Abstract
Problem
To determine if miR-203 mediates endothelial inflammatory response in preeclampsia.
Method of study
Maternal vessel miR-203 expression was assessed by in situ hybridization. Suppressor of cytokine signaling-3 (SOCS-3) and ICAM expression was determined by immunostaining. Subcutaneous fat tissue sections from normal and preeclamptic pregnant women were used. MiR-203 induced inflammatory response was evaluated by measurements of IL-6, IL-8, ICAM, and VCAM expression and production, and neutrophil adhesion in endothelial cells (EC) transfected with miR-203 precursor, pre-miR-203. SOCS3 expression was also determined.
Results
Up-regulation of miR-203 and ICAM expression and down-regulation of SOCS-3 expression was demonstrated in maternal vessel endothelium in preeclampsia. Over-expression of miR-203 resulted in down-regulation of SOCS-3 expression and increases in production of IL-6, IL-8, ICAM and VCAM, and neutrophil adhesion in ECs.
Conclusion
As miR-203 is an inflammatory microRNA, increased miR-203 production/ expression in ECs could diminish anti-inflammatory activity and increase endothelial inflammatory response in preeclampsia.
Keywords: miR-203, SOCS-3, endothelial cells, adhesion molecule, inflammatory response, preeclampsia
Introduction
Preeclampsia is a hypertensive and multi-system disorder in human pregnancy. Although the cause of preeclampsia is not clear, increased systemic endothelial inflammatory responsiveness is believed to be a major pathophysiological event of maternal vascular dysfunction in preeclampsia. Elevated maternal levels of inflammatory cytokines such as IL-6, IL-8, IL-16, and TNFα [1, 2] and endothelial adhesion molecules ICAM and VCAM [3] are all considered hallmarks of increased vascular inflammatory response in preeclampsia [4]. Moreover, increased inflammatory response also contributes to increased endothelial solute permeability [5, 6] and oxidative stress [7] in this pregnancy disorder. The impact of preeclampsia on women to their long-term health outcome is further emphasized by the findings of increased risk of cardiovascular diseases and intensiveness of inflammatory responses later in life in women who had preeclampsia during their pregnancy [8, 9]. However, the underlying cellular and molecular mechanism of increased inflammatory response in preeclampsia remains unclear.
Cytokine signaling is negatively regulated by a family of proteins named suppressor of cytokine signaling (SOCS). The SOCS family has 8 members, including SOCS-1 to SOCS-7 and cytokine-induced STAT inhibitor (CIS). These molecules modulate cytokine signaling through several mechanisms, which include inactivation of Janus kinases (JAKs), blocking access of STATs to receptor binding sites, and ubiquitination of signaling proteins and their subsequent targeting to the proteasome [10, 11]. Among the SOCS family members, SOCS-3 has been well characterized to regulate IL-6, leptin, and erythropoietin through binding to their receptors [12, 13]. We previously reported that SOCS-3 expression was down-regulated in both maternal leukocytes and vascular endothelium in women with preeclampsia [14]. SOCS-3 expression was also reduced in placental trophoblasts in preeclampsia [15]. Although down-regulation of SOCS-3 activity/expression may lead to alteration of cytokine signaling and increased inflammatory responses, little is known about the mechanism of SOCS-3 regulation in vascular endothelial cells.
Emerging evidence has shown that numerous microRNAs (miRNAs) are able to regulate immune and inflammatory responses and dysregulation of miRNA expression closely links to increased inflammatory responses [16]. For example, miR-146 regulates IL-2 expression and activation [17]. MiR-155 promotes Th17 relevant cytokines and is induced during macrophage inflammatory response [18]. Increased miR-203 expression is found to be associated with down-regulation of SOCS-3 expression in infected gingival epithelial cells [19]. In contrast, silencing miR-203 reverses the inhibition of SOCS-3 expression [19]. Moreover, over-expression of miR-203 significantly increases IL-6 and MMP-1 production in synovial fibroblasts [20]. However, the role of miR-203 associated with endothelial inflammatory response has never been studied in preeclampsia. To test the hypothesis that increased miR-203 expression may lead to down-regulation of SOCS-3 expression and result in increased endothelial inflammatory responses in preeclampsia, we determined miR-203 expression in maternal vessels from normal and preeclamptic pregnancies and assessed anti-inflammatory effects of SOCS-3 on endothelial cells. The role of miR-203 mediated endothelial inflammatory response was further evaluated by IL-6, IL-8 and ICAM expression and production, and neutrophil-endothelial adhesion in endothelial cells.
Materials and Methods
Sample collection
Maternal subcutaneous fat tissue was collected during cesarean section delivery from normal and preeclamptic pregnancies. Freshly obtained subcutaneous fat tissue was fixed immediately with 10% formalin and then embedded with paraffin. Tissue sections were used for detection of miR-203 expression, and SOCS-3 and ICAM expression. Collection of subcutaneous fat tissue was approved by IRB at Louisiana State University Health Sciences Center – Shreveport (LSUHSC-S), LA. Normal pregnancy was defined as pregnancy with blood pressure (<140/90mmHg), absence of proteinuria and obstetrical and medical complications. Diagnosis of preeclampsia was defined as follows: sustained systolic blood pressure of ≥ 140 mmHg or a sustained diastolic blood pressure of ≥ 90mmHg on two separate readings; proteinuria measurement of 1+ or more on dipstick, or 24 hour urine protein collection with ≥ 300mg in the specimen. Smokers were excluded. None of the study subjects had signs of infection. To avoid clinical phenotypic differences in preeclamptic patients, patients complicated with HELLP syndrome (hemolysis, elevated liver enzyme and low platelet count), diabetes and/or renal disease were excluded. Subcutaneous fat tissue from 5 normal and 5 preeclamptic women were used in this study and the clinical characteristics of study subjects are presented in Table 1.
Table 1.
Clinical information of study subjects from which maternal vessels were examined in the study
| Variables | Normal (n=5) | Preeclampsia (n=5) | p value |
|---|---|---|---|
| Maternal age (years) | 29 ± 7 (20–37) | 26 ± 5 (20–33) | 0.4633 |
| Racial Status | |||
| White | 1 | 0 | ND |
| Black | 3 | 5 | ND |
| Other | 1 | 0 | ND |
| BMI | 35 ± 11 (23–51) | 34 ± 8 (26–44) | 0.8683 |
| Blood Pressure (mmHg) | |||
| Systolic | 113 ± 11 (101–130) | 164 ± 15 (148–182) | 0.0003 |
| Diastolic | 70 ± 7 (60–78) | 102 ± 7 (93–113) | <0.0001 |
| Primigravida | 0 | 4 | ND |
| Proteinuria | N/A | 1 – 4+ | ND |
| Uric acid | N/A | 6.7±1.9 (4.1–9.4) | ND |
| Gestational Age | |||
| at delivery (weeks) | 37+3 ± 2+2 (34+3–39+5) | 31+6 ± 3+4 (28–36+2) | 0.0317 |
Data are expressed as mean ± SD (range). ND: not determined.
Detection of miR-203 expression by in situ hybridization (ISH)
MiR-203 expression was determined by in situ hybridization on formalin-fixed, paraffin-embedded subcutaneous fat tissue sections using the miRCURY LNA™ microRNA ISH Optimization Kit (FFPE) (product number 90010) purchased from Exiqon (Vedbaek, Denmark). The Kit contains 5′-DIG and 3′-DIG labeled LNA™ scrambled miRNA control probe (5′-DIG/gtgtaacacgtctatacgccca /DIG-3′) that was used as negative control and 5′-DIG labeled LNA™ U6 snRNA control probe (5′-DIG/cacgaatttgcgtgtcatcctt/DIG-3′) that was used as positive control. The miRCURY LNA™ 5′-DIG and 3′-DIG labeled detection probe specific to hsa-miR-203 (probe number 88079-15, 5′-DIG/tagtggtcctaaacatttca/DIG-3′) was also purchased from Exiqon. The staining procedure was performed following the manufacturer’s instruction. A concentration of 100nM for hsa-miR-203 probe was used in the assay. Counter staining was carried out with Nuclear Fast Red (Vector Laboratories, Burlingame, CA). Stained slides were then reviewed under an Olympus microscope (Olympus IX71), and images were captured by PictureFrame computer software (Uptronics, Sunnyvale, CA) and recorded to a microscope linked PC computer.
Immunohistochemistry
Maternal vessel endothelium expression for SOCS-3 and ICAM were examined by immunohistochemistry (IHC) of subcutaneous tissue sections as previously described [14]. Primary monoclonal antibody against SOCS-3 (ab16030) was purchased from Abcam Inc. (Cambridge, MA) and antibody for ICAM (SC-7891) was from Santa Cruz (San Diego, CA). The antibody concentration of 1.0μg/ml was used for both SOCS3 and ICAM staining. Stained slides were counterstained with Gill’s formulation hematoxylin. Tissue sections stained with secondary antibody only were used as controls. All slides stained with the same antibody were processed at the same time. Stained tissue slides were reviewed under an Olympus microscope, and images were captured.
Endothelial isolation and culture
Human umbilical cord vein endothelial cells (HUVECs) from normal term placentas were isolated as previously described [21, 22] and used in the study. Cells were cultured with endothelial growth medium (Lonza, Allendale, NJ) supplemented with hydrocortisone, ascorbic acid, bovine brain extract, epidermal growth factor (EGF), gentamicin sulfate amphotericin (GA-1000), and fetal bovine serum (FBS). HUVECs at passage 2 were used for pre-mir-203 transfection or SOCS-3 gene transfer experiments.
Pre-miR-203 transfection
miR-203 over-expression was carried out by transfection of pre-miR-203 (miR-203 precursor, AM17100) (Ambion) in endothelial cells using LipofectAMINE RNAiMAX transfection reagent (Invitrogen). Pre-miR-203 at a concentration of 50nM was used in the transfection assay. Cells transfected with pre-miR negative control (a random sequence miRNA) served as control. Total RNA was extracted by TRIzol reagent approximately 40h after transfection. miR-203 expression and mRNA expression for IL-6, IL-8, and ICAM were then determined by RT-PCR. Expression of U6 snRNA was determined and served as an endogenous control for miRNA-203 expression and GADPH was determined and served as an endogenous control for IL-6, IL-8, and ICAM mRNA expression. The primer sequences and accession number for miR-203, U6, IL-6, IL-8, ICAM, and GADPH are presented in Table 2. Primers for miR-203 (HP300235) and U6 (HP300001) were purchased from OriGene Technologies, Inc. (Rockville, MD). Primers for IL-6, IL-8, ICAM, and GADPH were synthesized by Integrated DNA Technologies (IDT, www.idtdna.com). For SOCS-3 expression, cells were lysed 24, 48, and 72hrs after pre-miR-203 transfection using protein lysis buffer containing 50mmol/L Tris, 0.5% NP40, 0.5% Triton X-100 with protease and phosphatase inhibitors. Total cellular protein was then collected and SOCS-3 protein expression was then determined.
Table 2.
Primer sequences used in the study
| Gene Name | Primer Sequences | Accession # |
|---|---|---|
| miR-203a-3p | Forward: 5′-GTGAAATGTTTAGGACCAC-3′ | MIMAT0000264 |
| Reverse: 5′-GAACATGTCTGCGTATCTC-3′ | ||
| U6 | Forward: 5′-CTCGCTTCGGCAGCACAT-3′ | NR_004394.1 |
| Reverse: 5′-TTTGCGTGTCATCCTTGCG-3′ | ||
| IL-6 | Forward: 5′-AAAGAGGCACTGGCAGAAAA-3′ | NM_000600 |
| Reverse: 5′-AGCTCTGGCTTGTTCCTCCTCAC-3′ | ||
| IL-8 | Forward: 5′-TCTGCAGCTCTGTGTGAAGG-3′ | NM_000584 |
| Reverse: 5′-ACTTCTCCACAACCCTCTGC-3′ | ||
| ICAM-1 | Forward: 5′-GCTTTCCGGCGCCCAACGTGATTCTGA-3′ | NM_000201 |
| Reverse: 5′-ACTCACACAGGACACGAA GCTCCCGGGTCT-3′ | ||
| GADPH | Forward: 5′-CAAAAGGGTCATCATCTCTGC-3′ | NM_002046 |
| Reverse: 5′-AGTTGTCATGGATGACCTTGG-3′ |
Protein expression
Protein expression for SOCS-3 was determined by Western blot. Briefly, 10μg of total cellular protein was subject to electrophoresis (Bio-Red, Hercules, CA) and then transferred to nitrocellulose membranes. After blocking, the membrane was probed with SOCS-3 antibody (same antibody used for IHC) and then followed by a matched secondary antibody. An enhanced chemiluminescent (ECL) detection kit (Amersham Corporation, Arlington Heights, IL) and X-ray film were used to visualize the bound antibody. The membrane was then stripped and re-probed with β-actin antibody (Sigma). After scanned, the density of bands was analyzed by NIH Image J analysis program. β-actin expression was used to normalize SOCS-3 expression. Data were presented as mean ± SE from 4 independent experiments.
Endothelial IL-6, IL-8, and ICAM production
Endothelial production of IL-6, IL-8, and ICAM were measured in cell culture medium by enzyme-linked immunosorbent assay (ELISA). ELISA kits of IL-6, IL-8, and ICAM were purchased from R&D Systems, Inc. (Minneapolis, MN). All samples were measured in duplicate in each assay. All assays were carried out according to the manufacturer’s instructions. Within assay variations were <7% for all the assays.
Myeloperoxidase (MPO) assay for neutrophil-endothelial adhesion
MPO activity was assessed as an index of neutrophil adhesion. Neutrophils were isolated from healthy non-pregnant volunteers as previously described [23]. Neutrophil-endothelial adhesion assay was performed approximately 40h after pre-mir-203 transfection or 48h after SOCS-3 gene transfer. Freshly isolated neutrophils were applied to endothelial cells 30min after treatment with TNFα. The plates were then washed twice with 1.0ml PBS, and reaction was initiated by adding following reagents to each well in order: 750μl 80mM K2HPO4 (pH=4.5), 50 μl fresh 10%HETAB, 100 μl 16mMTMB. MPO activity was then measured by a VERSAmax microplate reader (Molecular Devices, Walpole, MA) at a wavelength of 450nm.
Construction of SOCS-3/ZsGreen1 GFP vector
Plasmid pZsGreen1-N1 (Clontech, Mountain View, CA) was used to construct SOCS-3 vector, pSOCS-3. Briefly, open reading frame of human SOCS-3 was amplified from human cDNA by polymerase chain reaction (PCR) using oligonucleotide to create restriction sites for Nhe I and Kpn I at 5′ and 3′ end of SOCS-3 sequence using following primers: sense primer 5′-AGCGCTAGCACCATGGTCACCCACAGCAAGTTTCC-3′ and antisense primer 5′-GGTGGTACCCAAAGCGGGGCATCGTACTG-3′. Restriction sites for Nhe I (5′GCTAGC3′) and Kpn I (5′GGTACC3′) are underlined, respectively. PCR was performed using pfx Taq polymerase (Invitrogen). PCR product and the vector pZsGreen1-N1 were digested with Nhe I (New England BioLabs, Inc. Ipswich, MA) and Kpn I (New England BioLabs, Inc.). After ligation, competent Ecoli-Top10 (Invitrogen, Carlsbad, CA) was transformed with plasmid and selected positive clones were amplified. SOCS-3 sequence was verified by Mclab (South San Francisco, CA).
SOCS-3 gene transfer
Electroporation was performed for SOCS-3 gene transfer. HUVECs at passage 2 were used for all experiments. For SOCS-3 (pSOCS-3/ZsGreen1) gene transfer, an aliquot of 20μg of plasmid DNA mixed with 5×106 cells in 600μl buffer per 4mm electroporation cuvette. A fixed electroporation was given with a capacitance of 950uf and 250v using a Bio-Rad Gene Pulser instrument (Bio-Rad, Hercules, CA). Cells transfected with ZsGreen1 vector only were used as control. Cell surface adhesion molecule ICAM and VCAM expression (see below), and neutrophil-endothelial adhesion assay were performed 24 hours after SOCS-3 gene transfer. Cells were also lysed with lysis buffer and total cellular protein was also collected for SOCS-3 protein expression determined by Western blot.
Endothelial surface molecule ICAM and VCAM expression
Endothelial surface molecule ICAM and VCAM expression was determined in cells transfected with SOCS-3 gene as previously described [21]. Briefly, cells were grown in 24 well/plate and fixed with 1% paraformaldehyde and then incubated with a primary antibody (mouse anti-human) to ICAM-1 (CD54) or VCAM-1 (CD106). Horseradish peroxidase-goat anti-mouse immunoglobulin G (Sigma) was used as the secondary antibody. Hydrogen peroxide (0.003%) and 3,30,5,50-tetramethylbenzidine (TMB) (0.1mg/ml) were used as substrate and color generation. The reaction was terminated by addition of 100μl of 8N H2SO4 to each well. Cells that reacted with secondary antibody only were used as background. After reaction, plates were read at 450 nm by VERSAmax microplate reader (Molecular Devices, Sunnyvale, CA, USA). All samples were tested in triplicate.
Statistical Analysis
Statistical analysis was performed with ANOVA or paired t-test by computer software Prism 5 (GraphPad Software, Inc. La Jolla, CA). Student-Newman-Keuls test was used as post hoc tests. Data is expressed as mean ± SE. A probability level of less than 0.05 was considered statistically significant.
Results
Endothelial miR-203 expression is increased in preeclampsia
To determine if endothelial cells express miR-203 and whether altered miR-203 expression is present in preeclampsia, miR-203 expression was examined by in situ hybridization in subcutaneous fat tissue sections. Our results showed that miR-203 expression was weakly expressed in vessel endothelium in tissues from normotensive pregnant women, but strongly expressed in vessel endothelium in 4 out of 5 tissues from women with preeclampsia. Figure 1A shows representative miR-203 expression in maternal vessel endothelium from 2 normal and 2 preeclamptic subjects. An imaging panel showing miR-203 expression in maternal vessel endothelium from each study subject (5 normal and 5 preeclampsia) is presented in supplement Figure 1. These pictorial data clearly show miR-203 expression is up-regulated in maternal vessel endothelium in preeclampisa. Since SOCS-3 is a target of miR-203 and ICAM is an indicator of increased inflammatory response in endothelial cells, endothelial expression of SOCS-3 and ICAM was examined. As shown in Figure 1B, endothelial SOCS-3 expression was markedly reduced, which was consistent with our previous findings of reduced SOCS-3 expression in women with preeclampsia [14]. In contrast, endothelial ICAM expression was markedly increased in maternal vessels from women with preeclampsia compared to those from normal pregnant controls. These results suggest that up-regulation of miR-203 expression was associated with reduced SOCS-3 expression and increased ICAM expression in maternal vessel endothelium in women with preeclampsia.
Figure 1. Expression of miR-203, SOCS-3, and ICAM in maternal vessel endothelium in normal and preeclamptic pregnancies.
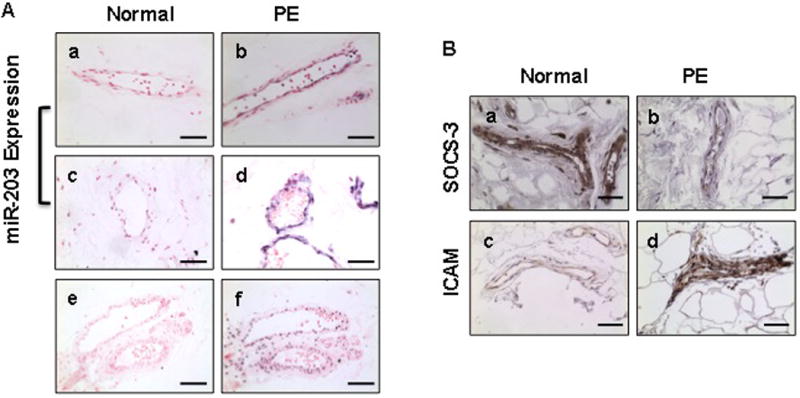
Subcutaneous fat tissue sections were used.
A: Representative images for miR-203 expression in maternal vessels from 2 normal and 2 preeclamptic pregnancies. miR-203 expression was not expressed in maternal vessels from normal pregnancies. However, strong miR-203 expression was observed in vessel endothelium in preeclampsia. a and c: normal; b and d: preeclampsia; e: negative control; and f: positive control U6 staining. a–d: bar = 50micron and e–f: bar = 100 micron.
B: Representative images for SOCS-3 and ICAM expression in maternal vessels from normal and preeclamptic pregnancies. SOCS-3 expression was markedly reduced and ICAM expression was markedly increased in maternal vessel endothelium from preeclampsia compared to normal pregnant controls. SOCS-3 and ICAM expression was undetectable in tissue sections stained with secondary antibody only (not shown). a and b: SOCS-3; c and d: ICAM. a and c: normal pregnancies; b and d: preeclampsia. bar = 50micron.
Over-expression of miR-203 results in down-regulation of SOCS-3 expression in endothelial cells
SOCS-3 is an important cellular anti-inflammatory regulator. miR-203 targets SOCS-3 [24]. To determine if increased miR-203 production/expression could down-regulate SOCS-3 expression in endothelial cells, endothelial cells were transfected with miR-203 precursor, pre-mir-203, and cells were collected 24, 48, and 72hrs after pre-mir-203 transfection. Total protein and RNA were extracted. As shown in Figure 2, mature miR-203 expression was markedly increased in cells transfected with pre-mir-203 compared to that in control cells (Figure 2A). In contrast, SOCS-3 expression was significantly reduced in cells transfected with pre-mir-203 and this inhibitory effect was in a time-dependent manner. The bar graph shows relative SOCS-3 protein expression in endothelial cells at 24, 48, and 72hrs after pre-mir-203 transfection, p<0.05, Figure 2B. Data are means ± SE from 4 independent experiments.
Figure 2. Transfection of miR-203 precursor results in up-regulation of miR-203 expression and down-regulation of SOCS-3 expression in endothelial cells.
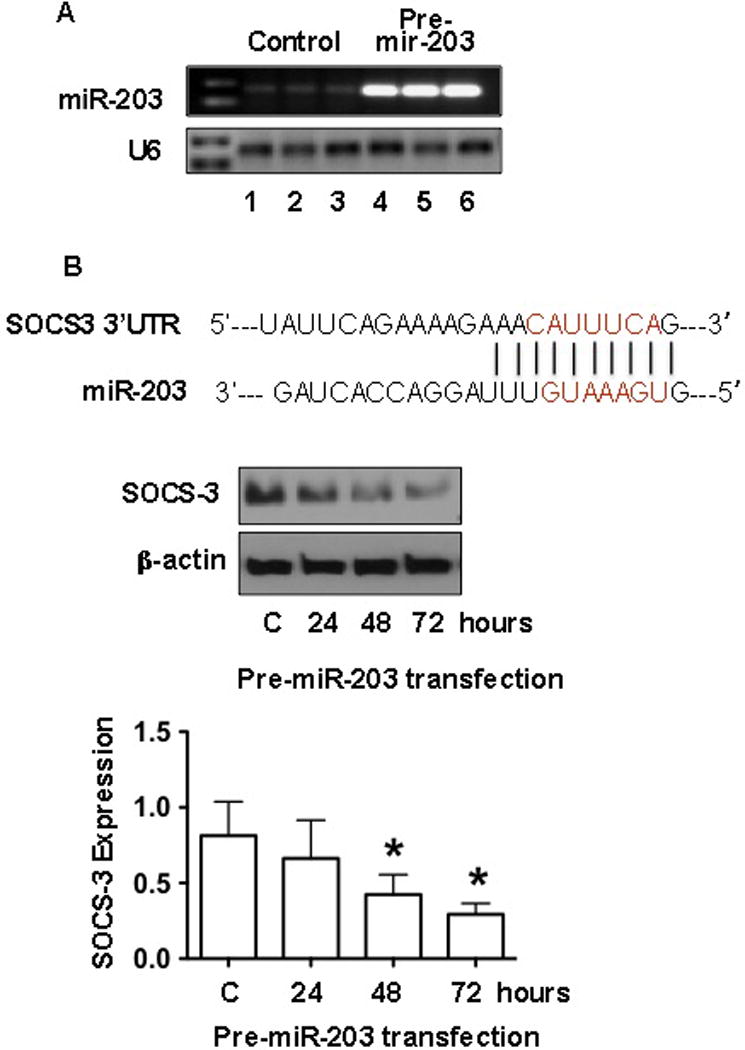
A: miR-203 expression was dramatically increased in endothelial cells transfected with pre-miR-203. U6 expression was determined as internal control for each sample. Lane 1–3: control cells were transfected with pre-mir control and lane 4–6: cells were transfected with pre-miR-203.
B: The upper sequences show the miRNA response elements (MREs) of miR-203 to the 3′ UTR of SOCS-3 mRNA that was predicted by TargetScan and miRBase Target. Red lined indicates the “seed” regions. The blot shows that SOCS-3 protein expression in endothelial cells transfected with pre-miR-203 at 24, 48, and 72hrs compared to untransfected control cells (C). Down-regulation of SOCS-3 expression was time-dependent in endothelial cells transfected with pre-miR-203. The bar graph was expressed as mean ± SE from 4 independent transfection experiments. * p<0.05: pre-miR-203 transfected cells vs. C. Statistical analysis was done by ANOVA and Newman-Keuls test was used as post hoc tests.
Over-expression of miR-203 promotes endothelial inflammatory response
As shown in Figure 1, increased miR-203 expression and increased ICAM expression were seen in maternal vessel endothelium. To further determine the consequences of increased miR-203 expression in endothelial cells, mRNA expression for IL-6, IL-8, and ICAM was examined in cells with or without transfection of pre-mir-203. Similar to miR-203 expression, mRNA expression for IL-6, IL-8 and ICAM was also significantly increased in cells transfected with pre-mir-203, Figure 3A. We further determined endothelial production of IL-6, IL-8, and ICAM by measuring IL-6, IL-8, and ICAM concentrations in cell culture medium. In this experiment, fresh medium was replaced 40 hours after pre-mir-203 transfection, and then medium was collected at 2, 4, and 8hrs and measured for IL-6, IL-8, and ICAM production by ELISA. Consistent with up-regulation of IL-6, IL-8 and ICAM mRNA expression, endothelial production of IL-6, IL-8 and ICAM were all significantly increased in cells transfected with pre-mir-203 compared to control cells and the increased IL-6, IL-8 and ICAM production was in a time-dependent manner, Figure 3B. These results clearly show that increase in miR-203 expression/production promotes endothelial expression and production of inflammatory cytokines and adhesion molecules.
Figure 3. Increased IL-6, IL-8, and ICAM expression and production, and increased neutrophil-endothelial adhesion in endothelial cells transfected with pre-miR-203.
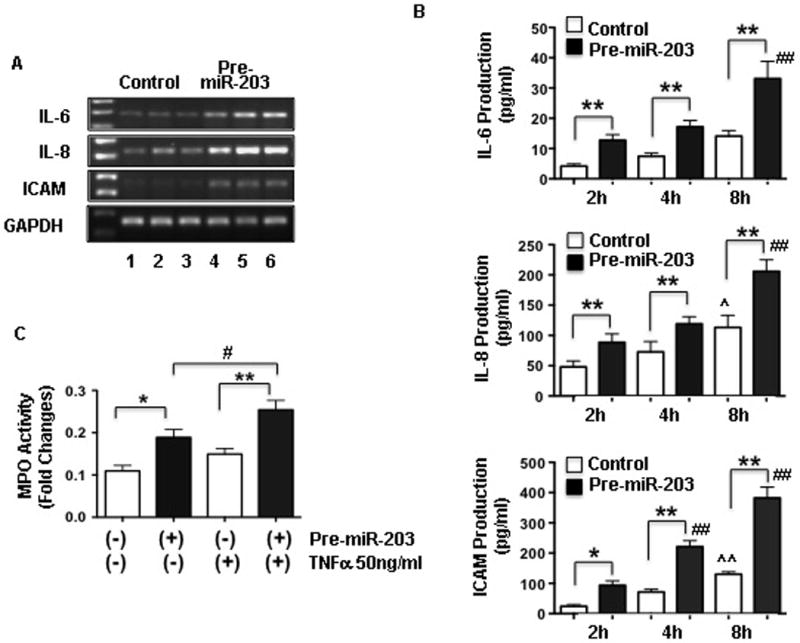
A: mRNA expression for IL-6, IL-8, and ICAM was increased in endothelial cells transfected with pre-miR-203 compared to control cells. Lane 1–3: control cells transfected with pre-miR control and lane 4–6: cells transfected with pre-miR-203.
B: Consistent with mRNA expression, IL-6, IL-8, and ICAM production was also significantly increased in cells transfected with pre-miR-203 compared to cells transfected with pre-miR controls. In this experiment, medium was changed 40hrs after transfection and newly added medium was then collected at 2, 4, and 8hrs and medium concentrations for IL-6, IL-8, and ICAM were measured by ELISA. IL-6, IL-8 and ICAM production was time-dependent increased in cells with or without pre-miR-203 transfection. However, cells transfected with pre-miR-203 produced significantly more IL-6, IL-8 and ICAM than cells transfected with control pre-miR. Data are presented as means ± SE from 6 independent experiments, * p<0.05 and ** p<0.01: pre-miR-203 transfected cells vs. control cells at each time point (tested by paired t-test). ˆ p<0.05 and ˆˆ p<0.01: control cells at 8hrs vs. 2hrs, and ## p<0.01: pre-miR-203 transfected cells at 4hrs or 8hrs vs. 2hrs (tested by ANOVA and Newman-Keuls test was used as post hoc tests), respectively.
C: MPO activity in endothelial cells transfected with pre-miR-203 with or without TNFα stimulation. Cells transfected with pre-miR control served as control. Neutrophil-endothelial adhesion was significantly increased in cells transfected with pre-miR-203 and further increased when TNFα was present in the culture. Data are expressed as mean ± SE from 3 independent experiments each in triplicate. * p<0.05 and ** p<0.01: pre-miR-203 transfected cells vs. controls; # p<0.05: pre-miR-203 transfected cells treated with TNFα vs. without TNFα treatment (by paired t-test), respectively.
Figure 3C shows neutrophil adhesion assessed by measuring of neutrophil myeloperoxidase (MPO) activity in endothelial cells transfected with pre-mir-203. Cells transfected with scramble pre-mir served as control. 40hrs after pre-mir-203 transfection, cells were treated with or without TNFα at a concentration of 50ng/ml for 2hrs and then freshly isolated neutrophils (1×105 cells/well in 24 well plate) were added to the cell culture. MPO activity was then determined. Our results showed that neutrophil adhesion was significantly increased in cells transfected with pre-mir-203 vs. control cells, p<0.05, Figure 3C. The increased neutrophil adhesion to endothelial cells was further increased in cells treated with TNFα. These results provide further evidence that up-regulation of miR-203 expression promotes endothelial inflammatory response.
SOCS-3 decreases endothelial inflammatory response
As shown in Figure 2 and 3, down-regulation of SOCS-3 expression was relevant to increased IL-6, IL-8, and ICAM expression and production in endothelial cells transfected with pre-miR-203. SOCS-3 is a target of miR-203. Reduced SOCS-3 expression has been demonstrated in maternal vessel endothelium in preeclampsia [14]. To further investigate if SOCS-3 modulates inflammatory response in endothelial cells, we constructed a SOCS-3/ZsGreen1 vector and transferred it into endothelial cells to determine if increase in SOCS-3 expression could increase anti-inflammatory activity in endothelial cells. Cells transfected with empty GFP vector were used as control. Cell adhesion molecule ICAM and VCAM expression and neutrophil-endothelial adhesion assay were performed 24hrs after SOCS3 gene transfer. Our results showed that SOCS-3 expression was increased in endothelial cells transfected with SOCS-3 gene (Figure 4A). In contrast, ICAM and VCAM expression were significantly reduced in cells transfected with SOCS-3 compared to control cells, p<0.01 (Figure 4B). Moreover, increased neutrophil-endothelial adhesion induced by TNFα were also significantly suppressed in cells transfected with SOCS-3 gene compared to those of controls, p<0.05 and p<0.01, respectively, Figure 4C.
Figure 4. Reduction of ICAM and VCAM expression and neutrophil-endothelial adhesion in endothelial cells transfected with SOCS-3 gene.
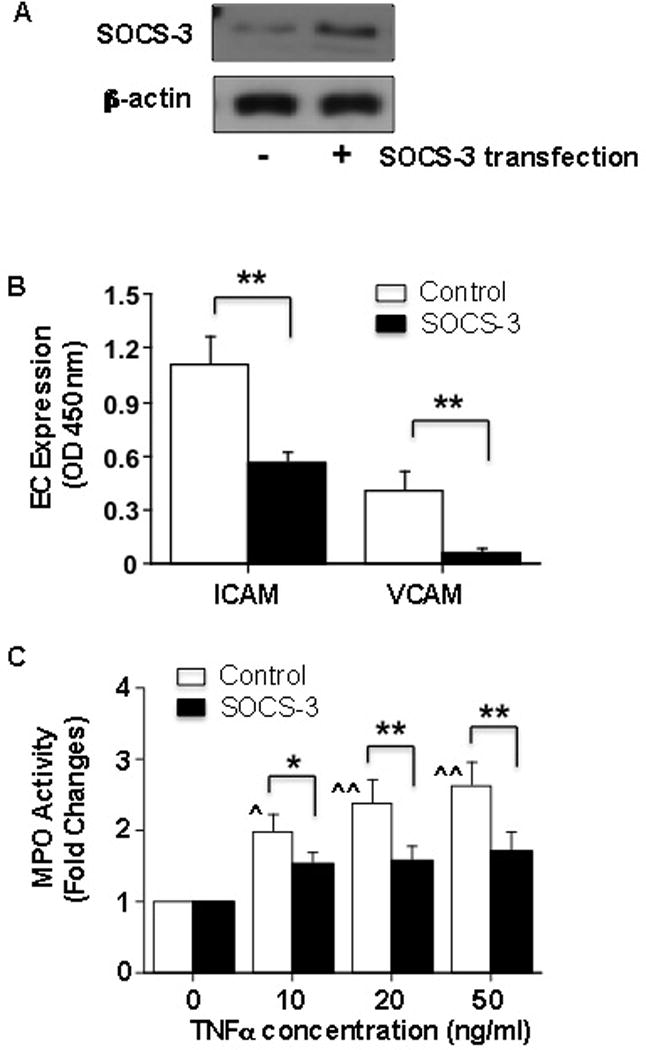
A: SOCS-3 protein expression is increased in ECs transfected with SOCS-3 gene compared to control cells. Total cellular protein was collected 48hrs after transfection.
B: Adhesion molecule ICAM and VCAM expression in ECs with or without transfection of SOCS-3 gene. Cells transfected with SOCS-3 expressed less ICAM and VCAM compared to the control cells, Data are presented as means ± SE from 4 independent experiments, ** p<0.01: SOCS-3 transfected cells vs. control cells.
C: Neutrophil-endothelial adhesion assessed by MPO activity in ECs with or without transfection of SOCS-3 gene. Data are presented as means ± SE from 4 independent experiments. In control cells, MPO activity was dose-dependently increased in cells treated with TNFα, ˆ p < 0.05 and ˆˆ p < 0.01: cells treated with TNFα vs. untreated cells. Data was analyzed by ANOVA with Student-Newman-Keuls test as post hoc test. MPO activity was significantly reduced when cells were transfected with SOCS-3 gene compared to the control cells treated with same dose of TNFα, * p<0.05 and ** p<0.01, respectively. Paired t-test was used for data analysis. MPO activity was not statistically significant in cells transfected with SOCS-3 with or without treatment of TNFα (analyzed by ANOVA).
Discussion
In the present study, we, for the first time, showed increased miR-203 expression in maternal vessel endothelium in women with preeclampsia compared to that in normal pregnant controls. We further found that over-expression of miR-203 expression could down-regulate cytokine suppressor SOCS-3 expression in endothelial cells, which was accompanied by increased endothelial inflammatory response. This was demonstrated by increased endothelial expression/production of IL-6, IL-8 and ICAM and increased neutrophil-endothelial adhesion in cells with over-expression of miR-203. The observation of up-regulation of ICAM expression in maternal vessel endothelium from preeclampsia is consistent as previously reported by Leik et al [25]. Thus, our findings are significant and provide new evidence that altered miR-203 expression could contribute to increased endothelial inflammatory response in preeclampsia. Although the number of maternal vessel samples in each group was small with different gestational age at delivery and primigravida between normal and preeclamptic pregnancies, and range of BMI within each of the groups, we believe that our ISH finding of increased miR-203 expression in maternal vessel endothelium from preeclampsia is reliable because the consistency of miRNA expression tested by ISH and real-time PCR was demonstrated in our previous published placental miRNA works [26].
MiRNAs have been recognized as a class of novel inflammatory regulators by modification of target genes at different levels through anti-inflammatory or pro-inflammatory signaling cascades in the cardiovascular system. Studies have shown that many miRNAs are involved in endothelial inflammation by directly or indirectly targeting the genes that regulate leukocyte recruitment. For example, miR-126 could inhibit VCAM-1 expression and limit leukocyte adherence to endothelial cells [27], while TNF-induced endothelial miR-31 and miR-17-3p expression could in turn suppress endothelial E-selectin and ICAM-1 expression induced by TNF stimulation [28]. An animal study has also shown that miR-181b inhibition exacerbated endotoxin-induced NF-κB activity, leukocyte influx, and lung injury [29]. Moreover, up-regulation of miR-146a, miR-9, miR-204 and miR-367 expression in senescent endothelial cells was also reported, and their predicted target genes include Toll-like receptor signaling (TLR) pathway, which is well known to play a pivotal role in inflammatory response, a key feature of senescence (inflammaging) [30]. It seems very likely that alteration of anti-inflammatory miRNA expression/production could directly disturb protective machinery of anti-inflammatory adaptive inflammatory responses.
MiR-203 was considered a skin- and keratinocyte-specific miRNA that was originally reported to be associated with common chronic inflammatory disorders in skin [31]. Up-regulation miR-203 expression was reported in psoriasis-affected skin plaques [32] and in diabetic foot ulcer skin tissues [33]. In the present study, we found increased miR-203 expression was in line with reduced SOCS-3 expression and increased ICAM expression in maternal vessel endothelium in women with preeclampsia compared to normal pregnant controls. Altered miR-203 expression was also reported in maternal plasma and placental tissue from preeclampsia [34]. It would be ideal to corroborate the findings of increased miR-203 in maternal vessel endothelium from preeclamptic pregnant women using a second approach such as real-time PCR. However, it is unlikely to obtain or isolate endothelial cells from systemic vasculature from pregnant women and to quantify the difference in miR-203 expression between normal and preeclamptic pregnancies, but our ISH results (supplement Figure 1) clearly showed that miR-203 expression is markedly increased in maternal vessel endothelium from preeclamptic pregnancies compared to that from normal pregnant women. Because SOCS-3 is a target of miR-203 [24] and SOCS-3 is an anti-inflammatory mediator, we then tested the consequences of miR-203 mediated inflammatory response in endothelial cells and determined effects of increased miR-203 expression on SOCS-3 and ICAM expression, and endothelial inflammatory response in cells transfected with miR-203 precursor, pre-miR-203. As we expected, miR-203 expression was dramatically increased in cells transfected with pre-mir-203. Moreover, cells transfected with pre-miR-203 resulted in a time-dependent down-regulation of SOCS-3 expression, which is inversely related to increased mRNA expression and production of inflammatory cytokine IL-6 and IL-8 and endothelial adhesion molecule ICAM in endothelial cells. These findings clearly showed consequences of miR-203 up-regulation mediated increased inflammatory responsiveness in endothelial cells.
MiR-203 mediated increased endothelial inflammatory response was further assessed by neutrophil-endothelial adhesion assay in endothelial cells transfected with miR-203 precursor pre-miR-203 in the presence or absence of TNFα. We found significant increases in neutrophil adhesion to endothelial cells, in which cells were transfected with pre-miR-203 compared to cells transfected with control pre-miRNA. We further found that pre-miR-203 could promote increased neutrophil adhesion to endothelial cells induced by TNFα. These data further demonstrate that increase in endothelial miR-203 expression could stimulate endothelial inflammatory response. We also noticed that pre-miR-203 transfection could induce TNFα expression in endothelial cells (data not shown). Although we did not validate the targeting effects of miR-203 on TNFα in endothelial cells, a study conducted by Primo et al, by screening a panel of cytokines that are up-regulated in psoriatic skin induced by miR-203, demonstrated that miR-203 could regulate inflammatory cytokine production of TNFα, IL-24, IL-15, and IL-17A, etc [35]. Their study suggests that miR-203 could serve to fine-tune cytokine signaling and may dampen skin immune responses by altering key pro-inflammatory cytokines [35].
SOCS-3 is an important anti-inflammatory mediator. Down-regulation of SOCS-3 expression by miR-203 is associated with increased endothelial inflammatory response. To further study endothelial anti-inflammatory activity mediated by SOCS-3, SOCS-3 gene transfer was used as a testing model, and endothelial ICAM and VCAM expression and neutrophil-endothelial adhesion were then determined. Our results clearly showed that endothelial ICAM and VCAM expression was significantly reduced in cells transfected with SOCS-3 gene. Moreover, over-expression of SOCS-3 could also suppress TNFα-induced increased neutrophil adhesion to endothelial cells. This data implies that down-regulation of SOCS-3 and increased inflammatory response in maternal vasculature could be consequences of increased miR-203 expression in preeclampsia.
Taken together, in this study we found that increased miR-203 expression is associated with reduced anti-inflammatory mediator SOCS-3 expression and increased endothelial adhesion molecule expression in maternal vessel endothelium in preeclampsia. We also demonstrated that over-expression of miR-203 resulted in increased inflammatory cytokines IL-6 and IL-8 and endothelial adhesion molecule ICAM expression/production and promoted neutrophil-endothelial adhesion. These findings provide considerable evidence that increased miR-203 expression could contribute to increased endothelial inflammatory response in preeclampsia.
Supplementary Material
Acknowledgments
This study was supported in part by grants from National Institute of Health, NHLBI R01 HL65997 and NICHD R21HD076289 to Yuping Wang.
References
- 1.Lyall F, Greer IA, Boswell F, Macara LM, Walker JJ, Kingdom JCP. The cell adhesion molecule, VCAM-1, is selectively elevated in serum in pre-eclampsia: does this indicate the mechanism of leucocyte activation? Br J Obstet Gynaecol. 1994;101:485–487. doi: 10.1111/j.1471-0528.1994.tb13146.x. [DOI] [PubMed] [Google Scholar]
- 2.Haller H, Ziegler E-M, Homuth V, Drab M, Eichhorn J, Nagy Z, Busjahn A, Vetter K, Luft FC. Endothelial adhesion molecules and leukocyte integrins in preeclamptic patients. Hypertension. 1997;29:291–296. doi: 10.1161/01.hyp.29.1.291. [DOI] [PubMed] [Google Scholar]
- 3.Taylor RN, Crombleholme WR, Friedman SA, Jones LA, Casal DC, Roberts JM. High plasma cellular fibronectin levels correlate with biochemical and clinical features of preeclampsia but cannot be attributed to hypertension alone. Am J Obstet Gynecol. 1991;165:895–901. doi: 10.1016/0002-9378(91)90435-t. [DOI] [PubMed] [Google Scholar]
- 4.Redman CWG, Sacks GP, Sargent IL. Preeclampsia: An excessive maternal inflammatory response to pregnancy. Am J Obstet Gynecol. 1999;180:499–506. doi: 10.1016/s0002-9378(99)70239-5. [DOI] [PubMed] [Google Scholar]
- 5.Wang Y, Gu Y, Granger DN, Roberts JM, Alexander JS. Endothelial junctional protein redistribution and increased monolayer permeability in HUVECs isolated during preeclampsia. Am J Obstet Gynecol. 2002;186:214–220. doi: 10.1067/mob.2002.119638. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Y, Gu Y, Li H, Lucas MJ, Wang Y. Increased endothelial monolayer permeability is induced by serum from women with preeclampsia but not by serum from women with normal pregnancy or that are not pregnant. Hypertens Pregn. 2003;22:121–131. doi: 10.1081/PRG-120017008. [DOI] [PubMed] [Google Scholar]
- 7.Davidge ST. Oxidative stress and altered endothelial cell function in preeclampsia. Sem Reprod Endocrinol. 1998;16:65–73. doi: 10.1055/s-2007-1016254. [DOI] [PubMed] [Google Scholar]
- 8.Bellamy L, Casas JP, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ. 2007;335(7627):974. doi: 10.1136/bmj.39335.385301.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Freeman DJ, McManus F, Brown EA, Cherry L, Norrie J, Ramsay JE, Clark P, Walker ID, Sattar N, Greer IA. Short- and long-term changes in plasma inflammatory markers associated with preeclampsia. Hypertension. 2004;44:708–714. doi: 10.1161/01.HYP.0000143849.67254.ca. [DOI] [PubMed] [Google Scholar]
- 10.Krebs DL, Hilton DJ. SOCS proteins: negative regulators of cytokine signaling. Stem Cells. 2001;19:378–387. doi: 10.1634/stemcells.19-5-378. [DOI] [PubMed] [Google Scholar]
- 11.Alexander WS, Starr R, Metcalf D, Nicholson SE, Farley A, Elefanty AG, Brysha M, Kile BT, Richardson R, Baca M, Zhang JG, Willson TA, Viney EM, Sprigg NS, Rakar S, Corbin J, Mifsud S, DiRago L, Cary D, Nicola NA, Hilton DJ. Suppressors of cytokine signaling (SOCS): negative regulators of signal transduction. J Leukoc Biol. 1999;66:588–592. doi: 10.1002/jlb.66.4.588. [DOI] [PubMed] [Google Scholar]
- 12.Alexander WS, Hilton DJ. The role of suppressors of cytokine signaling (SOCS) proteins in regulation of the immune response. Annu Rev Immunol. 2004;22:503–529. doi: 10.1146/annurev.immunol.22.091003.090312. [DOI] [PubMed] [Google Scholar]
- 13.Howard JK, Flier JS. Attenuation of leptin and insulin signaling by SOCS proteins. Trends Endocrinol Metab. 2006;17:365–371. doi: 10.1016/j.tem.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y, Lewis DF, Gu Y, Zhao S, Groome LJ. Elevated maternal soluble gp130 and IL-6 levels and reduced gp130 and SOCS-3 expressions in women with preeclampsia. Hypertension. 2011;57:336–342. doi: 10.1161/HYPERTENSIONAHA.110.163360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao S, Gu Y, Dong Q, Fan R, Wang Y. Altered IL-6 receptor, IL-6R and gp130, production and expression and decreased SOCS-3 expression in placentas from women with preeclampsia. Placenta. 2008;29:1024–1028. doi: 10.1016/j.placenta.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Connell RM, Rao DS, Baltimore D. microRNA regulation of inflamatory responses. Annu Rev Immunolm. 2012;30:295–312. doi: 10.1146/annurev-immunol-020711-075013. [DOI] [PubMed] [Google Scholar]
- 17.Sonkoly E, Ståhle M, Pivarcsi A. MicroRNAs and immunity: novel players in the regulation of normal immune function and inflammation. Semin Cancer Biol. 2008;18:131–140. doi: 10.1016/j.semcancer.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 18.O’Connell RM, Kahn D, Gibson WS, Round JL, Scholz RL, Chaudhuri AA, Kahn ME, Rao DS, Baltimore D. MicroRNA-155 promotes autoimmune inflammation by enhancing inflammatory T cell development. Immunity. 2010;33:607–619. doi: 10.1016/j.immuni.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moffatt CE, Lamont RJ. Porphyromonas gingivalis induction of microRNA-203 expression controls suppressor of cytokine signaling 3 in gingival epithelial cells. Infect Immun. 2011;79:2632–2637. doi: 10.1128/IAI.00082-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stanczyk J, Ospelt C, Karouzakis E, Filer A, Raza K, Kolling C, Gay R, Buckley CD, Tak PP, Gay S, Kyburz D. Altered expression of microRNA-203 in rheumatoid arthritis synovial fibroblasts and its role in fibroblast activation. Arthritis Rheum. 2011;63:373–381. doi: 10.1002/art.30115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Y, Adair CD, Coe L, Weeks JW, Lewis DF, Alexander JS. Activation of endothelial cells in preeclampsia: Increased neutrophil-endothelial adhesion correlates with up-regulation of adhesion molecule P-selectin in human umbilical vein endothelial cells isolated from preeclampsia. J Soc Gynecol Investig. 1998;5:237–243. doi: 10.1016/s1071-5576(98)00023-9. [DOI] [PubMed] [Google Scholar]
- 22.Jaffe EA, Nachman RL, Becker CG, Minick CR. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973;52:2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Y, Adair CD, Weeks JW, Lewis DF, Alexander JS. Increased neutrophil-endothelial adhesion induced by placental factors is mediated by platelet-activating factor in preeclampsia. J Soc Gynecol Investig. 1999;6:136–141. doi: 10.1016/s1071-5576(99)00004-0. [DOI] [PubMed] [Google Scholar]
- 24.Wei T, Orfanidis K, Xu N, Janson P, Ståhle M, Pivarcsi A, Sonkoly E. The expression of microRNA-203 during human skin morphogenesis. Exp Dermatol. 2010;19:854–856. doi: 10.1111/j.1600-0625.2010.01118.x. [DOI] [PubMed] [Google Scholar]
- 25.Leik CE, Walsh SW. Neutrophils infiltrate resistance-sized vessels of subcutaneous fat in women with preeclampsia. Hypertension. 2004;44:72–77. doi: 10.1161/01.HYP.0000130483.83154.37. [DOI] [PubMed] [Google Scholar]
- 26.Gu Y, Sun J, Groome LJ, Wang Y. Differential miRNA expression profiles between the first and third trimester human placentas. Am J Physiol Endocrinol Metab. 2013;304:E836–843. doi: 10.1152/ajpendo.00660.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harris TA, Yamakuchi M, Ferlito M, Mendell JT, Lowenstein CJ. MicroRNA-126 regulates endothelial expression of vascular cell adhesion molecule 1. Proc Natl Acad Sci U S A. 2008;105:1516–1521. doi: 10.1073/pnas.0707493105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suárez Y, Wang C, Manes TD, Pober JS. Cutting edge: TNF-induced microRNAs regulate TNF-induced expression of E-selectin and intercellular adhesion molecule-1 on human endothelial cells: feedback control of inflammation. J Immunol. 2010;184:21–25. doi: 10.4049/jimmunol.0902369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun X, Icli B, Wara AK, Belkin N, He S, Kobzik L, Hunninghake GM, Vera MP, MICU Registry. Blackwell TS, Baron RM, Feinberg MW. MicroRNA-181b regulates NF-κB-mediated vascular inflammation. J Clin Invest. 2012;122:1973–1990. doi: 10.1172/JCI61495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Olivieri F, Lazzarini R, Recchioni R, Marcheselli F, Rippo MR, Di Nuzzo S, Albertini MC, Graciotti L, Babini L, Mariotti S, Spada G, Abbatecola AM, Antonicelli R, Franceschi C, Procopio AD. MiR-146a as marker of senescence-associated pro-inflammatory status in cells involved in vascular remodelling. Age (Dordr) 2013;35:1157–1172. doi: 10.1007/s11357-012-9440-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sonkoly E, Ståhle M, Pivarcsi A. MicroRNAs: novel regulators in skin inflammation. Clin Exp Dermatol. 2008;33:312–315. doi: 10.1111/j.1365-2230.2008.02804.x. [DOI] [PubMed] [Google Scholar]
- 32.Sonkoly E, Wei T, Janson PC, Sääf A, Lundeberg L, Tengvall-Linder M, Norstedt G, Alenius H, Homey B, Scheynius A, et al. MicroRNAs: novel regulators involved in the pathogenesis of psoriasis? PLoS One. 2007;2:e610. doi: 10.1371/journal.pone.0000610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu J, Xu Y, Shu B, Wang P, Tang J, Chen L, Qi S, Liu X, Xie J. Quantification of the differential expression levels of microRNA-203 in different degrees of diabetic foot. Int J Clin Exp Pathol. 2015;8:13416–13420. [PMC free article] [PubMed] [Google Scholar]
- 34.Yang S, Li H, Ge Q, Guo L, Chen F. Deregulated microRNA species in the plasma and placenta of patients with preeclampsia. Mol Med Rep. 2015;12:527–534. doi: 10.3892/mmr.2015.3414. [DOI] [PubMed] [Google Scholar]
- 35.Primo MN, Bak RO, Schibler B, Mikkelsen JG. Regulation of pro-inflammatory cytokines TNFα and IL24 by microRNA-203 in primary keratinocytes. Cytokine. 2012;60:741–748. doi: 10.1016/j.cyto.2012.07.031. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


