Abstract
Introduction
Bone marrow is the soft tissue compartment inside the bones made up of hematopoietic cells, adipocytes, stromal cells, phagocytic cells, stem cells, and sinusoids. While [18F]-FLT has been utilized to image proliferative marrow, to date, there are no reports of particle based positron emission tomography (PET) imaging agents for imaging bone marrow. We have developed copper-64 labeled liposomal formulation that selectively targets bone marrow and therefore serves as an efficient PET probe for imaging bone marrow.
Methods
Optimized liposomal formulations were prepared with succinyl PE, DSPC, cholesterol, and mPEG-DSPE (69:39:1:10:0.1) with diameters of 90 and 140 nm, and were doped with DOTA-Bn-DSPE for stable 64Cu incorporation into liposomes.
Results
PET imaging and biodistribution studies with 64Cu-labeled liposomes indicate that accumulation in bone marrow was as high as 15.18 ± 3.69 %ID/g for 90 nm liposomes and 7.01 ± 0.92 %ID/g for 140 nm liposomes at 24 h post-administration. In vivo biodistribution studies in tumor-bearing mice indicate that the uptake of 90 nm particles is approximately 0.89 ± 0.48 %ID/g in tumor and 14.22 ± 8.07 %ID/g in bone marrow, but respective values for Doxil® like liposomes are 0.83 ± 0.49 %ID/g and 2.23 ± 1.00 %ID/g.
Conclusion
Our results indicate that our novel PET labeled liposomes target bone marrow with very high efficiency and therefore can function as efficient bone marrow imaging agents.
Keywords: bone marrow, liposome, DOTA-Bn-DSPE, PET, copper-64
INTRODUCTION
Bone marrow is the soft tissue compartment found inside bones (both hollow and spongy compartments) and is estimated to account for 2–5% of human body weight. Bone marrow comprises hematopoietic cells, adipocytes, stromal cells, phagocytic cells, stem cells, and sinusoids.[1] Regulation of hematopoiesis by resident hematopoietic stem cells is one of the essential functions of bone marrow, making it one of the most critical organs for survival. Bone marrow is dynamic in nature and its spatial distribution and composition changes with age. However, bone marrow disorders such as leukemias, lymphomas, myeloproliferative diseases, and myelosuppressive diseases lead to abnormal distribution of bone marrow compartments. In addition, certain pharmacological interventions such as chemotherapy and/or radiotherapy during cancer treatment or antibiotic treatments can interfere with marrow function, leading to reversible or irreversible suppression of bone marrow.[2] These conditions can cause significant changes in bone marrow distribution; thus, imaging of bone marrow is helpful in distinguishing among different hematological disorders.
To understand the behavior of bone marrow, sampling by aspiration is the gold standard, as it can provide detailed information about the different cell types. However, biopsies are not always feasible and are highly invasive in nature. Magnetic resonance imaging (MRI)-based techniques have been developed that take into account the presence of fatty tissue that leads to fast T1 relaxation time.[3] Nuclear medicine-based approaches have taken advantage of the phagocytic compartment to develop bone marrow imaging agents. Technetium-99m-labeled sulfur/albumin colloids phagocytosed by mononuclear phagocyte system (MPS) post-intravenous administration have been developed and successfully used for imaging bone marrow compartments in clinical practice.[4, 5] Though the functions of the hematopoietic system and phagocytic system are completely different, the distribution of these two systems is remarkably similar in bone marrow and thus utilizing either system to reflect overall bone marrow distribution has been highly successful for such applications.[6, 7] However, SPECT imaging is not quantitative and therefore has some limitations.
Iron-52 (t1/2 = 8.2 h; β+ = 56%), a PET isotope, can be used for labeling erythrocytes by simple i.v. administration of [52Fe]-Citrate to provide quantitative information about the erythropoietic compartment and thus imaging the bone marrow. However, the production of iron-52 (requiring ~70 MeV proton beam) is not feasible in routine biomedical cyclotrons and therefore limits its application in standard clinical settings. [18F]-3'-Fluoro-3'-deoxythymidine ([18F]-FLT) has been successfully used to image proliferating marrow and can aid in identifying hematological disorders that involve the disruption of proliferating bone marrow.[8, 9] To date, there have been no reports of PET-labeled particulate systems that can target the phagocytic compartment of the bone marrow. In the current manuscript, we report on the development of a copper-64 labeled liposomal system that can selectively target bone marrow and other organs of the MPS and can therefore function as a bone marrow PET probe. In addition to their imaging capability, the liposomes can function as drug carriers and can thus potentially be used for selective delivery of drugs to the bone marrow.
Liposomes are closed artificial spherical vesicles made of lipid bilayers that can range in size from 50–1,000 nm. For biological applications, the optimal size ranges from 50–500 nm and the liposomes can be employed for packaging and delivering different types of molecules, including small organics, peptides, RNA, DNA, and diagnostic and therapeutic agents.[10, 11] Indeed, liposomes are commonly referred to as nanocarriers and have been developed to mitigate side effects, enhance delivery, and reduce non-target toxicity based on either active or passive targeting mechanisms. The most famous example is the FDA-approved drug Doxil® (the trade name of the liposome-encapsulated topoisomerase inhibitor Doxorubicin), which is used to treat certain breast and pediatric cancers[12] and can target tumors due to long circulation time and enhanced permeability and retention (EPR) effect [13]. Liposomes have been developed to target bone marrow to deliver drugs. The utility of such liposomes was demonstrated through SPECT imaging of technetium-99m hexamethylpropyleneamine oxime ([99mTc]-HMPAO)-labeled liposomes by Phillips et al.[14] However, to date, no PET-labeled liposomes that can image bone marrow have been reported.
Our major goal is to develop a liposomal formulation that can be radiolabeled with a suitable PET isotope under mild conditions and can selectively target bone marrow with optimal pharmacokinetic behavior. The distribution of liposomes in vivo is highly dependent on the size, composition, and charge of the liposome. To achieve maximal bone marrow targeting (BMT) efficiency while minimizing circulation time, we incorporated three features in the liposomal design: 1) lower the polyethylene glycol (PEG) load to < 1% to minimize circulation time; 2) increase negative charge on the particle to facilitate phagocytic uptake in the bone marrow;[15, 16] and 3) minimize the particle size to reduce the trapping in the hepatic sinusoids.[17] Copper-64 (64Cu) was chosen as the isotope of choice because of its ideal 12.7 h half-life that matches the liposomal half-life, and since it is easy to chelate under mild conditions, forms stable complexes with chelators such as DOTA or 1,4,7-triazacyclononane-triacetic acid (NOTA), and exhibits little to no osseophilicity.[18] Additionally, studies from other groups have demonstrated that copper-64 labeled liposomes have good in vivo properties and accumulate in tumors by EPR effect.[19, 20]
Toward this goal, we have synthesized 90 nm DOTA-functionalized liposomes with 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC), cholesterol, 1,2-dipalmitoylsn-glycero-3-phosphoethanolamine-N-(succinyl) (succinyl DPPE), and 1,2-distearoyl-snglycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-2000] (mPEG2000-DSPE) and DOTA-Bn-DSPE (DSPE functionalized with bifunctional chelator DOTA) in the ratio of (60:30:10:1:0.1) and labeled them with 64Cu. The current manuscript describes the synthesis, evaluation, and biological characterization, including imaging and biodistribution studies, of 64Cu-radiolabeled bone marrow-targeting liposomes.
MATERIALS AND METHODS
Materials
All chemicals were used as received without further purification. DSPC, 1,2-dioctadecanoyl-sn-glycero-3-phosphoethanolamine (DSPE), Succinyl-DPPE, and mPEG2000-DSPE, along with cholesterol, were purchased from Avanti Polar Lipids (Alabaster, AL). The S-2-(4-Isothiocyanatobenzyl)-1,4,7,10-tetraazacyclododecane tetraacetic acid (p-SCN-Bn-DOTA) was purchased from Macrocyclics (Dallas, TX), and the 64Cu was purchased from Washington University (St. Louis, MO). PD-10 column was purchased from GE Healthcare Life Sciences (Pittsburgh, PA), and athymic male nude mice were purchased from Harlan Laboratories (Indianapolis, IN). Matrigel was purchased from BD Biosciences (San Jose, CA, USA). ITLC-SG chromatography paper was purchased from Agilent Technologies (SG10001, Santa Clara, CA).
Synthesis of DOTA-Bn-DSPE
Twelve µmoles of p-SCN-Bn-DOTA was dissolved in 1 mL of chloroform-methanol-water (65:35:8) mixture and 22 µmoles of DSPE was dissolved in 1 mL of the chloroform-methanol-water mixture. After mixing two solutions, 48 µmoles of triethylamine was added. The mixture was heated to 40 °C and stirred for 2 h, followed by stirring at room temperature for 16 h. The reaction progress was monitored using silica gel-coated TLC plates and product formation was confirmed by mass spectrum.
Preparation of Liposomes
The major component of bone marrow-targeting liposomes composed of DSPC and cholesterol in a molar ratio of 6:4. Depending on the formulation, the initial lipid mixture was supplemented with 1 or 2.5 mol% of mPEG2000-DSPE and/or 10% succinyl-DPPE. Doxil® like liposomes were composed of DSPC, cholesterol, succinyl-DPPE, and mPEG2000-DSPE in a molar ratio of 60:40:10:5. Additional 0.1 mol% DOTA-Bn-DSPE was added to all lipid composition for facilitating subsequent radiolabeling with copper-64 or other radiometals. All lipids were dissolved in chloroform and the solvent was evaporated under flowing nitrogen gas at 37 °C. Residual solvent was removed under reduced pressure (0.2 Torr) overnight. Lipid film was hydrated in PBS at 65 °C for 1 h and the crude lipid dispersion was extruded 11 times as specified in manufacturer's manual through either 0.1 µm or 0.03 µm pore size Whatman® Polycarbonate Membrane Filter using a mini-extruder system (Avanti Polar Lipids, Alabaster, AL) at 65 °C. After extrusion, the liposomes were purified using a PD-10 column (GE Life Sciences, Marlborough, MA) to remove unincorporated liposomal lipids and salts.
Characterization of Liposomes
Liposome size distribution and zeta potential at 25 °C in PBS pH 7.4 were determined by dynamic light scattering using Zetasizer Nano-ZS from Malvern Instruments (Malvern, Worcestershire, UK). Liposome particle concentration was measured at room temperature in PBS by light scattering using NanoSight NS500 from Malvern Instruments. Liposome stability under serum was determined after incubation in 50% FCS in PBS for 24 h. Long-term liposome stability at 4 °C was tested for one year by analyzing size distribution (Figure S5). DSPC concentrations in liposomes were determined by phopholipase D activity to hydrolyze the ester bond between the phosphate and the choline. The assay was performed using phosphatidylcholine assay kit by Sigma-Aldrich (Cat#: MAK049) following manufacturer protocol.
Radiolabeling of Liposome with copper-64
[64Cu]-CuCl2 (750 µCi in 0.1 N HCl) was added to 750 µL of PBS containing liposomes (20 µM total lipid concentration) and adjusted to pH 5.5 with 0.2 M sodium acetate buffer (pH 5.5). The reaction mixture was stirred at 50 °C for 1 h with constant shaking using an Eppendorf ThermoMixer®. Instant thin layer chromatography (ITLC) was performed on an ITLC-SG paper using 5 mM diethylenetriaminepentaacetic acid (DTPA) solution (pH 5.5) as eluant to monitor the progress of reaction and the liposomes are purified using PD-10 column to remove free 64Cu.
64Cu Stability on Liposome
The stability of 64Cu-labeled liposomes was measured in PBS, serum (50% FBS in PBS) and 10 mM EDTA. The 64Cu-labeled liposomes (30 µCi) were incubated with 150 µL of 50% FBS, or PBS or 10 mM EDTA at 37°C and small aliquots (0.2 µL) of samples were taken at different time points (2, 4, 8, 18, and 24 h) to perform ITLC analysis for measuring stability. ITLC was performed using 5 mM DTPA (pH 5.5) as eluant.
Biodistribution of 64Cu Liposome
For biodistribution studies, approximately 5.2 MBq of 64Cu-labeled liposome of 0.68 µmoles of total lipids was administered to athymic nude mice (n = 9) i.v. via tail vein injection and approximately 0.21 × 1012 liposomal particles determined by light scattering were injected into each mouse. Mice were sacrificed at 4 or 24 h post-injection and all the major organs were collected and placed in pre-weighed culture tubes or Eppendorf microcentrifuge tubes (for bone marrow collection). To collect bone marrow, the femur was dipped into liquid nitrogen and one of the epiphyses was carefully removed. A 30 mL syringe with 30½-gauge needles was inserted into open end of the femur and air was blown. The air escapes through the narrow crevices between the open femur bone and the needle. This movement of air also forces the marrow to accumulate in the needle, which was aspirated into microcentrifuge tubes, weighed immediately and radioactivity was measured using a Wizard 1470 gamma counter (PerkinElmer, Inc., Waltham, MA). Data were presented as percent injected dose per gram (%ID/g) of tissue.
Xenograft Model
All animal studies were reviewed and approved by the MSKCC’s Institutional Animal Care and Use Committee (IACUC). Briefly, 6–8-week-old female athymic nude mice were obtained from the vendor and allowed to acclimatize at the least for 1 week. PC9 cells were obtained from the laboratory of Dr. William Pao. To induce PC9 tumor xenografts, 5 × 106 cells suspended in 100 µL cold PBS and Matrigel (1:1) mixture were injected s.c. in the right flank using a 28-gauge needle. Tumors were allowed to grow for 2–3 weeks to reach a minimum size of 100 mm3 before performing biodistribution studies using 64Cu labeled liposomes.
MicroPET and PET/CT Imaging
PET imaging of mice administered with 64Cu-labeled liposomes was performed using either a microPET Focus120 small animal PET scanner (Siemens, Knoxville, TN) or Inveon small animal PET/CT scanner (Siemens, Knoxville, TN). 64Cu-labeled liposome (5.2 MBq) was administered into athymic nude mice by i.v. injection via tail vein and images were acquired at 4 and 24 h post-injection. Static scans were acquired for 5 min for 4 h post-injection images, and, for 20 min for 24 h post-injection images. Dynamic images were acquired for the first 65 min post-administration of the tracer. Regions of interest (ROIs) of heart, liver, spleen, and sacrum were drawn on the images and then quantified. ASIPro (Siemens Medical Solutions, Knoxville, TN) or Amide[21] software was used to visualize the PET data and generate images. Pharmacokinetic parameters were derived and calculated after data were fitted on two-compartment models[22] using non-linear curve fittings with a user-defined equation in Prism 6.0.
Software and Statistics
Prism 6.0 was used for plotting graph, fitting curve, and statistical analysis. ASIPro VM (Siemens Medical Solutions, Knoxville, TN) and Amide [21] was used for PET and PET/CT image analysis.
Institution Research Animal Approval
All animal experiments were approved by the Institutional Animal Care and Use Committee of Memorial Sloan Kettering Cancer Center under protocol 86-02-020.
RESULTS
DOTA-Bn-DSPE was easily synthesized by coupling p-SCN-Bn-DOTA and DSPE in presence of triethylamine as base. For synthesis, the mole ratio between DSPE and p-SCN-Bn-DOTA was set to 2:1 to ensure maximal consumption of p-SCN-Bn-DOTA during the coupling reaction with DSPE (Figure 1A). The final product formation was confirmed by mass spectrum, which indicated the presence of peak at 1,298 m/z corresponding to [M+H] peak and additional prominent peak at 747 m/z corresponding to unreacted DSPE (Figure S1).
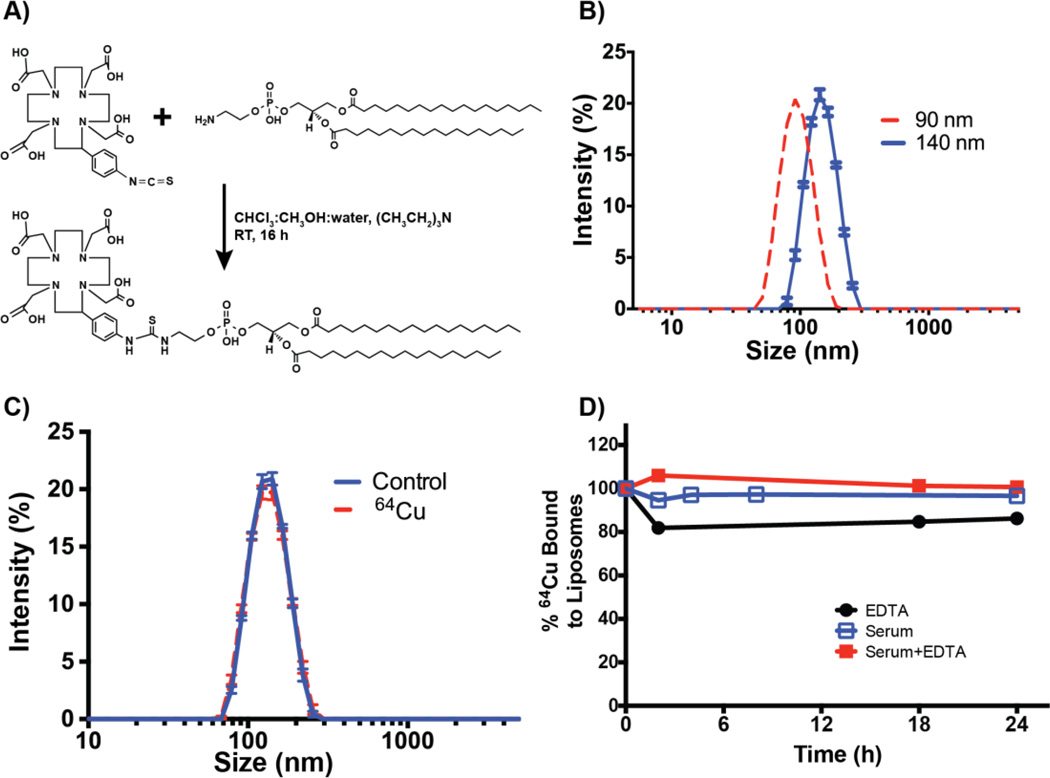
FIGURE 1. Synthesis, characterization, and stability studies of DOTA-Bn-DSPE liposomes.
A) The synthetic scheme of DOTA-Bn-DSPE, a critical liposomal component to chelate 64Cu or other radiometals, post-liposomal synthesis. B) Representative size distribution profiles of liposomes (140 nm and 90 nm diameter) as determined by dynamic light scattering. C) Size distribution profiles of liposomes are identical before and after chelation of 64Cu as determined by dynamic light scattering. D) Stability profile of 64Cu-labeled liposomes.
Liposomes of 140 and 90 nm containing different ratios of DSPE and succinyl DPPE, doped with 0.1% DOTA-Bn-DSPE, were prepared using extrusion method with 0.03 and 0.1 µm pore size membranes, respectively (Figure 1B). The size distribution characteristics of liposomes were analyzed using dynamic light scattering methods, which indicated that the liposomes were predominantly monodisperse with poly dispersity indices of 0.038 and 0.096 for 90 and 140 nm particles, respectively.
64Cu labeling was performed under standard mild conditions of incubation with [64Cu]-CuCl2 in acetate buffer (pH 5.5) at 50°C for 60 min. Analysis of the reaction mixture with instant thin layer chromatography (ITLC) data indicates that the labeling efficiency of 64Cu is nearly 100% (Figure S2). Labeling at room temperature was effective but the yields varied between 75–100% (data not shown) and therefore heating was adopted to expedite the labeling and generate consistent results. Dynamic light scattering studies have indicated that the size distribution of liposome does not change after 64Cu incorporation (Figure 1C). For the in vivo experiments, the stability of 64Cu-labeled liposome in serum is critical and therefore serum stability studies were performed by incubating the radiolabeled liposome in PBS and 50% serum with 10 mM EDTA at 37 °C for 24 h. The leaching of copper and/or loss of [64Cu]-DOTA-Bn-DSPE from the liposome was studied using ITLC. Figure 1D summarizes the stability data for 64Cu liposomes against serum and EDTA. From the figure it is clear that the copper label is relatively stable with little or no dissociation from DOTA-Bn-DSPE contacting liposome at room temperature for 24 h. Collectively the data indicates that the activity of copper-64 can be used as a marker for measuring liposomal concentration.
The organ distribution of the liposomes (Figure 2) was determined by measuring 64Cu activity distribution in major organs including MPS organs bone marrow, liver, and spleen. Liposomes 90 nm in size exhibited highest uptake in the bone marrow among the conditions studied, with 15.18 ± 3.69 %ID/g uptake at 24 h post-injection. Among other organs, the spleen displayed the highest amount of uptake (34.98 ± 3.16 %ID/g). ANOVAs of liposome accumulation to bone marrow were statistically significant with differences between the averages (p < 0.01), and consecutive multiple comparisons performed showed that accumulation of 90 nm liposome to bone marrow at 24 h was statistically significant in comparison to all the other conditions (p < 0.05).
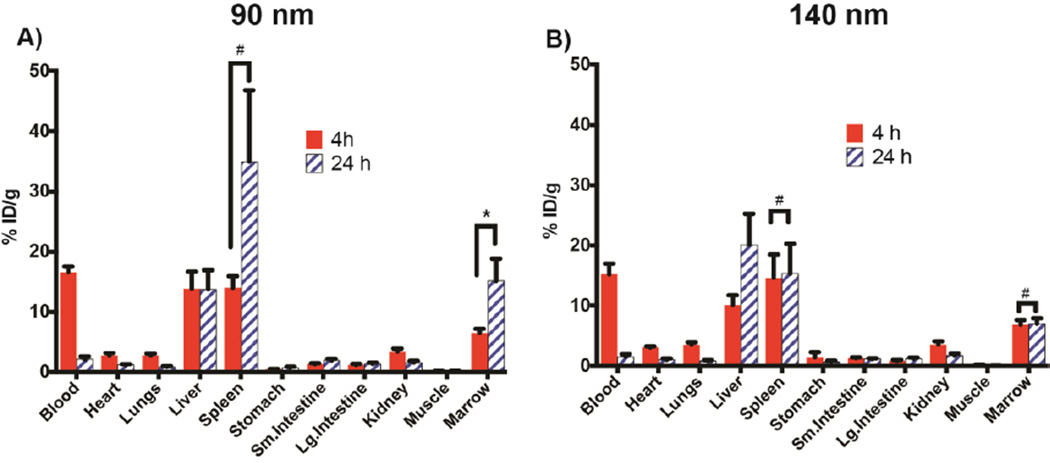
FIGURE 2. Ex vivo biodistribution data of 64Cu-labeled 90 and 140 nm BMT liposomes.
Biodistribution data (mean and SD) in athymic nude mice (n=9) of A) 90 nm liposomes and B) 140 nm liposomes at 4 and 24 h post-injection. * p < 0.5, # statistically insignificant.
The ex vivo biodistribution data of 64Cu-labeled liposomes sized 90 and 140 nm at 4 and 24 h, respectively, is presented in Table 1 and Figure 2. This data shows that both liposomes target the bone marrow, though with slightly different efficiencies. While at the 4 h time point the bone marrow targeting appears to be similar (about 6.5 %ID/g at 4 h) for both liposomes, at the 24 h time point the 90 nm liposomes exhibit preferential accumulation in the bone marrow in comparison to 140 nm liposomes. The corresponding %ID/g values are 15.18 ± 3.69 and 7.01 ± 0.92 for 90 and 140 nm liposomes, respectively. As expected with the particles, in addition to bone marrow, other MPS organs such as liver and spleen show high uptake of the liposomes. However, the time-dependent uptake in both the liver and spleen is different, depending on the size of the liposomes. For the 90 nm liposome, the liver uptake remains almost constant at 4 and 24 h with %ID/g values of 13.87 ± 2.81 and 13.78 ± 3.16, respectively. The spleen uptake reflects a pattern similar to bone marrow uptake, with increasing accumulation at later time points as indicated by the %ID/g values of 14.04 ± 1.90 and 34.98 ± 11.85 at 4 and 24 h, respectively. The corresponding values observed in the spleen for 140 nm liposomes were 14.61 ± 3.88 and 15.3 ± 4.95, respectively, indicating early targeting and retention in the spleen. On the other hand, the uptake of 140 nm liposomes in the liver shows a significant increase at 24 h with %ID/g values increasing from 10.12 ± 1.62 at 4 h to 20.11 ± 5.16 %ID/g at 24 h. It is important to note that the blood uptake values for both 90 and 140 nm liposomes are essentially the same. Based on this data, it is clear that the 90 nm liposome is better suited for bone marrow targeting applications.
Table 1.
Biodistribution of bone marrow-targeting liposome labeled with 64Cu in mouse. 64Cu-labeled liposome (5.2 MBq) was injected via tail vein. Nine mice per group were sacrificed at the indicated time and a gamma counter was used to measure radioactivity. The %ID/g (± SD) was calculated by measuring weight and decay corrected radioactivity.
| 90 nm | 140 nm | |||
|---|---|---|---|---|
| 4 h | 24 h | 4 h | 24 h | |
| Blood | 16.59 (0.95) | 2.17 (0.46) | 15.19 (1.82) | 1.56 (0.42) |
| Heart | 2.79 (0.43) | 1.22 (0.14) | 3.07 (0.20) | 1.06 (0.20) |
| Lung | 2.83 (0.30) | 0.87 (0.15) | 3.52 (0.40) | 0.85 (0.16) |
| Liver | 13.87 (2.81) | 13.78 (3.16) | 10.12 (1.62) | 20.11 (5.16) |
| Spleen | 14.04 (1.90) | 34.98 (11.85) | 14.61 (3.88) | 15.3 (4.95) |
| Stomach | 0.49 (0.06) | 0.74 (0.17) | 1.45 (0.84) | 0.73 (0.14) |
| S Intestine | 1.37 (0.13) | 1.97 (0.30) | 1.31 (0.11) | 1.18 (0.09) |
| L Intestine | 1.21 (0.19) | 1.4 (0.19) | 0.89 (0.14) | 1.2 (0.20) |
| Kidney | 3.43 (0.52) | 1.61 (0.27) | 3.54 (0.56) | 1.74 (0.36) |
| Muscle | 0.15 (0.02) | 0.16 (0.03) | 0.22 (0.05) | 0.11 (0.01) |
| Marrow | 6.5 (0.72) | 15.18 (3.69) | 6.86 (0.77) | 7.01 (0.92) |
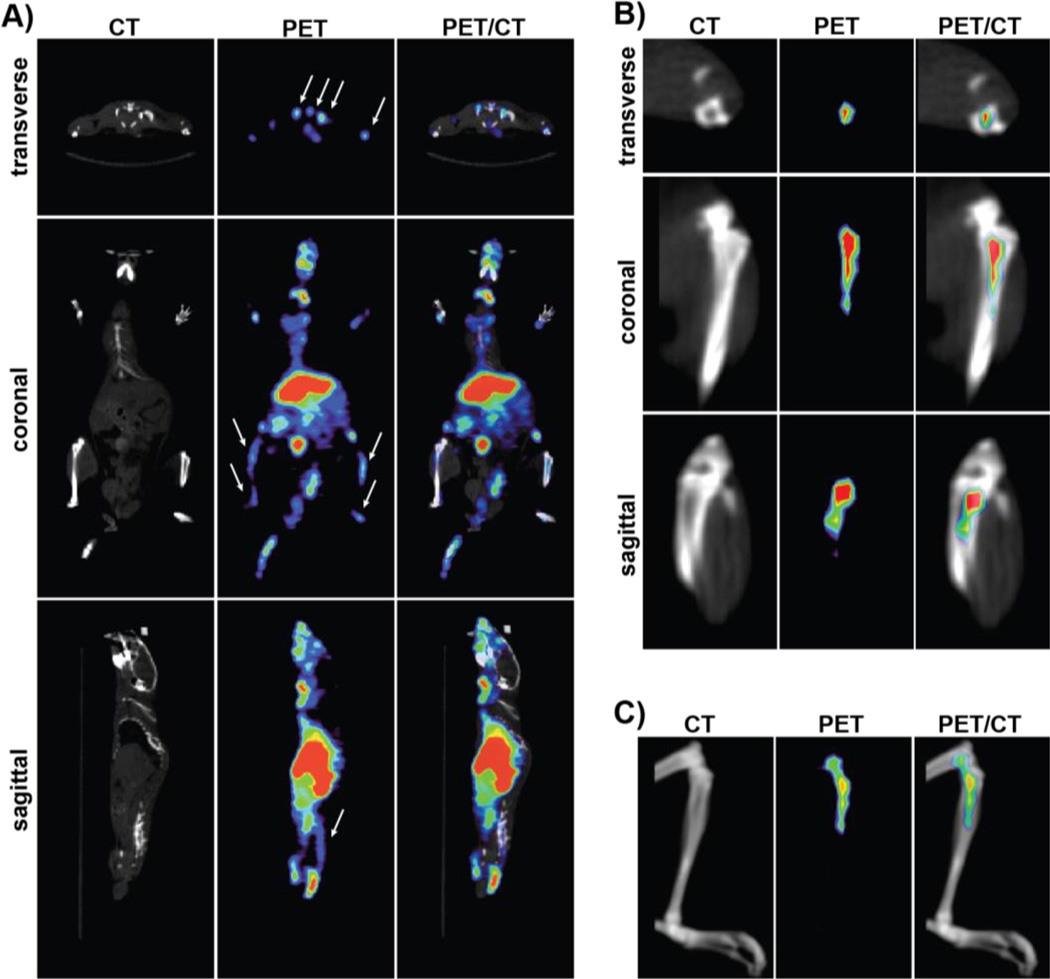
Figure 3 shows representative PET/CT images of 64Cu-DOTA-Bn-DSPE containing liposome (90 nm) 24 h post-administration. Reflecting the biodistribution data (Figure 2 and Table 1), the strongest signal was observed in the liver and spleen. Despite low marrow content (~5–10 mg) due to relatively high uptake in bone marrow (~15.8%ID/g), the signal from marrow can be visualized without corrections of partial volume effect [23]. Partial volume effect results in activity spillover to neighboring regions, which thereby reduces the observed intensity in bone marrow. Despite these limitations, the sacrum, spine (Figure S3), and femoral and tibial heads can be clearly delineated from the image.
FIGURE 3. Representative PET/CT images of mice administered with 64Culabeled BMT liposome (90 nm).
A) Representative CT, PET/CT fusion, and PET image slices of mice administered with 64Cu-liposome 24 h post-administration. Arrows indicate the bone marrow in PET images. B) Magnified image showing the CT, PET/CT fusion, and PET image slice of tibia (marrow-rich) of the same mice for clarity; and C) volume-rendered images showing CT, PET/CT fusion, and PET images of tibia demonstrate that accumulation of liposomes is specific to bone marrow.
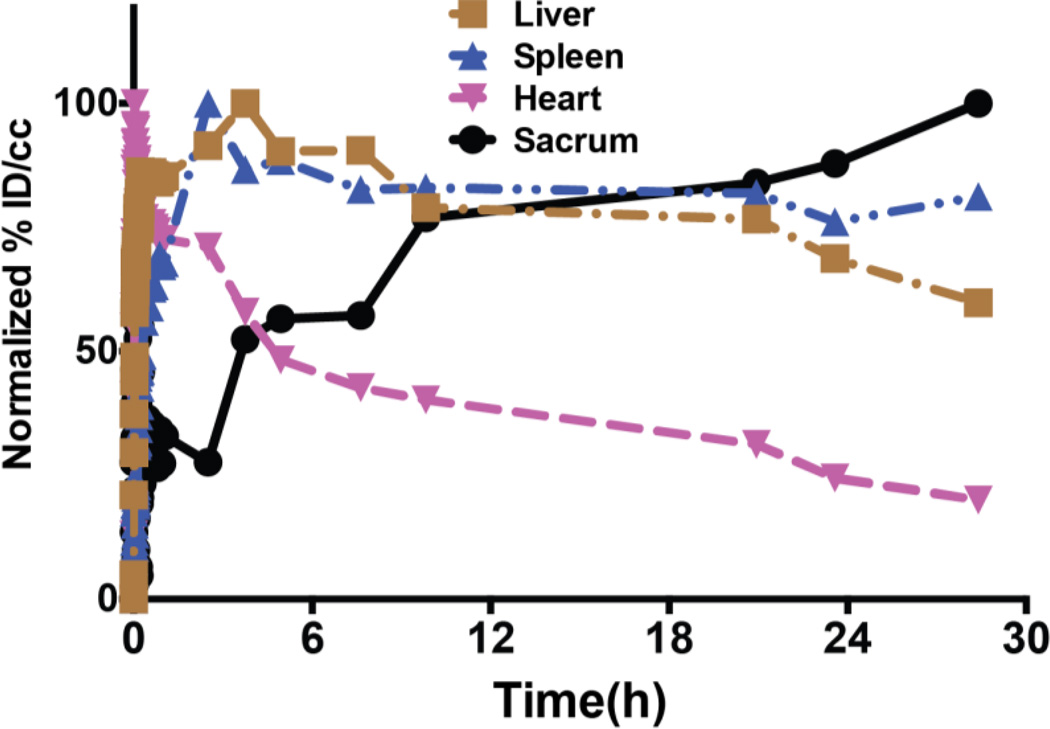
Dynamic microPET imaging set at 1 h was used to evaluate the early biodistribution profile of the 90 nm bone marrow-targeting liposomes. After tail vein injection, liposomes are rapidly distributed in the vascular compartment. As shown in Figure 4, blood activity reaches its maximum uptake at 200 sec after completion of the infusion of liposome, followed by clearance of radioactivity from the blood pool and redistribution in other organs. Liver uptake of liposomes reaches its maximum around 3–4 h and the activity accumulation in the liver remains similar until 28 h. Spleen uptake of liposome is rapid until 1 h after tail vein injection, when it slowly increases its activity until 28 h. Uptake of liposome in both the liver and spleen reflects the rapid initial clearance of liposomes from the blood circulation possibly by liver Kupffer cells and sinusoids and splenic trapping by macrophages and sinusoids[24, 25].
FIGURE 4. Normalized time-activity curves of 90 nm 64Cu-labeled liposome indicating biodistribution profile in mice.
Mice were administered with 64Culipsome via tail vein injection. Time-activity curve was constructed from mean activity concentrations of ROIs drawn around heart, liver, spleen, and sacrum from 1 h dynamic PET scanning image, combined with static PET images of 2.5, 3.7, 5, 7.5, 10, 20, 24, and 28 h. No attenuation correction or partial volume correction was applied.
Frequent arterial blood sampling is typically used to measure blood concentration and circulation of radioactive material, but arterial blood sampling is invasive and laborious with small animals. It is also difficult if the clearance is fast and requires frequent sampling at early time points post-injection. Therefore, the time activity curve derived by ROIs of large vascular structures, such as the heart from PET imaging, has been used to estimate the concentration of radioactivity in the blood[26]. Pharmacokinetic parameters of 64Cu-labeled bone marrow-targeting liposome after tail vein injection were derived using a two-compartment model.
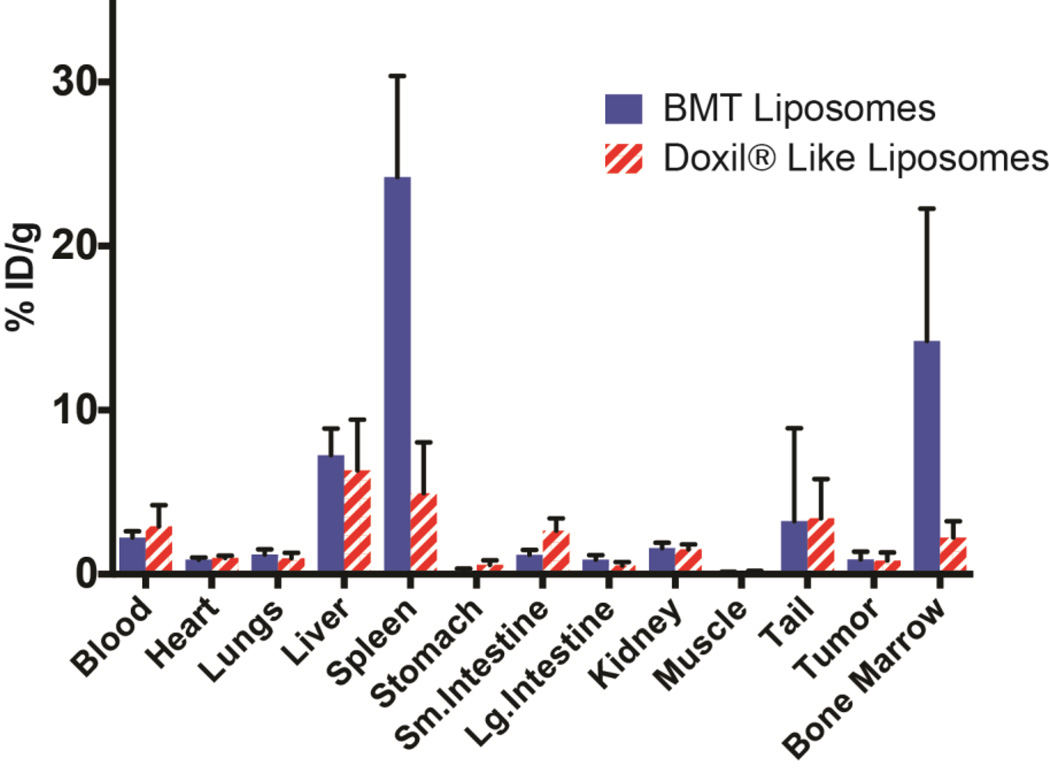
In addition to the effect of size on in vivo distribution of the liposomes in bone marrow and other organs, we wanted to demonstrate that the surface composition of the liposomes is equally important. Therefore we compared our marrow targeting 90 nm liposomes with the liposomes containing the composition of commonly used Doxil® of same size which we refer to as Doxil® like control liposomes. In addition, we wanted to evaluate the uptake of liposomes in tumors. Figure 5 shows the results of this comparative study. As expected at 24 h post administration, the 90 nm bone marrow targeting (BMT) liposomes targets bone marrow efficiently (14.22 ± 8.07 %ID/g) but show minimal uptake in PC9 xenografts (0.89 ± 0.48 %ID/g). The Doxil® like 90 nm liposomes exhibited marrow uptake of 2.23 ± 1.00 %ID/g which is significantly lower compared to BMT liposomes. The uptake in tumor was similar for Doxil® like liposomes accumulation g about. 0.83 ± 0.49 %ID/g at 24 h post-administration..
FIGURE 5. Ex vivo biodistribution data of bone BMT and Doxil® like liposomes.
Biodistribution data (mean and SD) of 64Cu labeled 90 nm liposomes in PC9 tumor bearing athymic nude mice (n=5) 24 h post i.v. administration.
DISCUSSION
Previous reports of radiolabeling BMT liposomes by Phillips and colleagues[14] involved remote loading by transchelation of [99mTc]-HMPAO complex with glutathione (loaded into the liposomes) without any specific chelator on the liposomal membrane. Our strategy was to synthesize a chelator on the surface using DOTA-DSPE conjugate (Figure 1), which can act as a lipid anchor for the bilayer. Synthesis of the DOTA-Bn-DSPE was straightforward, starting from p-SCN-Bn-DOTA as shown in Figure 1. Several different liposomes were formulated, characterized, and tested. The DOTA was chosen as the chelate because it can be radiolabeled with a variety of radiometals, including 68Ga (t1/2 = 68 min), 64Cu (t1/2 = 12.7 h), and 86Y (t1/2 = 14.7 h), among others, under mild conditions to yield stable complexes and since it permits non-invasive tracking and quantification of delivery to organs.
The in vivo distribution of the liposomes in the bone marrow, liver, spleen, and other organs is influenced by mechanical filtration, membrane fusion, and the interaction with serum proteins and their cellular receptors. Specifically, liposome clearance is mostly mediated by a complementary pathway in which liposomes are taken by the MPS. This complement-mediated pathway is governed by multiple factors, including surface negative charge, cholesterol content, acyl chain saturation and length, and the size of the liposome. When complement binding occurs in circulation, liposomes are attached and cleared by the MPS, particularly by Kupffer cells in the liver. By modulating the factors governing the complement binding system, the hepatic uptake of liposomes can be lowered, and the uptake by another MPS can be used. Liposomes are taken up by phagocytes, and the uptake is dependent on the surface charge and PEG content. Higher negative surface charge leads to efficient phagocytosis, whereas PEG negatively modulates opsonization efficiency but increases circulation time. To optimize the surface negative charge, succinyl DPPE content was adjusted. We wanted to optimize the balance between negative charge and PEG content. Sou et al.[27] reported that liposomes containing Dipalmitoylphosphatidylcholine (DPPC), cholesterol, PEG, and N-(3-carboxy-1-exopropyl)-1,5-dihexadecy ester (SAlipid) target bone marrow in rabbits with good efficiency. Based on the literature and our preliminary studies, we chose liposomal formulation containing DSPC, cholesterol, succinyl PE, and mPEG-DSPE and DOTA-Bn-DSPE in the ratio of (60:30:10:1:0.1) for further investigation.
Liposome size was controlled by the pore size of extrusion membrane, and dynamic light scattering was used to measure the properties of the liposomes. The liposomes were formulated using two different pore size membranes; 30 nm pore size membrane produces 90 nm hydrodynamic radius liposomes (small unilamellar vesicle), while 100 nm pore size membrane produces 140 nm hydrodynamic radius liposomes (large unilamellar vesicle). Two different sizes (90 nm and 140 nm) of liposomes were used to study the effect of size on the biodistribution of the liposome. As the results indicate, the 90 nm liposomes outperform 140 nm liposomes in targeting bone marrow. The uptake values are 15.18 ± 0.52 %ID/g and 7.01 ± 0.52 %ID/g, respectively, at 24 h.
PET imaging with 64Cu-labeled 90 nm liposomes clearly reveals that the major organs of liposomal accumulation are liver, spleen, and bone marrow. The liver and spleen can be easily visualized at 24 h post-administration. The %ID/g uptake of the marrow is very high, and this allows visualization of most marrow bearing bones, in the lumbar spine, sacrum and femora (Figure 3 and S4). For example in the femur, a relatively marrow-rich organ, the approximate volume of marrow is only 5 µL.[28], and yet it is well visualized in the images show because of the high uptake and exceptional contrast and sensitivity of imaging with PET. The EPR effect refers to how molecules of a specific size (nanoparticles or macromolecules) have the tendency to accumulate nonspecifically preferentially in the tumor tissue rather than healthy organs as a result of abnormal tumor vasculature.[29] It is assumed that the newly formed vessel structure within the tumors is leaky, and that 150–200 nm particles can escape from the vessel and accumulate in tumor tissue. We wanted to understand liposome distribution not only in bone marrow but also in the tumor to determine if any selectivity can be achieved. So, we compared our bone marrow imaging liposomes with a control liposome. The control liposome composition is relatively similar to BMT liposome but contains 5 mol% of mPEG to mimic stealthy liposome such as Doxil®. As shown in Figure 5, the data indicates that we can get as high as 16-fold higher uptake in marrow compared to tumor with BMT liposomes, while Doxil® like liposome show 2.7-fold higher uptake in the marrow compared to tumor.
The dynamic and rapidly proliferating nature of bone marrow makes it a very sensitive organ. The bone marrow is easily affected by radiation, chemotherapeutic agents, and other drugs. Therefore, a common side effect observed in cancer patients undergoing chemotherapy or radiotherapy is bone marrow depletion.[30] A potential additional application of our liposomes is to deliver drugs that can protect bone marrow from destruction or help aid in the recovery of bone marrow at much higher concentrations than what can be achieved by systemic delivery of naked drugs.[31] The bone marrow liposome's long stability (Figure S5) and near 100% labeling efficiency (Figure S2) enable us to develop a kit ready for immediate use with adjustment of pH, labeling of PET radiotracer, and neutralization.
Currently, 99mTc sulfur colloid [32] is used as a gold standard for imaging bone marrow using scintigraphy, which suffers from lower sensitivity, and the signal is not quantitative. Using copper-64 labeled BMT liposomes, will lead to increased sensitivity, and, PET being quantitative, it will aid the monitoring of changes in the bone marrow density as a result of therapeutic interventions. Therefore, copper-64 labeled BMT liposomes can potentially aid in measuring marrow depletion as a result of radiation or a chemotherapeutic treatment, or, estimate the efficacy of marrow engraftment in bone marrow transplant patients.
CONCLUSION
We have developed and optimized 64Cu-labeled liposomes that target bone marrow with high efficiency. As expected, our study confirms that the sizes, surface charges, and PEG content of the liposome can impact the bone marrow targeting efficiency. Among the liposomes studied, liposomes containing 10% succinyl DPPE, 1% PEG, and 90 nm diameter liposomes with zeta potential of -15.8 mV at pH 7.4 showed the best bone marrow targeting efficiency. Biodistribution and imaging data establish that these liposomes can target bone marrow with high efficiency (15.18 ± 3.69 %ID/g) with marrow-to-blood and marrow-to-muscle ratios of 7.0 and 94.9, respectively, at 24 h. Taken together, we have developed a very efficient particle-based PET imaging agent for imaging bone marrow distribution that can be used in patients to understand changes in bone marrow distribution as a function of disease or drug interactions.
Supplementary Material
Acknowledgments
This study was supported in part by the MSKCC Center for Molecular Imaging in Cancer grant from the National Cancer Institute (P50-CA086438). Technical services provided by the MSKCC Small-Animal Imaging Core Facility, supported in part by NIH Cancer Center Support Grant No. 2 (P30-CA008748-48), are gratefully acknowledged. NIH Shared Instrumentation Grants (S10-RR020892-01 and S10-OD016207-01) provided funding for the purchase of the Focus 120 microPET and Inveon PET/CT, respectively. The authors also thank support of NSF (award IGERT DGE 0965983) for their generous funding.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no competing financial interests.
REFERENCES
- 1.Hays K. Physiology of normal bone marrow. Seminars in oncology nursing. 1990;6:3–8. doi: 10.1016/s0749-2081(05)80127-5. [DOI] [PubMed] [Google Scholar]
- 2.Mintzer DM, Billet SN, Chmielewski L. Drug-induced hematologic syndromes. Advances in hematology. 2009;2009:495863. doi: 10.1155/2009/495863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blebea JS, Houseni M, Torigian DA, Fan C, Mavi A, Zhuge Y, et al. Structural and functional imaging of normal bone marrow and evaluation of its age-related changes. Seminars in nuclear medicine. 2007;37:185–194. doi: 10.1053/j.semnuclmed.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 4.Agool A, Glaudemans AW, Boersma HH, Dierckx RA, Vellenga E, Slart RH. Radionuclide imaging of bone marrow disorders. European journal of nuclear medicine and molecular imaging. 2011;38:166–178. doi: 10.1007/s00259-010-1531-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fordham EW, Ali A. Radionuclide imaging of bone marrow. Seminars in hematology. 1981;18:222–239. [PubMed] [Google Scholar]
- 6.Nelp WB, Larson SM, Lewis RJ. Distribution of the erythron and the RES in the bone marrow organ. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 1967;8:430–436. [PubMed] [Google Scholar]
- 7.Desai AG, Thakur ML. Radiopharmaceuticals for spleen and bone marrow studies. Seminars in nuclear medicine. 1985;15:229–238. doi: 10.1016/s0001-2998(85)80002-7. [DOI] [PubMed] [Google Scholar]
- 8.Agool A, Schot BW, Jager PL, Vellenga E. 18F-FLT PET in hematologic disorders: a novel technique to analyze the bone marrow compartment. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2006;47:1592–1598. [PubMed] [Google Scholar]
- 9.McGuire SM, Menda Y, Boles Ponto LL, Gross B, TenNapel M, Smith BJ, et al. Spatial mapping of functional pelvic bone marrow using FLT PET. Journal of applied clinical medical physics / American College of Medical Physics. 2014;15:4780. doi: 10.1120/jacmp.v15i4.4780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luk BT, Fang RH, Zhang L. Lipid- and polymer-based nanostructures for cancer theranostics. Theranostics. 2012;2:1117–1126. doi: 10.7150/thno.4381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Al-Jamal WT, Kostarelos K. Liposomes: from a clinically established drug delivery system to a nanoparticle platform for theranostic nanomedicine. Accounts of chemical research. 2011;44:1094–1104. doi: 10.1021/ar200105p. [DOI] [PubMed] [Google Scholar]
- 12.Chang HI, Yeh MK. Clinical development of liposome-based drugs: formulation, characterization, and therapeutic efficacy. International journal of nanomedicine. 2012;7:49–60. doi: 10.2147/IJN.S26766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khawar IA, Kim JH, Kuh HJ. Improving drug delivery to solid tumors: Priming the tumor microenvironment. Journal of controlled release : official journal of the Controlled Release Society. 2015;201C:78–89. doi: 10.1016/j.jconrel.2014.12.018. [DOI] [PubMed] [Google Scholar]
- 14.Sou K, Goins B, Takeoka S, Tsuchida E, Phillips WT. Selective uptake of surface-modified phospholipid vesicles by bone marrow macrophages in vivo. Biomaterials. 2007;28:2655–2666. doi: 10.1016/j.biomaterials.2007.01.041. [DOI] [PubMed] [Google Scholar]
- 15.Frohlich E. The role of surface charge in cellular uptake and cytotoxicity of medical nanoparticles. International journal of nanomedicine. 2012;7:5577–5591. doi: 10.2147/IJN.S36111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Allen TM, Austin GA, Chonn A, Lin L, Lee KC. Uptake of liposomes by cultured mouse bone marrow macrophages: influence of liposome composition and size. Biochimica et biophysica acta. 1991;1061:56–64. doi: 10.1016/0005-2736(91)90268-d. [DOI] [PubMed] [Google Scholar]
- 17.Sarin H. Physiologic upper limits of pore size of different blood capillary types and another perspective on the dual pore theory of microvascular permeability. Journal of angiogenesis research. 2010;2:14. doi: 10.1186/2040-2384-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCarthy DW, Shefer RE, Klinkowstein RE, Bass LA, Margeneau WH, Cutler CS, et al. Efficient production of high specific activity 64Cu using a biomedical cyclotron. Nuclear medicine and biology. 1997;24:35–43. doi: 10.1016/s0969-8051(96)00157-6. [DOI] [PubMed] [Google Scholar]
- 19.Hansen AE, Petersen AL, Henriksen JR, Boerresen B, Rasmussen P, Elema DR, et al. Positron Emission Tomography Based Elucidation of the Enhanced Permeability and Retention Effect in Dogs with Cancer Using Copper-64 Liposomes. ACS nano. 2015;9:6985–6995. doi: 10.1021/acsnano.5b01324. [DOI] [PubMed] [Google Scholar]
- 20.Petersen AL, Binderup T, Rasmussen P, Henriksen JR, Elema DR, Kjaer A, et al. 64Cu loaded liposomes as positron emission tomography imaging agents. Biomaterials. 2011;32:2334–2341. doi: 10.1016/j.biomaterials.2010.11.059. [DOI] [PubMed] [Google Scholar]
- 21.Loening AM, Gambhir SS. AMIDE: a free software tool for multimodality medical image analysis. Molecular imaging. 2003;2:131–137. doi: 10.1162/15353500200303133. [DOI] [PubMed] [Google Scholar]
- 22.Greenblatt DJ, Koch-Weser J. Clinical pharmacokinetics (second of two parts) The New England journal of medicine. 1975;293:964–970. doi: 10.1056/NEJM197511062931905. [DOI] [PubMed] [Google Scholar]
- 23.Dumouchel T, Thorn S, Kordos M, DaSilva J, Beanlands RS, deKemp RA. A three-dimensional model-based partial volume correction strategy for gated cardiac mouse PET imaging. Physics in medicine and biology. 2012;57:4309–4334. doi: 10.1088/0031-9155/57/13/4309. [DOI] [PubMed] [Google Scholar]
- 24.Rothkopf C, Fahr A, Fricker G, Scherphof GL, Kamps JA. Uptake of phosphatidylserine-containing liposomes by liver sinusoidal endothelial cells in the serum-free perfused rat liver. Biochimica et biophysica acta. 2005;1668:10–16. doi: 10.1016/j.bbamem.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 25.Bartneck M, Warzecha KT, Tacke F. Therapeutic targeting of liver inflammation and fibrosis by nanomedicine. Hepatobiliary surgery and nutrition. 2014;3:364–376. doi: 10.3978/j.issn.2304-3881.2014.11.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seo JW, Mahakian LM, Tam S, Qin S, Ingham ES, Meares CF, et al. The pharmacokinetics of Zr-89 labeled liposomes over extended periods in a murine tumor model. Nuclear medicine and biology. 2015;42:155–163. doi: 10.1016/j.nucmedbio.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sou K, Goins B, Oyajobi BO, Travi BL, Phillips WT. Bone marrow-targeted liposomal carriers. Expert opinion on drug delivery. 2011;8:317–328. doi: 10.1517/17425247.2011.553218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chung YR, Kim E, Abdel-Wahab O. Femoral bone marrow aspiration in live mice. Journal of visualized experiments : JoVE. 2014 doi: 10.3791/51660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang AZ, Langer R, Farokhzad OC. Nanoparticle delivery of cancer drugs. Annual review of medicine. 2012;63:185–198. doi: 10.1146/annurev-med-040210-162544. [DOI] [PubMed] [Google Scholar]
- 30.Manning G, Rothkamm K. Deoxyribonucleic acid damage-associated biomarkers of ionising radiation: current status and future relevance for radiology and radiotherapy. The British journal of radiology. 2013;86:20130173. doi: 10.1259/bjr.20130173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mendelson A, Frenette PS. Hematopoietic stem cell niche maintenance during homeostasis and regeneration. Nature medicine. 2014;20:833–846. doi: 10.1038/nm.3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Larson SM, Nelp WB. Radiopharmacology of a simplifield technetium-99mcolloid preparation for photoscanning. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 1966;7:817–826. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.