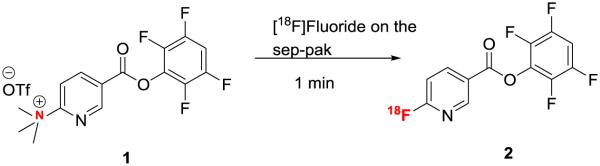
Abstract
Fluorine-18 labeled fluoronicotinic acid-2,3,5,6-tetrafluorophenyl ester has been successfully synthesized in an unprecedented way by flowing an acetonitrile solution of its quaternary ammonium salt precursor (N,N,N-trimethyl-5-((2,3,5,6-tetrafluorophenoxy)carbonyl)pyridin-2-aminium trifluoromethanesulfonate, 1) through an anion exchange cartridge. The fluorination reaction proceeded at room temperature without azeotropic drying of the fluoride. Over 75% conversion was observed with 10 mg of precursor in 2:8, acetonitrile: t-butanol in 1 min. The total synthesis time was 5 min which is ~30 min shorter than the current literature method.
1. Introduction
Positron emission tomography (PET) is one of the most powerful clinically established noninvasive imaging modalities, which provides not only information on biochemical, physiological and pharmacological processes but also offers the opportunity to study pharmacokinetics, metabolism, and mechanisms of action of novel and established drugs. Among the available PET radionuclides, fluorine-18 is favored for in vivo imaging for small molecules as it offers most suitable nuclear and chemical properties and its minimal perturbation to drug structure when substituted on to low molecular weight drugs.[1-5]
Fluorine-18 substitution can be done by electrophilic fluorination with 18F2 or by nucleophilic fluorination with [18F]fluoride. For electrophilic fluorination 18F2 is produced along with non-radioactive fluorine gas as a carrier,[6, 7] so radiopharmaceuticals prepared using 18F2 have low specific activities,[8, 9] while only half of the activity of 18F2 can be electrophilically substituted. The most useful route to obtain 18F-labeled compounds of high specific activity is through nucleophilic fluorination by no-carrier-added [18F]fluoride.[10-19] The first step of the nucleophilic fluorination is to pass the fluorine-18 containing target water through an anion exchange resin to trap the activity as [18F]fluoride. The activity can be eluted as [18F]-salt from the anion exchange resin with a base solution. Depending on the type of base used the eluted [18F]fluoride salt could be [18F]KF, [18F]CsF or [18F]TBAF.[2, 13] The next step is to dry the activity with acetonitrile (1 mL × 3, azeotropic drying). This azeotropic drying takes 15-20 min with some loss of activity due to normal decay and evaporation. Dried [18F]-salt and base mixture is then heated with the precursor at elevated temperature (40-180 °C) in an organic solvent medium to obtained fluorine-18 labeled tracers. Many precursors cannot withstand the temperature in highly basic medium. This multistep and harsh fluorine-18 labeling procedure restricts the access to the many useful fluorine-18 labeled PET imaging agents. Various modifications have been made to improve these standard protocols such as the use of ionic liquid media [20], the effect of additives [21, 22], green fluorination [23], titania-catalyzed radiofluorination [24], minimalist approach [25] or fluorination of diaryliodonium tosylates under aqueous-organic conditions [26] but these modifications have not been widely accepted by the PET community. Therefore, there is a clear need of the development of quicker and milder nucleophilic fluorination method for the extended use of fluorine-18 PET tracers in nuclear medicine.
Fluorine-18 radiolabeled fluoronicotinic acid-2,3,5,6-tetrafluorophenyl ester (2) is very useful synthon to radiolabel temperature sensitive biomolecules. This was first reported by Olberg et al.[27] Since then it has been used by our and other group to radiolabel protein and peptides.[28, 29] However, the precursor was not stable in K222/K2CO3. This issue was overcame by using less basic TBA-HCO3 but due to the limited amount of base used in this radiolabeling there is a significant amount of loss of radioactivity. While searching for a better procedure we have discovered an unprecedented fluorine-18 labeling technique to this precursor. In our surprise, we found out that [18F]fluoride activity from the sep-pak can be eluted by the quaternary ammonium triflate precursor (1), and the eluted compound is the fluorine-18 labeled product 2. Nucleophilic fluoride substitution happened inside the Sep-Pak instantly at room temperature. Herein we report the unique fluorine-18 labeling procedure to develop this useful fluorine-18 labeled prosthetic group.
2. Materials and Methods
Tetrabutylammonium hydrogen carbonate (0.075 M) used for radiochemical work was purchased from ABX (Radeberg, Germany). All other chemicals and solvents were received from Sigma Aldrich (St. Louis, MO, USA) and used without further purification. The precursor N,N,N-trimethyl-5-((2,3,5,6-tetrafluorophenoxy)carbonyl)pyridin-2-aminium fluoromethanesulfonate (1) and cold standard fluoronicotinic acid-2,3,5,6-tetrafluorophenyl ester were prepared according to a previously described method.[27, 28] Fluorine-18 was purchased from National Institutes of Health cyclotron facility (Bethesda, MD, USA). Chromafix 30-PS-HCO3 anion-exchange cartridge was purchased from Macherey-Nagel (Düren, Germany). Columns and all other the Sep-Pak cartridges used in this synthesis were obtained from Agilent Technologies (Santa Clara, CA, USA) and Waters (Milford, MA, USA), respectively. Oasis MCX Plus cartridge was conditioned by passing 5 mL ethanol, 10 mL air and 10 mL water. Analytical HPLC analyses for radiochemical work were performed on an Agilent 1200 Series instrument equipped with multi-wavelength detectors using an Agilent Eclipse plus C18 column (4.6 × 150 mm, 3.5 µm). Mobile phase: 20 - 80% acetonitrile (0.1% TFA) in water (0.1% TFA) in 12 min with a flow rate of 1.0 mL/min.
2.1. Radiosynthesis of [18F]fluoronicotinic acid-2,3,5,6-tetrafluorophenyl ester (2)
Fluorine-18 labeled target water was diluted with 2 mL water (10-25 mCi) and passed through an anion-exchange cartridge (Chromafix 30-PS-HCO3). Cartridge was washed with anhydrous acetonitrile and dried for 1 min. The [18F]fluoride from the sep-pak was eluted with its quaternary ammonium triflate precursor in 1 mL acetonitrile as fluorine-18 radiolabeled fluoronicotinic acid-2,3,5,6-tetrafluorophenyl ester. To the solution was added 5 mL water and passed through a preconditioned Oasis MCX Plus cartridge and the cartridge washed with 5 mL of water. The trapped [18F]fluoronicotinic acid-2,3,5,6-tetrafluorophenyl ester was eluted from the Sep-Pak cartridge with 2 mL 65% acetonitrile.
3. Results and Discussion
Although there are a few reports of direct fluorine-18 labeling of peptide,[30-33] fluorine-18 labeling of proteins are mostly done by an indirect approach using different fluorine-18 labeled small molecules.[34, 35] Therefore it is important to have a convenient synthetic method to prepare a labeled synthon in high yield in a short time. Fluorine-18 radiolabeled fluoronicotinic acid-2,3,5,6-tetrafluorophenyl ester (2) is one of the most useful synthons to radiolabel protein and peptide. It was prepared in one step without time consuming HPLC purification.
Precursor and cold standard were synthesized by literature method.[27, 28] Fluorine-18 labeling was achieved on the sep-pak (scheme 1). Specifically, fluorine-18 containing target water from cyclotron was passed through an anion exchange cartridge (PS-HCO3) and washed with 3 mL anhydrous acetonitrile. Over 70% activity was incorporated in to the product by passing 10 mg of quaternary ammonium triflate precursor (1) in 1 ml acetonitrile through the sep-pak in 1 min. Fluoride incorporation efficiency was tested using different conditions (Table 1). Better elution of the product was observed with mixture of solvents (2:8 acetonitrile, t-butanol). Slight improvement of yield was observed with increase in precursor amount (15 mg). No significant improvement of yield was observed with further dilution of the precursor (2 mL).
Scheme 1.
Synthesis of [18F]fluoronicotinic acid-2,3,5,6-tetrafluorophenyl ester (2)
Table 1.
Elution conditions from the Sep-Pak to prepare [18F]2
| Amount of precursor 1
(mg) |
Solvent (1 mL) |
Eluted from the Sep-Pak (%)a |
|---|---|---|
| Acetonitrile | 75 ± 3b | |
| 15 | 2:8, acetonitrile : t-butanol | 83 ± 2b |
| DMSO | 47 | |
|
| ||
| 10c | 2:8, acetonitrile : t-butanol | 67 |
|
| ||
| Acetonitrile | 72 ± 1b | |
| 10 | 2:8, acetonitrile : t-butanol | 78 ± 3b |
| DMSO | 34 | |
|
| ||
| Acetonitrile | 59 | |
| 5 | 2:8, acetonitrile : t-butanol | 57 |
| DMSO | 24 | |
|
| ||
| 3 | Acetonitrile | 30 ± 2b |
Radiolabeling was carried out with 10-20 mCi of fluorine-18
n = 3
Literature method
In this new method, fluorine-18 labeling was achieved without azeotropic drying of [18F]fluoride. It saved 15-20 min in comparison to conventional nucleophilic radiolabeling method. Therefore, the loss of activity due to evaporation and normal decay is negligible. Moreover, as no base is used and fluorination is happening at the room temperature, the stability of the precursor in basic medium and/or at high temperature will not be an issue.
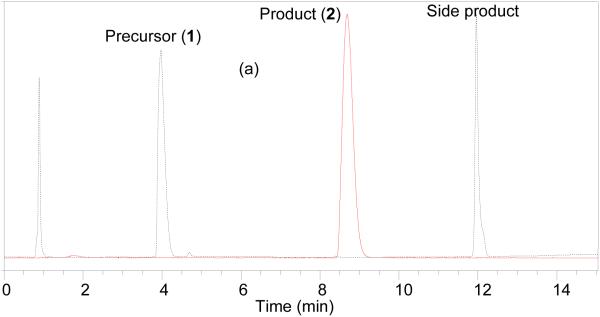
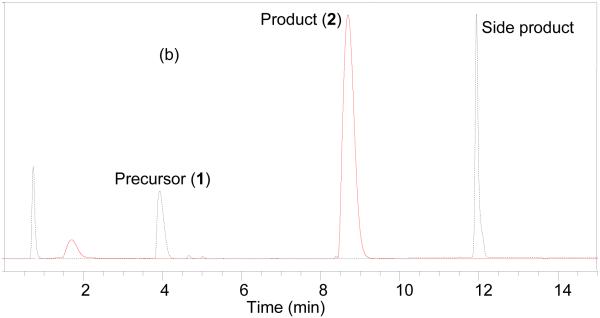
HPLC chromatogram of the crude product (Figure 1a) prepared using Sep-Pak reaction technique was almost identical with that of compound 2 prepared following the literature method (Figure 1b). The peak at ~4 min is for the precursor and ~12 min is the side product bis(2,3,5,6-tetrafluorophenyl) pyridine-2,5-dicarboxylate.[27] The quantification of the side product was not performed but from relative HPLC integration ratio of the precursor to side product it is obvious that side product is less for the current method compared to the literature method (1:0.6 vs. 1:2).
Figure 1.
HPLC analysis of the crude reaction mixture of a) 2 prepared by sep-pak method; b) 2 prepared following the literature method. Solid line, in-line radiodetector; dotted line, UV detector at 254 nm.
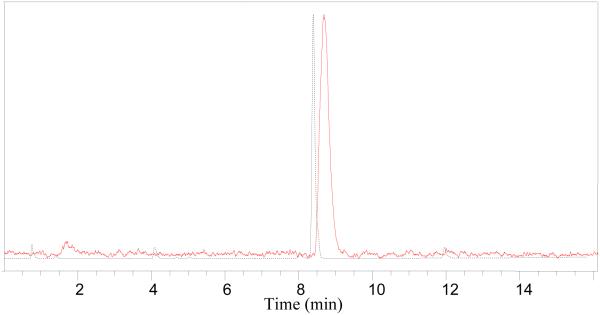
The overall radiochemical yield was 72 ± 3% (uncorrected, n = 3) in a 5 min synthesis time with a radiochemical purity of >98% by analytical HPLC. The identity of the product (2) was confirmed by comparing its HPLC retention time with co-injected, authentic nonradioactive fluoronicotinic acid-2,3,5,6-tetrafluorophenyl ester (Figure 2, supporting information). The formation of the product (2) was further confirmed by conjugation with N-(2-aminoethyl)maleimide to form a known compound [18F]N-(2-(2,5-dioxocyclopent-3-en-1-yl)ethyl)-6-fluoronicotinamide [36] (supporting information).
Figure 2.
HPLC analysis of 2, co-injected with the non-radioactive standard. Solid line, in-line radiodetector; dotted line, UV detector at 254 nm.
4. Conclusion
We have successfully developed the highly reproducible synthetic strategy for [18F]fluoronicotinic acid-2,3,5,6-tetrafluorophenyl ester (2) with in very short synthesis time (5 min). In a view of the importance of compound 2 as prosthetic group to prepare fluorine-18 labeled large biomolecules, this method is very attractive as it requires very short time with high radiochemical yields. Versatility of this method will be tested with other precursors.
Supplementary Material
Acknowledgements
This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. HHSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. This research was supported [in part] by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Le Bars D. Fluorine-18 and medical imaging: Radiopharmaceuticals for positron emission tomography. J Fluorine Chem. 2006;127:1488–93. [Google Scholar]
- 2.Cai L, Lu S, Pike VW. Chemistry with [18F]Fluoride Ion. Eur J Org Chem. 2008;2008:2853–73. [Google Scholar]
- 3.Ametamey SM, Honer M, Schubiger PA. Molecular Imaging with PET. Chem Rev. 2008;108:1501–16. doi: 10.1021/cr0782426. [DOI] [PubMed] [Google Scholar]
- 4.Papash AI, Alenitsky YG. Commercial cyclotrons. Part I: Commercial cyclotrons in the energy range 10–30 MeV for isotope production. Phys Part Nuclei. 2008;39:597–631. [Google Scholar]
- 5.Levin CS, Hoffman EJ. Calculation of positron range and its effect on the fundamental limit of positron emission tomography system spatial resolution. Phys Med Biol. 1999;44:781–99. doi: 10.1088/0031-9155/44/3/019. [DOI] [PubMed] [Google Scholar]
- 6.Bida GT, Ehrenkaufer RL, Wolf AP, Fowler JS, MacGregor RR, Ruth TJ. The Effect of Target-Gas Purity on the Chemical Form of F-18 during 18F-F2 Production Using the Neon/Fluorine Target. J Nucl Chem. 1980;21:758–62. [PubMed] [Google Scholar]
- 7.Miller PW, Long NJ, Vilar R, Gee AD. Synthesis of 11C, 18F, 15O, and 13N Radiolabels for Positron Emission Tomography. Angew Chem Int Ed. 2008;47:8998–9033. doi: 10.1002/anie.200800222. [DOI] [PubMed] [Google Scholar]
- 8.Adam MJ, Ruth TJ, Jivan S, Pate BD. Fluorination of aromatic compounds with F2 and acetyl hypofluorite: synthesis of 18F-aryl fluorides by cleavage of aryl-tin bonds [1] J Fluorine Chem. 1984;25:329–37. [Google Scholar]
- 9.Forsback S, Eskola O, Bergman J, Haaparanta M, Solin O. Alternative solvents for electrophilic synthesis of 6-[18F]fluoro-L-DOPA. J Labelled Compd Radiopharm. 2009;52:286–8. [Google Scholar]
- 10.Ryzhikov NN, Seneca N, Krasikova RN, Gomzina NA, Shchukin E, Fedorova OS, et al. Preparation of highly specific radioactivity [18F]flumazenil and its evaluation in cynomolgus monkey by positron emission tomography. Nucl Med Biol. 2005;32:109–16. doi: 10.1016/j.nucmedbio.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 11.Shen B, Ehrlichmann W, Uebele M, Machulla HJ, Reischl G. Automated synthesis of n.c.a. [18F]FDOPA via nucleophilic aromatic substitution with [18F]fluoride. Appl Radiat Isot. 2009;67:1650–3. doi: 10.1016/j.apradiso.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 12.Li Z, Conti PS. Radiopharmaceutical chemistry for positron emission tomography. Adv Drug Deliv Rev. 2010;62:1031–51. doi: 10.1016/j.addr.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 13.Jacobson O, Kiesewetter DO, Chen X. Fluorine-18 radiochemistry, labeling strategies and synthetic routes. Bioconjug Chem. 2015;26:1–18. doi: 10.1021/bc500475e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Preshlock S, Tredwell M, Gouverneur V. (18)F-Labeling of Arenes and Heteroarenes for Applications in Positron Emission Tomography. Chem Rev. 2016;116:719–66. doi: 10.1021/acs.chemrev.5b00493. [DOI] [PubMed] [Google Scholar]
- 15.Liang SH, Vasdev N. C(sp(3))-(1)(8)F bond formation by transition-metal-based [(1)(8)F]fluorination. Angew Chem Int Ed. 2014;53:11416–8. doi: 10.1002/anie.201407065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cole EL, Stewart MN, Littich R, Hoareau R, Scott PJ. Radiosyntheses using fluorine-18: the art and science of late stage fluorination. Curr Top Med Chem. 2014;14:875–900. doi: 10.2174/1568026614666140202205035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brooks AF, Topczewski JJ, Ichiishi N, Sanford MS, Scott PJ. Late-stage [18F]Fluorination: New Solutions to Old Problems. Chem Sci. 2014;5:4545–53. doi: 10.1039/C4SC02099E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Campbell MG, Ritter T. Late-Stage Fluorination: From Fundamentals to Application. Org Process Res Dev. 2014;18:474–80. doi: 10.1021/op400349g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neumann CN, Ritter T. Late-stage fluorination: fancy novelty or useful tool? Angew Chem Int Ed. 2015;54:3216–21. doi: 10.1002/anie.201410288. [DOI] [PubMed] [Google Scholar]
- 20.Kim HW, Jeong JM, Lee YS, Chi DY, Chung KH, Lee DS, et al. Rapid synthesis of [18F]FDG without an evaporation step using an ionic liquid. Appl Radiat Isot. 2004;61:1241–6. doi: 10.1016/j.apradiso.2004.02.027. [DOI] [PubMed] [Google Scholar]
- 21.Kim DW, Jeong HJ, Lim ST, Sohn MH. Tetrabutylammonium tetra(tert-butyl alcohol)-coordinated fluoride as a facile fluoride source. Angew Chem Int Ed. 2008;47:8404–6. doi: 10.1002/anie.200803150. [DOI] [PubMed] [Google Scholar]
- 22.Kim DW, Jeong HJ, Lim ST, Sohn MH, Chi DY. Facile nucleophilic fluorination by synergistic effect between polymer-supported ionic liquid catalyst and tert-alcohol reaction media system. Tetrahedron. 2008;64:4209–14. [Google Scholar]
- 23.Stewart MN, Hockley BG, Scott PJ. Green approaches to late-stage fluorination: radiosyntheses of (18)F-labelled radiopharmaceuticals in ethanol and water. Chem Commun. 2015;51:14805–8. doi: 10.1039/c5cc05919d. [DOI] [PubMed] [Google Scholar]
- 24.Sergeev ME, Morgia F, Lazari M, Wang C, Jr., van Dam RM. Titania-catalyzed radiofluorination of tosylated precursors in highly aqueous medium. J Am Chem Soc. 2015;137:5686–94. doi: 10.1021/jacs.5b02659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Richarz R, Krapf P, Zarrad F, Urusova EA, Neumaier B, Zlatopolskiy BD. Neither azeotropic drying, nor base nor other additives: a minimalist approach to (18)F-labeling. Org Biomol Chem. 2014;12:8094–9. doi: 10.1039/c4ob01336k. [DOI] [PubMed] [Google Scholar]
- 26.Chun JH, Telu S, Lu S, Pike VW. Radiofluorination of diaryliodonium tosylates under aqueous-organic and cryptand-free conditions. Org Biomol Chem. 2013;11:5094–9. doi: 10.1039/c3ob40742j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Olberg DE, Arukwe JM, Grace D, Hjelstuen OK, Solbakken M, Kindberg GM, et al. One Step Radiosynthesis of 6-[F-18]Fluoronicotinic Acid 2,3,5,6-Tetrafluorophenyl Ester ([F-18]F-Py-TFP): A New Prosthetic Group for Efficient Labeling of Biomolecules with Fluorine-18. J Med Chem. 2010;53:1732–40. doi: 10.1021/jm9015813. [DOI] [PubMed] [Google Scholar]
- 28.Basuli F, Li C, Xu B, Williams M, Wong K, Coble VL, et al. Synthesis of fluorine-18 radio-labeled serum albumins for PET blood pool imaging. Nucl Med Biol. 2015;42:219–25. doi: 10.1016/j.nucmedbio.2014.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen Y, Pullambhatla M, Foss CA, Byun Y, Nimmagadda S, Senthamizhchelvan S, et al. 2-(3-{1-Carboxy-5-[(6-[18F]fluoro-pyridine-3-carbonyl)-amino]-pentyl}-ureido)-pen tanedioic acid, [18F]DCFPyL, a PSMA-based PET imaging agent for prostate cancer. Clin Cancer Res. 2011;17:7645–53. doi: 10.1158/1078-0432.CCR-11-1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perrin DM. [18F]-Organotrifluoroborates as Radioprosthetic Groups for PET Imaging: From Design Principles to Preclinical Applications. Acc Chem Res. 2016 doi: 10.1021/acs.accounts.5b00398. [DOI] [PubMed] [Google Scholar]
- 31.McBride WJ, Sharkey RM, Goldenberg DM. Radiofluorination using aluminum-fluoride (Al18F) EJNMMI Research. 2013;3:1–11. doi: 10.1186/2191-219X-3-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Becaud J, Mu L, Karramkam M, Schubiger PA, Ametamey SM, Graham K, et al. Direct One-Step18F-Labeling of Peptides via Nucleophilic Aromatic Substitution. Bioconjug Chem. 2009;20:2254–61. doi: 10.1021/bc900240z. [DOI] [PubMed] [Google Scholar]
- 33.Cleeren F, Lecina J, Billaud EM, Ahamed M, Verbruggen A, Bormans GM. New Chelators for Low Temperature Al(18)F-Labeling of Biomolecules. Bioconjug Chem. 2016;27:790–8. doi: 10.1021/acs.bioconjchem.6b00012. [DOI] [PubMed] [Google Scholar]
- 34.Richter S, Wuest F. 18F-Labeled Peptides: The Future Is Bright. Molecules. 2014;19:20536. doi: 10.3390/molecules191220536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schirrmacher R, Wangler C, Schirrmacher E. Recent Developments and Trends in 18F-Radiochemistry: Syntheses and Applications. Mini Rev Org Chem. 2007;4:317–29. [Google Scholar]
- 36.Yue X, Yan X, Wu C, Niu G, Ma Y, Jacobson O, Shen B, Kiesewetter DO, Chen X. One-Pot Two-Step Radiosynthesis of a New 18F-Labeled Thiol Reactive Prosthetic Group and Its Conjugate for Insulinoma Imaging. Mol Pharmaceutics. 2014;11:3875–84. doi: 10.1021/mp5001857. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.