Abstract
Solid organ and allogeneic hematopoietic cell transplantation have become standard therapeutic interventions that save patient lives and improve quality of life. Our enhanced understanding of transplantation immunobiology has refined clinical management and improved outcomes. However, organ rejection and graft-versus-host disease remain major obstacles to the broader successful application of these therapeutic procedures. Notch signaling regulates multiple aspects of adaptive and innate immunity. Preclinical studies identified Notch signaling as a promising target in autoimmune diseases, as well as after allogeneic hematopoietic cell and solid organ transplantation. Notch was found to be a central regulator of alloreactivity across clinically relevant models of transplantation. Notch inhibition in T cells prevented graft-versus-host disease and organ rejection, establishing organ tolerance by skewing CD4+ T helper polarization away from a proinflammatory response towards suppressive regulatory T cells. Notch ligand blockade also dampened alloantibody deposition and prevented chronic rejection through humoral mechanisms. Toxicities of systemic Notch blockade were observed with γ-secretase inhibitors in preclinical and early clinical trials across different indications, but they did not arise upon preclinical targeting of Delta-like Notch ligands, a strategy sufficient to confer full benefits of Notch ablation in T cell alloimmunity. As multiple clinical grade reagents have been developed to target individual Notch ligands and receptors, the benefits of Notch blockade in transplantation are calling for translation of preclinical findings into human transplantation medicine.
Introduction
Since early successes of transplantation medicine in the mid-twentieth century,1,2 solid organ and hematopoietic cell transplantation have become mainstream therapeutic interventions. Almost 150,000 allogeneic transplants are performed annually in the world, including more than 115,000 solid organ3 (Global Observatory on Donation and Transplantation, produced by the World Health Organization and The Spanish National Transplant Organization collaboration, http://www.transplant-observatory.org/Pages/Data-Reports.aspx) and ~30,000 allogeneic hematopoietic cell transplants (allo-HCT).4 Our understanding of transplantation medicine has improved dramatically since initial concepts were first laid out. However, a major hindrance to a broader and more successful application of the procedures in their respective fields remains the lack of improved therapeutic options for transplant-related immune complications: rejection after solid organ transplantation, and graft-versus-host disease (GVHD) after allo-HCT. While broad immunosuppression remains a major strategy for controlling transplant complications, its efficacy remains limited. Current approaches lead to increased rates of opportunistic infections. In addition, they contribute to significant end-organ toxicities, such as kidney injury from chronic use of calcineurin inhibitors. Novel strategies of immunomodulation are necessary to harness the full therapeutic benefits of transplant procedures. Here, we review preclinical evidence identifying a role for Notch signaling in adaptive immune responses that defines the outcome of allo-HCT and solid organ transplantation, and we propose to consider Notch as a new therapeutic target in this area of unmet clinical need.
The Notch signaling pathway
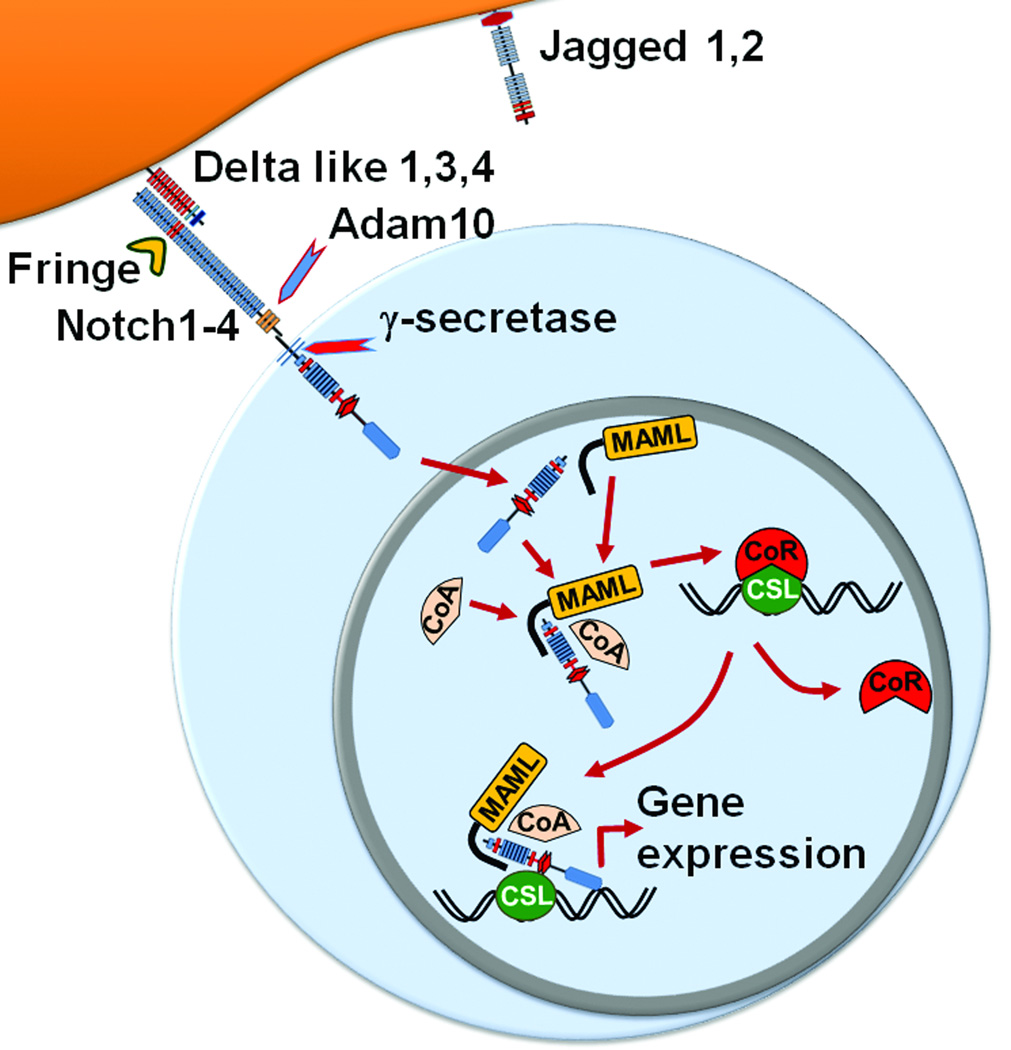
Notch is a highly conserved cell-to-cell surface signaling pathway with pleiotropic roles in multiple developmental processes, tissue homeostasis and disease.5–7 In mammals, Notch signaling is initiated by the interaction of Notch receptors (Notch1–4) with ligands of the Jagged (Jagged1–2) and Delta-like families (Dll1, Dll3 and Dll4) (Figure 1). Extensive O-fucose glycosylation of the multiple EGF-like repeats on the extracellular domain of Notch receptors is necessary for ligand recognition and thus for receptor function.8,9 Receptors are further modulated by the activity of Fringe family glycosyltransferases, which regulate their binding affinity to individual Notch ligands.10–12 Notch receptor-ligand interactions generate a physical force that unmasks an extracellular domain proteolytic site, allowing for cleavage by a disintegrin and metalloproteinase (ADAM10).13–15 This process rapidly leads to proteolysis within the transmembrane domain by the γ-secretase complex, releasing intracellular Notch (ICN).16,17 ICN translocates to the nucleus where it binds CSL/RBP-Jk (CBF-1, Suppressor of hairless, Lag-1; also called RBP-Jk and encoded by the Rbpj gene).18 ICN acts as a transcriptional activator that interacts with CSL/RBP-Jk and recruits a Mastermind-like family transcriptional coactivator (MAML), leading to target gene activation.19–22 To ensure tight Notch regulation and short-lived signals, the C-terminal PEST domain of ICN and other mechanisms target active Notch for rapid degradation.23–25 Altogether, Notch signaling connects cell surface signals induced by multiple Notch ligands and receptors to a common pathway of “canonical” transcriptional activation mediated by ICN, CSL and MAML at the core of a large multiprotein complex.
Figure 1. The Notch signaling pathway.
Four Notch receptors (Notch1–4) and 5 Notch ligands of the Jagged (Jagged1–2) and Delta-like (Dll1,3,4) families have been identified in mammals. Notch receptors are modified by Fringe glycosyltransferase, which modulates their interaction with Notch ligands. Receptor-ligand binding leads to extracellular cleavage of the receptor by the ADAM10 metalloprotease, followed by the intramembrane proteolysis via γ-secretase. Cleaved intracellular Notch (ICN) translocates into the nucleus. ICN associates with CSL (CBF1/Suppressor of Hairless/LAG-1; also known as RBPJ), a Mastermind-like (MAML) family coactivator, and other transcriptional co-activators (CoA) to displace co-repressors (CoR) and build a large multiprotein complex that activates target gene transcription.
Notch signaling in adaptive immunity
In the adaptive immune response, highly differentiated cellular elements arise after antigenic exposure in peripheral lymphoid organs. Adaptive immunity provides targeted and long-lasting protection, while memory formation facilitates rapid and focused responses upon subsequent antigen encounters. Antigen processing and presentation by professional antigen-presenting cells (APCs) to a limited number of mature T cells is the initial step in formation of adaptive immunity. The final T cell differentiation and effector response depend on the nature of the antigen and on the local priming environment, with regulatory impact from cytokine signals. Moreover, the field has been probing other inputs, including from the Notch signaling pathway, to account for the full spectrum of immune diversity and helper T cell (Th) plasticity.26
Notch has long been recognized to play a key role in early T cell development.27,28 In addition, immunologists have pursued investigations into Notch’s role in multiple aspects of adaptive immunity. Thus, a multifaceted impact of Notch signaling on adaptive immunity has surfaced over the past decade, including identification of its enabling role in many arms of Th polarization (including Th1,29–33,34 Th2,30,35–39 Th9,40 and Th1741,42); its effects in terminal effector vs. memory precursor CD8+ T cell differentiation43–46; its effects on regulatory T cell function47; and its synergy with B cell receptor signaling to promote class switching and terminal B cell differentiation and antibody production.48–50 How Notch mediates such a broad range of immune effects is progressively being elucidated.
Initial studies of Notch regulation in adaptive T cell responses reported upregulated expression of Delta-like but not Jagged ligands in APCs cultured in a Th1 polarizing environment.30 In vitro studies identified Notch signaling as a positive regulator of Th1 differentiation, with key inputs from TLR-Myd88 signaling.30,51–53 Notch1 and Notch2 receptors were identified as critical for Th1 polarization in some but not all models of Th1 differentiation.33 Forced expression of ICN or Dll ligands, or CD4+ T cell exposure to Dll1-Fc fusion protein promoted Th1 differentiation,29–32,53 which was inhibited by γ-secretase inhibitors (GSI).31 Notch was proposed to directly regulate Tbx21, encoding the master Th1 transcription factor Tbet.31 In this study, Notch1 was shown to directly bind to the Tbx21 locus and systemic Notch blockade with GSI alleviated experimental autoimmune encephalomyelitis (EAE), a mouse model of multiple sclerosis driven in part by pathogenic Th1 responses. A subsequent study, relying on Notch blockade via GSI and genetic pan-Notch inhibition with dominant negative MAML (DNMAML) expression also reported Tbx21 as a direct target of Notch signaling in addition to linking Notch to direct regulation of Ifng transcription.34 Other in vitro and in vivo studies, however, did not document Tbx21 regulation by canonical Notch signaling,54,55 despite the fact that in many cases Notch inhibition decreased IFN-γ production through other mechanisms.35,37,54–57 In addition, noncanonical effects of Notch1/2 receptors may exist in the regulation of IFN-γ production, although the significance of this finding remains unclear.33 How exactly Notch regulates Th1 responses remains to be fully clarified and could be context-dependent, with the bulk of current evidence supporting its role in Th1 function, rather than initial polarization.
Th2 responses, which are critical for parasite immunity and allergic responses, have also been studied for their regulation by Notch signaling. The initial observations of Th2 impairment upon Dll-driven Notch signaling30 have been further expanded to identify a key role for Notch signaling in development of Th2 responses in vivo, in part through direct regulation of Il4 and Gata3.35,36 In Th2 disease models, including infectious diseases, genetic Notch inhibition (Notch1/2 inactivation or DNMAML expression) and pharmacological interventions led to failure of Th2 polarization, reducing disease symptoms (asthma),38,39 or impairing protective Th2 responses (Trichuris muris).35 Conversely, forced Notch stimulation via Jagged, but not Dll ligands supported Th2 development.30,38,53,58
Regulatory T cells (Tregs) are major modulators of immune responses and maintain peripheral tolerance. Treg dysregulation and/or loss are associated with multiple disease states, in particular autoimmunity.59 While multiple studies have probed the effects of Notch on Treg homeostasis using in vitro assays and gain-of-function strategies, key information was obtained in recent studies using loss-of-function approaches in vivo. Charbonnier et al47 documented a direct antagonistic role of active Notch on Treg stability and function. Selective Notch inhibition only in Tregs allowed for emergence of a “super-regulatory” phenotype which was sufficient for long term protection in a GVHD model, whereas forced Notch activation impaired intrinsic Treg function. In addition, Notch overexpression decreased Treg suppressive capacity in a colitis model. While the destabilized and Th1 skewed Treg phenotype was predominantly the result of canonical Notch signaling, non-canonical Rictor-mediated effects contributed to the phenotype as well.47 The role of Notch signaling induced by Dll1/4 ligands in Treg homeostasis has been probed in various disease models, including experimental autoimmune encephalomyelitis and type 1 diabetes, supporting a role of Dll4-mediated Notch signals in decreasing Treg numbers and antagonizing their function.58,60,61 Given these emerging data, it is unclear how to interpret the positive regulatory role of Notch in Treg homeostasis that had been previously suggested, and it is possible that these findings were contextual or that they were artefacts of gain-of-function approaches.62–67
Notch ligand engagement was documented to impact cytotoxic CD8+ T cell differentiation, with Dll1 ligand enhancing Granzyme B production and cytotoxic effector responses.43,44,68 Notch was reported to regulate Eomes, which is required for the stability of CD8+ T cell memory and controls the expression of prototypic effector molecules such as IFN-γ and Granzyme B.69 Notch has been increasingly studied in adaptive CD8+ T cell immunity as well. In infectious disease models (Listeria monocytogenes, Influenza), Notch activity was critical to ensure short-lived effector CD8+ T cell differentiation, in part due to direct regulation of Il2ra expression.45,46 Upon Notch blockade, the acquisition of effector molecules (IFN-γ, Granzyme B, and Perforin) and the ultimate functional impact was variably affected in different models.45,46 Data on the role of Notch signaling in CD8+ memory responses are more limited, however Notch appeared to favor effector CD8+ T cell responses over memory formation.46
While the proposed impact of Notch signaling in immunity is broad, the exact mechanisms of Notch-mediated effects remain uncertain. Moreover, Notch signaling inputs, when it comes to ligand sources and precise receptor-ligand interactions still remain uncertain. A possible explanation for the versatility of Notch effects in Th differentiation has been proposed by Bailis et al.34 This study identified a facilitating Notch effect on Th polarization that rested upon its ability to simultaneously target multiple transcriptional Th programs, while sensitizing T cells to exogenous cytokines.34 Based on this model, Notch only enhanced Th lineage commitment defined by environmental cues, but did not act as lineage-defining switch. Further evidence supporting this facilitator role in adaptive immunity, focusing on the very early events after antigen exposure, was recently brought forward by Laky et al.70 In this study, antigen-specific responses were probed using transgenic T cells recognizing model antigens. A key facilitator role for active Dll4-driven Notch signaling emerged in shaping of CD4+ T cell priming, where Notch worked in concert with CD28-driven co-stimulation, augmenting the effects of the PI3K/Akt pathway.70 Notch enhanced T cell activation and proliferation, IL-2 production, and optimized the metabolic machinery, ultimately providing enhanced adaptive immune response. Altogether, these effects were reminiscent of costimulatory signals, suggesting that Notch may function as a new type of costimulatory modulator. In addition to early regulation of antigen-specific responses, metabolic Notch signature was also reported recently to sustain memory CD4+ T cells.71
In addition to T cell responses, Notch contributes to B cell immunity. Notch was reported to regulate the development of marginal zone B cells in the spleen in a dosage-specific manner,72–75 with recent data showing that continuous Notch signals are also required for the maintenance of these cells.76 In addition, Notch was described to enhance follicular B cell activation and differentiation into antibody-producing cells.48 Notch signaling was reported to induce B cell differentiation and class switching,49 and in synergy with BAFF signaling promote survival of germinal center B cells via Jagged1-Notch1/2 interactions.77
Beyond adaptive immunity, functions of Notch signaling are also being discovered in the regulation of myeloid cell subsets (including macrophages and dendritic cells), as well as innate lymphoid cells.78–87 The mechanisms and significance of these effects remain to be fully explored. Notch signaling can also play a role in the homeostasis of adult organs, as well as in tissue repair. For example, canonical Notch signaling is dispensable for the maintenance of adult hematopoietic stem cells,88,89 but Notch2 was reported to play a role in the speed of hematopoietic regeneration after transplantation.89 In the intestine, a major target organ of GVHD, Notch signaling promotes differentiation into absorptive enterocytes and regulates intestinal stem cell homeostasis, while supporting epithelial regeneration after injury.90–92 Whether these effects and other potential functions of Notch signaling in tissue repair are relevant in the context of allogeneic transplantation remains to be fully investigated. So far, investigations into the immune functions of Notch signaling during transplantation have produced evidence supporting its consideration as a potential therapeutic target (Figure 2). Furthermore, targeting individual Notch ligands and receptors has emerged as a strategy to avoid the side effects of systemic pan-Notch inhibition, preserving intestinal and hematopoietic homeostasis and thus offering a therapeutic window.57 Here, we will outline findings about the role of Notch signaling in allogeneic hematopoietic cell and solid organ transplantation, and propose new perspectives highlighting common features of Notch signaling in alloimmunity.
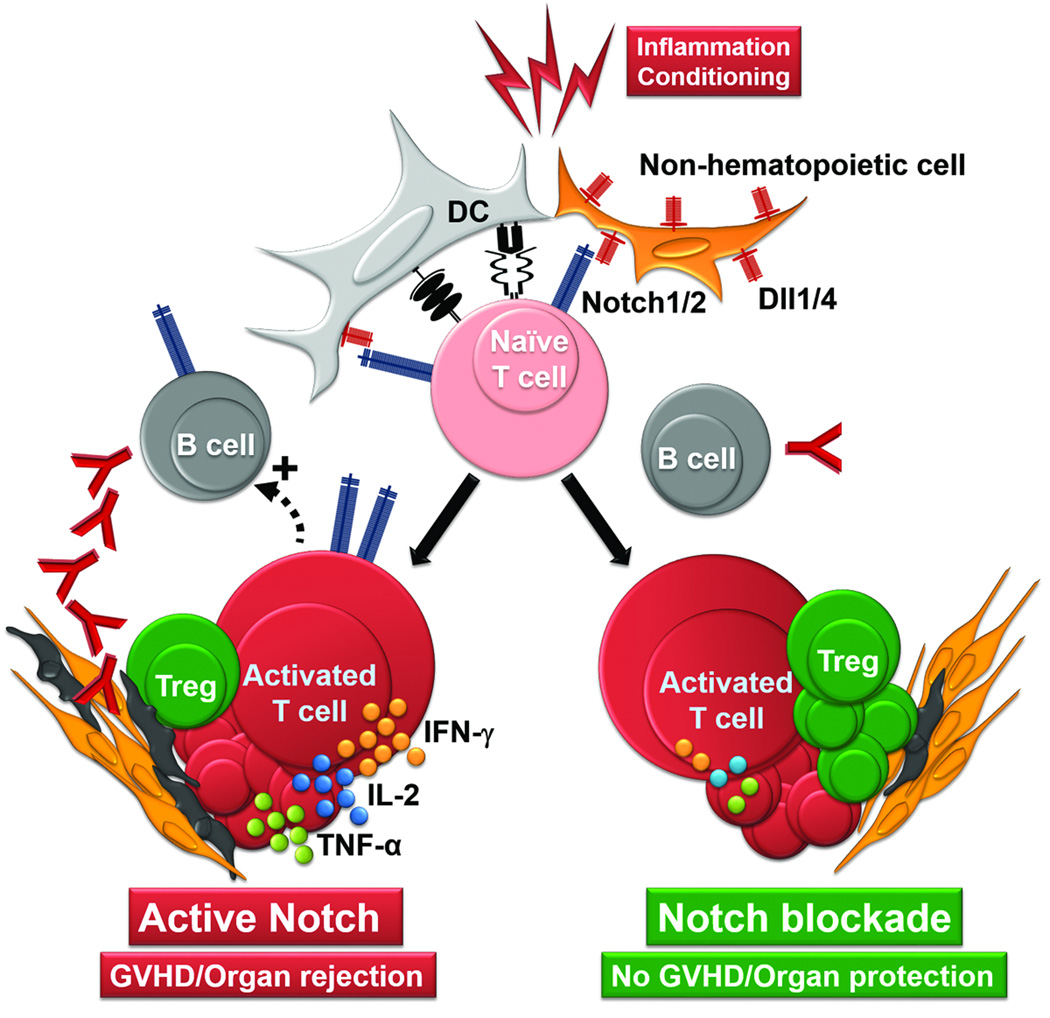
Figure 2. Effects of Notch signaling in the alloresponse.
Inflammation and use of conditioning regimens in allo-HCT activate dendritic cells (DC) and nonhematopoietic cells, which express Dll1 and Dll4 Notch ligands. In concert with TCR and costimulatory molecules, Notch activates naïve T cells, leading to proliferation, Th polarization, proinflammatory cytokine production, and acquisition of effector functions, thus promoting GVHD or solid organ rejection (left). In addition, T cell help and Notch engagement in B cells facilitate alloreactive antibody production, contributing to tissue deposition and enhanced alloimmune tissue injury. Upon genetic or biochemical inhibition of Notch signaling (right), cytokine production is decreased and Treg expansion enhanced, despite preserved overall T cell activation and proliferation. Decreased antibody production by B cells adds to an environment of enhanced tolerance characterized by diminished GVHD and long term solid organ transplant survival.
Notch signaling in alloimmunity
Notch in graft-versus-host disease
Mouse models of allo-HCT were instrumental in defining the critical role that Notch signaling plays in the development of alloimmune injury during GVHD, a major hindrance to the most widely used immunotherapeutic strategy in human medicine. After allo-HCT, transplanted naïve donor T cells recognize host antigens presented by APCs activated by conditioning injury and its cytokine storm.93,94 Additional co-stimulatory cues lead to T cell activation, proliferation, and acquisition of effector functions. Activated T cells exert anti-tumor responses, a major therapeutic benefit of allo-HCT. However, alloantigen recognition also leads to host tissue injury in the form of GVHD, blunting the therapeutic efficacy of the procedure. The negative impact of GVHD is related not only to the severe morbidity and mortality of GVHD itself, but also to the need for global immunosuppression that increases risks of disease relapse, causes end-organ damage, and dramatically increases the odds of severe opportunistic infections in vulnerable hosts.93,94
Using multiple mouse models of allo-HCT, our group documented GVHD protective effects of genetic strategies to block Notch activity in mature T cells.55–57 In these studies spanning multiple MHC-mismatched and matched models of allo-HCT, recipients of Notch-deprived T cells (via DNMAML expression vs. Rbpj or Notch1/2 inactivation) experienced long-term GVHD-free survival.55–57 When compared to wild-type T cells, Notch-deprived T cells showed profound defects in multiple cytokines (including IFN-γ, TNF-α, IL-2, IL-17 and IL-4), but preserved Tbx21 and enhanced Foxp3 expression. Notch inhibition only had a minor impact on overall proliferation of allogeneic T cells in vivo, but it significantly enhanced Treg expansion. Most importantly, Notch deprivation preserved graft-vs-tumor effects, as DNMAML T cells exerted potent antitumor activity against A20 host-type lymphoma.56,57 Other studies in alternate models of mouse allo-HCT replicated the GVHD-protective benefits of Notch ablation. Protective effects of Notch inhibition were also reported in a mouse model of aplastic anemia induced by an allogeneic response.95 Sandy et al55 showed that Notch-deprived allogeneic T cells acquired impaired Ras/MAPK and NFκB activity and expressed multiple negative regulators of T cell activation. Subsequent studies dissected the role of individual Notch receptors and ligands to facilitate therapeutic targeting of Notch signaling in GVHD.57,96 Using humanized neutralizing antibodies to individual Notch ligands and receptors as well as genetic strategies, Tran et al identified key functions for Notch1 and Notch2 as well as Dll1 and Dll4 Notch ligands in the pathogenic functions of Notch signaling during acute GVHD.57 The Notch1-Dll4 axis was identified as dominant. Pan-Notch inhibition, either via use of GSI or combined Notch1/2 receptor blockade with neutralizing antibodies, however, was accompanied by severe intestinal damage. This on-target toxicity of systemic pan-Notch antagonism was due to the impaired intestinal regeneration, with conditioning by total body irradiation exacerbating the milder phenotype seen with steady-state Notch1 blockade.97 Combined blockade of individual Dll1 and Dll4 ligands, however, avoided gastrointestinal side effects and led to long-lasting GVHD protection, with immunophenotypic T cell changes mimicking the effects seen with genetic Notch ablation. Simultaneously, Notch blockade via Dll1/4 targeting had no adverse impact on hematopoietic reconstitution, while anti-tumor efficacy remained potent.57 Thus, Dll1/4 blockade in the peri-transplant period emerged as a promising new therapeutic approach to prevent GVHD.
Sandy et al documented that enhanced Treg recovery after Notch blockade was due to the expansion of preexisting donor Tregs, although it remained unclear if these expanded Tregs were important to mediate the protective effects of Notch inhibition after allo-HCT.55 The role for Tregs in GVHD-mediated pathology has been well documented across multiple mouse models, in which adoptive Treg therapies mitigate, and Treg depletion aggravates GVHD.98 Tregs are major effectors of emerging GVHD preventative strategies,99 and their depletion is well described during both acute and chronic GVHD.100–102 A recent study by Charbonnier et al.47 provided additional insights into Notch regulation of peri-transplant Treg homeostasis. In their study, using a MHC-mismatched allo-HCT model, the authors identified a critical inhibitory role of active Notch signaling on posttransplant Treg homeostasis. Despite unperturbed Notch signaling in conventional T cells, conditional Notch ablation in Tregs alone was identified as sufficient to provide GVHD protection. Proposed mechanisms included induction of an enhanced suppressive phenotype with increased stability in the inflammatory environment.47 Interestingly, a dominant role of Notch-inhibited Tregs was not observed in past work using DNMAML-mediated pan-Notch inhibition, since co-transplantation of Notch-sufficient and Notch-deficient T cells failed to control GVHD.56 The precise role of Notch signaling in posttransplant Tregs vs. conventional T cells thus remains to be clarified. Nevertheless, existing observations all highlight the robust therapeutic potential of Notch inhibition in acute GVHD.
Recent evidence suggesting a role for hematopoietic APCs in providing Notch ligands driving alloreactivity were provided by Mochizuki and collaborators.96 This study identified a subset of host-derived Dll4-expressing dendritic cells (DCs) in recipient animals. The same group recently identified a putative human counterpart of mouse Dll4-expressing inflammatory DCs.103 In both settings, these cells were found early after transplant (week 3 onwards in humans) and promoted in vitro Th1 and Th17 polarization more effectively than naïve DCs. While these data were suggestive of a role for professional APCs in driving Notch signaling in allogeneic T cells, it is difficult to confirm directly the importance of these cells as a source of Notch ligands in vivo. Using genetic approaches allowing for Dll1/4 inactivation in specific compartments and complementary biochemical strategies, our group demonstrated that both host and donor hematopoietic APCs were dispensable sources of Notch ligands to drive GVHD.104,105 Indeed, transplant recipients lacking Dll1/4 within the hematopoietic system were not protected from GVHD and remained susceptible to systemic Dll1/4 blockade, while nonhematopoietic elements106,107 emerged as key sources of Dll1/4. Moreover, Dll1/4 inactivation in fibroblastic reticular cells replicated all the benefits of pan-T cell and systemic Notch blockade.105 Thus, the cellular source of Dll1/4 in GVHD may overlap with recently identified fibroblastic niches in secondary lymphoid organs that provide critical differentiation inputs to marginal zone B cells, subsets of Notch-dependent DCs and follicular helper T cells.108
Our studies in multiple models of acute GVHD identified a narrow timing of posttransplant Notch activity that was essential for GVHD initiation. We found that Notch blockade conferred protective benefits when pursued within the 2-day immediate posttransplant period (105 and unpublished). Together with the fact that hematopoietic cells are dispensable as a source of Notch ligands in GVHD,96,103–105 this finding provides an impetus to study the regulation of Notch ligand expression in nonhematopoietic cells, and their early interactions with incoming T cells. Mechanistically, another important line of investigation will be to identify the relevant transcriptional targets responsible for the broad immune effects of Notch signaling in allogeneic T cells, as well as in other immune contexts. The importance of putative non-canonical effects of Notch signaling remains unclear, given that the full functional benefits of Notch blockade are observed upon interference with the Notch transcriptional complex.55–57 Altogether, it is unlikely that non-canonical Notch effects play a major role in alloreactivity, as all major effects of Notch inhibition were observed upon interference with the ICN-CSL-MAML canonical transcriptional activation complex.55–57
Published work identified active Notch as a master regulator of acute GVHD in mouse models of allo-HCT. Recent evidence of active Notch signaling in pathogenic T cells mediating GVHD in Rhesus macaques109 adds to the growing knowledge supporting a translational potential for therapeutic Notch inhibition after allo-HCT. Whether there is a role for Notch ligands other than Dll1/4 in GVHD, and if active Notch contributes to posttransplant events beyond acute GVHD, remains to be seen. Chronic GVHD is another major cause of late morbidity and mortality after allo-HCT that is characterized by multi-faceted immune dysfunction. Virtually all arms of Th immunity, humoral immunity, innate immune activation, as well as tissue responses, have been described to contribute to chronic GVHD pathogenesis.110–114 Given that a footprint of Notch activity has been identified to function in all these elements, future studies will help define the effects of Notch signaling in this area of unmet clinical need.
Notch in solid organ rejection
Acute cellular and chronic humoral rejection remain a major hindrance to the broader successful application of solid organ transplantation. If untreated, they can cause life-threatening organ failure that may require repeat organ transplantation into vulnerable patients. Treatment options bring limited success and are associated with high rates of opportunistic infections and can cause significant organ damage, both major contributors to the increased morbidity and mortality, and possible transplant failure. Key immunological events in acute and chronic rejection can be compared to the pathogenesis of acute and chronic GVHD, respectively. The role of Notch signaling in solid organ rejection was probed in mouse models of heterotopic heart allograft transplantation. Early findings in this field relied on artificial gain-of-function approaches and paradoxically suggested a role of Notch signaling as an inducer of transplant tolerance.64,68,115,116 However, recent observations using in vivo loss-of-function approaches have uncovered a proinflammatory effect of Notch signals in vivo that bears many similarities to its effects in GVHD.117,118
To test the role of Notch in solid organ rejection, Riella et al117 used the BALB/c→C57BL/6 MHC-mismatched model of heart transplantation. The authors identified upregulation of Dll1 in professional hematopoietic cells and targeted Dll1 systemically with neutralizing antibodies. Dll1 inhibition, particularly when combined with co-stimulation blockade (use of CTLA4-Ig or Cd28 knockouts), significantly prolonged allograft survival. The effects of Dll1 blockade were associated with Th skewing towards Th2 responses and decreased cytotoxic T cells, and they were dependent upon intact IL-4 production and STAT6 signaling.
Our group applied strategies of Notch blockade that had shown benefit in models of allo-HCT.56,57 In the same BALB/c→C57BL/6 model used by Riella et al,117 genetic pan-Notch inhibition in mature T cells significantly prolonged heart allograft survival, an effect significantly enhanced by peri-transplant CD8+ T cell depletion.118 In Notch-deprived recipients, a dramatic decrease of graft-infiltrating effector T cells, and in situ, but not systemic Treg expansion was noted. We tested the individual contributions of Dll1 and Dll4 ligands. Individual Dll1 or Dll4 neutralization via peri-transplant antibody-mediated blockade induced protective effects indistinguishable from those seen with genetic pan-Notch inhibition in T cells. Dual blockade conferred enhanced benefit and improved organ survival beyond that seen with genetic Notch ablation with DNMAML. Systemic Dll1/4 blockade conferred the same T cell modulating benefits by decreasing graft infiltration and skewing in situ T cell responses towards Tregs, but also significantly delayed humoral alloimmune responses. While DNMAML recipients still developed delayed organ rejection accompanied by donor-directed antibody generation and in situ complement deposition, this effect was profoundly inhibited with systemic Dll1/4 blockade and correlated with decreased plasmablast and germinal center formation.118 These effects suggested that Notch inhibition may exert beneficial effects both on cellular and humoral mechanisms of transplant rejection.
In solid organ transplant models, much less is known about cellular sources of Dll1/4 than in allo-HCT. However, our preliminary data suggest a role for fibroblastic reticular cells as a source of Notch ligands, as seen in GVHD (Maillard, unpublished). Whether the temporal requirement for active Notch signaling via Dll1/4 is as narrow as seen in allo-HCT, remains to be seen. Altogether, our studies established key role for Notch ligands Dll1/4 in mediating solid organ rejection via effects that extend beyond T cell modulation, thus opening the door to new translational strategies.
Conclusions
Notch plays an essential role in alloimmune responses driving GVHD and solid organ rejection (Figure 2), 2 complications that limit the success of transplantation medicine. Notch-driven alloreactivity is controlled by Dll1/4 Notch ligands provided through cellular sources that extend beyond conventional professional APCs. Therapeutic targeting of Notch signaling in mouse models of allo-HCT and solid organ transplantation provides durable benefits, contingent upon early peri-transplant intervention. The therapeutic potential of Notch blockade is substantial, with early and transient targeting allowing for long-term protection without additional immunosuppression in preclinical models. This could provide new strategies to pave the way towards transplantation medicine free of calcineurin inhibitors, a long desired goal in the field.
Abbreviations
- Allo-HCT
allogeneic hematopoietic cell transplantation
- APC
antigen-presenting cell
- CSL
CBF1/Suppressor of Hairless/Lag-1
- Dll
Delta-like ligand
- DC
dendritic cell
- DNMAML
dominant negative Mastermind-like
- GVHD
graft-versus-host disease
- HCT
hematopoietic cell transplantation
- ICN
intracellular Notch
- MAML
Mastermind-like family transcriptional coactivator
- Th
helper T cell
- EAE
experimental autoimmune encephalomyelitis
- Treg
regulatory T cell
References
- 1.Thomas ED, Lochte HL, Jr, Lu WC, Ferrebee JW. Intravenous infusion of bone marrow in patients receiving radiation and chemotherapy. N Engl J Med. 1957;257(11):491–496. doi: 10.1056/NEJM195709122571102. [DOI] [PubMed] [Google Scholar]
- 2.Murray JE, Merrill JP, Harrison JH, Wilson RE, Dammin GJ. Prolonged survival of human-kidney homografts by immunosuppressive drug therapy. N Engl J Med. 1963;268:1315–1323. doi: 10.1056/NEJM196306132682401. [DOI] [PubMed] [Google Scholar]
- 3.2013 Organ Donation and Transplantation Activities. [Accessed 3/6/2015];2015 http://www.transplant-observatory.org/Pages/Data-Reports.aspx. [Google Scholar]
- 4.Niederwieser D, Baldomero H. Global Perspectives of Hematopoetic Stem Cell Transplantation including Macroeconomics; Paper presented at: 3. WBMT Workshop and 3. Scientific Symposium; November 14–15, 2014; Cape Town, South Africa. [Google Scholar]
- 5.Artavanis-Tsakonas S, Muskavitch MA, Yedvobnick B. Molecular cloning of Notch, a locus affecting neurogenesis in Drosophila melanogaster. Proc Natl Acad Sci U S A. 1983;80(7):1977–1981. doi: 10.1073/pnas.80.7.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kopan R, Ilagan MX. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell. 2009;137(2):216–233. doi: 10.1016/j.cell.2009.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Louvi A, Artavanis-Tsakonas S. Notch and disease: A growing field. Semin Cell Dev Biol. 2012;23(4):473–480. doi: 10.1016/j.semcdb.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yao D, Huang Y, Huang X, et al. Protein O-fucosyltransferase 1 (Pofut1) regulates lymphoid and myeloid homeostasis through modulation of Notch receptor ligand interactions. Blood. 2011;117(21):5652–5662. doi: 10.1182/blood-2010-12-326074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takeuchi H, Haltiwanger RS. Significance of glycosylation in Notch signaling. Biochemical and Biophysical Research Communications: in press. 2014 doi: 10.1016/j.bbrc.2014.05.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moloney DJ, Panin VM, Johnston SH, et al. Fringe is a glycosyltransferase that modifies Notch. Nature. 2000;406(6794):369–375. doi: 10.1038/35019000. [DOI] [PubMed] [Google Scholar]
- 11.Bruckner K, Perez L, Clausen H, Cohen S. Glycosyltransferase activity of Fringe modulates Notch-Delta interactions. Nature. 2000;406(6794):411–415. doi: 10.1038/35019075. [DOI] [PubMed] [Google Scholar]
- 12.Visan I, Yuan JS, Tan JB, Cretegny K, Guidos CJ. Regulation of intrathymic T-cell development by Lunatic Fringe- Notch1 interactions. Immunol Rev. 2006;209:76–94. doi: 10.1111/j.0105-2896.2006.00360.x. [DOI] [PubMed] [Google Scholar]
- 13.Gordon WR, Vardar-Ulu D, Histen G, Sanchez-Irizarry C, Aster JC, Blacklow SC. Structural basis for autoinhibition of Notch. Nat Struct Mol Biol. 2007;14(4):295–300. doi: 10.1038/nsmb1227. [DOI] [PubMed] [Google Scholar]
- 14.Tian L, Wu X, Chi C, Han M, Xu T, Zhuang Y. ADAM10 is essential for proteolytic activation of Notch during thymocyte development. Int Immunol. 2008;20(9):1181–1187. doi: 10.1093/intimm/dxn076. [DOI] [PubMed] [Google Scholar]
- 15.Gordon WR, Zimmerman B, He L, et al. Mechanical Allostery: Evidence for a Force Requirement in the Proteolytic Activation of Notch. Dev Cell. 2015;33(6):729–736. doi: 10.1016/j.devcel.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schroeter EH, Kisslinger JA, Kopan R. Notch-1 signalling requires ligand-induced proteolytic release of intracellular domain. Nature. 1998;393(6683):382–386. doi: 10.1038/30756. [DOI] [PubMed] [Google Scholar]
- 17.De Strooper B, Annaert W, Cupers P, et al. A presenilin-1-dependent -secretase-like protease mediates release of Notch intracellular domain. Nature. 1999;398:518–522. doi: 10.1038/19083. [DOI] [PubMed] [Google Scholar]
- 18.Honjo T. The shortest path from the surface to the nucleus: RBP-J kappa/Su(H) transcription factor. Genes Cells. 1996;1(1):1–9. doi: 10.1046/j.1365-2443.1996.10010.x. [DOI] [PubMed] [Google Scholar]
- 19.Wu L, Aster JC, Blacklow SC, Lake R, Artavanis-Tsakonas S, Griffin JD. MAML1, a human homologue of drosophila mastermind, is a transcriptional co-activator for NOTCH receptors. Nat Genet. 2000;26(4):484–489. doi: 10.1038/82644. [DOI] [PubMed] [Google Scholar]
- 20.Maillard I, Weng AP, Carpenter AC, et al. Mastermind critically regulates Notch-mediated lymphoid cell fate decisions. Blood. 2004;104(6):1696–1702. doi: 10.1182/blood-2004-02-0514. [DOI] [PubMed] [Google Scholar]
- 21.Nam Y, Sliz P, Song L, Aster JC, Blacklow SC. Structural basis for cooperativity in recruitment of MAML coactivators to Notch transcription complexes. Cell. 2006;124(5):973–983. doi: 10.1016/j.cell.2005.12.037. [DOI] [PubMed] [Google Scholar]
- 22.Oyama T, Harigaya K, Sasaki N, et al. Mastermind-like 1 (MamL1) and mastermind-like 3 (MamL3) are essential for Notch signaling in vivo. Development. 2011;138(23):5235–5246. doi: 10.1242/dev.062802. [DOI] [PubMed] [Google Scholar]
- 23.Weng AP, Ferrando AA, Lee W, et al. Activating mutations of NOTCH1 in human T cell acute lymphoblastic leukemia. Science. 2004;306(5694):269–271. doi: 10.1126/science.1102160. [DOI] [PubMed] [Google Scholar]
- 24.Fryer CJ, Lamar E, Turbachova I, Kintner C, Jones KA. Mastermind mediates chromatin-specific transcription and turnover of the Notch enhancer complex. Genes Dev. 2002;16(11):1397–1411. doi: 10.1101/gad.991602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fryer CJ, White JB, Jones KA. Mastermind recruits CycC:CDK8 to phosphorylate the Notch ICD and coordinate activation with turnover. Mol Cell. 2004;16(4):509–520. doi: 10.1016/j.molcel.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 26.Nakayamada S, Takahashi H, Kanno Y, O'Shea JJ. Helper T cell diversity and plasticity. Curr Opin Immunol. 2012;24(3):297–302. doi: 10.1016/j.coi.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Radtke F, Wilson A, Stark G, et al. Deficient T cell fate specification in mice with an induced inactivation of Notch1. Immunity. 1999;10(5):547–558. doi: 10.1016/s1074-7613(00)80054-0. [DOI] [PubMed] [Google Scholar]
- 28.Pui JC, Allman D, Xu L, et al. Notch1 expression in early lymphopoiesis influences B versus T lineage determination. Immunity. 1999;11(3):299–308. doi: 10.1016/s1074-7613(00)80105-3. [DOI] [PubMed] [Google Scholar]
- 29.Maekawa Y, Tsukumo S, Chiba S, et al. Delta1-Notch3 interactions bias the functional differentiation of activated CD4+ T cells. Immunity. 2003;19(4):549–559. doi: 10.1016/s1074-7613(03)00270-x. [DOI] [PubMed] [Google Scholar]
- 30.Amsen D, Blander JM, Lee GR, Tanigaki K, Honjo T, Flavell RA. Instruction of distinct CD4 T helper cell fates by different notch ligands on antigen-presenting cells. Cell. 2004;117(4):515–526. doi: 10.1016/s0092-8674(04)00451-9. [DOI] [PubMed] [Google Scholar]
- 31.Minter LM, Turley DM, Das P, et al. Inhibitors of gamma-secretase block in vivo and in vitro T helper type 1 polarization by preventing Notch upregulation of Tbx21. Nat Immunol. 2005;6(7):680–688. [PubMed] [Google Scholar]
- 32.Skokos D, Nussenzweig MC. CD8- DCs induce IL-12-independent Th1 differentiation through Delta 4 Notch-like ligand in response to bacterial LPS. J Exp Med. 2007;204(7):1525–1531. doi: 10.1084/jem.20062305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Auderset F, Schuster S, Coutaz M, et al. Redundant Notch1 and Notch2 Signaling Is Necessary for IFNgamma Secretion by T Helper 1 Cells During Infection with Leishmania major. PLoS pathogens. 2012;8(3):e1002560. doi: 10.1371/journal.ppat.1002560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bailis W, Yashiro-Ohtani Y, Fang TC, et al. Notch simultaneously orchestrates multiple helper T cell programs independently of cytokine signals. Immunity. 2013;39(1):148–159. doi: 10.1016/j.immuni.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tu L, Fang TC, Artis D, et al. Notch signaling is an important regulator of type 2 immunity. J Exp Med. 2005;202(8):1037–1042. doi: 10.1084/jem.20050923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Amsen D, Antov A, Jankovic D, et al. Direct regulation of Gata3 expression determines the T helper differentiation potential of Notch. Immunity. 2007;27(1):89–99. doi: 10.1016/j.immuni.2007.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fang TC, Yashiro-Ohtani Y, Del Bianco C, Knoblock DM, Blacklow SC, Pear WS. Notch directly regulates Gata3 expression during T helper 2 cell differentiation. Immunity. 2007;27(1):100–110. doi: 10.1016/j.immuni.2007.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Okamoto M, Matsuda H, Joetham A, et al. Jagged1 on dendritic cells and Notch on CD4+ T cells initiate lung allergic responsiveness by inducing IL-4 production. J Immunol. 2009;183(5):2995–3003. doi: 10.4049/jimmunol.0900692. [DOI] [PubMed] [Google Scholar]
- 39.Kang JH, Kim BS, Uhm TG, et al. Gamma-secretase inhibitor reduces allergic pulmonary inflammation by modulating Th1 and Th2 responses. Am J Respir Crit Care Med. 2009;179(10):875–882. doi: 10.1164/rccm.200806-893OC. [DOI] [PubMed] [Google Scholar]
- 40.Elyaman W, Bassil R, Bradshaw EM, et al. Notch receptors and Smad3 signaling cooperate in the induction of interleukin-9-producing T cells. Immunity. 2012;36(4):623–634. doi: 10.1016/j.immuni.2012.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ito T, Schaller M, Hogaboam CM, et al. TLR9 regulates the mycobacteria-elicited pulmonary granulomatous immune response in mice through DC-derived Notch ligand delta-like 4. J Clin Invest. 2009;119(1):33–46. doi: 10.1172/JCI35647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mukherjee S, Schaller MA, Neupane R, Kunkel SL, Lukacs NW. Regulation of T cell activation by Notch ligand, DLL4, promotes IL-17 production and Rorc activation. J Immunol. 2009;182(12):7381–7388. doi: 10.4049/jimmunol.0804322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maekawa Y, Minato Y, Ishifune C, et al. Notch2 integrates signaling by the transcription factors RBP-J and CREB1 to promote T cell cytotoxicity. Nat Immunol. 2008;9(10):1140–1147. doi: 10.1038/ni.1649. [DOI] [PubMed] [Google Scholar]
- 44.Sugimoto K, Maekawa Y, Kitamura A, et al. Notch2 signaling is required for potent antitumor immunity in vivo. J Immunol. 2010;184(9):4673–4678. doi: 10.4049/jimmunol.0903661. [DOI] [PubMed] [Google Scholar]
- 45.Backer RA, Helbig C, Gentek R, et al. A central role for Notch in effector CD8(+) T cell differentiation. Nat Immunol. 2014;15(12):1143–1151. doi: 10.1038/ni.3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mathieu M, Duval F, Daudelin JF, Labrecque N. The Notch signaling pathway controls short-lived effector CD8+ T cell differentiation but is dispensable for memory generation. J Immunol. 2015;194(12):5654–5662. doi: 10.4049/jimmunol.1402837. [DOI] [PubMed] [Google Scholar]
- 47.Charbonnier LM, Wang S, Georgiev P, Sefik E, Chatila TA. Control of peripheral tolerance by regulatory T cell-intrinsic Notch signaling. Nat Immunol. 2015;16(11):1162–1173. doi: 10.1038/ni.3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thomas M, Calamito M, Srivastava B, Maillard I, Pear WS, Allman D. Notch activity synergizes with B-cell-receptor and CD40 signaling to enhance B-cell activation. Blood. 2007;109(8):3342–3350. doi: 10.1182/blood-2006-09-046698. [DOI] [PubMed] [Google Scholar]
- 49.Santos MA, Sarmento LM, Rebelo M, et al. Notch1 engagement by Delta-like-1 promotes differentiation of B lymphocytes to antibody-secreting cells. Proc Natl Acad Sci U S A. 2007;104(39):15454–15459. doi: 10.1073/pnas.0702891104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kang JA, Kim WS, Park SG. Notch1 is an important mediator for enhancing of B-cell activation and antibody secretion by Notch ligand. Immunology. 2014;143(4):550–559. doi: 10.1111/imm.12333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Napolitani G, Rinaldi A, Bertoni F, Sallusto F, Lanzavecchia A. Selected Toll-like receptor agonist combinations synergistically trigger a T helper type 1-polarizing program in dendritic cells. Nat Immunol. 2005;6(8):769–776. doi: 10.1038/ni1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rudd BD, Schaller MA, Smit JJ, et al. MyD88-mediated instructive signals in dendritic cells regulate pulmonary immune responses during respiratory virus infection. J Immunol. 2007;178(9):5820–5827. doi: 10.4049/jimmunol.178.9.5820. [DOI] [PubMed] [Google Scholar]
- 53.Sun J, Krawczyk CJ, Pearce EJ. Suppression of Th2 cell development by Notch ligands Delta1 and Delta4. J Immunol. 2008;180(3):1655–1661. doi: 10.4049/jimmunol.180.3.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sandy AR, Stoolman J, Malott K, Pongtornpipat P, Segal BM, Maillard I. Notch signaling regulates T cell accumulation and function in the central nervous system during experimental autoimmune encephalomyelitis. J Immunol. 2013;191(4):1606–1613. doi: 10.4049/jimmunol.1301116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sandy A, Chung J, Toubai T, et al. T cell-specific Notch inhibition blocks graft-versus-host disease by inducing a hyporesponsive program in alloreactive CD4+ and CD8+ T cells. J Immunol. 2013;190(11):5818–5828. doi: 10.4049/jimmunol.1203452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang Y, Sandy AR, Wang J, et al. Notch signaling is a critical regulator of allogeneic CD4+ T-cell responses mediating graft-versus-host disease. Blood. 2011;117(1):299–308. doi: 10.1182/blood-2010-03-271940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tran IT, Sandy AR, Carulli AJ, et al. Blockade of individual Notch ligands and receptors controls graft-versus-host disease. J Clin Invest. 2013;123(4):1590–1604. doi: 10.1172/JCI65477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Elyaman W, Bradshaw EM, Wang Y, et al. JAGGED1 and delta1 differentially regulate the outcome of experimental autoimmune encephalomyelitis. J Immunol. 2007;179(9):5990–5998. doi: 10.4049/jimmunol.179.9.5990. [DOI] [PubMed] [Google Scholar]
- 59.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133(5):775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 60.Bassil R, Zhu B, Lahoud Y, et al. Notch ligand delta-like 4 blockade alleviates experimental autoimmune encephalomyelitis by promoting regulatory T cell development. J Immunol. 2011;187(5):2322–2328. doi: 10.4049/jimmunol.1100725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Billiard F, Lobry C, Darrasse-Jeze G, et al. Dll4-Notch signaling in Flt3-independent dendritic cell development and autoimmunity in mice. J Exp Med. 2012;209(5):1011–1028. doi: 10.1084/jem.20111615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hoyne GF, Le Roux I, Corsin-Jimenez M, et al. Serrate1-induced notch signalling regulates the decision between immunity and tolerance made by peripheral CD4(+) T cells. Int Immunol. 2000;12(2):177–185. doi: 10.1093/intimm/12.2.177. [DOI] [PubMed] [Google Scholar]
- 63.Anastasi E, Campese AF, Bellavia D, et al. Expression of activated Notch3 in transgenic mice enhances generation of T regulatory cells and protects against experimental autoimmune diabetes. J Immunol. 2003;171(9):4504–4511. doi: 10.4049/jimmunol.171.9.4504. [DOI] [PubMed] [Google Scholar]
- 64.Vigouroux S, Yvon E, Wagner HJ, et al. Induction of antigen-specific regulatory T cells following overexpression of a Notch ligand by human B lymphocytes. J Virol. 2003;77(20):10872–10880. doi: 10.1128/JVI.77.20.10872-10880.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kared H, Adle-Biassette H, Fois E, et al. Jagged2-expressing hematopoietic progenitors promote regulatory T cell expansion in the periphery through notch signaling. Immunity. 2006;25(5):823–834. doi: 10.1016/j.immuni.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 66.Samon JB, Champhekar A, Minter LM, et al. Notch1 and TGFbeta1 cooperatively regulate Foxp3 expression and the maintenance of peripheral regulatory T cells. Blood. 2008;112(5):1813–1821. doi: 10.1182/blood-2008-03-144980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Asano N, Watanabe T, Kitani A, Fuss IJ, Strober W. Notch1 signaling and regulatory T cell function. J Immunol. 2008;180(5):2796–2804. doi: 10.4049/jimmunol.180.5.2796. [DOI] [PubMed] [Google Scholar]
- 68.Wong KK, Carpenter MJ, Young LL, et al. Notch ligation by Delta1 inhibits peripheral immune responses to transplantation antigens by a CD8+ cell-dependent mechanism. J Clin Invest. 2003;112(11):1741–1750. doi: 10.1172/JCI18020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cho OH, Shin HM, Miele L, et al. Notch regulates cytolytic effector function in CD8+ T cells. J Immunol. 2009;182(6):3380–3389. doi: 10.4049/jimmunol.0802598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Laky K, Evans S, Perez-Diez A, Fowlkes BJ. Notch signaling regulates antigen sensitivity of naive CD4+ T cells by tuning co-stimulation. Immunity. 2015;42(1):80–94. doi: 10.1016/j.immuni.2014.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Maekawa Y, Ishifune C, Tsukumo S, Hozumi K, Yagita H, Yasutomo K. Notch controls the survival of memory CD4+ T cells by regulating glucose uptake. Nat Med. 2015;21(1):55–61. doi: 10.1038/nm.3758. [DOI] [PubMed] [Google Scholar]
- 72.Tanigaki K, Han H, Yamamoto N, et al. Notch-RBP-J signaling is involved in cell fate determination of marginal zone B cells. Nat Immunol. 2002;3(5):443–450. doi: 10.1038/ni793. [DOI] [PubMed] [Google Scholar]
- 73.Saito T, Chiba S, Ichikawa M, et al. Notch2 is preferentially expressed in mature B cells and indispensable for marginal zone B lineage development. Immunity. 2003;18(5):675–685. doi: 10.1016/s1074-7613(03)00111-0. [DOI] [PubMed] [Google Scholar]
- 74.Hozumi K, Negishi N, Suzuki D, et al. Delta-like 1 is necessary for the generation of marginal zone B cells but not T cells in vivo. Nat Immunol. 2004;5(6):638–644. doi: 10.1038/ni1075. [DOI] [PubMed] [Google Scholar]
- 75.Wu L, Maillard I, Nakamura M, Pear WS, Griffin JD. The transcriptional coactivator Maml1 is required for Notch2-mediated marginal zone B-cell development. Blood. 2007;110(10):3618–3623. doi: 10.1182/blood-2007-06-097030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Simonetti G, Carette A, Silva K, et al. IRF4 controls the positioning of mature B cells in the lymphoid microenvironments by regulating NOTCH2 expression and activity. J Exp Med. 2013;210(13):2887–2902. doi: 10.1084/jem.20131026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yoon SO, Zhang X, Berner P, Blom B, Choi YS. Notch ligands expressed by follicular dendritic cells protect germinal center B cells from apoptosis. J Immunol. 2009;183(1):352–358. doi: 10.4049/jimmunol.0803183. [DOI] [PubMed] [Google Scholar]
- 78.Fung E, Tang SM, Canner JP, et al. Delta-like 4 induces notch signaling in macrophages: implications for inflammation. Circulation. 2007;115(23):2948–2956. doi: 10.1161/CIRCULATIONAHA.106.675462. [DOI] [PubMed] [Google Scholar]
- 79.Caton ML, Smith-Raska MR, Reizis B. Notch-RBP-J signaling controls the homeostasis of CD8-dendritic cells in the spleen. J Exp Med. 2007;204(7):1653–1664. doi: 10.1084/jem.20062648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lewis KL, Caton ML, Bogunovic M, et al. Notch2 receptor signaling controls functional differentiation of dendritic cells in the spleen and intestine. Immunity. 2011;35(5):780–791. doi: 10.1016/j.immuni.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wong SH, Walker JA, Jolin HE, et al. Transcription factor RORalpha is critical for nuocyte development. Nat Immunol. 2012;13(3):229–236. doi: 10.1038/ni.2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Xu H, Zhu J, Smith S, et al. Notch-RBP-J signaling regulates the transcription factor IRF8 to promote inflammatory macrophage polarization. Nat Immunol. 2012;13(7):642–650. doi: 10.1038/ni.2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yang Q, Monticelli LA, Saenz SA, et al. T cell factor 1 is required for group 2 innate lymphoid cell generation. Immunity. 2013;38(4):694–704. doi: 10.1016/j.immuni.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Franklin RA, Liao W, Sarkar A, et al. The cellular and molecular origin of tumor-associated macrophages. Science. 2014;344(6186):921–925. doi: 10.1126/science.1252510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xu J, Chi F, Guo T, et al. NOTCH reprograms mitochondrial metabolism for proinflammatory macrophage activation. J Clin Invest. 2015;125(4):1579–1590. doi: 10.1172/JCI76468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chea S, Perchet T, Petit M, et al. Notch signaling in group 3 innate lymphoid cells modulates their plasticity. Sci Signal. 2016;9(426):ra45. doi: 10.1126/scisignal.aaf2223. [DOI] [PubMed] [Google Scholar]
- 87.Viant C, Rankin LC, Girard-Madoux MJ, et al. Transforming growth factor-beta and Notch ligands act as opposing environmental cues in regulating the plasticity of type 3 innate lymphoid cells. Sci Signal. 2016;9(426):ra46. doi: 10.1126/scisignal.aaf2176. [DOI] [PubMed] [Google Scholar]
- 88.Maillard I, Koch U, Dumortier A, et al. Canonical Notch signaling is dispensable for the maintenance of adult hematopoietic stem cells. Cell Stem Cell. 2008;2(4):356–366. doi: 10.1016/j.stem.2008.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Varnum-Finney B, Halasz LM, Sun M, Gridley T, Radtke F, Bernstein ID. Notch2 governs the rate of generation of mouse long- and short-term repopulating stem cells. J Clin Invest. 2011;121(3):1207–1216. doi: 10.1172/JCI43868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Riccio O, van Gijn ME, Bezdek AC, et al. Loss of intestinal crypt progenitor cells owing to inactivation of both Notch1 and Notch2 is accompanied by derepression of CDK inhibitors p27Kip1 and p57Kip2. EMBO Rep. 2008;9(4):377–383. doi: 10.1038/embor.2008.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.VanDussen KL, Carulli AJ, Keeley TM, et al. Notch signaling modulates proliferation and differentiation of intestinal crypt base columnar stem cells. Development. 2012;139(3):488–497. doi: 10.1242/dev.070763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pellegrinet L, Rodilla V, Liu Z, et al. Dll1- and dll4-mediated notch signaling are required for homeostasis of intestinal stem cells. Gastroenterology. 2011;140(4):1230–1240. e1231–e1237. doi: 10.1053/j.gastro.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ferrara JL, Levine JE, Reddy P, Holler E. Graft-versus-host disease. Lancet. 2009;373(9674):1550–1561. doi: 10.1016/S0140-6736(09)60237-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Blazar BR, Murphy WJ, Abedi M. Advances in graft-versus-host disease biology and therapy. Nat Rev Immunol. 2012;12(6):443–458. doi: 10.1038/nri3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Roderick JE, Gonzalez-Perez G, Kuksin CA, et al. Therapeutic targeting of NOTCH signaling ameliorates immune-mediated bone marrow failure of aplastic anemia. Journal of Experimental Medicine. 2013;210(7):1311–1329. doi: 10.1084/jem.20112615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mochizuki K, Xie F, He S, et al. Delta-like Ligand 4 Identifies a Previously Uncharacterized Population of Inflammatory Dendritic Cells That Plays Important Roles in Eliciting Allogeneic T Cell Responses in Mice. J Immunol. 2013;190(7):3772–3782. doi: 10.4049/jimmunol.1202820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wu Y, Cain-Hom C, Choy L, et al. Therapeutic antibody targeting of individual Notch receptors. Nature. 2010;464(7291):1052–1057. doi: 10.1038/nature08878. [DOI] [PubMed] [Google Scholar]
- 98.Beres AJ, Drobyski WR. The role of regulatory T cells in the biology of graft versus host disease. Frontiers in immunology. 2013;4:163. doi: 10.3389/fimmu.2013.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ganguly S, Ross DB, Panoskaltsis-Mortari A, et al. Donor CD4+ Foxp3+ regulatory T cells are necessary for post-transplantation cyclophosphamide-mediated protection against GVHD in mice. Blood. 2014 doi: 10.1182/blood-2013-10-525873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zorn E, Kim HT, Lee SJ, et al. Reduced frequency of FOXP3+ CD4+CD25+ regulatory T cells in patients with chronic graft-versus-host disease. Blood. 2005;106(8):2903–2911. doi: 10.1182/blood-2005-03-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Magenau JM, Qin X, Tawara I, et al. Frequency of CD4(+)CD25(hi)FOXP3(+) regulatory T cells has diagnostic and prognostic value as a biomarker for acute graft-versus-host-disease. Biol Blood Marrow Transplant. 2010;16(7):907–914. doi: 10.1016/j.bbmt.2010.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bremm M, Huenecke S, Lehrnbecher T, et al. Advanced flowcytometric analysis of regulatory T cells: CD127 downregulation early post stem cell transplantation and altered Treg/CD3(+)CD4(+)-ratio in severe GvHD or relapse. J Immunol Methods. 2011;373(1–2):36–44. doi: 10.1016/j.jim.2011.07.018. [DOI] [PubMed] [Google Scholar]
- 103.Meng L, Bai Z, He S, et al. The Notch Ligand DLL4 Defines a Capability of Human Dendritic Cells in Regulating Th1 and Th17 Differentiation. J Immunol. 2016;196(3):1070–1080. doi: 10.4049/jimmunol.1501310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chung J, Koch U, Tran IT, et al. Delta-Like Notch Ligands Expressed By Host Non-Hematopoietic Radioresistant Cells Regulate Graft-Versus-Host Disease and Extrathymic T Cell Development After Bone Marrow Transplantation. Blood. 2013;122(21):2003–2003. [Google Scholar]
- 105.Chung J, Ebens CL, Radojcic V, et al. Delta-like Ligands Expressed By Stromal Cells in Secondary Lymphoid Organs Deliver an Early Pulse of Notch Signaling and Drive T Cell Pathogenicity in Acute Graft-Versus-Host Disease. Blood. 2014;124(21):841–841. [Google Scholar]
- 106.Koyama M, Kuns RD, Olver SD, et al. Recipient nonhematopoietic antigen-presenting cells are sufficient to induce lethal acute graft-versus-host disease. Nat Med. 2011;18(1):135–142. doi: 10.1038/nm.2597. [DOI] [PubMed] [Google Scholar]
- 107.Toubai T, Tawara I, Sun Y, et al. Induction of acute GVHD by sex-mismatched H-Y antigens in the absence of functional radiosensitive host hematopoietic-derived antigen-presenting cells. Blood. 2012;119(16):3844–3853. doi: 10.1182/blood-2011-10-384057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Fasnacht N, Huang HY, Koch U, et al. Specific fibroblastic niches in secondary lymphoid organs orchestrate distinct Notch-regulated immune responses. Journal of Experimental Medicine. 2014;211(11):2265–2279. doi: 10.1084/jem.20132528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Furlan SN, Watkins B, Tkachev V, et al. Transcriptome analysis of GVHD reveals aurora kinase A as a targetable pathway for disease prevention. Sci Transl Med. 2015;7(315):315ra191. doi: 10.1126/scitranslmed.aad3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhang C, Todorov I, Zhang Z, et al. Donor CD4+ T and B cells in transplants induce chronic graft-versus-host disease with autoimmune manifestations. Blood. 2006;107(7):2993–3001. doi: 10.1182/blood-2005-09-3623. [DOI] [PubMed] [Google Scholar]
- 111.Young JS, Wu T, Chen Y, et al. Donor B cells in transplants augment clonal expansion and survival of pathogenic CD4+ T cells that mediate autoimmune-like chronic graft-versus-host disease. J Immunol. 2012;189(1):222–233. doi: 10.4049/jimmunol.1200677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zerr P, Palumbo-Zerr K, Distler A, et al. Inhibition of hedgehog signaling for the treatment of murine sclerodermatous chronic graft-versus-host disease. Blood. 2012;120(14):2909–2917. doi: 10.1182/blood-2012-01-403428. [DOI] [PubMed] [Google Scholar]
- 113.Flynn R, Du J, Veenstra RG, et al. Increased T follicular helper cells and germinal center B cells are required for cGVHD and bronchiolitis obliterans. Blood. 2014;123(25):3988–3998. doi: 10.1182/blood-2014-03-562231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Alexander KA, Flynn R, Lineburg KE, et al. CSF-1-dependant donor-derived macrophages mediate chronic graft-versus-host disease. J Clin Invest. 2014;124(10):4266–4280. doi: 10.1172/JCI75935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yvon ES, Vigouroux S, Rousseau RF, et al. Over expression of the Notch ligand, Jagged-1 induces alloantigen-specific human regulatory T cells. Blood. 2003;102(10):3815–3821. doi: 10.1182/blood-2002-12-3826. [DOI] [PubMed] [Google Scholar]
- 116.Lin Y, Chen W, Li J, et al. Overexpression of Jagged-1 combined with blockade of CD40 pathway prolongs allograft survival. Immunol Cell Biol. 2015;93(2):213–217. doi: 10.1038/icb.2014.84. [DOI] [PubMed] [Google Scholar]
- 117.Riella LV, Ueno T, Batal I, et al. Blockade of Notch ligand delta1 promotes allograft survival by inhibiting alloreactive Th1 cells and cytotoxic T cell generation. J Immunol. 2011;187(9):4629–4638. doi: 10.4049/jimmunol.1004076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wood S, Feng J, Chung J, et al. Transient blockade of delta-like Notch ligands prevents allograft rejection mediated by cellular and humoral mechanisms in a mouse model of heart transplantation. J Immunol. 2015;194(6):2899–2908. doi: 10.4049/jimmunol.1402034. [DOI] [PMC free article] [PubMed] [Google Scholar]