Abstract
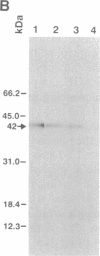
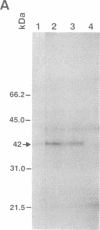
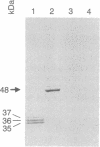
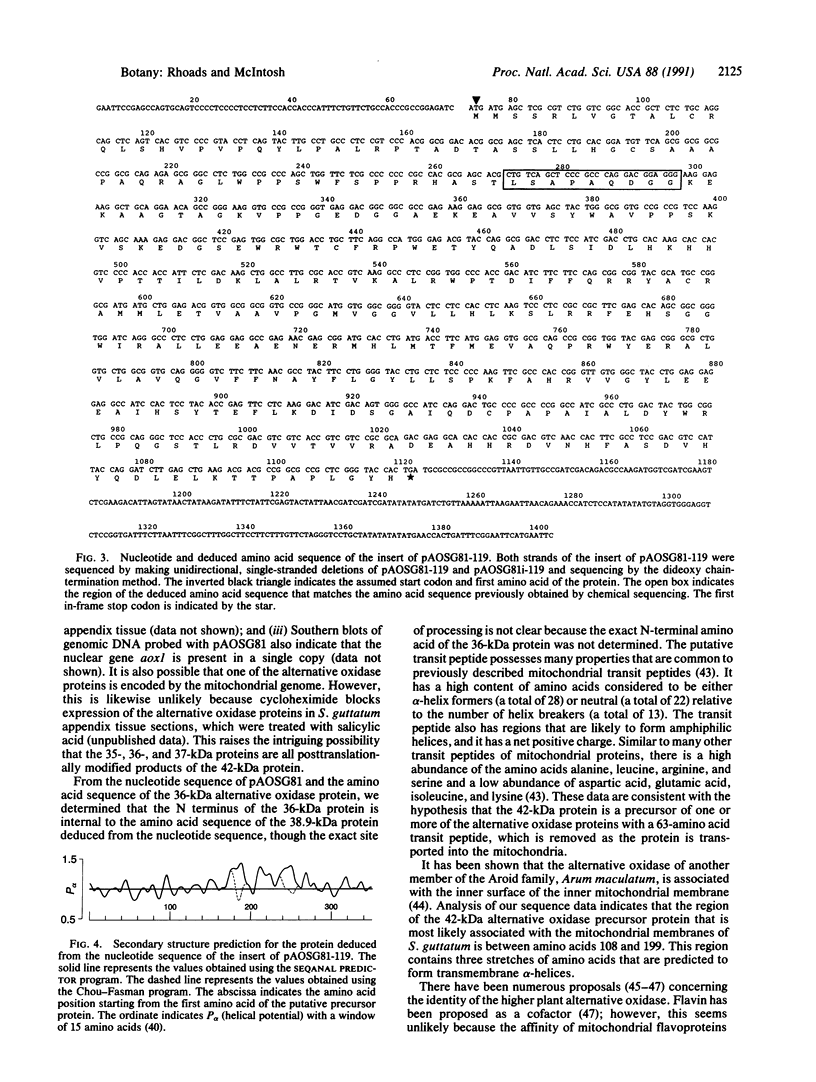
Polyclonal and monoclonal antibodies that recognize the 35-, 36-, and 37-kDa alternative oxidase proteins of Sauromatum guttatum (Schott) were used to isolate a cDNA clone, pAOSG81, from an S. guttatum cDNA expression library. A fusion protein with an apparent molecular mass of 48 kDa was expressed from a pUC119 derivative of pAOSG81 (pAOSG81-119) in Escherichia coli cells and was recognized by the monoclonal antibodies. When the in vitro translated and immunoprecipitated products made from mRNA hybrid-selected by pAOSG81 were analyzed, a single band corresponding to a protein with an apparent molecular mass of 42 kDa was observed. DNA sequence characterization showed that pAOSG81 contains the entire coding region of a protein with a calculated molecular mass of 38.9 kDa, a putative 63-amino acid transit peptide, and a 9-amino acid match to the authentic N-terminal sequence of the 36-kDa alternative oxidase protein. Analyses of the deduced amino acid sequence indicate: (i) that the transit peptide is predicted to form amphiphilic helices, and (ii) that three regions of the processed protein are likely to form transmembrane alpha-helices. We conclude from these data that pAOSG81 represents a nuclear gene, aox1, encoding a precursor protein of one or more of the alternative oxidase proteins of S. guttatum.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. J., Blobel G. Immunoprecipitation of proteins from cell-free translations. Methods Enzymol. 1983;96:111–120. doi: 10.1016/s0076-6879(83)96012-3. [DOI] [PubMed] [Google Scholar]
- BENDALL D. S. Cytochromes and some respiratory enzymes in mitochondria from the spadix of Arum maculatum. Biochem J. 1958 Nov;70(3):381–390. doi: 10.1042/bj0700381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendall D. S., Bonner W. D. Cyanide-insensitive Respiration in Plant Mitochondria. Plant Physiol. 1971 Feb;47(2):236–245. doi: 10.1104/pp.47.2.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake M. S., Johnston K. H., Russell-Jones G. J., Gotschlich E. C. A rapid, sensitive method for detection of alkaline phosphatase-conjugated anti-antibody on Western blots. Anal Biochem. 1984 Jan;136(1):175–179. doi: 10.1016/0003-2697(84)90320-8. [DOI] [PubMed] [Google Scholar]
- Bower T. G. The visual world of infants. Sci Am. 1966 Dec;215(6):80–passim. doi: 10.1038/scientificamerican1266-80. [DOI] [PubMed] [Google Scholar]
- Chamberlain J. P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979 Sep 15;98(1):132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Conformational parameters for amino acids in helical, beta-sheet, and random coil regions calculated from proteins. Biochemistry. 1974 Jan 15;13(2):211–222. doi: 10.1021/bi00699a001. [DOI] [PubMed] [Google Scholar]
- Cohen F. E., Abarbanel R. M., Kuntz I. D., Fletterick R. J. Secondary structure assignment for alpha/beta proteins by a combinatorial approach. Biochemistry. 1983 Oct 11;22(21):4894–4904. doi: 10.1021/bi00290a005. [DOI] [PubMed] [Google Scholar]
- Dale R. M., McClure B. A., Houchins J. P. A rapid single-stranded cloning strategy for producing a sequential series of overlapping clones for use in DNA sequencing: application to sequencing the corn mitochondrial 18 S rDNA. Plasmid. 1985 Jan;13(1):31–40. doi: 10.1016/0147-619x(85)90053-8. [DOI] [PubMed] [Google Scholar]
- Dry I. B., Moore A. L., Day D. A., Wiskich J. T. Regulation of alternative pathway activity in plant mitochondria: nonlinear relationship between electron flux and the redox poise of the quinone pool. Arch Biochem Biophys. 1989 Aug 15;273(1):148–157. doi: 10.1016/0003-9861(89)90173-2. [DOI] [PubMed] [Google Scholar]
- Elthon T. E., McIntosh L. Characterization and Solubilization of the Alternative Oxidase of Sauromatum guttatum Mitochondria. Plant Physiol. 1986 Sep;82(1):1–6. doi: 10.1104/pp.82.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elthon T. E., McIntosh L. Identification of the alternative terminal oxidase of higher plant mitochondria. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8399–8403. doi: 10.1073/pnas.84.23.8399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elthon T. E., Nickels R. L., McIntosh L. Monoclonal antibodies to the alternative oxidase of higher plant mitochondria. Plant Physiol. 1989 Apr;89(4):1311–1317. doi: 10.1104/pp.89.4.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry M. F., Nyns E. D. Cyanide-insensitive respiration. An alternative mitochondrial pathway. Subcell Biochem. 1975 Mar;4(1):1–65. [PubMed] [Google Scholar]
- Hiser C., McIntosh L. Alternative Oxidase of Potato Is an Integral Membrane Protein Synthesized de Novo during Aging of Tuber Slices. Plant Physiol. 1990 May;93(1):312–318. doi: 10.1104/pp.93.1.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huq S., Palmer J. M. Isolation of a cyanide-resistant duroquinol oxidase from Arum maculatum mitochondria. FEBS Lett. 1978 Nov 15;95(2):217–220. doi: 10.1016/0014-5793(78)80997-1. [DOI] [PubMed] [Google Scholar]
- Konigsberg W. H., Henderson L. Removal of sodium dodecyl sulfate from proteins by ion-pair extraction. Methods Enzymol. 1983;91:254–259. doi: 10.1016/s0076-6879(83)91022-4. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lambowitz A. M., Sabourin J. R., Bertrand H., Nickels R., McIntosh L. Immunological identification of the alternative oxidase of Neurospora crassa mitochondria. Mol Cell Biol. 1989 Mar;9(3):1362–1364. doi: 10.1128/mcb.9.3.1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambowitz A. M., Slayman C. W. Cyanide-resistant respiration in Neurospora crassa. J Bacteriol. 1971 Dec;108(3):1087–1096. doi: 10.1128/jb.108.3.1087-1096.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh L., Cattolico R. A. Preservation of algal and higher plant ribosomal RNA integrity during extraction and electrophoretic quantitation. Anal Biochem. 1978 Dec;91(2):600–612. doi: 10.1016/0003-2697(78)90546-8. [DOI] [PubMed] [Google Scholar]
- Moore A. L., Bonner W. D. Measurements of membrane potentials in plant mitochondria with the safranine method. Plant Physiol. 1982 Nov;70(5):1271–1276. doi: 10.1104/pp.70.5.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore A. L., Rich P. R., Bonner W. D., Jr, Ingledew W. J. A complex EPR signal in mung bean mitochondria and its possible relation to the alternate pathway. Biochem Biophys Res Commun. 1976 Oct 4;72(3):1099–1107. doi: 10.1016/s0006-291x(76)80245-8. [DOI] [PubMed] [Google Scholar]
- Raskin I., Ehmann A., Melander W. R., Meeuse B. J. Salicylic Acid: a natural inducer of heat production in arum lilies. Science. 1987 Sep 25;237(4822):1601–1602. doi: 10.1126/science.237.4822.1601. [DOI] [PubMed] [Google Scholar]
- Rich P. R., Moore A. L. The involvement of the protonmotive ubiquinone cycle in the respiratory chain of higher plants and its relation to the branchpoint of the alternate pathway. FEBS Lett. 1976 Jun 15;65(3):339–344. doi: 10.1016/0014-5793(76)80142-1. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwitzguebel J. P., Siegenthaler P. A. Purification of peroxisomes and mitochondria from spinach leaf by percoll gradient centrifugation. Plant Physiol. 1984 Jul;75(3):670–674. doi: 10.1104/pp.75.3.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short J. M., Fernandez J. M., Sorge J. A., Huse W. D. Lambda ZAP: a bacteriophage lambda expression vector with in vivo excision properties. Nucleic Acids Res. 1988 Aug 11;16(15):7583–7600. doi: 10.1093/nar/16.15.7583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart C. R., Martin B. A., Reding L., Cerwick S. Respiration and Alternative Oxidase in Corn Seedling Tissues during Germination at Different Temperatures. Plant Physiol. 1990 Mar;92(3):755–760. doi: 10.1104/pp.92.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey B. T., Bahr J. T. The respiratory chain of plant mitochondria. II. Oxidative phosphorylation in skunk cabbage mitochondria. Plant Physiol. 1969 Jan;44(1):126–134. doi: 10.1104/pp.44.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey B. T. Respiratory Chain of Plant Mitochondria: XVIII. Point of Interaction of the Alternate Oxidase with the Respiratory Chain. Plant Physiol. 1976 Oct;58(4):521–525. doi: 10.1104/pp.58.4.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G., Steppuhn J., Herrmann R. G. Domain structure of mitochondrial and chloroplast targeting peptides. Eur J Biochem. 1989 Apr 1;180(3):535–545. doi: 10.1111/j.1432-1033.1989.tb14679.x. [DOI] [PubMed] [Google Scholar]