Abstract
Objectives
To evaluate the relation between life-space mobility (extent, frequency, and independence of movement) and mortality in older women.
Design
Prospective cohort study.
Setting
Four U.S. clinical sites.
Participants
Women (N=1,498) aged 75–102 years (mean 87.6) followed from 2006–2015.
Measurements
Life-space during the past four weeks was assessed by interview, scored from 0 (daily restriction to bedroom) to 120 (daily trips outside town without assistance), and categorized (0–20, 21–40, 41–60, 61–80, 81–120). All-cause mortality was the primary outcome; noncancer, cardiovascular, cancer, and noncardiovascular noncancer mortality were secondary outcomes.
Results
Over mean 5.2 years, 842 (56.2%) women died. Unadjusted risk of all-cause mortality was 82.6% among women with the lowest level of life-space (0–20 points) compared to 36.2% among women with the highest level (81–120 points). In multivariable proportional hazards models, there was a strong relation between decreasing life-space and increasing risk of all-cause mortality (Ptrend<0.001). Women with the lowest level of life-space (0–20 points) had 2.4 times higher risk (95% confidence interval (CI) = 1.5,4.0) of all-cause mortality than women with the highest level (81–120 points); women with life-space scores between 21–60 had 1.5 times higher risk of all-cause mortality. Each SD decrease in life-space was associated with a 1.2 times higher risk (95% CI=1.1,1.4) of all-cause mortality. Women unable to travel beyond their neighborhood without assistance had 1.4 times higher (95% CI=1.1,1.7) risk of all-cause mortality. Results were similar for noncancer, cardiovascular, and other mortality and did not change after control for underlying disease or living arrangement.
Conclusion
Life-space scores of 60 or less were associated with mortality in older women independent of other strong risk factors.
Keywords: aged, mobility, death, independence, survival
INTRODUCTION
Aging in humans and other species is marked by declining mobility. While mobility limitations among older adults are often invisible and overlooked in clinical practice1, they are common and costly. In the U.S. an estimated 15.4 million older Medicare beneficiaries report limited ability to walk 2–3 blocks, which has been estimated to add $42 billion to annual health care costs2. Notably, individuals with compromised mobility have an elevated risk of mortality3–6.
Life-space is an integrated measure of mobility that assesses the extent, frequency, and independence of an individual’s movement in the four weeks preceding assessment7, 8. In contrast to commonly used performance-based (e.g., gait speed) or self-report (e.g., ability to walk 2–3 blocks) measures of mobility, which assess physical capacity for mobility at a given point in time, the life-space assessment captures enacted mobility – what individuals actually do in their daily life – and is thus a multidimensional construct, influenced by a range of demographic, biological, medical, psychological, sociological, and environmental factors7–13. In prospective studies, life-space has been shown to predict frailty14, decline in cognitive function15, 16, mild cognitive impairment16, incident Alzheimer’s Disease16, and nursing home admission17.
Because life-space is a comprehensive measure of enacted mobility, it may also be particularly well suited for discriminating risk for death in older adults. If shown to be a reliable predictor of mortality, life-space could be used in research to characterize study populations and as an endpoint in intervention studies. Moreover, life-space could help to guide clinical decision-making for the care of older adults by helping to refine survival estimates and target timely interventions to extend independent mobility and prevent premature mortality.
A previous prospective study of older men (mean age 79.3 years) with relatively limited follow-up time (mean 2.7 years) found that life-space independently predicted mortality18. At this point, replication studies are needed in other populations, including cohorts of women in very late life. Accordingly, the primary aim of this study was to test the hypothesis that lower levels of life-space mobility are associated with greater all-cause mortality in older women. A secondary and exploratory aim was to test the hypotheses that lower levels of life-space mobility are (i) associated with greater noncancer, cardiovascular, and other (noncardiovascular, noncancer) mortality, and (ii) not associated with cancer mortality18, 19 in older women.
METHODS
Study Population
Participants were from the Study of Osteoporotic Fractures (SOF), a prospective observational study. During the baseline examination from 1986 to 1988, 9704 community-dwelling white women aged 65 years or older were enrolled from population-based listings in 4 areas of the United States: Baltimore, Maryland; Minneapolis, Minnesota; Portland, Oregon; and Monongahela Valley near Pittsburgh, Pennsylvania. Between February 1997 and February 1998, an additional 662 African American women 65 years or older were enrolled at the same 4 sites. Race/ethnicity was self-reported by participants, using investigator-defined categories. The protocol and consent forms were approved by the institutional review boards at all of the participating institutions. All participants provided written informed consent.
Trained clinic staff administered the University of Alabama at Birmingham Life-Space Assessment8 tool via interview either at the clinic site or at the participant’s residence during the ninth follow-up visit for SOF between November 2006 and September 2008 to ascertain daily movement in five life-space levels (described below) during the prior 4 weeks. The Life-Space Assessment was conducted with the assistance of a proxy if necessary; previous studies have demonstrated the construct and concurrent validity and test-retest reliability of the Life-Space Assessment in older adults7, 8 and that administration of the life-space assessment to a familiar proxy informant is suitable and provides valid responses20. Participants with non-missing values for life-space were included in the analysis data set for this study (N=1,498; 97.7% of 1534 active participants who had a clinic or home visit at the ninth visit; 58.8% of 2548 total active participants who had a clinic visit, home visit, or self-administered questionnaire at the ninth visit).
Life-Space Assessment
At the ninth follow-up visit for SOF, participants were asked “During the past 4 weeks, have you been to: other rooms of your home besides the room where you sleep? (level 1); an area outside your home, such as your porch, deck, or patio, hallway (of an apartment building), or garage, in your own yard or driveway? (level 2); places in your neighborhood, other than your own yard or apartment building? (level 3); places outside your neighborhood, but within your town? (level 4), and places outside your town? (level 5)” For each level, participants were asked how often they traveled to that area (1=less than 1/wk, 2= 1–3/wk, 3= 4–6/wk, 4=daily), and whether they needed assistance from equipment or another person to travel to that level (2=no assistance, 1.5=use of equipment only, 1=use of another person with or without assistance). Individual level scores were calculated by multiplying the level number by the scores for independence and frequency. In turn, composite life-space scores were calculated by summing the individual level scores and could range from 0 (restricted to one’s bedroom) to 120 (daily travel outside one’s town without assistance).
Mortality
After life-space assessment, women were contacted every 4 months; when they did not return these questionnaires and could not be reached by telephone, next of kin were contacted. Death certificates and medical records (where possible) were collected for all deaths. Centralized physician adjudicators reviewed date and cause of death from death certificates. Cause of death was classified by International Classification of Diseases, Ninth Revision (ICD-9) codes as cardiovascular (401–444.9), cancer (140–239.9), or other causes (codes not in previous categories). Follow-up for vital status was more than 95% complete over a mean 5.2 +/− 2.2 years after life-space assessment through June 2015. The primary outcome was all-cause mortality as conceptualization of a single underlying cause of death is not appropriate for the majority of deaths occurring in late life since these deaths are usually due to multiple coexisting diseases and conditions21. Secondary and exploratory outcomes were non-cancer mortality, cardiovascular mortality, cancer mortality, and other mortality (noncancer, noncardiovascular), consistent with other studies18, 22.
Other Measurements
At the ninth follow-up visit for SOF, participants self-reported marital status (married vs. other), race (white non-hispanic vs. other), education (college or greater vs. other), overall self-rated health compared to others of same age (excellent/good vs. very poor/poor/fair), smoking status (current, past, never), overnight hospitalization in the past year, living arrangement (assisted living/nursing home vs. other), and walking for exercise via questionnaire. Height was measured with a wall-mounted stadiometer and body weight was measured with a balance beam or digital scale. Body mass index (BMI) was calculated as weight (kg) / height (m2). Global cognitive function was assessed by interview using the Teng modified Mini-Mental State Examination (3MS)23, 24. Gait speed was assessed as the fastest of two 6-meter over-ground trials at usual pace from standing25. Depressive symptoms were assessed using the Geriatric Depression Scale (score ≥ 6)26.
Participants reported whether a clinician had ever told them they had the following medical conditions: diabetes mellitus, Parkinson’s Disease, chronic obstructive pulmonary disease (COPD), myocardial infarction/coronary heart disease, hypertension, congestive heart failure, stroke, peripheral vascular disease, non-skin cancer, and Dementia/Alzheimer’s Disease.
Participants indicated if they had body pain or back pain on most days for one month in the past 12 months (severe/extreme vs. none/mild/moderate). They were categorized as having an activity of daily living (ADL) limitation if they reported much difficulty with any of the following five activities or not doing an activity because of a health problem: walking 2–3 blocks; climbing up 10 stairs; getting in and out of bed and chairs; dressing; bathing or showering27. Similarly, they were classified as having an instrumental activity of daily limitation (IADL) limitation if they reported much difficulty with any of the following three activities or not doing an activity because of a health problem: meal preparation; shopping; or heavy housework27.
Statistical Analysis
Characteristics of women were compared according to level of life-space using analysis of variance for normally distributed continuous variables, Kruskal-Wallis for skewed continuous variables, and chi-square for categorical variables.
Life-space was analyzed as a continuous variable (per standard deviation (SD) decrease), and as a categorical variable using previously defined 20-point intervals (0–20, 21–40, 41–60, 61–80, 81–100, 101–120)7, 18. Due to the small sample size in the 101–120 group (n=43), we combined the two highest categories into a single reference category (scores from 81–120, n=229). Restricted independent life-space was defined as confinement to one’s neighborhood if assistance (equipment or personal) was not used or not available; life-space scores ranged from 0 to 87 (mean 39.2 +/− 14.1) for participants with restricted independent life-space.
We plotted Kaplan-Meier product-limit survival curves to illustrate survival according to life-space level. We used Cox proportional hazards models to estimate hazard ratios (HRs) and corresponding 95% confidence intervals (CIs) of mortality according to life-space level. We adjusted base models for age. Then we added adjustment for gait speed because it measures capacity for daily movement (and thus supplements actual amount of daily movement assessed by life-space) and is a strong predictor of mortality in older adults6. To construct final multivariable models we screened a set of potential confounders: clinic site; season of life-space assessment; body mass index; walk for exercise; Teng 3MS; race; marital status; education; self-rated health; hospitalization in past year; smoking status; body pain; back pain; ADL limitation; IADL limitation; and each of the medical conditions mentioned previously. If covariates were associated with life-space and at least one mortality outcome in age-adjusted models at P<0.10, they were retained and included in initial multivariable models. Next, if covariates were associated with at least one mortality outcome at P<0.05 in initial multivariable models, they were retained and included in the final multivariable models.
We performed several sensitivity analyses to examine the robustness of associations between life-space and mortality. To examine the extent to which life-space reflects underlying disease we performed three analyses. First, we excluded women (n=769) who reported a history of diabetes mellitus, COPD, coronary heart disease, congestive heart failure, stroke, peripheral vascular disease, or dementia/Alzheimer’s Disease at the time of life-space assessment; then we re-ran age-adjusted models on the resulting “healthy” subset of participants. We did not exclude women who reported a history of hypertension or non-skin cancer, as we did not find these conditions to be associated with life-space (Table 1). Second, we excluded women (n=33) who died within 6 months of the life-space assessment and re-ran multivariable models. Third, we adjusted multivariable models for number of self-reported medical conditions (index of chronic disease burden) rather than individual medical conditions. To examine the impact of living arrangement on the association between life-space and mortality, we excluded women (n=79) who reported living in a nursing home and re-ran multivariable models, as opportunities for daily travel likely differs between nursing home residents and community-dwelling older adults.
Table 1.
Baseline Characteristics of Participants in the Study of Osteoporotic Fractures by Category of Life-space (LS).
|
Characteristic N(%) or mean (SD) |
0–20 (n=69) |
21–40 (n=387) |
41–60 (n=435) |
61–80 (n=378) |
81–100 (n=186) |
101–120 (n=43) |
P |
|---|---|---|---|---|---|---|---|
| Age, yrs | 89.2 (3.7) | 88.9 (3.4) | 87.7 (3.3) | 86.5 (3.0) | 86.4 (2.8) | 86.6 (3.0) | <0.001 |
| BMI, kg/m2 | 26.7 (5.8) | 26.8 (5.2) | 26.5 (4.9) | 26.1 (4.6) | 26.6 (4.1) | 26.0 (4.8) | 0.570 |
| 6 meter gait speed, m/s | 0.42 (0.16) | 0.55 (0.20) | 0.70 (0.21) | 0.83 (0.19) | 0.86 (0.19) | 0.96 (0.21) | <0.001 |
| Walk for exercise | 4 (6.0) | 112 (29.9) | 176 (41.6) | 178 (48.2) | 114 (61.6) | 25 (58.1) | <0.001 |
| Teng 3MS score (0–100) | 76.1 (14.8) | 84.0 (12.0) | 86.9 (9.7) | 90.4 (6.8) | 91.7 (6.4) | 91.1 (8.6) | <0.001 |
| Season of LS assessment | 0.070 | ||||||
| Fall | 14 (20.3) | 80 (20.7) | 76 (17.5) | 94 (24.9) | 51 (27.4) | 4 (9.3) | |
| Winter | 25 (36.2) | 134 (34.6) | 137 (31.5) | 111 (29.4) | 48 (25.8) | 19 (44.2) | |
| Spring | 19 (27.5) | 93 (24.0) | 125 (28.7) | 83 (22.0) | 46 (24.7) | 13 (30.2) | |
| Summer | 11 (15.9) | 80 (20.7) | 97 (22.3) | 90 (23.8) | 41 (22.0) | 7 (16.3) | |
| White, non-Hispanic | 62 (89.9) | 355 (91.7) | 383 (88.1) | 323 (85.5) | 167 (89.8) | 34 (79.1) | 0.025 |
| Married | 7 (10.3) | 59 (15.3) | 70 (16.2) | 79 (20.9) | 36 (19.4) | 13 (30.2) | 0.002 |
| Excellent/good self-rated health |
38 (55.9) | 255 (66.2) | 332 (76.7) | 331 (87.8) | 161 (86.6) | 39 (90.7) | <0.001 |
| Greater than college education |
17 (24.6) | 137 (35.4) | 160 (36.8) | 154 (40.7) | 87 (46.8) | 20 (47.6) | <0.001 |
| Assisted living/nursing home |
22 (32.4) | 41 (10.7) | 14 (3.2) | 2 (0.5) | 0 (0.0) | 0 (0.0) | <0.001 |
| Current/Past smoker | 22 (32.4) | 126 (32.7) | 144 (33.3) | 134 (35.5) | 69 (37.1) | 8 (18.6) | 0.800 |
| Never smoker | 46 (67.6) | 259 (67.3) | 289 (66.7) | 244 (64.5) | 117 (62.9) | 35 (81.4) | |
| Severe/extreme body pain | 42 (62.7) | 201 (52.6) | 203 (47.5) | 143 (38.2) | 70 (37.6) | 15 (34.9) | <0.001 |
| Severe/extreme back pain | 24 (36.4) | 183 (47.9) | 203 (47.1) | 134 (35.7) | 66 (35.7) | 13 (30.2) | 0.001 |
| ADL limitation | 67 (98.5) | 318 (83.3) | 232 (54.0) | 82 (22.0) | 19 (9.8) | 5 (11.6) | <0.001 |
| IADL limitation | 63 (92.7) | 290 (75.5) | 212 (49.7) | 73 (19.5) | 28 (15.1) | 5 (11.6) | <0.001 |
| Hospitalization past 12 mo. | 29 (42.7) | 143 (37.1) | 125 (28.9) | 61 (16.1) | 35 (18.8) | 8 (18.6) | <0.001 |
| Medical conditions | |||||||
| Diabetes mellitus | 14 (20.6) | 74 (19.2) | 63 (14.6) | 42 (11.1) | 22 (11.8) | 2 (4.7) | <0.001 |
| Parkinson’s Disease | 2 (2.9) | 6 (1.6) | 6 (1.4) | 2 (0.5) | 1 (0.5) | 0 (0.0) | 0.037 |
| COPD | 10 (14.7) | 53 (13.8) | 41 (9.5) | 44 (11.6) | 14 (7.5) | 2 (4.7) | 0.016 |
| Myocardial infarction/CHD |
13 (19.1) | 94 (24.4) | 80 (18.5) | 58 (15.3) | 36 (19.4) | 6 (14.0) | 0.023 |
| Hypertension | 43 (63.2) | 271 (70.4) | 290 (67.0) | 236 (62.4) | 122 (65.6) | 30 (69.8) | 0.250 |
| Congestive heart failure | 13 (19.1) | 69 (17.9) | 51 (11.8) | 29 (7.7) | 17 (9.1) | 3 (7.0) | <0.001 |
| Stroke | 16 (23.5) | 65 (16.9) | 69 (15.9) | 28 (7.4) | 12 (6.5) | 1 (2.3) | <0.001 |
| Peripheral
vascular disease |
6 (8.8) | 29 (7.5) | 24 (5.5) | 20 (5.3) | 7 (3.8) | 3 (7.0) | 0.070 |
| Non-skin cancer | 14 (20.9) | 84 (21.9) | 100 (23.2) | 88 (23.4) | 39 (21.0) | 4 (9.3) | 0.470 |
| Dementia/Alzheimer’s | 12 (17.7) | 24 (6.2) | 22 (5.1) | 6 (1.6) | 0 (0.0) | 1 (2.3) | <0.001 |
| # of medical conditions | 2.1 (1.4) | 2.0 (1.4) | 1.7 (1.2) | 1.5 (1.2) | 1.5 (1.1) | 1.2 (1.1) | <0.001 |
| Geriatric Depression Scale score ≥ 6 |
35 (51.5) | 78 (21.1) | 39 (9.2) | 16 (4.3) | 9 (4.9) | 0 (0.0) | <0.001 |
P values from ANOVA for normally distributed continuous variables, Kruskal-Wallis for skewed continuous variables, and chi-square for categorical variables).
Abbreviations: ADL, activities of daily living; BMI, body mass index; CHD, coronary heart disease; COPD, chronic obstructive pulmonary disease; IADL, instrumental activities of daily living.
RESULTS
Participants ranged in age from 75 to 102 years with mean +/− SD of 87.6 +/− 3.4 years. Life-space scores were approximately normally distributed (mean +/− SD 55.4 +/− 23.6, median 52, interquartile range 37–72). Lower levels of life-space were associated with older age, slower gait speed, less walking for exercise, and worse cognitive function (Table 1). Women with lower levels of life-space were also more likely to be white, to live in an assisted living or nursing home setting, to report severe or extreme body pain, to have an ADL or IADL impairment, to have had an overnight hospitalization in the past 12 months, to have depressive symptoms, and to have a variety of medical conditions. Women with lower levels of life-space were also less likely to be married, to report excellent or good health compared to others their age, and to have a college education.
Over a mean 5.2 +/− 2.2 years (range 7 days to 7.9 years) of follow-up, 842 (56.2%) women died (unadjusted rate 109.0 deaths/1,000 person years, 95% CI=101.6 to 116.3). Of these, 244 (29.0%) were classified as cardiovascular deaths, 117 (13.9%) as cancer deaths, and 481 (57.1%) as other (noncardiovascular, noncancer). Among cardiovascular deaths, the most common subtype was congestive heart failure (n=87, ICD-9 codes 428, 429.2). Among cancer deaths, the most common subtype was colon cancer (n=21, ICD-9 codes 153–154.9). Among other deaths, the most common subtype was neurological disease including Dementias, Alzheimer’s and Parkinson’s Disease (N=114, ICD-9 codes 290–290.9, 331–331.9, 332–332.1).
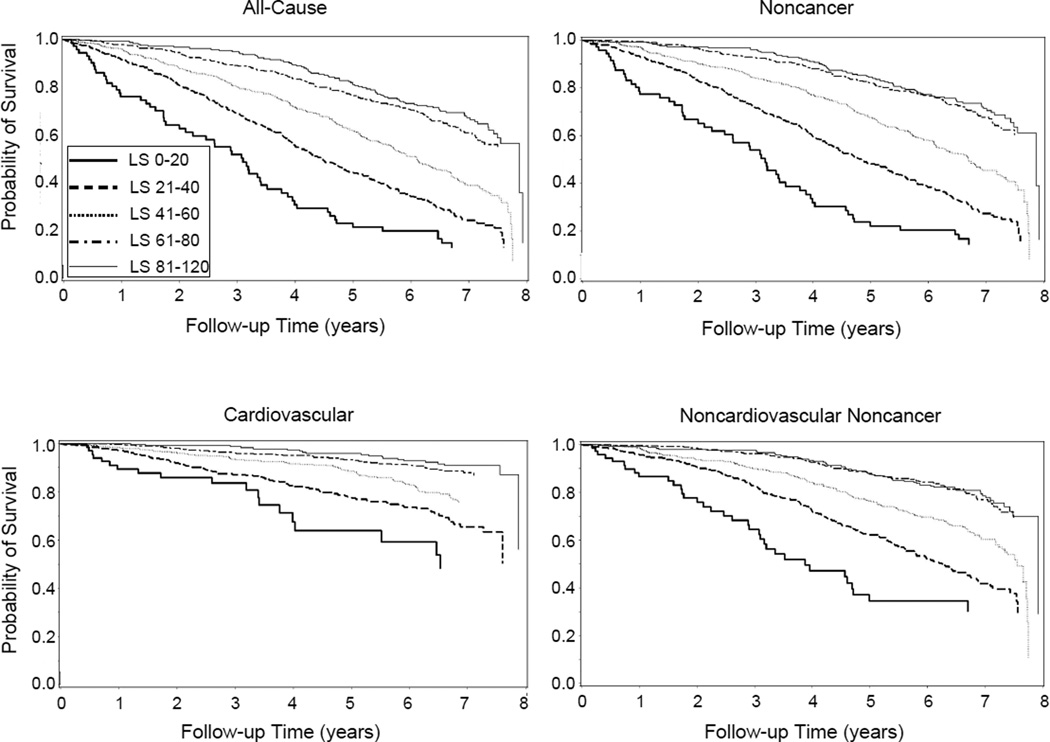
Unadjusted Kaplan-Meier curves for all-cause, noncancer, cardiovascular, and other mortality are shown in Figure 1 according to category of life-space.
Figure 1.
Kaplan-Meier survival plots according to life-space in the Study of Osteoporotic Fractures, 2006 to 2015.
In age-adjusted models, there was a strong relation between decreasing level of life-space and increasing risk of all-cause mortality (Ptrend<0.001) (Table 2). In age-adjusted models, risk of all-cause mortality was 4.90 times higher among women with the lowest level of life-space (0–20 points) than in those with the highest level of life-space (81–120 points). Women with life-space scores between 21 and 60 also had 2–3 times higher risk of all-cause mortality. Each SD (24 point) decrease in life-space was associated with a 1.61 times higher risk of all-cause mortality. For women with restricted independent life-space, risk of all-cause mortality was 2.23 times higher than for women without restricted independent life-space.
Table 2.
Event Rates and Hazard Ratios with 95% Confidence Intervals for Mortality According to Life-space (LS) in the U.S. Study of Osteoporotic Fractures, 2006 to 2015.
| n | Deaths, n | Risk, % | Events/1000 person years |
Model 1 Age Adjusted |
Model 2 Age and Gait Speed Adjusted |
Model
3 Multivariable Adjusted |
|
|---|---|---|---|---|---|---|---|
| All-cause mortality | |||||||
| LS 0–20 | 69 | 57 | 82.6 | 259.9 | 4.90 (3.47, 6.91) | 2.87 (1.84, 4.48) | 2.44 (1.49, 3.99) |
| LS 21–40 | 387 | 287 | 74.2 | 173.6 | 2.92 (2.27, 3.77) | 1.83 (1.38, 2.44) | 1.54 (1.12, 2.12) |
| LS 41–60 | 435 | 267 | 61.4 | 119.1 | 2.06 (1.60, 2.65) | 1.61 (1.24, 2.09) | 1.45 (1.09, 1.93) |
| LS 61–80 | 378 | 148 | 39.2 | 67.3 | 1.22 (0.93, 1.60) | 1.14 (0.87, 1.50) | 1.14 (0.86, 1.52) |
| LS 81–120 | 229 | 83 | 36.2 | 58.7 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| P for trend | <0.001 | <0.001 | <0.001 | ||||
| Per SD decrease in LS | 1.61 (1.49, 1.75) | 1.32 (1.20, 1.45) | 1.22 (1.09, 1.36) | ||||
| Restricted LS | 814 | 571 | 70.2 | 155.2 | 2.23 (1.92, 2.59) | 1.52 (1.27, 1.81) | 1.36 (1.11, 1.66) |
| Model N | 1498 | 1379 | 1284 | ||||
| Noncancer mortality | |||||||
| LS 0–20 | 69 | 54 | 78.3 | 246.2 | 5.43 (3.78, 7.80) | 3.10 (1.95, 4.93) | 2.74 (1.63, 4.58) |
| LS 21–40 | 387 | 261 | 67.4 | 157.9 | 3.08 (2.34, 4.05) | 1.87 (1.38, 2.54) | 1.60 (1.13, 2.25) |
| LS 41–60 | 435 | 223 | 51.3 | 99.5 | 2.01 (1.53, 2.64) | 1.52 (1.14, 2.02) | 1.40 (1.03, 1.91) |
| LS 61–80 | 378 | 116 | 30.7 | 52.7 | 1.13 (0.84, 1.53) | 1.05 (0.78, 1.42) | 1.09 (0.80, 1.48) |
| LS 81–120 | 229 | 71 | 31.0 | 50.3 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| P for trend | <0.001 | <0.001 | 0.001 | ||||
| Per SD decrease in LS | 1.69 (1.55, 1.85) | 1.36 (1.22, 1.51) | 1.27 (1.12, 1.43) | ||||
| Restricted LS | 814 | 507 | 62.3 | 137.8 | 2.43 (2.06, 2.86) | 1.59 (1.31, 1.93) | 1.46 (1.17, 1.82) |
| Model N | 1498 | 1379 | 1284 | ||||
| Cardiovascular mortality | |||||||
| LS 0–20 | 69 | 19 | 27.5 | 86.6 | 6.11 (3.21, 11.66) | 4.04 (1.86, 8.80) | 3.70 (1.53, 8.96) |
| LS 21–40 | 387 | 91 | 23.5 | 55.0 | 3.47 (2.09, 5.76) | 2.09 (1.19, 3.69) | 1.96 (1.05, 3.64) |
| LS 41–60 | 435 | 75 | 17.2 | 33.5 | 2.32 (1.40, 3.84) | 1.82 (1.07, 3.10) | 1.78 (1.01, 3.15) |
| LS 61–80 | 378 | 39 | 10.3 | 17.7 | 1.33 (0.77, 2.31) | 1.29 (0.74, 2.27) | 1.46 (0.82, 2.57) |
| LS 81–120 | 229 | 20 | 8.7 | 14.2 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| P for trend | <0.001 | <0.001 | 0.007 | ||||
| Per SD decrease in LS | 1.71 (1.47, 1.99) | 1.38 (1.15, 1.65) | 1.33 (1.08, 1.64) | ||||
| Restricted LS | 814 | 170 | 20.9 | 46.2 | 2.25 (1.69, 2.98) | 1.44 (1.04, 2.01) | 1.46 (1.01, 2.11) |
| Model N | 1498 | 1379 | 1284 | ||||
| Cancer mortality | |||||||
| LS 0–20 | 69 | 3 | 4.4 | 13.7 | 1.78 (0.50, 6.36) | 0.87 (0.11, 6.97) | 0.77 (0.09, 6.60) |
| LS 21–40 | 387 | 26 | 6.7 | 15.7 | 1.96 (0.97, 3.93) | 1.38 (0.63, 3.07) | 1.10 (0.45, 2.67) |
| LS 41–60 | 435 | 44 | 10.1 | 19.6 | 2.37 (1.25, 4.50) | 2.18 (1.12, 4.24) | 1.81 (0.88, 3.70) |
| LS 61–80 | 378 | 32 | 8.5 | 14.6 | 1.72 (0.88, 3.33) | 1.64 (0.84, 3.19) | 1.47 (0.74, 2.92) |
| LS 81–120 | 229 | 12 | 5.2 | 8.5 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| P for trend | 0.030 | 0.230 | 0.580 | ||||
| Per SD decrease in LS | 1.23 (1.01, 1.50) | 1.12 (0.88, 1.43) | 1.02 (0.77, 1.36) | ||||
| Restricted LS | 814 | 64 | 7.9 | 17.4 | 1.39 (0.95, 2.02) | 1.18 (0.75, 1.85) | 0.93 (0.55, 1.58) |
| Model N | 1498 | 1379 | 1284 | ||||
|
Noncardiovascular, noncancer mortality |
|||||||
| LS 0–20 | 69 | 35 | 50.7 | 159.6 | 5.17 (3.33, 8.01) | 2.72 (1.52, 4.88) | 2.32 (1.23, 4.40) |
| LS 21–40 | 387 | 170 | 43.9 | 102.8 | 2.93 (2.12, 4.06) | 1.80 (1.25, 2.60) | 1.47 (0.97, 2.23) |
| LS 41–60 | 435 | 148 | 34.0 | 66.0 | 1.89 (1.37, 2.62) | 1.42 (1.01, 1.99) | 1.26 (0.87, 1.83) |
| LS 61–80 | 378 | 77 | 20.4 | 35.0 | 1.05 (0.74, 1.51) | 0.96 (0.67, 1.38) | 0.95 (0.66, 1.38) |
| LS 81–120 | 229 | 51 | 22.3 | 36.1 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| P for trend | <0.001 | <0.001 | 0.005 | ||||
| Per SD decrease in LS | 1.68 (1.51, 1.87) | 1.35 (1.19, 1.53) | 1.24 (1.07, 1.44) | ||||
| Restricted LS | 814 | 337 | 41.4 | 91.6 | 2.52 (2.06, 3.09) | 1.68 (1.33, 2.13) | 1.47 (1.12, 1.93) |
| Model N | 1498 | 1379 | 1284 |
Model 1, adjusted for age. Model 2, adjusted for factors in Model 1 plus gait speed (fastest 6–m gait speed from two trials).
Model 3, adjusted for factors in Model 2 plus others (clinic site, season of assessment, BMI, walking for exercise, Teng 3MS, race, self-reported health, smoking status, body pain, ADL impairment, IADL impairment, prior hospitalization, COPD, CHF, and cancer).
These associations were somewhat attenuated but remained strong and significant after adding gait speed and full multivariable adjustment (Table 2). In the multivariable model, the relation between decreasing level of life-space and increasing risk of all-cause mortality remained strong (Ptrend<0.001). After multivariable adjustment, women with the lowest level of life-space (0–20 points) had a 2.44 times higher risk of all-cause mortality than women with the highest level (81–120 points), and women with life-space scores between 21–60 had 1.45–1.54 times higher risk of all-cause mortality. Each SD decrease in life-space was associated with a 1.22 times higher risk of all-cause mortality. For women with restricted independent life-space, risk of all-cause mortality was 1.36 times higher than for women without restricted independent life-space.
In multivariable models, associations between life-space and the outcomes of non-cancer and other (noncardiovascular, noncancer) mortality were similar in magnitude to those for all-cause mortality (Table 2). Life-space was more strongly associated with cardiovascular mortality than with all-cause mortality, and life-space was not associated with cancer mortality.
Sensitivity analyses showed that life-space was more strongly associated with all-cause, noncancer, cardiovascular, and other mortality in age-adjusted models in the subset of “healthy” women (n=729) who were free of a variety of medical conditions at baseline (Table 3). In contrast, adjusting for number of medical conditions (index of comorbidity burden) rather than individual medical conditions did not alter the main multivariable results. Similarly, excluding the 33 women who died within 6 months of life-space assessment or the 79 women who lived in nursing homes did not alter the main multivariable results (data not shown).
Table 3.
Event Rates and Hazard Ratios with 95% Confidence Intervals for Mortality According to Life-space (LS) in the U.S. Study of Osteoporotic Fractures, 2006 to 2015 in the “Healthy Subset” of Participants Free of Chronic Conditions at Time of Life-space Assessment.
| n | Deaths, n | Risk, % | Events/1000 person years | Healthy Subset Age Adjusted | |
|---|---|---|---|---|---|
| All-cause mortality | |||||
| LS 0–20 | 21 | 20 | 95.2 | 357.8 | 7.60 (4.44, 12.99) |
| LS 21–40 | 153 | 109 | 71.2 | 158.3 | 2.81 (1.95, 4.04) |
| LS 41–60 | 199 | 105 | 52.8 | 93.7 | 1.79 (1.26, 2.55) |
| LS 61–80 | 218 | 83 | 38.1 | 65.2 | 1.35 (0.93, 1.94) |
| LS 81–120 | 138 | 45 | 32.6 | 52.0 | 1.00 (ref) |
| P for trend | <0.001 | ||||
| Per SD decrease in LS | 1.55 (1.37, 1.75) | ||||
| Restricted LS | 336 | 222 | 66.1 | 137.4 | 2.18 (1.75, 2.72) |
| Model N | 729 | ||||
| Noncancer mortality | |||||
| LS 0–20 | 21 | 17 | 81.0 | 304.1 | 7.72 (4.31, 13.82) |
| LS 21–40 | 153 | 98 | 64.1 | 142.3 | 2.86 (1.93, 4.23) |
| LS 41–60 | 199 | 86 | 43.2 | 76.7 | 1.72 (1.17, 2.53) |
| LS 61–80 | 218 | 66 | 30.3 | 51.8 | 1.29 (0.86, 1.93) |
| LS 81–120 | 138 | 38 | 27.5 | 43.9 | 1.00 (ref) |
| P for trend | <0.001 | ||||
| Per SD decrease in LS | 1.57 (1.38, 1.80) | ||||
| Restricted LS | 336 | 194 | 57.7 | 120.1 | 2.33 (1.82, 2.97) |
| Model N | 729 | ||||
| Cardiovascular mortality | |||||
| LS 0–20 | 21 | 9 | 42.9 | 161.0 | 12.62 (5.14, 30.99) |
| LS 21–40 | 153 | 26 | 17.0 | 37.8 | 2.30 (1.10, 4.77) |
| LS 41–60 | 199 | 31 | 15.6 | 27.7 | 2.01 (1.00, 4.01) |
| LS 61–80 | 218 | 20 | 9.2 | 15.7 | 1.27 (0.61, 2.65) |
| LS 81–120 | 138 | 11 | 8.0 | 12.7 | 1.00 (ref) |
| P for trend | <0.001 | ||||
| Per SD decrease in LS | 1.61 (1.27, 2.04) | ||||
| Restricted LS | 336 | 60 | 17.9 | 37.1 | 2.03 (1.32, 3.12) |
| Model N | 729 | ||||
| Cancer mortality | |||||
| LS 0–20 | 21 | 3 | 14.3 | 53.7 | 7.52 (1.91, 29.61) |
| LS 21–40 | 153 | 11 | 7.2 | 16.0 | 2.26 (0.86, 5.96) |
| LS 41–60 | 199 | 19 | 9.5 | 17.0 | 2.16 (0.91, 5.16) |
| LS 61–80 | 218 | 17 | 7.8 | 13.4 | 1.63 (0.68, 3.94) |
| LS 81–120 | 138 | 7 | 5.1 | 8.1 | 1.00 (ref) |
| P for trend | 0.009 | ||||
| Per SD decrease in LS | 1.41 (1.05, 1.88) | ||||
| Restricted LS | 336 | 28 | 8.3 | 17.3 | 1.55 (0.91, 2.65) |
| Model N | 729 | ||||
|
Noncardiovascular,
noncancer mortality |
|||||
| LS 0–20 | 21 | 8 | 38.1 | 143.1 | 5.39 (2.42, 12.00) |
| LS 21–40 | 153 | 72 | 47.1 | 104.6 | 3.14 (1.97, 5.01) |
| LS 41–60 | 199 | 55 | 27.6 | 49.1 | 1.59 (0.99, 2.54) |
| LS 61–80 | 218 | 46 | 21.1 | 36.1 | 1.30 (0.80, 2.10) |
| LS 81–120 | 138 | 27 | 19.6 | 31.2 | 1.00 (ref) |
| P for trend | <0.001 | ||||
| Per SD decrease in LS | 1.56 (1.33, 1.83) | ||||
| Restricted LS | 336 | 134 | 39.9 | 82.9 | 2.48 (1.84, 3.34) |
| Model N | 729 |
DISCUSSION
In this prospective study, unadjusted life-space mobility discriminated among older women with low/moderate to very high risk for all-cause, noncancer, cardiovascular, and other (noncardiovascular, noncancer) mortality. After adjustment for strong and established risk factors for mortality (including age, gait speed, body mass index, physical activity, cognitive function, self-rated health, smoking, pain, ADL and IADL impairment, history of hospitalization, and chronic medical conditions), life-space scores of 60 or less were significantly associated with elevated risk of mortality. In addition, a 24-point reduction in life-space was associated with a 22–33% higher risk of mortality, and women with restricted life-space (confined to their neighborhoods if assistance was not used or not available) had a 36–47% higher risk of mortality than their unrestricted counterparts.
Importantly, life-space was associated with mortality in older women after accounting for our measures of underlying illness and gait speed. To control for the association between chronic conditions and mortality, we conducted analyses where we (i) adjusted for individual medical conditions, (ii) adjusted for number of medical conditions, (iii) excluded women who died within 6 months of life-space assessment, and (iv) excluded women who had one or more chronic conditions at the time of life-space assessment. Further, we adjusted multivariable models for gait speed, which is one of the strongest physical performance based predictor of mortality in older adults6. Across all of these permutations, life-space remained significantly associated with mortality, which suggests that enacted daily mobility contributes to health and survival separately and distinctly from medical status, disability, and physical capacity; these results also point to the importance of spatial contributions to health28.
Life-space likely has some direct influence on mortality through downstream effects on physical activity and fitness that result in early mortality3, 29. Life-space likely also reflects underlying unmeasured biological, social, psychological, and environmental factors. Biological factors may include pre-clinical disease burden, lack of physiological reserve, frailty, the energy cost of mobility, and disturbances to organ systems. Social factors may include lack of personal assistance and social support30, limited transportation options31, and changing cognitive and emotional functioning. Psychological factors may include personality, motivation, and mental health10. Environmental factors may include built environment features such as presence of sidewalks, green and blue spaces, destinations of interest, weather, and neighborhood safety32, 33. To this end, the life-space assessment would be a simple, quick, and easy-to-administer proxy for a host of other risk factors for mortality. To target interventions to prevent premature mortality, future research is warranted to examine biological, social, psychological, and environmental mechanisms underlying the association between life-space and mortality.
The current study extends our understanding of the relation between life-space and mortality in older adults. A previous study showed that lower levels of life-space were associated with higher risk of mortality in older men independent of other established risk factors18. It remained uncertain, however, if life-space predicted mortality in women; moreover, as mean duration of mortality follow-up in that study was 2.7 years, the authors could not exclude the possibility that life-space was a marker for existing disease. The current study shows that life-space is associated with mortality in women in very late life (mean age was 87.6 years, and most women were in their 9th and 10th decades of life). Furthermore, mean duration of mortality follow-up in the current study was much longer at 5.2 years; thus, the results of the current study more clearly suggest that limited life-space is a risk factor for future mortality, even among the oldest old, rather than simply a marker of existing disease.
Average composite life-space scores among older women in the current study were considerably lower than average scores among older men in our previous study (55.4 vs. 84.9)18. This likely reflects the older age distribution of women compared to men (mean age 87.6 vs. 79.3 years), but also suggests a sex-based difference in life-space mobility, as has been noted in previous studies8, 10, 34 and may point to greater risk for social isolation among older women, and thus greater need among older women for social and environmental supports to enable mobility.
The strengths of this study are the large and well-characterized study population, long duration of mortality follow-up, and central physician adjudication of cause of death. This study also has certain limitations. First, as the average age of participants was 87.6 years (range: 75 to 102 years), this study does not provide knowledge about the association between life-space and mortality in younger populations who may have ceiling effects for the life-space assessment. Second, while the assumption of one underlying cause of death is reasonable for individuals with a single disease or little comorbidity, assigning a single underlying cause of death is not appropriate for the vast majority of deaths occurring in very late life, as these deaths are usually due to multiple coexisting diseases and conditions. Thus, to avoid bias due to misclassification, the primary outcome for this study was all-cause mortality, and the cause-specific analyses we reported were exploratory in nature. Of note, the pattern of associations we observed between life-space and cause-specific mortality was previously observed between a variety of biomarkers and cause-specific mortality in a younger (65–69 years) subgroup of women from the SOF cohort22, and between life-space and cause-specific mortality in men of mean age 79.3 years18. Therefore, in situations when it is possible to assign cause of death with more certainty (less error), we would expect even stronger associations between life-space and cause-specific mortality than found in the current study. Furthermore, as hypothesized, we found that life-space was not associated with cancer mortality. This finding is consistent with and reinforces how cancer death has a different association with biomarkers of aging and frailty than other causes of death18, 19, 22. A minority proportion (11.5%, n=157) of participants who completed the life-space assessment without any assistance from a proxy scored <80 on the 3MS, indicative of some degree of cognitive impairment. Life-space scores from these individuals may not be accurate and would likely lead to underestimation of the association between life-space and mortality. Finally, we adjusted the multivariable models for gait speed, ADL and IADL limitations, and physical activity because we postulated that reductions in the capacity for movement captured by these variables drive subsequent reductions in enacted mobility behaivour. Thus, we conceived of these variables as potential confounders of the association between life-space and mortality, which necessitated statistical adjustment. If these adjustments were unnecessary, the multivariable adjusted associations reported in this paper may be underestimated35.
In summary, life-space predicts all-cause, noncancer, cardiovascular, and other (noncardiovascular, noncancer) mortality in older women. Life-space scores of 60 or less are associated with these types of mortality independent of other strong risk factors. The life-space assessment may prove to be a useful tool in clinical practice and geriatric research.
Acknowledgments
Funding Sources
The Study of Osteoporotic Fractures (SOF) is supported by National Institutes of Health funding. The National Institute on Aging (NIA) provides support under the following grant numbers: R01 AG005407, R01 AR35582, R01 AR35583, R01 AR35584, R01 AG005394, R01 AG027574, and R01 AG027576.
Sponsor’s Role
The sponsor had no role in the design, methodology, participant recruitment, data collection, analysis or preparation of the paper.
Footnotes
| Elements
of Financial/Personal Conflicts |
DCM | LYL | PMC | KE | KY | SRC | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | |
| Employment or Affiliation |
X | X | X | X | X | X | ||||||
| Grants/Funds | X | X | X | X | X | X | ||||||
| Honoraria | X | X | X | X | X | X | ||||||
| Speaker Forum | X | X | X | X | X | X | ||||||
| Consultant | X | X | X | X | X | X | ||||||
| Stocks | X | X | X | X | X | X | ||||||
| Royalties | X | X | X | X | X | X | ||||||
| Expert Testimony | X | X | X | X | X | X | ||||||
| Board Member | X | X | X | X | X | X | ||||||
| Patents | X | X | X | X | X | X | ||||||
| Personal Relationship |
X | X | X | X | X | X | ||||||
Author Contributions
All authors made substantial contributions to the study and article. Specifically, DCM conceived and designed the study, analyzed and interpreted the data, drafted the article and revised it critically for important intellectual content. LYL analyzed and interpreted the data, revised the article critically for important intellectual content. PMC, KE, SRC acquired data, interpreted data, revised the article critically for important intellectual content. KY interpreted data, revised the article critically for important intellectual content. All authors approved the final version to be published.
REFERENCES
- 1.Cummings SR, Studenski S, Ferrucci L. A diagnosis of dismobility--giving mobility clinical visibility: a Mobility Working Group recommendation. JAMA. 2014;311:2061–2062. doi: 10.1001/jama.2014.3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hardy SE, Kang Y, Studenski SA, Degenholtz HB. Ability to walk 1/4 mile predicts subsequent disability, mortality, and health care costs. J Gen Intern Med. 2011;26:130–135. doi: 10.1007/s11606-010-1543-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blair SN, Kampert JB, Kohl HW, 3rd, et al. Influences of cardiorespiratory fitness and other precursors on cardiovascular disease and all-cause mortality in men and women. JAMA. 1996;276:205–210. [PubMed] [Google Scholar]
- 4.Manini TM, Everhart JE, Patel KV, et al. Daily activity energy expenditure and mortality among older adults. JAMA. 2006;296:171–179. doi: 10.1001/jama.296.2.171. [DOI] [PubMed] [Google Scholar]
- 5.Newman AB, Simonsick EM, Naydeck BL, et al. Association of long-distance corridor walk performance with mortality, cardiovascular disease, mobility limitation, and disability. JAMA. 2006;295:2018–2026. doi: 10.1001/jama.295.17.2018. [DOI] [PubMed] [Google Scholar]
- 6.Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA. 2011;305:50–58. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baker PS, Bodner EV, Allman RM. Measuring life-space mobility in community-dwelling older adults. J Am Geriatr Soc. 2003;51:1610–1614. doi: 10.1046/j.1532-5415.2003.51512.x. [DOI] [PubMed] [Google Scholar]
- 8.Peel C, Sawyer Baker P, Roth DL, Brown CJ, Brodner EV, Allman RM. Assessing mobility in older adults: the UAB Study of Aging Life-Space Assessment. Phys Ther. 2005;85:1008–1119. [PubMed] [Google Scholar]
- 9.Brown CJ, Roth DL, Allman RM, Sawyer P, Ritchie CS, Roseman JM. Trajectories of life-space mobility after hospitalization. Ann Intern Med. 2009;150:372–378. doi: 10.7326/0003-4819-150-6-200903170-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Byles JE, Leigh L, Vo K, Forder P, Curryer C. Life space and mental health: a study of older community-dwelling persons in Australia. Aging Ment Health. 2015;19:98–106. doi: 10.1080/13607863.2014.917607. [DOI] [PubMed] [Google Scholar]
- 11.Lo AX, Brown CJ, Sawyer P, Kennedy RE, Allman RM. Life-space mobility declines associated with incident falls and fractures. J Am Geriatr Soc. 2014;62:919–923. doi: 10.1111/jgs.12787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Webber SC, Porter MM, Menec VH. Mobility in older adults: a comprehensive framework. Gerontologist. 2010;50:443–450. doi: 10.1093/geront/gnq013. [DOI] [PubMed] [Google Scholar]
- 13.Portegijs E, Rantakokko M, Mikkola TM, Viljanen A, Rantanen T. Association between physical performance and sense of autonomy in outdoor activities and life-space mobility in community-dwelling older people. J Am Geriatr Soc. 2014;62:615–621. doi: 10.1111/jgs.12763. [DOI] [PubMed] [Google Scholar]
- 14.Xue QL, Fried LP, Glass TA, Laffan A, Chaves PH. Life-space constriction, development of frailty, and the competing risk of mortality: the Women's Health And Aging Study I. Am J Epidemiol. 2008;167:240–248. doi: 10.1093/aje/kwm270. [DOI] [PubMed] [Google Scholar]
- 15.Crowe M, Andel R, Wadley VG, Okonkwo OC, Sawyer P, Allman RM. Life-space and cognitive decline in a community-based sample of African American and Caucasian older adults. J Gerontol A Biol Sci Med Sci. 2008;63:1241–1245. doi: 10.1093/gerona/63.11.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.James BD, Boyle PA, Buchman AS, Barnes LL, Bennett DA. Life space and risk of Alzheimer disease, mild cognitive impairment, and cognitive decline in old age. Am J Geriatr Psychiatry. 2011;19:961–969. doi: 10.1097/JGP.0b013e318211c219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sheppard KD, Sawyer P, Ritchie CS, Allman RM, Brown CJ. Life-space mobility predicts nursing home admission over 6 years. J Aging Health. 2013;25:907–920. doi: 10.1177/0898264313497507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mackey DC, Cauley JA, Barrett-Connor E, et al. Life-space mobility and mortality in older men: a prospective cohort study. J Am Geriatr Soc. 2014;62:1288–1296. doi: 10.1111/jgs.12892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dumurgier J, Elbaz A, Ducimetiere P, Tavernier B, Alperovitch A, Tzourio C. Slow walking speed and cardiovascular death in well functioning older adults: prospective cohort study. BMJ. 2009;339:b4460. doi: 10.1136/bmj.b4460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cavanaugh JT, Crawford K. Life-Space Assessment and Physical Activity Scale for the Elderly: validity of proxy informant responses. Arch Phys Med Rehabil. 2014;95:1527–1532. doi: 10.1016/j.apmr.2014.03.027. [DOI] [PubMed] [Google Scholar]
- 21.Tinetti ME, McAvay GJ, Murphy TE, Gross CP, Lin H, Allore HG. Contribution of individual diseases to death in older adults with multiple diseases. J Am Geriatr Soc. 2012;60:1448–1456. doi: 10.1111/j.1532-5415.2012.04077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Swindell WR, Ensrud KE, Cawthon PM, et al. Indicators of "healthy aging" in older women (65–69 years of age). A data-mining approach based on prediction of long-term survival. BMC Geriatr. 2010;10:55. doi: 10.1186/1471-2318-10-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48:314–318. [PubMed] [Google Scholar]
- 24.Yaffe K, Middleton LE, Lui LY, et al. Mild cognitive impairment, dementia, and their subtypes in oldest old women. Arch Neurol. 2011;68:631–636. doi: 10.1001/archneurol.2011.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cummings SR, Nevitt MC, Browner WS, et al. Risk factors for hip fracture in white women. Study of Osteoporotic Fractures Research Group. NEJM. 1995;332:767–773. doi: 10.1056/NEJM199503233321202. [DOI] [PubMed] [Google Scholar]
- 26.Almeida OP, Almeida SA. Short versions of the geriatric depression scale: a study of their validity for the diagnosis of a major depressive episode according to ICD-10 and DSM-IV. Int J Geriatr Psychiatry. 1999;14:858–865. doi: 10.1002/(sici)1099-1166(199910)14:10<858::aid-gps35>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 27.Cawthon PM, Fink HA, Barrett-Connor E, et al. Alcohol use, physical performance, and functional limitations in older men. J Am Geriatr Soc. 2007;55:212–220. doi: 10.1111/j.1532-5415.2007.01062.x. [DOI] [PubMed] [Google Scholar]
- 28.Winters M, Voss C, Ashe MC, Gutteridge K, McKay H, Sims-Gould J. Where do they go and how do they get there? Older adults' travel behaviour in a highly walkable environment. Soc Sci Med. 2015;133:304–312. doi: 10.1016/j.socscimed.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 29.Blair SN, Kohl HW, 3rd, Paffenbarger RS, Jr, Clark DG, Cooper KH, Gibbons LW. Physical fitness and all-cause mortality. A prospective study of healthy men and women. JAMA. 1989;262:2395–2401. doi: 10.1001/jama.262.17.2395. [DOI] [PubMed] [Google Scholar]
- 30.Rutledge T, Matthews K, Lui LY, Stone KL, Cauley JA. Social networks and marital status predict mortality in older women: prospective evidence from the Study of Osteoporotic Fractures (SOF) Psychosom Med. 2003;65:688–694. doi: 10.1097/01.psy.0000041470.25130.6c. [DOI] [PubMed] [Google Scholar]
- 31.Shah RC, Maitra K, Barnes LL, James BD, Leurgans S, Bennett DA. Relation of driving status to incident life space constriction in community-dwelling older persons: a prospective cohort study. J Gerontol A Biol Sci Med Sci. 2012;67:984–989. doi: 10.1093/gerona/gls133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Finlay J, Franke T, McKay H, Sims-Gould J. Therapeutic landscapes and wellbeing in later life: Impacts of blue and green spaces for older adults. Health Place. 2015;34:97–106. doi: 10.1016/j.healthplace.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 33.Rantakokko M, Iwarsson S, Portegijs E, Viljanen A, Rantanen T. Associations between environmental characteristics and life-space mobility in community-dwelling older people. J Aging Health. 2015;27:606–621. doi: 10.1177/0898264314555328. [DOI] [PubMed] [Google Scholar]
- 34.Hjorthol R. Transport resources, mobility and unmet transport needs in old age. Ageing Soc. 2013;33:1190–1211. [Google Scholar]
- 35.Schisterman EF, Cole SR, Platt RW. Overadjustment bias and unnecessary adjustment in epidemiologic studies. Epidemiology. 2009;20:488–495. doi: 10.1097/EDE.0b013e3181a819a1. [DOI] [PMC free article] [PubMed] [Google Scholar]