Abstract
There is a large body of research supporting the association between disrupted physiological reactivity to negative stimuli and depression. The present study aimed to examine whether physiological reactivity to emotional stimuli, assessed via pupil dilation, served as a biological marker of risk for depression recurrence among individuals who are known to be at a higher risk due to having previous history of depression. Participants were 57 women with a history of Major Depressive Disorder (MDD). Pupil dilation to angry, happy, sad, and neutral faces was recorded. Participant’s diagnoses and symptoms were assessed 24 months after the initial assessment. We found that women’s pupillary reactivity to negative (sad or angry faces) but not positive stimuli prospectively predicted MDD recurrence. Additionally, we found that both hyper and hypo pupillary reactivity to angry faces predicted risk for MDD recurrence. These findings suggest that disrupted physiological response to negative stimuli indexed via pupillary dilation, could serve as a physiological marker of MDD risk, thus presenting clinicians with a convenient and inexpensive method to predict which of the at-risk women are more likely to experience depression recurrence.
Keywords: depression recurrence, pupillometry, physiological reactivity, biomarker, emotion processing
Major depressive disorder (MDD) is the leading cause of disability worldwide with an estimated 350 million of people affected (“WHO | Depression,” 2012). Women are nearly twice as likely to experience MDD than men, thus representing a population that is particularly vulnerable to developing MDD (Kessler et al., 1993). Depression is a highly recurrent disorder and up to 60% of individuals who experience an initial MDD episode are expected to relapse within 5 years following recovery (Bulloch et al., 2014; Burcusa and Lacono, 2007; Solomon et al., 2000). Recurrent MDD is characterized by distinct genetic, neurobiological and hormonal profiles (Admon et al., 2014; Holsen et al., 2013; Levinson et al., 2003), transmits a greater risk for more severe and chronic consequences (Burcusa and Lacono, 2007; Lewinsohn et al., 1999), and is less responsive to antidepressant medication (Kaymaz et al., 2008). Thus, the identification of reliable and easily measurable markers of risk is critical for aiding clinicians in predicting which women who have recovered from an initial MDD episode are at greatest risk for relapse. This will allow clinicians and policy makers to channel limited resources to those most in need.
A rapidly growing body of research highlights the role of dysregulated neurophysiological reactivity to negative stimuli as a potential biological marker of depression risk (e.g., Price et al., 2015; Siegle et al., 2001). Both hyper- and hypo-reactivity in brain regions involved in visual emotive processing, including the anterior cingulate cortex (ACC) ventromedial prefrontal cortex (VMPFC), and the dorsolateral prefrontal cortex (DLPFC) in response to emotional stimuli in patients with MDD have been reported by neuroimaging studies (Bermpohl et al., 2009; Davidson et al., 2003; Fales et al., 2009; Gotlib et al., 2005; Grimm et al., 2009; Guo et al., 2015; Keedwell et al., 2005; Kumari et al., 2003; Tao et al., 2012). These patterns of hyper- and hypo-reactivity have also been linked to differential antidepressant treatment response (Williams et al., 2015). Specifically, Williams et al. found that nonresponders showed pre-treatment hyper-reactivity in the amygdala, followed by post-treatment hypo-reactivity to sad stimuli, compared to controls. In contrast, responders showed hypo-reactivity compared to controls at baseline, which normalized to levels similar to those observed in controls by the end of treatment, suggesting that both hypo and hyper amygdala reactivity could be associated with MDD (Williams et al., 2015). Additionally, findings from studies that used measures of emotional reactivity other than fMRI, provide support for both decreased and increased reactivity among participants with current MDD diagnosis and those who are at a greater risk for developing MDD (Bylsma et al., 2008; Cohen et al., 2005; Kovacs et al., 2009; Kujawa et al., 2012; Lethbridge and Allen, 2008; Pine et al., 2001).
Providing a peripheral measure of cognitive and emotion processing, there is evidence that the pupil dilates in response to stimuli associated with cognitive load and emotional intensity (Hess and Polt, 1960; Siegle et al., 2003a). Pupil dilation has also been linked to activity in brain regions associated with the processing and regulation of emotion, including DLPFC, VMPFC, amygdala, inferior frontal gyrus (IFG), superior frontal gyrus (SFG), and ACC, such that greater blood oxygen level dependent (BOLD) activity in those areas was associated with greater pupil dilation (Siegle, Steinhauer, Stenger, Konecky, & Carter, 2003; Urry et al., 2006). Specifically, modulation of negative affect corresponded to changes in pupil diameter, such that participants who displayed higher activation in the left IFG and SFG clusters when they were instructed to decrease negative emotions evidenced higher pupil dilation (Urry et al., 2006). Additionally, activity in ACC, which is thought to be related to tasks that require heightened control due to emotional and cognitive interference, was reported to be positively associated with pupil dilation during the Stroop task, and higher magnitude of ACC activation predicted increased pupil dilation during error-related trials (Critchley et al., 2005). There is also evidence that pupil dilation can serve as a reliable index of activity in the locus coeruleus-noradrenergic (LC-NA) neuromodulatory system, which is essential for a broad range of cognitive and emotional processes (Gilzenrat et al., 2012; Hermans et al., 2013; Murphy et al., 2014, 2011). Both hypo- and hyper-activity of the LC-NA system are thought to be associated with emotion dysregulation and affective disorders, including depression (Aston-Jones et al., 1999). Moreover, disrupted LC-NA transmission is associated with depression-like behaviors, including learned helplessness and insomnia, in animals (Pavcovich and Ramirez, 1993).
Notably, pupillometry is a relatively inexpensive and accessible measure of cognitive-affective processing that could be easily used in clinicians’ offices for assessing risk. Importantly, there is a small but growing body of research suggesting that pupil dilation to emotional stimuli may be associated with depression risk. Importantly, there is evidence for the association of both increased and decreased pupillary reactivity to negative emotional stimuli and depression diagnosis, treatment success, and risk. For example, there is evidence that individuals with current MDD evidence increased pupil dilation to negative stimuli compared to never depressed participants (Siegle, Steinhauer, Carter, et al., 2003). Pre-treatment sustained pupil dilation in response to negative stimuli, in combination with initial symptom severity, has also been shown to predict remission rates following cognitive-behavioral therapy among patients with MDD (Siegle, Steinhauer, Friedman, Thompson, & Thase, 2011). Specifically, the findings suggested that remission was associated with low sustained pupil dilation to negative stimuli and high initial severity of depression. Decreased pupil dilation to emotional stimuli has been reported among never depressed individuals who are at a higher risk for MDD due to having a parent with MDD (Bistricky et al., 2014). Lastly, increased pupil dilation to sad faces has been found to prospectively predict depression onset among children of mothers with a history of MDD (Burkhouse et al., 2015). Despite the strengths of these studies, it is still unclear whether pupil dilation in response to negative information predicts MDD recurrence risk in adults.
In this study, we sought to examine whether pupil dilation to emotional stimuli prospectively predicts depression risk in a group of individuals known to be at heightened risk: those with previous history of MDD. Given that women are at a higher risk for MDD than men, (Kessler et al., 1993), we focused exclusively on women. Based on previous research suggesting that the association between depression and changes in pupil dilation was stronger for negative stimuli, compared to positive information (Burkhouse et al., 2015; Siegle et al., 2011) we hypothesized that pupillary reactivity to negative (sad or angry faces) but not positive stimuli would prospectively predict MDD recurrence. We also sought to examine the potential role of hyper- versus hypo-reactivity to emotional stimuli. In addition to examining whether high versus low pupillary reactivity predicted risk for MDD recurrence, we also examined whether both high and low pupillary reactivity may be a marker of risk. Given evidence that emotion dysregulation and depression are characterized by both increased and decreased activity in DLPFC, VMPFC, and ACC, as well as hypo and hyper LC-NA activation, all of which are indexed by pupil dilation, we hypothesized that both hyper- and hypo-pupillary reactivity would predict risk for MDD recurrence.
Method
Procedure
Potential participants were recruited from the community through a variety of means (e.g. newspaper and TV advertisements), as part of a larger study of intergenerational transmission of depression. Women responding to the advertisements were screened over the phone and eligible participants were invited to participate with their children. Upon arrival to the laboratory, participants provided informed consent, were administered the SCID-I, and completed computer-based tasks. Follow-up assessment occurred every 6 months for 24 months (5 assessments total), during which participants were administered the SCID-I to assess for new episodes of MDD. Of the 57 participants who completed the Time 1 assessment, 51 (89.5%) completed Time 2, 48 (84.2%) completed Time 3, 41 (71.9%) completed Time 4, and 38 (66.6%) completed Time 5. If a participant missed an appointment, the following appointment covered any diagnoses that occurred since their last completed assessment. Importantly, our use of survival analysis accommodates differing duration of follow-up by focusing both on whether and when the event (diagnosis) occurred during follow-up. Participants who did not experience an MDD recurrence during the follow-up, regardless of the duration of the follow-up for that individual were treated as right censored in the analysis (cf. Tabachnick & Fidell, 2007). For example, for participants who dropped out after Time 4 assessment, we coded the time variable as 18 months, signifying that those participants did not relapse during the participation in our study1.
Participants who completed all of the assessments did not differ significantly from those who missed one or more assessments on any of the baseline demographic, clinical, or pupil variables (lowest p = .07), except annual family income. Participants with missing data had significantly lower annual family income (35,001–40,000), compared to participants with no missing data (55,001–60,000). Participants were compensated $275 for their participation in all 5 assessments. All study procedures were approved by the Binghamton University’s Institutional Review Board.
Participants
Participants were 57 women with a past history of MDD recruited from the community. The average age of women in the study was 39.21 (SD = 6.99, Range = 24–55). The majority of participants were Caucasians (85.5%) and the rest were African America (3.6%), American Indian (1.8%), Multiracial (3.6%) or from other racial/ethnic groups (5.5%). The median annual family income was $40,001–45,000.
Measures
Lifetime histories of psychiatric disorders were assessed using the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I; First, Spitzer, Gibbon, & Williams, 2002). A subset of 20 SCID-I interviews was coded by a second interviewer and inter-rater reliability for diagnoses of MDD was excellent (κ = 1.00). Exclusion criteria included the presence of schizophrenia, a history of alcohol or substance dependence within the last six months, or lifetime history of bipolar disorder. All of the participants had a past history of one or more episodes of MDD. None of the participants had current MDD, psychotic, obsessive-compulsive, or eating disorders. Twenty one percent of the participants (n = 12) met criteria for current anxiety disorder. During the follow up, 19 women met criteria for a new MDD episode.
Participants’ current symptoms of depression and anxiety were assessed via the Beck Depression Inventory-II (BDI-II; Beck, Steer, & Brown, 1996) and the Beck Anxiety Inventory (BAI; Beck & Steer, 1993), respectively. In this sample, both measures showed excellent internal consistency (BDI-II: α = .91; BAI: α = .92).
Pupil dilation was assessed in a moderately lit room using Tobii T60 & T60XL eye-trackers2 (Falls Church, VA), while participants viewed full-color images of actors displaying various emotions (angry, happy, sad, and neutral) taken from a standardized stimulus set (Matsumoto and Ekman, 1988). The stimuli consisted of emotional and neutral photographs from each actor, morphed to form a continuum of 10% increments between the two photographs. Each emotion is represented by four continua (two male and two female actors), for a total of 12 continua. Eleven morphed images were used from each continuum, representing 10% increments of the two emotions ranging from 100% neutral (0% target emotion) to 100% target emotion (e.g., 90% neutral, 10% sad; 80% neutral, 20% sad; and so on). Each trial started with a fixation cross, presented for 500ms. The pictures were then presented, one at a time in random order, in the middle of the screen for 3 s and the participant was asked to indicate which emotion was being displayed (angry, happy, sad, and neutral) by pressing a corresponding button on a keypad. The intertrial interval randomly varied between 750 ms and 1000 ms. The stimuli were 16.51 cm high and 20.32 cm wide and 16.51 cm high and participants sat 65 cm for the screen, yielding a visual angle of 14.48° × 17.77°. Participants completed 264 trials (88 trials per emotion) with a break after 132 trials. To provide an adequate number of trials for pupillary analyses within each morph level, images were binned into three separate morph conditions for analyses: low (0%, 10%, 20%, and 30%), medium (40%, 50%, and 60%), and high (70%, 80%, 90%, and 100%). Because our previous findings from children in this study suggested that the strongest pupillary effects are observed at the highest morph level (Burkhouse et al., 2015, 2014), the present study focused solely on images at the highest morph level (29.33 trials)3. Descriptive statistics for participants’ responses to each facial emotion type at the highest morph level are shown in Table 1. Change in pupil dilation in response to angry, sad, and happy faces is depicted in Figure 1. During this task, pupil size was recorded using the eye trackers, which took measurements every 16.7 ms (60 Hz) for 3 s following the onset of each facial stimulus. Data were cleaned using Siegle et al.’s (2008) recommended procedures. Trials comprised of over 50% blinks were omitted, which resulted in the exclusion of an average of 10% of trials per participant (Range 0–41%; SD = 11%). Following standard procedures, linear interpolation was used to replace blinks throughout the data set and the data were smoothed using a 10-point weighted average filter. The total number of rejected trials and the percentage of pupil data that was replaced with linear interpolations were not significantly correlated with women’s depressive symptoms at baseline or with their likelihood of developing a depressive episode during the follow-up (lowest p = .20). The relative luminance of the images of angry faces (120.33 arbitrary units [AU]) was significantly higher than the relative luminance of the images of happy (110.15 AU) and sad (114.84 AU) faces. The relative luminance of the images in the latter two groups also differed significantly. Effects associated with a light-reflex that were independent of stimulus type were removed by subtracting the mean waveform across all three valences from the average waveform for each valence (Franzen et al., 2009). The average pupil diameter over the 333 ms preceding the onset of the stimulus was subtracted from pupil diameter after stimulus onset to produce stimulus-related pupil dilation. Peak stimulus-related pupil dilation was calculated by taking the maximum pupil response on average across all trials for each valence (angry, happy, and sad). No outliers were identified using boxplots for peak pupil dilation to angry, happy, or sad faces. Peak pupil dilation to angry faces was correlated with peak pupil dilation to sad, r = .26, p = .05 and happy faces, r = .31, p = .02, with no correlation between peak pupil dilation to happy and sad faces, r = .04, p = .76.
Table 1.
Means (Standard Deviations) for the Emotional Faces Paradigm
| Components | Angry faces | Happy faces | Sad faces |
|---|---|---|---|
| Peak pupil dilation (mm) | .025 (.018) | .025 (.019) | .046 (.031) |
| Detection accuracy | .998 (.007) | .995 (.014) | .971 (.057) |
| Response time (ms) | 1882 (703) | 1663 (792) | 2000 (663) |
Note: Means with different superscripts differ significantly.
Figure 1.
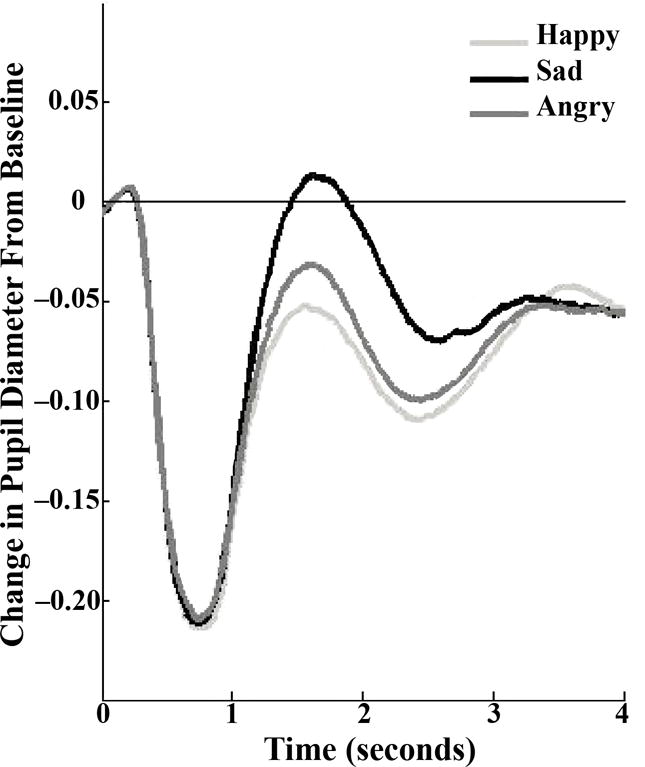
Participants’ change in pupil diameter in response to angry, happy, and sad faces.
Results
We conducted a survival analyses to assess whether peak pupil dilation to emotional stimuli assessed at baseline predicted MDD recurrence during the 2-year follow-up period. As noted earlier, we examined both linear and nonlinear effects of peak pupil dilation to emotional faces at high levels of Morph.2 Specifically, in the first step of the model, we entered the linear effect of peak pupil dilation. To examine nonlinear effects, we added a quadratic peak pupil dilation variable in the second step of the Cox regression model for survival analysis, which allowed us to determine whether this quadratic effect explained significant unique variance in MDD risk beyond that accounted for by the linear effect. Focusing first on the predictive validity of peak pupil dilation to sad faces, we found that the linear effect of peak significantly predicted women’s time to MDD recurrence, β = −1.66, Wald = 3.78, p = .05. This suggests that women who evidenced decreased peak pupil dilation to sad faces experienced MDD recurrence sooner during a two-year follow-up, compared to women who exhibited moderate or increased peak pupil dilation. Specifically, the odds of participants with low peak pupil dilation to sad faces experiencing MDD recurrence were approximately five times higher than participants with higher peak pupil dilation to sad faces. When the quadratic effect was added in the second step, it was not significant, β = −5.43, Wald = 1.88, p = .17. To visually depict the significant linear effect, we plotted the results for women in the upper and lower quartiles of pupillary reactivity to sad faces. As shown in Figure 2, women who evidenced lower, compared to higher, peak dilation to sad faces experienced a shorter time to MDD recurrence over the follow-up. To examine the robustness of these findings and the predictive validity of peak pupil dilation beyond baseline symptoms, we tested whether the effect would be maintained when we statistically controlled for the influence of baseline levels of depression and anxiety. Although these results were maintained when we statistically controlled for the impact of anxiety symptoms (BAI), β = −1.82, Wald = 4.16, p = .04, they were reduced to a nonsignificant trend when we statistically controlled for the baseline depressive symptoms (BDI-II), β = −1.50, Wald = 2.78, p = .10.
Figure 2.
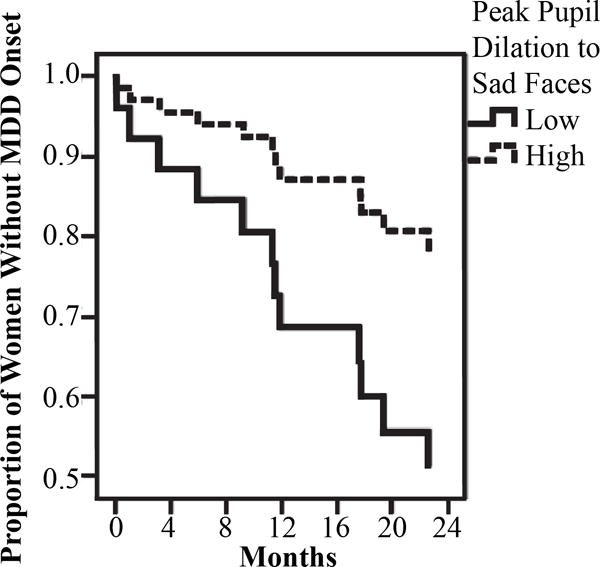
Results of the survival analysis using Cox regression model predicting prospective onset of MDD based on peak pupil dilation to sad faces.
Focusing next on the predictive validity of peak pupil dilation to angry faces, we found that the linear effect of participants’ pupil peak dilation to angry faces was not significant in the first step of the survival analysis, β = −.90, Wald = .37, p = .54. However, when the quadratic effects of peak pupil dilation was added in the second step, it was significant, β = 16.32, Wald = 8.60, p = .003. To visually depict these findings, we plotted the results for women in the upper and lower tertiles of peak pupil dilation to angry faces, as well as those at the median. As can be seen in Figure 3, women exhibiting either high or low peak pupil dilation to angry faces, compared to those exhibiting more moderate peak pupil dilation, were at greatest risk for MDD recurrence during the follow-up. This result was maintained when we statistically controlled for the impact of current depressive, β = 12.34, Wald = 9.81, p = .002, or anxiety, β = 9.22, Wald = 5.55, p = .02, symptoms suggesting that it was at least partially independent of participants’ current mood at the baseline assessment. Given the significant results observed for both the linear effect of peak pupil dilation to sad faces and the quadratic effect of peak pupil dilation to angry faces, we included both in the same survival analysis (as well as the linear effect of peak pupil dilation to angry faces) to determine if both were uniquely predictive of MDD risk. In this analysis, the quadratic effect of peak pupil dilation to angry faces remained significant, β = 15.65, Wald = 8.49, p = .004, but the linear effect of peak pupil dilation to sad faces was reduced to nonsignificant, β = −1.61, Wald = 3.30, p = .07, suggesting that peak pupil dilation to angry faces was a stronger predictor of MDD risk in this sample. In regards to participants’ peak pupil dilation to happy faces, the results showed no significant linear, β = .69, Wald = .44, p = .51, or quadratic, β = −5.74, Wald = 1.27, p = .26, effects on MDD recurrence.
Figure 3.
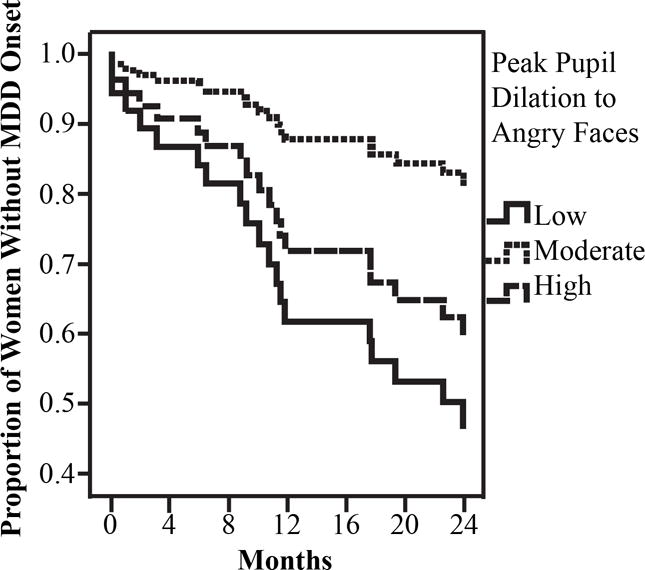
Results of the survival analysis using Cox regression model predicting prospective onset of MDD based on peak pupil dilation to angry faces.
To examine the unique contribution of peak pupil dilation to each of the three emotions, we conducted two additional survival analyses, one that included linear effects for each emotion type (to examine the robustness of our finding for pupil dilation to sad faces) and one that included both linear and quadratic effects of peak pupil dilation to all three emotions (to examine the robustness of findings for pupil dilation to angry faces). We found that, when only linear effects of peak pupil dilation were included in the survival analysis, decreased peak pupil dilation to sad faces was the only significant predictor of shorter time to MDD recurrence (β = −1.74, Wald = 3.71, p = .05), but not peak pupil dilation to angry (β = −.72, Wald = .21, p = .65) or happy (β = 1.24, Wald = 1.33, p = .25) faces. However, when quadratic effects of peak pupil dilation to all three emotions were included in the second analysis, the quadratic effect of peak pupil dilation to angry faces was the only significant predictor of time to depression recurrence (β = 15.31, Wald = 7.14, p = .008), but not the quadratic effects of peak pupil dilation to happy (β = −8.85, Wald = 2.26, p = .13) or sad (β = −5.13, Wald = 1.58, p = .21) faces. We should also note that, once the quadratic effects were added to the model, the significant linear effect of peak pupil dilation to sad faces was reduced to nonsignificant (β = −1.30, Wald = 1.12, p = .29). To provide a more practical example of the predictive validity of women’s peak pupil dilation to angry faces, a total of 47.4% of women who evidenced low peak pupil dilation to angry faces (lowest tertile) and 36.8% of women who evidenced high peak pupil dilation to angry faces (highest tertile) experienced MDD recurrence during the 2-year follow-up, compared to 15.8% of women who evidenced moderate peak pupil dilation to angry faces (middle tertile).
Discussion
In this study, we examined whether pupillary reactivity to negative stimuli could be used to determine which women with a prior history of MDD are at greatest risk for recurrence. Building from evidence that MDD and MDD risk may be associated with both hyper and hypo reactivity to negative stimuli, we examined both linear and nonlinear effects of peak pupil dilation. We found that, among women with a history of prior MDD, risk of MDD recurrence was greatest among those who exhibited either hyper or hypo peak pupillary reactivity angry facial stimuli, compared to those who exhibited a more moderate peak pupillary response during a two-year follow up. These findings were maintained when we statistically controlled for the influence of depressive and anxiety symptom levels at baseline, suggesting that peak pupil dilation to angry faces contributes unique risk for MDD recurrence beyond current symptom elevations. It was also maintained when we statistically controlled for the influence of peak pupil dilation to sad faces, suggesting that it is uniquely predictive of MDD risk. Although we also found that decreased peak pupil dilation to sad stimuli prospectively predicted MDD recurrence, this finding appeared to be less robust. Specifically, although the findings were maintained when we statistically controlled for baseline anxiety symptoms, it was reduced to a nonsignificant trend when we statistically controlled for baseline depressive symptoms and when we controlled for peak pupil dilation to angry faces. Future research is needed, therefore, to determine the role of pupillary reactivity to sad faces in (women’s) risk for MDD recurrence. Finally, consistent with previous research (Burkhouse et al., 2015) and our prediction, peak pupil dilation in response to viewing happy faces was not a significant predictor of depression onset.
The current findings extend previous research in several important ways. First, the findings are consistent with research showing that depression is associated with dysregulated physiological reactivity to negative stimuli (e.g., Mayberg, 2003). Although the precise neural mechanisms underlying these effects cannot be determined because the pupil is enervated by the connections from multiple brain regions (Critchley et al., 2005; Siegle et al., 2011; Urry et al., 2006), they are consistent with the a priori hypothesis that both hypo and hyper reactivity in DLPFC, VMPFC, ACC, and the LC-NA system would be associated with depression. Specifically, findings from neuroimaging studies reported that both hypo (Fales et al., 2009; Ritchey et al., 2011; Siegle et al., 2007) and hyper (Bermpohl et al., 2009; Elliott et al., 2002; Grimm et al., 2009) activity in the DLPFC was linked to MDD. Findings from the studies that examined activity in VMPFC were also similar and suggest that MDD association with both hyper (Keedwell et al., 2005) and hypo (Guo et al., 2015) activity during emotive processing. Similarly, both hypo (Davidson et al., 2003; Kumari et al., 2003) and hyper (Gotlib et al., 2005; Tao et al., 2012) activity in the ACC was associated with MDD. Additionally, previous studies have suggested that both hypo and hyper LC-NA activity was associated with depression (Ressler and Nemeroff, 2000). Relying on previous research that employed pupil dilation as a peripheral marker of LC-NA system functioning (Gilzenrat et al., 2012; Murphy et al., 2014), the current report provides initial support that, to the extend that pupil dilation reflects LC-NA functioning and activation of DLPFC, VMPFC, and ACC, both hypo- and hyper-activity in response to angry facial stimuli prospectively predict risk for MDD relapse among at-risk individuals.
The current findings also suggest that pupillary reactivity to negative stimuli (perhaps specifically angry faces) may be a promising biomarker of risk for MDD recurrence among women with a history of MDD. Specifically, in the current study, peak pupil dilation in response to angry faces was a more robust predictor of depression onset than peak pupil dilation to sad faces. Most studies that found differences in pupillary reactivity in individuals with and without depression employed negative words without assigning them to sad or angry categories (e.g. Steidtmann, Ingram, & Siegle, 2010). Studies that used images of faces found that pupillary reactivity specifically to sad faces predicted depressive symptoms and onset among at risk children (Burkhouse et al., 2015). However, resent research on attention biases among individuals with remitted depression found evidence for selective attention specific to angry faces (Sears et al., 2011; Woody et al., 2015). Thus, together with previous research, our findings suggest that, among individual with a history of MDD, physiological reactivity to stimuli that evoke feelings of guilt or indicate interpersonal rejection (e.g. angry faces) may be a better prediction of recurrence than physiological reactivity to self-referential stimuli (e.g. sad faces). If replicated, these findings could aid clinicians in identifying which individuals with a prior MDD history are at a greater risk of recurrence using an inexpensive and convenient methodology. This could help focus the limited clinical resources and direct aid to the population who are at the highest risk of MDD recurrence.
The present study had several strengths, including the longitudinal design and focus on a readily accessible and easily measurable physiological marker (Siegle et al., 2003b). Although firm conclusions must await replication, the current findings provide promising initial evidence that peak to negative stimuli could serve as a biological marker of depression risk in adult women with a history of MDD and may help identify which of those individuals are at greatest risk for MDD recurrence. Despite these strengths, the current study has a number of limitations, which could provide direction for future research. First, the current study focused only on risk for MDD relapse and did not examine whether pupillary reactivity may also predict first onsets. Thus, future studies are needed to determine whether peak pupil dilation to negative stimuli may be a biomarker of risk for both recurrence and first onsets of MDD. Second, only 19 women experienced MDD recurrence during follow-up in our sample and the findings need to be replicated by future studies using larger samples. Third, the current sample was primarily Caucasian, middle-class women, and future research is needed to determine whether the results will generalize to other populations. Additionally, the images were not equated on luminance and there were significant differences in relative luminance based on emotion category. However, the effects associated with light-reflex were removed prior to analyses. Moreover, the main research question of this study was focused on individual differences in peak pupil dilation to images within each emotion category. Lastly, given that pupil is enervated by connections from multiple brain areas, we are unable to clarify a specific brain mechanism underlying the findings. Additionally, it remains unclear whether the same brain areas are involved in the association between both hypo and hyper peak pupil reactivity and MDD recurrence or whether different brain areas underlie the link between hypo versus hypo peak pupil reactivity to angry faces and MDD recurrence.
In summary, the current findings extend previous research and suggest that pupillary hypo- or hyper-reactivity to angry faces stimuli may be a promising biomarker of risk for MDD recurrence in at-risk populations. This is the first study that has examined whether disrupted physiological reactivity to negative stimuli prospectively predicted depression recurrence among women with a prior history of MDD. If replicated, these findings suggest that pupillary reactivity could be used as a biomarker to determine which at risk individuals are at greatest risk for recurrence so that limited intervention resources could be targeted to those most at need.
We examined whether peak pupil dilation predicted depression recurrence in women
We show that hypo and hyper reactivity to angry faces predicted risk for recurrence
Findings support pupillometry as a way to predict depression risk in at-risk women
Acknowledgments
This project was supported by National Institute of Child Health and Human Development grant HD057066 and National Institute of Mental Health grant MH098060 awarded to B.E. Gibb. We would like to thank Ashley Johnson, Lindsey Stone, Andrea Hanley, Sydney Meadows, Michael Van Wie Ariel Ravid, Devra Alper, Cope Feurer, Eric Funk, and Effua Sosoo for their help in conducting assessments for this project.
Footnotes
There were no significant differences in pupil findings across the two eye trackers.
The findings of the study were maintained when we only focused on participants who completed Time 5 assessment.
There were no significant linear or quadratic effects of peak pupil dilation to angry, happy, or sad faces at low and medium morph levels (lowest p >.12).
Conflict of Interest
The authors declare no conflict of interest.
References
- Admon R, Holsen LM, Aizley H, Remington A, Whitfield-Gabrieli S, Goldstein JM, Pizzagalli Da. Striatal Hypersensitivity During Stress in Remitted Individuals with Recurrent Depression. Biol Psychiatry. 2014:67–76. doi: 10.1016/j.biopsych.2014.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Rajkowski J, Cohen J. Role of locus coeruleus in attention and behavioral flexibility. Biol Psychiatry. 1999;46:1309–1320. doi: 10.1016/S0006-3223(99)00140-7. [DOI] [PubMed] [Google Scholar]
- Beck A, Steer R. Beck Anxiety Inventory Manual. Psychological Corporation; San Antonio, TX: 1993. [Google Scholar]
- Beck A, Steer R, Brown G. Manual for the Beck Depression Inventory-II. Psychological Corporation; San Antonio, TX: 1996. [Google Scholar]
- Bermpohl F, Walter M, Sajonz B, Lücke C, Hägele C, Sterzer P, Adli M, Heinz A, Northoff G. Attentional modulation of emotional stimulus processing in patients with major depression–alterations in prefrontal cortical regions. Neurosci Lett. 2009;463:108–13. doi: 10.1016/j.neulet.2009.07.061. [DOI] [PubMed] [Google Scholar]
- Bistricky SL, Ingram RE, Siegle GJ, Short M. Parental Depression Risk and Reduced Physiological Responses During a Valence Identification Task. Cognit Ther Res. 2014;39:318–331. doi: 10.1007/s10608-014-9660-6. [DOI] [Google Scholar]
- Bulloch A, Williams J, Lavorato D, Patten S. Recurrence of major depressive episodes is strongly dependent on the number of previous episodes. Depress Anxiety. 2014;31:72–76. doi: 10.1002/da.22173. [DOI] [PubMed] [Google Scholar]
- Burcusa S, Lacono W. Risk for Recurrence in Depression. Clin Psychol Rev. 2007;27:959–985. doi: 10.1016/j.cpr.2007.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhouse KL, Siegle GJ, Gibb BE. Pupillary reactivity to emotional stimuli in children of depressed and anxious mothers. J Child Psychol Psychiatry Allied Discip. 2014;55:1009–1016. doi: 10.1111/jcpp.12225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhouse KL, Siegle GJ, Woody ML, Kudinova AY, Gibb BE. Pupillary Reactivity to Sad Stimuli as a Biomarker of Depression Risk : Evidence From a Prospective Study of Children. J Abnorm Psychol. 2015;124:498–506. doi: 10.1037/abn0000072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bylsma LM, Morris BH, Rottenberg J. A meta-analysis of emotional reactivity in major depressive disorder. Clin Psychol Rev. 2008;28:676–91. doi: 10.1016/j.cpr.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Cohen LH, Gunthert KC, Butler AC, O’Neill SC, Tolpin LH. Daily affective reactivity as a prospective predictor of depressive symptoms. J Pers. 2005;73:1687–713. doi: 10.1111/j.0022-3506.2005.00363.x. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Tang J, Glaser D, Butterworth B, Dolan RJ. Anterior cingulate activity during error and autonomic response. Neuroimage. 2005;27:885–895. doi: 10.1016/j.neuroimage.2005.05.047. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Irwin W, Anderle MJ, Kalin NH. The neural substrates of affective processing in depressed patients treated with venlafaxine. Am J Psychiatry. 2003;160:64–75. doi: 10.1176/appi.ajp.160.1.64. [DOI] [PubMed] [Google Scholar]
- Elliott R, Rubinsztein JS, Sahakian BJ, Dolan RJ. The neural basis of mood-congruent processing biases in depression. Arch Gen Psychiatry. 2002;59:597–604. doi: 10.1001/archpsyc.59.7.597. [DOI] [PubMed] [Google Scholar]
- Fales CL, Barch DM, Rundle MM, Mintun MA, Mathews J, Snyder AZ, Sheline YI. Antidepressant treatment normalizes hypoactivity in dorsolateral prefrontal cortex during emotional interference processing in major depression. J Affect Disord. 2009;112:206–11. doi: 10.1016/j.jad.2008.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. Biometrics Research. New York State Psychiatric Institute; New York: 2002. Structured clinical interview for DSM-IV-TR axis I disorders, research version, non-patient edition (SCID-I/NP) [Google Scholar]
- Franzen PL, Buysse DJ, Dahl RE, Thompson W, Siegle GJ. Sleep deprivation alters pupillary reactivity to emotional stimuli in healthy young adults. Biol Psychol. 2009;80:300–5. doi: 10.1016/j.biopsycho.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilzenrat MS, Nieuwenhuis S, Cohen JD. NIH Public Access. 2012;10:252–269. doi: 10.3758/CABN.10.2.252.Pupil. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlib IH, Sivers H, Gabrieli JDE, Whitfield-Gabrieli S, Goldin P, Minor KL, Canli T. Subgenual anterior cingulate activation to valenced emotional stimuli in major depression. Neuroreport. 2005;16:1731–4. doi: 10.1097/01.wnr.0000183901.70030.82. [DOI] [PubMed] [Google Scholar]
- Grimm S, Boesiger P, Beck J, Schuepbach D, Bermpohl F, Walter M, Ernst J, Hell D, Boeker H, Northoff G. Altered negative BOLD responses in the default-mode network during emotion processing in depressed subjects. Neuropsychopharmacology. 2009;34:932–43. doi: 10.1038/npp.2008.81. [DOI] [PubMed] [Google Scholar]
- Guo CC, Nguyen VT, Hyett MP, Parker GB, Breakspear MJ. Out-of-sync: disrupted neural activity in emotional circuitry during film viewing in melancholic depression. Sci Rep. 2015;5:11605. doi: 10.1038/srep11605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans EJ, Henckens MJAG, Roelofs K, Fernández G. Fear bradycardia and activation of the human periaqueductal grey. Neuroimage. 2013;66:278–287. doi: 10.1016/j.neuroimage.2012.10.063. [DOI] [PubMed] [Google Scholar]
- Hess EH, Polt JM. Pupil size as related to interest value of visual stimuli. Science (80-) 1960;132:349–350. doi: 10.1126/science.132.3423.349. [DOI] [PubMed] [Google Scholar]
- Holsen LM, Lancaster K, Klibanski a, Whitfield-Gabrieli S, Cherkerzian S, Buka S, Goldstein JM. HPA-axis hormone modulation of stress response circuitry activity in women with remitted major depression. Neuroscience. 2013;250:732–742. doi: 10.1016/j.neuroscience.2013.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaymaz N, van Os J, Loonen AJM, Nolen W. Evidence That Patients With Single Versus Recurrent Depressive Episodes Are Differentially Sensitive to Treatment Discontinuation: A Meta-Analysis of Placebo-Controlled Randomized Trials. J Clin Psychiatry. 2008;69:1424–1437. doi: 10.4088/jcp.v69n0910. [DOI] [PubMed] [Google Scholar]
- Keedwell PA, Andrew C, Williams SCR, Brammer MJ, Phillips ML. The neural correlates of anhedonia in major depressive disorder. Biol Psychiatry. 2005;58:843–53. doi: 10.1016/j.biopsych.2005.05.019. [DOI] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Swartz M, Blazer DG, Nelson CB. Sex and depression in the National Comorbidity Survey. I: Lifetime prevalence, chronicity and recurrence. J Affect Disord. 1993;29:85–96. doi: 10.1016/0165-0327(93)90026-g. [DOI] [PubMed] [Google Scholar]
- Kovacs M, Rottenberg J, George C. Maladaptive mood repair responses distinguish young adults with early-onset depressive disorders and predict future depression outcomes. Psychol Med. 2009;39:1841–54. doi: 10.1017/S0033291709005789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa A, Hajcak G, Torpey D, Kim J, Klein DN. Electrocortical reactivity to emotional faces in young children and associations with maternal and paternal depression. J Child Psychol Psychiatry. 2012;53:207–15. doi: 10.1111/j.1469-7610.2011.02461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari V, Mitterschiffthaler MT, Teasdale JD, Malhi GS, Brown RG, Giampietro V, Brammer MJ, Poon L, Simmons A, Williams SCR, Checkley SA, Sharma T. Neural abnormalities during cognitive generation of affect in treatment-resistant depression. Biol Psychiatry. 2003;54:777–91. doi: 10.1016/s0006-3223(02)01785-7. [DOI] [PubMed] [Google Scholar]
- Lethbridge R, Allen NB. Mood induced cognitive and emotional reactivity, life stress, and the prediction of depressive relapse. Behav Res Ther. 2008;46:1142–50. doi: 10.1016/j.brat.2008.06.011. [DOI] [PubMed] [Google Scholar]
- Levinson DF, Zubenko GS, Crowe RR, DePaulo RJ, Scheftner WS, Weissman MM, Holmans P, Zubenko WN, Boutelle S, Murphy-Eberenz K, MacKinnon D, McInnis MG, Marta DH, Adams P, Sassoon S, Knowles Ja, Thomas J, Chellis J. Genetics of recurrent early-onset depression (GenRED): design and preliminary clinical characteristics of a repository sample for genetic linkage studies. Am J Med Genet B Neuropsychiatr Genet. 2003;119B:118–130. doi: 10.1002/ajmg.b.20009. [DOI] [PubMed] [Google Scholar]
- Lewinsohn PM, Allen NB, Seeley JR, Gotlib IH. First onset versus recurrence of depression: differential processes of psychosocial risk. J Abnorm Psychol. 1999;108:483–9. doi: 10.1037//0021-843x.108.3.483. [DOI] [PubMed] [Google Scholar]
- Matsumoto D, Ekman P. Japanese and Caucasian Facial Expressions of Emotion (JACFEE) San Francisco State University; San Francisco, CA: 1988. [Google Scholar]
- Mayberg HS. Modulating dysfunctional limbic-cortical circuits in depression: Towards development of brain-based algorithms for diagnosis and optimised treatment. Br Med Bull. 2003;65:193–207. doi: 10.1093/bmb/65.1.193. [DOI] [PubMed] [Google Scholar]
- Murphy PR, O’Connell RG, O’Sullivan M, Robertson IH, Balsters JH. Pupil diameter covaries with BOLD activity in human locus coeruleus. Hum Brain Mapp. 2014;35:4140–4154. doi: 10.1002/hbm.22466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy PR, Robertson IH, Balsters JH, O’connell RG. Pupillometry and P3 index the locus coeruleus-noradrenergic arousal function in humans. Psychophysiology. 2011;48:1532–1543. doi: 10.1111/j.1469-8986.2011.01226.x. [DOI] [PubMed] [Google Scholar]
- Pavcovich LA, Ramirez OA. Effects of chronic desipramine administration on the locus coeruleus neuronal activity in the learned helplessness paradigm. Brain Res Bull. 1993;32:83–86. doi: 10.1016/0361-9230(93)90059-K. [DOI] [PubMed] [Google Scholar]
- Pine D, Cohen P, Brook J. Emotional reactivity and risk for psychopathology among adolescents. PubMed – NCBI CNS Spectr. 2001;6:27–35. doi: 10.1017/s1092852900022860. [DOI] [PubMed] [Google Scholar]
- Price RB, Rosen D, Siegle GJ, Ladouceur CD, Tang K, Allen KB, Ryan ND, Dahl RE, Forbes EE, Silk JS. From Anxious Youth to Depressed Adolescents: Prospective Prediction of 2-Year Depression Symptoms via Attentional Bias Measures. J Abnorm Psychol. 2015 doi: 10.1037/abn0000127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ressler KJ, Nemeroff CB. Role of serotonergic and noradrenergic systems in thepathophysiology of depression and anxiety disorders. Depress Anxiety. 2000;12:2–19. doi: 10.1002/1520-6394(2000)12. [DOI] [PubMed] [Google Scholar]
- Ritchey M, Dolcos F, Eddington KM, Strauman TJ, Cabeza R. Neural correlates of emotional processing in depression: Changes with cognitive behavioral therapy and predictors of treatment response. J Psychiatr Res. 2011;45:577–587. doi: 10.1016/j.jpsychires.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sears CR, Newman KR, Ference JD, Thomas CL. Attention to emotional images in previously depressed individuals: An eye-tracking study. Cognit Ther Res. 2011;35:517–528. doi: 10.1007/s10608-011-9396-5. [DOI] [Google Scholar]
- Siegle GJ, Granholm E, Ingram RE, Matt GE. Pupillary and reaction time measures of sustained processing of negative information in depression. Biol Psychiatry. 2001;49:624–36. doi: 10.1016/s0006-3223(00)01024-6. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Ichikawa N, Steinhauer S. Blink before and after you think: blinks occur prior to and following cognitive load indexed by pupillary responses. Psychophysiology. 2008;45:679–687. doi: 10.1111/j.1469-8986.2008.00681.x. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Steinhauer SR, Carter CS, Ramel W, Thase ME. Do the Seconds Turn Into Hours ? Relationships Between Sustained Pupil Dilation in Response to Emotional Information and Self-Reported Rumination. Cognit Ther Res. 2003a;27:365–382. [Google Scholar]
- Siegle GJ, Steinhauer SR, Friedman ES, Thompson WS, Thase ME. Remission prognosis for cognitive therapy for recurrent depression using the pupil: Utility and neural correlates. Biol Psychiatry. 2011;69:726–733. doi: 10.1016/j.biopsych.2010.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegle GJ, Steinhauer SR, Friedman ES, Thompson WS, Thase ME. Remission prognosis for cognitive therapy for recurrent depression using the pupil: utility and neural correlates. Biol Psychiatry. 2011;69:726–33. doi: 10.1016/j.biopsych.2010.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegle GJ, Steinhauer SR, Stenger VA, Konecky R, Carter CS. Use of concurrent pupil dilation assessment to inform interpretation and analysis of fMRI data. 2003b;20:114–124. doi: 10.1016/S1053-8119(03)00298-2. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Thompson W, Carter CS, Steinhauer SR, Thase ME. Increased amygdala and decreased dorsolateral prefrontal BOLD responses in unipolar depression: related and independent features. Biol Psychiatry. 2007;61:198–209. doi: 10.1016/j.biopsych.2006.05.048. [DOI] [PubMed] [Google Scholar]
- Solomon DA, Keller MB, Leon AC, Mueller TI, Lavori PW, Shea MT, Coryell W, Warshaw M, Turvey C, Maser JD, Endicott J. Multiple recurrences of major depressive disorder. Am J Psychiatry. 2000;157:229–233. doi: 10.1176/appi.ajp.157.2.229. [DOI] [PubMed] [Google Scholar]
- Steidtmann D, Ingram RE, Siegle GJ. Pupil response to negative emotional information in individuals at risk for depression. Cogn Emot. 2010;24:480–496. doi: 10.1080/02699930902738897. [DOI] [Google Scholar]
- Tabachnick BG, Fidell LS. Using multivariate statistics. 5th. New York, NY: Pearson; 2007. [Google Scholar]
- Tao R, Calley CS, Hart J, Mayes TL, Nakonezny PA, Lu H, Kennard BD, Tamminga CA, Emslie GJ. Brain activity in adolescent major depressive disorder before and after fluoxetine treatment. Am J Psychiatry. 2012;169:381–8. doi: 10.1176/appi.ajp.2011.11040615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urry HL, van Reekum CM, Johnstone T, Kalin NH, Thurow ME, Schaefer HS, Jackson Ca, Frye CJ, Greischar LL, Alexander AL, Davidson RJ. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. J Neurosci. 2006;26:4415–4425. doi: 10.1523/JNEUROSCI.3215-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. Depression. 2012 [Google Scholar]
- Williams LM, Korgaonkar MS, Song YC, Paton R, Eagles S, Goldstein-Piekarski A, Grieve SM, Harris AWF, Usherwood T, Etkin A. Amygdala Reactivity to Emotional Faces in the Prediction of General and Medication-Specific Responses to Antidepressant Treatment in the Randomized iSPOT-D Trial. Neuropsychopharmacology. 2015;40:2398–2408. doi: 10.1038/npp.2015.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woody ML, Owens M, Burkhouse KL, Gibb BE. Selective Attention Toward Angry Faces and Risk for Major Depressive Disorder in Women: Converging Evidence From Retrospective and Prospective Analyses. Clin Psychol Sci. 2015 doi: 10.1177/2167702615581580. [DOI] [PMC free article] [PubMed] [Google Scholar]


