Abstract
Introduction
Osteosarcoma overall survival has plateaued around 70%, without meaningful improvements in over 30 years. Outcomes for patients with overt metastatic disease at presentation or who relapse are dismal. In this study we investigated a novel osteosarcoma therapy utilizing radioimmunotherapy (RIT) targeted to IGF2R, which is widely expressed in OS.
Methods
Binding efficiency of the Rhenium-188(188Re)-labeled IGF2R-specific monoclonal antibody (mAb) to IGF2R on OS17 OS cells was assessed with Scatchard plot analysis. Biodistribution studies were performed in heterotopic murine osteosarcoma xenografts. Tumor growth was compared over a 24-day period post-treatment between mice randomized to receive 188Re-labeled IGF2R-specific murine mAb MEM-238 (188Re-MEM-238) or one of three controls—188Re-labeled isotype control mAb, unlabeled MEM-238,or no treatment.
Results
Results demonstrate the radioimmunoconjugate had a high binding constant to IGF2R. Both 188Re-MEM-238 and the isotype control had similar initial distribution in normal tissue. After 48 hours 188Re-MEM-238 exhibited a 1.8 fold selective uptake within tumor compared to the isotype control (p=0.057). Over 24 days, the tumor growth ratio was suppressed in animals treated with RIT compared to unlabeled and untreated controls (p=0.005) as demonstrated by a 38% reduction of IGF2R expressing osteosarcoma cells in the RIT group (p=0.002).
Conclusions
In conclusion, given the lack of new effective therapies in osteosarcoma, additional investigation into this target is warranted.
Advances in Knowledge
High expression of IGF2R on osteosarcoma tumors, paired with the specificity and in vivo anti-cancer activity of 188Re-labeled IGF2R-specific mAb suggests that IGF2R may represent a novel therapeutic target in the treatment of osteosarcoma.
Implications for Patient Care
This targeted approach offers the benefits of being independent of a specific pathway, a resistance mechanism, and/or an inherent biologic tumor trait and therefore is relevant to all OS tumors that express IGF2R.
Keywords: OS, Radioimmunotherapy, Insulin-like Growth Factor-2 Receptor (IGF2R), Rhenium-188
INTRODUCTION
Osteosarcoma (OS) is the most common non-hematologic primary bone malignancy [2, 3]. Additionally it is the most common primary malignant bone tumor and the fifth most common primary malignancy among adolescents and young adults [4]. Unfortunately, overall 5 year survival has plateaued at approximately 70%, with no meaningful improvement realized in over 30 years [3-6]. Patients with metastatic disease have poor outcomes, with pulmonary and osseous metastases portending an overall survival of less than 40% and 20%, respectively. Recent studies have demonstrated an inability to improve outcomes using conventional chemotherapy strategies, underscoring the need for novel treatment approaches [7].
Overexpression of the cation-independent mannose-6-phosphate/insulin-like growth factor-2 receptor (IGF2R) has been demonstrated across a panel of OS cell lines [1]. Additionally, a single nucleotide polymorphism (SNP) within a haplotype block in IGF2R has been associated with an increased risk of developing OS [8], however, the role of IGF2R in the pathogenesis of OS is still being investigated. Although IGF2R is expressed normally across a wide array of tissue types, the consistent overexpression of IGF2R in OS suggests it may serve as a valuable therapeutic target.
Radioimmunotherapy (RIT) is a method of delivering cytotoxic radiation therapy in a targeted fashion whereby an antigen-specific antibody is bound to either an α- or β-emitting radioisotope [9, 10]. The technique has been successfully employed in a number of challenging oncologic settings [11, 12]. RIT delivers cytocidal radiation to the targeted cell, is less affected by the multidrug resistance ensuing mechanisms than chemotherapy, and does not depend on the immune status of a patient or microenvironmental fluctuations the way immunotherapy with naked antibodies or T cells does. It permits for systemic administration, antibody-mediated specificity, and physical cytocidal damage in a manner which is well-tolerated by the patient.
The purpose of this study was to investigate a novel therapeutic approach to OS, using IGF2R-targeted RIT. We have selected 188-Rhenium (188Re), a powerful β-emitter (Emax=2.01 meV) with relatively short physical half-life of 16.9 hrs for radiolabeling the IGF2R-specific antibody. 188Re has been successfully used in both pre-clinical and clinical RIT with insignificant side effects [12 and references therein]. We hypothesized that 188Re-labeled IGF2R-specific mAb will serve to effectively target OS tumor cells and that 188Re will be able to deliver high tumoricidal doses to the tumors without toxicity to healthy tissues.
EXPERIMENTAL DESIGN
Cell lines
OS-17 is a well-characterized pediatric preclinical testing program patient-derived OS xenograft model, obtained from a primary femoral tumor and grown continuously in SCID mice [13, 14]. Tumor authentication was performed on the cell morphologically and via differentiation markers. Cell lines were grown in Eagle’s Minimum Essential Medium and supplemented with 10% FBS and a combination of 100 U penicillin with 0.1 mg/mL streptomycin (P/S). Cells were grown in a humidified condition of 95% air and 5% CO2 at 37°C. Once confluent, cells were washed with PBS twice, trypsinized and re-suspended in media. Approximately 3 million cells in 150 μL of media were implanted per animal.
Animal Model
Experiments were performed with the approval of the Albert Einstein College of Medicine Institutional Animal Care and Use Committee and in accordance with the institutional animal welfare policy. Six- to eight-week old female CB17 SCID mice weighing approximately 20 g (Taconic Biosciences) were housed in a pathogen-free barrier facility until tumor implantation. Twenty mice per experiment underwent heterotopic implantation of OS-17 in the right flank [14, 15]. Tumors were allowed to grow until palpable, measuring approximately 5 mm in diameter.
Radioisotopes and Radiolabeling of Antibodies
The IGF2R-specific murine mAb MEM-238 and the isotype matching control mAb MOPC21 (Abnova, Novus Biologics) were reduced using 75 molar excess of dithiothreitol (DTT) over the antibody by adding 5-10 μL of 3 mg/mL DTT in water to the mAbs to generate SH groups on the mAbs [16]. The mixture was left to react for 40 minutes at 37°C. The mAb was washed to remove excess dithiothreitol with 2×1.5 mL of ammonium acetate buffer in a Centricon-30 microconcentrator (Amicon). The β-emitting isotope 188Re in the form of Na188ReO4, was eluted with normal saline from a 188W/188Re radionuclide generator (Isotope Technologies). The 188ReO4− was reduced for 60 minutes at 37°C with 20 mg/mL SnCl2 in 0.1 M HCl in the presence of 50 mg/mL sodium gluconate. The reduced 188Re was then incubated with the DTT-treated antibodies for 60 min at 37°C as in [16]. The radiolabeling yields for 188Re-mAbs were measured by instant thin layer chromatography (ITLC) by developing silica gel (SG) 10 cm strips in saline. In this system the radiolabeled antibodies stay at the point of application while free 188Re moves with the solvent front. The average radiolabeling yield for either 188Re-mAbs was 85±5%. The radiolabeled mAbs were purified by HPLC using a TSK gel G4000SW size exclusion column (Tosoh Biosciences, Japan) eluted with PBS at 1 ml/min using Waters HPLC system equipped with UV (Waters 2487) and radiation (Bioscan Flow-Count) flow-through detectors.
Biodistribution
As murine and human IGF2Rs are not homologous, the mAb MEM-238 to human IGF2R can only bind specifically to IGF2R in OS17 xenografts. Thus, the biodistribution was performed to assess whether IGF2R-specific mAb will localize in the tumor preferentially when compared to non-specific isotype matching control MOPC21. The in vivo stability of the radiolabeled mAbs could also be assessed in such experiment. . The IGF2R-specific murine mAb MEM-238 and isotype matching control mAb MOPC21 were radiolabeled with 188Re as above. Mice were randomized and administered 50 μCi via an intraperitoneal injection, with ten mice receiving 188Re-MEM-238 and ten receiving 188Re -MOPC21. At 24 hours post-injection, 5 mice from each group were sacrificed and their tumors, blood and major organs were harvested, weighed and counted for radioactivity in a gamma counter using a dosimetry approach developed specifically for the laboratory rodent models, accounting for both for γ- and β-radiation. [17, 18] At 48 hours post-injection, the remaining 5 mice were sacrificed and similar harvesting and measurements were performed. The percentage of the injected radiolabeled mAb dose per gram of tissue (ID/g, %) was calculated for each animal.
Binding Sites
The Scatchard transformation was used to assess the number of IGF2R binding sites expressed on OS-17 cells [19]. OS17 cells were cultured and for each concentration of radiolabeled mAb, approximately 8 million cells were included. The experiment was performed twice using 0.0025, 0.005, 0.010, and 0.020 nM concentrations of radiolabeled 188Re MEM-238 and MOPC21 mAbs. The calculation of association constant Ka and the number of IGF2R binding sites per OS-17 cell were performed as previously reported. [19]
In Vivo RIT Studies
Following OS-17 implantation and growth to 5 mm in diameter, the mice were randomized into 4 groups. Group 1 received 300 μCi of 188Re-MEM-238, group 2 received 300 μCi of 188Re-MOPC21, group 3 received unlabeled (cold) MEM-238, and group 4 was left untreated. This dose level was chosen as it proved to be effective for other 188Re-antibody combinations in RIT of experimental cervical cancer [20]. Intraperitoneal injections were performed as previously described. Tumor volume was calculated every 3 days. Electronic calipers were utilized to measure the 3 largest diameters of the mass. Volume was calculated utilizing the equation for spheroid volume (V=Π·d1·d2·d3/6). To normalize the variability in tumor size between each animal, tumor volume ratio was utilized. Tumor growth ratio (TGR) was calculated by dividing the difference between the initial tumor volume at the start of the experiment (Vo) and the calculated tumor volume (Vn), divided by the initial tumor volume: TGR=(Vo-Vn)/Vo [21]. The calculated TGRs were plotted against time. The experiment was performed twice, initially over a 14-day period (Supplemental Figure 1s.) and thereafter over a 24-day period.
Histologic Analysis
The tumors were harvested at the conclusion of the RIT study. Tumors were fixed in 4% paraformaldehyde, decalcified, and embedded in paraffin blocks. Paraffin-embedded tissue slides were heated at 60°C for 1 hour, de-paraffinized using xylenes and rehydrated using graded alcohols. Endogenous peroxidase activity was quenched using 0.3% hydrogen peroxide in methanol. Antigenic proteins were unmasked by heat induced antigen retrieval method using sodium citrate buffer. The tissue was blocked with 10% normal goat serum in 1% bovine serum albumin (BSA) in tris-buffered saline (TBS), and stained with 10 μg/mL MEM-238 IGF2R mAb diluted in 1% BSA in TBS overnight at 4°C. Commercially available paraffin-embedded placenta tissue (BioChain Institute, Hayward, CA) was used as the positive control, and MOPC-21 IgG1 diluted in the same diluent as above was used instead of the primary antibody as the isotype negative control. Detection of the antibody-binding reaction was performed with biotinylated secondary antibody coupled with streptavidin-horseradish peroxidase. Avidin biotinylated enzyme complex (Vectastain ABC System; Vector Laboratories) was used according to the manufacturer’s instructions. The tissue was treated with 3,3’-diaminobenzidine (Vector Laboratories) to visualize the antibody binding and counterstained with hematoxylin. The tissue was then dehydrated with alcohol, permeated with xylenes and mounted with Permount organic mounting solution (Fisher Scientific). Staining technique was optimized by comparison with positive and negative controls. Stained slides were viewed using a PerkinElmer P250 High Capacity Slide Scanner (SIG #1S10OD019961-01, TissueScope™, Huron Digital Pathology) and staining patterns were contrasted quantitatively utilizing 3-D imaging analysis software (Volocity® 6.3, PerkinElmer). The total pixels of the tissue and the total number of brown pixels on each slide were counted. The total number of brown pixels was divided by the total number of pixels of tissue on each slide. Three slides per group were evaluated and results for each group were averaged.
Statistical Methodology
Power analysis for the in vivo cytotoxicity studies was estimated using PASS version 11 (NCSS, Inc.) using simulations of different tumor volumes based on pilot data and conservative assumptions regarding the groups treated with the radiolabeled antibodies. All simulations showed power of at least 83% with only five animals per group because of the large differences between treated and untreated animals. Thus, 5 mice per group were utilized in the in vivo studies. The differences between the biodistribution groups were analyzed using the Kruskal-Wallis and/or the Mann-Whitney tests. Differences between the treatment groups were similarly analyzed using the Kruskal-Wallis and/or the Mann-Whitney tests. Immunohistochemical analysis was performed by comparing the fraction of stained pixels using an overdispersed logistic model. A two-sided p value <0.05 was considered statistically significant for all studies.
RESULTS
OS-17 cells demonstrate a high binding efficacy for IGF2R-specific mAb
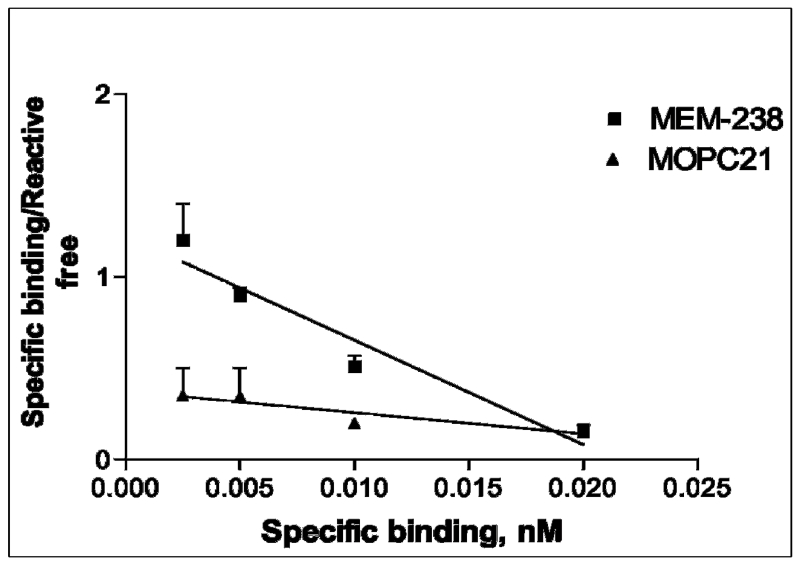
The results of the Scatchard plot used to characterize and validate the binding affinity of the IFG2R specific murine mAb and the isotype matching control mAb demonstrated that there are 1,800 binding sites for 188Re-MEM-238 mAb with an association constant, ka, of 5.8 × 1010 M−1 (Figure 1). The slope for 188Re-MOPC21 mAb was not significantly different from zero, indicating there was no observed specific binding to OS-17 cells.
Figure 1.
Scatchard plot demonstrating non-specific binding of MOPC21 and specific binding for MEM-238.
IGF2R labeled mAb preferentially localizes to tumor cells
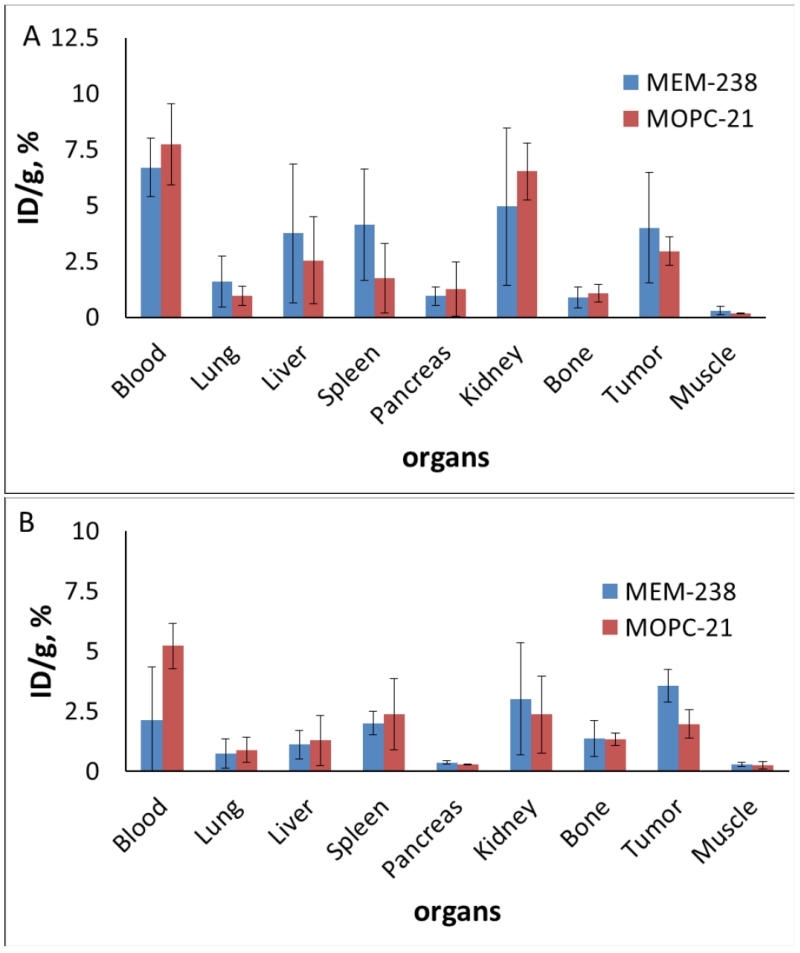
The distribution of 188Re -MEM-238 in normal tissue was not significantly different from that of the isotype control, indicating that it was stable in vivo and as a result did not preferentially pool within any specific anatomic location. Importantly, when compared with the isotype control, 188Re-MEM-238 preferentially localized within the tumor after 48 hours, at which point complete separation of the two groups was apparent. The radiolabeled IGF2R antibody exhibited a 1.8 fold selective uptake within tumor tissue compared to the isotype control. This difference approached statistical significance (p=0.057, Figure 2). The clearance of 188Re-MEM-238 from blood was faster than that of the control 188Re-MOPC21.
Figure 2.
The biodistribution of radioactivity post-injection across selected SCID mouse organs following the administration of 188Re-labeled IGF2R-specific mAb (MEM-238) and 188Re-labeled control mAb (MOPC21): A) 24 hrs; B) 48 hrs
Treatment with 188Re-labeled IGF2R-specific mAb results in tumor growth suppression in vivo
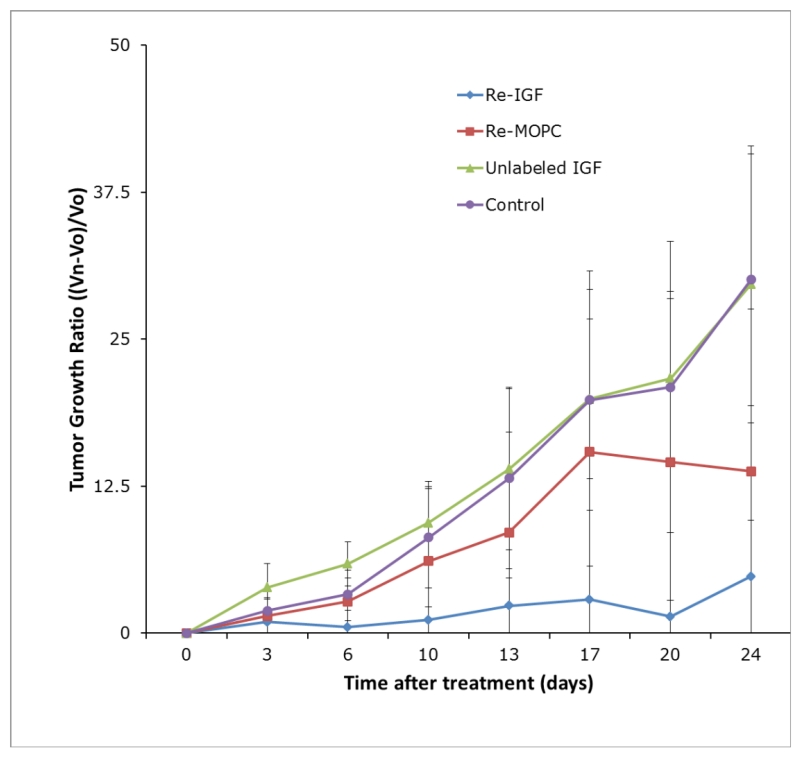
The effect of both specific and non-specific radionuclide therapy was evident, with Group 1 (188Re-MEM-238) and Group 2 (188Re-MOPC-21) demonstrating a clear decrease in both absolute tumor volume (p<0.001) and tumor growth ratio (p=0.001) compared with that of the unlabeled control groups, (unlabeled MEM-238 and untreated) in which tumor grew aggressively. The pilot RIT experiment, performed over a 14-day period (Figure 1S), demonstrated a strong trend to suppress tumor growth in the 188Re-MEM-238 treated group compared to the control groups. In the follow-up 24-day RIT experiment (Figure 3) the average tumor growth ratio (TGR) was 4.8 for the 188Re-MEM-238 group, as compared to 13.7 for the 188Re-MOPC-21 group, 29.6 for the unlabeled MEM-238 group, and 30.4 for the untreated group. A whisker plot of day 24 TGR demonstrates the range of values for each treatment arm of the experiment (Figure 2S). The TGR of Group 1 (188Re-MEM-238) was compared to that of Group 3 (unlabeled MEM-238) and Group 4 (untreated), demonstrating suppression of tumor growth in Group 1 (188Re-MEM-238, p=0.005). The specific effect of 188Re-MEM-238 utilized in Group 1 appeared more profound than that of control Group 2 (188Re-MOPC-21), reaching a non-significant trend (p=0.057).
Figure 3.
Tumor growth ratio over a 24 day-period demonstrated marked suppression of tumor growth by 188Re-MEM-238 (Group 1). The non-specific control 188Re-MOPC21 mAb (Group 2) produced a lesser effect on tumor growth suppression. The tumors treated with unlabeled mAb (Group 3) and untreated tumors (Group 4) demonstrate uninhibited growth.
Treatment with 188Re-labeled IGF2R-specific mAb induces cytotoxicity in OS cells expressing IGF2R
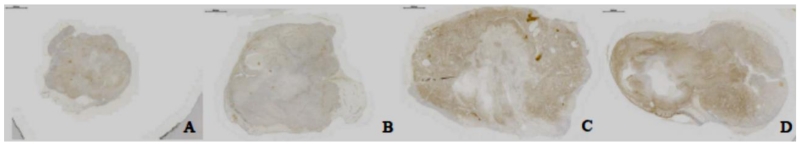
Treatment with 188Re-MEM-238 (Group 1) resulted in 37.4% staining of total pixels after 24 days, (Figure 4A), suggesting a substantial tumoricidal effect on IGF2R-expressing OS cells. Treatment with 188Re-MOPC21 (Group 2) resulted in 44.4% staining of total pixels (Figure 4B), suggesting a partial effect on IGF2R-expressing OS cells. Robust IGF2R staining was visualized in tumor treated with unlabeled MEM-238 (Group 3, Figure 4C) and in untreated tumor (Group 4, Figure 4D), in which 60.8% and 59.6% of total pixels were stained respectively, suggesting minimal to no effect on IGF2R-expressing OS cells. Staining of 188Re-MEM-238 (Group 1) was significantly different from that of unlabeled MEM-238 (Group 3) and untreated (Group 4, p=0.002). The difference between the188Re-MOPC21 (Group 2) and the 188Re-MEM-238 (Group 1) did not reach significance.
Figure 4.
Immunohistochemical staining demonstrated minimal IGF2R-staining intensity following treatment with 188Re-MEM-238 (A), moderate IGF2R-staining following treatment with 188Re-MOPC21 (B), and robust IGF2R-staining in both tumors treated with unlabeled MEM-238 (C) or in tumor untreated (D). Findings suggest that OS cells expressing IGF2R have been killed by the RIT treatment.
DISCUSSION
This study demonstrates the feasibility of targeting IGF2R with RIT in a heterotopic murine OS model. Scatchard plot analysis demonstrated a moderate number of available IGF2R binding sites and tight Ab binding, reflected by the high binding constant [19]. Since efficacy of RIT is directly proportional to the mAb’s binding constant, high RIT efficacy was predicted. It should be noted that in RIT studies where similar antigen levels on the surface of the targeted cells were observed in vitro [22,23], the RIT was nevertheless successful in killing the targeted cells. In the future, use of αemitters should also be explored for IGF2R-targeted RIT of osteosarcoma to counteract the relatively low number of binding sites. The radioimmunoconjugate demonstrated preferential localization to tumor tissue at 48 hours when compare to the isotype control. Finally, the use of RIT for the treatment of OS resulted in the suppression of tumor growth within the described model. The summation of these findings suggests that IGF2R has the potential to be an effective target for the treatment of OS and that 188Re-labeled IGF2R-specific mAb may offer a useful approach for the treatment of OS, particularly in patients with metastatic or refractory disease.
Currently, there is a paucity of therapeutic options available to patients who fail first-line therapy and the addition of conventional chemotherapeutic agents to the backbone of methotrexate, doxorubicin, and cisplatin is unlikely to significantly improve overall survival. Novel approaches are needed for patients who demonstrate local or distant relapse and arguably for patients who respond poorly to standard chemotherapy.
Techniques such as stereotactic body radiotherapy, proton therapy, and immune-modulated radiotherapy have changed the landscape of radiation therapy and overcome some of its historical challenges [24]. To date, the gold standard treatment for OS unequivocally remains complete resection and cytotoxic chemotherapy [2, 5]. However, radiotherapy is utilized in patients for whom complete resection is not feasible, such as in patients with tumors in the axial skeleton. In such cases, radiotherapy, in combination with standard chemotherapy, has been reported to result in higher overall survival then with chemotherapy alone [25, 26]. Newer radiation modalities are proving to be important for palliative management of pulmonary and osseous disease [24, 27, 28]. Although radiation therapy is not currently a part of first-line therapy, there is precedence for its use in scenarios where conventional strategies are insufficient.
Radionuclide therapy for the treatment of osseous metastases from carcinoma and OS has been previously explored. Samarium-153 and Radium-223 (Alpharadin) act as calcium mimetics targeting bone cortical surfaces and areas of high bone turnover. Samarium-153 lexidronan is approved as a palliative agent for pain associated with osteoblastic metastases in hormone resistant prostate carcinoma [29] while Alpharadin has improved median overall survival by 3.6 months in patients with metastatic prostate cancer [30]. Lastly, there is an open phase I trial in OS patients with osteoblastic tumors [29], underscoring its relevance as a therapeutic modality.
The effectiveness of combining radionuclides and monoclonal antibodies [31] has been well-documented for both hematopoietic and solid tumors [32-34]. The incorporation of RIT in the treatment of OS is founded on its reliable surface overexpression of IGF2R. IGF2R’s consistent overexpression on the tumor’s surface and mAb localization within the tumor at 48 hours both suggest it is a useful target. Binding studies additionally demonstrate high efficacy of IGF2R, further supporting this contention. RIT acts through the direct-targeted mechanism and also exerts a “cross-fire” effect, whereby proximate non-IGF2R binding cells are impacted as well. This may prove very relevant in the setting of a genetically variable and unstable entity such as OS, as it obviates the need for all tumor cells to possess the target and could rely on a more heterogeneous expression pattern. The current study demonstrates the effect of utilizing IGF2R as a reliable target as demonstrated by 188Re-labeled IGF2R-specific mAb mediated growth suppression and decreased expression of IGF2R immunohistochemical staining. Regardless of whether RIT ultimately develops into a clinically relevant modality, the current study serves as a proof of concept for IGF2R-targeted therapy.
Limitations of this study include the relatively short experimental time course. As an initial study, a 24-day time course was felt to be sufficient to characterize preliminary results. However, a longer time course may yield additional information relevant to toxicity, resistance, and durability. The evaluation of human OS was accomplished by using immunocompromised animals. Although the model is well-described and validated, it is difficult to know to what extent the immune response, or lack thereof, contributed to these results. While dictated by the described power analysis, the relatively small sample size utilized likely contributes to the lack of significance in the difference seen between targeted and non-specific RIT treatment, particularly since one animal in each group died at an early time-point. Future studies should incorporate more OS cell lines and should evaluate biodistribution across a greater number of time points. Finally, our antibody was specific only to human IGF2R and future use of an antibody with human and murine specificity will permit for a more comprehensive assessment of anti-tumor effect and associated toxicity. Based on the limitations of the current study, a broader and longer-term study is warranted. Current findings serve as proof-of-principles and the foundation for a more comprehensive investigation.
This targeted approach offers the benefits of being independent of a specific pathway, a resistance mechanism, and/or an inherent biologic tumor trait and therefore is relevant to all OS tumors that express IGF2R. It is recognized that this is an entirely different approach to treating this malignancy, but given the lack of effective new treatments it may prove to be of tremendous value, in particular to patients with metastatic disease for whom there is often no useful therapy. The current frustration with unimproved survival rates and the absence of a clearly beneficial second line therapy strongly stand in favor of alternative approaches that may provide a subset of patients a meaningful, though incremental, improvement.
Supplementary Material
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: Richard Gorlick is a member of the Scientific Advisory Board of Oncolytics, which has no relationship to the subject of the current paper. No potential conflicts of interest were disclosed by any of the other authors associated with this manuscript.
REFERENCES
- 1.Hassan SE, et al. Cell surface receptor expression patterns in OS. Cancer. 2012;118(3):740–9. doi: 10.1002/cncr.26339. [DOI] [PubMed] [Google Scholar]
- 2.Mirabello L, Troisi RJ, Savage SA. International OS incidence patterns in children and adolescents, middle ages and elderly persons. Int J Cancer. 2009;125(1):229–34. doi: 10.1002/ijc.24320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mirabello L, Troisi RJ, Savage SA. OS incidence and survival rates from 1973 to 2004: data from the Surveillance, Epidemiology, and End Results Program. Cancer. 2009;115(7):1531–43. doi: 10.1002/cncr.24121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaatsch P. Epidemiology of childhood cancer. Cancer Treat Rev. 2010;36(4):277–85. doi: 10.1016/j.ctrv.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 5.Ferrari S, et al. Neoadjuvant chemotherapy with high-dose Ifosfamide, high-dose methotrexate, cisplatin, and doxorubicin for patients with localized OS of the extremity: a joint study by the Italian and Scandinavian Sarcoma Groups. J Clin Oncol. 2005;23(34):8845–52. doi: 10.1200/JCO.2004.00.5785. [DOI] [PubMed] [Google Scholar]
- 6.Meyers PA, et al. OS: a randomized, prospective trial of the addition of ifosfamide and/or muramyl tripeptide to cisplatin, doxorubicin, and high-dose methotrexate. J Clin Oncol. 2005;23(9):2004–11. doi: 10.1200/JCO.2005.06.031. [DOI] [PubMed] [Google Scholar]
- 7.Whelan JS, et al. EURAMOS-1, an international randomised study for OS: results from pre-randomisation treatment. Ann Oncol. 2015;26(2):407–14. doi: 10.1093/annonc/mdu526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Savage SA, et al. Analysis of genes critical for growth regulation identifies Insulin-like Growth Factor 2 Receptor variations with possible functional significance as risk factors for OS. Cancer Epidemiol Biomarkers Prev. 2007;16(8):1667–74. doi: 10.1158/1055-9965.EPI-07-0214. [DOI] [PubMed] [Google Scholar]
- 9.Milenic DE, Brady ED, Brechbiel MW. Antibody-targeted radiation cancer therapy. Nat Rev Drug Discov. 2004;3(6):488–99. doi: 10.1038/nrd1413. [DOI] [PubMed] [Google Scholar]
- 10.Sharkey RM, Goldenberg DM. Perspectives on cancer therapy with radiolabeled monoclonal antibodies. J Nucl Med. 2005;46(Suppl 1):115S–27S. [PubMed] [Google Scholar]
- 11.Kaminski MS, et al. 131I-tositumomab therapy as initial treatment for follicular lymphoma. N Engl J Med. 2005;352(5):441–9. doi: 10.1056/NEJMoa041511. [DOI] [PubMed] [Google Scholar]
- 12.Klein M, et al. Safety and efficacy of 188-rhenium-labeled antibody to melanin in patients with metastatic melanoma. J Skin Cancer. 2013;2013:828329. doi: 10.1155/2013/828329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Whiteford CC, et al. Credentialing preclinical pediatric xenograft models using gene expression and tissue microarray analysis. Cancer Res. 2007;67(1):32–40. doi: 10.1158/0008-5472.CAN-06-0610. [DOI] [PubMed] [Google Scholar]
- 14.Houghton PJ, et al. The pediatric preclinical testing program: description of models and early testing results. Pediatr Blood Cancer. 2007;49(7):928–40. doi: 10.1002/pbc.21078. [DOI] [PubMed] [Google Scholar]
- 15.Peterson JK, et al. In vivo evaluation of ixabepilone (BMS247550), a novel epothilone B derivative, against pediatric cancer models. Clin Cancer Res. 2005;11(19 Pt 1):6950–8. doi: 10.1158/1078-0432.CCR-05-0740. [DOI] [PubMed] [Google Scholar]
- 16.Dadachova E, Mirzadeh S. The role of tin in the direct labelling of proteins with Rhenium-188. Nucl Med Biol. 1997;24(6):605–8. doi: 10.1016/s0969-8051(97)00079-6. [DOI] [PubMed] [Google Scholar]
- 17.Thompson S, et al. 166Ho and 90Y labeled 6D2 monoclonal antibody for targeted radiotherapy of melanoma: comparison with 188Re radiolabel. Nucl Med Biol. 2014;41(3):276–81. doi: 10.1016/j.nucmedbio.2013.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quispe-Tintaya W, et al. Nontoxic radioactive Listeria(at) is a highly effective therapy against metastatic pancreatic cancer. Proc Natl Acad Sci U S A. 2013;110(21):8668–73. doi: 10.1073/pnas.1211287110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dadachova E, et al. Interaction of radiolabeled antibodies with fungal cells and components of the immune system in vitro and during radioimmunotherapy for experimental fungal infection. J Infect Dis. 2006;193(10):1427–36. doi: 10.1086/503369. [DOI] [PubMed] [Google Scholar]
- 20.Wang X-G, et al. Treating cancer as an infectious disease - viral antigens as novel targets for treatment and potential prevention of tumors of viral etiology. PLOS One. 2007;2(10):e1114. doi: 10.1371/journal.pone.0001114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Revskaya E, et al. Radioimmunotherapy of experimental human metastatic melanoma with melanin-binding antibodies and in combination with dacarbazine. Clin Cancer Res. 2009;15(7):2373–9. doi: 10.1158/1078-0432.CCR-08-2376. [DOI] [PubMed] [Google Scholar]
- 22.Vervoordeldonk SF, et al. Preclinical studies with radiolabeled monoclonal antibodies for treatment of patients with B-cell malignancies. Cancer. 1994;73(3 Suppl):1006–1011. doi: 10.1002/1097-0142(19940201)73:3+<1006::aid-cncr2820731339>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 23.Miederer M, et al. Comparison of the radiotoxicity of two alpha-particle-emitting immunoconjugates, terbium-149 and bismuth-213, directed against a tumor-specific, exon 9 deleted (d9) E-cadherin adhesion protein. Radiat Res. 2003;159(5):612–620. doi: 10.1667/0033-7587(2003)159[0612:cotrot]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 24.Lo SS, et al. Stereotactic body radiation therapy: a novel treatment modality. Nat Rev Clin Oncol. 2010;7(1):44–54. doi: 10.1038/nrclinonc.2009.188. [DOI] [PubMed] [Google Scholar]
- 25.Ozaki T, et al. OS of the spine: experience of the Cooperative OS Study Group. Cancer. 2002;94(4):1069–77. [PubMed] [Google Scholar]
- 26.Ozaki T, et al. OS of the pelvis: experience of the Cooperative OS Study Group. J Clin Oncol. 2003;21(2):334–41. doi: 10.1200/JCO.2003.01.142. [DOI] [PubMed] [Google Scholar]
- 27.Whelan JS, et al. A systematic review of the role of pulmonary irradiation in the management of primary bone tumours. Ann Oncol. 2002;13(1):23–30. doi: 10.1093/annonc/mdf047. [DOI] [PubMed] [Google Scholar]
- 28.Schwarz R, et al. The role of radiotherapy in oseosarcoma. Cancer Treat Res. 2009;152:147–64. doi: 10.1007/978-1-4419-0284-9_7. [DOI] [PubMed] [Google Scholar]
- 29.Anderson PM, Subbiah V, Rohren E. Bone-seeking radiopharmaceuticals as targeted agents of OS: samarium-153-EDTMP and radium-223. Adv Exp Med Biol. 2014;804:291–304. doi: 10.1007/978-3-319-04843-7_16. [DOI] [PubMed] [Google Scholar]
- 30.Parker C, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369(3):213–23. doi: 10.1056/NEJMoa1213755. [DOI] [PubMed] [Google Scholar]
- 31.Palmedo H, et al. Repeated bone-targeted therapy for hormone-refractory prostate carcinoma: tandomized phase II trial with the new, high-energy radiopharmaceutical rhenium-188 hydroxyethylidenediphosphonate. J Clin Oncol. 2003;21(15):2869–75. doi: 10.1200/JCO.2003.12.060. [DOI] [PubMed] [Google Scholar]
- 32.Kraeber-Bodere F, et al. Radioimmunoconjugates for the treatment of cancer. Semin Oncol. 2014;41(5):613–22. doi: 10.1053/j.seminoncol.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 33.Dadachova E, et al. Dead cells in melanoma tumors provide abundant antigen for targeted delivery of ionizing radiation by a mAb to melanin. Proc Natl Acad Sci U S A. 2004;101(41):14865–70. doi: 10.1073/pnas.0406180101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jain M, et al. Emerging trends for radioimmunotherapy in solid tumors. Cancer Biother Radiopharm. 2013;28(9):639–50. doi: 10.1089/cbr.2013.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.