Abstract
Rearrangement of MYC is associated with a poor prognosis in patients with diffuse large B cell lymphoma (DLBCL) and B cell lymphoma unclassifiable (BCLU), particularly in the setting of double hit lymphoma (DHL). Yet, little is known about outcomes of patients who demonstrate MYC rearrangement without evidence of BCL2 or BCL6 rearrangement (single hit) or amplification (>4 copies) of MYC. We identified 87 patients with single hit lymphoma (SHL), 22 patients with MYC-amplified lymphoma (MYC amp) as well as 127 DLBCL patients without MYC rearrangement or amplification (MYC normal) and 45 patients with double hit lymphoma (DHL), all treated with either R-CHOP or intensive induction therapy. For SHL and MYC amp patients, the 2 year progression free survival rate (2yPFS) was 49% and 48% and 2 year overall survival rate (2yOS) was 59% and 71%, respectively. SHL patients receiving intensive induction experienced higher 2yPFS (59% vs. 23%, P=0.006) but similar 2yOS as compared with SHL patients receiving R-CHOP. SHL DLBCL patients treated with R-CHOP, but not intensive induction, experienced significantly lower 2yPFS and 2yOS (p<0.001 for both) when compared with MYC normal patients. SHL patients appear to have a poor prognosis, which may be improved with receipt of intensive induction.
Keywords: MYC, Gene rearrangement, Gene amplification, B cell lymphoma, CHEMOTHERAPY
Introduction
The transcription factor MYC is responsible for many cellular functions including regulation of cell cycle activity and protein synthesis (Dang, et al 2006, Meyer and Penn 2008). Translocation of MYC with the immunoglobulin heavy chain promotor (IgH) or less commonly light chain promoters (IgK and IgL) is a defining feature of Burkitt lymphoma, which is frequently curable with immunochemotherapy. However, rearrangement of MYC is reported to occur in approximately 10% of diffuse large B cell lymphoma (DLBCL) cases (Lin and Medeiros 2013, Slack and Gascoyne 2011) and up to 50% of cases of B cell lymphoma unclassifiable with features intermediate between diffuse large B cell lymphoma and Burkitt lymphoma (BCLU)/Burkitt-like lymphoma (Boerma, et al 2009), and is associated with a poor prognosis. For DLBCL patients treated with either front-line cyclophosphamide, doxorubicin, vincristine and prednisone (CHOP) with or without etoposide, or rituximab-CHOP (R-CHOP), inferior overall survival (OS) was noted for those with evidence of MYC rearrangement as compared with those without MYC rearrangement (Barrans, et al 2010, Klapper, et al 2008, Savage, et al 2009). Similarly, for patients with BCLU, receipt of front-line R-CHOP was associated with a short duration of progression free survival (PFS) if MYC-rearranged (Lin, et al 2012). Additionally, patients with relapsed/refractory DLBCL with MYC rearrangement appear to respond poorly to salvage immunochemotherapy and autologous stem cell transplantation as compared with those without MYC rearrangement (Cuccuini, et al 2012).
A subset of MYC-rearranged lymphomas known as double hit lymphoma (DHL), which demonstrate rearrangement of BCL2 and/or BCL6 in addition to MYC (Aukema, et al 2011), are associated with a particularly poor prognosis, although this may be improved for patients treated with front-line intensive immunochemotherapy (Howlett, et al 2015, Petrich, et al 2014). However, the prognosis for patients with DLBCL/BCLU demonstrating rearrangement of MYC with negative testing for rearrangements of BCL2 and BCL6, known as “single hit” lymphoma (SHL), is unclear. Outcomes for this subset of patients are typically not distinguished from those of DLBCL/BCLU patients harboring MYC rearrangement whose tissue samples have not been tested for both BCL2 and BCL6 rearrangements, for whom the possibility of DHL has not been excluded. SHL is prevalent within cases of non-Burkitt MYC-rearranged B cell lymphomas, representing 40% of cases of MYC-rearranged DLBCL (Copie-Bergman, et al 2015, Horn, et al 2013) and potentially up to 50% of cases of MYC-rearranged BLCU (Lin, et al 2012, Perry, et al 2013).
Another alteration in MYC that has been detected in patients with high-grade B cell non-Hodgkin lymphomas is increased copy number or amplification, although the prognostic value of this finding is unclear (Stasik, et al 2010, Testoni, et al 2011, Valera, et al 2013, Yoon, et al 2008), which may be due in part to small numbers of these patients studied.
Through a multicenter analysis, we aimed to determine the impact of clinicopathologic characteristics and treatment received on survival of DLBCL and BCLU patients with SHL as well as MYC-amplified lymphoma (MYC amp). We also compared the outcomes of these patients to those with lymphomas not demonstrating MYC rearrangement or amplification by fluorescence in situ hybridization (FISH) (MYC normal) and DHL.
Methods
Patients
Cases of SHL, defined as the presence of MYC rearrangement and/or translocation by conventional cytogenetics or FISH along with the absence of BCL2 and BCL6 rearrangement by FISH, as well as cases of MYC amp lymphoma, defined per prior publications (Mossafa, et al 2006, Valera, et al 2013) as >4 copies of MYC by FISH regardless of BCL2 and BCL6 FISH results, were collected from 13 United States-based academic centers. For the purpose of comparing survival outcomes, all known cases of MYC normal lymphoma and DHL were also collected from the University of Pennsylvania and Northwestern University. Inclusion criteria included age 18–90 years, DLBCL or BCLU histologic classifications and front-line treatment with either R-CHOP or intensive induction therapy, defined as either rituximab-EPOCH (etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin), rituximab-hyper-CVAD (hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone alternating with methotrexate and cytarabine), or rituximab-CODOX-M/IVAC (cyclophosphamide, vincristine, doxorubicin, and high-dose methotrexate, alternating with ifosfamide, etoposide, and cytarabine). All MYC normal patients included received R-CHOP. Patients with inadequate clinicopathologic and survival data as well as those known to have human immunodeficiency virus (HIV) were excluded. Cases with both MYC rearrangement and >4 copies of MYC as detected by FISH (n=3) were also excluded. Only one case of MYC normal BCLU meeting inclusion criteria was identified and was excluded due to small sample size. Cell of origin classification was defined per Hans algorithm (Hans, et al 2004). When staging bone marrow aspiration and biopsy was not performed, patients with lymphomatous involvement of the peripheral blood by high-grade lymphoma were considered to have bone marrow involvement, while patients with no abnormal FDG uptake in the bones were considered not to have bone marrow involvement by high grade lymphoma (Cheson, et al 2014). Cases were reviewed by hematopathologists at each academic medical center. Criteria for performance of FISH for cases of DLBCL and BLCU were per the policy of each center. Therapy was prescribed at the discretion of the treating physician. Data were censored in March 2016.
Statistical analysis
PFS was defined as the time from diagnosis until disease progression or death and OS was defined as the time from diagnosis until death from any cause. Disease progression was defined as either evidence of relapse or increase in disease burden for a patient with prior disease response, or change in therapy due to lack of disease response in patients without prior disease response. Disease response was defined by the Revised Response Criteria for Malignant Lymphoma (Cheson, et al 2007). Categorical data were analyzed by Fisher’s exact test and continuous variables by the Wilcoxon-Mann-Whitney test. PFS and OS curves were plotted using Kaplan Meier estimates and survival analysis performed using the log-rank test. Univariate analysis was performed using Cox proportional-hazards regression. Statistical significance was defined as a two-tailed p value of <0.05. All statistical analyses were performed using Stata version 13 (StataCorp, College Station, TX, USA). This protocol was approved by the institutional review board of each participating center.
Results
SHL patients
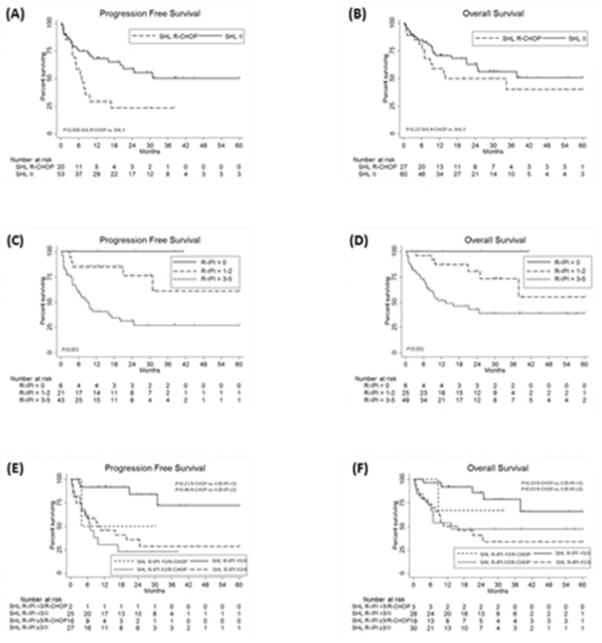
Baseline characteristics for SHL patients are shown in Table I. For 87 SHL patients with a median length of follow-up of 28.9 months (range 0.5–84.5 months), the rate of 2 year (2y) PFS (n=73) was 49% and 2yOS 59%. Front-line therapy received was R-CHOP in 27 patients and intensive induction in 60 patients. As depicted in Fig. 1, 2yPFS was 23% for SHL patients receiving R-CHOP as compared with 59% for those receiving intensive induction (P=0.006) and 2yOS 50% and 63%, respectively (P=0.22). When classifying SHL patients by R-IPI score of 0, 1–2 and 3–5 (Sehn, et al 2007), 2yPFS was 100%, 77% and 31%, while 2yOS was 100%, 81% and 43%, respectively (P<0.001 and P=0.002, respectively) (Fig. 1). Given similar 2yPFS and 2yOS for patients with R-IPI scores of 0 and 1–2 (P=0.27 and P=0.36, respectively), patients were further classified as R-IPI score of <3 and ≥3. For patients with R-IPI <3, 2yPFS was 82% and 2yOS 84% and did not differ significantly based on receipt of R-CHOP as compared with intensive induction (50% vs. 84%, P=0.20 for 2yPFS and 67% vs. 86%, P=0.34 for 2yOS). For patients with R-IPI ≥3, 2yPFS was 31% and 2yOS 43% and also did not differ significantly based on receipt of R-CHOP as compared with intensive induction (23% vs. 36%, P=0.46 for 2yPFS and 47% vs. 41% for 2yOS, P=0.93) (Fig. 1).
Table I.
Patient clinicopathologic characteristics.
| MYC normal (n = 127) | SHL (n = 87) | MYC amp (n = 22) | DHL (n = 45) | |
|---|---|---|---|---|
| Sex | ||||
| Male | 64 (50%) | 57 (66%) | 13 (59%) | 24 (53%) |
| Female | 63 (50%) | 30 (34%) | 9 (41%) | 21 (47%) |
| Age | ||||
| Median | 62 | 57 | 53 | 60 |
| ≤60 | 59 (46%) | 48 (55%) | 14 (64%) | 20 (44%) |
| > 60 | 68 (54%) | 39 (45%) | 8 (36%) | 25 (56%) |
| Lactate dehydrogenase | ||||
| Normal | 44 (35%) | 17 (20%) | 5 (23%) | 7 (16%) |
| >Normal | 62 (49%) | 59 (68%) | 13 (59%) | 31 (68%) |
| Unknown | 21 (16%) | 11 (12%) | 4 (18%) | 7 (16%) |
| Stage | ||||
| I–II | 59 (46%) | 20 (23%) | 2 (9%) | 11 (24%) |
| III–IV | 67 (53%) | 67 (77%) | 20 (91%) | 34 (76%) |
| Unknown | 1 (1%) | |||
| ECOG performance status | ||||
| <2 | 111 (87%) | 57 (66%) | 18 (82%) | 36 (80%) |
| ≥2 | 9 (7%) | 20 (23%) | 3 (14%) | 7 (16%) |
| Unknown | 7 (4%) | 10 (11%) | 1 (4%) | 2 (4%) |
| Bone marrow involvement | ||||
| No | 106 (83%) | 44 (51%) | 14 (64%) | 27 (60%) |
| Yes | 18 (14%) | 40 (46%) | 7 (32%) | 14 (31%) |
| Unknown | 3 (3%) | 3 (3%) | 1(4%) | 4 (9%) |
| Extranodal sites of disease ≥2 | ||||
| No | 87 (69%) | 37 (43%) | 13 (59%) | 29 (64%) |
| Yes | 38 (30%) | 50 (57%) | 9 (41%) | 16 (36%) |
| Unknown | 2 (1%) | |||
| B symptoms | ||||
| No | 91 (72%) | 47 (54%) | 15 (68%) | 29 (64%) |
| Yes | 32 (25%) | 38 (44%) | 7 (32%) | 14 (31%) |
| Unknown | 4 (3%) | 2 (2%) | 2 (5%) | |
| Histologic classification | ||||
| DLBCL | 127 (100%) | 54 (62%) | 17 (77%) | 37 (82%) |
| BCLU | 33 (38%) | 5 (23%) | 8 (18%) | |
| Ki67 % expression | ||||
| Median | 80 | 90 | 80 | 80 |
| <90 | 61 (48%) | 16 (18%) | 10 (46%) | 30 (67%) |
| ≥90 | 36 (28%) | 56 (64%) | 8 (36%) | 10 (22%) |
| Unknown | 30 (24%) | 15 (18%) | 4 (18%) | 5 (11%) |
| Cell of origin | ||||
| Non-germinal center | 41 (32%) | 12 (14%) | 7 (32%) | 5 (11%) |
| Germinal center | 71 (56%) | 62 (71%) | 12 (55%) | 39 (87%) |
| Unknown | 15 (12%) | 13 (15%) | 3 (13%) | 1 (2%) |
| R-IPI score | ||||
| 0 | 16 (12%) | 6 (7%) | 1 (4%) | 3 (7%) |
| 1–2 | 56 (44%) | 25 (29%) | 11 (50%) | 18 (40%) |
| 3–5 | 45 (35%) | 49 (56%) | 9 (42%) | 19 (42%) |
| Unknown | 10 (9%) | 7 (8%) | 1 (4%) | 5 (11%) |
DLBCL, diffuse large B cell lymphoma; BCLU, B cell lymphoma unclassifiable with features intermediate between diffuse large B cell lymphoma and Burkitt lymphoma; R-IPI, Revised-International Prognostic Index
Fig. 1. Survival outcomes for SHL patients.
Progression free survival (A) and overall survival (B) for SHL patients by front-line therapy received. Progression free survival (C) and overall survival (D) for SHL patients by R-IPI score. Progression free survival (E) and overall survival (F) for SHL patients by R-IPI score and front-line therapy received. II indicates intensive induction.
Univariate analysis of baseline characteristics for SHL patients is shown in Table II. Characteristics predictive of 2yPFS were elevated LDH, stage III–IV disease, ECOG performance status (PS) ≥2, ≥2 sites of extranodal disease, age >60 and bone marrow involvement. Characteristics predictive of 2yOS were stage III–IV disease, ECOG PS ≥2, elevated LDH, bone marrow involvement, ≥2 sites of extranodal disease, age >60 and presence of B symptoms. Of note, receipt of R-CHOP as compared with intensive induction was associated with a HR of 2.5 for 2yPFS (95% CI 1.3–5.1, P=0.009) but was not predictive of 2yOS.
Table II.
Univariate Cox Regression Analysis for SHL patients.
| Progression at 2 years | Survival at 2 years | |||
|---|---|---|---|---|
| P-value | HR (95% CI) | P-value | HR (95% CI) | |
| Sex (male vs. female) | 0.37 | 0.44 | ||
| Age ≥60 (yes vs. no) | 0.007 | 2.6 (1.3–5.3) | 0.006 | 2.8 (1.3–5.8) |
| ECOG PS ≥2 (yes vs. no) | <0.001 | 3.9 (1.8–8.3) | <0.001 | 5.2 (2.3–11.6) |
| Bone marrow involvement (yes vs. no) | 0.01 | 2.5 (1.2–5.2) | <0.001 | 4.6 (2.0–10.8) |
| ≥2 sites of extranodal disease (yes vs. no) | 0.01 | 2.8 (1.2–6.2) | 0.008 | 3.1 (1.3–7.3 |
| B symptoms (yes vs. no) | 0.07 | 0.03 | 2.3 (1.1–4.9) | |
| Ki67 % expression at least 90 (yes vs. no) | 0.68 | 0.35 | ||
| Histologic classification (BCLU vs. DLBCL) | 0.34 | 0.31 | ||
| Cell of origin (GCB vs. non-GCB) | 0.85 | 0.31 | ||
| LDH >normal (yes vs. no) | 0.03 | 9.6 (1.3–71.0) | 0.04 | 4.7 (1.1–19.6) |
| Stage (I–II vs. III–IV) | 0.005 | 7.7 (1.9–32.4) | 0.02 | 5.6 (1.3–23.4) |
HR, hazard ratio; CI, confidence interval; BCLU, B cell lymphoma unclassifiable with features intermediate between diffuse large B cell lymphoma and Burkitt lymphoma; DLBCL, diffuse large B cell lymphoma; GCB, germinal center B cell
Thirty-six SHL patients demonstrated evidence of MYC translocation with IgH (n=34) or IgK (n=2) and were further classified as MYCIg. For MYCIg patients, 2yPFS (n=29) was 29% and similar for those receiving R-CHOP as compared with intensive induction (29% for both, P=0.94), while 2yOS was 42% and also did not differ by receipt of R-CHOP as compared with intensive induction (32% vs. 47% respectively, P=0.27) (Fig. 2).
Fig. 2. Survival outcomes for SH MYCIg patients.
Progression free survival (A) and overall survival (B) for SHL MYCIg patients by front-line therapy received. II indicates intensive induction.
MYC amp patients
Baseline characteristics for MYC amp patients are shown in Table I. For 22 MYC amp patients with a median length of follow-up of 28.0 months (range 0.5–132.4 months), 2yPFS (n=22) was 48% and 2yOS 71%. Front-line therapy received was R-CHOP in 12 patients and intensive induction in 10 patients. As depicted in Fig. 3, receipt of R-CHOP as compared with intensive induction resulted in similar 2yPFS (47% vs. 49%, P=0.93) and 2yOS (71 vs. 73%, P=0.91) for MYC amp patients. When analyzing the subgroup of MYC amp patients who also demonstrated rearrangement of BCL2 and/or BCL6 (n=8), the 2yPFS was 16% and 2yOS 71% for this cohort, and did not differ significantly based on treatment with R-CHOP (n=3) or intensive induction (n=5), (2yPFS 0% vs. 33%, P=0.41 and 2yOS 67% for both, P=0.89).
Fig. 3. Survival outcomes for MYC amp patients.
Progression free survival (A) and overall survival (B) for MYC amp patients by front-line therapy received. II indicates intensive induction.
Univariate analysis of baseline characteristics for MYC amp patients revealed that only ECOG PS ≥2 was predictive of 2yPFS as well as 2yOS (data not shown).
All patients
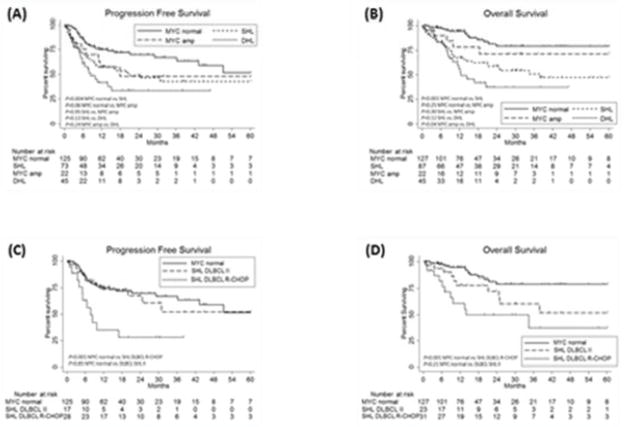
Baseline characteristics for MYC normal and DHL patients are shown in Table I. The median length of follow-up for all patients was 19.9 months (range 0.5–156.1 months). MYC normal patients experienced a 2yPFS of 70% (n=125) and 2yOS of 79% and DHL patients (36% treated with R-CHOP and 64% with intensive induction) experienced a 2yPFS of 33% (n=45) and 2yOS of 37%. As depicted in Fig. 4, both 2yPFS and 2yOS were significantly lower for SHL as compared with MYC normal patients (P=0.004 and P<0.001, respectively) but similar for MYC amp as compared with MYC normal patients (P=0.06 and P=0.25, respectively). When compared with DHL patients, 2yPFS and 2yOS were similar for both SHL (P=0.13 and P=0.12, respectively) and 2yPFS for MYC amp patients (p=0.24); however, 2yOS was significantly higher for MYC amp patients (P=0.04). For SHL patients with DLBCL histologic classification, 2yPFS was 53% (28% for R-CHOP patients and 67% for intensive induction patients) and 2yOS was 62% (49% for R-CHOP patients and 72% for intensive induction patients). When compared with MYC normal patients, 2yPFS and 2yOS were significantly lower for SHL DLBCL patients receiving R-CHOP (P<0.001 for both) but similar for SHL DLBCL patients receiving intensive induction (P=0.85 and P=0.21, respectively) (Fig. 4). For SHL patients with BCLU histologic classification, 2yPFS was 41% (0% for R-CHOP patients and 49% for intensive induction patients, P=0.03) and 2yOS was 47% (50% for R-CHOP patients and 53% for intensive induction patients). As previously mentioned, only one case of BCLU without MYC rearrangement was identified, so a survival analysis of BCLU patients based on MYC rearrangement status could not be performed.
Fig. 4. Survival outcomes for all patients.
Progression free survival (A) and overall survival (B) for all patients by front-line therapy received. Progression free survival (C) and overall survival (D) for MYC normal and SHL DLBCL patients by front-line therapy received. II indicates intensive induction.
Discussion
Here we report the largest series of SHL and MYC amp patients and demonstrate the poor prognosis for patients with SHL. The 2yPFS and 2yOS for these patients was similar to that of DHL patients and inferior to that of MYC normal patients, even when excluding SHL patients with BCLU to allow for comparison of SHL DLBCL patients to MYC normal patients (all with DLBCL and received R-CHOP). While 2yPFS and 2yOS were significantly lower for SHL DLBCL patients receiving R-CHOP as compared to MYC normal patients, 2yPFS and 2yOS were similar for SHL DLBCL patients receiving intensive induction as compared to MYC normal patients. The latter finding suggests that receipt of intensive induction therapy may overcome the poor prognosis associated with SHL in DLBCL patients. Another important finding is that while SHL patients treated with intensive induction achieved a significantly higher 2yPFS and a higher (but non-statistically significant) 2yOS as compared to SHL patients treated with R-CHOP, SHL patients with R-IPI score ≥3 and evidence of MYC translocation with an Ig partner appear to have a poor prognosis which is not significantly improved by receipt of intensive induction.
Our findings regarding SHL patients are similar to those in other published pathologic series also incorporating patients with both DLBCL and BCLU histologic classifications. Sixty-one DLBCL/BCLU SHL patients treated at the MD Anderson Cancer Center experienced a 2yOS of 41% which was not significantly different from that of DHL patients (Li, et al 2015). Additionally, 31 SHL patients captured within the Molecular Mechanisms in Malignant Lymphomas Network were reported to have similar survival to that of DHL patients (Aukema, et al 2014). However, a report of 17 SHL DLBCL patients treated at the University of Nebraska suggests a similar prognosis to that of a comparison group of DLBCL patients without MYC rearrangement, and both of these cohorts experienced prolonged survival as compared with DHL patients (Caponetti, et al 2015). In the context of these reports, our series is of importance due to analysis of a larger cohort of SHL patients as well as reporting of outcomes based on clinical characteristics and therapy received. Similarities in genomic complexity between SHL and DHL patients as previously suggested (Aukema, et al 2014) may explain the similar survival outcomes experienced by SHL and DHL patients in our series. Future pathologic studies of SHL and DHL patients should focus on molecular analysis of these subtypes of MYC-rearranged lymphoma.
The poor prognosis of MYCIg patients demonstrated in our series is validated by other published reports. A Danish series identified 12 DLBCL patients with MYC translocated to an immunoglobulin gene partner who experienced significantly reduced OS when compared with patients with MYC translocated to a non-immunoglobulin gene partner or without MYC translocation (Pedersen, et al 2014). A publication from the GELA/LYSA describing 24 DLBCL/BCLU patients with MYC translocated to an immunoglobulin gene partner reported inferior OS for these patients as compared with patients demonstrating MYC translocated to a non-immunoglobulin gene partner, the latter of whom experienced similar OS to that of patients without MYC translocation (Copie-Bergman, et al 2015). Our series is of importance due to the inclusion of a larger number of patients with an identifiable MYC translocation partner (N=36) as well as analysis of survival outcomes based on therapy received.
In regard to MYC amp patients (n=22), our analysis demonstrated a 2yPFS of 48% and 2yOS of 71% for this cohort which did not differ significantly by front-line therapy received. The 2yOS rate of patients captured in our analysis is higher than that of 48% reported for 8 DLBCL patients with MYC increased copy number reported by the University of Arizona (Stasik, et al 2010). Also, our finding of similar 2yOS for MYC amp as compared with MYC normal patients differs from that of a South Korean analysis, which demonstrated significantly shorter OS for 11 DLBCL patients with MYC increased copy number as compared with those without MYC rearrangement or increased copy number (Yoon, et al 2008). To the contrary, results from a European series suggest that MYC increased copy number does not predict for reduced survival in the absence of concurrent del(8p) (Testoni, et al 2011). Interestingly, we demonstrate a relatively low 2yPFS (18%) but high 2yOS (67%) for 8 MYC amp patients with concurrent rearrangement of BCL2 and/or BCL6, which differs from a prior report demonstrating a median OS of approximately 1 year for these patients (Li, et al 2012). Even though our analysis of MYC amp patients is the largest reported, we feel that the prognosis of increased copy number of MYC for DLBCL and BCLU patients remains unclear, and a larger sample size of patients would need to be studied to determine the prognostic significance of factors such as degree of copy number increase, histologic classification and therapy received.
Our study is not without limitations. First, as screening of all DLBCL and BCLU cases for MYC rearrangement and/or copy number change was not the practice of all participating centers, we cannot exclude the possibility that selection bias influenced the decision to test specific cases. We are unaware of any large series of SHL and MYC amp patients who were identified by random testing to compare to our series. However, the distribution of the baseline characteristics of age >60, stage III–IV disease, LDH >normal and ≥2 sites of extranodal disease in our DHL cohort are very similar to those of a previously-published unselected DHL cohort (Johnson, et al 2009). Similarly, distribution of these characteristics seen in our MYC normal cohort also appears similar when compared to previously-published unselected cohorts of DLBCL patients not demonstrating MYC rearrangement (Klapper, et al 2008, Savage, et al 2009). Second, diagnostic tissue specimens were not centrally reviewed. Nevertheless, cases were reviewed locally by hematopathologists at academic medical centers which we believe indicates high-quality interpretation and reporting of results. Finally, front-line therapy received by patients was not dictated by a specific protocol.
In conclusion, treatment with front-line intensive induction therapy may improve survival outcomes for SHL patients, and in those with DLBCL, overcome the poor prognosis associated with the presence of sole MYC rearrangement. The prognosis for MYC amp patients appears more favorable and not altered by receipt of intensive induction therapy, although a relatively small sample size of these patients analyzed in our series may limit the accuracy of this finding. It is apparent that certain clinicopathologic features, such as elevated R-IPI score and evidence of MYC translocation to an Ig partner, may predict for poor prognosis in SHL patients, even when treated with intensive induction. Ongoing research efforts to identify patients with MYC-altered lymphomas at risk for treatment failure with available front line immunochemotherapy regimens, as well as the feasibility and benefit of incorporating novel non-cytotoxic agents into such regimens for these patients, are warranted.
Footnotes
Disclosure
The authors have declared no conflicts of interest.
D.J.L. designed the study, collected data, analyzed data and wrote the manuscript
A.P. designed the study, collected data and reviewed and approved the manuscript
C.N. collected data, contributed to manuscript writing and reviewed and approved the manuscrip
M.F., B.C., A.B., S.L., L.J.M., R.C., N.R., M.B., J.V., K.K., S.S., P.P., F.J.H., R.K, S.R., D.T.Y., J.J.M., K.A.B., W.Z. and C.V collected data and reviewed and approved the manuscript
Contributor Information
Daniel Landsburg, University of Pennsylvania, Hematology/Oncology.
Marissa Falkiewicz, Rutgers Robert Wood Johnson Medical School, School of Medicine.
Adam Petrich, Northwestern University Feinberg School of Medicine, Hematology/Oncology; AbbVie Inc, Oncology Development.
Benjamin Chu, Northwestern University Feinberg School of Medicine, School of Medicine.
Amir Behdad, Northwestern Memorial Hospital, Pathology.
Shaoying Li, The University of Texas MD Anderson Cancer Center, Hematopathology.
L. Medeiros, UT MD Anderson Cancer Center, Hematopathology
Ryan Cassaday, Fred Hutchinson Cancer Research Center, Hematology/Oncology.
Nishitha Reddy, Vanderbilt University, Medicine.
Martin Bast, University of Nebraska Medical Center, Medicine.
Julie Vose, Nebraska Medical Center, Hematology/Oncology.
Kimberly Kruczek, Loyola University Chicago, Hematology/Oncology.
Scott Smith, Loyola University Chicago, Medicine.
Priyank Patel, Roswell Park Cancer Institute, Hematology/Oncology.
Francisco Hernandez-Ilizaliturri, Roswell Park Cancer Institute, Medicine and Immunology.
Reem Karmali, Rush University Medical Center, Medicine, Hematology.
Saurabh Rajguru, University of Wisconsin Madison, Hematology/Oncology.
David Yang, University of Wisconsin, Pathology and Laboratory Medicine.
Joseph Maly, The Ohio State University - James Comprehensive Cancer Center, Internal Medicine.
Kristie Blum, The Ohio State University, Division of Hematology Oncology.
Weiqiang Zhao, The Ohio State University, Department of Pathology.
Charles VanSlambrouck, University of Chicago, Pathology.
Chadi Nabhan, University of Chicago, Hematology/Oncology.
References
- Aukema SM, Kreuz M, Kohler CW, Rosolowski M, Hasenclever D, Hummel M, Kuppers R, Lenze D, Ott G, Pott C, Richter J, Rosenwald A, Szczepanowski M, Schwaenen C, Stein H, Trautmann H, Wessendorf S, Trumper L, Loeffler M, Spang R, Kluin PM, Klapper W, Siebert R. Biological characterization of adult MYC-translocation-positive mature B-cell lymphomas other than molecular Burkitt lymphoma. Haematologica. 2014;99:726–735. doi: 10.3324/haematol.2013.091827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aukema SM, Siebert R, Schuuring E, van Imhoff GW, Kluin-Nelemans HC, Boerma EJ, Kluin PM. Double-hit B-cell lymphomas. Blood. 2011;117:2319–2331. doi: 10.1182/blood-2010-09-297879. [DOI] [PubMed] [Google Scholar]
- Barrans S, Crouch S, Smith A, Turner K, Owen R, Patmore R, Roman E, Jack A. Rearrangement of MYC is associated with poor prognosis in patients with diffuse large B-cell lymphoma treated in the era of rituximab. J Clin Oncol. 2010;28:3360–3365. doi: 10.1200/JCO.2009.26.3947. [DOI] [PubMed] [Google Scholar]
- Boerma EG, Siebert R, Kluin PM, Baudis M. Translocations involving 8q24 in Burkitt lymphoma and other malignant lymphomas: a historical review of cytogenetics in the light of todays knowledge. Leukemia. 2009;23:225–234. doi: 10.1038/leu.2008.281. [DOI] [PubMed] [Google Scholar]
- Caponetti GC, Dave BJ, Perry AM, Smith LM, Jain S, Meyer PN, Bast M, Bierman PJ, Bociek RG, Vose JM, Armitage JO, Aoun P, Fu K, Greiner TC, Chan WC, Sanger WG, Weisenburger DD. Isolated MYC cytogenetic abnormalities in diffuse large B-cell lymphoma do not predict an adverse clinical outcome. Leuk Lymphoma. 2015:1–8. doi: 10.3109/10428194.2015.1034699. [DOI] [PubMed] [Google Scholar]
- Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, Lister TA. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32:3059–3068. doi: 10.1200/JCO.2013.54.8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, Coiffier B, Fisher RI, Hagenbeek A, Zucca E, Rosen ST, Stroobants S, Lister TA, Hoppe RT, Dreyling M, Tobinai K, Vose JM, Connors JM, Federico M, Diehl V. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579–586. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- Copie-Bergman C, Cuilliere-Dartigues P, Baia M, Briere J, Delarue R, Canioni D, Salles G, Parrens M, Belhadj K, Fabiani B, Recher C, Petrella T, Ketterer N, Peyrade F, Haioun C, Nagel I, Siebert R, Jardin F, Leroy K, Jais JP, Tilly H, Molina TJ, Gaulard P. MYC-IG rearrangements are negative predictors of survival in DLBCL patients treated with immunochemotherapy: a GELA/LYSA study. Blood. 2015 doi: 10.1182/blood-2015-05-647602. [DOI] [PubMed] [Google Scholar]
- Cuccuini W, Briere J, Mounier N, Voelker HU, Rosenwald A, Sundstrom C, Cogliatti S, Hirchaud E, Ysebaert L, Bron D, Soulier J, Gaulard P, Houlgatte R, Gisselbrecht C, Thieblemont C. MYC+ diffuse large B-cell lymphoma is not salvaged by classical R-ICE or R-DHAP followed by BEAM plus autologous stem cell transplantation. Blood. 2012;119:4619–4624. doi: 10.1182/blood-2012-01-406033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang CV, O’Donnell KA, Zeller KI, Nguyen T, Osthus RC, Li F. The c-Myc target gene network. Semin Cancer Biol. 2006;16:253–264. doi: 10.1016/j.semcancer.2006.07.014. [DOI] [PubMed] [Google Scholar]
- Hans CP, Weisenburger DD, Greiner TC, Gascoyne RD, Delabie J, Ott G, Muller-Hermelink HK, Campo E, Braziel RM, Jaffe ES, Pan Z, Farinha P, Smith LM, Falini B, Banham AH, Rosenwald A, Staudt LM, Connors JM, Armitage JO, Chan WC. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103:275–282. doi: 10.1182/blood-2003-05-1545. [DOI] [PubMed] [Google Scholar]
- Horn H, Ziepert M, Becher C, Barth TF, Bernd HW, Feller AC, Klapper W, Hummel M, Stein H, Hansmann ML, Schmelter C, Moller P, Cogliatti S, Pfreundschuh M, Schmitz N, Trumper L, Siebert R, Loeffler M, Rosenwald A, Ott G. MYC status in concert with BCL2 and BCL6 expression predicts outcome in diffuse large B-cell lymphoma. Blood. 2013;121:2253–2263. doi: 10.1182/blood-2012-06-435842. [DOI] [PubMed] [Google Scholar]
- Howlett C, Snedecor SJ, Landsburg DJ, Svoboda J, Chong EA, Schuster SJ, Nasta SD, Feldman T, Rago A, Walsh KM, Weber S, Goy A, Mato A. Front-line, dose-escalated immunochemotherapy is associated with a significant progression-free survival advantage in patients with double-hit lymphomas: a systematic review and meta-analysis. Br J Haematol. 2015;170:504–514. doi: 10.1111/bjh.13463. [DOI] [PubMed] [Google Scholar]
- Johnson NA, Savage KJ, Ludkovski O, Ben-Neriah S, Woods R, Steidl C, Dyer MJ, Siebert R, Kuruvilla J, Klasa R, Connors JM, Gascoyne RD, Horsman DE. Lymphomas with concurrent BCL2 and MYC translocations: the critical factors associated with survival. Blood. 2009;114:2273–2279. doi: 10.1182/blood-2009-03-212191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klapper W, Stoecklein H, Zeynalova S, Ott G, Kosari F, Rosenwald A, Loeffler M, Trumper L, Pfreundschuh M, Siebert R. Structural aberrations affecting the MYC locus indicate a poor prognosis independent of clinical risk factors in diffuse large B-cell lymphomas treated within randomized trials of the German High-Grade Non-Hodgkin’s Lymphoma Study Group (DSHNHL) Leukemia. 2008;22:2226–2229. doi: 10.1038/leu.2008.230. [DOI] [PubMed] [Google Scholar]
- Li S, Lin P, Fayad LE, Lennon PA, Miranda RN, Yin CC, Lin E, Medeiros LJ. B-cell lymphomas with MYC/8q24 rearrangements and IGH@BCL2/t(14;18)(q32;q21): an aggressive disease with heterogeneous histology, germinal center B-cell immunophenotype and poor outcome. Mod Pathol. 2012;25:145–156. doi: 10.1038/modpathol.2011.147. [DOI] [PubMed] [Google Scholar]
- Li S, Weiss VL, Wang XJ, Desai PA, Hu S, Yin CC, Tang G, Reddy NM, Medeiros LJ, Lin P. High-grade B-cell Lymphoma With MYC Rearrangement and Without BCL2 and BCL6 Rearrangements Is Associated With High P53 Expression and a Poor Prognosis. Am J Surg Pathol. 2015 doi: 10.1097/PAS.0000000000000542. [DOI] [PubMed] [Google Scholar]
- Lin P, Dickason TJ, Fayad LE, Lennon PA, Hu P, Garcia M, Routbort MJ, Miranda R, Wang X, Qiao W, Medeiros LJ. Prognostic value of MYC rearrangement in cases of B-cell lymphoma, unclassifiable, with features intermediate between diffuse large B-cell lymphoma and Burkitt lymphoma. Cancer. 2012;118:1566–1573. doi: 10.1002/cncr.26433. [DOI] [PubMed] [Google Scholar]
- Lin P, Medeiros LJ. The impact of MYC rearrangements and “double hit” abnormalities in diffuse large B-cell lymphoma. Curr Hematol Malig Rep. 2013;8:243–252. doi: 10.1007/s11899-013-0169-y. [DOI] [PubMed] [Google Scholar]
- Meyer N, Penn LZ. Reflecting on 25 years with MYC. Nat Rev Cancer. 2008;8:976–990. doi: 10.1038/nrc2231. [DOI] [PubMed] [Google Scholar]
- Mossafa H, Damotte D, Jenabian A, Delarue R, Vincenneau A, Amouroux I, Jeandel R, Khoury E, Martelli JM, Samson T, Tapia S, Flandrin G, Troussard X. Non-Hodgkin’s lymphomas with Burkitt-like cells are associated with c-Myc amplification and poor prognosis. Leuk Lymphoma. 2006;47:1885–1893. doi: 10.1080/10428190600687547. [DOI] [PubMed] [Google Scholar]
- Pedersen MO, Gang AO, Poulsen TS, Knudsen H, Lauritzen AF, Nielsen SL, Klausen TW, Norgaard P. MYC translocation partner gene determines survival of patients with large B-cell lymphoma with MYC- or double-hit MYC/BCL2 translocations. Eur J Haematol. 2014;92:42–48. doi: 10.1111/ejh.12212. [DOI] [PubMed] [Google Scholar]
- Perry AM, Crockett D, Dave BJ, Althof P, Winkler L, Smith LM, Aoun P, Chan WC, Fu K, Greiner TC, Bierman P, Gregory Bociek R, Vose JM, Armitage JO, Weisenburger DD. B-cell lymphoma, unclassifiable, with features intermediate between diffuse large B-cell lymphoma and burkitt lymphoma: study of 39 cases. Br J Haematol. 2013;162:40–49. doi: 10.1111/bjh.12343. [DOI] [PubMed] [Google Scholar]
- Petrich AM, Gandhi M, Jovanovic B, Castillo JJ, Rajguru S, Yang DT, Shah KA, Whyman JD, Lansigan F, Hernandez-Ilizaliturri FJ, Lee LX, Barta SK, Melinamani S, Karmali R, Adeimy C, Smith S, Dalal N, Nabhan C, Peace D, Vose J, Evens AM, Shah N, Fenske TS, Zelenetz AD, Landsburg DJ, Howlett C, Mato A, Jaglal M, Chavez JC, Tsai JP, Reddy N, Li S, Handler C, Flowers CR, Cohen JB, Blum KA, Song K, Sun HL, Press O, Cassaday R, Jaso J, Medeiros LJ, Sohani AR, Abramson JS. Impact of induction regimen and stem cell transplantation on outcomes in double-hit lymphoma: a multicenter retrospective analysis. Blood. 2014;124:2354–2361. doi: 10.1182/blood-2014-05-578963. [DOI] [PubMed] [Google Scholar]
- Savage KJ, Johnson NA, Ben-Neriah S, Connors JM, Sehn LH, Farinha P, Horsman DE, Gascoyne RD. MYC gene rearrangements are associated with a poor prognosis in diffuse large B-cell lymphoma patients treated with R-CHOP chemotherapy. Blood. 2009;114:3533–3537. doi: 10.1182/blood-2009-05-220095. [DOI] [PubMed] [Google Scholar]
- Sehn LH, Berry B, Chhanabhai M, Fitzgerald C, Gill K, Hoskins P, Klasa R, Savage KJ, Shenkier T, Sutherland J, Gascoyne RD, Connors JM. The revised International Prognostic Index (R-IPI) is a better predictor of outcome than the standard IPI for patients with diffuse large B-cell lymphoma treated with R-CHOP. Blood. 2007;109:1857–1861. doi: 10.1182/blood-2006-08-038257. [DOI] [PubMed] [Google Scholar]
- Slack GW, Gascoyne RD. MYC and aggressive B-cell lymphomas. Adv Anat Pathol. 2011;18:219–228. doi: 10.1097/PAP.0b013e3182169948. [DOI] [PubMed] [Google Scholar]
- Stasik CJ, Nitta H, Zhang W, Mosher CH, Cook JR, Tubbs RR, Unger JM, Brooks TA, Persky DO, Wilkinson ST, Grogan TM, Rimsza LM. Increased MYC gene copy number correlates with increased mRNA levels in diffuse large B-cell lymphoma. Haematologica. 2010;95:597–603. doi: 10.3324/haematol.2009.012864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testoni M, Kwee I, Greiner TC, Montes-Moreno S, Vose J, Chan WC, Chiappella A, Baldini L, Ferreri AJ, Gaidano G, Mian M, Zucca E, Bertoni F. Gains of MYC locus and outcome in patients with diffuse large B-cell lymphoma treated with R-CHOP. Br J Haematol. 2011;155:274–277. doi: 10.1111/j.1365-2141.2011.08675.x. [DOI] [PubMed] [Google Scholar]
- Valera A, Lopez-Guillermo A, Cardesa-Salzmann T, Climent F, Gonzalez-Barca E, Mercadal S, Espinosa I, Novelli S, Briones J, Mate JL, Salamero O, Sancho JM, Arenillas L, Serrano S, Erill N, Martinez D, Castillo P, Rovira J, Martinez A, Campo E, Colomo L. MYC protein expression and genetic alterations have prognostic impact in patients with diffuse large B-cell lymphoma treated with immunochemotherapy. Haematologica. 2013;98:1554–1562. doi: 10.3324/haematol.2013.086173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon SO, Jeon YK, Paik JH, Kim WY, Kim YA, Kim JE, Kim CW. MYC translocation and an increased copy number predict poor prognosis in adult diffuse large B-cell lymphoma (DLBCL), especially in germinal centre-like B cell (GCB) type. Histopathology. 2008;53:205–217. doi: 10.1111/j.1365-2559.2008.03076.x. [DOI] [PubMed] [Google Scholar]