Abstract
Objectives
To evaluate the nonclinical outcomes of a proactive palliative care program funded and operated by a health system for Medicare Advantage plan beneficiaries.
Design
Observational, retrospective study using propensity‐based matching.
Setting
A health system in southern California.
Participants
Individuals who received the intervention between 2007 and 2014 (n = 368) were matched with 1,075 comparison individuals within each of four disease groups: cancer, chronic obstructive pulmonary disease, heart failure, and dementia. All were known to be dead at the time of the retrospective study, were Medicare Advantage beneficiaries, and had 2 years of usage data before death. Median age at death for each disease group was older than 80.
Intervention
Home‐ and clinic‐based palliative care (PC) services provided by a multidisciplinary team.
Measurements
Outcomes included hospital costs, other healthcare costs, readmission rates, hospital admissions and bed days, intensive care unit use in final 30 days of life, and death within 30 days of an admission.
Results
Intervention participants in all four disease groups had less hospital use and lower hospital costs nonintervention participants, which drove lower overall healthcare costs. In the final 6 months of life, healthcare costs for the intervention groups stayed largely the same from month to month, whereas costs for comparison participants increased dramatically.
Conclusion
In the context of an alternative payment model in which the provider was “at risk” of bearing the costs of care, a proactive PC program helped to avoid the escalation in hospital use and costs commonly seen in the final months of life.
Keywords: palliative care, utilization, costs, Medicare Advantage, serious illness
Palliative care (PC) delivered proactively in ambulatory and community‐based settings to individuals with a variety of progressive, life‐limiting diseases concurrently with disease‐focused care is becoming more common.1, 2 This is due to several factors, including the increase in understanding of the needs and preferences of these individuals and their families,3, 4, 5, 6 research demonstrating the positive effects of earlier PC offered outside of inpatient hospital settings,2, 7, 8, 9 and pressure from payers to reduce overuse of hospitals.10, 11 The evaluation of one such program, the Transitions program at Sharp HealthCare in southern California, is described in this article.12
Program Description
Transitions is a concurrent care home‐based program designed for individuals with advanced chronic illness who would benefit from support provided by a trained specialty PC team comprising doctors, nurses, spiritual care providers, and social workers. The Transitions program has four components: in‐home medical consultation, ongoing evidence‐based prognostication of further survival, caregiver support, and advance healthcare planning. The team provides pain and nonpain symptom management, education to promote individual and family awareness of illness trajectory and treatment choices, and psychosocial and spiritual support.
In this concurrent care approach, PC is added to traditional disease‐focused care. The program has acute and maintenance phases. In the acute phase, a registered nurse helps the individual and family to develop structured medical goals, and a social worker helps them to develop a structured list of caregiver and family goals. During the acute phase, individuals receive four to six weekly home visits from the registered nurses, one to three home visits from the social workers, and home visits from spiritual care provider if needed.12 When the identified goals have been achieved, individuals move into the maintenance phase, in which they continue to receive home visits, although usually less frequently, supplemented with scheduled telephone calls for case management. All staff are trained in PC and aim to address advanced illness and end‐of‐life needs. The palliative physician role is primarily supervisory, with infrequent need for physicians to visit individuals’ homes. Chaplain services are provided when requested.
Individuals are identified through primary care providers, specialists, case managers, home health, or Sharp Extended Care (skilled nursing care program) staff using general and disease‐specific criteria. There are no automated triggers, but referring providers are encouraged to use a wide range of assessments before referral, such as assessment of functional abilities, disease‐specific and general laboratory results (e.g., best compensated N‐terminal pro brain‐type natriuretic peptide, blood urea nitrogen, creatinine), and the question, “Would you be surprised if the patient started to use the hospital to manage their direct disease issues?” Thus, referrals depend upon established providers’ prognostic tendencies, their relationships with their patients, and their understanding of the Transitions program. When identified by case managers or hospitalists as appropriate for Transitions, the individual's primary care provider must also agree to the referral because he or she will need to sign orders for the individual. The program mandates that Transitions participants continue to see their primary care provider and specialists as needed.
Participants are not required to have a Medicare Part A skilled need, to be homebound, or to have a life expectancy of less than 6 months, and they can refuse Transitions enrollment or services. The program focuses on Medicare Advantage beneficiaries seen in medical groups affiliated with Sharp Healthcare, for which Sharp is at full risk for hospital costs. Under such a payment mechanism, the clinical and business imperatives for earlier, proactive PC involvement are aligned.13 The program has been open to Pioneer accountable care organization and Medicare fee‐for‐service beneficiaries, but those populations have not widely used it, and this study focused on the Medicare Advantage beneficiaries and the costs of care for which Sharp was at full risk.
Methods
Data Source and Scope of Evaluation
Transitions participants’ responses to a brief survey of their experience with the program were summarized. Medical records data and billing and claims data were extracted and de‐identified for this evaluation. Sharp administrative systems provided costs per year for operating the Transitions programs, which were divided by the number of individuals served per year for a per‐person cost; detailed cost‐accounting of specific Transitions services per participant were not available. All participants in the intervention and comparison groups had Medicare Advantage insurance coverage, and almost all had at least one of four diseases that the Transitions program focused on during the study period: cancer, chronic obstructive pulmonary disease (COPD), heart failure (HF), and dementia. (A fifth participant group, geriatric frailty, was more recently added but not included in this evaluation.) The evaluation was limited to Transitions participants and comparison participants who had Medicare Advantage and one or more of those four diseases and 2 years of usage data before death. Participants with more than one disease were assigned to a single disease category based on the disease associated with the greatest healthcare charges in the 2 years of data. Data on Transitions enrollment, health plan enrollment, and healthcare usage was available from 2007 forward, so the sample was limited to individuals who died between 2009 and 2014. Sources of death data were medical records and linkage to the Social Security Death Index limited death master file. Based on these inclusion criteria, the initial dataset had 495 Transitions participants and 2,749 potential comparison participants (all deceased).
Exclusions and Matching
Because the study was using the first 6 months of the 24 months of usage data as a basis for matching, and it was desired that the intervention not contaminate the data, 76 intervention participants who had used Transitions services for longer than 18 months before death were excluded. Forty‐nine participants who enrolled in Transitions in the final 30 days of life were also excluded because some of the outcome measures focused on this period. The potential sample was thus reduced to 370 Transitions participants (38 cancer, 66 COPD, 174 HF, 92 dementia).
Matching and most analyses were conducted according to disease group because it was not assumed that trajectories of functional decline,14 participant characteristics, treatments, hospital usage, duration of Transitions enrollment, or magnitude of effect of Transitions would necessarily be equivalent across disease groups.
For observational research that is potentially subject to selection effects, various methods exist to attempt to control for heterogeneity between the intervention and comparison groups.15 The current study used propensity score matching.16, 17 Propensity scores were derived using logistic regression separately for each of the four diagnostic groups, with Transitions enrollment as the outcome. Variables in the propensity score were age, race (white, nonwhite), sex, and hospital and nonhospital charges in the first 6 months of the 2‐year period. The latter were used to represent tendency toward usage and severity of illness. Matching was performed based on the logit of the propensity score. This was greedy matching, nearest neighbor, with a tolerance of 0.2*SD(logit), as others have recommended.18 An attempt was made to match three controls for each intervention (Transitions) participant; all but two intervention participants were matched to at least one comparison participant.
Outcome variables focused on usage and costs for which the healthcare system was at risk. To be able to assess the effect of the Transitions program, usage needed to be assessed once the individual had entered the program. For comparison participants, an index date was created that was the same number of days before death as the matching intervention participant. Thus the time period of outcome measurement was defined according to a participant's enrollment date in Transitions relative to death date, and that period of time was also applied to that participants’ three matched comparison cases; the outcome measurement time period could range from 1 to 18 months. Because the number of days from index date to death varied for each matched set, variables that represented usage per month were also created. In other words, the outcome evaluation timeframe was from Transitions enrollment to death (and matching time frame for matched comparison participants), regardless of whether a participant used hospice during that time.
Costs were analyzed from the perspective of the expenditures for which the health system (Sharp HealthCare) would be at risk. This includes hospitalizations, outpatient care, home health, transportation services, diagnostic services, durable medical equipment, injectable drugs, chemotherapy agents, and professional services. It does not include hospice care, because Medicare Advantage beneficiaries revert to the traditional Medicare hospice benefit when they elect hospice. This cost measure is appropriate given the design of the Transitions program and the fact that it is self‐funded by Sharp HealthCare and not reimbursed by third‐party payers.
Statistical Analysis
Data were managed and analyzed using Microsoft SQL Server (Microsoft Corp., Redmond, WA) and SAS (SAS Institute, Inc., Cary, NC). Costs were standardized to 2014 values to control for medical inflation.19 For several outcomes represented by binary variables (death in hospital, hospitalized in last 30 days, intensive care unit use in last 30 days, and admission within 30 days of death), Cochran–Mantel–Haenszel methods were used to compare groups. For analysis of the cost variables, mixed‐model analysis of variance was used on the per‐month values. Generalized estimating equations (GEEs) (SAS Proc Genmod) with a negative binomial link were used for analysis of number of hospitalizations and number of hospital days. GEE Poisson regression was used for analysis of readmissions.
Results
Matching
Adequacy of matching was assessed by calculating the standardized differences between intervention and comparison beneficiaries for the matching variables.20 Standardized differences of 10% or less are preferred. For the 20 standardized differences calculated across the four disease groups, the range was 0.4–12.6%. Only two of 20 of these standardized differences exceeded 10% (10.6% for hospital charges in the COPD group, 12.6% for sex in the dementia group).
Program Costs
During the timeframe of the study (2007–14), the cost of operating the Transitions program was $4,585 per participant standardized to 2014 dollars. With an average time from enrollment to death of 7.14 months, the cost was $642 per participant per month. Because detailed cost accounting for Transitions services was not available at the encounter level, only this summary‐level of program costs is available.
Participant Experience
Table 1 shows the percentage of participants who chose very good in response to the seven questions asked regarding their experience with Transitions; other response options included good, fair, poor very poor, and not applicable. For six of the questions, 83–88% gave a response of very good. The lowest response was for improvement in quality of life, with 74% responding very good.
Table 1.
Responses to Participant Experience Survey
| Question | Very Good | All Valid Answers | Very Good, % |
|---|---|---|---|
| n | |||
| 1. The extent to which you were taught to manage your medications and symptoms related to your diagnosis | 313 | 377 | 83 |
| 2. The education you received regarding contacting the Transitions team at any time for assistance in managing your symptoms | 350 | 401 | 87 |
| 3. The assistance you received with long‐term care planning and advanced directives | 303 | 362 | 84 |
| 4. Improvement in your quality of life | 268 | 364 | 74 |
| 5. Effectiveness in reducing hospitalization and emergency room visits | 306 | 366 | 84 |
| 6. Assistance received from the nurse or medical social worker when problems occurred | 304 | 354 | 86 |
| 7. Likelihood of recommending the Sharp Transitions program to others for managing advanced chronic illness | 354 | 403 | 88 |
Participant Characteristics
The average age was greater than 81 for each disease group. The sample was predominantly Caucasian. Individuals with cancer were in Transitions an average of 4.8 months, and those in the other disease groups were enrolled for an average of slightly more than 7 months (Table 2).
Table 2.
Sample Characteristics of Matched Analysis Cohort According to Disease Group
| Cancer | COPD | CHF | Neuro | |||||
|---|---|---|---|---|---|---|---|---|
| Trans (n = 37) | Control (n = 111) | Trans (n = 65) | Control (n = 189) | Tran (n = 174) | Control (n = 499) | Trans (n = 92) | Control (n = 276) | |
| Age | 81.9 (7.4) | 82.4 (7.9) | 82.8 (7.7) | 83.4 (8.1) | 87.5 (6.6) | 87.1 (6.4) | 87.0 (5.9) | 87.0 (6.1) |
| White | 26 (70.3) | 81 (73.0) | 53 (81.5) | 150 (80.2) | 136 (78.2) | 393 (78.8) | 64 (69.6) | 191 (69.2) |
| Male | 22 (59.5) | 63 (56.8) | 26 (40) | 76 (40.6) | 77 (44.3) | 218 (43.7) | 35 (38.0) | 91 (33.0) |
| Hospital charges 19–24 months prior to death | 17,121 (36,447) | 17,431 (36,313) | 24,751 (88,782) | 18,381 (48,090) | 29,322 (91,199) | 23,796 (107,924) | 11,623 (32,577) | 9,182 (46,470) |
| Non‐hospital charges 19–24 months prior to death | 25,573 (38,612) | 24,408 (49,121) | 13,184 (16,292) | 11,631 (15,278) | 15,942 (32,949) | 17,240 (47,394) | 7,460 (12,978) | 7,065 (16,270) |
| Days to death | 144.6 | 144.6 | 221.6 | 220.4 | 223.2 | 222.5 | 215.3 | 215.3 |
Outcomes
For each disease, hospital costs and total costs per month were lower for Transitions participants (all P ≤ .002) (Table 3). For three of the four disease groups, there was not a significant difference in nonhospital costs (P = .32 for cancer, P = .08 for COPD, P = .09 for HF). For each disease, the percentage of participants hospitalized and the number of hospital days were lower for Transitions than for controls (all P ≤ .001). For each disease, the percentage being admitted in the final 30 days of life (P < .001), using the intensive care unit in the final 30 days of life (P < .001), and dying in the hospital (P < .001) were lower for Transitions than controls. The mean 30‐day readmission rate was lower for Transitions participants with COPD (P = .005), HF (P < .001), and dementia (P = .01) but not those with cancer (P = .08).
Table 3.
Outcomes for Transitions and Comparison Participants
| Outcome | Cancer | Chronic Obstructive Pulmonary Disease | Heart Failure | Dementia | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Transitions Participants, n = 37 | Comparison Participants, n = 111 | P‐Value | Transitions Participants, n = 65 | Comparison Participants, n = 189 | P ‐Value | Transitions Participants, n = 174 | Comparison Participants, n = 499 | P ‐Value | Transitions Participants, n = 92 | Comparison Participants, n = 276 | P‐Value | |
| Costs/month for hospital care, $, mean ± SD | 983.8 ± 2,776.3 | 5,194.8 ± 7,353.1 | .002 | 1,696.7 ± 4,424.7 | 5,859.1 ± 8,686.0 | <.001 | 1,432.8 ± 3,724.5 | 5,137.1 ± 7,077.1 | <.001 | 885.3 ± 3,098.8 | 3,574.6 ± 5,485.9 | <.001 |
| Costs/month for other care, $, mean±SD | 1,392.8 ± 2,810.7 | 2,081.5 ± 3,812.0 | .32 | 1,108.4 ± 1,721.4 | 1,604.8 ± 2,004.7 | .08 | 1,305.5 ± 1,746.1 | 1,690.4 ± 2,772.9 | .09 | 648.8 ± 822.8 | 1,291.2 ± 1,847.7 | .002 |
| All costs/month, $, mean ± SD | 2,376.6 ± 4,653.6 | 7,276.2 ± 8,566.9 | .002 | 2,805.1 ± 5,943.1 | 7,463.9 ± 9,640.5 | <.001 | 2,738.3 ± 4,917.6 | 6,827.5 ± 8,527.4 | <.001 | 1,534.1 ± 3,632.8 | 4,865.8 ± 6,541.0 | <.001 |
| In hospital at least once, % | 35.1 | 73.9 | <.001 | 40.0 | 84.0 | <.001 | 44.3 | 85.0 | <.001 | 33.7 | 76.1 | <.001 |
| Number of hospitalizations/month, mean ± SD | 0.14 ± 0.33 | 0.39 ± 0.40 | <.001 | 0.15 ± 0.30 | 0.35 ± 0.38 | <.001 | 0.14 ± 0.24 | 0.34 ± 0.35 | <.001 | 0.11 ± 0.27 | 0.27 ± 0.32 | <.001 |
| Number of hospital days/month, mean ± SD | 0.69 ± 1.84 | 2.62 ± 3.44 | .001 | 0.90 ± 1.73 | 1.89 ± 2.31 | .001 | 0.72 ± 1.58 | 2.17 ± 2.76 | <.001 | 0.75 ± 2.11 | 1.68 ± 2.56 | <.001 |
| Admitted within 30 days of death, % | 21.6 | 66.7 | <.001 | 23.1 | 73.8 | <.001 | 21.8 | 74.7 | <.001 | 17.4 | 63.0 | <.001 |
| Dying in hospital, % | 5.4 | 56.8 | <.001 | 7.7 | 63.1 | <.001 | 10.9 | 58.5 | <.001 | 5.4 | 51.1 | <.001 |
| 30‐day readmission rate, mean | 0.15 | 0.45 | .08 | 0.16 | 0.44 | .005 | 0.14 | 0.40 | <.001 | 0.11 | 0.35 | .01 |
| In intensive care unit during admission within 30 days of death, % | 10.8 | 45.0 | <.001 | 13.8 | 54.0 | <.001 | 12.6 | 57.7 | <.001 | 8.7 | 34.4 | <.001 |
All comparisons are within disease group and within the evaluation period (number of days from Transitions enrollment to death and same number of days before death for matched comparison cases). Transition program costs not included. SD = standard deviation.
Transitions participants were less likely to be admitted to the hospital during the evaluation period and in the final 30 days of life. The cost of hospital care for Transitions participants was a fraction of the cost for comparison participants. For example, the per‐participant per‐month expenditure for hospital care for participants with dementia was $885 for Transitions participants and $3,575 for comparison participants.
Trends in Usage in Final Months of Life
Across the disease groups, the healthcare cost per participant was analyzed for each of the 6 final months of life for Transitions participants who were enrolled for at least those final 6 months and their matched comparisons. Transitions participants’ costs increased only slightly in the final months of life (from $1,550 4 months before death to $3,711 in final month), whereas comparison participants’ costs increased dramatically (from $2,631 4 months before death to $17,006 in final month) (Figure 1).
Figure 1.
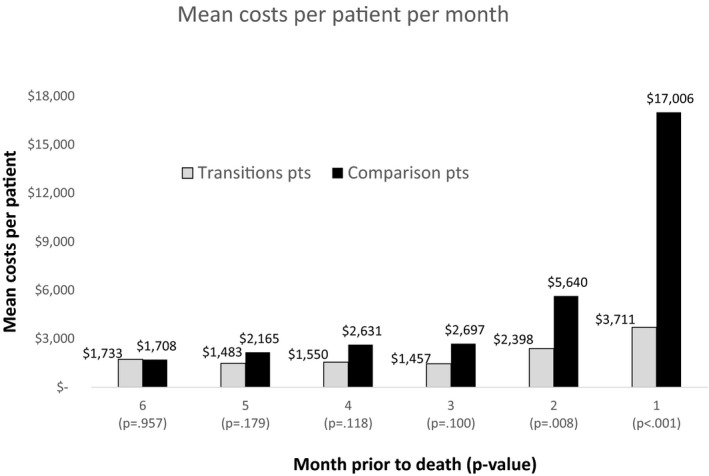
Costs per participant per month for final 6 months of life. Costs per participant per month limited to Transitions participants enrolled for at least the last 6 months before death (n = 178; n = 10 cancer, n = 35 chronic obstructive pulmonary disease (COPD), n = 91 heart failure (HF), n = 42 dementia) and their matched controls (n = 515; n = 30 cancer, n = 99 COPD, n = 260 HF, n = 126 dementia). The differences were statistically significant for the final 2 months of life. Transition program costs are not included.
Cost Reduction with Program Costs Included
Adding the $642 in costs per month for Transitions services to the Transitions group, the net savings per participant per month were $4,258 for cancer, $4,017 for COPD, $3,447 for HF, and $2,690 for dementia. The return on investment (net cost reduction divided by program costs) thus ranged from 4.2 for dementia to 6.6 for cancer.
Use of Hospice
Reliable data on hospice use for Transitions participants were available (almost all of whom used Sharp Hospice) but not for non‐Transitions participants. Therefore hospice use is reported only for Transitions participants in Table 4 according to disease group; 87% of Transitions participants used hospice.
Table 4.
Hospice Use of Transitions Participants
| Hospice Use | Cancer, n = 37 | Chronic Obstructive Pulmonary Disease, n = 65 | Heart Failure, n = 174 | Dementia, n = 92 |
|---|---|---|---|---|
| Using hospice, n (%) | 33 (89.2) | 59 (90.8) | 147 (84.4) | 81 (87.0) |
| Days from hospice enrollment to death, median | 15 | 39 | 44 | 46 |
| Days in hospice, mean ± standard deviation | 41.0 ± 64.5 | 91.4 ± 102.7 | 79.3 ± 92.2 | 82.5 ± 95.3 |
Discussion
Nationally, more Medicare beneficiaries are accessing hospice before death, but for many, enrollment does not occur until the final few days of life.4 Some health systems have created ways to offer specialized PC earlier in the course of their illness to individuals who are in need of an extra layer of support but who do not want or are not yet eligible for hospice.
In Medicare Advantage plans, the payer and the provider shared the financial risk for overuse of hospital care, rather than the payer alone, as is the case in fee‐for‐service or traditional Medicare. In this context, Sharp HealthCare invested resources to change the treatment of individuals, aligning a person‐centered PC approach with financial exposure.13 Among other things, the Sharp program encourages referring providers to identify individuals who are likely to start using the hospital to manage their disease process.
In this study, a proactive, community‐based PC program engaged participants several months before death, resulting in dramatically lower hospital usage and lower healthcare expenditures, achieved with modest program costs. Participants’ ratings of the program quality were high, and quality metrics such as death within 30 days of hospital admission and readmission rates also improved.
The effect on costs in this study is similar to what was found in a randomized controlled trial of home‐based PC services for individuals with HF, COPD, or cancer in a health maintenance organization payment model.7 In that study, the cost differential per participant per day was $118, or approximately $4,535 per month in 2014 dollars.19 In the current study, the net reduction in costs per participant per month in 2014 dollars ranged from $2,690 for dementia to $4,258 for cancer (Table 3), similar to the cost effect found in the earlier randomized controlled trial. In both studies, reduction in hospital usage and its costs determined the overall cost reduction. The results were also similar in effect to those reported in home‐based primary care studies.21, 22 The current study also included a dementia group, which a number of other studies have excluded (see 2 for review).
Benchmark data on hospice use for Medicare Advantage beneficiaries are not available at the regional level. Using Dartmouth Atlas of Health Care data from Medicare fee‐for‐service beneficiaries for 2012,23 the San Diego Health Referral Region had 52.5% of Medicare decedents enrolled in hospice at time of death and a mean of 27.2 days in hospice per decedent (median days not available). These provide some context for interpreting hospice use for Transitions participants, 87% of whom used hospice ranging from a mean of 41.0 days for cancer to 91.4 days for COPD.
Transitions participants with cancer spent fewer days in hospice than those averages, but the Transitions participants in the other disease groups had longer hospice lengths of service. Some of the effect of Transitions may be the result of high hospice referral rates, but this does not negate the importance of the Transitions program. The program is meant to provide a reasonable transition from fully disease‐focused care to fully comfort care (hospice) by providing concurrent PC in the interim. The interplay between the various forms of care could be explored in more depth in future studies if reliable data are available for intervention and comparison participants regarding hospice use.
Introducing PC earlier in the disease course may lead to a variety of positive person‐centered outcomes, including longer survival,8, 24 greater satisfaction with healthcare,1, 7 and fewer symptoms and better quality of life.1, 8, 25 For the vast majority of health systems, a positive financial return needs to accompany the positive clinical effect for sustained and widespread implementation.13, 26, 27 Medicare Advantage, as in this study, health maintenance organizations,7 or alternative payment models such as accountable care organizations better align the “clinical case” and the “business case.”13
Limitations
This was a retrospective, observational, data‐based study and as such is subject to concerns about potential bias in selection. R referring providers did not track refusals of individuals to whom they suggested the program. That said, the propensity‐based matching approach resulted in reasonably well‐balanced intervention and comparison groups. There are costs that were not captured in the methods, including some nonchemotherapeutic pharmacy costs and out‐of‐pocket costs, but all of the costs for which the program funder (the health system) was at full risk were captured. The four diseases do not necessarily represent the potential effect of this intervention on other diseases such as chronic kidney disease, although they represent a mixture of disease trajectories as depicted in a previous study.14 The current study focused only on Medicare Advantage beneficiaries in a single health system and thus may or may not be generalizable to other individuals in other settings and regions. It was not possible to gather reliable data on hospice enrollment for non‐Transitions participants; future studies could address this by purchasing data from Medicare (or other payers) regarding the duration and types of hospice services provided to both groups. For some participants, time in the intervention was limited, perhaps not providing much opportunity to change usage behavior, although individuals who were enrolled for fewer than 30 days were excluded. Clinical outcomes were not measured, and future studies of Transitions and similar programs should strive to do so.
Conclusions
Proactive PC provided in the home led to earlier hospice use and greatly reduced the escalation in hospital use and costs commonly seen in the final months of life. The results are similar in magnitude to those demonstrated in previously published controlled studies of community‐based PC and home‐based primary care28, 29 and demonstrate a reasonable return on investment for health systems at risk for healthcare costs. Community health systems that cannot afford the resources necessary for prospective, randomized trials can adopt the retrospective, claims‐based methods of this study.
Acknowledgments
The research team thanks Sharp and VCU staff who assisted with this project, including Scott Rogers, Fahd Benabdeljalil, John Rinaldi, and R. Michael Sarkissian. An oral presentation of this study was made at the annual assembly of the American Academy of Hospice and Palliative Medicine and the Hospice and Palliative Nurses Association, Chicago, Illinois, March 11, 2016.
This work was supported in part by National Cancer Institute Cancer Center Support Grant P30CA016059 to VCU Massey Cancer Center and Grants 17686 and 17373 from the California Healthcare Foundation. The funders did not play any role in the content of this paper.
Author Contributions: Cassel J. B., Hoefer D.: conception, design, interpretation of data, drafting manuscript, final approval for publication. Kerr K. M.: conception, design, interpretation of data, drafting manuscript, final approval for publication. McClish D. K.: design, analysis and interpretation of data, drafting manuscript, final approval for publication. Skoro N.: design, acquisition and analysis of data, drafting manuscript, final approval for publication. Johnson S.: conception, design, acquisition and interpretation of data, drafting manuscript; final approval for publication. Wanke C.: design, acquisition of data, drafting manuscript, final approval for publication.
J Am Geriatr Soc 64:2288‐2295, 2016.
References
- 1. Rabow MW, Smith AK, Braun JL et al. Outpatient palliative care practices. Arch Intern Med 2010;170:654–655. [DOI] [PubMed] [Google Scholar]
- 2. Rabow M, Kvale E, Barbour L et al. Moving upstream: A review of the evidence of the impact of outpatient palliative care. J Palliat Med 2013;16:1540–1549. [DOI] [PubMed] [Google Scholar]
- 3. Teno JM, Clarridge BR, Casey V et al. Family perspectives on end‐of‐life care at the last place of care. JAMA 2004;291:88–93. [DOI] [PubMed] [Google Scholar]
- 4. Teno JM, Gozalo PL, Bynum JP et al. Change in end‐of‐life care for Medicare beneficiaries: Site of death, place of care, and health care transitions in 2000, 2005, and 2009. JAMA 2013;309:470–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kirchhoff KT, Hammes BJ, Kehl KA et al. Effect of a disease‐specific advance care planning intervention on end‐of‐life care. J Am Geriatr Soc 2012;60:946–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pecanac KE, Repenshek MF, Tennenbaum D et al. Respecting choices® and advance directives in a diverse community. J Palliat Med 2014;17:282–287. [DOI] [PubMed] [Google Scholar]
- 7. Brumley RD, Enguidanos S, Jamison P et al. Increased satisfaction with care and lower costs: Results of a randomized trial of in‐home palliative care. J Am Geriatr Soc 2007;55:993–1000. [DOI] [PubMed] [Google Scholar]
- 8. Temel JS, Greer JA, Muzikansky A et al. Early palliative care for patients with metastatic non small‐cell lung cancer. N Engl J Med 2010;363:733–742. [DOI] [PubMed] [Google Scholar]
- 9. Lukas L, Foltz C, Paxton H. Hospital outcomes for a home‐based palliative medicine consulting service. J Palliat Med 2013;16:179–184. [DOI] [PubMed] [Google Scholar]
- 10. Kahn CN 3rd, Ault T, Potetz L et al. Assessing Medicare's hospital pay‐for‐performance programs and whether they are achieving their goals. Health Aff (Millwood) 2015;34:1281–1288. [DOI] [PubMed] [Google Scholar]
- 11. Carey K, Lin MY. Readmissions to New York hospitals fell for three target conditions from 2008 to 2012, consistent with Medicare goals. Health Aff (Millwood) 2015;34:978–985. [DOI] [PubMed] [Google Scholar]
- 12. Hoefer DR, Johnson SK, Binder M. Development and preliminary evaluation of an innovative advanced chronic disease care model. J Clin Outcomes Manag 2013;20:408–418. [Google Scholar]
- 13. Cassel JB, Kerr KM, Kalman NS et al. The business case for palliative care: Translating research into program development in the US. J Pain Symptom Manage 2015;50:741–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lunney JR, Lynn J, Foley DJ et al. Patterns of functional decline at the end of life. JAMA 2003;289:2387–2392. [DOI] [PubMed] [Google Scholar]
- 15. Starks H, Diehr P, Curtis JR. The challenge of selection bias and confounding in palliative care research. J Palliat Med 2009;12:181–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Garrido MM. Propensity scores: A practical method for assessing treatment effects in pain and symptom management research. J Pain Symptom Manage 2014;48:711–718. [DOI] [PubMed] [Google Scholar]
- 17. Garrido MM. Propensity scores and palliative care. J Palliat Med 2014;17:261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res 2011;46:399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bureau of Labor Statistics: Databases, Tables and Calculators by Subject. Available at http://data.bls.gov/timeseries/CUUR0000SAM?output_view=pct_12mths Accessed August 25, 2015.
- 20. Austin PC. Balanced diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity‐score matched samples. Stat Med 2009;28:3083–3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. DeJonge KE, Jamshed N, Daniel GD et al. Effects of home‐based primary care on Medicare costs in high‐risk elderly adults. J Am Geriatr Soc 2014;62:1825–1831. [DOI] [PubMed] [Google Scholar]
- 22. Edes T, Kinosian B, Vuckovic N et al. Better access, quality and cost for clinically complex veterans with home based primary care. J Am Geriatr Soc 2014;62:1954–1961. [DOI] [PubMed] [Google Scholar]
- 23. Dartmouth Atlas of Healthcare [on‐line]. Available at http://www.dartmouthatlas.org/ Accessed August 25, 2015.
- 24. Bakitas MA, Tosteson TD, Li Z et al. Early versus delayed initiation of concurrent palliative oncology care: Patient outcomes in the ENABLE III randomized controlled trial. J Clin Oncol 2015;33:1438–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bakitas M, Lyons KD, Hegel MT et al. Effects of a palliative care intervention on clinical outcomes in patients with advanced cancer: The project ENABLE II randomized controlled trial. JAMA 2009;302:741–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kerr CW, Donohue KA, Tangeman JC et al. Cost savings and enhanced hospice enrollment with a home‐based palliative care program implemented as a hospice‐private payer partnership. J Palliat Med 2014;17: 1328–1335. [DOI] [PubMed] [Google Scholar]
- 27. Kerr CW, Tangeman JC, Rudra CB et al. Clinical impact of a home‐based palliative care program: A hospice‐private payer partnership. J Pain Symptom Manage 2014;48:883–892.e1. [DOI] [PubMed] [Google Scholar]
- 28. Boling PA, Leff B. Comprehensive longitudinal health care in the home for high‐cost beneficiaries: A critical strategy for population health management. J Am Geriatr Soc 2014;62:1974–1976. [DOI] [PubMed] [Google Scholar]
- 29. Leff B, Weston CM, Garrigues S et al. Home‐based primary care practices in the United States: Current state and quality improvement approaches. J Am Geriatr Soc 2015;63:963–969. [DOI] [PubMed] [Google Scholar]


