Abstract
Orexin neurons are known to augment the sympathetic control of cardiovascular function, however the role of orexin neurons in parasympathetic cardiac regulation remains unclear. To test the hypothesis that orexin neurons contribute to parasympathetic control we selectively expressed channelrhodopsin-2 (ChR2) in orexin neurons in orexin-Cre transgenic rats and examined postsynaptic currents in cardiac vagal neurons (CVNs) in the dorsal motor nucleus of the vagus (DMV). Simultaneous photostimulation and recording in ChR2-expressing orexin neurons in the lateral hypothalamus resulted in reliable action potential firing as well as large whole-cell currents suggesting a strong expression of ChR2 and reliable optogenetic excitation. Photostimulation of ChR2-expressing fibers in the DMV elicited short-latency (ranging from 3.2 ms to 8.5 ms) postsynaptic currents in 16 out of 44 CVNs tested. These responses were heterogeneous and included excitatory glutamatergic (63%) and inhibitory GABAergic (37%) postsynaptic currents. The results from this study suggest different sub-population of orexin neurons may exert diverse influences on brainstem CVNs and therefore may play distinct functional roles in parasympathetic control of the heart.
Keywords: Optogenetic, neurons, orexin, brainstem, cardiac, parasympathetic
Preganglionic cardiac vagal neurons (CVNs) in the dorsal motor nucleus of the vagus (DMV) project directly to the cardiac ganglia and play a substantial role in the cardiac regulation (Sullivan and Connors, 1981, Ciriello and Calaresu, 1982, Cheng et al., 1999). Electrical stimulation of the cardiac branches of the vagus nerve antidromically activates neurons in the DMV and electrical stimulation of the DMV reduces heart rate and myocardial contractility (Calaresu and Pearce, 1965, Nosaka et al., 1979, Ciriello and Calaresu, 1982). Increasing the activity CVNs in the DMV protects the heart from ischemia/reperfusion injury independent of changes in heart rate (Mastitskaya et al., 2012). The activity of CVNs in the DMV are strongly influenced by neurotransmission from other neurons in the brainstem, as well as pathways from the locus coeruleus and oxytocin neurons in the hypothalamus (DePuy et al., 2013, Dergacheva et al., 2014, Pinol et al., 2014, Wang et al., 2014). The results from immunohistochemical studies indicate orexin neurons could be another important source of innervation to neurons in the DMV (Peyron et al., 1998, Date et al., 1999). However, the DMV is a heterogeneous nucleus and it is unknown whether there are direct connections between orexin neurons and the selective population of CVNs in the DMV that play a major role in controlling heart rate and cardiac function.
Compelling evidence indicates orexin neurons and receptors play an important role in the regulation of cardiovascular function (Peyron et al., 1998, Ciriello and de Oliveira, 2003, Ciriello et al., 2003, Dergacheva et al., 2005, Carrive, 2013, Dergacheva et al., 2013). Orexin is well known to exert sympathoexcitatory effects such as increases in heart rate, blood pressure and sympathetic nerve activity (Samson et al., 1999, Shirasaka et al., 1999, Chen et al., 2000, Antunes et al., 2001, Matsumura et al., 2001). However, little is known about the role of orexin neurons in parasympathetic control of the heart and the few studies that have examined this issues are conflicting. For example, microinjection of orexin-A into the rostral ventral medulla produces tachycardia mediated in part by inhibition of parasympathetic activity to the heart (Ciriello et al., 2003), whereas microinjection of orexin-A into the nucleus ambiguus elicits bradycardia mediated by excitation of parasympathetic activity to the heart (Ciriello and de Oliveira, 2003).
Thus, this study was undertaken to identify and characterize the synaptic pathway from orexin neurons to CVNs in the DMV. To accomplish this goal, we utilized a transgenic strain of orexin-Cre rats that allows us to photoexcite channelrhodopsin-2 (ChR2) in orexin fibers in the brainstem DMV while recording synaptic events in fluorescently identified CVNs in the DMV in an in-vitro slice preparation.
EXPERIMENTAL PROCEDURES
Animals
Orexin-EGFP-2A-Cre rats (both males and females) were used in this study. The generation of these transgenic animals in which Cre recombinase and green fluorescent protein are exclusively expressed in hypothalamic orexin neurons has been described previously (Dergacheva et al., 2016). Rats were housed in the George Washington University animal care facility. All animal surgeries and experiments were approved by the George Washington University Institutional Animal Care and Use Committee. We made all efforts to minimize number of rats used in this study and reduce animal discomfort.
Cardiac neuron labeling and viral injections into the lateral hypothalamus
Parasympathetic CVNs were labeled as described previously (Dergacheva et al., 2013, Dergacheva, 2015). At postnatal days 4–5 orexin-Cre rats were anesthetized with hypothermia, the heart was exposed and 0.05 ml of 1–5% rhodamine (XRITC; Molecular Probes, Eugene, OR) was injected into the pericardial sac. The previous study showed the specificity of the cardiac vagal labeling (Bouairi et al., 2004).
ChR2 fused to enhanced yellow fluorescent protein (EYFP) was targeted to the plasma membrane of orexin neurons and axons as shown in Fig. 1. Adeno-associated viral vector with “FLEX-switch” ChR2 construct (AAV1-ChR2-EYFP, Penn Vector Core, Philadelphia, PA, catalog number AV-1-20298P) was injected into the lateral hypothalamus of orexin-Cre rats using a stereotactic apparatus with a neonatal adapter (Stoelting, Wood Dale, IL). A pulled calibrated pipette (VWR, Radnor, PA) containing viral vector was positioned at the following coordinates: 1.7-1.9 mm posterior and 0.4 mm lateral relative to bregma. The viral vector (60 nL) was slowly injected 5.2 mm lower the dorsal surface of the brain. The pipette was left in the lateral hypothalamus for 5 minutes after injection, then slowly retracted. To reduce pain and discomfort caused by the surgery, buprenorphine was administered after surgery, and animals were carefully monitored until ambulatory.
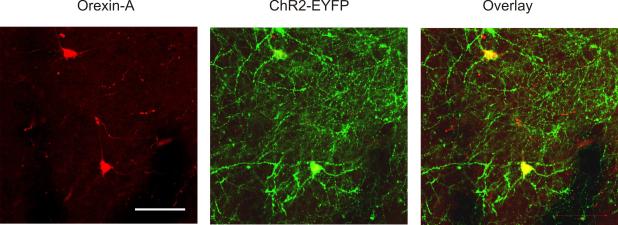
Figure 1.
Localization of orexin-A immunoreactivity (red, left panel) with ChR2-EYFP (green, middle panel) driven by orexin-Cre selective expression in the lateral hypothalamus. Co-localization is shown in the right panel. Representative example of n=4 animals. Scale bar, 100 μm.
Slice Preparation and Electrophysiology
Animals (21-24 days old) were anesthetized with isoflurane and sacrifice by transcardial perfusion of glycerol-based artificial cerebrospinal fluid (aCSF, 4°C) that contained (in mM): 252 glycerol, 1.6 KCl, 1.2 NaH2PO4, 1.2 MgCl, 2.4 CaCl2, 26 NaHCO3, and 11 glucose. Coronal slices of the brainstem (300-μm) were obtained with a vibratome. In another set of experiments 300-μm-thick coronal slices of the hypothalamus that included orexin neurons were made using a vibratome. The slices were then allowed to recover for 15 min in a solution containing (in mM): 110 N-methyl-d-glucamine (NMDG), 2.5 KCl, 1.2 NaH2PO4, 25 NaHCO3, 25 glucose, 110 HCl, 0.5 CaCl2, and 10 mM MgSO4 equilibrated with 95% O2-5% CO2 (pH 7.4, at 34°C). For performing electrophysiological experiments, the slices were transferred to a recording chamber containing (in mM) 125 NaCl, 3 KCl, 2 CaCl2, 26 NaHCO3, 5 glucose, 5 HEPES and equilibrated with 95% O2-5% CO2 (pH 7.4, at 25°C).
CVNs in the DMV and orexin neurons in the lateral hypothalamus were identified by the presence of the retrograde tracer and EYFP-expression, respectfully. Infrared-sensitive video detection cameras and differential interference contrast optics were then used to image these identified CVNs and orexin cells. We patched CVNs with patch pipettes (2.5–3.5 MΩ) filled with a solution consisting of (in mM) 150 KCl, 2 MgCl2, 2 EGTA, 10 HEPES, and 2 Mg-ATP, pH 7.3. In experiments that examined activity in orexin cells, the patch pipettes were filled with a solution consisting of 135 mM K-gluconic acid, 10 mM HEPES, 10 mM EGTA, 1 mM CaCl2, and 1 mM MgCl2, pH 7.3. We performed voltage clamp whole-cell recordings at a holding potential of −80 mV. Firing activity of orexin neurons was examined in current-clamp whole-cell configuration. All recordings were made with an Axopatch 200 B and pClamp 8 software (Axon Instruments, Union City, CA). Drugs used in this electrophysiological study were purchased from Sigma-Aldrich Chemical Co (St. Louis, MO).
For selective photostimulation of ChR2 expressing fibers surrounding CVNs we used a CrystaLaser (473-nm blue light, Reno, NV, USA) which was attached to the microscope using a dual housing adapter (Nikon). A series of 60 consecutive single stimulations (3 ms, at a frequency of 1 Hz) were applied to each neuron. Laser light intensity was kept at an output of 10 mW across all experiments.
Immunohistochemistry and Imaging Study
We utilized immunostaining to determine the specificity of ChR2-EYFP expression in orexin neurons in the lateral hypothalamus. Three weeks after AAV1-ChR2-EYFP viral injections into the lateral hypothalamus, hypothalamic slices (100 μm) were prepared and soaked 3 hours in 10% formalin and were then processed for orexin immunoreactivity. Rabbit anti-orexin-A was used as a primary antibody (1: 15,000 dilution, overnight incubation, Phoenix Pharmaceuticals, Inc., Burlingame, CA) and anti-rabbit Alexa Fluor 633 was used as a secondary antibody (1:200, 4 hours, Life Technologies, Carlsbad, CA). For analysis of co-localization of orexin immunoreactivity and ChR2-EYFP-expression the Zeiss 710 confocal imaging system was used. Orexin immunoreactivity was found in 83±3% of ChR2-EYFP neurons whereas 68±3% of orexin-containing neurons expressed ChR2-EYFP, (n=4 rats, see Fig. 1).
Data Analysis and Statistics
Postsynaptic currents were measured with pClamp 8 software (Molecular Devices, Sunnyvale, CA). The time between the onset of the optogenetic stimulation and the onset of synaptic current evoked by the stimulation was determined as a synaptic latency of the responses. The mean value for each neuron was created by averaging responses to 60 consecutive photostimulations. A summary of results for each population was created by averaging the mean value from each neuron in the population. The results were statistically compared using GraphPad Prism 5 software and using Student's paired T-test. The data is presented as mean ± SE with significant difference set at p < 0.05.
RESULTS
Selective photostimulation of orexin neurons
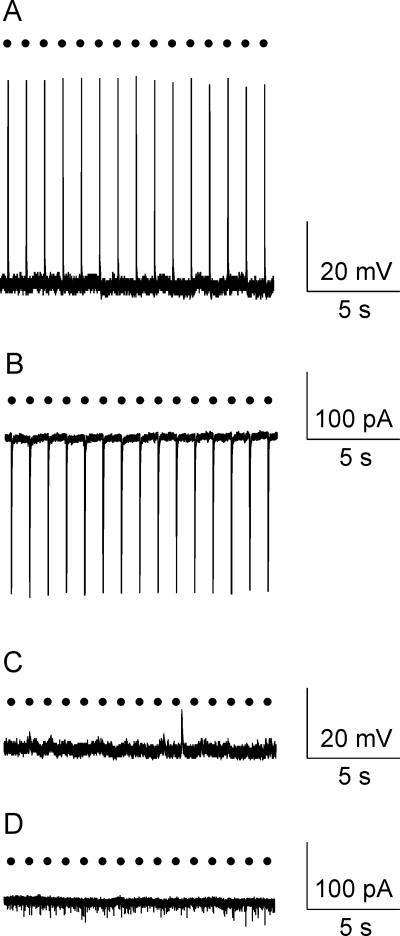
Optogenetic photostimulation (3 ms, 1 Hz) of orexin neurons fluorescently identified by ChR2-EYFP expression in the lateral hypothalamus generated large inward current (in voltage clamp configuration, 521±1842 pA, n=8, Fig. 2, B,) and reliable action potentials with each photostimulation (n=8 cells, Fig. 2, A). This robust excitation upon photoactivation indicates there is a strong expression of ChR2-EYFP (Schoenenberger et al., 2011). No electrophysiological responses in orexin neurons in the lateral hypothalamus were observed in orexin-Cre rats that did not receive AAV1-ChR2-EYFP viral injections (n=5 cells, Fig. 2, C and D), indicating that the responses were not due to non-specific cellular activation by light pulses.
Figure 2.
Optogenetic stimulation of orexin cell bodies in the lateral hypothalamus evoked reliable action potentials (A) as well as large inward whole-cell currents (B) in all orexin neurons tested (n=8) in rats that received AAV1-ChR2-EYFP viral injections. In contrast, neither light-triggered action potential firing (C) nor large inward currents (D) were observed in animals that did not receive AAV1-ChR2-EYFP viral injections (n=5 orexin neurons). Black circles represent light pulses (3 ms, 1 Hz).
Direct projections from orexin-containing neurons to CVNs in the DMV
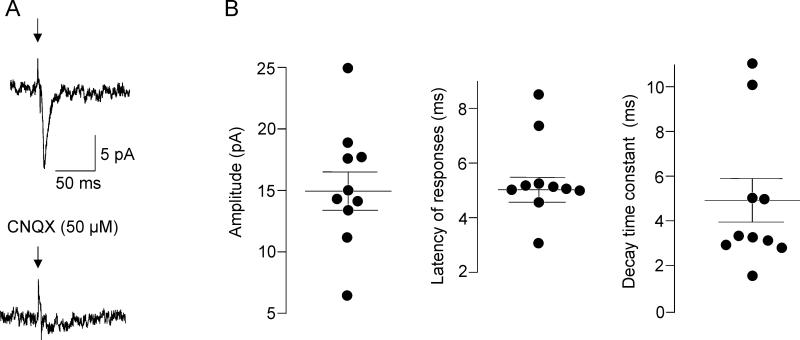
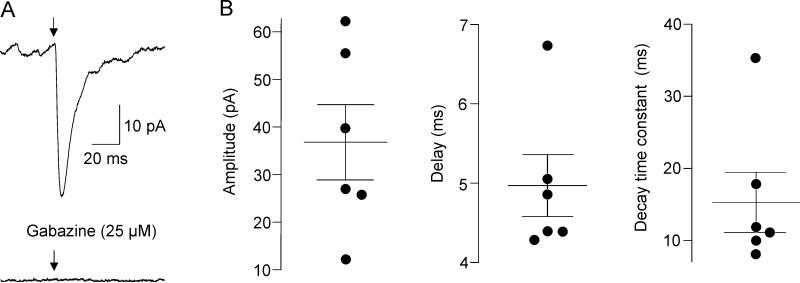
To identify and characterize functional projection from orexin neurons to CVNs in the DMV optogenetic stimulation of ChR2-containing fibers from orexin neurons was combined with patch-clamp recordings from CVNs. Although brainstem slices that contained CVNs in the DMV did not include the cell bodies of ChR2-EYFP-expressing orexin neurons it has been previously shown that light-triggered transmitter release from ChR2-EYFP-expressing fibers occurs in the absence of the ChR2-EYFP-expressing cell bodies (Pinol et al., 2012, Schone et al., 2012, Dergacheva et al., 2014). Photostimulation of ChR2-EYFP fibers with brief light pulses (3 ms) generated fast post-synaptic responses in 16 out of 44 CVNs tested (36%). A majority of responses (63%, n=10 neurons) were blocked by application of CNQX (50 μM, from 14.9±1.6 pA to 2.9±0.4 pA, p< 0.001, Student's paired t-test, Fig. 3) indicating these excitatory postsynaptic currents (EPSCs) were mediated via glutamatergic AMPA receptor activation. The latency of excitatory currents was 5.5±0.5 ms (ranging from 3.2 ms to 8.5 ms) while the decay time constant was 4.9±1.0 ms (ranging from 2.8 ms to 11 ms, n=10, see Fig. 3). GABAergic inhibitory postsynaptic currents (IPSCs) were detected in 6 out of 16 CVNs (37%) as these responses were not abolished by CNQX, but were successfully blocked by application of gabazine at a concentration of 25 μM (pick amplitude diminished from 36.8±7.9 pA to 3.3±0.9 pA, p< 0.005, Student's paired t-test). The latency of these GABAergic IPSCs was 4.9±0.4 ms (ranging from 4.3 ms to 6.8 ms) and the decay time constant was 15.3±4.2 ms (ranging from 7.6 ms to 35.2 ms, n=6, see Fig. 4).
Figure 3.
Glutamatergic EPSCs in CVNs. Representative example of optogenetically-evoked glutamatergic postsynaptic current in an individual CVN shown in A, left. Abolishment of this this synaptic response by application of CNQX (50 μM) is demonstrated in A, right. Properties of glutamatergic synaptic events in CVNs are illustrated in B. Black circles represent average from 60 sweeps in individual CVNs (3 ms stimulation at 1 Hz) while horizontal bars are population means ± SEM. Amplitude, latency and decay time constant of the glutamatergic responses are shown from left to right.
Figure 4.
GABAergic IPSCs in CVNs. Representative example of optogenetically-evoked GABAergic postsynaptic current in an individual CVN shown in A, left. Abolishment of this this current by application of gabazine (25 μM) is demonstrated in A, right. Properties of GABAergic responses in CVNs are illustrated in B. Black circles represent average from 60 sweeps in individual CVNs (3 ms stimulation at 1 Hz) while horizontal bars are population means ± SEM. Amplitude, latency and decay time constant of the GABAergic responses are shown from left to right.
In addition to establishing functional connectivity between orexin neurons and the DMV, we examined whether orexin neurons project to another important region of cardiac parasympathetic control – CVNs in the nucleus ambiguus. Although ChR2-EYFP fibers from orexin neurons were detectable within the nucleus ambiguus no direct electrophysiological responses were found in CVNs upon optogenetic stimulation of orexin fibers (n=17 cells).
DISCUSSION
To the best of our knowledge, this is the first report identifying and characterizing neurotransmission from orexin neurons to CVNs in the DMV. There are 2 major findings in this study. 1) Optogenetic stimulation of ChR2-EYFP-expressing orexin neurons in the lateral hypothalamus results in reliable excitation of these orexin neurons, observed both by action potential firing and large inward currents. 2) Optogenetic stimulation of ChR2-EYFP-expressing orexin fibers in the DMV evokes short-latency postsynaptic responses in 35% of CVNs tested in the DMV. Of the CVNs that responded to photostimulation of orexin fibers 63% received glutamatergic and 37% received GABAergic neurotransmission.
Orexin has previously been shown to contribute substantially to central cardiovascular and respiratory regulation (Peyron et al., 1998, Ciriello and de Oliveira, 2003, Ciriello et al., 2003, Dergacheva et al., 2005, Iigaya et al., 2012, Carrive, 2013, Dergacheva et al., 2013). Intraventricular administration of orexin elicits tachycardia, hypertension and hyperventilation (Samson et al., 1999, Shirasaka et al., 1999, Chen et al., 2000, Matsumura et al., 2001, Zhang et al., 2005), while orexin knock-out animals have reduced blood pressure and diminished cardiovascular response to bicuculline injections in the hypothalamus (Kayaba et al., 2003). These data point to a sympathoexcitatory role of orexin, however microinjection of orexin into various regions of the central autonomic network has been shown to produce unexpected and differential effects. For instance, pressor and tachycardiac effects have been demonstrated after injections of orexin into medullary raphe area (Luong and Carrive, 2012), rostral ventrolateral medulla (Chen et al., 2000, Machado et al., 2002, Huang et al., 2010) and commissural nucleus of the solitary tract(Smith et al., 2002). However, in contrast, orexin administrated into caudal dorsolateral and medial subnuclei of the solitary tract elicits depressor and bradycardia responses (de Oliveira et al., 2003). Microinjection of orexin into the nucleus ambiguus, an area also involved in parasympathetic regulation of heart rate, results in a dose-related decrease in heart rate and activated arterial baroreflex (Ciriello and de Oliveira, 2003). The results from these previous studies, therefore, strongly point to involvement of orexin in the cardiovascular regulation, however these results suggest orexin may play complex differential (or even opposite) roles in different regions of the brain.
One important target of orexin neurons are the CVNs in the DMV. The DMV is innervated by orexin-containing fibers (Peyron et al., 1998, Date et al., 1999) and superfusion of orexin depolarizes neurons in this nucleus (Hwang et al., 2001). However, the pathways and mechanisms underlying cardiac effects of orexin in CVNs in the DMV have not been previously established. The results from this study indicate that ChR2-EYFP expression in orexin neurons is sufficient for optogenetic stimulations of both soma of orexin neurons and their distal axons surrounding CVNs in the DMV. Photostimulation of each orexin neurons results in reliable action potential firing and large inward currents. Optogenetic stimulation of orexin neuron fibers in the brainstem evokes short-latency responses in CVNs in the DMV indicating a monosynaptic connection (Petreanu et al., 2007). These responses include both glutamatergic and GABAergic postsynaptic currents. A majority of the responses (63%) are mediated by glutamate release and postsynaptic AMPA receptor activation as these responses are completely blocked by application of the AMPA receptor antagonist CNQX (50 μM). These results provide an evidence that orexin neurons monosynaptically excite, via glutamatergic neurotransmission, CVNs in the DMV. Similar data have been reported that optogenetic stimulation of ChR2-containing fibers from orexin neurons elicits glutamatergic postsynaptic currents in histaminergic tuberomammillary neurons (Schone et al., 2012). In addition, our data support previous work from immunohistochemical studies indicating that orexin neurons use glutamate as their main neurotransmitter (Torrealba et al., 2003, Henny et al., 2010, Carrive, 2013). A minority (37%) of the responses include GABAergic currents as these responses are not abolished by CNQX, but blocked by application of gabazine (25 μM). In accordance with these data, GABA-like immunoreactivity has been found in 10-25% of orexin neurons (Apergis-Schoute et al., 2015).
Thus, our data suggest there are different subpopulations of orexin neurons including those that differentially release glutamate and GABA, with excitatory and inhibitory inputs, respectively, to CVNs in the DMV. These subpopulations of orexin-containing cells could be differently influenced by various environmental stimuli and/or internal factors and may therefore play differential roles in parasympathetic control of cardiovascular function. Since the majority of the inputs from orexin neurons to CVNs are excitatory it is likely that activation of orexin cells would increase the activity of parasympathetic CVNs in the DMV and decrease heart rate. Orexin neuron inactivation, in turn, may lead to diminished cardioprotective parasympathetic activity from CVNs to the heart. Supporting this hypothesis, individuals with narcolepsy, the disease characterized by orexin neurons deficiency, have increased heart rate during wakefulness (Grimaldi et al., 2012, Sorensen et al., 2013). Similar, tachycardia has been reported in orexin-deficient mice (Bastianini et al., 2011, Silvani et al., 2014).
In conclusion, this report is the first demonstration of heterogeneous projections from orexin neurons to CVNs in the DMV. This study elucidates the network mechanisms by which orexin neurons contribute to parasympathetic regulation of cardiovascular function. Further work is needed to understand the differences between subpopulations of orexin neurons and their pathways to CVNs and how they may serve disparate roles in cardiovascular regulation and cardiovascular abnormalities associated with narcolepsy.
Highlights.
-
●
Photoactivation of orexin neurons by channelrhodopsin-2 expression
-
●
Orexin neurons monosynaptically project to brainstem cardiac vagal neurons
-
●
Orexin cells release GABA and glutamate to modulate cardiac vagal neuron activity
Acknowledgements
Supported by NIH grant HL 72006 (D.M.)
ABBREVIATIONS
- aCSF
Artificial cerebrospinal fluid
- CVNs
cardiac vagal neurons
- ChR2
channelrhodopsin-2
- DMV
dorsal motor nucleus of the vagus
- EYFP
enhanced green fluorescent protein
- EPSCs
excitatory postsynaptic currents
- IPSCs
inhibitory postsynaptic currents
- NMDG
110 N-methyl-d-glucamine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Antunes VR, Brailoiu GC, Kwok EH, Scruggs P, Dun NJ. Orexins/hypocretins excite rat sympathetic preganglionic neurons in vivo and in vitro. American journal of physiology Regulatory, integrative and comparative physiology. 2001;281:R1801–1807. doi: 10.1152/ajpregu.2001.281.6.R1801. [DOI] [PubMed] [Google Scholar]
- Apergis-Schoute J, Iordanidou P, Faure C, Jego S, Schone C, Aitta-Aho T, Adamantidis A, Burdakov D. Optogenetic evidence for inhibitory signaling from orexin to MCH neurons via local microcircuits. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2015;35:5435–5441. doi: 10.1523/JNEUROSCI.5269-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastianini S, Silvani A, Berteotti C, Elghozi JL, Franzini C, Lenzi P, Lo Martire V, Zoccoli G. Sleep related changes in blood pressure in hypocretin-deficient narcoleptic mice. Sleep. 2011;34:213–218. doi: 10.1093/sleep/34.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouairi E, Neff R, Evans C, Gold A, Andresen MC, Mendelowitz D. Respiratory sinus arrhythmia in freely moving and anesthetized rats. J Appl Physiol (1985) 2004;97:1431–1436. doi: 10.1152/japplphysiol.00277.2004. [DOI] [PubMed] [Google Scholar]
- Calaresu FR, Pearce JW. Effects on Heart Rate of Electrical Stimulation of Medullary Vagal Structures in the Cat. The Journal of physiology. 1965;176:241–251. doi: 10.1113/jphysiol.1965.sp007547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrive P. Orexin, orexin receptor antagonists and central cardiovascular control. Frontiers in neuroscience. 2013;7:257. doi: 10.3389/fnins.2013.00257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CT, Hwang LL, Chang JK, Dun NJ. Pressor effects of orexins injected intracisternally and to rostral ventrolateral medulla of anesthetized rats. American journal of physiology Regulatory, integrative and comparative physiology. 2000;278:R692–697. doi: 10.1152/ajpregu.2000.278.3.R692. [DOI] [PubMed] [Google Scholar]
- Cheng Z, Powley TL, Schwaber JS, Doyle FJ., 3rd Projections of the dorsal motor nucleus of the vagus to cardiac ganglia of rat atria: an anterograde tracing study. The Journal of comparative neurology. 1999;410:320–341. [PubMed] [Google Scholar]
- Ciriello J, Calaresu FR. Medullary origin of vagal preganglionic axons to the heart of the cat. Journal of the autonomic nervous system. 1982;5:9–22. doi: 10.1016/0165-1838(82)90086-8. [DOI] [PubMed] [Google Scholar]
- Ciriello J, de Oliveira CV. Cardiac effects of hypocretin-1 in nucleus ambiguus. American journal of physiology Regulatory, integrative and comparative physiology. 2003;284:R1611–1620. doi: 10.1152/ajpregu.00719.2002. [DOI] [PubMed] [Google Scholar]
- Ciriello J, Li Z, de Oliveira CV. Cardioacceleratory responses to hypocretin-1 injections into rostral ventromedial medulla. Brain research. 2003;991:84–95. doi: 10.1016/j.brainres.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Date Y, Ueta Y, Yamashita H, Yamaguchi H, Matsukura S, Kangawa K, Sakurai T, Yanagisawa M, Nakazato M. Orexins, orexigenic hypothalamic peptides, interact with autonomic, neuroendocrine and neuroregulatory systems. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:748–753. doi: 10.1073/pnas.96.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira CV, Rosas-Arellano MP, Solano-Flores LP, Ciriello J. Cardiovascular effects of hypocretin-1 in nucleus of the solitary tract. American journal of physiology Heart and circulatory physiology. 2003;284:H1369–1377. doi: 10.1152/ajpheart.00877.2002. [DOI] [PubMed] [Google Scholar]
- DePuy SD, Stornetta RL, Bochorishvili G, Deisseroth K, Witten I, Coates M, Guyenet PG. Glutamatergic neurotransmission between the C1 neurons and the parasympathetic preganglionic neurons of the dorsal motor nucleus of the vagus. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:1486–1497. doi: 10.1523/JNEUROSCI.4269-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dergacheva O. Chronic intermittent hypoxia alters neurotransmission from lateral paragigantocellular nucleus to parasympathetic cardiac neurons in the brain stem. Journal of neurophysiology. 2015;113:380–389. doi: 10.1152/jn.00302.2014. [DOI] [PubMed] [Google Scholar]
- Dergacheva O, Boychuk CR, Mendelowitz D. Developmental changes in GABAergic neurotransmission to presympathetic and cardiac parasympathetic neurons in the brainstem. Journal of neurophysiology. 2013;110:672–679. doi: 10.1152/jn.01054.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dergacheva O, Dyavanapalli J, Pinol RA, Mendelowitz D. Chronic intermittent hypoxia and hypercapnia inhibit the hypothalamic paraventricular nucleus neurotransmission to parasympathetic cardiac neurons in the brain stem. Hypertension. 2014;64:597–603. doi: 10.1161/HYPERTENSIONAHA.114.03603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dergacheva O, Wang X, Huang ZG, Bouairi E, Stephens C, Gorini C, Mendelowitz D. Hypocretin-1 (orexin-A) facilitates inhibitory and diminishes excitatory synaptic pathways to cardiac vagal neurons in the nucleus ambiguus. The Journal of pharmacology and experimental therapeutics. 2005;314:1322–1327. doi: 10.1124/jpet.105.086421. [DOI] [PubMed] [Google Scholar]
- Dergacheva O, Yamanaka A, Schwartz AR, Polotsky VY, Mendelowitz D. Hypoxia and hypercapnia inhibit hypothalamic orexin neurons in rats. Journal of neurophysiology jn. 2016:00196 02016. doi: 10.1152/jn.00196.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimaldi D, Calandra-Buonaura G, Provini F, Agati P, Pierangeli G, Franceschini C, Barletta G, Plazzi G, Montagna P, Cortelli P. Abnormal sleep-cardiovascular system interaction in narcolepsy with cataplexy: effects of hypocretin deficiency in humans. Sleep. 2012;35:519–528. doi: 10.5665/sleep.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henny P, Brischoux F, Mainville L, Stroh T, Jones BE. Immunohistochemical evidence for synaptic release of glutamate from orexin terminals in the locus coeruleus. Neuroscience. 2010;169:1150–1157. doi: 10.1016/j.neuroscience.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang SC, Dai YW, Lee YH, Chiou LC, Hwang LL. Orexins depolarize rostral ventrolateral medulla neurons and increase arterial pressure and heart rate in rats mainly via orexin 2 receptors. The Journal of pharmacology and experimental therapeutics. 2010;334:522–529. doi: 10.1124/jpet.110.167791. [DOI] [PubMed] [Google Scholar]
- Hwang LL, Chen CT, Dun NJ. Mechanisms of orexin-induced depolarizations in rat dorsal motor nucleus of vagus neurones in vitro. The Journal of physiology. 2001;537:511–520. doi: 10.1111/j.1469-7793.2001.00511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iigaya K, Horiuchi J, McDowall LM, Lam AC, Sediqi Y, Polson JW, Carrive P, Dampney RA. Blockade of orexin receptors with Almorexant reduces cardiorespiratory responses evoked from the hypothalamus but not baro- or chemoreceptor reflex responses. American journal of physiology Regulatory, integrative and comparative physiology. 2012;303:R1011–1022. doi: 10.1152/ajpregu.00263.2012. [DOI] [PubMed] [Google Scholar]
- Kayaba Y, Nakamura A, Kasuya Y, Ohuchi T, Yanagisawa M, Komuro I, Fukuda Y, Kuwaki T. Attenuated defense response and low basal blood pressure in orexin knockout mice. American journal of physiology Regulatory, integrative and comparative physiology. 2003;285:R581–593. doi: 10.1152/ajpregu.00671.2002. [DOI] [PubMed] [Google Scholar]
- Luong LN, Carrive P. Orexin microinjection in the medullary raphe increases heart rate and arterial pressure but does not reduce tail skin blood flow in the awake rat. Neuroscience. 2012;202:209–217. doi: 10.1016/j.neuroscience.2011.11.073. [DOI] [PubMed] [Google Scholar]
- Machado BH, Bonagamba LG, Dun SL, Kwok EH, Dun NJ. Pressor response to microinjection of orexin/hypocretin into rostral ventrolateral medulla of awake rats. Regulatory peptides. 2002;104:75–81. doi: 10.1016/s0167-0115(01)00351-2. [DOI] [PubMed] [Google Scholar]
- Mastitskaya S, Marina N, Gourine A, Gilbey MP, Spyer KM, Teschemacher AG, Kasparov S, Trapp S, Ackland GL, Gourine AV. Cardioprotection evoked by remote ischaemic preconditioning is critically dependent on the activity of vagal pre-ganglionic neurones. Cardiovascular research. 2012;95:487–494. doi: 10.1093/cvr/cvs212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura K, Tsuchihashi T, Abe I. Central orexin-A augments sympathoadrenal outflow in conscious rabbits. Hypertension. 2001;37:1382–1387. doi: 10.1161/01.hyp.37.6.1382. [DOI] [PubMed] [Google Scholar]
- Nosaka S, Yamamoto T, Yasunaga K. Localization of vagal cardioinhibitory preganglionic neurons with rat brain stem. The Journal of comparative neurology. 1979;186:79–92. doi: 10.1002/cne.901860106. [DOI] [PubMed] [Google Scholar]
- Petreanu L, Huber D, Sobczyk A, Svoboda K. Channelrhodopsin-2-assisted circuit mapping of long-range callosal projections. Nature neuroscience. 2007;10:663–668. doi: 10.1038/nn1891. [DOI] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinol RA, Bateman R, Mendelowitz D. Optogenetic approaches to characterize the long-range synaptic pathways from the hypothalamus to brain stem autonomic nuclei. Journal of neuroscience methods. 2012;210:238–246. doi: 10.1016/j.jneumeth.2012.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinol RA, Jameson H, Popratiloff A, Lee NH, Mendelowitz D. Visualization of oxytocin release that mediates paired pulse facilitation in hypothalamic pathways to brainstem autonomic neurons. PloS one. 2014;9:e112138. doi: 10.1371/journal.pone.0112138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson WK, Gosnell B, Chang JK, Resch ZT, Murphy TC. Cardiovascular regulatory actions of the hypocretins in brain. Brain research. 1999;831:248–253. doi: 10.1016/s0006-8993(99)01457-2. [DOI] [PubMed] [Google Scholar]
- Schoenenberger P, Scharer YP, Oertner TG. Channelrhodopsin as a tool to investigate synaptic transmission and plasticity. Experimental physiology. 2011;96:34–39. doi: 10.1113/expphysiol.2009.051219. [DOI] [PubMed] [Google Scholar]
- Schone C, Cao ZF, Apergis-Schoute J, Adamantidis A, Sakurai T, Burdakov D. Optogenetic probing of fast glutamatergic transmission from hypocretin/orexin to histamine neurons in situ. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:12437–12443. doi: 10.1523/JNEUROSCI.0706-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirasaka T, Nakazato M, Matsukura S, Takasaki M, Kannan H. Sympathetic and cardiovascular actions of orexins in conscious rats. The American journal of physiology. 1999;277:R1780–1785. doi: 10.1152/ajpregu.1999.277.6.R1780. [DOI] [PubMed] [Google Scholar]
- Silvani A, Bastianini S, Berteotti C, Cenacchi G, Leone O, Lo Martire V, Papa V, Zoccoli G. Sleep and cardiovascular phenotype in middle-aged hypocretin-deficient narcoleptic mice. Journal of sleep research. 2014;23:98–106. doi: 10.1111/jsr.12081. [DOI] [PubMed] [Google Scholar]
- Smith PM, Connolly BC, Ferguson AV. Microinjection of orexin into the rat nucleus tractus solitarius causes increases in blood pressure. Brain research. 2002;950:261–267. doi: 10.1016/s0006-8993(02)03048-2. [DOI] [PubMed] [Google Scholar]
- Sorensen GL, Knudsen S, Petersen ER, Kempfner J, Gammeltoft S, Sorensen HB, Jennum P. Attenuated heart rate response is associated with hypocretin deficiency in patients with narcolepsy. Sleep. 2013;36:91–98. doi: 10.5665/sleep.2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan JM, Connors NA. An orthograde labelling study of axons of the dorsal motor nucleus of the vagus to rat cardiac ganglia as demonstrated by autoradiography. Acta anatomica. 1981;110:128–135. doi: 10.1159/000145422. [DOI] [PubMed] [Google Scholar]
- Torrealba F, Yanagisawa M, Saper CB. Colocalization of orexin a and glutamate immunoreactivity in axon terminals in the tuberomammillary nucleus in rats. Neuroscience. 2003;119:1033–1044. doi: 10.1016/s0306-4522(03)00238-0. [DOI] [PubMed] [Google Scholar]
- Wang X, Pinol RA, Byrne P, Mendelowitz D. Optogenetic stimulation of locus ceruleus neurons augments inhibitory transmission to parasympathetic cardiac vagal neurons via activation of brainstem alpha1 and beta1 receptors. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34:6182–6189. doi: 10.1523/JNEUROSCI.5093-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Fukuda Y, Kuwaki T. Respiratory and cardiovascular actions of orexin-A in mice. Neuroscience letters. 2005;385:131–136. doi: 10.1016/j.neulet.2005.05.032. [DOI] [PubMed] [Google Scholar]