in salivary cortisol with all-cause and cardiovascular mortality: findings from the Whitehall II study. The Journal of Clinical Endocrinology & Metabolism. 2011;96(5):1478–1485. doi: 10.1210/jc.2010-2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BY, Newberg AB. Religion and health: a review and critical analysis. Zygon. 2005;40(2):443–468. [Google Scholar]
- Lutgendorf SK, Russell D, Ullrich P, Harris TB, Wallace R. Religious participation, interleukin-6, and mortality in older adults. Health Psychology. 2004;23(5):465. doi: 10.1037/0278-6133.23.5.465. [DOI] [PubMed] [Google Scholar]
- Magyar-Russell G, Brown IT, Edara IR, Smith MT, Marine JE, Ziegelstein RC. In Search of Serenity: Religious Struggle Among Patients Hospitalized for Suspected Acute Coronary Syndrome. Journal of religion and health. 2014;53(2):562–578. doi: 10.1007/s10943-013-9713-2. [DOI] [PubMed] [Google Scholar]
- Newport F. Americans’ church attendance inches up in 2010. Gallup. 2010 Jun;25 [Google Scholar]
- Pargament K, Feuille M, Burdzy D. The Brief RCOPE: Current Psychometric Status of a Short Measure of Religious Coping. Religions. 2011;2(1):51–76. [Google Scholar]
- Pargament KI. Religious methods of coping: Resources for the conservation and transformation of significance. In: Shafranske EP, editor. Religion and the clincial practice of psychology. Washington, DC: American Psychological Association; 1996. pp. 215–239. [Google Scholar]
- Pargament KI. The psychology of religion and coping: Theory, research, practice. Guilford Press; 2001. [Google Scholar]
- Pargament KI, Koenig HG, Tarakeshwar N, Hahn J. Religious struggle as a predictor of mortality among medically ill elderly patients: a 2-year longitudinal study. Archives of Internal Medicine. 2001;161(15):1881–1885. doi: 10.1001/archinte.161.15.1881. [DOI] [PubMed] [Google Scholar]
- Pargament KI, Murray-Swank NA, Magyar GM, Ano GG. Spiritual struggle: A phenomenon of interest to psychology and religion. In: Miller WR, Delaney HD, editors. Judeo-Christian perspectives on psychology: Human nature, motivation, and change. Washington, DC: American Psychological Association; 2005. pp. 245–268. [Google Scholar]
- Pargament KI, Smith BW, Koenig HG, Perez L. Patterns of positive and negative religious coping with major life stressors. Journal for the scientific study of religion. 1998:710–724. [Google Scholar]
- Polk DE, Cohen S, Doyle WJ, Skoner DP, Kirschbaum C. State and trait affect as predictors of salivary cortisol in healthy adults. Psychoneuroendocrinology. 2005;30:261–272. doi: 10.1016/j.psyneuen.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Powell LH, Shahabi L, Thoresen CE. Religion and spirituality: Linkages to physical health. American Psychologist. 2003;58(1):36. doi: 10.1037/0003-066x.58.1.36. [DOI] [PubMed] [Google Scholar]
- Ramirez SP, Macêdo DS, Sales PMG, Figueiredo SM, Daher EF, Araújo SM, Carvalho AF. The relationship between religious coping, psychological distress and quality of life in hemodialysis patients. Journal of psychosomatic research. 2012;72(2):129–135. doi: 10.1016/j.jpsychores.2011.11.012. [DOI] [PubMed] [Google Scholar]
- Raudenbush SW, Bryk AS, Congdon R. HLM 7: Hierarchical linear and nonlinear modeling. Lincolnwood, IL: Scientific Software International; 2013. [Google Scholar]
- Robles TF, Slatcher RB, Trombello JM, McGinn MM. Marital quality and health: A meta-analytic review. Psychological Bulletin. 2014;140(1):140. doi: 10.1037/a0031859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selig JP, Preacher KJ. Mediation models for longitudinal data in developmental research. Research in Human Development. 2009;6(2–3):144–164. [Google Scholar]
- Sephton SE, Koopman C, Schaal M, Thoresen C, Spiegel D. Spiritual Expression and Immune Status in Women with Metastatic Breast Cancer: An Exploratory Study. The Breast Journal. 2001;7(5):345–353. doi: 10.1046/j.1524-4741.2001.20014.x. [DOI] [PubMed] [Google Scholar]
- Sephton SE, Sapolsky RM, Kraemer HC, Spiegel D. Diurnal Cortisol Rhythm as a Predictor of Breast Cancer Survival. Journal of the National Cancer Institute. 2000;92(12):994–1000. doi: 10.1093/jnci/92.12.994. [DOI] [PubMed] [Google Scholar]
- Sherman AC, Simonton S, Latif U, Spohn R, Tricot G. Religious struggle and religious comfort in response to illness: Health outcomes among stem cell transplant patients. Journal of Behavioral Medicine. 2005;28:359–367. doi: 10.1007/s10865-005-9006-7. [DOI] [PubMed] [Google Scholar]
- Taylor EJ. Spirituality, culture, and cancer care. Seminars in oncology nursing. 2001;17(3):197–205. doi: 10.1053/sonu.2001.25949. [DOI] [PubMed] [Google Scholar]
- Tomiyama AJ, O'Donovan A, Lin J, Puterman E, Lazaro A, Chan J, Blackburn E. Does cellular aging relate to patterns of allostasis?: An examination of basal and stress reactive HPA axis activity and telomere length. Physiology & behavior. 2012;106(1):40–45. doi: 10.1016/j.physbeh.2011.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walen HR, Lachman ME. Social Support and Strain from Partner, Family, and Friends: Costs and Benefits for Men and Women in Adulthood. Journal of Social and Personal Relationships. 2000;17(1):5–30. [Google Scholar]