Abstract
Objective
This study determined whether having minor children in the home was associated with the Teachable Moment (TM) constructs of lung cancer worry, perceived risk, health-related self-concept, and the novel construct of synergistic risk.
Design
Secondary data analysis of baseline data from a randomized controlled trial of an intervention to reduce home exposure to radon and secondhand smoke (SHS).
Sample
Quota sample of adults recruited at a Central Kentucky academic medical center (N = 556).
Measurements
Survey items assessed lung cancer worry, perceived risk, synergistic risk perception, and health-related self-concept.
Results
The presence of children in the home was not a significant predictor of any construct needed to create a teachable moment for lung cancer prevention. Individuals with children living in the home were more likely to be younger, a racial/ethnic minority, a current smoker, and live with a smoker compared to those without children in the home.
Conclusions
There is a critical need to raise parental awareness on child health inequities related to the home exposure to radon and SHS. Public health nurses can create teachable moments for lung cancer prevention through greater awareness of the risks posed by radon and SHS along with promoting home testing and low cost resources to reduce risk.
Keywords: child, adolescent, radon, tobacco smoke pollution, lung neoplasms
Background
Concern for a child’s health can motivate parents to address environmental exposures (Escoffery, Kegler, & Butler, 2008; Jones et al., 2011). The home is a significant source of environmental exposures including radon and secondhand smoke (SHS), which are known to cause lung cancer (U.S. Department of Health and Human Services, 2005; United States Department of Health and Human Services, 2010). These environmental exposures are particularly relevant for children and adolescents, who spend as many as 15 hours a day indoors (U.S. Environmental Protection Agency, 2011; Weitzman et al., 2013). Children are considered more sensitive to the decay products of radon than adults (Kendall & Smith, 2005). Although, lung cancer is a disease of adulthood, exposure to radon in childhood may increase the lifetime risk for lung cancer (Chen, 2013). To decrease lung cancer risk for adults and children in the home environment, interventions are needed to reduce tobacco use, exposure to radon, and SHS.
The burden of lung cancer in the United States is substantial. In 2015, there were an estimated 224,390 new cases of lung cancer along with 158,080 lung cancer deaths (American Cancer Society, 2016). The leading cause of lung cancer is firsthand smoking, followed by radon and SHS (Henley et al., 2014; National Cancer Institute, 2014). Approximately 40,000 of the annual lung cancer deaths are radon-induced (Kim, Hwang, Cho, & Kang, 2016; National Research Council, 1999). Radon is a colorless, tasteless, odorless gas that results from the breakdown of uranium in soil or ground water and it can seep into homes without detection. The threat of radon is present in all 50 states. The risk for developing lung cancer is heightened when individuals are exposed to a combination of radon gas and tobacco smoke, referred to as synergistic risk (National Research Council, 1999). Research is needed to determine if the presence of children and adolescents in the home supports health behavior change to address indoor environmental exposures such as radon and SHS.
A health-related event such as a cancer diagnosis, a stroke, or a pregnancy can serve as a teachable moment (TM) to cue health behavior change (McBride, Emmons, & Lipkus, 2003). The TM model posits that cueing events are associated with three major psychosocial factors: (a) perceived risk, (b) prompting emotions such as worry and (c) health-related self-concept (see Figure 1). To date, research on the TM has been a challenge given that health events, such as cancer diagnoses, are typically not predicted or randomly assigned (McBride, et al., 2003). Therefore, the TM model has been tested only with existing conditions (e.g., cancer diagnosis) using retrospective designs. A better understanding of how to create and amplify teachable moments to increase awareness of indoor environmental exposures is needed in order to prevent lung cancer.
Figure 1.
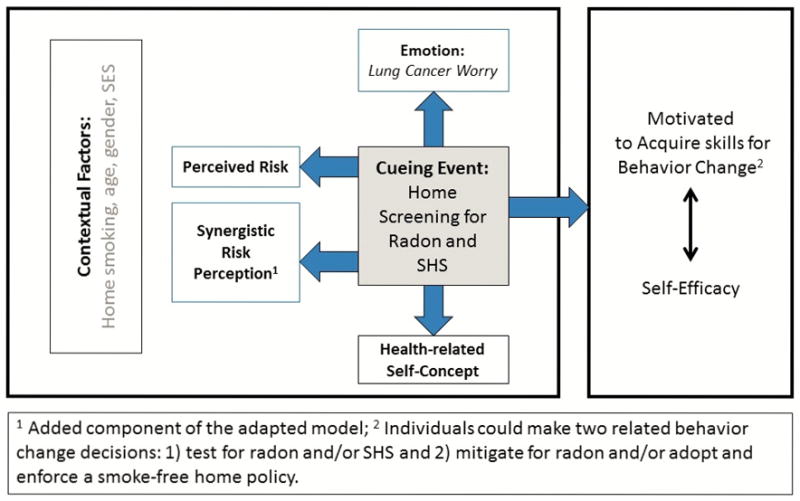
Adapted Teachable Moment™ Model (McBride, 2003 & 2008)
The purposes of this study were to: 1) determine whether having minor children in the home, as well as other demographic and personal characteristics, were associated with lung cancer worry, perceived risk, synergistic risk perception (radon plus smoking), and health-related self-concept; and 2) assess whether those with and without minor children in the home differed on demographic or personal characteristics related to lung cancer prevention. Although the presence of children in the home is not a traditional TM cueing event, those with children in the home may respond differently to the TM constructs. We hypothesized that having children in the home would be associated with lung cancer worry, perceived risk of lung cancer, synergistic risk, and health-related self-concept. Understanding whether or not the presence of children in the home is associated with the TM constructs will contribute to the development of targeted interventions to reach those most at risk for lung cancer.
Methods
Design and Sample
A quota sample comprised of 556 adults who completed a baseline survey as part of a randomized controlled trial to test an intervention to reduce home exposure to radon and SHS. Participants were recruited in the waiting areas of a family medicine clinic, pediatric clinic, and a pharmacy located in a Central Kentucky academic medical center between January 2013 and September 2015. Study recruiters approached potential participants by inquiring if they were interested in learning about a research study to reduce the risk of lung cancer through home testing for radon and SHS. Through quota sampling, participants over 18 years of age were recruited into one of four categories: 1) renter with at least one smoker in the home; 2) renter without a smoker in the home; 3) homeowner with at least one smoker in the home; and 4) homeowner without a smoker in the home. Electronic surveys were administered via iPads to assess sociodemographic factors and the TM constructs of lung cancer worry, lung cancer risk, synergistic risk and health-related self-concept. The academic medical center Institutional Review Board approved all study protocols and informed consent procedures.
Measures
Lung cancer worry
The Lung Cancer Worry Scale (Lerman et al., 1991) contains four questions including: ‘How much do you currently worry about getting lung cancer some day?,’ ‘How much do worries about lung cancer impact your mood?,’ ‘How much do worries about lung cancer impact your daily activities?,’ and ‘When you worry about lung cancer, how difficult is it to control these worries?’ The first item was rated on a 5-point scale ranging from (1) ‘Not at all’ to (5) ‘Almost all of the time.’ The remaining items had response options on a 4-point scale ranging from (1) ‘Not at all’ to (4) ‘A lot.’ So that each item contributed the same weight to the summary score, the first item was rescaled to a maximum possible score of 4. The total score was the sum of the four items, with a potential range from 4–16; higher scores indicate greater worry. Cronbach’s alpha for this scale was 0.83.
Lung cancer risk
One item measured a participant’s perceived lung cancer risk. Participants were asked to rate their risk of developing lung cancer in their lifetime on a scale of 0–10, with 10 representing the highest perceived risk.
Synergistic risk
Since the risk factors of radon and tobacco smoke are related, with more radon-related lung cancers among those with a history of smoking (National Research Council, 1999), we added synergistic risk perception as a novel component of the TM model. Participants were asked to rate the risk from being exposed to radon AND smoking a pack of cigarettes per day, compared to the risk of smoking only a pack of cigarettes a day with no radon exposure. Responses followed a 5-point Likert scale ranging from (1) ‘Much less risky’ to (5) ‘Much more risky.’
Health-related self-concept
The health-protective motivation subscale of the 8-item HRSC-76 measured health-related self-concept including health-enhancing behaviors, behavioral intention, attitudes towards health-protective behavior, and self-efficacy (Wiesmann, Niehorster, Hannich, & Hartmann, 2008). Sample items from the subscale include, “I actively take care of my health” and “Practicing healthy behaviors is good for me.” Participants are asked to rate the degree they believe the statement applies to them, ranging from (1) ‘Disagree entirely’ to (7) ‘Agree entirely.’ All but one item are positively worded so higher scores reflect greater beliefs; the one negatively worded item was reverse coded prior to creating the summary score. The summary score was calculated by summing the 8 items; the potential range is 8–56, with higher scores indicating greater health-related self-concept. Cronbach’s alpha for this scale was 0.91.
Sociodemographic and personal characteristics
Age (in years), gender (male/female/transgender), and race/ethnicity were assessed. The race variable included five racial categories and ‘more than one race.’ Ethnicity was recorded as ‘Hispanic’ or ‘non-Hispanic.’ Since each minority category included a relatively small percentage of participants, the race/ethnicity variable was categorized as ‘White/non-Hispanic’ versus ‘Other’ for the purpose of this analysis. Education was recorded with an 8-category ordinal variable ranging from ‘Never attended/only Kindergarten’ to ‘Postgraduate education.’ This variable was dichotomized to ‘At most high school’ versus ‘At least some college’ prior to analysis.
Participants were asked when was the last time they smoked a cigarette and response options ranged from ‘today’ to ‘more than 1 year ago;’ ‘I have never smoked cigarettes’ was an option. Current smokers were those who had smoked in the last 30 days; all other responses were coded as non-smokers. We used the binary form of smoking status since the majority of non-smokers in the sample had never smoked (63%); in addition, 91% of former smokers had last smoked more than one year ago. Participants were asked whether anyone in their family had ever been diagnosed with lung cancer (yes/no), whether they owned or rented their home, whether there were any cigarette smokers living in the home (yes/no), and whether there were any children under the age of 18 living in the home (yes/no).
Analytic Strategy
Descriptive statistics, including mean and standard deviation or frequency distribution, were used to summarize the study variables. Two-sample t-tests or chi-square tests of association were used to compare demographic and personal characteristics between those with and without minor children in the home. Multiple linear regression was used to determine the predictors of the Teachable Moment constructs, with a model for each of the four outcomes. To assess the effect of interactions among demographic and personal characteristics on the outcomes, all relevant two-way terms were included in the initial models and then removed if they did not meet the alpha level for significance. Contrasts were used to discern the differences among combinations of the significant interaction term. Variance inflation factors assessed the presence of multicollinearity. Data analysis was conducted using SAS, v. 9.3; an alpha level of .01 was used throughout to protect against Type I error in light of multiple comparisons.
Results
The average age of the participants was 50.4 years; those with children in the home were younger (M=43.2) than those without children (M=54.5; see Table 1). In the full sample of 556 participants, 67% were female and 83% had completed at least some college; there was no association between gender or education and whether or not there were children in the home. Those with children in the home were less likely to be White/non-Hispanic (79%) compared to those without minor children (87%). There was a larger percentage of current smokers among participants with children (33%) compared to those without (21%). In the full sample, 24% had a family history of lung cancer and 92% were homeowners; there was no association between having a child in the home and either of these two variables. Those with children in a home were more likely to have one or more smokers living there (57%), compared to those without children (45%).
Table 1.
Descriptive summary of demographic and personal characteristics by whether there are children in the home, with group comparisons using chi-square test or two-sample t-test (N = 556)
| Full sample (N = 556) | Children in the Home
|
Comparison between those with and without children in home | ||
|---|---|---|---|---|
| Yes (n = 203) | No (n = 353) | |||
| Variable | Mean (SD) or n (%) | Mean (SD) or n (%) | Mean (SD) or n (%) | χ2 or t (p) |
| Age | 50.4 (13.0) | 43.2 (10.8) | 54.5 (12.4) | 11.3 (<.001)a |
| Gender | ||||
| Male | 182 (32.7%) | 64 (31.5%) | 118 (33.4%) | 0.2 (.64) |
| Female | 374 (67.3%) | 139 (68.5%) | 235 (66.6%) | |
| Race/ethnicity | ||||
| White/non-Hispanic | 465 (83.9%) | 159 (78.7%) | 306 (86.9%) | 6.4 (.011) |
| Other race/ethnicity | 89 (16.1%) | 43 (21.3%) | 46 (13.1%) | |
| Education | ||||
| High school or below | 92 (16.6%) | 35 (17.2%) | 57 (16.2%) | 0.1 (.75) |
| At least some college | 463 (83.4%) | 168 (82.8%) | 295 (83.8%) | |
| Smoking status | ||||
| Smoker | 142 (25.6%) | 67 (33.0%) | 75 (21.3%) | 9.2 (.002) |
| Non-smoker | 413 (74.4%) | 136 (67.0%) | 277 (78.7%) | |
| Family history of lung cancer | ||||
| Yes | 132 (23.7%) | 48 (23.7%) | 84 (23.8%) | <0.1 (.97) |
| No | 424 (76.3%) | 155 (76.3%) | 269 (76.2%) | |
| Homeowner status | ||||
| Homeowner | 509 (91.6%) | 190 (93.6%) | 319 (90.4%) | 1.7 (.19) |
| Renter | 47 (8.4%) | 13 (6.4%) | 34 (9.6%) | |
| Smoker(s) in the home | ||||
| Yes | 274 (49.3%) | 116 (57.1%) | 158 (44.8%) | 7.9 (.005) |
| No | 282(50.7%) | 87 (42.9%) | 195 (55.2%) | |
two-sample t-test; all other comparisons used chi-square test
The average score on the Lung Cancer Worry Scale was 6.2 (SD=2.5) out of a possible total of 16, representing 39% of the maximum possible score. The average perceived lung cancer risk was 3.9 (SD=2.5), or 39% of the maximum possible score of 10. The mean score for synergistic risk was 3.9 (SD=1.0), representing 78% of the maximum score possible. Finally, health-related self-concept scores were relatively high: out of a possible total score of 56, the average score was 46.4 (SD=8.1), representing 83% of the maximum total score.
Predictors of TM constructs
The regression models for lung cancer worry, lung cancer risk, synergistic risk and health-related self-concept were all significant (Tables 2 and 3). For the outcomes of lung cancer worry, synergistic risk, and health related self-concept, the variance inflation factors were less than 1.7. For the model that had lung cancer risk as the outcome, which included the interaction between gender and education, the VIFs for the gender main effect and the interaction were between 7 and 8, but this is consistent with inclusion of an interaction term; the remaining VIFs were less than 2. Thus for all models, there is no evidence to suggest the presence of multicollinearity among predictors. Having children in the home was not a significant predictor of any of the TM constructs.
Table 2.
Multiple linear regressions modeling lung cancer worry and perceived lung cancer risk
| Lung cancer worry (n = 551)
|
Perceived lung cancer risk (n = 551)
|
|||||
|---|---|---|---|---|---|---|
| Est. β [95% CI*] | Std. β | p | Est. β [95% CI*] | Std. β | p | |
| Age | 0.005 [−0.012, 0.021] | 0.024 | .58 | −0.006 [−0.022, 0.009] | −0.033 | .42 |
| Male | −0.091 [−0.487, 0.305] | −0.017 | .65 | 1.138 [0.121, 2.156] | 0.209 | .028 |
| White/non-Hispanic | −0.568 [−1.078, −0.058] | −0.083 | .029 | −0.093 [−0.592, 0.407] | −0.013 | .72 |
| At least some college | −1.532 [−2.054, −1.009] | −0.226 | <.001 | −0.613 [−1.194, −0.033] | −0.089 | .038 |
| Current smoker | 1.107 [0.572, 1.642] | 0.192 | <.001 | 1.486 [0.963, 2.008] | 0.254 | <.001 |
| Family history of lung cancer | 0.605 [0.174, 1.035] | 0.102 | .006 | 1.122 [0.702, 1.541] | 0.187 | <.001 |
| Homeowner | −0.601 [−1.292, 0.090] | −0.066 | .088 | −0.177 [−0.850, 0.497] | −0.019 | .61 |
| Smoker in the home | 1.063 [0.599, 1.527] | 0.211 | <.001 | 1.231 [0.778, 1.684] | 0.241 | <.001 |
| Children in the home | 0.184 [−0.240, 0.608] | 0.035 | .39 | −0.041 [−0.455, 0.373] | −0.008 | .85 |
| Male x at least some college | −1.452 [−2.557, 0.347] | −0.257 | .010 | |||
| Male | −2.066 [−3.038, −1.093] | <.001 | ||||
| Female | −0.614 [−1.194, −0.033] | .038 | ||||
CI = Confidence Interval
Table 3.
Multiple linear regressions modeling synergistic risk and health-related self-concept
| Synergistic risk (n = 549)
|
Health-related self-concept (n = 540)
|
|||||
|---|---|---|---|---|---|---|
| Est. β (95% CI*) | Std. β | p | Est. β (95% CI*) | Std. β | p | |
| Age | −0.002 [−0.009, 0.005] | −0.022 | .64 | 0.046 [−0.011, 0.102] | 0.074 | .11 |
| Male | −0.158 [−0.329, 0.013] | −0.077 | .070 | −2.869 [−4.231, −1.506] | −0.166 | <.001 |
| White/non-Hispanic | 0.029 [−0.192, 0.250] | 0.011 | .80 | −0.976 [−2.735, 0.782] | −0.044 | .28 |
| At least some college | 0.309 [0.081, 0.535] | 0.118 | .008 | 3.079 [1.253, 4.904] | 0.139 | .001 |
| Current smoker | −0.398 [−0.628, −0.167] | −0.179 | <.001 | −3.821 [−5.664, −1.979] | −0.205 | <.001 |
| Family history of lung cancer | 0.139 [−0.047, 0.324] | 0.061 | .14 | −0.576 [−2.051, 0.899] | −0.030 | .44 |
| Homeowner | −0.075 [−0.373, 0.222] | −0.022 | .62 | 0.3447 [−2.011, 2.700] | 0.012 | .77 |
| Smoker in the home | −0.042 [−0.242, 0.158] | −0.022 | .68 | −2.211 [−3.801, −0.621] | −0.137 | .006 |
| Children in the home | −0.016 [−0.199, 0.167] | −0.008 | .86 | 0.482 [−0.993, 1.957] | 0.029 | .52 |
CI = Confidence Interval
Significant predictors of lung cancer worry included education, current smoking, family history of lung cancer, and having at least one smoker living in the home. Participants with at least some college had lower lung cancer worry, while current smokers, those with a family history of lung cancer, and those with at least one smoker living in the home had elevated lung cancer worry. On average, those with at least some college scored 1.53 points lower on lung cancer worry than those with less education. Current smokers scored an average of 1.11 points higher than nonsmokers on this outcome and compared to those without a family history, those with one or more family members with lung cancer scored an average of 1.06 points higher on lung cancer worry. Those with a smoker in the home scored 0.61 points higher, on average, for lung cancer worry than those without.
Perceived lung cancer risk was predicted by current smoking status, family history of lung cancer, having one or more smokers in the home, and the interaction between gender and education. Current smokers scored an average of 1.49 points higher than nonsmokers on perceived lung cancer risk, while those with a family history scored 1.12 points higher, on average, than those without on this outcome. Those with one or more smokers living in the home scored 1.23 points higher on perceived lung cancer risk than those without. Among males, those with at least some college education rated their risk of lung cancer 2.07 points lower than those with less education, while the difference in perceived risk by education level was not significant for females.
The model for synergistic risk (radon exposure plus cigarette smoking) revealed that education and current smoking were associated with this outcome. Those with college-level education rated the synergistic risk of combined exposure to radon and tobacco smoke as higher than those with at most a high school degree; those with more education rated synergistic risk an average of 0.31 points higher than did those without any post-secondary education. Current smokers rated synergistic risk lower than did nonsmokers; the average rating of synergistic risk was 0.40 points lower for smokers compared with nonsmokers. Significant predictors of health-related self-concept were gender, education, current smoking, and living with at least one smoker. Participants who had a college education rated their health-related self-concept higher than those who were less educated. Males reported lower health-related self-concept, as did smokers and those living with at least one smoker. On average, males rated their health-related self-concept 2.87 points lower than females, and those with at least some post-secondary education rated their health-related self-concept 3.08 points higher, on average, than those without any college coursework. Current smokers rated their health-related self-concept an average of 3.82 points lower than nonsmokers, and those living with at least one smoker rated this outcome an average of 2.21 points lower than those with no smokers in their household.
Discussion
In contrast to our hypotheses, the presence of children living in the home was not a significant predictor of the three teachable moment constructs for lung cancer prevention (lung cancer worry, perceived risk, or health-related self-concept) or the novel construct of synergistic risk. These findings point to a critical need to raise parental awareness on home environmental exposures related to lung cancer and their lifetime impact on children. Parents who actively rear children may be more attuned to immediate concerns such as infectious disease and/or injury prevention and less aware of the daily threat posed by environmental exposures of radon and SHS. Further, a diagnosis of lung cancer is a long-term consequence of radon and tobacco smoke exposure; thus, parents may not think to take action to address potentially chronic, carcinogenic exposures. There is an inherent challenge with communicating risk related to environmental exposures; the threat to health is often invisible and may not be perceived as imminent.
Public health nurses can increase awareness of indoor air pollutants by educating families and connecting them with resources to encourage testing of the home environment. Families can purchase inexpensive test kits for radon at home improvement stores or obtain them from health departments; however, a comparably inexpensive, commercially available test kit for SHS in the home is currently unavailable. Families who rent a house or apartment can also test for radon but may be powerless to take action on their test results. To address this environmental justice issue, public health nurses can engage local, state and federal government officials to advocate for policy level solutions to protect families and children from radon and SHS in public and rental housing. In addition to the goal of radon mitigation as a solution to radon exposure, public health nurses can inform families of the risk reduction strategies to decrease the impact of radon in the home. A few of these radon risk reduction strategies include moving children’s rooms and play areas out of the basement to higher levels in the home and considering spending less time indoors (Larsson, 2014).
Those living with children in the home in our study were distinctly different from those with no children in the home. Individuals with children living in the home were more likely to be younger, a racial/ethnic minority, and a current smoker compared to those without children in the home. Those with minor children in the home were also more likely to live with a smoker in the home. Early, chronic exposure to SHS impacts health over the life course, leading to negative health outcomes including sudden infant death syndrome, asthma, chronic obstructive pulmonary disease and lung cancer (United States Department of Health and Human Services, 2006). Nurses are well positioned to engage parents in brief, tailored discussions on SHS exposure during well child examinations and vaccination appointments. SHS exposure must be targeted as a crucial child health disparity.
There is limited research on the constructs that contribute to a teachable moment for health behavior change (Lawson & Flocke, 2009; McBride, et al., 2003). This study sought to explore which demographic and personal characteristics were associated with lung cancer worry, perceived risk, synergistic risk perception (radon plus smoking), and health-related self-concept. Education level and current smoking were associated with all of the TM constructs, as well as synergistic risk. While post-secondary education was associated with lower scores on lung cancer worry and perceived risk of lung cancer (the latter for males only), it was associated with higher perception of synergistic risk and health-related self-concept. This finding aligns with previous research stating those with post-secondary education are more likely to implement smoke-free home policies (Butler et al., 2013). Focused efforts are needed to increase awareness of lung cancer risks among individuals with no post-secondary education. Being a current smoker was associated with greater lung cancer worry and perception of lung cancer risk, but lower perception of synergistic risk and health-related self-concept. A study examining the TM constructs among relatives of those diagnosed with lung cancer reported similar findings of smokers reporting moderately high risk of acquiring lung cancer as well (McBride, Blocklin, Lipkus, Klein, & Brandon, 2015). Public health nurses can develop and advocate for targeted lung cancer prevention interventions with smokers that integrate SHS and radon awareness into tobacco treatment programs.
For those with children in the home, 57% reported having a smoker in the home. Although concerning, this finding was expected as the state of Kentucky has one of the highest adult smoking rates (25.6%) in the United States (McClave, Rock, Thorne, & Malarcher, 2010). A study in a bordering state found nearly half (48%) of adolescents reported smoking among parents, stepparents and siblings (Huntington-Moskos, Turner-Henson, & Rice, 2014). Having a smoker living in the home was associated with greater lung cancer worry and perceived risk. Similarly, those with a smoker in the home had poorer health-related self-concept than those who do not live with a smoker. Although previous research has found that smoke-free policies are less likely to be implemented when a smoker is present in the home (Butler, et al., 2013), public health nurses can connect individuals and families to the resources needed to address smoking in the home. Family history of lung cancer was associated with greater lung cancer worry and perceived lung cancer risk, but it was not associated with perception of synergistic risk or health-related self-concept. An individual’s lung cancer diagnosis can serve as a cueing event for a relative (McBride, et al., 2015). Similarly, our findings reveal that family history of lung cancer may also play an important role in the development of a cueing event for lung cancer prevention.
There are a few study limitations to discuss. The participants were selected using nonrandom quota sampling and were all from a specific geographic region; in addition, there was an overrepresentation of college-educated females, relative to the population in the region. We had relatively few former smokers who had smoked more recently than a year prior to the survey; thus, former smokers were included with never smokers in the non-smoker group as it was not possible to consider this subgroup separately in the analysis due to sample size. In addition to the potential for selection bias, the cross-sectional study design does not allow for causal inferences and the use of self-report measures can raise validity concerns. Potential participants were recruited for the study by mentioning home testing to reduce lung cancer risk; this may have introduced selection bias by engaging participants with greater self-efficacy to address environmental exposures in the home. Related to self-efficacy to address home exposures, the sample comprised participants who were relatively well educated and largely homeowners, which may make these findings difficult to generalize in more diverse populations. When using the TM constructs in research, the definitions and methods used to study the phenomenon are largely untested and not standardized (Lawson & Flocke, 2009). Health-related self-concept is a key element of the TM heuristic and, yet, few studies have operationalized and examined the impact of health-related self-concept on behavior change (McBride et al., 2008). Finally, the item used to assess whether or not children lived in the home simply asked if any children under the age of 18 resided in the home. Future studies need to quantify how many adolescents (ages 10–17) and children under the age of 10 are present in the home as this information may provide different findings related to TM constructs. Knowing the age of children in the home may help target a period of time when parents may have increased motivation to address environmental exposures in the home.
Further research on home environmental exposures is needed including efforts to understand health behavior change regarding radon exposure, tobacco use and secondhand smoke exposure. Secondhand smoke exposure in the home is an important child health disparity issue that requires greater attention. Future studies need to quantify the number of smokers in the home to better characterize the threat. Future studies may also consider integrating the concept of genetic testing for susceptibility; there is evidence to suggest that certain genetic polymorphisms increase the risk of lung cancer due to radon exposure (Ruano-Ravina et al., 2014).
Practical and effective lung cancer prevention interventions are critically needed to address the child health inequities that exist related to both radon and SHS exposure. Unfortunately, neither radon nor SHS is addressed in the Guide to Clinical Preventive Services (Agency for Healthcare Research and Quality, 2014). Therefore, healthcare professionals do not have the necessary guidance for how to talk with patients about risk for radon and SHS exposure in their homes. There is substantially more public awareness about SHS and smoke-free policies, and ample science to demonstrate that smoke-free environments not only reduce the harm caused by SHS but they also promote quitting (Institute of Medicine, 2010). Even with this pervasive messaging, far too many nonsmokers, including children and adolescents, remain exposed to SHS in the home (Marano, Schober, Brody, & Zhang, 2009). Public health nurses can create teachable moments for lung cancer prevention through education about the risks posed by radon and SHS along with 1) promoting home testing (Hahn et al., 2014); 2) low cost resources to reduce risk (Larsson, 2014); and 3) radon and SHS messaging integrated into tobacco treatment programs (Denman et al., 2015; Lantz, Mendez, & Philbert, 2013). Creating teachable moments for lung cancer prevention, especially among those who are actively rearing children, will improve health equity and promote environmental justice for children, adolescents and families.
Acknowledgments
A supplement award from the National Institute of Environmental Health Sciences (NIEHS-funded parent grant 5R01ES021502; 09/01/2012 – 05/31/2017; PI: E. Hahn) provided support for this research.
References
- Agency for Healthcare Research and Quality. The guide to clinical preventive services. 2014 Retrieved September, 2015, from http://www.ahrq.gov/professionals/clinicians-providers/guidelines-recommendations/guide/index.html. [PubMed]
- American Cancer Society. Cancer Facts & Figures 2016. Atlanta, GA: American Cancer Society; 2016. [Google Scholar]
- Butler KM, Rayens MK, Ashford K, Adkins S, Gombeski B, Britt J, Hahn EJ. Smoke-Free Homes, Strength of Smoke-Free Law, and Children in the Home. Nicotine & Tobacco Research. 2013 doi: 10.1093/ntr/ntt191. [DOI] [PubMed] [Google Scholar]
- Chen J. Canadian lung cancer relative risk from radon exposure for short periods in childhood compared to a lifetime. International Journal of Environmental Research and Public Health. 2013;10(5):1916–1926. doi: 10.3390/ijerph10051916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denman AR, Rogers S, Timson K, Phillips PS, Crockett RG, Groves-Kirkby CJ. Future initiatives to reduce lung cancer incidence in the United Kingdom: smoking cessation, radon remediation and the impact of social change. Perspectives in Public Health. 2015;135(2):92–101. doi: 10.1177/1757913914522785. [DOI] [PubMed] [Google Scholar]
- Escoffery C, Kegler MC, Butler S. Formative research on creating smoke-free homes in rural communities. Health Education Research. 2008;24(1):76–86. doi: 10.1093/her/cym095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn EJ, Kercsmar SE, Adkins SM, Wright AP, Robertson HE, Rinker G. Dual home screening and tailored environmental feedback to reduce radon and secondhand smoke: An exploratory study. Journal of Environmental Health. 2014;76(6):156–161. [PubMed] [Google Scholar]
- Henley SJ, Richards TB, Underwood JM, Eheman CR, Plescia M, McAfee TA. Lung cancer incidence trends among men and women- United States, 2005 – 2009. Morbidity and Mortality Weekly Report. 2014;63(1):1–5. [PMC free article] [PubMed] [Google Scholar]
- Huntington-Moskos L, Turner-Henson A, Rice M. Tobacco exposure, weight status, and elevated blood pressure in adolescents. Journal of Community Health. 2014;39(4):653–659. doi: 10.1007/s10900-014-9839-5. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine. Secondhand smoke exposure and cardiovascular effects. Washington DC: The National Academies Press; 2010. [PubMed] [Google Scholar]
- Jones LL, Atkinson O, Longman J, Coleman T, McNeill A, Lewis SA. The motivators and barriers to a smoke-free home among disadvantaged caregivers: identifying the positive levers for change. Nicotine & Tobacco Research. 2011;13(6):479–486. doi: 10.1093/ntr/ntr030. [DOI] [PubMed] [Google Scholar]
- Kendall GM, Smith TJ. Doses from radon and its decay products to children. Journal of Radiological Protection. 2005;25(3):241–256. doi: 10.1088/0952-4746/25/3/002. [DOI] [PubMed] [Google Scholar]
- Kim SH, Hwang WJ, Cho JS, Kang DR. Attributable risk of lung cancer deaths due to indoor radon exposure. Annals of Occupational and Environmental Medicine. 2016;28:8. doi: 10.1186/s40557-016-0093-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lantz PM, Mendez D, Philbert MA. Radon, smoking, and lung cancer: The need to refocus radon control policy. American Journal of Public Health. 2013;103(3):443–447. doi: 10.2105/AJPH.2012.300926. doi:10.2105/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson LS. Risk-reduction strategies to expand radon care planning with vulnerable groups. Public Health Nursing. 2014;31(6):526–536. doi: 10.1111/phn.12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson PJ, Flocke SA. Teachable moment for health behavior change: A concept analysis. Patient Education and Counseling. 2009;76:25–30. doi: 10.1016/j.pec.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerman C, Trock B, Rimer BK, Boyce A, Jepson C, Engstrom PF. Psychological and behavioral implications of abnormal mammograms. Annals of Internal Medicine. 1991;114(8):657–661. doi: 10.7326/0003-4819-114-8-657. [DOI] [PubMed] [Google Scholar]
- Marano C, Schober SE, Brody DJ, Zhang C. Secondhand tobacco smoke exposure among children and adolescents: United States, 2003–2006. Pediatrics. 2009;124(5):1299–1305. doi: 10.1542/peds.2009-0880. [DOI] [PubMed] [Google Scholar]
- McBride CM, Blocklin M, Lipkus IM, Klein WM, Brandon TH. Patient’s lung cancer diagnosis as a cue for relatives’ smoking cessation: evaluating the constructs of the teachable moment. Psycho-Oncology. 2015 doi: 10.1002/pon.4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride CM, Emmons KM, Lipkus IM. Understanding the potential of teachable moments: the case of smoking cessation. Health Education Research. 2003;18(2):156–170. doi: 10.1093/her/18.2.156. [DOI] [PubMed] [Google Scholar]
- McBride CM, Puleo E, Pollak KI, Clipp EC, Woolford S, Emmons KM. Understanding the role of cancer worry in creating a “teachable moment” for multiple risk factor reduction. Social Science Medicine. 2008;66(3):790–800. doi: 10.1016/j.socscimed.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClave A, Rock V, Thorne S, Malarcher A. State-specific prevalence of cigarette smoking and smokeless tobacco use among adults - United States, 2009. MMWR Morbidity and Mortality Weekly Report. 2010;59(43):1400–1406. [PubMed] [Google Scholar]
- National Cancer Institute. A snapshot of lung cancer Retrieved November. 2014;30:2015. from http://www.cancer.gov/research/progress/snapshots/lung. [Google Scholar]
- National Research Council. Health effects of exposure to radon: BEIR VI. Washington DC: The National Academies Press; 1999. [PubMed] [Google Scholar]
- Ruano-Ravina A, Pereyra MF, Castro MT, Perez-Rios M, Abal-Arca J, Barros-Dios JM. Genetic susceptibility, residential radon, and lung cancer in a radon prone area. Journal of Thoracic Oncology. 2014;9(8):1073–1080. doi: 10.1097/JTO.0000000000000205. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. Surgeon General releases national health advisory on radon. 2005 from http://www.surgeongeneral.gov/pressreleases/sg01132005.html.
- U.S. Environmental Protection Agency. Exposure Factors Handbook. Washington DC: U.S. Environmental Protection Agency; 2011. Final ed. [Google Scholar]
- United States Department of Health and Human Services. The health consequences of involuntary exposure to tobacco smoke: A report of the surgeon general executive summary. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, Coordinating Center for Health Promotion, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2006. [Google Scholar]
- United States Department of Health and Human Services. How tobacco smoke causes disease: The biology and behavioral basis for smoking-attributable disease: A report of the surgeon general. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2010. [PubMed] [Google Scholar]
- Weitzman M, Baten A, Rosenthal DG, Hoshino R, Tohn E, Jacobs DE. Housing and child health. Current Problems in Pediatric and Adolescent Health Care. 2013;43(8):187–224. doi: 10.1016/j.cppeds.2013.06.001. [DOI] [PubMed] [Google Scholar]
- Wiesmann U, Niehorster G, Hannich HJ, Hartmann U. Dimensions and profiles of the generalized health-related self-concept. British Journal of Health Psychology. 2008;13(Pt 4):755–771. doi: 10.1348/135910707X256699. [DOI] [PubMed] [Google Scholar]


