Abstract
Objectives
Few studies have examined the association of diet with specific cognitive domains. We examined the association of diet with executive function, episodic memory and global cognition in the Einstein Aging Study (EAS) cohort and determined whether race modifies this relationship.
Design
Cross-sectional.
Setting
Community.
Participants
492 non-demented EAS participants completed the Rapid Eating and Activity Assessment for Patients (REAP).
Measurements
The previously-validated REAP is based on the 2000 U.S. dietary guidelines. REAP scores were dichotomized as less healthy diet (<median) or healthier diet (≥median). Nine neurocognitive tests were subjected to principle component analysis revealing three components: episodic memory, executive function, and global cognition. Impaired cognitive function in each domain was defined as ≥2 standard deviations (SD) below the mean on any task or a total score ≥1.5 SD below the mean. Using logistic regression, we assessed the association of diet with cognitive impairment, adjusting for age, education, sex, cardiovascular comorbidities, hypertension, BMI, diabetes, depressive symptoms, race and a race by diet group interaction.
Results
The sample was 60% female and 74% White with a mean age of 80. In the entire sample, impaired executive function was associated with the race by diet group interaction (p=0.08) while other cognitive domains were not. In race-stratified analyses, healthier diet was associated with reduced odds of impaired executive function among Whites (OR=0.44, 95%CI=0.21–0.93, p=0.03), as were healthier scores on the saturated fat sub-scale (OR=0.34, 95%CI=0.16–0.71, p=0.004). Among Blacks, REAP scores were not associated with cognitive domains.
Conclusion
Healthy diet was associated with reduced risk of executive dysfunction in whites. Race differences may be due to increased vascular risk among Blacks or to differences in generalizability of the REAP.
Keywords: Diet, cognition, executive function, race
INTRODUCTION
With the demographic shift in the US towards older age groups, prevalence of age-associated cognitive impairment is expected to rise1. Without established treatments for cognitive impairment, enhancing modifiable protective factors offers a potential approach to quelling progression. Diet quality represents a possible target for prevention as suggested by studies showing associations between dietary characteristics and cognitive performance2–8. Early work reported relationships between the intake of individual vitamins and minerals and cognitive function2–3. Recent work suggests that overall dietary patterns are better predictors of health outcomes9. Dietary patterns such as the Mediterranean diet are linked to reduced risk for cognitive impairment or reduced rates of cognitive decline5–8.
Several studies have investigated the relationship between diet and global cognitive function in older adults2–8. However, few have examined specific cognitive domains to establish whether the effect of diet is global or domain-specific2,5, and, results are variable.
We examined the cross-sectional association of an index of overall diet quality with cognitive performance in the Einstein Aging Study (EAS), a community-dwelling cohort of older adults. The Rapid Eating and Activity Assessment for Patients (REAP) was developed by the Nutrition Academic Award Program as a brief, user-friendly questionnaire that assesses dietary intake relative to the 1996 Food Guide Pyramid and the 2000 U.S. Dietary Guidelines10–11. We examined the REAP in relation to performance in three cognitive domains: episodic memory, executive function and global verbal cognition. We hypothesized that a healthier dietary pattern would be associated with better cognitive function, particularly executive function, given the relation of dietary patterns to cardiovascular disease and evidence that vascular mechanisms are associated with executive function12,13. Because of differences in rates of dementia and cognitive decline between Whites and Blacks14 and the paucity of data addressing the generalizability of dietary assessments to minority populations11, we also explored the relationship between dietary patterns and cognitive impairment according to race.
METHODS
Subjects
Data is from the EAS, a longitudinal community-based cohort of adults aged 70 years or older who were systematically recruited from Bronx County, NY beginning in 1993. Participants complete annual clinical, neuropsychological and psychosocial evaluations15. This cross-sectional analysis is based on cognitive and diet data from EAS assessments in 2006–2007, when the REAP was incorporated as part of an ancillary study. Of 627 participants asked to self-administer the REAP, 549 agreed and completed the REAP. To facilitate an examination by race, we excluded 31 participants who self-identified as race other than White or Black, and 26 were excluded for missing covariate data leaving 492 in the analysis sample. Written informed consent was obtained using a protocol approved by the local institutional review board.
Neurocognitive Measures
A standard neurocognitive test battery was administered by trained neuropsychological assistants at each clinic visit. The Blessed Information-Memory-Concentration test (BIMC) measured global cognitive function16. Measures of episodic memory included the Free and Cued Selective Reminding Test (FCSRT)17 and the Logical Memory (LM) test from the Wechsler Memory Scale–Revised18. The Category Fluency test is a measure of verbal semantic production19. Tests from the Wechsler Adult Intelligence Scale–III20 included Block Design, a measure of visuospatial abilities and abstract reasoning; Digit Span, a measure of auditory attention and working memory; Digit Symbol, a measure of psychomotor speed; and Vocabulary, a measure of reading comprehension. Trail Making Test part A21 is a measure of attention and visual scanning and sequencing with a motor component. Trail Making Test part B21 is a measure of executive function involving mental flexibility and set-shifting. The Boston Naming Task is a measure of confrontational naming22.
Dementia diagnoses are assigned by consensus at clinical case-conferences using standardized clinical criteria from the Diagnostic and Statistical Manual of Mental Disorders IV.
The REAP assessment of diet quality
The REAP is a brief, dietary questionnaire that enables physicians to rapidly evaluate a patient’s dietary habits and physical activity10,11. The REAP assesses diet pattern based on national nutrition priorities for adults related to prevention of chronic diseases such as cardiovascular disease, cancer and diabetes11. The REAP has demonstrated reliability and validity among community-dwelling adults, correlating strongly with the Healthy Eating Index Scores11. The REAP is comprised of 27 questions that assess intake of whole grains, fruits and vegetables, lean meats, saturated fat, cholesterol, sugary beverages, sodium, alcoholic beverages and physical activity. Responses were assigned a point value: “Usually/Often”=1, “Sometimes”=2, and “Rarely/Never”=3. All questions were worded such that a response of “Rarely/Never” reflects optimal dietary habit, whereas “Usually/Often” reflects poor dietary habit. The REAP score is the average score across all REAP questions (range: 1.00–3.00) and represents a measure of overall adherence to a healthy diet, with higher scores reflecting healthier diet. REAP developers separated questions into 10 sub-scales to assess specific dietary components including total fats, saturated fats, cholesterol, grains, dairy, fruits, vegetables, meat, sodium and dietary variety. Individual sub-scales represent the average of scores for questions related to each component.
Because current dietary guidelines no longer consider total fat as a significant factor in diet quality23 and because we are interested in diet alone, analyses are based on a modified REAP score that excludes questions pertaining solely to total fat (5 questions) and physical activity (2 questions). Supplemental analyses demonstrate that results were similar using either this version or the original REAP and when analyses were adjusted for the physical activity subscale.
Covariates
EAS clinic visits include assessments of sociodemographic characteristics and self-reported medical history that includes history of physician diagnosis of diabetes or hypertension. A cardiovascular comorbidity index was computed as the sum of the following conditions: heart failure, angina, myocardial infarction, stroke (CV index; range 0–4). Body mass index (BMI) was calculated using self-reported weight and height (kg/m2)24. The Geriatric Depression Scale (GDS) assessed depressive symptoms over the past month (range 0–15)25.
Statistical Analysis
Principle Component Analysis of Cognitive Tests
To minimize the number of statistical tests, we performed principle component analysis (PCA) of the EAS neurocognitive battery to identify tests that tap specific domains of cognitive function. Our PCA methods and results resembled those of other cohort studies with comprehensive neurocognitive batteries26. Regression scores generated by the PCA were used to form cognitive component scores. A test was included in a factor if the coefficient loading was >0.45. The PCA resulted in three significant orthogonal components that were labelled: episodic memory (LM, FCSRT and Category Fluency), executive function (Block Design, Digit Symbol, Digit Span, Trail Making Tests A and B) global verbal cognition (Vocabulary and Boston Naming). Component scores were used to define domain-specific cognitive impairment. Cognitive impairment was defined as ≥2 standard deviations (SD) below the mean on any task within the domain or an overall cognitive domain score ≥1.5 SDs below the mean. Task-specific cutoffs were more conservative so that borderline impairment on one task did not weigh too heavily when defining impairment in a domain.
Analysis of REAP scores in relation to cognitive performance
The REAP score and subscale scores were dichotomized at the median because the data indicated a threshold effect at the REAP median rather than a linear relationship with executive function. Scores ≤ the median represented less healthy diet and scores ≥ the median represented a healthier diet. Demographic and clinical characteristics were compared between the diet groups using analysis of variance for continuous variables and chi-square tests for categorical variables.
Nested logistic regression models examined the relation of diet group to cognitive impairment in each domain. The less healthy diet group served as the reference. Initial models adjusted for demographic characteristics (age, sex, years of education). The second model additionally adjusted for cardiovascular risk (CV index, hypertension, BMI and history of diabetes) and depressive symptoms (GDS scores). The third model added race and a race by diet group interaction to the second model. Interactions were considered significant at p<0.10 and further explored in race-stratified analyses. Secondary analyses of REAP subscale scores were conducted to see if the overall association was driven by specific dietary components. Analyses were performed using SPSS (version 22.0, SPSS Inc., Chicago, Illinois).
RESULTS
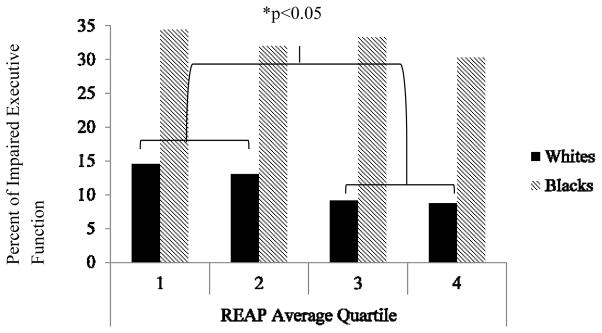
The analysis sample (N=492) was 60.4% female and 74.0% White. Mean age at REAP assessment was 80.1 years and mean years of education was 13.9 years. Comparisons of participants who completed the REAP with those that did not showed that REAP completers were younger, had less depressive symptoms, were more likely to have a history of hypertension and demonstrated better global cognitive function (p’s<0.05). Within our analysis sample, 81(17%) met criteria for executive function impairment (11% of Whites; 33% of Blacks); 106 (21%) met criteria for episodic memory impairment (20% of Whites; 26% of Blacks); and 71 (14%) met criteria for global verbal cognitive impairment (8.5% of Whites; 31.3% of Blacks). REAP scores ranged from 1.29 to 3.00 with a mean of 2.38 (SD=0.30) and a median of 2.36. Notably, when the sample was sex-stratified, the median was the same for men and women indicating similar diet patterns between sexes. The healthier diet group included more women (p=0.01) and Whites (p=0.02) and those with higher levels of education (p=0.05) and a lower BMI (p=0.09) (Table 1). Among Whites, the healthier diet group included a significantly higher proportion of women (p=0.01). Among Blacks, the healthier diet group had significantly greater years of education (p=0.03), a significantly higher proportion women (p=0.01) and a significantly lower CV index (p=0.05) (Table 1). All REAP sub-scale scores were significantly higher in the healthier diet group (p’s<0.05). In logistic regression models, diet group was not significantly associated with impairment in episodic memory or global verbal cognition (Table 2). However, those in the healthier diet group had significantly lower odds of impairment in executive function after adjusting for age, sex, and years of education than the less healthy diet group. This association was not changed after further adjustment in model two for cardiovascular risk (CV index, hypertension, BMI and history of diabetes) and depressive symptoms (GDS scores) (p=0.03). When race and the race by diet interaction were added to this model, the association of diet with executive function was eliminated (p=0.67), and the race by diet group interaction was a statistical trend (p=0.08) suggesting effect modification by race. To explore this interaction, we performed race-stratified analyses. Impaired executive function was more prevalent among Blacks (33%) than among Whites (10.4%; p<0.001; Figure 1). The proportion with impaired executive function was similar across quartiles of REAP scores in Blacks (p’s>0.05), whereas, the proportion with impaired executive function was significantly lower in the highest two REAP quartiles (healthier diet) among Whites (p<0.05).
Table 1.
Characteristics of the overall sample and Whites and Blacks by REAP diet group.
| Characteristic | Total M (SD) |
Less healthy diet (REAP average < 2.36) M (SD) |
Healthier diet (REAP average ≥ 2.36) M (SD) |
Less vs. healthier diet p-value |
|---|---|---|---|---|
| Overall Sample (N=492) | ||||
| Age (years) | 80.1 (5.3) | 79.8 (5.4) | 80.3 (5.1) | 0.32 |
| Education (years) | 13.9 (3.4) | 13.7 (3.2) | 14.3 (3.6) | 0.05 |
| Sex (% female) | 60.4 | 54.1 | 66.0 | 0.01 |
| Race (% White) | 74.0 | 69.1 | 78.4 | 0.02 |
| GDS score | 2.2 (2.1) | 2.3 (2.1) | 2.1 (2.1) | 0.34 |
| Global cognition (BIMC) | 1.7 (1.9) | 1.9 (2.0) | 1.6 (1.7) | 0.13 |
| BMI, kg/m2 | 26.5 (4.4) | 26.9 (4.3) | 26.2 (4.4) | 0.09 |
| History of hypertension (% yes) | 63.6 | 64.8 | 62.5 | 0.60 |
| History of diabetes (% yes) | 17.7 | 18.9 | 16.6 | 0.51 |
| CV index | 0.3 (0.7) | 0.4 (0.7) | 0.3 (0.6) | 0.15 |
| Whites (n=364) | ||||
| Age (years) | 80.2 (5.2) | 79.8 (5.3) | 80.6 (5.1) | 0.12 |
| Education (years) | 14.3 (3.3) | 14.2 (3.0) | 14.4 (3.6) | 0.57 |
| Sex (% female) | 54.4 | 47.2 | 60.1 | 0.01 |
| GDS score | 2.1 (1.9) | 2.1 (1.9) | 2.1 (2.1) | 0.91 |
| Global cognition (BIMC) | 1.4 (1.7) | 1.5 (1.8) | 1.4 (1.6) | 0.44 |
| BMI, kg/m2 | 26.0 (4.0) | 26.4 (4.1) | 25.7 (3.8) | 0.11 |
| History of hypertension (% yes) | 58.8 | 59.6 | 58.1 | 0.77 |
| History of diabetes (% yes) | 15.1 | 17.4 | 13.3 | 0.28 |
| CV index | 0.3 (0.7) | 0.4 (0.7) | 0.3 (0.7) | 0.50 |
| Blacks (n=128) | ||||
| Age (years) | 79.7 (5.4) | 80.1 (5.5) | 79.3 (5.0) | 0.43 |
| Education (years) | 13.1 (3.6) | 12.5 (3.3) | 13.8 (3.8) | 0.03 |
| Sex (% female) | 77.3 | 69.4 | 87.5 | 0.01 |
| GDS score | 23 (2.3) | 2.6 (2.5) | 2.0 (2.0) | 0.18 |
| Global cognition (BIMC) | 2.6 (2.1) | 2.7 (2.2) | 2.5 (2.0) | 0.67 |
| BMI, kg/m2 | 27.9 (5.2) | 27.9 (4.6) | 27.8 (5.9) | 0.93 |
| History of hypertension (% yes) | 77.3 | 76.4 | 78.6 | 0.77 |
| History of diabetes (% yes) | 25.0 | 22.2 | 28.6 | 0.41 |
| CV index | 0.3 (0.6) | 0.4 (0.7) | 0.2 (0.4) | 0.05 |
Note. M = mean; SD = standard deviation; REAP = Rapid Eating and Activity Assessment for Patients (score range: 1.00–3.00); GDS = 15-item Geriatric Depression Scale (score range: 0–15); BIMC = Blessed Information Memory Concentration Test; BMI = body mass index; CV index = cardiovascular index (score range: 0–4).
Table 2.
Results of multiple logistic regressions examining the relationship between diet group and cognitive impairment in the domains of executive function, episodic memory and global verbal cognition.
| Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|
| Outcome/Parameter | Odds Ratio (95% CI) | p-value | Odds Ratio (95% CI) | p-value | Odds Ratio (95% CI) | p-value |
| Executive Function | ||||||
| Age | 1.13 (1.08–1.19) | <0.001 | 1.14 (1.08–1.20) | <0.001 | 1.15 (1.09–1.22) | <0.001 |
| Education | 0.76 (0.69–0.83) | <0.001 | 0.77 (0.70–0.84) | <0.001 | 0.77 (0.70–0.85) | <0.001 |
| Sex | 1.46 (0.83–2.55) | 0.18 | 1.46 (0.81–2.63) | 0.18 | 1.15 (0.62–2.13) | 0.66 |
| Healthy vs. less healthy group | 0.54 (0.31–0.92) | 0.03 | 0.54 (0.31–0.94) | 0.03 | 1.21 (0.50–2.91) | 0.67 |
| CV index | - | - | 1.08 (0.75–1.56) | 0.58 | 1.17 (0.79–1.74) | 0.42 |
| Hypertension | - | - | 1.37 (0.74–2.53) | 0.26 | 1.17 (0.62–2.22) | 0.63 |
| BMI | - | - | 1.01 (0.95–1.07) | 0.68 | 0.99 (0.92–1.05) | 0.63 |
| History of diabetes | - | - | 1.53 (0.79–2.94) | 0.21 | 1.38 (0.70–2.74) | 0.35 |
| GDS score | - | - | 1.09 (0.96–1.23) | 0.18 | 1.09 (0.96–1.24) | 0.18 |
| Race | - | - | - | - | 1.12 (0.19–6.45) | 0.90 |
| Diet group X race | - | - | - | - | 0.35 (0.11–1.12) | 0.08 |
|
| ||||||
| Memory Impairment | ||||||
| Age | 1.11 (1.06–1.16) | <0.001 | 1.10 (1.05–1.15) | <0.001 | 1.10 (1.05–1.15) | <0.001 |
| Education | 0.89 (0.83–0.96) | 0.001 | 0.90 (0.84–0.96) | 0.003 | 0.90 (0.84–0.97) | 0.004 |
| Sex | 0.72 (0.46–1.15) | 0.17 | 0.17 (0.44–1.15) | 0.17 | 0.66 (0.40–1.08) | 0.10 |
| Healthy vs. less healthy group | 0.93 (0.59–1.47) | 0.76 | 0.93 (0.59–1.48) | 0.76 | 1.31 (0.55–3.14) | 0.54 |
| CV index | - | - | 1.08 (0.79–1.49) | 0.62 | 1.10 (0.80–1.53) | 0.54 |
| Hypertension | - | - | 0.90 (0.55–1.47) | 0.67 | 0.86 (0.52–1.42) | 0.56 |
| BMI | - | - | 0.96 (0.91–1.02) | 0.20 | 0.96 (0.90–1.01) | 0.14 |
| History of diabetes | - | - | 1.37 (0.77–2.46) | 0.28 | 1.32 (0.74–2.38) | 0.35 |
| GDS score | - | - | 1.16 (1.04–1.28) | 0.005 | 1.16 (1.05–1.28) | 0.005 |
| Race | - | - | - | - | 1.22 (0.25–6.00) | 0.80 |
| Diet group X race | - | - | - | - | 0.66 (0.24–1.84) | 0.43 |
|
| ||||||
| Global Verbal Cognitive Impairment | ||||||
| Age | 1.01 (0.96–1.06) | 0.68 | 1.02 (0.97–1.08) | 0.50 | 1.03 (0.97–1.08) | 0.30 |
| Education | 0.74 (0.67–0.81) | <0.001 | 0.75 (0.68–0.83) | <0.001 | 0.76 (0.68–0.84) | <0.001 |
| Sex | 1.27 (0.71–2.27) | 0.42 | 1.37 (0.74–2.52) | 0.31 | 1.03 (0.54–1.96) | 0.94 |
| Healthy vs. less healthy group | 1.02 (0.59–1.76) | 0.94 | 1.04 (0.60–1.82) | 0.88 | 1.86 (0.79–4.38) | 0.16 |
| CV index | - | - | 1.13 (0.78–1.63) | 0.52 | 1.22 (0.83–1.78) | 0.32 |
| Hypertension | - | - | 1.76 (0.91–3.40) | 0.09 | 1.47 (0.74–2.92) | 0.27 |
| BMI | - | - | 1.03 (0.97–1.09) | 0.29 | 1.01 (0.95–1.07) | 0.85 |
| History of diabetes | - | - | 1.87 (0.98–3.56) | 0.06 | 1.66 (0.85–3.24) | 0.14 |
| GDS score | - | - | 1.00 (0.87–1.14) | 0.96 | 0.99 (0.86–1.13) | 0.88 |
| Race | - | - | - | - | 0.61 (0.10–4.00) | 0.61 |
| Diet group X race | - | - | - | - | 0.53 (0.17–1.72) | 0.29 |
Note. CV index = cardiovascular index (score range: 0–4). BMI = body mass index. GDS = Geriatric Depression Scale (range=0–15).
Figure 1.
Prevalence of impaired executive function across quartiles of the Rapid Eating and Activity Assessment for Patients (REAP) score by race.
A healthier diet was significantly associated with reduced odds of impaired executive function among Whites but not Blacks in the fully-adjusted model (OR=0.43, 95%CI=0.20–0.92, p=0.03). Analyses of REAP subscales among Whites indicated that those in the healthier diet group for saturated fat intake had decreased odds of impaired executive function (OR=0.32, 95%CI=0.15–0.69, p=0.004). No other subscale score significantly related to executive function impairment (p’s>0.05). When all subscale scores were modeled simultaneously, saturated fat continued to be the only subscale significantly related to executive function impairment (p<0.01).
DISCUSSION
As hypothesized, we found that a less healthy diet, as measured by a brief dietary assessment, was associated with impairment in executive function in older adults, particularly among Whites. We found no association between diet quality and impairment in memory or global verbal cognition. This study is among the first to assess the relationship between diet quality and specific cognitive domains.
Poor diet plays a critical role in cardiovascular health and disease risk27. Our findings are consistent with prior evidence linking impairments in executive function to cardiovascular risk factors and disease12,13. Cardiovascular disease is a risk factor for cognitive impairment and dementia, particularly vascular dementia which primarily impacts frontal lobe structures that mediate executive processes13. Herein, the diet and executive function relationship was significant despite adjustment for cardiovascular co-morbidities, hypertension, BMI and history of diabetes. Thus, the presence of clinical cardiovascular disease does not appear to explain our findings. However, the association of diet with executive function may be related to the negative impact of less healthy diet on subclinical vascular disease. Alternatively, diet has been linked to inflammation, oxidative stress and neurotransmitter signaling7. Thus, dietary health may directly impact brain processes underlying executive function.
Healthy diet was related to executive dysfunction specifically in Whites. It is possible that statistical power was limited among the smaller sub-sample of Blacks; however, there was no evidence for a trend relating diet quality to executive function in Blacks. Consistent with prior reports28,29, impaired executive function and cardiovascular risk factors (e.g. hypertension, diabetes) were significantly more prevalent in Blacks versus Whites (p’s≤0.01). Therefore, the cumulative effect of vascular risk factors may increase risk of impaired executive function and mask the influence of diet quality within the Black subsample. Given that the REAP was validated in a community-based sample that was 94% non-Hispanic White11, another possibility is that the REAP is not a sensitive measure of dietary health in minority populations. Additionally, race-specific results may be due to differences in genetic and environmental factors that impact diet and cognition and to race differences in the validity of cognitive tests.
The effect of diet quality on executive function in Whites was specific to saturated fat intake. Excessive saturated fat consumption has been associated with reduced overall cognitive function in the elderly3,4. Greater saturated fat intake has been associated with higher low-density lipoprotein (LDL) cholesterol, release of reactive free radicals, oxidative stress and inflammation30 which can lead to cognitive impairment either indirectly through cardiovascular effects or directly through neural damage31. Our results suggest that the saturated fats and cognitive function relationship is specific to executive function.
Our study had limitations. The cross-sectional analyses cannot assess the temporality of the observed associations. Our classification of impaired executive function was not a clinical diagnosis but a relative categorization within a sample of independent, dementia-free individuals. While poor diet quality may lead to executive dysfunction, reverse causality is also plausible. That is, below-average executive function may lead to poor dietary habits. In addition, cognitive impairment may compromise the reliability of self-reported REAP data. We minimized this risk by excluding participants with dementia. Additionally, global cognition (BIMC score) was within the normal range (0–8)16. The REAP has not specifically been validated among older adults, which may limit interpretation of results. Although the brevity of the REAP is advantageous for clinical assessment of overall diet quality, we were unable to estimate intake of specific food groups or nutrients. Lastly, it is possible that the findings are due to unmeasured factors that similarly influence both diet and cognition.
In sum, lower scores on a brief, clinical assessment of diet quality were associated with executive function impairment specifically in older White adults. Studies suggest that the beneficial effects of diet manifest in the early prodromal phase of dementia, after which neurodegenerative processes overwhelm any effects of diet5. Thus, our results may be clinically relevant to an older population of dementia-free individuals who may have early signs of cognitive impairment. Additionally, executive processes (e.g. planning, organization, self-control) are critical for functioning in everyday life and maintaining independence. Findings highlight the importance of regular assessment to identify those who may benefit from nutritional counseling or interventions to reduce risk of executive function impairment.
Acknowledgments
Funding Source: This research was supported by the Einstein Aging Study (PO1AG03949) from the National Institutes on Aging program; the National Institutes of Health CTSA (1UL1TR001073) from the National Center for Advancing Translational Sciences, the Sylvia and Lenard Marx Foundation, the Resnick Gerontology Center at the Albert Einstein College of Medicine (pilot grant for aging related research), and the Czap Foundation. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Footnotes
This research will be presented as a poster at the 2015 Annual Meeting of the Alzheimer’s Association International Conference in Washington D.C.
Conflicts of interest: All authors have no conflicts of interest to disclose regarding the current study.
Author contributions: Erin E. Sundermann, Carol A. Derby, and Mindy J. Katz, K conceived and designed the research project; Carol A. Derby and Mindy J. Katz, collected data; Erin E. Sundermann, Carol A. Derby, Mindy J. Katz, and Alice H. Lichtenstein designed statistical methods; ES conducted statistical analyses; Erin E. Sundermann, wrote the paper; all authors edited the paper and contributed to the final version; Erin E. Sundermann, has primary responsibility for final content. All authors read and approved the final manuscript.
Sponsor’s role: The sponsor, Carol A. Derby, had a role in study design, methods, statistical analysis and preparation of paper.
References
- 1.Comas-Herrera A, Wittenberg R, Pickard L, et al. Cognitive impairment in older people: future demand for long-term care services and the associated costs. Int J Geriatr Psychiatry. 2007;22:1037–1045. doi: 10.1002/gps.1830. [DOI] [PubMed] [Google Scholar]
- 2.La Rue A, Koehler KM, Wayne SJ, et al. Nutritional status and cognitive functioning in a normally aging sample: A 6-y reassessment. Am J Clin Nutr. 1997;65:20–29. doi: 10.1093/ajcn/65.1.20. [DOI] [PubMed] [Google Scholar]
- 3.Ortega RM, Requejo AM, Andrés P, et al. Dietary intake and cognitive function in a group of elderly people. Am J Clin Nutr. 1997;66:803–809. doi: 10.1093/ajcn/66.4.803. [DOI] [PubMed] [Google Scholar]
- 4.Requejo AM, Ortega RM, Robles F, et al. Influence of nutrition on cognitive function in a group of elderly, independently living people. Eur J Clin Nutr. 2003;57(Suppl 1):S54–S57. doi: 10.1038/sj.ejcn.1601816. [DOI] [PubMed] [Google Scholar]
- 5.Féart C, Samieri C, Rondeau V, et al. Adherence to a Mediterranean diet, cognitive decline, and risk of dementia. JAMA. 2009;302:638–648. doi: 10.1001/jama.2009.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scarmeas N, Stern Y, Mayeux R, et al. Mediterranean diet and mild cognitive impairment. Arch Neurol. 2009;66:216–225. doi: 10.1001/archneurol.2008.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lourida I, Soni M, Thompson-Coon J, et al. Mediterranean diet, cognitive function, and dementia: a systematic review. Epidemiology. 2013;24:479–489. doi: 10.1097/EDE.0b013e3182944410. [DOI] [PubMed] [Google Scholar]
- 8.Martinez-Lapiscina EH, Clavero P, Toledo E, et al. Mediterranean diet improves cognition: The PREDIMED-NAVARRA randomised trial. J Neurol Neurosurg Psychiatry. 2013;84:1318–1325. doi: 10.1136/jnnp-2012-304792. [DOI] [PubMed] [Google Scholar]
- 9.Alles B, Samieri C, Feart C, et al. Dietary patterns: A novel approach to examine the link between nutrition and cognitive function in older individuals. Nutr Res Rev. 2012;25:207–222. doi: 10.1017/S0954422412000133. [DOI] [PubMed] [Google Scholar]
- 10.Gans KM, Ross E, Barner CW, et al. REAP and WAVE: New tools to rapidly assess/discuss nutrition with patients. J Nutr. 2003;133:556S–562S. doi: 10.1093/jn/133.2.556S. [DOI] [PubMed] [Google Scholar]
- 11.Gans KM, Risica PM, Wylie-Rosett J, et al. Development and evaluation of the nutrition component of the Rapid Eating and Activity Assessment for Patients (REAP): A new tool for primary care providers. J Nutr Educ Behav. 2006;38:286–292. doi: 10.1016/j.jneb.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 12.O’Brien JT, Erkinjuntti T, Reisberg B, et al. Vascular cognitive impairment. Lancet Neurol. 2003;2:89–98. doi: 10.1016/s1474-4422(03)00305-3. [DOI] [PubMed] [Google Scholar]
- 13.Traykov L, Baudic S, Thibaudet MC, et al. Neuropsychological deficit in early subcortical vascular dementia: Comparison to Alzheimer’s disease. Dement Geriatr Cogn Disord. 2002;14(1):26–32. doi: 10.1159/000058330. [DOI] [PubMed] [Google Scholar]
- 14.Alzheimer’s Association. Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2012;8:131–168. doi: 10.1016/j.jalz.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 15.Katz MJ, Lipton RB, Hall CB, et al. Age-specific and sex-specific prevalence and incidence of mild cognitive impairment, dementia, and Alzheimer dementia in blacks and whites: A report from the Einstein Aging Study. Alzheimer Dis Assoc Disord. 2012;26:335–343. doi: 10.1097/WAD.0b013e31823dbcfc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blessed G, Tomlinson BE, Roth M. The association between quantitative measures of dementia and of senile change in the cerebral grey matter of elderly subjects. Br J Psychiatry. 1968;114:797–811. doi: 10.1192/bjp.114.512.797. [DOI] [PubMed] [Google Scholar]
- 17.Grober E, Lipton RB, Hall C, et al. Memory impairment on free and cued selective reminding predicts dementia. Neurology. 2000;54:827–832. doi: 10.1212/wnl.54.4.827. [DOI] [PubMed] [Google Scholar]
- 18.Wechsler D. Wechsler Memory Scale - Revised Manual. San Antonio, TX: The Psychological Corporation; 1987. [Google Scholar]
- 19.Monsch AU, Bondi MW, Butters N, et al. Comparisons of verbal fluency tasks in the detection of dementia of the Alzheimer type. Arch Neurol. 1992;49:1253–1258. doi: 10.1001/archneur.1992.00530360051017. [DOI] [PubMed] [Google Scholar]
- 20.Wechsler D. Wechsler Adult Intelligence Scale 3. New York: The Psychological Corporation; 1997. [Google Scholar]
- 21.Army Individual Test Battery. Manual of Directions and Scoring. Washington, DC: War Department, Adjutant General’s Office; 1944. [Google Scholar]
- 22.Kaplan EF, Goodglass H, Weintraub S. The Boston Naming Test. Philadelphia, PA: Lea and Febiger; 1983. [Google Scholar]
- 23.US Department of Agriculture and US Department of Health and Human Services. Dietary [Google Scholar]
- 24.Guidelines for Americans, 2010. 7. Washington, DC: Government Printing Office; National Heart Lung, and Blood Institute; 2010. [Accessed February 15, 2015]. Calculate your Body Mass Index (online). Available at: http://www.nhlbi.nih.gov/health/educational/lose_wt/BMI/bmicalc.htm. [Google Scholar]
- 25.Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: A preliminary report. J Psychiatr Res. 1982–1983;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 26.Wilson R, Barnes L, Bennett D. Assessment of lifetime participation in cognitively stimulating activities. J Clin Exp Neuropsychol. 2003;25(5):634–642. doi: 10.1076/jcen.25.5.634.14572. [DOI] [PubMed] [Google Scholar]
- 27.Scientific Report of the 2015 Dietary Guidelines Advisory Committee. [Accessed 6 April 2015];Advisory Report to the Secretary of Health and Human Services and the Secretary of Agriculture (online) Available at: http://health.gov/dietaryguidelines/2015-scientific-report/PDFs/Scientific-Report-of-the-2015-Dietary-Guidelines-Advisory-Committee.pdf.
- 28.Norman MA, Moore DJ, Taylor M, et al. Demographically corrected norms for African Americans and Caucasians on the Hopkins Verbal Learning Test-Revised, Brief Visuospatial Memory Test-Revised, Stroop Color and Word Test, and Wisconsin Card Sorting Test 64-Card Version. J Clin Exp Neuropsychol. 2011;33:793–804. doi: 10.1080/13803395.2011.559157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rooks RN, Simonsick EM, Miles T, et al. The association of race and socioeconomic status with cardiovascular disease indicators among older adults in the Health ABC study. J Gerontol B Psychol Sci Soc Sci. 2002;57:S247–S256. doi: 10.1093/geronb/57.4.s247. [DOI] [PubMed] [Google Scholar]
- 30.Fang YZ, Yang S, Wu G. Free radicals, antioxidants, and nutrition. Nutrition. 2002;18:872–879. doi: 10.1016/s0899-9007(02)00916-4. [DOI] [PubMed] [Google Scholar]
- 31.Frisardi V, Panza F, Seripa D, et al. Nutraceutical properties of Mediterranean diet and cognitive decline: Possible underlying mechanisms. J Alzheimers Dis. 2010;22:715–740. doi: 10.3233/JAD-2010-100942. [DOI] [PubMed] [Google Scholar]