Abstract
Obesity increases the risk of a number of chronic diseases in humans including several cancers. Biological mechanisms responsible for such increased risks are not well understood at present. Increases in systemic inflammation and oxidative stress, endogenous production of mutagenic metabolites, altered signaling in proliferative pathways, and increased sensitivity to exogenous mutagens and carcinogens are some of the potential contributing factors. We hypothesize that obesity creates an endogenously mutagenic environment in addition to increasing the sensitivity to environmental mutagens. To test this hypothesis, we examined two in vivo genotoxicity endpoints. Pig-a mutant frequencies and micronucleus frequencies were determined in blood cells in two independent experiments in 30-week old male mice reared on either a high-fat diet (60% calories from fat) that exhibit an obese phenotype or a normal-fat diet (10% calories from fat) that do not exhibit an obese phenotype. Mice were assayed again at 52 weeks of age in one of the experiments. N-ethyl-N-nitrosourea (ENU) was used as a positive mutation control in one experiment. ENU induced a robust Pig-a mutant and micronucleus response in both phenotypes. Obese, otherwise untreated mice, did not differ from non-obese mice with respect to Pig-a mutant frequencies in reticulocytes or micronucleus frequencies. However, such mice, had significantly higher and sustained Pig-a mutant frequencies (increased 2.5–3.7-fold, p<0.02) in erythrocytes as compared to non-obese mice (based on measurements collected at 30 weeks or 30 and 52 weeks of age). This suggests that obesity, in the absence of exposure to an exogenous mutagen, is itself mutagenic.
Keywords: obesity, high-fat diet, Pig-a mutant, micronucleus
Introduction
Obesity is a major risk factor for the development of a number of chronic diseases including several cancers (Irigaray et al. 2007a; Irigaray et al. 2007b; Lavie et al. 2009; Roberts et al. 2010; Flegal et al. 2012; Regnier and Sargis 2014; Lauby-Secretan et al. 2016). Mechanistically, it is unclear what factors contributing to obesity drive the increased risk of developing cancer (Roberts et al. 2010; Lichtman 2012; Yoshimoto et al. 2013; Lauby-Secretan et al. 2016). Obesity and consequent metabolic disorder may independently increase cancer risk through the production of endogenous reactive metabolites, systemic oxidative stress, and/or systemic inflammation. Thus, a high-fat, high-calorie diet that induces obesity may itself be carcinogenic. Obesity may also increase sensitivity to environmental mutagens and carcinogens. It is likely that multiple factors interact to increase cancer risk.
While genetic and environmental factors contribute to the development of obesity, the primary cause of obesity stems from an energy imbalance due to the excess caloric intake of primarily fats and carbohydrates in conjunction with reduced energetic expenditure (Casazza et al. 2013; Malik et al. 2013). Medical consequences of an obese physiological state manifest as dysfunctional metabolism as well as chronic, systemic inflammation and oxidative stress (Matsuda and Shimomura 2013; Aye et al. 2014). This can lead to increased mutagenesis and carcinogenesis by chronically elevating the levels of reactive oxygen species.
Recent research indicates that microbes in the gastrointestinal (GI) tract and the composition of the microbial community in the GI tract itself can produce mutagenic and possibly carcinogenic metabolites (Yoshimoto et al. 2013). This may be a function of the high fat and/or high carbohydrate and low fiber diet that promotes obesity. Additionally, obesity may affect the hepatic metabolism of some chemicals by altering the expression of essential drug metabolizing enzymes (O’Shea et al. 1994). For example, obese individuals are more sensitive to the toxic effects of acetaminophen, and this appears to be modulated through increased expression or activity of the bioactivating enzyme, CYP2E1 (O’Shea et al. 1994; Aubert et al. 2012; Brill et al. 2012). This supports the hypothesis that obesity alters the metabolism of drugs and xenobiotics in the liver and likely other organs as well. It is probable that chronic systemic inflammation, oxidative stress, and altered multi-organ xenobiotic metabolism compound mutagenic and toxicological risk in obese individuals. Determining the molecular mechanisms that cause these changes and using biomarkers to delineate increased mutagenicity and increased risk from eobiotics, endobiotics, and xenobiotics is needed to fill these important gaps in our knowledge regarding obesity and cancer risk.
This set of experiments was designed to determine if age-matched, male C57BL/6J mice reared on a high-fat diet (60% fat) that exhibit an obese phenotype have a higher frequency of somatic Pig-a mutants and/or an increase in the formation of micronuclei when compared to control mice reared on a source-matched normal-fat diet (10% fat) that do not exhibit an obese phenotype. Mice with an obese phenotype reared on this high-fat diet exhibit a number of biochemical and phenotypic markers (e.g. insulin resistance, glucose intolerance, excess adiposity/body mass) consistent with human obesity (Surwit et al. 1988; Petro et al. 2004).
We hypothesize that obesity and the high-fat diet that induces and maintains an obese phenotype in these mice increase the risk of somatic mutagenesis independent of exposures to exogenous mutagens effectively creating an endogenous mutagenic environment. To test this hypothesis, we used the Pig-a gene mutation and micronucleus assays in a mouse model of diet-induced obesity (DIO). The Pig-a gene mutation assay is a relatively new platform for studying somatic mutations and has been used in recent rodent studies including mice (Bryce et al. 2008; Phonethepswath et al. 2008; Bhalli et al. 2011; Dertinger et al. 2011; Kimoto et al. 2011a; Kimoto et al. 2011b; Lemieux et al. 2011; Dobrovolsky et al. 2012; Ohtani et al. 2012; Cao et al. 2014; Labash et al. 2016). Furthermore, observations in the Pig-a mutation biomarker in the mouse have the potential to be confirmed in analyses of the human PIG-A gene. An explicit assumption that we make in this study is that the underlying cause of the cell surface CD24-negative phenotype among cells analyzed are mutations in the X-linked Pig-a gene and that observed mutant cells are the product of independent mutational events. Without DNA sequence analysis of the Pig-a gene itself from mutant cells, this cannot be verified in this study. However, the vast literature in which the Pig-a gene and other Pig protein encoding genes have been sequenced either as a function of mutational biomarker analysis or studies investigating paroxysmal nocturnal hemoglobinuria find, with extremely rare exception, that mutations in the Pig-a/PIG-A gene are the cause of the mutant Pig/PIG phenotype (Chen et al. 2001; Araten et al. 2005; Araten and Luzzatto 2006; Brodsky and Hu 2006; Miura et al. 2008; Brodsky 2009; Kimoto et al. 2011b; Nicklas et al. 2015). That said, without DNA sequence and mutational analysis of observed mutants, we cannot be unequivocally certain in this study that these cells are the product of independent mutational events (our assumption) and not the product(s) of clonal expansion from a mutant progenitor cell(s) as is the case with PNH.
Materials and Methods
Pig-a mutant and micronucleus frequencies were examined in two independent groups of mice described in more detail below. All mice in both groups were male C57BL/6J mice. All mice in both groups were obtained from the Jackson Laboratory at 24 weeks of age and had been maintained on either a high-fat calorie content (60%) diet (D12492) or a source-matched normal-fat calorie content (10%) diet (D12450B) starting at 6 weeks of age (Research Diets, Inc., NJ). All mice in both groups were maintained on a 12-hour light:dark cycle and were fed and watered ad libitum. Mice maintained on the high-fat diet exhibit a diet-induced obese phenotype by week 24 (DIO) compared with mice maintained on the normal-fat diet (non-DIO). All DIO and non-DIO mice were maintained on their respective research diets throughout both group experiments and were housed individually. All procedures involving these mice were approved by Tulane University’s IACUC (Protocols #4303 and 4374). Body mass (in grams) was obtained from untreated or vehicle control mice prior to initiation, throughout, and at the end of each experiment.
Experimental Group 1 Mice
A total of 36 mice were used in this experimental group. There were 18 DIO mice and 18 non-DIO mice. In this particular experiment, mice were either untreated, treated with a vehicle control, or treated with N-ethyl-N-nitrosourea (ENU), a potent, direct-acting, alkylating mutagen, as described in more detail in the following subsections.
ENU treatment as a positive control for the Pig-a gene mutation and micronucleus endpoints
On day 21 post-arrival, 24 mice (n=12 DIO, n=12 non-DIO controls) were randomly selected for either ENU or vehicle control (phosphate-buffered saline, pH 6.0) treatment. Positive mutational control mice were randomly selected for gavage treatment for 3 consecutive days to 40 mg/kg ENU (n=6 DIO, n=6 non-DIO controls) in vehicle totaling 120 mg/kg ENU cumulative exposure. Mice treated with ENU for chemical induction of Pig-a gene mutants (n=3 DIO, n=3 non-DIO controls) were treated at the beginning of the exposure experiment over the first 3 days to allow for a 3.5-week mutant manifestation period. Mice treated with ENU for micronucleus (MN) induction (n=3 DIO, n=3 non-DIO controls) were treated near the end of the exposure experiment over the last 3 days prior to euthanasia and blood sampling. Vehicle control mice were randomly selected to receive an appropriately adjusted volume of vehicle control (n=6 DIO and n=6 non-DIO controls). All gavage volumes in ENU-treated or vehicle-treated mice were ≤100 μl. ENU and vehicle control-treated mice were 30 weeks of age at the time of blood sampling for Pig-a mutant cell and MN analysis.
Background Pig-a mutant frequency and micronucleus formation
Twelve additional mice (n=6 DIO and n=6 non-DIO controls) were maintained on their respective diets throughout experimental time period. These mice were chemically untreated and were used to establish the spontaneous frequencies of Pig-a mutant cells and MN frequency at 30 weeks of age in the absence of any treatment other than dietary fat content.
Experimental Group 2A and 2B Mice
Fourteen mice were used in these experiments (n=7 DIO and n=7 non-DIO controls). Mice were maintained on their respective diets and blood samples were taken non-lethally (submandibular blood collection) at week 30 (2A) for Pig-a and MN analysis. No mutagenic chemical treatments were administered in any of these mice throughout this experiment. Exposures were only to caloric content from fat in the respective diets. Mice were sampled again for Pig-a and micronucleus analysis at 52 weeks of age (2B) by cardiac exsanguination following euthanasia.
Blood collection, shipment, and data analysis
For mice in the experimental groups 2A and 2B, the first blood samples were obtained non-lethally by submandibular blood collection at 30 weeks of age. Samples, 60–150 μl of blood, were collected in 1.7 ml microtubes containing K2-EDTA as an anticoagulant. Samples were then packaged and shipped as described below. In terminal experiments with both groups of mice (group 1 at 30 weeks of age, group 2B at 52 weeks of age), mice were euthanized using CO2 asphyxiation and immediately exsanguinated by cardiac puncture collecting blood into heparin-coated syringes. Blood samples were transferred to vacutainer tubes containing K2-EDTA and packed into Exakt-Pak® shipping containers. Samples were shipped priority overnight to Litron Laboratories (Rochester, NY) for further processing, preparation, and flow cytometric analysis using the MutaFlow® method for Pig-a mutant cell analysis and enumeration and the MicroFlow® method for micronucleus analysis and enumeration.
Statistical analysis
Statistical and post hoc power analyses were conducted using Prism 6 for Windows (ver. 6.07, GraphPad, La Jolla, CA) and G*Power (ver. 3.1.9.2, freeware that can be accessed at http://www.gpower.hhu.de/en.html), respectively (Faul et al. 2007; Faul et al. 2009). Biological endpoints for statistical analyses included the frequency of Pig-a mutant RBCs, the frequency of Pig-a mutant RETs, fold-differences in Pig-a mutant frequencies, the percentage of RETs (%RET), the percentage of micronucleated normochromatic erythrocytes (%MN-NCE), and the percentage of micronucleated reticulocytes (%MN-RET) as a function of dietary fat exposure and phenotype in untreated mice and exposure to ENU in treated mice. Data were tested for normality, where possible, using the D’Agostino & Pearson omnibus normality test. Parametric statistical testing methods included the Student’s t-test applying a Welch’s correction when variances were determined to be unequal and Analysis of Variance (ANOVA) followed by Bonferroni-corrected post-hoc mean comparisons when the results of the ANOVA were significant. If the assumption of normality for any data was not met or could not be adequately tested, non-parametric testing methods including the Mann-Whitney and Kruskal-Wallis tests were used to test for statistical significance. All tests were conducted as two-tailed tests and differences were considered statistically significant at p<0.05. G*Power was used for power analyses and for estimating minimum sample sizes based on observed effect sizes when statistical tests did not reach the level of significance for select cases.
Results
All mice survived and were sampled for Pig-a and micronucleus endpoints in both experimental groups except for 1 DIO mouse at the 52-week time-point in group 2. This mouse was found dead at week 48 and was therefore not included in the blood sampling for mutant analysis for the 52-week time-point. On gross necropsy, this mouse had substantial visceral, gonadal, and cardiopulmonary fat deposits, an enlarged liver, and a visibly atrophied, diseased spleen.
Body Mass
Body masses for mice in the experimental groups and time-points are presented in Table I as arithmetic means ± standard deviations. Only body mass measurements taken at the end of each experiment are included in Table I. No ENU-treated mice in group 1 were included in the body mass estimates for that group as there were notable losses of body mass likely related to treatment at 30 days post-treatment in the mice treated for Pig-a mutant frequency analysis. Male mice in both groups 1 and 2 maintained on the high-fat diet for 30 weeks were significantly heavier than male mice maintained on the normal-fat diet for the same time period (Group 1, t=12.1, df=17.1, p<0.0001; Group 2, t=9.8, df=12.0, p<0.0001), but mice on their respective diets among groups were not significantly different from one another at this time-point (Non-DIO, t=1.7, df=17.0, p<0.11; DIO, t=2.1, df=17.0, p<0.06). Mice in group 2 on the high-fat diet were significantly heavier than mice on the normal-fat diet at 52 weeks (t=11.0, df=11.0, p<0.0001). Group 2 mice on their respective diets at 52 weeks were significantly heavier than they were at 30 weeks (Non-DIO, t=5.5, df=24.0, p<0.0001; DIO, t=3.4, df=23.0, p<0.003).
Table I.
Body mass (in grams) of age-matched male mice at experimental sampling times for Pig-a mutation and micronucleus analysis.
| Diet (% Calories from Fat) and Body Mass (in grams) | ||
|---|---|---|
| Experimental Group (age in weeks) | Normal Fat (10%) | High Fat (60%) |
| Group 1a (30 weeks) | 33.5 (±2.7) | 52.9 (±4.9)b |
| Group 2A (30 weeks) | 35.6 (±2.4)c | 48.8 (±2.6)b,c |
| Group 2B (52 weeks) | 40.8 (±2.5)d | 58.2 (±3.2)d,e |
Does not include ENU-treated mice for either dietary regimen
Significantly higher body mass than normal-fat diet mice at 30 weeks (p < 0.0001)
Not significantly different from mice on respective diets among groups at 30 weeks
Significantly higher body mass than mice on respective diets at 30 weeks (p < 0.0001 for normal fat diet, p < 0.003 for high fat diet)
Significantly higher body mass than normal-fat diet mice at 52 weeks (p < 0.0001)
Experimental Group 1 Mice
Background/Spontaneous Pig-a mutant frequencies and MN frequencies
To explore whether or not it was reasonable to combine untreated mice with vehicle control treated mice in both dietary groups, we analyzed the frequencies of Pig-a mutant RBCs and RETs as well as %MN-NCEs and %MN-RETs using non-parametric Mann-Whitney tests. Sample sizes in these groups (maximum n=6) were too small for normality testing.
Two DIO mice were not included in the Pig-a analyses because of cellular aggregation that prevented mutant frequency analysis on these two samples. Therefore 12 non-DIO mice and 10 DIO mice (n=6 untreated, n=4 vehicle control) were available for Pig-a analyses. In a comparison of untreated control and vehicle-treated mice, we found no significant differences in mutant RBCs (non-DIO, Mann-Whitney U=9, p<0.17; DIO, Mann-Whitney U=7.5, p<0.36) or mutant RETs (non-DIO, Mann-Whitney U=12.5, p<0.40; DIO, Mann-Whitney U=9.5, p<0.65). All mice were available for %MN analyses. In a comparison of untreated control and vehicle-treated mice, we found no significant differences in %MN-NCEs (non-DIO, Mann-Whitney U=15, p<0.67; DIO, Mann-Whitney U=18, p<0.99) or %MN-RETs (non-DIO, Mann-Whitney U=9, p<0.17; DIO, Mann-Whitney U=16, p<0.78).
We chose to combine the data from untreated and vehicle control treated mice in their respective dietary groups and respective mutant endpoints going forward.
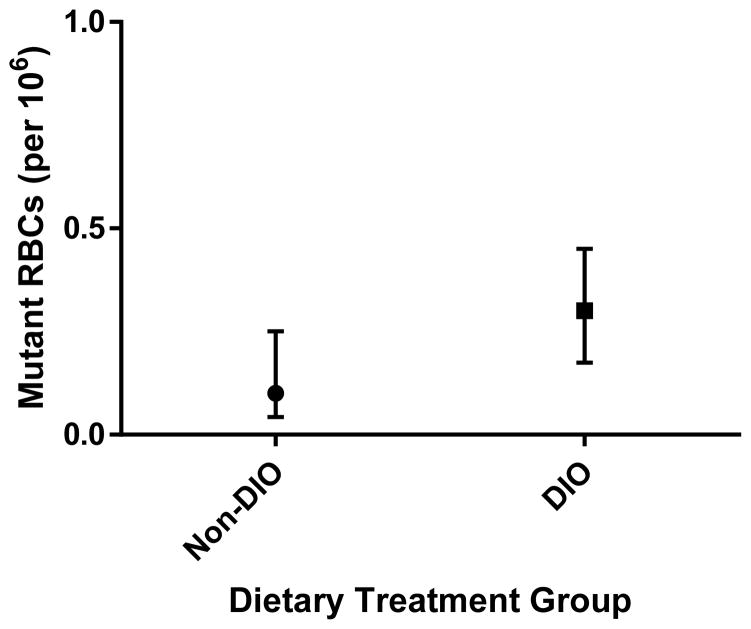
After combining data from untreated and vehicle control treated mice in both dietary groups, tests for normality indicated that frequencies of Pig-a mutant RBCs and RETs were not normally distributed. Therefore, Mann-Whitney non-parametric testing was conducted. Though on average the frequency of Pig-a mutant RETs appeared elevated in DIO mice, this difference was not statistically significant between non-DIO and DIO mice in this experimental group (Mann-Whitney U=47, p<0.41). The frequency of Pig-a mutant RBCs was significantly higher in DIO mice when compared to non-DIO mice (Mann-Whitney U=26.5, p<0.025). Frequencies of Pig-a mutant RBCs in DIO mice were on average 2.5-fold higher than in non-DIO mice (Figure 1).
Figure 1.
Background or spontaneous frequencies of RBCs phenotypically mutant for Pig-a in Experimental Group 1 mice. Non-DIO mice (n = 12, 6 untreated, 6 vehicle control treated) were reared on a normal fat diet and DIO mice (n = 12, 6 untreated, 6 vehicle control treated) were reared on a high-fat diet. Mice assayed at 30 weeks of age. The frequency of mutant RBCs is significantly higher in DIO mice (p<0.025). Presented as median ± interquartile range.
After combining data from untreated and vehicle control treated mice in both dietary groups for %MN-NCEs and %MN-RETs, Mann-Whitney non-parametric testing was conducted. No significant differences were observed between dietary groups with respect to %MN-NCEs (Mann-Whitney U=65.5, p<0.73) or %MN-RETs (Mann-Whitney U=68, p<0.83). No significant difference was observed regarding the %RETs between dietary groups (Mann-Whitney U=43, p<0.10).
ENU-induced Pig-a mutant frequencies
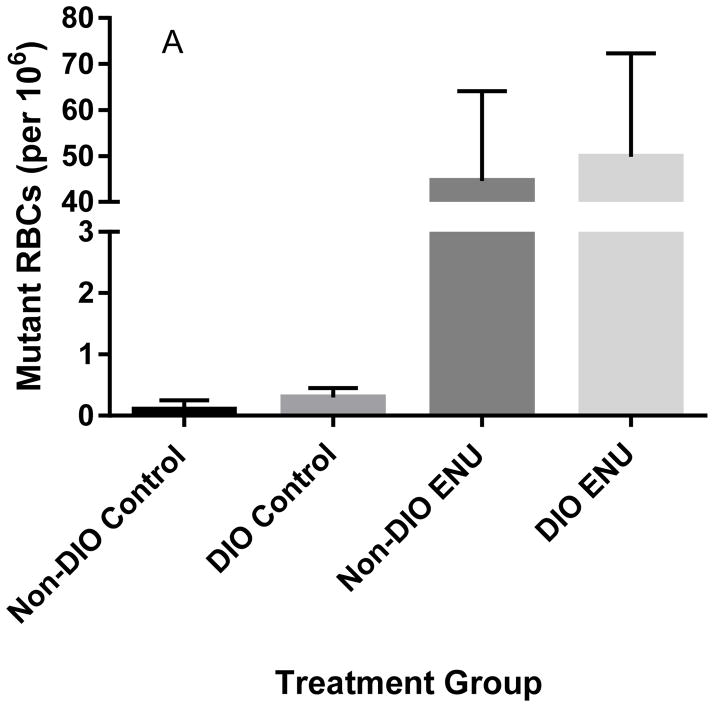
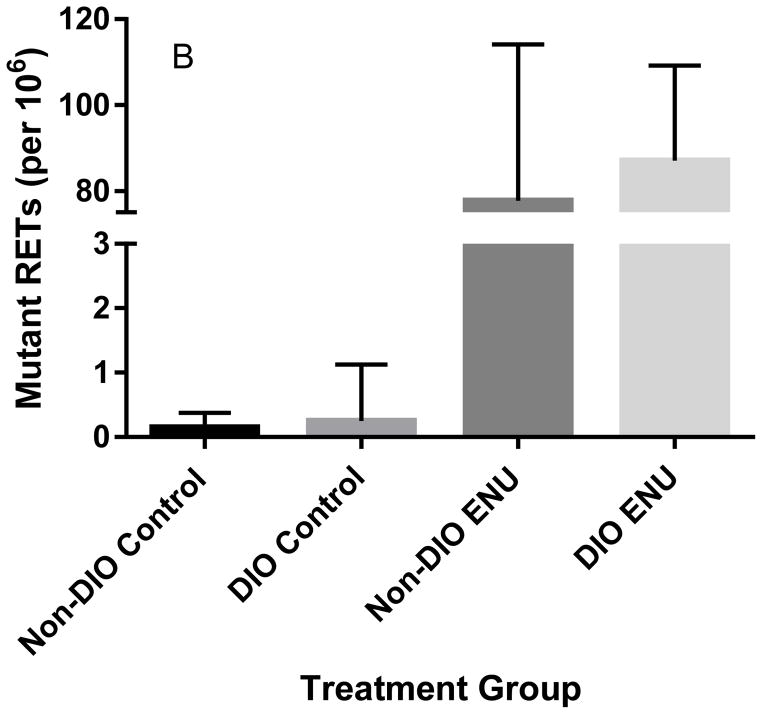
One ENU-treated (120 mg/kg/3 days) DIO mouse and one ENU-treated (120 mg/kg/3 days) non-DIO mouse were unusable for the Pig-a assay because of cellular aggregation. As expected, ENU induced a robust, significant mutagenic response as measured by Pig-a mutant frequencies in RBCs (Kruskal-Wallis statistic=13.6, p<0.002) and RETs (Kruskal-Wallis statistic=10.5, p<0.02) in both DIO and non-DIO mice (Figures 2A and 2B), but there was no significant difference between phenotypes.
Figure 2.
Frequencies of Pig-a mutant RBCs (A) and RETs (B) in untreated mice on either a normal-fat diet (Non-DIO) or a high-fat diet (DIO) compared to ENU-treated (120 mg/kg/3 days) mice on their respective diets in Experimental Group 1 mice. Mutant RBCs and RETs are significantly higher in ENU-treated mice. Presented as median ± interquartile range.
ENU-induced micronucleus frequencies
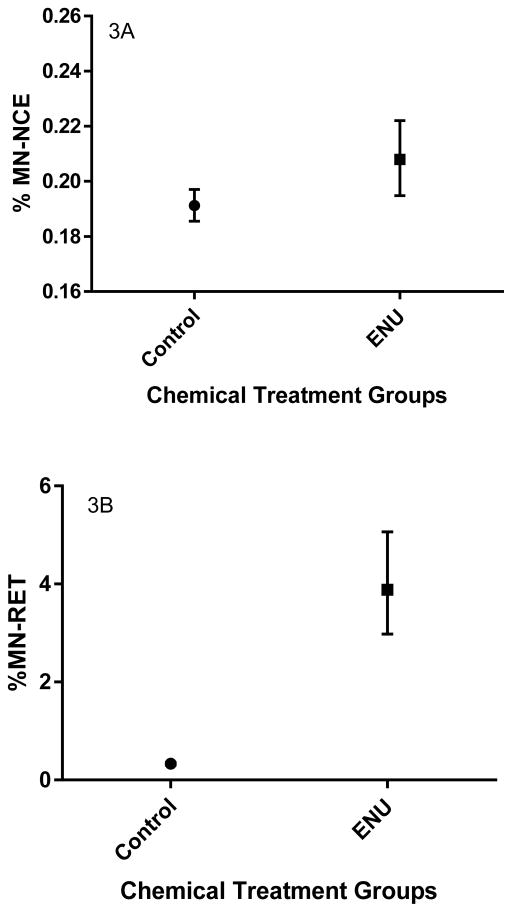
ENU treatment significantly increased both %MN-NCEs (Mann-Whitney U=25, p<0.01) and %MN-RETs (Mann-Whitney U=0, p<0.0001) in both non-DIO and DIO mice (Figures 3a and 3b) when compared to untreated controls. ENU-induced MN frequencies were not significantly different between treated non-DIO and treated DIO mice (%MN-NCE, Mann-Whitney U=1.5, p<0.4; %MN-RET, Mann-Whitney U=4, p<0.99). The %RETs was significantly lower in ENU-treated mice compared to untreated mice (Mann-Whitney U=0, p<0.0001).
Figure 3.
Frequencies of micronucleated normochromatic erythrocytes (%MN-NCEs; 3A) and micronucleated reticulocytes (%MN-RETs; 3B) in untreated mice on either a normal-fat diet (Non-DIO) or a high-fat diet (DIO) compared to ENU-treated (120 mg/kg/3 days) mice on their respective diets in Experimental Group 1 mice. %MN-NCEs and %MN-RETs are significantly higher in ENU-treated mice. Presented as geometric means ± 95% confidence intervals.
Experimental Group 2 Mice
Background/Spontaneous Pig-a mutant frequencies and MN frequencies
One DIO animal was not used in the analysis at 30 weeks because the blood sample was unusable due to clotting. This same animal was not used in the analysis at 52 weeks because this animal expired at 48 weeks of age.
30 Weeks of Age
The frequency of Pig-a mutant RBCs was significantly higher in the DIO mice (n=6) when compared to the non-DIO mice (n=7) confirming what was observed in the prior, independent experiment (Mann-Whitney U=4, p<0.02; Figure 4). The difference was on average 3-fold higher in the DIO mice. The frequency of Pig-a mutant RETs was again on average higher (~5-fold in this experiment) in the DIO mice, but this difference was not statistically significant (Mann Whitney U=11, p<0.17). Fold-differences normalized to each respective control (non-DIO) group were combined for mice in both experiments, and on average, DIO mice at 30 weeks of age (n=16) had combined frequencies of Pig-a mutant RETs that were 3-fold higher than age-matched non-DIO mice (n=19) but this difference was not statistically significant (Mann-Whitney U=116, p<0.24; Figure 5). A post-hoc power analysis indicated that at this effect size (d=0.63) and an α<0.05, the power (1-β error probability) of the experiment was 0.55. An a-priori power analysis using this same effect size in this slightly imbalanced design and a power of 0.80 indicated that sample sizes would need to be doubled (N=70; n=38 non-DIO, n=32 DIO) to achieve statistical significance at α<0.05. Setting the power at 0.95 indicated that the total sample size needed to achieve statistical significance would minimally require 120 animals (n=65 non-DIO, n=55 DIO).
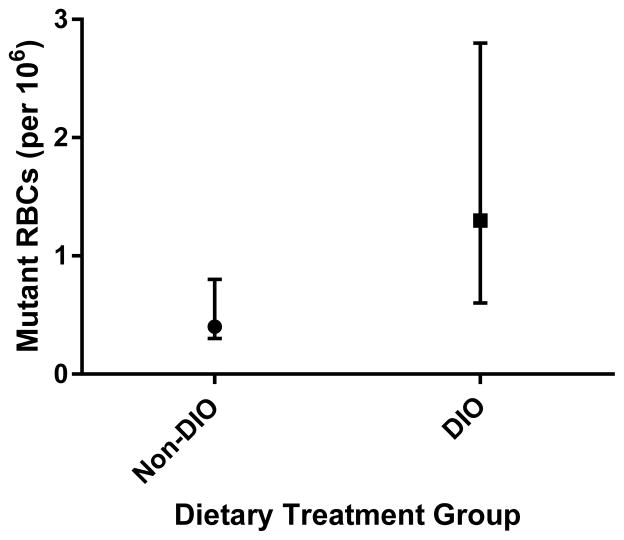
Figure 4.
Background or spontaneous frequencies of RBCs phenotypically mutant for Pig-a in Experimental Group 2 mice at 30 weeks of age. Non-DIO mice (n = 7) were reared on a normal fat diet and DIO mice (n = 6) were reared on a high-fat diet. The frequency of mutant RBCs is significantly higher in DIO mice (p<0.02). Presented as median ± interquartile range.
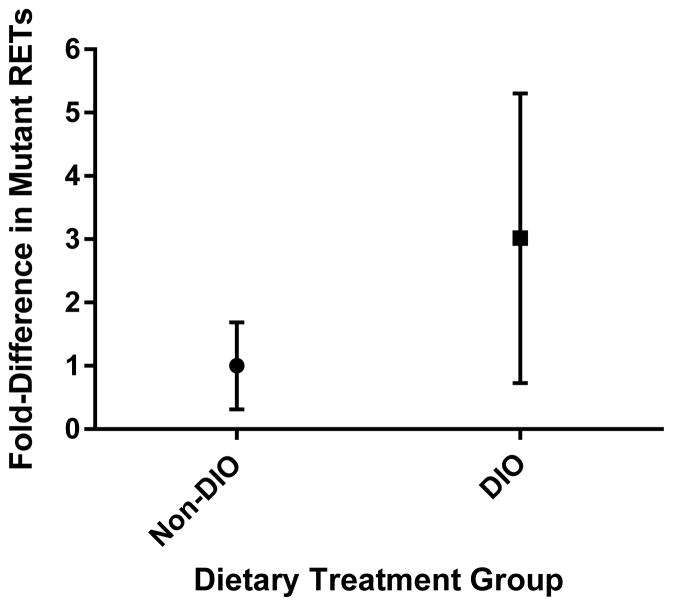
Figure 5.
Background or spontaneous frequencies of RETs phenotypically mutant for Pig-a in Experimental Groups 1 and 2 mice at 30 weeks of age combined. Non-DIO mice (n = 19) were reared on a normal fat diet and DIO mice (n = 16) were reared on a high-fat diet. The frequency of mutant RETs is not significantly higher in DIO mice (p<0.24). Presented as median ± interquartile range.
No significant differences were observed between dietary groups and body mass phenotypes for %MN-NCEs (Mann-Whitney U=16, p<0.50) or %MN-RETs (Mann-Whitney U=17.5, p<0.65).
52 Weeks of Age
The frequency of Pig-a mutant RBCs was significantly greater in the aged DIO mice when compared to the aged non-DIO mice (Mann-Whitney U=4, p<0.02), confirming what was observed in the same animals at 30 weeks of age. The difference was on average 3.7-fold higher in the DIO mice. The frequency of Pig-a mutant RETs was again on average higher (2-fold in this experiment) in the DIO mice, but this difference was not statistically significant (Mann Whitney U=20, p<0.92).
No significant differences were observed between dietary groups and body mass phenotypes for %MN-NCEs (Mann-Whitney U=20, p<0.93) or %MN-RETs (Mann-Whitney U=14, p<0.36).
After combining dietary groups at each sampling time-point, we conducted matched pairs analyses of %RETs, %MN-NCEs, and %MN-RETs using a Wilcoxon matched-pairs signed rank test. Samples from the mice at 52 weeks had significantly lower %RETs (median difference=-1.49, p<0.01), significantly higher %MN-NCEs (median difference=0.04, p<0.001), and significantly higher %MN-RETs (median difference=0.05, p<0.02) than the samples from 30 week old mice.
Mutant Frequencies in RBCs-Numerical Differences and Fold-Differences
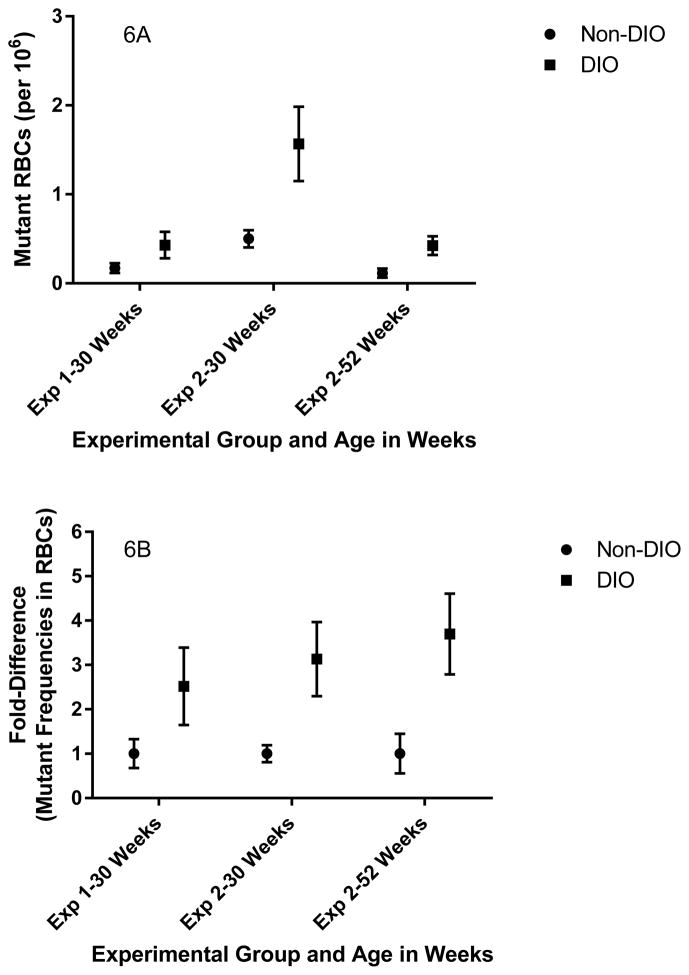
Numerical estimates of the frequency of Pig-a mutants in RBCs (adjusted per million RBCs) and fold-differences in Pig-a mutant frequencies are presented in Figures 6a and 6b. Spontaneous mutant frequencies in the Pig-a gene in mice are exceptionally low. As such, the procedure uses an immunomagnetic separation/enrichment step in order to increase the total number of cells analyzed. This or other processing steps may contribute to the modest day-to-day variation observed in spontaneous Pig-a mutant RBC frequencies for these studies. Variation among experiments both among and within laboratories has been observed and documented in studies of Pig-a mutagenesis in rats though differences are considered modest and results are considered quite reproducible (Godin-Ethier et al. 2015; Johnson et al. 2016). Reducing such variation among rodent Pig-a mutation assays, especially for spontaneous mutant frequencies, remains an active area of research and development. In any event, fold-differences which serve to normalize this variability across experimental time-points quite clearly show that Pig-a mutant frequencies in RBCs are consistently elevated in DIO mice compared to non-DIO mice, and these differences are maintained as mice age up to one year (Figures 6a and 6b).
Figure 6.
Numerical estimates of the frequencies of Pig-a mutant RBCs (6A) and fold-differences (6B) in the frequencies of mutant RBCs normalized to their respective non-DIO samples in untreated mice on either a normal-fat diet (Non-DIO) or a high-fat diet (DIO). Mutant RBCs and associated fold-differences are significantly higher in DIO mice in both experiments and at both time points in experiment 2. Presented as arithmetic averages ± standard error of the mean.
Data on Pig-a mutant cell analysis and micronucleus analysis for all animals used in this study are available in Supplemental Tables I–III.
Discussion
Robust induction of Pig-a mutant phenotype cells by orally administered ENU (40 mg/kg/day for 3 days) was expected. A significant 50-fold increase in Pig-a mutant RBCs and RETs was induced by ENU. No significant difference with respect to obesity phenotype and ENU-induced mutagenesis was noted. Thus, the Pig-a biomarker reported expected mutant induction by this model exogenous, direct-acting genotoxicant in both non-DIO and DIO mice. As mentioned previously, a few samples were not amenable to Pig-a flow cytometric analysis because of cellular aggregation. This likely occurred due to some clotting of blood at the time of blood sampling that may affect Pig-a processing but not during the processing of samples for Pig-a staining and flow cytometric analysis. With a relatively small number of mice, conclusions are difficult, but in our limited experience, the DIO mice are more difficult to exsanguinate by cardiac puncture and tended to take more time to do so than their non-DIO counterparts. Opening the chest cavity immediately following euthanasia and exsanguinating the cardiac compartment directly was more effective as that was the approach we took in closing out the second experiment at 52 weeks.
As expected, ENU also induced a significant increase in the formation of micronucleated NCEs and RETs and a decrease in the %RETs indicating some degree of acute hematopoietic toxicity in both non-DIO and DIO mice. No significant difference was observed between phenotypes with respect to ENU-induced MN frequencies, but the sample size was probably too small for a compelling comparison.
Untreated and vehicle control treated male mice maintained on a high-fat diet over the time-course of experiment 1 (24 weeks) had a significantly higher Pig-a mutant frequency (2.5-fold) in RBCs than age-, strain-, and gender-matched mice that were maintained on a normal fat diet at 30 weeks of age. Mice in independent experiment 2 reared on the same source-matched diets exhibited the same pattern in that male DIO mice had a significantly higher frequency (3.1-fold) of Pig-a mutants in RBCs compared to non-DIO mice. Therefore, the same outcome in terms of increased Pig-a mutant RBCs has been observed in untreated, gender- and age-matched mice from 2 independent experiments. The same mice in experiment 2 assayed again at 52 weeks had significantly higher frequencies (3.7-fold) of Pig-a mutants in RBCs. Thus, obesity in this mouse model appears to sustain this apparent increase in Pig-a mutants in RBCs at least up to 1 year. This increase in somatic mutant frequency is evident in the absence of any explicit exposure to mutagenic toxicants. The only exposure is to different proportions of calories driven by dietary fat content (10% fat non-DIO vs 60% fat DIO) that causes an obese phenotype in mice maintained on the high-fat diet. The trends in RETs were similar to those in the RBCs but did not reach statistical significance. This is likely a function of scoring error and statistical power, in that far fewer RETs (5–7×106 RET equivalents in these experiments) are assayed in comparison to RBCs (130–200×106 RBC equivalents in these experiments).
DIO mice reared and maintained on a high-fat diet did not exhibit an age-matched increase in the frequency of micronucleated NCEs or RETs. This suggests that this phenotype is not associated with any substantial increase in the types of DNA damage that would be responsive to the micronucleus assay. We observed a small but significant age-related increase in micronucleus formation in Group 2 mice comparing %MN in these mice at 30 weeks of age and again at 52 weeks of age. This has been demonstrated previously in mice including the C57BL/6J strain (Sato et al. 1995; Dass et al. 1997).
At 30 weeks of age, the mice reared and maintained on the high-fat diet were obese and approximately 45% heavier in terms of body mass compared with the non-DIO, matched mice. Though body mass increased up to the 52 week time-point in both non-DIO and DIO groups, DIO mice were still on average approximately 42% heavier by mass than their non-DIO counterparts. Taken together, this suggests that obesity as expressed in this mouse model is mutagenic as measured by the Pig-a mutation assay and that this mutant phenotype is maintained up to 1 year of age.
We hypothesize that biochemical and phenotypic obesity essentially creates an endogenous mutagenic environment in the absence of an overt exposure to exogenous mutagens. It is also plausible that the excess caloric intake of fats in the diet used in this study is genotoxic and mutagenic. Fats are important and necessary dietary macronutrients but under conditions of excessive intake, it is possible that increased lipid peroxidation and excess fatty acid metabolism through β-oxidation or by phase I metabolic enzymes increases the systemic content of genotoxic intermediates (Refsgaard et al. 2000; Robertson et al. 2001; Day 2002; Gonzalez 2005; Reddy and Sambasiva Rao 2006; Porubsky et al. 2008; Toshiro et al. 2009; Pizzimenti et al. 2013). Interestingly, a recent study in rats has found that malnutrition appears to increase the frequency of Pig-a mutants (Pacheco-Martínez et al. 2016). In light of our study, this might suggest that caloric and nutrient imbalance is mutagenic under malnourishment as well as overnourishment.
This hypothesis remains to be thoroughly tested using appropriate experimental designs and a range of genotoxicity endpoints as utilized herein. This includes using assays and/or other cell types amenable to DNA sequence and mutational analysis to verify whether or not observed mutant cells are in fact primarily the products of independent mutational events and not clonal expansion from a mutant progenitor(s). Even so, the current experiments suggest that obesity and/or a major dietary factor (high-fat content) contribute to a small but significant increase in somatic mutant frequencies as reported by the Pig-a gene mutation assay without exposure to a known exogenous mutagen. These initial data are consistent with the observation that obesity increases the risk of developing somatic mutation-based diseases such as cancer.
Supplementary Material
Acknowledgments
Funding for this research was provided by the Tulane University 170th Annual Early Career Professorship awarded to J. K. Wickliffe. Analyses conducted at Litron Laboratories were supported in part by a grant from NIH-NIEHS, R44ES021973. In addition, this publication was made possible by the Deepwater Horizon Research Consortia grant numbers U01/U19 1U19ES20677-01 (Tulane University) from the National Institute of Environmental Health Sciences (NIEHS). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIEHS or NIH. We also acknowledge support from the Baton Rouge Area Foundation, the Tulane Cancer Center, and the Louisiana Cancer Research Consortium.
Footnotes
Disclosure
SDD, DKT, and SLA are employees of Litron Laboratories. Litron holds patents covering flow cytometric methods for scoring micronucleated reticulocytes and sells kits based on this technology (In Vivo MicroFlow®). Litron holds patents covering flow cytometric methods for scoring GPI anchor-deficient erythrocytes as described herein, and sells kits based on this technology (In Vivo MutaFlow®).
Statement of Author Contributions
All authors played a critical role in the design and conduct of the study being responsible for animal husbandry (Drs. Wickliffe and Wilson), selection of mutagen and exposures (Drs. Wickliffe, Wilson, Dertinger, and Ms. Torous), animal processing (Drs. Wickliffe, Wilson and Simon-Friedt), and sample collection (Drs. Wickliffe, Wilson and Simon-Friedt). Drs. Dertinger, and Ms. Torous, and Avlasevich were responsible for the Pig-a analysis and quality assurance. Dr. Wickliffe was responsible for statistical analysis. Drs. Wickliffe, Wilson, and Simon-Friedt were primarily responsible for drafting the manuscript. All authors shared responsibility for proofing and finalizing the manuscript.
References
- Araten DJ, Golde DW, Zhang RH, Thaler HT, Gargiulo L, Notaro R, Luzzatto L. A quantitative measurement of the human somatic mutation rate. Cancer Research. 2005;65(18):8111–8117. doi: 10.1158/0008-5472.CAN-04-1198. [DOI] [PubMed] [Google Scholar]
- Araten DJ, Luzzatto L. The mutation rate in PIG-A is normal in patients with paroxysmal nocturnal hemoglobinuria (PNH) Blood. 2006;108(2):734–736. doi: 10.1182/blood-2006-01-0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubert J, Begriche K, Delannoy M, Morel I, Pajaud J, Ribault C, Lepage S, McGill MR, Lucas-Clerc C, Turlin B, Robin M-A, Jaeschke H, Fromenty B. Differences in early acetaminophen hepatotoxicity between obese ob/ob and db/db Mice. J Pharmacol Exp Ther. 2012;342(3):676–687. doi: 10.1124/jpet.112.193813. [DOI] [PubMed] [Google Scholar]
- Aye ILMH, Lager S, Ramirez VI, Gaccioli F, Dudley DJ, Jansson T, Powell TL. Increasing maternal body mass index is associated with systemic inflammation in the mother and the activation of distinct placental inflammatory pathways. Biol Reprod. 2014;90(6):1–9. doi: 10.1095/biolreprod.113.116186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhalli JA, Pearce MG, Dobrovolsky VN, Heflich RH. Manifestation and persistence of Pig-a mutant red blood cells in C57BL/6 mice following single and split doses of N-ethyl-N-nitrosourea. Environ Mol Mutagen. 2011;52(9):766–773. doi: 10.1002/em.20682. [DOI] [PubMed] [Google Scholar]
- Brill ME, Diepstraten J, Rongen A, Kralingen S, Anker J, Knibbe CJ. Impact of obesity on drug metabolism and elimination in adults and children. Clin Pharmacokinet. 2012;51(5):277–304. doi: 10.2165/11599410-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Brodsky RA. How I treat paroxysmal nocturnal hemoglobinuria. Blood. 2009;113(26):6522–6527. doi: 10.1182/blood-2009-03-195966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodsky RA, Hu R. PIG-A mutations in paroxysmal nocturnal hemoglobinuria and in normal hematopoiesis. Leuk Lymphoma. 2006;47(7):1215–1221. [Google Scholar]
- Bryce SM, Bemis JC, Dertinger SD. In vivo mutation assay based on the endogenous Pig-a locus. Environ Mol Mutagen. 2008;49(4):256–264. doi: 10.1002/em.20379. [DOI] [PubMed] [Google Scholar]
- Cao X, Mittelstaedt RA, Pearce MG, Allen BC, Soeteman-Hernández LG, Johnson GE, Bigger CAH, Heflich RH. Quantitative dose–response analysis of ethyl methanesulfonate genotoxicity in adult gpt-delta transgenic mice. Environ Mol Mutagen. 2014;55(5):385–399. doi: 10.1002/em.21854. [DOI] [PubMed] [Google Scholar]
- Casazza K, Fontaine KR, Astrup A, Birch LL, Brown AW, Bohan Brown MM, Durant N, Dutton G, Foster EM, Heymsfield SB, McIver K, Mehta T, Menachemi N, Newby PK, Pate R, Rolls BJ, Sen B, Smith DL, Thomas DM, Allison DB. Myths, presumptions, and facts about obesity. N Engl J Med. 2013;368(5):446–454. doi: 10.1056/NEJMsa1208051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Eshleman JR, Brodsky RA, Medof ME. Glycophosphatidylinositol-anchored protein deficiency as a marker of mutator phenotypes in cancer. Cancer Research. 2001;61(2):654–658. [PubMed] [Google Scholar]
- Dass SB, Ali SF, Heflich RH, Casciano DA. Frequency of spontaneous and induced micronuclei in the peripheral blood of aging mice. Mutat Res. 1997;381(1):105–110. doi: 10.1016/s0027-5107(97)00156-5. [DOI] [PubMed] [Google Scholar]
- Day CP. Pathogenesis of steatohepatitis. Best Practice & Research Clinical Gastroenterology. 2002;16(5):663–678. doi: 10.1053/bega.2002.0333. [DOI] [PubMed] [Google Scholar]
- Dertinger SD, Bryce SM, Phonethepswath S, Avlasevich SL. When pigs fly: Immunomagnetic separation facilitates rapid determination of Pig-a mutant frequency by flow cytometric analysis. Mutat Res. 2011;721(2):163–170. doi: 10.1016/j.mrgentox.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrovolsky VN, Heflich RH, Ferguson SA. The frequency of Pig-a mutant red blood cells in rats exposed in utero to N-ethyl-N-nitrosourea. Environ Mol Mutagen. 2012;53(6):440–450. doi: 10.1002/em.21704. [DOI] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Buchner A, Langridge JM. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behavior Research Methods. 2009;41:1149–1160. doi: 10.3758/BRM.41.4.1149. [DOI] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Lang A-G, Buchner A. G*Power 3: A flexible statistical power analysis for the social, behaviorial, and biomedical sciences. Behavior Research Methods. 2007;39:175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA. 2012;307(5):491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- Godin-Ethier J, Leroux F, Wang N, Thébaud S, Merah F, Nelson A. Characterisation of an in vivo Pig-a gene mutation assay for use in regulatory toxicology studies. Mutagenesis. 2015;30(3):359–363. doi: 10.1093/mutage/gev005. [DOI] [PubMed] [Google Scholar]
- Gonzalez FJ. Role of cytochromes P450 in chemical toxicity and oxidative stress: studies with CYP2E1. Mutat Res. 2005;569(1–2):101–110. doi: 10.1016/j.mrfmmm.2004.04.021. [DOI] [PubMed] [Google Scholar]
- Irigaray P, Newby JA, Clapp R, Hardell L, Howard V, Montagnier L, Epstein S, Belpomme D. Lifestyle-related factors and environmental agents causing cancer: An overview. Biomed Pharmacother. 2007a;61(10):640–658. doi: 10.1016/j.biopha.2007.10.006. [DOI] [PubMed] [Google Scholar]
- Irigaray P, Newby JA, Lacomme S, Belpomme D. Overweight/obesity and cancer genesis: More than a biological link. Biomed Pharmacother. 2007b;61(10):665–678. doi: 10.1016/j.biopha.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Johnson GE, Yamamoto M, Suzuki Y, Adachi H, Kyoya T, Takasawa H, Horibata K, Tsutsumi E, Wada K, Kikuzuki R, Yoshida I, Kimoto T, Maeda A, Narumi K. Measuring reproducibility of dose response data for the Pig-a assay using covariate benchmark dose analysis. Mutat Res. 2016 doi: 10.1016/j.mrgentox.2016.04.004. In Press. [DOI] [PubMed] [Google Scholar]
- Kimoto T, Chikura S, Suzuki K, Kobayashi Xm, Itano Y, Horibata K, Honma M, Dobrovolsky VN, Heflich RH, Miura D, Kasahara Y. Further development of the rat Pig-a mutation assay: Measuring rat Pig-a mutant bone marrow erythroids and a high throughput assay for mutant peripheral blood reticulocytes. Environ Mol Mutagen. 2011a;52(9):774–783. doi: 10.1002/em.20677. [DOI] [PubMed] [Google Scholar]
- Kimoto T, Suzuki K, Kobayashi Xm, Dobrovolsky VN, Heflich RH, Miura D, Kasahara Y. Manifestation of Pig-a mutant bone marrow erythroids and peripheral blood erythrocytes in mice treated with N-ethyl-N-nitrosourea: Direct sequencing of Pig-a cDNA from bone marrow cells negative for GPI-anchored protein expression. Mutat Res. 2011b;723(1):36–42. doi: 10.1016/j.mrgentox.2011.03.016. [DOI] [PubMed] [Google Scholar]
- Labash C, Avlasevich SL, Carlson K, Berg A, Torous DK, Bryce SM, Bemis JC, MacGregor JT, Dertinger SD. Mouse Pig-a and micronucleus assays respond to N-ethyl-N-nitrosourea, benzo[a]pyrene, and ethyl carbamate, but not pyrene or methyl carbamate. Environ Mol Mutagen. 2016;57(1):28–40. doi: 10.1002/em.21965. [DOI] [PubMed] [Google Scholar]
- Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K. Body fatness and cancer — Viewpoint of the IARC working group. N Engl J Med. 2016;375(8):794–798. doi: 10.1056/NEJMsr1606602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavie CJ, Milani RV, Ventura HO. Obesity and cardiovascular disease: Risk factor, paradox, and impact of weight loss. J Am Coll Cardiol. 2009;53(21):1925–1932. doi: 10.1016/j.jacc.2008.12.068. [DOI] [PubMed] [Google Scholar]
- Lemieux CL, Douglas GR, Gingerich J, Phonethepswath S, Torous DK, Dertinger SD, Phillips DH, Arlt VM, White PA. Simultaneous measurement of benzo[a]pyrene-induced Pig-a and lacZ mutations, micronuclei and DNA adducts in MutaMouse. Environ Mol Mutagen. 2011;52(9):756–765. doi: 10.1002/em.20688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtman MA. Obesity and the risk of chronic myelogenous leukemia: Is this another example of the neoplastic effects of increased body fat? Leukemia. 2012;26(1):183–184. doi: 10.1038/leu.2011.190. [DOI] [PubMed] [Google Scholar]
- Malik VS, Willett WC, Hu FB. Global obesity: trends, risk factors and policy implications. Nat Rev Endocrinol. 2013;9(1):13–27. doi: 10.1038/nrendo.2012.199. [DOI] [PubMed] [Google Scholar]
- Matsuda M, Shimomura I. Increased oxidative stress in obesity: Implications for metabolic syndrome, diabetes, hypertension, dyslipidemia, atherosclerosis, and cancer. Obes Res Clin Pract. 2013;7(5):e330–e341. doi: 10.1016/j.orcp.2013.05.004. [DOI] [PubMed] [Google Scholar]
- Miura D, Dobrovolsky VN, Mittelstaedt RA, Kasahara Y, Katsuura Y, Heflich RH. Development of an in vivo gene mutation assay using the endogenous Pig-A gene: II. Selection of Pig-A mutant rat spleen T-cells with proaerolysin and sequencing Pig-A cDNA from the mutants. Environ Mol Mutagen. 2008;49(8):622–630. doi: 10.1002/em.20413. [DOI] [PubMed] [Google Scholar]
- Nicklas JA, Carter EW, Albertini RJ. Both PIGA and PIGL mutations cause GPI-a deficient isolates in the Tk6 cell line. Environ Mol Mutagen. 2015;56(8):663–673. doi: 10.1002/em.21953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Shea D, Davis SN, Kim RB, Wilkinson GR. Effect of fasting and obesity in humans on the 6-hydroxylation of chlorzoxazone: A putative probe of CYP2E1 activity. Clin Pharmacol Ther. 1994;56(4):359–367. doi: 10.1038/clpt.1994.150. [DOI] [PubMed] [Google Scholar]
- Ohtani S, Unno A, Ushiyama A, Kimoto T, Miura D, Kunugita N. The in vivo Pig-a gene mutation assay is useful for evaluating the genotoxicity of ionizing radiation in mice. Environ Mol Mutagen. 2012;53(8):579–588. doi: 10.1002/em.21724. [DOI] [PubMed] [Google Scholar]
- Pacheco-Martínez MM, Cortés-Barberena E, Cervantes-Ríos E, del Carmen García-Rodríguez M, Rodríguez-Cruz L, Ortiz-Muñiz R. Moderate malnutrition in rats induces somatic gene mutations. Mutat Res. 2016;789:26–32. doi: 10.1016/j.mrfmmm.2016.01.008. [DOI] [PubMed] [Google Scholar]
- Petro AE, Cotter J, Cooper DA, Peters JC, Surwit SJ, Surwit RS. Fat, carbohydrate, and calories in the development of diabetes and obesity in the C57BL/6J mouse. Metabolism. 2004;53(4):454–457. doi: 10.1016/j.metabol.2003.11.018. [DOI] [PubMed] [Google Scholar]
- Phonethepswath S, Bryce SM, Bemis JC, Dertinger SD. Erythrocyte-based Pig-a gene mutation assay: Demonstration of cross-species potential. Mutat Res. 2008;657(2):122–126. doi: 10.1016/j.mrgentox.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzimenti S, Ciamporcero ES, Daga M, Pettazzoni P, Arcaro A, Cetrangolo G, Minelli R, Dianzani C, Lepore A, Gentile F, Barrera G. Interaction of aldehydes derived from lipid peroxidation and membrane proteins. Front Physiol. 2013;4:1–17. doi: 10.3389/fphys.2013.00242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porubsky PR, Meneely KM, Scott EE. Structures of human cytochrome P-450–2E1: Insights into the binding of inhibitors and both small molecular weight and fatty acid substrates. Journal of Biological Chemistry. 2008;283(48):33698–33707. doi: 10.1074/jbc.M805999200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy JK, Sambasiva Rao M. Lipid Metabolism and Liver Inflammation. II. Fatty liver disease and fatty acid oxidation. American Journal of Physiology - Gastrointestinal and Liver Physiology. 2006;290(5):G852–G858. doi: 10.1152/ajpgi.00521.2005. [DOI] [PubMed] [Google Scholar]
- Refsgaard HHF, Tsai L, Stadtman ER. Modifications of proteins by polyunsaturated fatty acid peroxidation products. Proceedings of the National Academy of Sciences. 2000;97(2):611–616. doi: 10.1073/pnas.97.2.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regnier SM, Sargis RM. Adipocytes under assault: Environmental disruption of adipose physiology. Biochim Biophys Acta. 2014;1842(3):520–533. doi: 10.1016/j.bbadis.2013.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts DL, Dive C, Renehan AG. Biological mechanisms linking obesity and cancer risk: New perspectives. Annual Review of Medicine. 2010;61(1):301–316. doi: 10.1146/annurev.med.080708.082713. [DOI] [PubMed] [Google Scholar]
- Robertson G, Leclercq I, Farrell GC. II. Cytochrome P-450 enzymes and oxidative stress. American Journal of Physiology - Gastrointestinal and Liver Physiology. 2001;281(5):G1135–G1139. doi: 10.1152/ajpgi.2001.281.5.G1135. [DOI] [PubMed] [Google Scholar]
- Sato S-i, Taketomi M, Nakajima M, Kitazawa M, Shimada H, Itoh S, Igarashi M, Higashikuni N, Sutou S, Sasaki YF, Hayashi M, Sofuni T, Higashiguchi T, Nito S, Kondo Y, Honda S, Hayashi M, Shinagawa Y, Nakajima E, Oka Y, Shimoi K, Hokabe Y, Morita A, Kinae N, Takeuchi M, Hirono H, Yamamura E, Tamai K. Effect of aging on spontaneous micronucleus frequencies in peripheral blood of nine mouse strains: the results of the 7th collaborative study organized by CSGMT/JEMS · MMS. Mutation Research/DNAging. 1995;338(1–6):51–57. doi: 10.1016/0921-8734(95)00011-t. [DOI] [PubMed] [Google Scholar]
- Surwit RS, Kuhn CM, Cochrane C, McCubbin JA, Feinglos MN. Diet-induced type II diabetes in C57BL/6J mice. Diabetes. 1988;37(9):1163–1167. doi: 10.2337/diab.37.9.1163. [DOI] [PubMed] [Google Scholar]
- Toshiro N, Norie M, Hiroshi Y. Oxidation of endobiotics mediated by xenobiotic-metabolizing forms of human cytochrome P450. Current Drug Metabolism. 2009;10(7):700–712. doi: 10.2174/138920009789895525. [DOI] [PubMed] [Google Scholar]
- Yoshimoto S, Loo TM, Atarashi K, Kanda H, Sato S, Oyadomari S, Iwakura Y, Oshima K, Morita H, Hattori M, Honda K, Ishikawa Y, Hara E, Ohtani N. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature. 2013;499(7456):97–101. doi: 10.1038/nature12347. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.