Abstract
Purpose
To evaluate differences in pathologic complete response (pCR) rates and overall survival among patients receiving either neoadjuvant chemotherapy or chemoradiation prior to esophagectomy for locally advanced esophageal cancer.
Patients and methods
Esophageal cancer patients receiving either neoadjuvant chemotherapy or chemoradiation prior to esophagectomy were identified using the National Cancer Data Base (NCDB). Univariate analysis compared patient, tumor, and postoperative outcome characteristics. Logistic regression was performed to identify variables associated with achieving pCR. Kaplan-Meier analysis was performed to compare overall median survival by neoadjuvant therapy type and pCR status. Finally, a Cox proportional hazards model was fitted to identify variables associated with increased mortality hazard.
Results
From 2006 – 2012, 916/7,338 (12.5%) of patients received neoadjuvant chemotherapy while 6,422 (87.5%) received neoadjuvant chemoradiation. Neoadjuvant chemoradiation patients were more likely to achieve pCR (17.2% versus 6.4%, p<0.001) and less likely to have positive margins (5.6% versus 11.5%, p<0.001) than neoadjuvant chemotherapy patients, with no difference in 30- or 90-day mortality. Achieving pCR was associated with improved overall median survival (59.5 months ± 4.0 versus 30.1 months ± 0.76 for those with persistent disease, p<0.001). On logistic regression, neoadjuvant chemoradiation therapy was independently associated with achieving pCR (Odds Ratio 2.75, 2.01 – 3.77, p<0.001). Despite improvement in pCR rate with neoadjuvant chemoradiation, neoadjuvant therapy type was not independently associated with long-term survival (HR 1.12, 95% CI 0.97 – 1.30, p=0.12).
Conclusion
While neoadjuvant chemoradiation is more successful in downstaging esophageal cancer prior to esophagectomy, this therapy was not independently prognostic for improved long-term survival. Other factors affecting long-term survival among pathologic complete responders and among patients with persistent disease should be investigated to clarify this association.
Introduction
Esophagectomy is a well-established tool in the arsenal of therapies for patients with locally advanced esophageal cancer, with reports demonstrating improved rates of local control. [1–3] Unfortunately, five year overall survival in this patient population is often reported at less than 25%, driven primarily by regional and distant failure. [2,3] As such, adjunctive therapies such as chemotherapy and radiation hold great appeal as a means to improve outcomes in this population.
Over the past 25 years, more than a dozen randomized controlled trials with over 2,000 locally advanced esophageal cancer patients have compared neoadjuvant chemotherapy to upfront esophagectomy. [4] A recently published comprehensive metaanalysis found that neoadjuvant chemotherapy improved the likelihood of complete (R0) resection and was associated with improved overall survival compared to surgical resection alone with a 12% decrease in the mortality hazard. [4] In parallel, studies evaluating neoadjuvant chemoradiation therapy have demonstrated high rates of R0 resection and improved survival as well. The CROSS trial is the most recent randomized controlled trial to evaluate the outcomes of neoadjuvant chemoradiation versus upfront esophagectomy and also documented a substantial survival benefit, with a 35% decrease in overall mortality hazard in the chemoradiation and surgery arm. [5] However, no completed randomized controlled trial has compared long-term survival outcomes of patients receiving neoadjuvant chemotherapy to chemoradiation therapy directly. Currently, many centers practice the method of neoadjuvant therapy per their institutional standard and local consensus.
Nationally, the usage of neoadjuvant therapy by modality and its associated outcomes in locally advanced esophageal cancer are not well characterized. Our goal was to use the National Cancer Data Base (NCDB) to: 1) investigate practice patterns of neoadjuvant chemotherapy and chemoradiation therapy in the United States 2) determine if neoadjuvant chemoradiation therapy is associated with improved rates of pathologic complete response (pCR) and 3) evaluate for differences in overall survival between neoadjuvant chemotherapy versus chemoradiation therapy.
Patients and Methods
The NCDB participant user file (PUF) for esophageal cancer was used to abstract patients who had received either neoadjuvant chemotherapy or chemoradiation therapy prior to esophagectomy. The NCDB, which is a joint effort of the American College of Surgeons (ACoS) and the American Cancer Society (ACS), was established in 1989 and represents approximately 70% of patients receiving treatment at Commission on Cancer (CoC) accredited centers. This study was deemed exempt by the Washington University School of Medicine Institutional Review Board. From the NCDB data dictionary, patient characteristics were dichotomized to either Caucasian or non-Caucasian, population type ≥250,000 versus <250,000 individuals, average income of patient’s zip code area <$38,000 or ≥$38,000, academic or community cancer center type, private or non-private insurance status, and having a Charlson/Deyo comorbidity score of 0, 1, or ≥2 (excluding their current cancer diagnosis). Using ICD-0–3 codes, patients were dichotomized into either adenocarcinoma or squamous cell histologic subtype. Patients were coded as having a complete pathological response if their final pathology included T0 and N0 status. Patients with no lymph nodes examined were labeled as having an unknown pathologic response status.
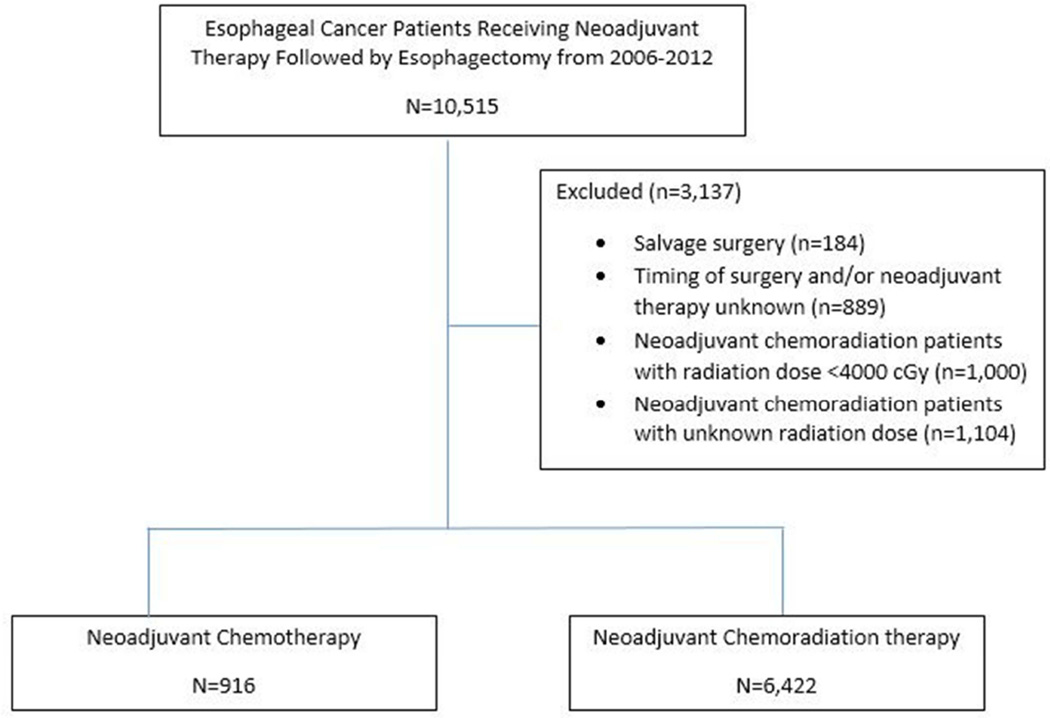
Patients excluded from analysis included: clinical M1, M1A, or M1B metastatic disease, tumors located in the cervical esophagus, and those that did not receive an esophagectomy. Patients with ≥200 days from the time of initiation of neoadjuvant therapy and surgery were excluded as likely salvage therapy patients. Patients documented as receiving neoadjuvant chemoradiation therapy, but receiving less than 4000 cGy or an unknown dosage were also excluded. A Consolidated Standards of Reporting Trials (CONSORT) flow diagram is shown in Figure 1.
Figure 1.
Consolidated Standards of Reporting Trials (CONSORT) flow diagram for NCDB esophageal cancer patients receiving neoadjuvant therapy followed by esophagectomy from 2006–2012.
Descriptive statistics of continuous variables were calculated as mean ± standard deviation. Univariate analysis included independent sample t-tests to compare continuous variables and chi-square analysis for comparisons of normally distributed categorical data. Stepwise backwards logistic regression was used to identify variables associated with pathologic complete response. Criteria for entry into the logistic regression model included a p-value of <0.10 on univariate analysis. Kaplan-Meier analysis was performed to compare overall median survival outcomes by neoadjuvant therapy type and pCR status, with log-rank testing. A Cox-proportional hazards model was created to identify variables independently associated with increased risk of mortality. P values of less than 0.05 were considered statistically significant. All statistical analyses were performed in SPSS 22.0 for Windows (SPSS Inc, Chicago, IL, 2013).
Results
Neoadjuvant Therapy Type Characteristics and Outcomes
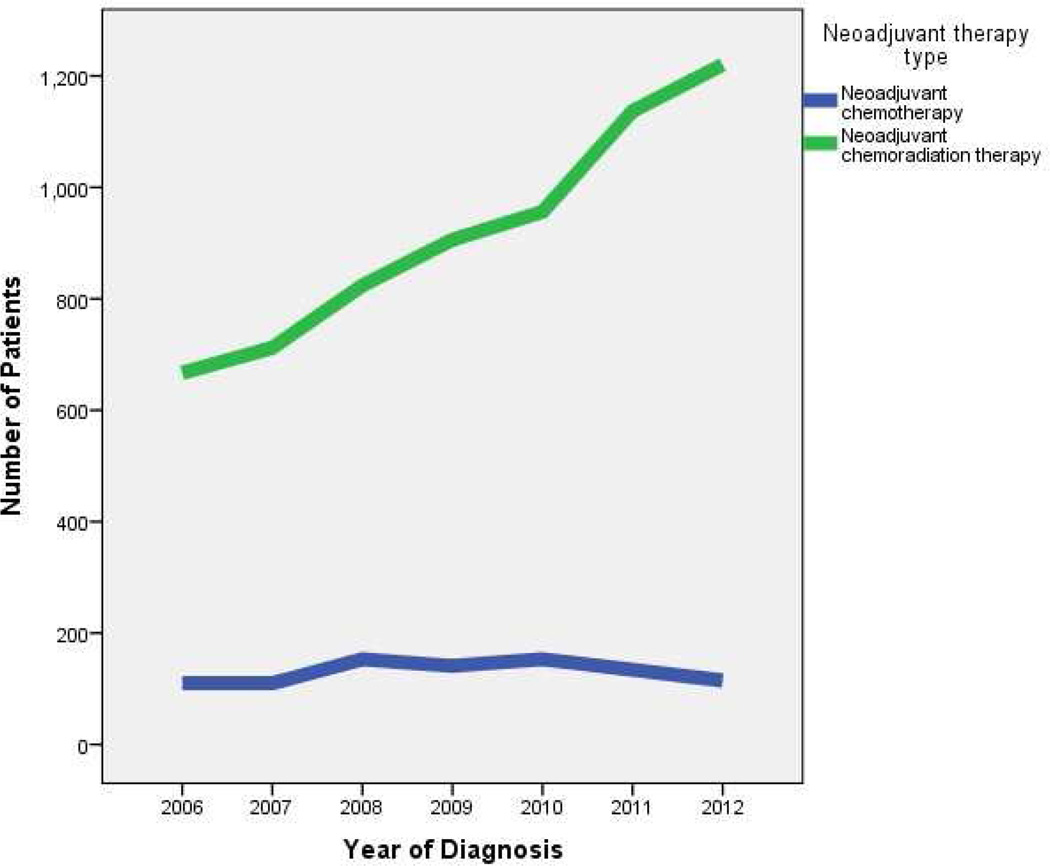
Of 7,338 patients undergoing neoadjuvant therapy prior to esophagectomy from 2006 to 2012, 916 (12.5%) received neoadjuvant chemotherapy, while 6,422 (87.5%) received neoadjuvant chemoradiation therapy. While the use of neoadjuvant chemoradiation therapy increased from 2006 to 2012, the use of neoadjuvant chemotherapy has remained steady (Figure 2). Characteristics of patients receiving either neoadjuvant chemotherapy or chemoradiation therapy are compared in Table 1. Patient and tumor characteristics independently associated with increased likelihood of receiving neoadjuvant chemoradiation therapy on logistic regression included being treated in 2010 – 2012 (reference 2006 – 2009): odds ratio (OR) 1.24 (95% CI 1.07 – 1.45, p=0.006), having a clinical T2 (OR 1.83, 1.35 – 2.48, p<0.001), T3 (OR 1.84, 1.41 – 2.41, p<0.001), or T4 tumor (OR 2.26, 1.28 – 4.01, p=0.005), reference clinical T0-T1, and having clinical N1 disease (OR 1.19, 1.01 – 1.41, p=0.036). Variables associated with a decreased likelihood of receiving neoadjuvant chemoradiation therapy included increasing age (by year: OR 0.99, 0.98 – 0.99, p=0.004) and living in an urban area (0.67, 0.57 – 0.80, p<0.001).
Figure 2.
Neoadjuvant therapy trends by year for locally advanced esophageal cancer patients receiving esophagectomy, from the NCDB.
Table 1.
Univariate analysis of patients in the NCDB receiving either neoadjuvant chemotherapy or neoadjuvant chemoradiation therapy prior to esophagectomy for esophageal cancer.
| Variable | Induction chemotherapy (n=916, 12.5%) |
Induction chemoradiation therapy (n=6422, 87.5%) |
P value |
|---|---|---|---|
| Age | 62.3 ± 10.0 | 61.5 ± 9.4 | 0.02 |
| Gender | |||
| Male | 769 (84.0%) | 5483 (85.4%) | 0.26 |
| Race | |||
| Caucasian | 840 (92.8%) | 6021 (94.4%) | 0.049 |
| Non-Caucasian | 65 (7.2%) | 354 (5.6%) | |
| Population type | |||
| <250,000 | 222 (25.6%) | 2047 (33.1%) | <0.001 |
| ≥250,000 | 645 (74.4%) | 4144 (66.9%) | |
| Distance from treatment center (miles) |
43.2 ± 126.3 | 45.9 ± 136.3 | 0.58 |
| Income (US dollars) | |||
| <$38,000 | 117 (13.2%) | 928 (14.7%) | 0.22 |
| ≥$38,000 | 770 (86.8%) | 5370 (85.3%) | |
| Insurance status | |||
| Private | 452 (49.6%) | 3370 (53.0%) | 0.06 |
| Non-private | 459 (50.4%) | 2989 (47.0%) | |
| Treatment center type | |||
| Academic | 445 (48.6%) | 3199 (49.9%) | 0.45 |
| Nonacademic | 471 (51.4%) | 3208 (50.1%) | |
| Treatment year | |||
| 2006 – 2009 | 514 (56.1%) | 3109 (48.4%) | <0.001 |
| 2010 – 2012 | 402 (43.9%) | 3313 (51.6%) | |
| Charlson/Deyo Score | |||
| 0 | 703 (76.7%) | 4881 (76.0%) | 0.78 |
| 1 | 171 (18.7%) | 1260 (19.6%) | |
| ≥2 | 42 (4.6%) | 281 (4.4%) | |
| Tumor size (mm) | 40.9 ± 45.2 | 44.3 ± 40.6 | 0.05 |
| Histology | |||
| Adenocarcinoma | 674 (81.1%) | 4725 (80.3%) | 0.57 |
| Squamous cell | 157 (18.9%) | 1162 (19.7%) | |
| Clinical T stage | |||
| T0, Tis, T1 | 81 (9.0%) | 363 (5.7%) | <0.001 |
| T2 | 147 (16.3%) | 1157 (18.1%) | |
| T3 | 481 (53.2%) | 4054 (63.3%) | |
| T4 | 19 (2.1%) | 177 (2.8%) | |
| TX | 176 (19.5%) | 655 (10.2%) | |
| Clinical N stage | |||
| N0 | 344 (38.1%) | 2256 (35.2%) | <0.001 |
| N1 | 394 (43.6%) | 3296 (51.5%) | |
| ≥N2 | 37 (4.1%) | 409 (6.4%) | |
| NX | 129 (14.3%) | 445 (6.9%) | |
| Interval from start of chemotherapy to surgery (days) |
100.0 ± 30.6 | 98.1 ± 26.8 | 0.07 |
| Number of nodes examined | 15.1 ± 11.1 | 12.3 ± 9.5 | <0.001 |
| Number of positive nodes | 2.2 ± 4.2 | 1.1 ± 2.4 | <0.001 |
| Surgical Margins | |||
| ≥R1 | 102 (11.5%) | 349 (5.6%) | <0.001 |
| Pathologic complete response (pT0N0) |
50 (6.4%) | 816 (17.2%) | <0.001 |
| Inpatient length of stay (days) | 12.4 ± 12.1 | 12.6 ± 10.7 | 0.61 |
| 30-day Readmission | 47 (5.3%) | 397 (6.5%) | 0.19 |
| 30-day mortality | 27 (3.4%) | 183 (3.6%) | 0.84 |
| 90-day mortality | 61 (7.8%) | 434 (8.5%) | 0.50 |
Patients receiving neoadjuvant chemoradiation therapy were less likely to have positive surgical margins (≥R1), 349 (5.6%) versus 102 (11.5%) and more likely to achieve pCR, 816 (17.2%) versus 50 (6.4%) than patients receiving neoadjuvant chemotherapy, respectively, both p<0.001. No significant difference in thirty-day readmission, thirty-day mortality, or ninety-day mortality after esophagectomy was detected between the two neoadjuvant modalities. There was no significant difference in overall median survival between the two neoadjuvant therapy types (34.0 ± 2.0 months for neoadjuvant chemotherapy versus 34.4 ± 0.8 months for neoadjuvant chemoradiation therapy, p =0.87). While the number of patients censored in both arms was substantial, the proportions were similar (371/801, 46.3% in the neoadjuvant chemotherapy arm and 2,439/5,202, 46.9% in the neoadjuvant chemoradiation group).
pCR Patient Characteristics and Survival
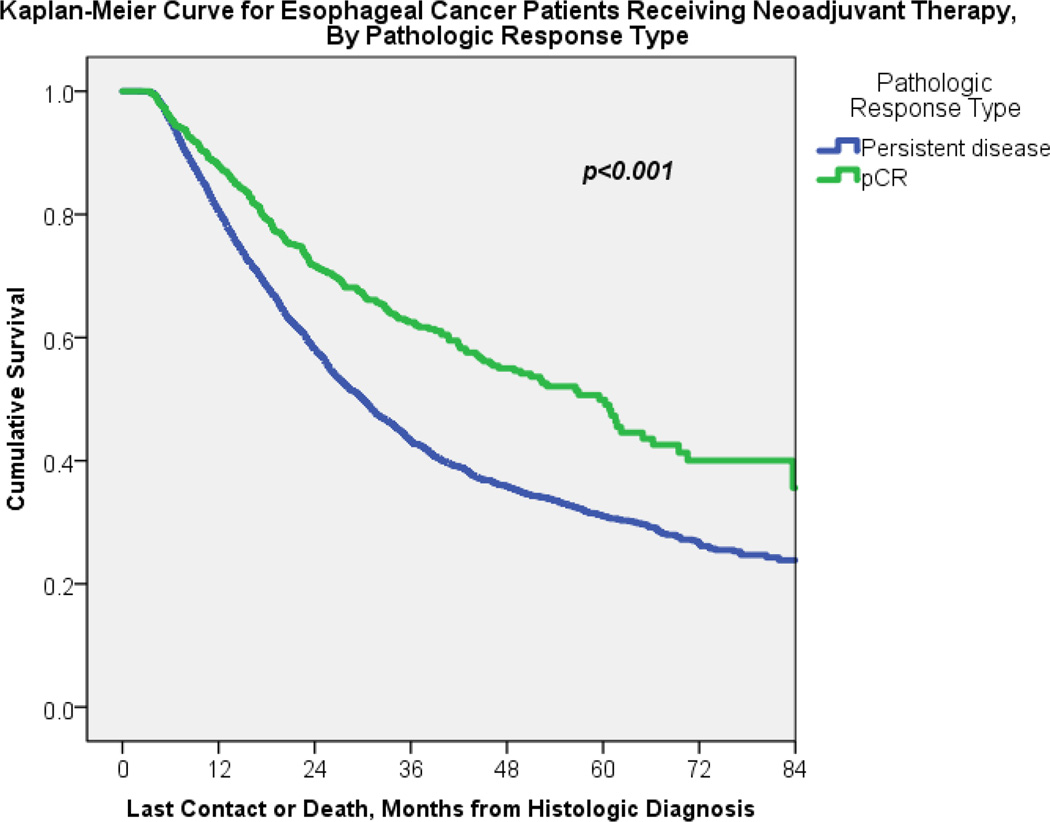
Given the significant and substantial improvement that neoadjuvant chemoradiation patients had in obtaining pCR at the time of esophagectomy, univariate analysis was performed to compare characteristics of pCR patients, versus those with residual disease (Table 2). 5,523/7,338 (75%) of patients in this analysis had pathologic response data available for analysis. 866/5,523 (15.7%) of patients achieved pCR upon completion of induction therapy and esophagectomy. Variables that were independently associated with an increased likelihood of obtaining pCR included urban population (OR 1.31, 1.10 – 1.56, p=0.003), treatment from 2010–2012 (OR 1.31, 1.11 – 1.55, p=0.001), and neoadjuvant chemoradiation therapy (2.75, 2.01 – 3.77, p<0.001). Variables associated with a decreased likelihood of obtaining a pathologic complete response after neoadjuvant therapy and esophagectomy included adenocarcinoma histology (0.44, 0.37 – 0.53, p=<0.001), clinical Tx status (0.47, 0.29 – 0.78, p=0.003), and clinical Nx status (0.41, 0.23 – 0.72, p=0.002), reference categories T0-T1 and N0, respectively. On Kaplan-Meier analysis, achieving pCR was significantly associated with improved overall median survival (59.5 months ± 4.0 versus 30.1 months ± 0.76 for those with residual disease, p<0.001), Figure 3.
Table 2.
Univariate analysis of patients that underwent neoadjuvant therapy followed by esophagectomy, by pathologic complete response (pT0N0) versus residual disease.
| Variable | Pathologic persistent disease on esophagectomy (n=4,657, 84.3%) |
Pathologic complete response (pT0N0) (n=866, 15.7%) |
P value |
|---|---|---|---|
| Age | 61.7 ± 9.5 | 61.7 ± 9.2 | 0.92 |
| Gender | |||
| Male | 4003 (84.9%) | 712 (15.1%) | 0.004 |
| Female | 654 (80.9%) | 154 (19.1%) | |
| Race | |||
| Caucasian | 4383 (84.5%) | 801 (15.5%) | 0.06 |
| Non-Caucasian | 238 (80.4%) | 58 (19.6%) | |
| Population type | |||
| <250,000 | 1448 (86.1%) | 234 (13.9%) | 0.009 |
| ≥250,000 | 3024 (83.3%) | 608 (16.7%) | |
| Distance from treatment center (miles) |
46.8 ± 143.2 | 52.7 ± 131.0 | 0.26 |
| Income (US dollars) | |||
| <$38,000 | 640 (84.1%) | 121 (15.9%) | 0.92 |
| ≥$38,000 | 3915 (84.2%) | 732 (15.8%) | |
| Insurance status | |||
| Private | 2232 (84.6%) | 407 (15.4%) | 0.54 |
| Non-private | 2385 (84.0%) | 455 (16.0%) | |
| Treatment center type | |||
| Academic | 2455 (83.2%) | 369 (14.4%) | 0.016 |
| Nonacademic | 2192 (85.6%) | 495 (16.8%) | |
| Treatment year | |||
| 2006 – 2009 | 2193 (87.1%) | 326 (12.9%) | <0.001 |
| 2010 – 2012 | 2464 (82.0%) | 540 (18.0%) | |
| Charlson/Deyo Score | |||
| 0 | 3503 (84.0%) | 668 (16.0%) | 0.25 |
| 1 | 955 (85.9%) | 157 (14.1%) | |
| ≥2 | 199 (82.9%) | 41 (17.1%) | |
| Neoadjuvant therapy type | |||
| Chemotherapy | 726 (93.6%) | 50 (6.4%) | <0.001 |
| Chemoradiation therapy | 3931 (82.8%) | 816 (17.2%) | |
| Tumor size (mm) | 42.8 ± 44.4 | 44.5 ± 24.4 | 0.38 |
| Histology | |||
| Adenocarcinoma | 3534 (86.1%) | 247 (26.6%) | <0.001 |
| Squamous cell | 682 (73.4%) | 570 (13.9%) | |
| Clinical T stage | |||
| T0, Tis, T1 | 275 (82.1%) | 60 (17.9%) | <0.001 |
| T2 | 773 (79.9%) | 195 (20.1%) | |
| T3 | 2906 (84.1%) | 548 (15.9%) | |
| T4 | 118 (81.4%) | 27 (18.6%) | |
| TX | 566 (94.2%) | 35 (5.8%) | |
| Clinical N stage | |||
| N0 | 1643 (82.9%) | 339 (17.1%) | <0.001 |
| N1 | 2287 (83.6%) | 448 (16.4%) | |
| ≥ N2 | 292 (83.0%) | 60 (17.0%) | |
| NX | 416 (95.6%) | 19 (4.4%) | |
| Interval from start of induction therapy to esophagectomy (days) |
97.5 ± 27.4 | 99.6 ± 26.0 | 0.04 |
| Number of lymph nodes examined |
13.7 ± 9.6 | 14.1 ± 9.4 | 0.27 |
| Inpatient length of stay (days) |
12.7 ± 11.1 | 12.3 ± 9.0 | 0.28 |
| 30-day Readmission | 286 (6.4%) | 51 (6.1%) | 0.76 |
| 30-day mortality | 135 (3.6%) | 28 (4.4%) | 0.35 |
| 90-day mortality | 333 (9.0%) | 41 (6.5%) | 0.04 |
Figure 3.
Kaplan-Meier curve for neoadjuvant therapy patients followed by esophagectomy by either complete response or residual disease on final pathology, p<0.001.
Residual Disease Patient Characteristics and Outcomes
Of the patients with pathologic staging data, 4,657/5,523 (84.3%) of patients had persistent disease after neoadjuvant therapy and esophagectomy. 4,135/4,657 (88.8%) did not go on to receive adjuvant systemic therapy for their persistent disease, while 522/4,657 (11.2%) did. Characteristics of patients with persistent disease receiving adjuvant therapy are listed in Table 3. On multivariate logistic regression, variables that were independently associated with a decreased likelihood of receiving adjuvant therapy included increasing age (by year, OR 0.98, 95% CI 0.97 – 0.99, p=0.004), receiving neoadjuvant chemoradiation (OR 0.30, 95% CI 0.24 – 0.39, p<0.001), and increasing length of inpatient stay after esophagectomy (by day, OR 0.98, 95% CI 0.97 – 0.99, p=0.004). Variables associated with an increased likelihood of receiving adjuvant therapy included private insurance status (OR 1.41, 95% CI 1.09 – 1.83, p=0.01), adenocarcinoma histology (OR 1.50, 95% CI 1.04 – 2.17, p=0.03), and increasing pathologic nodal stage (ref: N0, N1 OR 2.37, 95% CI 1.85 – 3.04, N2 OR 3.08, 95% CI 2.08 – 4.57, and N3 OR 3.48, 95% CI 1.89 – 6.41, all p<0.001). The overall median survival for patients with persistent disease receiving adjuvant systemic therapy was greater than for those that did not (35.2 months ± 2.3 versus 29.0 ± 0.77, p=0.001).
Table 3.
Univariate analysis of patients with persistent disease after neoadjuvant therapy, by adjuvant systemic therapy status.
| Variable | Residual Disease, Did Not Receive Adjuvant Therapy (n=4135, 88.8%) |
Residual Disease, Received Adjuvant Therapy (n=522, 11.2%) |
P value |
|---|---|---|---|
| Age (years) | 62.0 ± 9.5 | 59.5 ± 9.4 | <0.001 |
| Gender | |||
| Male | 3530 (88.2%) | 473 (11.8%) | 0.001 |
| Female | 605 (92.5%) | 49 (9.4%) | |
| Race | |||
| Caucasian | 3886 (88.7%) | 497 (11.3%) | 0.32 |
| Non-Caucasian | 216 (90.8%) | 22 (9.2%) | |
| Greatest circle distance (miles) |
47.8 ± 149.6 | 38.4 ± 75.8 | 0.02 |
| Proportion in community | |||
| without high school diploma |
|||
| <21% no high school degree |
3570 (88.4%) | 470 (11.6%) | 0.06 |
| ≥21% no high school degree |
472 (91.1%) | 46 (8.9%) | |
| Income | |||
| ≥$38,000 | 3458 (88.3%) | 457 (11.7%) | 0.07 |
| <$38,000 | 581 (90.8%) | 59 (9.2%) | |
| Population type | |||
| <250,000 | 2667 (88.2%) | 1296 (89.5%) | 0.20 |
| ≥250,000 | 357 (11.8%) | 152 (10.5%) | |
| Insurance Status | |||
| No private insurance | 2036 (91.2%) | 196 (8.8%) | <0.001 |
| Private insurance | 2065 (86.6%) | 320 (13.4%) | |
| Charlson/Deyo Score | |||
| 0 | 3095 (88.4%) | 408 (11.6%) | 0.18 |
| 1 | 864 (90.5%) | 91 (9.5%) | |
| ≥2 | 176 (88.4%) | 23 (11.6%) | |
| Facility Type | |||
| Academic | 2173 (88.5%) | 282 (11.5%) | 0.53 |
| Community | 1953 (89.1%) | 239 (10.9%) | |
| Histology | |||
| Adenocarcinoma | 3129 (88.5%) | 405 (11.5%) | <0.001 |
| Squamous Cell | 635 (93.1%) | 47 (6.9%) | |
| Neoadjuvant type | |||
| Chemotherapy | 551 (75.9%) | 175 (24.1%) | <0.001 |
| Chemoradiation therapy | 3584 (91.2%) | 347 (8.8%) | |
| Path T | |||
| T0, Tis, T1 | 1083 (91.5%) | 100 (8.5%) | <0.001 |
| T2 | 1040 (90.9%) | 104 (9.1%) | |
| T3 | 1940 (86.1%) | 314 (13.9%) | |
| T4 | 71 (95.9%) | 3 (4.1%) | |
| Path N | |||
| N0 | 2214 (93.3%) | 159 (6.7%) | <0.001 |
| N1 | 1507 (84.9%) | 267 (15.1%) | |
| N2 | 258 (81.9%) | 57 (18.1%) | |
| N3 | 76 (68.5%) | 35 (31.5%) | |
| Positive Margins (≥R1) | 317 (7.8%) | 57 (11.2%) | 0.009 |
| Tumor size (mm) | 42.8 ± 46.4 | 43.1 ± 22.8 | 0.87 |
| Number of nodes examined |
13.4 ± 9.5 | 15.7 ± 10.1 | <0.001 |
| Number of positive nodes | 1.36 ± 2.66 | 2.64 ± 3.83 | <0.001 |
| Inpatient Length of Stay (days) |
13.0 ± 11.4 | 10.9 ± 7.5 | <0.001 |
| 30-day readmission | 260 (6.5%) | 26 (5.2%) | 0.24 |
Cox Proportional Hazards Model
Finally, a Cox-proportional hazards model was created (Table 4). Factors independently associated with a decreased overall mortality hazard included female gender, zip code area average income ≥ $35,000/year, private insurance status, and receiving adjuvant systemic therapy. Factors independently associated with an increased overall mortality hazard included increasing age, Charlson/Deyo comorbidity score of 1, clinical T4 stage, clinical ≥N1 or NX stage, positive surgical margins, increasing positive lymph node burden, and 30-day readmission after esophagectomy. Of note, despite a significant improvement in pCR rate, receiving neoadjuvant chemoradiation therapy did not significantly impact the overall mortality hazard (HR 1.12, 95% CI 0.97 – 1.30, p=0.12).
Table 4.
Cox Proportional Hazards Model (using backwards stepwise regression, with final model step shown). Variables entered into the model included age, gender, race, distance from treatment center, insurance status, population type, income, facility type, Charlson/Deyo comorbidity score, clinical T and N stage, histologic type, neoadjuvant therapy type, pathologic tumor size (mm), margin status, number of positive lymph nodes, readmission history, and adjuvant therapy status.
| Variable | Hazard Ratio (95% CI) | P value |
|---|---|---|
| Age (by year) | 1.01 (1.01 – 1.02) | <0.001 |
| Female Gender | 0.83 (0.72 – 0.91) | 0.01 |
| Income ≥ $35,000 | 0.79 (0.69 – 0.91) | 0.01 |
| Private Insurance | 0.86 (0.77 – 0.97) | 0.01 |
| Academic cancer center | 0.87 (0.80 – 0.98) | 0.02 |
| Charlson/Deyo Score (ref: 0) | ||
| 1 | 1.17 (1.03 – 1.32) | 0.02 |
| ≥2 | 1.09 (0.86 – 1.38) | 0.48 |
| Clinical T stage (ref: T0-T1) | ||
| T2 | 0.93 (0.73 – 1.19) | 0.57 |
| T3 | 0.99 (0.80 – 1.24) | 0.97 |
| T4 | 1.55 (1.12 – 2.16) | 0.01 |
| TX | 1.16 (0.88 – 1.54) | 0.30 |
| Clinical N stage (ref: N0) | ||
| N1 | 1.18 (1.05 – 1.32) | 0.01 |
| ≥ N2 | 1.38 (1.06 – 1.80) | 0.02 |
| NX | 1.39 (1.10 – 1.74) | 0.01 |
| Positive margin status (R1) | 1.85 (1.56 – 2.21) | <0.001 |
| Positive lymph nodes (per node) | 1.08 (1.06 – 1.09) | <0.001 |
| 30-day readmission | 1.47 (1.21 – 1.78) | <0.001 |
| Adjuvant chemotherapy | 0.74 (0.62 – 0.89) | 0.001 |
Discussion
The main findings of this NCDB study are that (1) neoadjuvant chemoradiation improves the pCR rate compared with chemotherapy alone, (2) patients with pCR at the time of esophagectomy demonstrate improved overall survival, but (3) there is no improvement in overall median survival with use of chemoradiation compared with chemotherapy. These findings from a large national dataset suggest that the relationship between the type of therapy, the pathologic response rate, and ultimate survival may be complex and perhaps multifactorial.
National guidelines currently recommend either neoadjuvant chemotherapy or chemoradiation therapy prior to esophagectomy in locally advanced squamous cell or adenocarcinoma of the esophagus. [6] From this analysis, it is notable that the majority of Commission on Cancer (CoC) accredited centers in the United States currently use neoadjuvant chemoradiation as their induction approach, with this percentage reaching approximately 90% in the most recent cohort of patients from 2010–2012. While a randomized controlled trial directly comparing the long-term outcomes of these neoadjuvant therapy approaches has never been completed, the national practice preference of neoadjuvant chemoradiation likely gained momentum by the improvement in pCR rates and impressive survival benefits seen in national Phase III randomized controlled trials such as CROSS (Netherlands) and CALGB 9781 (United States) which evaluated neoadjuvant chemoradiation followed by resection versus upfront esophagectomy. [5,7] Specifically, CROSS (which evaluated a regimen of weekly carboplatin, paclitaxel, and concurrent daily radiotherapy of 41.4 Gy in 23 fractions, followed by esophagectomy) demonstrated a pCR rate of 29%, and improvement in median OS of 49.4 months versus 24.0 months in the surgery only group. [5] CALGB 9781 (which used cisplatin, fluorouracil, and 50.4 Gy of radiation therapy over 5 weeks, followed by esophagectomy, but closed secondary to poor accrual) had a 40% pCR rate, and improvement in median OS of 4.5 years versus 1.8 years for surgery alone patients. [7] In comparison, earlier trials of neoadjuvant chemotherapy and surgery versus upfront resection reported pCR ranges of only 3–13%, although still demonstrating improved survival benefits. [8–10]
Previous institutional retrospective analyses have similarly found that neoadjuvant chemoradiation therapy improves pCR rates when compared to neoadjuvant chemotherapy. In contrast, however, several of these studies demonstrating an improved pCR rate with chemoradiotherapy did not demonstrate a significant difference in survival (at 1-, 3- or 5 years) when analyzed by neoadjuvant therapy type. [11] However, when neoadjuvant chemoradiation patients alone were analyzed by pCR status, a significant increase in median OS was detected (32.7 months versus 16.9 months for patients with residual disease, p=0.01). [11] Of note, a recent multicenter randomized clinical trial of neoadjuvant chemotherapy versus chemoradiation therapy for esophageal cancer patients (T1N1–3 or T2–3, any N) in Europe found that those receiving neoadjuvant chemoradiation therapy experienced higher rates of pCR (28% versus 9%), increased R0 resection (87% versus 74%), and lower rate of lymph node metastases (35% versus 62%). [12] However similar to our analysis, these improvements in tumor control at the time of esophagectomy did not translate into long-term survival benefits, with no difference in progression-free 3-year survival or 3-year overall survival. [12]
Of note, in the European RCT comparing neoadjuvant chemoradiation therapy to chemotherapy alone, there was no significant difference detected in 30- or 90-day mortality, or in the postoperative complication rate. [13] While there was no difference in the frequency of complications after esophagectomy, the severity of the complications in the neoadjuvant chemoradiation group were significantly higher than in the chemotherapy group, as measured on the Clavien-Dindo complication severity index, which factors in the need for medical, procedural, or surgical intervention, and ICU care. [13] Many reports comparing neoadjuvant chemoradiotherapy to upfront esophagectomy have been conflicting, with some institutions reporting increased rates of pneumonia, arrhythmias, sepsis, and respiratory failure, while others have reported no difference in postoperative complications. [14–16] Of note, a retrospective analysis at MD Anderson identified that the likelihood of thirty-day pulmonary and gastrointestinal postoperative complications following esophagectomy increased two- to threefold for patients that received 3-dimensional conformal radiation therapy (3D-CRT) when compared to those neoadjuvant chemoradiation patients that received either intensity modulated radiation therapy (IMRT) or proton beam therapy (PBT), respectively. [17] While the NCDB does not capture specific postoperative complications, we found no significant difference in inpatient length of stay, thirty-day readmission, thirty-day mortality, or ninety-day mortality by neoadjuvant therapy type.
Reconciling the lack of improvement in overall survival with neoadjuvant chemoradiation therapy despite increased pCR rates is likely multifactorial. From this NCDB analysis, we did see that while neoadjuvant chemoradiation therapy patients had improved pCR rates, for those that had persistent esophageal cancer, the use of adjuvant therapy was much lower than for neoadjuvant chemotherapy patients (8.8% versus 24.1%). Part of the decreased use of adjuvant systemic therapy in neoadjuvant chemoradiation patients could be due to lower mean pathologic positive lymph nodes and positive margin rate. On multivariate logistic regression, which accounted for these variables, neoadjuvant chemoradiation was still independently associated with a decreased likelihood of receiving adjuvant systemic therapy (OR 0.30). This was despite no difference in thirty day readmission or length of stay between the two neoadjuvant modalities. However, other post-esophagectomy complications, reintervention and frailty factors not captured by the NCDB could be influencing the willingness to give additional systemic therapy. Additionally it is possible that while neoadjuvant chemoradiation improves local tumor response as evidenced in the pathologic esophagectomy specimen, distant mechanisms of tumor escape and growth after neoadjuvant therapy is completed may still occur.
While this study demonstrated that pCR patients had an improved overall survival compared to non-pCR patients, an overall survival benefit with neoadjuvant chemoradiation could be obscured by the improved survival of neoadjuvant chemotherapy patients with persistent disease that go on to receive adjuvant therapy (whom also demonstrated a survival benefit in this analysis). While the NCDB does not record cancer recurrence or disease free survival, a previous pooled analysis of pCR patients (after neoadjuvant chemoradiation and esophagectomy) documented cancer recurrence in approximately 30% of ypT0N0M0 patients, with a median time to recurrence of 12 months, and median disease-free survival of 48 months. [18] If almost one-third of pCR patients are recurring, but are underutilizing adjuvant therapy, it could be a factor in the lack of apparent overall survival difference between the two neoadjuvant modalities seen in our analysis. While a comparison of neoadjuvant chemoradiation patients with pCR and subsequent recurrence to neoadjuvant chemotherapy patients with persistent disease that receive adjuvant therapy is not possible with this dataset, it is possible that these particular patient groups may demonstrate similar long-term survival profiles.
Another consideration for future prospective study could include monitoring of late appearing radiation complications that may compromise a long-term survival benefit. For example, radiation induced cardiac toxicity with standard 3D-CRT have been well-described in other chest malignancies (particularly those that have longer periods of cancer-free survival such as breast cancer and Hodgkin’s lymphoma), while data specific to esophageal cancer is emerging. [19–23] It has recently been suggested that the survival benefit imparted by the improved locoregional sterilization of disease by neoadjuvant chemoradiation therapy is allowing trimodality esophageal cancer patients to live long enough to develop and succumb to radiation induced cardiac comorbidities such as ischemic heart disease and heart failure. [24] It is plausible that using radiation therapies that have improved tumor targeting profiles with lower doses delivered to surrounding tissues, such as IMRT and PBT, could decrease the incidence of cardiac and pulmonary toxicities [25,26].
There are important limitations to this study that should be mentioned. This is a retrospective analysis of a large national database, and as such, may reflect selection biases for neoadjuvant therapy type that we cannot control for. Despite being a robust database in terms of years of treatment and number of patients, no data is available on the methods of clinical staging, presence or absence of esophageal cancer recurrence, or disease-free survival. We hypothesize that given the recent years of this analysis (2006–2012), multimodality staging with CT, PET, and EUS would likely have been used for a majority of patients, however we have no method to confirm that assumption. Even with multimodality staging, many patients may still be discordant from their actual stage, and therefore, our matching on clinical T and N status is not perfect. If patients that were actually higher T and N stages are disproportionally assigned to neoadjuvant chemoradiation therapy (i.e. patients that also presented with symptoms such as dysphagia), this could lead to a less than expected survival benefit among chemoradiation patients, despite a high initial pCR rate. Additionally, we are unable to account for patients that were started on a neoadjuvant regimen and became too ill, died, or no longer wanted surgical intervention during their treatment. From previous phase III trials, studies have shown that approximately 9–12% of patients are unable to finish their neoadjuvant course of chemotherapy or chemoradiation therapy, and these percentages may be higher in clinical practice (5, 27, 28). If the rates of neoadjuvant therapy completion are actually lower among chemoradiation patients compared to chemotherapy patients, this could potentially influence long-term survival.
In conclusion, the most reliable study to compare the short- and long-term performance of neoadjuvant chemotherapy versus chemoradiation therapy for locally advanced esophageal cancer, followed by esophagectomy, remains a randomized controlled trial. However, at this time the majority of centers in the United States are utilizing neoadjuvant chemoradiation therapy for locally advanced esophageal cancer, so such a trial may not be feasible in the current practice environment. Further efforts to try to study long-term outcomes among these two treatment modalities should include prospective analysis of adjuvant therapy use among a) neoadjuvant chemoradiation patients with persistent disease and b) neoadjuvant chemoradiation pCR patients that demonstrate recurrence, as well as monitoring and characterizing late-term radiation effects in these patients. If one (or all) of these factors are influencing the maximum potential benefit of neoadjuvant chemoradiation therapy, then tailored interventions and trials could be developed.
Acknowledgments
Pamela Samson, MD has grant support through NIH T32HL07776.
Varun Puri, MD, MSCI has grant funding through NIH K07CA178120 and K12CA167540-02.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Murphy CC, Correa AM, Ajani JA, Komaki RU, et al. Surgery is an essential component of multimodality therapy for patients with locally advanced esophageal adenocarcinoma. J Gastrointest Surg. 2013;17(8):1359–1369. doi: 10.1007/s11605-013-2223-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paul S, Altorki N. Outcomes in the Management of Esophageal Cancer. J Surg Oncol. 2014;110(5):599–610. doi: 10.1002/jso.23759. [DOI] [PubMed] [Google Scholar]
- 3.Dorth JA, Pura JA, Palta M, Willett CG, et al. Patterns of recurrence after trimodality therapy for esophageal cancer. Cancer. 2014;120(14):2099–2105. doi: 10.1002/cncr.28703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kidane B, Coughlin S, Vogt K, et al. Preoperative chemotherapy for resectable thoracic esophageal cancer. Cochrane Database Syst Rev. 2015 doi: 10.1002/14651858.CD001556.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Hagen P, Hulshof MC, van Lanschott JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366(22):2074–2084. doi: 10.1056/NEJMoa1112088. [DOI] [PubMed] [Google Scholar]
- 6.Little AG, Lerut AE, Harpole DH, Hofstetter WL, et al. The Society of Thoracic Surgeons Practice Guidelines on the Role of Multimodality Treatment for Cancer of the Esophagus and Gastroesophageal Junction. Ann Thorac Surg. 2014;98:1880–1885. doi: 10.1016/j.athoracsur.2014.07.069. [DOI] [PubMed] [Google Scholar]
- 7.Tepper J, Krasna MJ, Niedzwiecki D, Hollis D, et al. Phase III Trial of Trimodality Therapy with Cisplatin, Fluorouracil, Radiotherapy, and Surgery Compared With Surgery Alone for Esophageal Cancer: CALGB 9781. J Clin Oncol. 2008;26:1086–1092. doi: 10.1200/JCO.2007.12.9593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Korst RJ, Kansler AL, Port JL, Lee PC, et al. Downstaging of T or N Predicts Long-Term Survival After Preoperative Chemotherapy and Radical Resection for Esophageal Carcinoma. Ann Thorac Surg. 2006;82:480–485. doi: 10.1016/j.athoracsur.2006.03.072. [DOI] [PubMed] [Google Scholar]
- 9.Kelson DP, Ginsberg R, Pajak TF, et al. Chemotherapy followed by surgery compared with surgery alone for localized esophageal cancer. N Engl J Med. 1998;339:1979–1984. doi: 10.1056/NEJM199812313392704. [DOI] [PubMed] [Google Scholar]
- 10.Darton SJ, Archer VR, Stocken DD, Mulholland PJ, et al. Preoperative mitomycin, ifosfamide, and cisplatin followed by esophagectomy in squamous cell carcinoma of the esophagus: pathologic complete response induced by chemotherapy leads to long-term survival. J Clin Oncol. 2003;21:4009–4015. doi: 10.1200/JCO.2003.01.236. [DOI] [PubMed] [Google Scholar]
- 11.Luu TD, Gaur P, Force SD, Staley CA, et al. Neoadjuvant Chemoradiation Versus Chemotherapy for Patients Undergoing Esophagectomy for Esophageal Cancer. Ann Thorac Surg. 2008;85:1217–1224. doi: 10.1016/j.athoracsur.2007.11.070. [DOI] [PubMed] [Google Scholar]
- 12.Klevebro F, Alexandersson von Dobeln G, Wang G, et al. A randomized clinical trial of neoadjuvant chemotherapy versus neoadjuvant chemoradiotherapy for cancer of the oesophagus or gasto-oesophageal junction. Ann Oncol. 2016;27:660–667. doi: 10.1093/annonc/mdw010. [DOI] [PubMed] [Google Scholar]
- 13.Klevebro F, Johnsen G, Johnson E, et al. Morbidity and mortality after surgery for cancer of the oesophagus and gastro-oesophageal junction: A randomized clinical trial of neoadjuvant chemotherapy versus neoadjuvant chemoradiation. EJSO. 2015;41:920–926. doi: 10.1016/j.ejso.2015.03.226. [DOI] [PubMed] [Google Scholar]
- 14.Bosch DJ, Muijs CT, Mul VEM, Beukema JC, et al. Impact of Neoadjuvant Chemoradiotherapy on Postoperative Course after Curative-intent Transthoracic Esophagectomy in Esophageal Cancer Patients. Ann Surg Oncol. 2014;21:605–611. doi: 10.1245/s10434-013-3316-8. [DOI] [PubMed] [Google Scholar]
- 15.Reynolds JV, Ravi N, Hollywood D, Kennedy J, et al. Neoadjuvant chemoradiation may increase the risk of respiratory complications and sepsis after transthoracic esophagectomy. J Thorac Cardiovasc Surg. 2006;132:549–555. doi: 10.1016/j.jtcvs.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 16.Merritt RE, Whyte RI, D’Arcy NT, Hoang CD, Shrager JB. Morbidity and Mortality After Esophagectomy Following Neoadjuvant Chemoradiation. Ann Thorac Surg. 2011;92:2034–2040. doi: 10.1016/j.athoracsur.2011.05.121. [DOI] [PubMed] [Google Scholar]
- 17.Wang J, Wei C, Tucker SL, Myles B, et al. Predictors of Postoperative Complications After Trimodality Therapy for Esophageal Cancer. Int J Radiation Oncol Biol Phys. 2013;86(5):885–891. doi: 10.1016/j.ijrobp.2013.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luc G, Gronnier C, Lebreton G, Brigand C, et al. Predictive Factors of Recurrence in Patients with Pathological Complete Response After Esophagectomy Following Neoadjuvant Chemoradiotherapy for Esophageal Cancer: A Multicenter Study. Ann Surg Oncol. 2015 doi: 10.1245/s10434-015-4619-8. [DOI] [PubMed] [Google Scholar]
- 19.Paszat LF, Mackillop WJ, Groome PA, et al. Mortality from myocardial infarction after adjuvant radiotherapy for breast cancer in the surveillance, epidemiology, and end-results cancer registries. J Clin Oncol. 1998;16:2625–2631. doi: 10.1200/JCO.1998.16.8.2625. [DOI] [PubMed] [Google Scholar]
- 20.Darby SC, Ewertz M, McGale P, Bennet AM, et al. Risk of Ischemic Heart Disease in Women after Radiotherapy for Breast Cancer. N Engl J Med. 2013;368:987–998. doi: 10.1056/NEJMoa1209825. [DOI] [PubMed] [Google Scholar]
- 21.Aleman BM, van den Belt-Dusebout AW, Klokman WJ, et al. Long-term cause-specific mortality of patients treated for Hodgkin’s disease. J Clin Oncol. 2003;21:3431–3439. doi: 10.1200/JCO.2003.07.131. [DOI] [PubMed] [Google Scholar]
- 22.Kumekawa Y, Kaneko K, Ito H, Kurahashi T, et al. Late toxicity in complete response cases after definitive chemoradiotherapy for esophageal squamous cell carcinoma. J Gastroenterol. 2006;41:425–432. doi: 10.1007/s00535-006-1771-8. [DOI] [PubMed] [Google Scholar]
- 23.Gayed IW, Liu HH, Yusuf SW, Komaki R, et al. The prevalence of myocardial ischemia after concurrent chemoradiation therapy as detected by gated myocardial perfusion imaging in patients with esophageal cancer. J Nucl Med. 2006;47(11):1756–1762. [PubMed] [Google Scholar]
- 24.Beukema JC, van Luijk P, Widder J, Langendijk JA, Muijs CT. Is cardiac toxicity a relevant issue in the radiation treatment of esophageal cancer? Radiotherapy and Oncology. 2015;114:80–85. doi: 10.1016/j.radonc.2014.11.037. [DOI] [PubMed] [Google Scholar]
- 25.Kole TP, Aghayere O, Kwah J, Yorke ED, Goodman KA. Comparison of Heart and Coronary Artery Doses Associated with Intensity-Modulated Radiotherapy Versus Three-Dimensional Conformal Radiotherapy for Distal Esophageal Cancer. Int J Radiation Oncol Biol Phys. 2012;83(5):1580–1586. doi: 10.1016/j.ijrobp.2011.10.053. [DOI] [PubMed] [Google Scholar]
- 26.Lin SH, Komaki R, Liao Z, Wei C, et al. Proton Beam Therapy and Concurrent Chemotherapy for Esophageal Cancer. Int J Radiation Oncol Biol Phys. 2012;83(3):345–351. doi: 10.1016/j.ijrobp.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Allum WH, Stenning SP, Bancewicz J, Clark PI, et al. Long-Term Results of a Randomized Trial of Surgery With or Without Preoperative Chemotherapy in Esophageal Cancer. J Clin Oncol. 2009;27(30):5062–5067. doi: 10.1200/JCO.2009.22.2083. [DOI] [PubMed] [Google Scholar]
- 28.Boonstra JJ, Kok TC, Wijnhoven BP, van Heijl M, et al. Chemotherapy followed by surgery versus surgery alone in patients with resectable oesophageal squamous cell carcinoma: Long-term results of a randomized controlled trial. BMC Cancer. 2011;11(181) doi: 10.1186/1471-2407-11-181. [DOI] [PMC free article] [PubMed] [Google Scholar]