Abstract
Hemolysis is a key feature of sickle cell anemia (HbSS). Direct quantitation of hemolysis could be used as an objective outcome in clinical trials of new therapeutics for HbSS and would also enable better human studies of the pathogenesis of complications of HbSS that are ostensibly hemolysis-related, such as pulmonary hypertension. However, contemporary human studies in HbSS have used only surrogate markers of hemolysis rather than direct measurements of RBC survival. We directly quantified hemolysis in HbSS by measuring survival of an age cohort of RBCs labeled with a stable isotope, administered orally as 15N-glycine, a metabolic precursor of heme. The atomic excess of 15N in heme extracted from blood was monitored by mass spectrometry over time. We performed 13 labeling experiments in 11 individuals with HbSS. Mean RBC survival was 31.9 days (range 14.1 – 53.6). Both HbF level, a known determinant of hemolysis, and absolute reticulocyte count (ARC), an index of the marrow’s response to hemolysis, correlated with directly measured RBC survival (r=0.61, P<0.002; r=−0.84, P<0.001). However, commonly used biochemical surrogates of hemolysis (LDH, AST, bilirubin and plasma free hemoglobin) did not correlate with directly measured RBC survival. These biochemical surrogates should be interpreted cautiously, at best, in clinical trials and human physiologic studies in HbSS. ARC was the best correlate of total hemolysis, but only 70% of the variation in RBC survival was reflected in this marker. If greater accuracy is required in human studies, 15N-glycine RBC labeling can directly and accurately quantify hemolysis.
Keywords: erythrocyte survival, erythrocyte lifespan, erythrocyte age, erythrocyte turnover, hemolysis, hemolytic rate, stable isotope, cohort label, sickle cell anemia, sickle cell disease
Introduction
The two main pathophysiologic features of sickle cell anemia (HbSS) are hemolysis and vaso-occlusion. Hemolysis, defined as abnormal shortening of red blood cell (RBC) survival, is the accepted cause of some complications of HbSS, such as anemia and pigment gallstones. Recently, intravascular hemolysis has been proposed as a cause of or contributor to a number of other complications of HbSS, including pulmonary hypertension, priapism, leg ulcers and stroke.1–3 The proposed mechanism has been called hemolysis-associated endothelial dysfunction, wherein cell-free hemoglobin and other RBC contents released by intravascular hemolysis decrease the bioavailability of nitric oxide that regulates endothelial function (in concert with other mechanisms).1–3 Additionally, novel therapeutics that can attenuate the degree of hemolysis in HbSS are being developed and tested in humans.4–7 These human physiologic studies and clinical trials have all relied on surrogate markers, such as reticulocyte count, LDH, and AST, rather than direct measurements of hemolysis. Surrogate markers have multiple confounders that may limit causal inferences about hemolysis.8–10 One example is LDH, which derives from multiple tissues (heart, kidney, muscle, liver, leukocytes, RBCs and others). When elevated, LDH could indicate hemolysis, tissue injury, or both. To appropriately interpret studies that use surrogate markers of RBC survival to study hemolysis in HbSS, it is necessary to evaluate the degree of association between surrogate and direct measurements.
Direct measurements of hemolysis employ a label to track RBC survival.11 This label may be placed ex vivo on a sample population of circulating RBCs of all ages (e.g., biotin or 51Cr). Alternatively, a label may be incorporated metabolically into normoblasts in the bone marrow to create an age cohort of RBCs (e.g., 15N or 13C stable isotopes). One such metabolic label is glycine, a precursor of both heme and globin. Following oral administration, glycine is rapidly absorbed, transported into hemoglobin-synthesizing normoblasts in the bone marrow, and incorporated into heme. When glycine is enriched with a stable isotope (e.g., 15N-glycine), heme becomes highly labeled by 15N, because the 4 nitrogen atoms in the heme ring derive directly from the nitrogen atoms of 4 glycine molecules. The cells are labeled over a period of several hours, and they are subsequently released into the circulation as an approximate age cohort that can be tracked over time.
The stable isotope method was pioneered on a small scale in the 1940s and 1950s.12–14 Despite its safety and relative ease of administration, it was largely replaced by biotin and 51Cr labeling even though these ex vivo population labeling methods are labor-intensive, expensive, and moderately invasive, involving sterile manipulation and re-infusion of labeled (sometimes radiolabeled) RBCs. The preference for ex vivo labeling was driven by the cost of manufacturing stable isotopes with high percentage of enrichment and the cumbersome mass spectrometry of the time. Manufacturing processes and measurement technology have since improved, overcoming these obstacles. We recently demonstrated that the stable isotope method, using modern technology, correlates well with the gold-standard biotin method in a mixed population of healthy and diabetic individuals,15 and we propose that it has utility for direct measurements of hemolysis in clinical studies.
The aims of the current study were (1) to demonstrate that a modern stable isotope labeling method to measure RBC survival (i.e., hemolysis) directly is practical for use in clinical studies in HbSS and (2) to evaluate the degree of association between commonly used surrogate markers of hemolysis and directly measured RBC survival in HbSS.
Methods
Study Design and Participants
This was a non-therapeutic study using oral 15N-glycine as an age cohort label to measure RBC survival directly in individuals with HbSS. The inclusion criteria were: a diagnosis of homozygous sickle cell anemia (HbSS); age ≥ 11 years; and having no medical issues needing acute intervention in the 1 month preceding enrollment. The exclusion criteria were: RBC transfusion in the past 3 months; known, symptomatic hepatic or biliary disease; and pregnancy or nursing. Hydroxyurea therapy was not an exclusion criterion, but the hydroxyurea dose must have been stable for at least 3 months prior to enrolment. Participants were recruited from the sickle cell programs at Cincinnati Children’s Hospital Medical Center and the University of Cincinnati Medical Center. This study was approved by a local institutional review board. Adult participants provided written informed consent. Minors provided written assent and their parents or legal guardians provided written consent. Most participants were also enrolled in a separate (“parent”) cardiac physiology study (clinicaltrials.gov: NCT02410811).
Clinical Assessments and Procedures
Participants had 26 study visits over approximately 4 months. Pre-ingestion blood samples for complete blood counts, reticulocyte counts, HbF quantitation, clinical laboratory tests (AST, ALT, LDH, bilirubin fractions, plasma free hemoglobin), and heme extraction were obtained on visits 1 and 2. After collection of baseline blood samples on visit 2, 2 grams of 15N-glycine [15N glycine (98%) (H2*NCH2COOH), Catalog # NLM-202, Cambridge Isotope Laboratories, Inc. Andover, MA] dissolved in 30 mL of H2O were given orally.15 Post-ingestion blood samples for heme extraction were obtained twice weekly for 8 weeks, weekly for 6 weeks, then every 2 weeks for 4 weeks. Post-ingestion blood samples for complete blood counts, reticulocyte counts, and clinical laboratory tests were obtained approximately monthly. For each participant, blood was stored at −80°C until the end of his or her study period, and heme isolation for each participant was performed in a single batch. Demographic and clinical data were obtained from participants’ medical and research records, including echocardiographic measurements of tricuspid regurgitant jet velocity (TRV).
Isolation of Heme and Mass Spectrometry
Heme was extracted from whole blood hemolysates using a method modified from Egyed16 and described previously in detail.15 Briefly, 1 ml of venous blood collected in EDTA was subjected to an acetone, ethylacetate, and glacial acetic acid extraction process. Undissolved proteins were removed by filtration followed by precipitation of heme with the addition of ddH2O. A heme pellet was washed, lyophilized, and stored at room temperature protected from light under dry conditions. Heme samples from each participant were analyzed by mass spectrometry in a single batch. The ratio of 15N/14N released by combustion of RBC heme samples was measured by a commercial laboratory (Metabolic Solutions, Inc., Nashua, NH) using a Europa Scientific 20/20 gas isotope ratio mass spectrometer.17 Starting with participant 7, a new mass spectrometer was used (Supporting Information Figure 1): a Thermo Finnigan Delta V gas isotope ratio mass spectrometer connected to a continuous flow Thermo Flash elemental analyzer (EA 1112). Both instruments were calibrated against international standards available from the International Atomic Energy Agency (15N-labeled ammonium sulfate). The instruments’ analytical precision is 0.0002 atom %15N. 15N-urea secondary standards, at three different enrichments, were run before and after each daily run to check instrument performance. A control heme sample was run with 54% of batches; the coefficient of variation for the heme control was less than 1%.
Survival Curves and Statistical Analysis
The atom percent excess (APE) of the sample was calculated relative to 15N in air (15N/14N = 0.0036765). The baseline 15N/14N ratios were subtracted from ratios obtained after administration of stable isotope to calculate the APE. A relative APE (%APE) was derived for each time point, defining the maximum value as 100% (Supporting Information Figure 1). From the APE, normalized RBC survival curves were generated for each participant and RBC survival was calculated as described previously.15,18 Briefly, the starting point for all RBC survival calculations was the time at which the rising APE reached 50% of the maximum value. The endpoint was the time at which the rate of decrement of APE had decreased to <0.5% per day. APE was corrected for any residual component at the endpoint by assuming a linear increase in the residual from the starting point to the endpoint. Normalized RBC survival curves were fit using a 5-order polynomial expression. Mean and median RBC survival and mean RBC age were calculated from these curves.15,18
Summary statistics, Pearson correlation, and linear modeling were performed with SPSS v23.0 (IBM Corp., Somers, NY). The mean of each participant’s clinical laboratory values during the study period was used for correlation with RBC survival. Analyses were pre-specified and no corrections were made for multiple comparisons. Figures were generated with Prism 7.0a (GraphPad Software, Inc., La Jolla, CA).
Results
Characteristics of Participants and RBC Survival
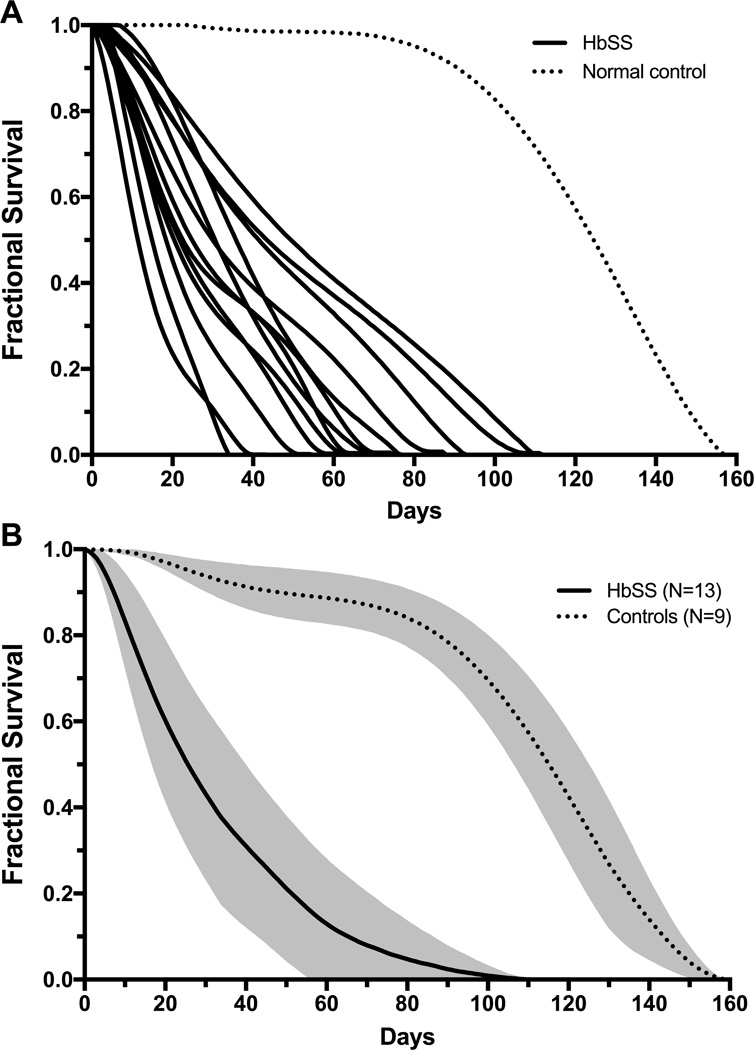
There were 5 males and 6 females who had a mean age of 23 years (Table). Sequencing of the β-globin genes (HBB) and copy number variation analysis of the β-globin gene cluster demonstrated that all had homozygous sickle cell anemia; none had compound heterozygosity for HbS and gene-deletion hereditary persistence of fetal hemoglobin (HbS/HPFH). Hydroxyurea was prescribed to 7 participants for clinical indications. The range of HbF values was 1.0 – 33.8% across all participants (Table). Two participants completed the study twice (#5 and #6) for a total of 13 labeling studies. Representative %APE curves are shown in Supporting Information Figure 1. Individual and composite RBC survival curves for all participants are shown in Figure 1. Mean RBC survival was 31.9 days (S.D. 12.0 days) with a range of 14.1 – 53.6 days (Supporting Information Table).
Table.
Characteristics and mean RBC survival of participants.
| Participant | Age (years) |
Sex | α-globin genotype |
HU therapy |
HbF (%) |
Hb (g/dL) |
ARC (/mm3) |
Mean RBC Survival (days)* |
|---|---|---|---|---|---|---|---|---|
| 1 | 21 | M | N/A | Yes | 16.1 | 9.7 | 399,800 | 25.2 |
| 2 | 26 | F | –α/–α | Yes | 9.1 | 9.7 | 177,100 | 25.3 |
| 3 | 31 | F | αα/–α | Yes | 13.7 | 7.5 | 243,300 | 30.5 |
| 4 | 22 | F | αα/–α | No | 24.3 | 11.8 | 288,800 | 44.9 |
| 5 | 18 | F | αα/αα | Yes | 19.0 | 10.1 | 109,500 | 36.0 |
| 5 (repeat) | 20 | " | " | Yes | 23.7 | 9.6 | 81,600 | 48.7 |
| 6 | 16 | M | αα/αα | Yes | 25.0 | 10.9 | 140,300 | 53.6 |
| 6 (repeat) | 18 | " | " | Yes | 26.9 | 10.5 | 141,600 | 37.7 |
| 7 | 41 | F | αα/αα | No | 33.8 | 11.8 | 117,200 | 16.4 |
| 8 | 23 | F | –α/–α | No | 2.1 | 9.2 | 204,700 | 32.3 |
| 9 | 19 | M | αα/–α | No | 1.0 | 10.0 | 358,700 | 21.4 |
| 10 | 24 | M | –α/–α | Yes | 1.4 | 10.2 | 265,900 | 25.2 |
| 11 | 20 | M | αα/–α | Yes | 9.6 | 9.1 | 302,700 | 25.3 |
Abbreviations: ARC, absolute reticulocyte count; Hb, total hemoglobin concentration; HbF, fetal hemoglobin; HU, hydroxyurea; N/A, not available; RBC, red blood cell.
Mean RBC survival in normal individuals (N=6) measured using the same 15N-glycine stable isotope method as the study participants: 111 days (standard deviation: 9 days).11
Figure 1. RBC survival curves.
Panel A shows individual RBC survival curves for HbSS patients (solid lines; N=13) and a representative control with normal hemoglobin composition (dotted line) from a prior study.15 Panel B shows a composite RBC survival curve for HbSS patients (solid line, N=13) and controls with normal hemoglobin composition (dotted line, N=9). Mean fractional RBC survival is indicated by the line, and the shaded region depicts ± 1 standard deviation for mean fractional survival.
It is important to note the distinction between cohort-labeled survival curves as seen in Figure 1 and population-labeled (e.g., biotin) curves reported previously.19,20 In the representative normal control sample in Figure 1, the cohort of labeled cells remains fully viable (fractional survival ≈ 1.0) for almost 100 days, at which point fractional survival begins to decline. Fifty percent of the label remains at about 120 days, defining the median survival (lifespan) of normal RBCs. The label persists for almost 160 days, reflecting heterogeneity of the lifespan of individual cells in the population. The survival curves for HbSS RBCs are markedly different, showing rapid decline in the label, reflecting the destruction of some RBCs at a very young age. Some curves are biphasic, consistent with differential survival of F-cell and non-F-cells. Overall, mean RBC survival was shortened in study participants by 50–85% compared to normal controls (Table, Figure 1, Supporting Information Table).
Correlation of Mean RBC Survival with Hematologic Parameters
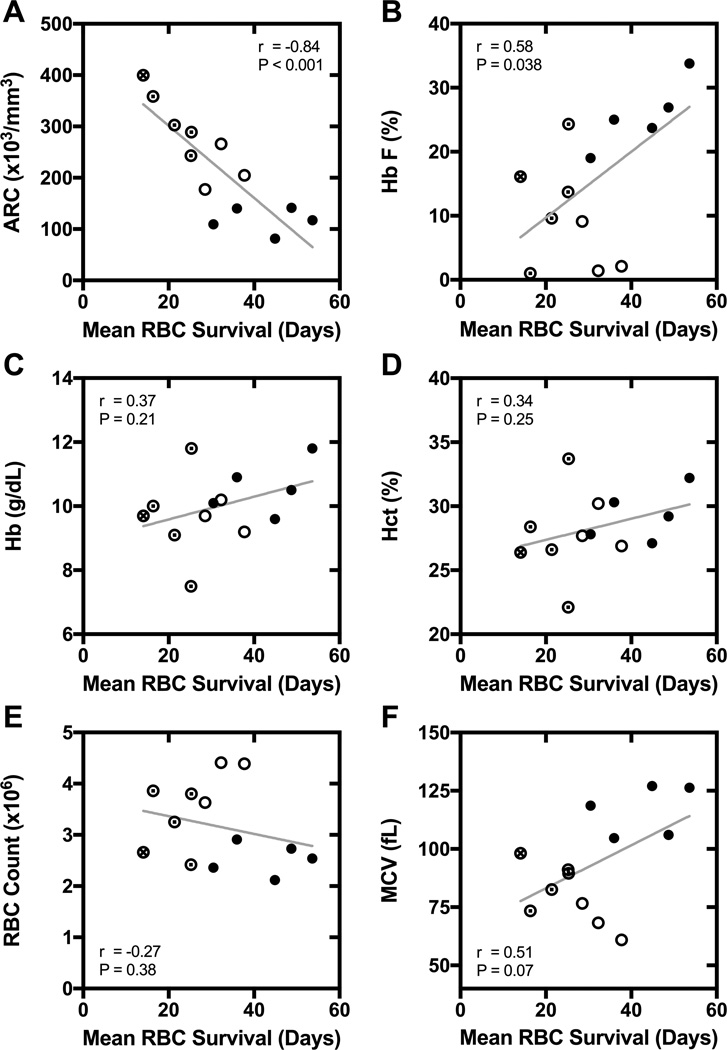
Mean RBC survival had a strong inverse correlation with absolute reticulocyte count (r = −0.84, P < 0.0001) and percentage of reticulocytes (r = −0.78, P = 0.002) (Figure 2, Panel A). The coefficients of determination (r2) for absolute reticulocyte count (ARC) and percentage of reticulocytes were 0.71 (P < 0.001) and 0.61 (P < 0.002), respectively. There was also a positive correlation between mean RBC survival and percent HbF (r = 0.58, P = 0.038; Figure 2, Panel B). The slope of the regression line of HbF on mean RBC survival was 0.52 ± 0.22 (slope ± standard error), indicating that every 1% increase in HbF (e.g., 20 to 21%) was associated with a 1.9-day increase in mean RBC survival. No significant correlations were observed between mean RBC survival and any other complete blood count parameter (Figure 2, Panels C–F).
Figure 2. Correlation of mean RBC survival with hematologic parameters.
Panel A: absolute reticulocyte count (ARC). Panel B: percent fetal hemoglobin (HbF). Panel C: total hemoglobin (Hb) concentration. Panel D: hematocrit. Panel E: RBC count. Panel F: mean cell volume (MCV). For each panel, the r and P-value for Pearson correlation are specified, and a linear regression line is shown. The α-globin genotype is also indicated for each participant (● αα/αα;⊙ αα/–α; ○ –α/–α; ⊗ genotype not available).
Hemolytic rate in HbSS is known to be modified by coinherited α-thalassemia.21,22 As expected, we found that relationships between mean RBC survival and several hematologic parameters, e.g., HbF and MCV, appeared to be modified by α-globin genotype. Participants with 2-gene-deletion α-thalassemia had longer mean RBC survival than would be predicted from HbF level alone (Figure 2, Panel B). Indeed, in a linear model of RBC survival, there was a significant interaction between HbF level and α-thalassemia status (interaction term β = 2.2, P < 0.001). The degree of linear correlation between mean RBC survival and HbF was higher (r = 0.82, P = 0.003) when the participants with 2-gene-deletion α-thalassemia were excluded (compared to the whole group in whom r = 0.58, P = 0.038). The relationship between mean RBC survival and MCV is complex (Figure 2, Panel F), in that participants with the highest (>100 fL) and lowest (<75 fL) values for MCV had the longest mean RBC survival. This relationship is likely explained by the attenuation of hemolysis by α-thalassemia in microcytic participants and by hydroxyurea therapy in macrocytic participants, while normocytic participants with lower mean RBC survival had neither 2-gene-deletion α-thalassemia nor maximal hydroxyurea therapy.
Correlation of Mean RBC Survival with Biochemical Surrogate Markers of Hemolysis and TRV
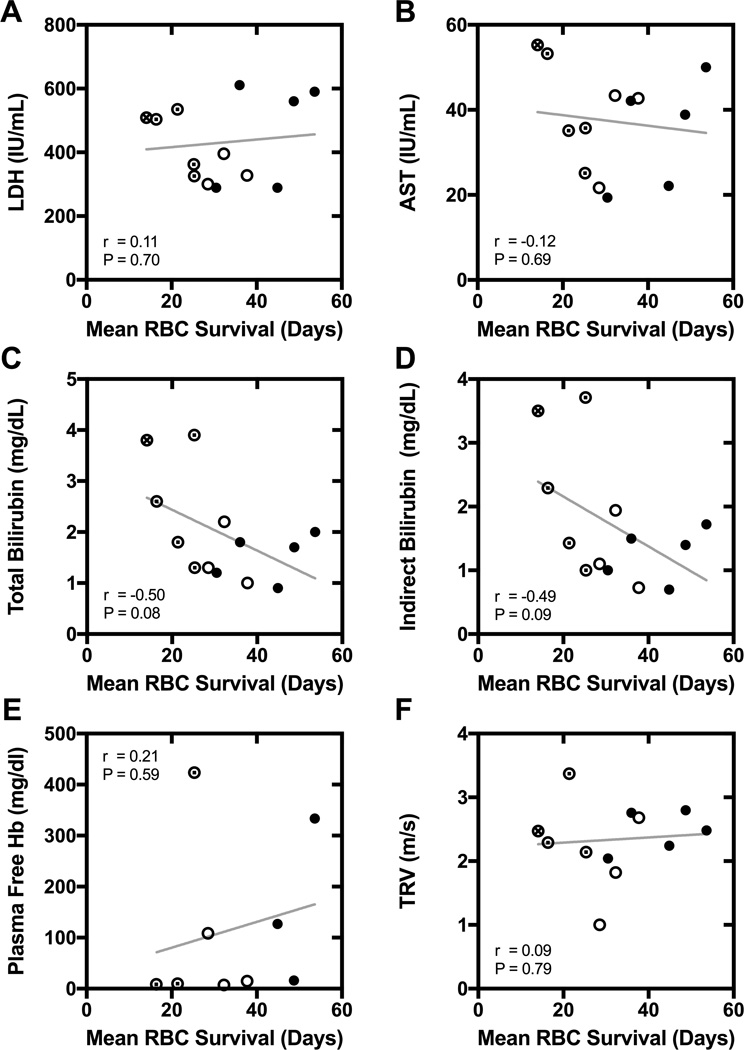
The commonly used biochemical surrogate markers of hemolysis, AST, LDH, total bilirubin, indirect bilirubin, and plasma free hemoglobin, had no significant correlation with directly measured RBC survival (Figure 3). Among these, total and indirect bilirubin concentration had the highest correlation coefficients (r ≈ 0.50, P ≈ 0.09). All but one participant had an echocardiographic measurement of TRV, which has been associated with biochemical surrogate markers of hemolysis, such as LDH, in prior studies (specifically, higher values of surrogate markers were associated with higher TRV).23–25 We also found a correlation between TRV and LDH (r = 0.62, P = 0.03), but there was no correlation between TRV and directly measured RBC survival in the same participants (r = 0.09, P = 0.79; Figure 3, Panel F; Supporting Information Figure 2).
Figure 3. Correlation of mean RBC survival with biochemical surrogate markers of hemolysis and tricuspid jet regurgitant velocity (TRV).
Panel A: lactate dehydrogenase (LDH). Panel B: aspartate aminotransferase (AST). Panel C: total bilirubin. Panel D: indirect bilirubin. Panel E: plasma free hemoglobin (Hb). Panel F: TRV. For each panel, the r and P-value for Pearson correlation are specified, and a linear regression line is shown. The α-globin genotype is also indicated for each participant (● αα/αα;⊙ αα/–α; ○ –α/–α; ⊗ genotype not available).
Repeated Mean RBC Survival Measurements
Two participants (#5 and #6) completed two labeling studies with intervals of 20 and 23 months, respectively, between ingestions of 15N-glycine. For comparison of these paired measurements, we repeated the extractions of heme concurrently from frozen aliquots of blood for all 4 studies and reanalyzed the samples concurrently on the same, newer instrument (Thermo Finnigan Delta V gas isotope ratio mass spectrometer). These participants received standard clinical care by hematologists who were not involved in this study. Participant #5 (Supporting Information Figure 3, Panel A) was not at the maximal tolerated dose (MTD) of hydroxyurea based on peripheral blood counts at the time of first study. Her dose of hydroxyurea was increased to MTD in the interval between the first and second studies, and this is reflected by increases in HbF (19 to 24%) and MCV (119 to 127 fL) and decreases in total white blood cell count (WBC), absolute neutrophil count (ANC), and platelet count. This participant had a 20.9-day increase in RBC lifespan on the second labeling study. Participant #6 (Supporting Information Figure 3, Panel B) remained at the same dose of hydroxyurea during and between both labeling studies. His hematologic parameters were similar across studies. His HbF level increased modestly from 25 to 26.9%, and there was a 3.3-day increase in RBC lifespan on the second study.
Discussion
Direct measurements of RBC survival to quantify hemolysis have been used in several clinical studies in HbSS.8,26–32 However, none of the contemporary human studies of treatments for HbSS or human physiological studies exploring the role of hemolysis in ostensibly hemolysis associated complications of HbSS (e.g., pulmonary hypertension, priapism, leg ulcers, and stroke) have directly measured hemolysis, relying instead on surrogate markers.2,4,7,25,33–39 Here we show that RBC labeling with orally administered 15N-glycine is a safe and practicable method to quantify total hemolysis and that biochemical surrogate markers of hemolysis (LDH, AST, bilirubin and plasma free hemoglobin) do not have a robust relationship with directly measured RBC survival in HbSS. Original 15N-glycine methods typically administered 40 – 50 g of approximately 30% purity 15N-glycine in multiple doses over 2 days and required about 25 mL of blood for isolation of heme at each time point.13 We used a single 2 g dose of 15N-glycine of 98% purity and 1 mL of blood for isolation of heme at each time point. This method is suitable for multi-institutional clinical studies because whole blood can be frozen, unprocessed, at clinical sites and stored for later shipment in batches to a central laboratory for processing and analysis.
In this study, the only clinical laboratory measurements that correlated with directly measured RBC survival were HbF level and reticulocyte count, consistent with prior studies.26–29,31,32 HbF is best considered a determinant of hemolysis (by reducing sickling) rather than a marker of hemolysis. HbF would also not be expected to change in studies of many treatments that might improve RBC lifespan (e.g., modifiers of hemoglobin oxygen affinity or RBC hydration), so HbF would not be a useful marker of hemolysis for such studies. This leaves reticulocyte count as the single surrogate marker with utility. Reticulocyte count is a measure of the marrow response to hemolysis. Hence, it is indirect and modifiable by inter-individual differences in reticulocyte maturation time19 and the ability of the marrow to respond to anemia, which may be limited by nutritional deficiencies, comorbid sickle nephropathy, hydroxyurea therapy, and even ineffective erythropoiesis.40 We found that about 70% of the variation in RBC survival can be explained by ARC (r2 = 0.71). Accordingly, ARC could be useful as a surrogate marker of hemolytic rate in population studies. However, it is not sufficiently accurate to quantify the rate of hemolysis in individual patients. For example, an ARC of 300,000/µL could be consistent with an RBC lifespan in the range of 18 to 29 days (Supporting Information Figure 4). This represents as much as a 61% difference in RBC lifespan for a given ARC.
In contrast to ARC and HbF, the commonly used biochemical markers of hemolysis, LDH, AST, and plasma free hemoglobin, did not correlate with directly quantified hemolysis. Even the correlation with bilirubin was weak. Prior studies also showed weak or absent correlation between bilirubin concentration and RBC survival in HbSS.26,31, 32 Because these biochemical markers do not correlate well with directly quantified hemolysis individually, composite measures of these same markers, such as the “hemolytic index” or “hemolytic component” (derived from the combination of LDH, AST, total bilirubin and reticulocyte count)25,35–37 may need to be reassessed. Indeed, we found that the hemolytic component (calculated according to Gordeuk et al.36; data not shown) performed no better than ARC as a surrogate of mean RBC survival (correlation of hemolytic component with mean RBC survival, r = −0.73, P = 0.004; correlation of ARC with mean RBC survival, r = −0.84, P < 0.001). That is, in our data, the hemolytic component provided, at best, no more information about mean RBC survival than ARC alone.
Why biochemical surrogates may not be robust markers of hemolysis in HbSS, specifically, has been discussed elsewhere.8 For example, LDH has many tissue sources, and different tissues contain different proportions of the 5 isoenzymes of LDH. A study of sickle cell disease showed that increases in the LD-1 and LD-2 isozymes of LDH mainly explained the elevation in total serum LDH.2 While LD-1 and LD-2 are the predominant isozymes in RBCs, they are also the predominant isozymes in cardiac muscle, kidneys, leukocytes, and the brain, all of which may be affected in HbSS. That is, there are no LDH isozymes expressed only in RBCs. We did not study LDH isozymes here, so the relationship between directly measured RBC survival and LD-1 and LD-2 levels is not known in HbSS and may be better than we observed for total LDH. Moreover, the relationship between LDH and RBC survival that has been established for hemolysis (presumably purely intravascular) due to cardiac valves,41 has not been established for HbSS. Indeed, we found no such relationship—neither linear nor non-linear. Given this lack of relationship, if LDH hypothetically reflected only intravascular hemolysis, but not total hemolysis (intravascular plus extravascular), then the contribution of intravascular hemolysis to total hemolysis in HbSS must be relatively small and/or sporadic in HbSS. These biochemical markers, however, are not likely to reflect intravascular hemolysis only. For example, patients with predominantly extravascular hemolysis, such as those with hereditary spherocytosis, may have elevated LDH and decreased haptoglobin.42 Taken together, our data reinforce the notion that LDH may be a better marker of multiple tissue injury than rate of hemolysis in HbSS.10
Elevations of LDH and other biochemical surrogate markers of hemolysis have also been associated with elevated TRV, which is one argument for a role of hemolysis in the genesis of pulmonary hypertension in HbSS.43,44 We also found a positive linear correlation between TRV and LDH. However, we found no correlation between TRV and directly measured RBC survival in these same individuals (Supporting Information Figure 2). Our data indicate that the relationship between TRV and LDH, while valid and reproducible, is not readily explained by total hemolytic rate. We did not directly address the role of intravascular hemolysis in the development of pulmonary arterial hypertension. Instead, we examined the performance of the biomarkers that have been used, in part, to support a causal link between hemolysis and complications of HbSS in human studies, including pulmonary hypertension. There are, however, explanations for pulmonary hypertension in HbSS instead of—or in addition to—intravascular hemolysis. Several groups have shown that elevated TRV in at least half of patients with HbSS can be largely explained by left heart disease, which produces secondary, post-capillary pulmonary hypertension, rather than primary pulmonary vascular disease.45–48 We have confirmed this finding in a meta-analysis and also demonstrated that a cardiomyopathy with restrictive physiology is at least one cause of this left heart disease in both humans with HbSS49,50 and sickle mice.51,52 In contrast, intravascular hemolysis could predispose to pre-capillary pulmonary hypertension, but even pre-capillary pulmonary hypertension can be a secondary complication of left heart disease.53–55
The main limitation of the 15N-glycine RBC labeling method is that serial blood sampling is required (26 time-points for this study). However, the number of time-points can be reduced depending on the precision in measurement of RBC lifespan that is required for a particular application (Supporting Information Figure 5). The main limitation of this study is sample size. We studied a small sample of non-randomly selected individuals. The participants in this study had a wide range of clinical characteristics (Table) that could mitigate the degree of hemolysis (e.g, hydroxyurea therapy, α-globin genotype and HbF level), but all had marked hemolysis as indicated by 50–85% shortening of mean RBC survival (Table, Figure 1, Supporting Table). We also present only 13 observations to study the relationship between directly measured RBC survival and surrogate markers of hemolysis. Our findings of correlation of RBC survival with reticulocyte count and HbF and no correlation with bilirubin are consistent with prior studies.26–29,31,32 We are not aware of any other systematic studies exploring the association of LDH, AST or plasma free hemoglobin with directly measured RBC survival in HbSS. There certainly could be weaker correlations that we could not detect in this small sample, but there were no robust correlations (as found with reticulocyte count and HbF). At least until more studies are done, the preponderance of evidence indicates that biochemical surrogate markers of hemolysis do not have a robust relationship with directly measured RBC survival in HbSS. We caution that past inferences about hemolytic rate in HbSS, specifically, based only on these biochemical surrogates may need to be reconsidered. While elevated LDH levels have predictive value and are associated with certain clinical complications of HbSS,56–58 our data suggest that the biological link between levels of LDH and outcomes may not be mediated by hemolysis.
When designing studies using this stable isotope method, it is important to consider two points about its practical application and the interpretation of its results. First, it not an instantaneous measure of hemolytic rate, so it cannot be used to measure rapid changes in RBC survival that might occur after only a few days of an intervention. However, it can be applied to therapeutic trials of longer duration, such as the comparison of preventive therapies. Illustrating the utility of this method for clinical trials, the participants in this study who had two RBC survival measurements had interval increases in HbF that were associated with proportional increases in RBC survival. Second, neither this nor any other current method can quantify the relative contributions of intravascular and extravascular hemolysis in HbSS. The notion that one-third of total hemolysis in HbSS occurs in the intravascular compartment and two-thirds occurs in the extravascular compartment stems from a single mathematical estimate put forth in a review article 60 years ago.59 There are likely to be untoward manifestations of HbSS disease that result specifically from intravascular hemolysis. Unfortunately, we can only quantify total hemolysis in humans.11,60 We cannot directly measure the relative contributions of the intravascular and extravascular compartments of hemolysis. This is an important and unmet need that needs to be addressed before the consequences of intravascular hemolysis in humans with HbSS can be properly studied. This problem also needs to be solved to determine which biomarkers of hemolysis correlate best with intravascular hemolysis, extravascular hemolysis, both, or neither.
In summary, RBC labeling with orally administered 15N-glycine is a safe and practicable method to measure RBC survival directly, and thereby quantify total hemolysis. Commonly used biochemical surrogate markers of hemolytic rate (LDH, AST, bilirubin, and plasma free hemoglobin) do not correlate with directly measured RBC survival and need to be interpreted cautiously in therapeutic and physiologic studies in HbSS. Moreover, a measurement of intravascular hemolysis is lacking yet needed to advance the filed. In contrast to these biochemical markers, ARC and HbF level did correlate with directly measured RBC survival. ARC was the best correlate of total hemolysis, but only 70% of the variation in RBC survival was reflected in this marker. If greater accuracy is required for physiological studies or clinical trials, 15N-glycine RBC labeling can directly and accurately quantify total hemolysis.
Supplementary Material
Acknowledgments
This study was supported by the NIH Clinical and Translational Science Award (CTSA) program (1UL1TR001425) and the NIH-NHLBI Excellence in Hemoglobinopathy Research Award (EHRA) program (U01HL117709), and a VA Merit Award (1 I01 CX000121).
We are indebted to Courtney Little, Amy Shova, and Megan Reynolds for assistance with recruitment of participants and collection of clinical data.
Footnotes
Authorship
Design of research: CTQ, CHJ, RMC, RSF
Recruitment of participants: CTQ, ON
Collection of clinical data: CTQ, ON
Sample processing: EPS, SA, PKK
Analysis of data: CJL, CTQ
Writing and editing of manuscript: CTQ, EPS, AS, PKK, CJL, ON, CHJ, RSF, RMC
The authors have no conflicts of interest to declare.
References
- 1.Rother RP, Bell L, Hillmen P, Gladwin MT. The clinical sequelae of intravascular hemolysis and extracellular plasma hemoglobin: a novel mechanism of human disease. JAMA. 2005;293(13):1653–1662. doi: 10.1001/jama.293.13.1653. [DOI] [PubMed] [Google Scholar]
- 2.Kato GJ. Lactate dehydrogenase as a biomarker of hemolysis-associated nitric oxide resistance, priapism, leg ulceration, pulmonary hypertension, and death in patients with sickle cell disease. Blood. 2006;107(6):2279–2285. doi: 10.1182/blood-2005-06-2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morris CR, Kato GJ, Poljakovic M, et al. Dysregulated arginine metabolism, hemolysis-associated pulmonary hypertension, and mortality in sickle cell disease. JAMA. 2005;294(1):81–90. doi: 10.1001/jama.294.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ataga KI, Smith WR, De Castro LM, et al. Efficacy and safety of the Gardos channel blocker, senicapoc (ICA-17043), in patients with sickle cell anemia. Blood. 2008;111(8):3991–3997. doi: 10.1182/blood-2007-08-110098. [DOI] [PubMed] [Google Scholar]
- 5.Ataga KI, Reid M, Ballas SK, et al. Improvements in haemolysis and indicators of erythrocyte survival do not correlate with acute vaso-occlusive crises in patients with sickle cell disease: a phase III randomized, placebo-controlled, double-blind study of the Gardos channel blocker senicapoc (ICA-17043) Br J Haematol. 2011;153(1):92–104. doi: 10.1111/j.1365-2141.2010.08520.x. [DOI] [PubMed] [Google Scholar]
- 6.Oksenberg D, Dufu K, Patel MP, et al. GBT440 increases haemoglobin oxygen affinity, reduces sickling and prolongs RBC half-life in a murine model of sickle cell disease. Br J Haematol. 2016 doi: 10.1111/bjh.14214. [DOI] [PubMed] [Google Scholar]
- 7.Lehrer-Graiwer J, Howard J, Hemmaway CJ, et al. GBT440, a Potent Anti-Sickling Hemoglobin Modifier Reduces Hemolysis, Improves Anemia and Nearly Eliminates Sickle Cells in Peripheral Blood of Patients with Sickle Cell Disease. Blood. 2015;126(23):542. [Google Scholar]
- 8.Hebbel RP. Reconstructing sickle cell disease: A data-based analysis of the “hyperhemolysis paradigm” for pulmonary hypertension from the perspective of evidence-based medicine. Am J Hematol. 2011;86(2):123–154. doi: 10.1002/ajh.21952. [DOI] [PubMed] [Google Scholar]
- 9.Bunn HF, Nathan DG, Dover GJ, et al. Pulmonary hypertension and nitric oxide depletion in sickle cell disease. Blood. 2010;116(5):687–692. doi: 10.1182/blood-2010-02-268193. [DOI] [PubMed] [Google Scholar]
- 10.Ballas SK. Lactate dehydrogenase and hemolysis in sickle cell disease. Blood. 2013;121(1):243–244. doi: 10.1182/blood-2012-10-462135. [DOI] [PubMed] [Google Scholar]
- 11.Franco RS. The measurement and importance of red cell survival. Am J Hematol. 2009;84(2):109–114. doi: 10.1002/ajh.21298. [DOI] [PubMed] [Google Scholar]
- 12.Shemin D, Rittenberg D. The life span of the human red blood cell. J Biol Chem. 1946;166(2):627–636. [PubMed] [Google Scholar]
- 13.London IM, Shemin D. Heme synthesis and red blood cell dynamics in normal humans and in subjects with polycythemia vera, sickle-cell anemia, and pernicious anemia. J Biol Chem. 1949;179(1):463–484. [PubMed] [Google Scholar]
- 14.Berlin NI, Waldmann TA, Weissman SM. Life span of red blood cell. Physiol Rev. 1959;39(3):577–616. doi: 10.1152/physrev.1959.39.3.577. [DOI] [PubMed] [Google Scholar]
- 15.Khera PK, Smith EP, Lindsell CJ, et al. Use of an oral stable isotope label to confirm variation in red blood cell mean age that influences HbA1c interpretation. Am J Hematol. 2015;90(1):50–55. doi: 10.1002/ajh.23866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Egyed A. A simple method for heme isolation. Experientia. 1972;28(11):1396–1397. doi: 10.1007/BF01965368. [DOI] [PubMed] [Google Scholar]
- 17.Browne TR, Szabo GK, Ajami A, Wagner D. Performance of human mass balance/metabolite identification studies using stable isotope (13C, 15N) labeling and continuous-flow isotope-ratio mass spectrometry as an alternative to radioactive labeling methods. J Clin Pharmacol. 1993;33(3):246–252. doi: 10.1002/j.1552-4604.1993.tb03951.x. [DOI] [PubMed] [Google Scholar]
- 18.Lindsell CJ, Franco RS, Smith EP, Joiner CH, Cohen RM. A method for the continuous calculation of the age of labeled red blood cells. Am J Hematol. 2008;83(6):454–457. doi: 10.1002/ajh.21148. [DOI] [PubMed] [Google Scholar]
- 19.Franco RS, Lohmann J, Silberstein EB, et al. Time-dependent changes in the density and hemoglobin F content of biotin-labeled sickle cells. J Clin Invest. 1998;101(12):2730–2740. doi: 10.1172/JCI2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Franco RS, Yasin Z, Palascak MB, et al. The effect of fetal hemoglobin on the survival characteristics of sickle cells. Blood. 2006;108(3):1073–1076. doi: 10.1182/blood-2005-09-008318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Higgs DR, Aldridge BE, Lamb J, et al. The interaction of alpha-thalassemia and homozygous sickle-cell disease. N Engl J Med. 1982;306(24):1441–1446. doi: 10.1056/NEJM198206173062402. [DOI] [PubMed] [Google Scholar]
- 22.Embury SH, Dozy AM, Miller J, et al. Concurrent sickle-cell anemia and alpha-thalassemia: effect on severity of anemia. N Engl J Med. 1982;306(5):270–274. doi: 10.1056/NEJM198202043060504. [DOI] [PubMed] [Google Scholar]
- 23.Lin EE, Rodgers GP, Gladwin MT. Hemolytic anemia-associated pulmonary hypertension in sickle cell disease. Curr Hematol Rep. 2005;4(2):117–125. [PubMed] [Google Scholar]
- 24.Liem RI, Young LT, Thompson AA. Tricuspid regurgitant jet velocity is associated with hemolysis in children and young adults with sickle cell disease evaluated for pulmonary hypertension. Haematologica. 2007;92(11):1549–1552. doi: 10.3324/haematol.11576. [DOI] [PubMed] [Google Scholar]
- 25.Minniti CP, Sable C, Campbell A, et al. Elevated tricuspid regurgitant jet velocity in children and adolescents with sickle cell disease: association with hemolysis and hemoglobin oxygen desaturation. Haematologica. 2009;94(3):340–347. doi: 10.3324/haematol.13812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Serjeant GR, Serjeant BE, Milner PF. The irreversibly sickled cell; a determinant of haemolysis in sickle cell anaemia. Br J Haematol. 1969;17(6):527–533. doi: 10.1111/j.1365-2141.1969.tb01403.x. [DOI] [PubMed] [Google Scholar]
- 27.McCurdy PR. 32-DFP and 51-Cr for measurement of red cell life span in abnormal hemoglobin syndromes. Blood. 1969;33(2):214–224. [PubMed] [Google Scholar]
- 28.Gillette PN, Manning JM, Cerami A. Increased survival of sickle-cell erythrocytes after treatment in vitro with sodium cyanate. Proc Natl Acad Sci U S A. 1971;68(11):2791–2793. doi: 10.1073/pnas.68.11.2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Milner PF, Charache S. Life span of carbamylated red cells in sickle cell anemia. J Clin Invest. 1973;52(12):3161–3171. doi: 10.1172/JCI107516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bensinger TA, Gillette PN. Hemolysis in sickle cell disease. Arch Intern Med. 1974;133(4):624–631. [PubMed] [Google Scholar]
- 31.McCurdy PR, Mahmood L, Sherman AS. Red cell life span in sickle cell-hemoglobin C disease with a note about sickle cell-hemoglobin O ARAB. Blood. 1975;45(2):273–279. [PubMed] [Google Scholar]
- 32.McCurdy PR, Sherman AS. Irreversibly sickled cells and red cell survival in sickle cell anemia: a study with both DF32P and 51CR. Am J Med. 1978;64(2):253–258. doi: 10.1016/0002-9343(78)90053-0. [DOI] [PubMed] [Google Scholar]
- 33.Olnes M, Chi A, Haney C, et al. Improvement in hemolysis and pulmonary arterial systolic pressure in adult patients with sickle cell disease during treatment with hydroxyurea. Am J Hematol. 2009;84(8):530–532. doi: 10.1002/ajh.21446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gladwin MT, Sachdev V, Jison ML, et al. Pulmonary hypertension as a risk factor for death in patients with sickle cell disease. N Engl J Med. 2004;350(9):886–895. doi: 10.1056/NEJMoa035477. [DOI] [PubMed] [Google Scholar]
- 35.Gordeuk VR, Sachdev V, Taylor JG, et al. Relative systemic hypertension in patients with sickle cell disease is associated with risk of pulmonary hypertension and renal insufficiency. Am J Hematol. 2008;83(1):15–18. doi: 10.1002/ajh.21016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gordeuk VR, Campbell A, Rana S, et al. Relationship of erythropoietin, fetal hemoglobin, and hydroxyurea treatment to tricuspid regurgitation velocity in children with sickle cell disease. Blood. 2009;114(21):4639–4644. doi: 10.1182/blood-2009-04-218040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gordeuk VR, Minniti CP, Nouraie M, et al. Elevated tricuspid regurgitation velocity and decline in exercise capacity over 22 months of follow up in children and adolescents with sickle cell anemia. Haematologica. 2011;96(1):33–40. doi: 10.3324/haematol.2010.030767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Milton JN, Rooks H, Drasar E, et al. Genetic determinants of haemolysis in sickle cell anaemia. Br J Haematol. 2013;161(2):270–278. doi: 10.1111/bjh.12245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nouraie M, Lee JS, Zhang Y, et al. The relationship between the severity of hemolysis, clinical manifestations and risk of death in 415 patients with sickle cell anemia in the US and Europe. Haematologica. 2013;98(3):464–472. doi: 10.3324/haematol.2012.068965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu CJ, Krishnamurti L, Kutok JL, et al. Evidence for ineffective erythropoiesis in severe sickle cell disease. Blood. 2005;106(10):3639–3645. doi: 10.1182/blood-2005-04-1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Myhre E, Rasmussen K, Andersen A. Serum lactic dehydrogenase activity in patients with prosthetic heart valves: a parameter of intravascular hemolysis. Am Heart J. 1970;80(4):463–468. doi: 10.1016/0002-8703(70)90192-4. [DOI] [PubMed] [Google Scholar]
- 42.Gallagher PG. In: Red blood cell membrane disorders, in Hematology: Basic Principles and Practice. 6th. Hoffman, Benz, Silberstein, Heslop, Weitz, Anastasi, editors. Elsevier Health Sciences; 2012. [Google Scholar]
- 43.Kato GJ, Gladwin MT, Steinberg MH. Deconstructing sickle cell disease: reappraisal of the role of hemolysis in the development of clinical subphenotypes. Blood Rev. 2007;21(1):37–47. doi: 10.1016/j.blre.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gladwin MT. Revisiting the hyperhemolysis paradigm. Blood. 2015;126(6):695–696. doi: 10.1182/blood-2015-06-649491. [DOI] [PubMed] [Google Scholar]
- 45.Sachdev V, Machado RF, Shizukuda Y, et al. Diastolic Dysfunction Is an Independent Risk Factor for Death in Patients With Sickle Cell Disease. J Am Coll Cardiol. 2007;49(4):472–479. doi: 10.1016/j.jacc.2006.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dham N, Ensing G, Minniti C, et al. Prospective echocardiography assessment of pulmonary hypertension and its potential etiologies in children with sickle cell disease. Am J Cardiol. 2009;104(5):713–720. doi: 10.1016/j.amjcard.2009.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Parent F, Bachir D, Inamo J, et al. A hemodynamic study of pulmonary hypertension in sickle cell disease. N Engl J Med. 2011;365(1):44–53. doi: 10.1056/NEJMoa1005565. [DOI] [PubMed] [Google Scholar]
- 48.Fonseca GHH, Souza R, Salemi VMC, Jardim CVP, Gualandro SFM. Pulmonary hypertension diagnosed by right heart catheterisation in sickle cell disease. Eur. Respir. J. 2012;39(1):112–118. doi: 10.1183/09031936.00134410. [DOI] [PubMed] [Google Scholar]
- 49.Niss O, Quinn CT, Lane A, et al. Cardiomyopathy with Restrictive Physiology in Sickle Cell Disease. JACC Cardiovasc Imaging. 2016;9(3):243–252. doi: 10.1016/j.jcmg.2015.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Caughey MC, Ataga KI, Hinderliter AL. Sickle Cardiomyopathy: The Missing Forest in the Trees. JACC Cardiovasc Imaging. 2016;9(3):253–254. doi: 10.1016/j.jcmg.2015.06.024. [DOI] [PubMed] [Google Scholar]
- 51.Bakeer N, James J, Roy S, et al. Sickle cell anemia mice develop a unique cardiomyopathy with restrictive physiology. Proc Natl Acad Sci U S A. 2016;113(35):E5182–E5191. doi: 10.1073/pnas.1600311113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wood JC. The heart in sickle cell disease, a model for heart failure with preserved ejection fraction. Proc Natl Acad Sci U S A. 2016;113(35):9670–9672. doi: 10.1073/pnas.1611899113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lam CSP, Roger VL, Rodeheffer RJ, et al. Pulmonary hypertension in heart failure with preserved ejection fraction: a community-based study. J Am Coll Cardiol. 2009;53(13):1119–1126. doi: 10.1016/j.jacc.2008.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guazzi M, Borlaug BA. Pulmonary hypertension due to left heart disease. Circulation. 2012;126(8):975–990. doi: 10.1161/CIRCULATIONAHA.111.085761. [DOI] [PubMed] [Google Scholar]
- 55.Vachiéry J-L, Adir Y, Barbera JA, et al. Pulmonary hypertension due to left heart diseases. J Am Coll Cardiol. 2013;62(25 Suppl):D100–D108. doi: 10.1016/j.jacc.2013.10.033. [DOI] [PubMed] [Google Scholar]
- 56.O'Driscoll S, Height SE, Dick MC, Rees DC. Serum lactate dehydrogenase activity as a biomarker in children with sickle cell disease. Br J Haematol. 2008;140(2):206–209. doi: 10.1111/j.1365-2141.2007.06894.x. [DOI] [PubMed] [Google Scholar]
- 57.Stankovic Stojanovic K, Steichen O, Lefevre G, et al. High lactate dehydrogenase levels at admission for painful vaso-occlusive crisis is associated with severe outcome in adult SCD patients. Clin Biochem. 2012;45(18):1578–1582. doi: 10.1016/j.clinbiochem.2012.07.114. [DOI] [PubMed] [Google Scholar]
- 58.Gurkan S, Scarponi KJ, Hotchkiss H, Savage B, Drachtman R. Lactate dehydrogenase as a predictor of kidney involvement in patients with sickle cell anemia. Pediatr Nephrol. 2010;25(10):2123–2127. doi: 10.1007/s00467-010-1560-8. [DOI] [PubMed] [Google Scholar]
- 59.Crosby WH. The metabolism of hemoglobin and bile pigment in hemolytic disease. Am J Med. 1955;18(1):112–122. doi: 10.1016/0002-9343(55)90208-4. [DOI] [PubMed] [Google Scholar]
- 60.Franco RS. Measurement of Red Cell Lifespan and Aging. Transfus Med Hemother. 2012;39(5):302–307. doi: 10.1159/000342232. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.