Abstract
Dispositional negativity—the propensity to experience and express more frequent, intense, or enduring negative affect—is a fundamental dimension of childhood temperament and adult personality. Elevated levels of dispositional negativity can have profound consequences for health, wealth, and happiness, drawing the attention of clinicians, researchers, and policy makers. Here, we highlight recent advances in our understanding of the psychological and neurobiological processes linking stable individual differences in dispositional negativity to momentary emotional states. Self-report data suggest that three key pathways—increased stressor reactivity, tonic increases in negative affect, and increased stressor exposure—explain most of the heightened negative affect that characterizes individuals with a more negative disposition. Of these three pathways, tonically elevated, indiscriminate negative affect appears to be most central to daily life and most relevant to the development of psychopathology. New behavioral and biological data provide insights into the neural systems underlying these three pathways and motivate the hypothesis that seemingly ‘tonic’ increases in negative affect may actually reflect increased reactivity to stressors that are remote, uncertain, or diffuse. Research focused on humans, monkeys, and rodents suggests that this indiscriminate negative affect reflects trait-like variation in the activity and connectivity of several key brain regions, including the central extended amygdala and parts of the prefrontal cortex. Collectively, these observations provide an integrative psychobiological framework for understanding the dynamic cascade of processes that bind emotional traits to emotional states and, ultimately, to emotional disorders and other kinds of adverse outcomes.
Humans, monkeys, and other animals show marked individual differences in temperament, the tendency to experience fear, anger, disgust, joy, and other fleeting emotional states (Gosling, 2008)1. This distinction between emotional traits and emotional states has its origins in antiquity. More than 2,000 years ago, the Roman intellectual Cicero drew a sharp distinction between anxious temperament (anxietas) and anxiety (angor) (Eysenck, 1983). Like many contemporary researchers, he characterized traits as the proneness to experience particular emotional states (proclivitas). Our own working definition is that traits represent enduring emotional and cognitive biases that first emerge early in life, but continue to evolve and grow in complexity across the lifespan (Shiner, in press-b). Emotional traits account, in a probabilistic manner, for consistency in thoughts, feelings, physiology, and actions across time and situations (Caspi, Roberts, & Shiner, 2005; Fleeson, 2001; Shiner, in press-a; Shiner et al., 2012). Like other psychological constructs that vary across individuals, emotional traits reflect the combined influence of genes and experience on brain structure and function (Polderman et al., in press).
The study of temperament has proven theoretically informative and practically important. Individual differences in temperament and personality have profound consequences for health, wealth, and happiness. Accordingly, temperament has increasingly drawn the attention of educators, social scientists, neurobiologists, clinicians, economists, and public policy makers (Duckworth & Allred, 2012; Ferguson, Heckman, & Corr, 2011; Lahey, 2009; Moffitt, Poulton, & Caspi, 2013; Roberts, Kuncel, Shiner, Caspi, & Goldberg, 2007). Despite this growing interest, fundamental questions about the nature and origins of temperament have remained unresolved. One of the most basic questions concerns the nature of the relations between trait-like differences in temperament and more transient emotional experiences and behaviors (Barlow, Sauer-Zavala, Carl, Bullis, & Ellard, 2013; Epstein, 1994). As the pioneering psychologist David Funder noted, traits “describe patterns and consistencies in behavior, but they don’t explain where those patterns and consistencies come from” (Funder, 1994).
Here, we highlight recent advances in our understanding of the psychological and neurobiological processes linking emotional traits to emotional states, focusing on dispositional negativity, one of the most intensively studied dimensions of temperament and personality. We begin by describing the nature of dispositional negativity and surveying its association with well-being and disease. Next, we review self-report and behavioral data suggesting that three key pathways—increased stressor reactivity, tonic increases in negative affect, and increased stressor exposure—explain most of the heightened negative affect characteristic of individuals with a negative disposition (Figure 1). In the third section of the review, we explore recent advances in our understanding of the neural systems underlying these three pathways, focusing especially on studies of fear and anxiety. This work motivates the hypothesis that seemingly ‘tonic’ increases in negative affect may actually reflect increased reactivity to stressors that are uncertain, temporally remote, or perceptually diffuse (e.g., an unfamiliar experimental context, a pitch-black room; Figure 2). These observations raise a number of interesting new questions. We conclude by outlining several strategies for addressing them and for developing a deeper understanding of the pathways linking emotional traits to momentary emotional states and, ultimately, to psychopathology and other kinds of adverse outcomes.
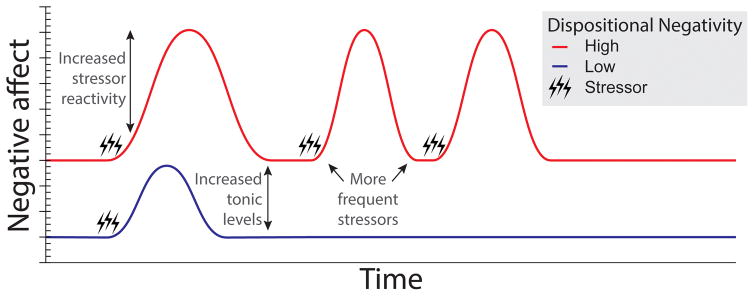
Figure 1. Pathways linking dispositional negativity (trait) to increased momentary negative affect (state).
Questionnaire and behavioral data suggest that three key pathways—increased stressor reactivity, increased tonic levels of negative affect, and more frequent stressors—explain most of the heightened negative affect characteristic of individuals with a negative disposition. Lines depict hypothesized fluctuations in momentary negative affect in individuals with high (red) and low (blue) levels of dispositional negativity, respectively. Acute stressors (e.g., daily hassles, social conflict, and negative life events) are indicated by black lightning bolts.
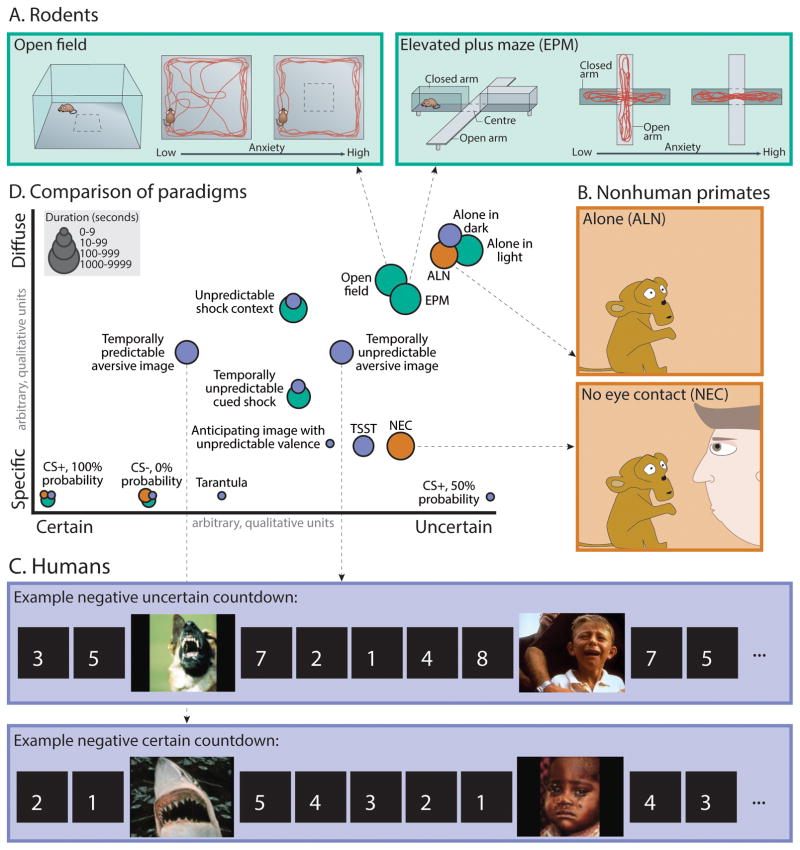
Figure 2. Different kinds of threat.
A. Rodents (Green). In rats and mice, the open field test and the elevated plus maze (EPM) are commonly used to assess emotional responses to diffuse threat. In the open field, rodents are placed into a relatively large, brightly lit, and unfamiliar context. In the elevated plus maze, rodents are placed in a maze with two open arms and two arms enclosed by walls. Freezing and avoidance of the center of the open field or the open arms of the maze provide behavioral ‘read-outs’ of negative affect. Figure adapted with permission from (Tovote et al., 2015). B. Nonhuman primates (Orange). In monkeys, the Human ‘Intruder’ Paradigm (HIP) can be used to quantify naturalistic defensive behaviors, neuroendocrine activity, and brain metabolic activity associated with exposure to a range of threats. In the ‘Alone’ (ALN) condition, the monkey is simply placed in the testing cage. This novel, diffusely threatening context elicits low levels of freezing and cortisol and a moderate frequency of alarm and separation calls. In the ‘No Eye Contact’ (NEC) condition (A, left), the intruder presents his or her profile while avoiding making eye contact. This elicits passive defenses, including freezing and vocal reductions, similar to procedures used for quantifying behavioral inhibition in children. Panel adapted with permission from (A. S. Fox & Kalin, 2014). C. Humans (Purple). In humans, a wide variety of paradigms have been used to probe responses to uncertain, diffuse, or remote threat. Often, these involve the unpredictable presentation of electric shocks or, as shown in the accompanying figure, aversive images. For example, Somerville and colleagues assessed neural activity associated with the temporally certain or uncertain presentation of aversive images. Figure adapted with permission from (Somerville et al., 2013). D. Comparison of paradigms (Scatter plot). Threats differ along several key dimensions, including certainty (x-axis), physical or temporal imminence, diffuseness (y-axis; specific cues vs. real or virtual contexts), and duration (dot size). Here we present some common paradigms used in rodents (green), nonhuman primates (organge), and humans (purple). Studies were chosen for illustrative purposes. We did not attempt a comprehensive review of the literature and, of necessity, the locations of particular paradigms along the two dimensions of the scatter plot are approximate and somewhat arbitrary. Interestingly, many paradigms confound multiple dimensions (e.g., if vs. when threat will occur) and, because of temporal constraints imposed by conventional fMRI techniques, human imaging studies have focused on the relatively brief (<2 min) anticipation of uncertain threat. Studies [duration in sec.]—Rodents: Alone in brightly lit cage [900] (D. L. Walker & Davis, 1997); CS+, 100% probability [30] (Duvarci et al., 2009); CS−, 0% probability [30] (Duvarci et al., 2009); Elevated plus maze (EPM) [900] (Tye et al., 2011); Open field [1080] (Tye et al., 2011); Temporally unpredictable cued shock [mean=162] (Miles, Davis, & Walker, 2011); Unpredictable shock context [1200] (Luyten et al., 2012). Monkeys: Alone (ALN) [1800] (A. S. Fox et al., 2008); CS+, 100% probability [4] (Winslow, Noble, & Davis, 2007); CS−, 0% probability [10] (Kalin, Shelton, Davidson, & Lynn, 1996); No eye contact (NEC) [1800] (A. S. Fox et al., 2008). Humans: Alone in dark room [120] (Grillon, Pellowski, Merikangas, & Davis, 1997); Anticipating image with unpredictable valence [5] (Grupe et al., 2013); CS+, 100% probability [8] (Gazendam et al., 2013); CS+, 50% probability [3] (Buchel, Morris, Dolan, & Friston, 1998); CS−, 0% shock probability (conditioned safety cue) [8] (Gazendam et al., 2013); Tarantula (video clip of approach) [4] (Mobbs et al., 2010); Temporally unpredictable aversive image [115] (Somerville et al., 2013); Temporally unpredictable cued shock [mean=140] (Moberg & Curtin, 2009); Trier Social Stress Test (TSST) [780] (http://topics.sciencedirect.com/topics/page/Trier_social_stress_test); Virtual reality context paired with unpredictable shock [40] (Alvarez, Chen, Bodurka, Kaplan, & Grillon, 2011).
Method
The goal of our review was to integrate psychological and biological perspectives on dispositional negativity into a coherent theoretical framework. Accordingly, it includes 579 citations covering the period between 1966 and 2016, with the major emphasis on recent research (median year of publication = 2011, SD = 8.1; ‘in press’ publications were coded as 2016). This body of research encompasses laboratory, field (e.g., daily diary, ecological momentary assessments), and epidemiological research. It includes published studies of mice, rats, monkeys, and humans, including studies of children, adolescents, adults, and elders. Although most of the work is focused on unselected individuals, we also highlight relevant evidence gleaned from studies of psychiatric and neurological patients. Of necessity, we draw on published articles and reviews from a broad spectrum of scholarly disciplines, from psychology and psychiatry to genetics and neuroscience. For all articles, backward citation checks were used to increase coverage. Although our aims and scope precluded the use of formal meta-analytic techniques, in order to maximize reproducibility and generalizability we place special emphasis on evidence gleaned from meta-analyses2 and large-scale studies, including prospective longitudinal studies and nationally representative samples.
The Nature and Consequences of Elevated Dispositional Negativity
Dispositional negativity or ‘negative emotionality’—the propensity to experience and express more frequent, intense, or enduring negative affect—is a fundamental dimension of childhood temperament and adult personality. Individuals with a more negative disposition tend to be anxious, guilt-prone, insecure, moody, critical, angry, and dissatisfied. They tend to perceive the world as dangerous and threatening and themselves as inadequate (Barlow et al., 2013; Caspi et al., 2005; L. A. Clark & Watson, 2008; Lahey, 2009). Dispositional negativity is sometimes parsed into two mid-level dimensions: anxious distress, encompassing feelings of anxiety, fear, and depression; and irritable distress, encompassing feelings of anger, frustration, and hostility (Caspi et al., 2005). At present, there is greater theoretical consensus about the nature and significance of anxious distress (Caspi et al., 2005; Ormel et al., 2013; Soto & John, in press) and it has received more empirical attention (Barlow et al., 2013). Anxious distress, in turn, subsumes a variety of narrower facet traits, including anxious temperament, anxiety sensitivity, behavioral inhibition, harm avoidance, neuroticism, and trait anxiety (Barlow et al., 2013; Caspi et al., 2005; Markon, Krueger, & Watson, 2005; van den Berg et al., 2014; Widiger, 2009)3.
We conceptualize dispositional negativity as an extended family of closely related phenotypes that first emerge early in development, persist into adulthood, and reflect a combination of heritable and non-heritable factors (A. S. Fox & Kalin, 2014; Lake, Eaves, Maes, Heath, & Martin, 2000; Ormel et al., 2013; Power & Pluess, 2015; Smith et al., 2015; Soto & John, 2014; Turkheimer et al., 2014; Vukasovic & Bratko, 2015). Among adults, concordance between self- and informant-reported (e.g., friends, family members, co-workers) dispositional negativity is substantial (Connolly, Kavanagh, & Viswesvaran, 2007), particularly when multiple informants are employed (McCrae & Costa, 1987), suggesting that it is more than just a negative response bias. Core features of this phenotypic family—including increased behavioral inhibition, heightened vigilance, and other signs of fear and anxiety—are expressed similarly across mammalian species, enabling mechanistic studies to be performed in rodents and monkeys (Boissy, 1995; Mobbs & Kim, 2015; Oler, Fox, Shackman, & Kalin, 2016). Although the molecular underpinnings of dispositional negativity and its neural substrates remain poorly understood (Bastiaansen et al., 2014; Bogdan, Pagliaccio, Baranger, & Hariri, 2016; Christian et al., 2009), some promising candidates have recently been identified in humans (Buckholtz et al., 2008; Okbay et al., in press), monkeys (Alisch et al., 2014; A. S. Fox et al., 2012; Kalin et al., in press; Oler et al., 2009; Rogers et al., 2013; Roseboom et al., 2014), and rodents (Turner, Clinton, Thompson, Watson, & Akil, 2011).
Dispositional negativity is stable, but not immutable, and like other emotional traits continues to develop and change across the lifespan (Fraley & Roberts, 2005; Roberts & DelVecchio, 2000; Roberts & Mroczek, 2008; Roberts et al., 2006). In fact, mean levels of dispositional negativity show substantial fluctuations—equivalent to T-scores of 2 in males and 5 in females—between the ages of 10 and 65, peaking in adolescence (Soto, John, Gosling, & Potter, 2011). Several large (n = 4,850 – 1,267,218) international studies indicate that, from about age 14 on, women tend to report substantially higher levels of dispositional negativity than men (De Bolle et al., 2015; Schmitt, Realo, Voracek, & Allik, 2008; Soto et al., 2011).
A range of evidence indicates that dispositional negativity can be increased by stress, trauma, and negative life events (e.g., death of a spouse, birth of a child, chronic disease; Barlow et al., 2013; Hutteman, Bleidorn, Kerestes, et al., 2014; Jeronimus, Riese, Sanderman, & Ormel, 2014; Jokela, Hakulinen, Singh-Manoux, & Kivimaki, 2014; Jokela, Kivimaki, Elovainio, & Keltikangas-Jarvinen, 2009; Laceulle, Nederhof, Karreman, Ormel, & Van Aken, 2011; Ludtke, Roberts, Trautwein, & Nagy, 2011; Parker, Ludtke, Trautwein, & Roberts, 2012; Roberts, Caspi, & Moffitt, 2003; Robins, Caspi, & Moffitt, 2002). But importantly it can also be decreased by cognitive-behavioral (Barlow et al., 2013; Bennett et al., 2015; Mihalopoulos et al., 2015) and pharmacological interventions for anxiety and depression (Barlow et al., 2013; Soskin, Carl, Alpert, & Fava, 2012), raising the possibility of developing strategies for identifying high-risk individuals and preventing the onset of more severe sequelae. Identifying the psychological and neurobiological mechanisms governing the malleability of temperament is a particularly important avenue for future research, one that promises to provide new targets for intervention (A. S. Fox et al., 2012).
There is clear evidence that dispositionally negative individuals tend to experience heightened levels of momentary negative affect. Self-report measures of dispositional negativity (trait) and negative affect (state) are strongly correlated (Lieberman et al., in press; Matthews et al., 2009; Watson & Clark, 1984, 1992). In fact, a recent meta-analysis incorporating data from more than 30,000 individuals showed that dispositional negativity explains 30–50% of the variance in negative affect (Steel et al., 2008). Ratings obtained from other informants, such as clinicians and spouses, yield similar conclusions, indicating that these trait-state relations are not an artifact of response biases (Lieberman et al., in press; McCrae & Costa, 1991; Soto & John, in press; Steel et al., 2008; Watson & Clark, 1984).
It merits comment that the impact of dispositional negativity on momentary emotional experience is not limited to negative affect. Individuals with a more negative disposition are also prone to lower levels of positive affect (Aldinger et al., 2014; Gable, Reis, & Elliot, 2000; Jacobs et al., 2011; Soto & John, in press; Watson & Clark, 1984; Zautra, Affleck, Tennen, Reich, & Davis, 2005) and subjective wellbeing (Steel et al., 2008), perhaps reflecting a suppressive consequence of negative affect on reward and appetitive motivation (i.e., sometimes termed ‘stress-induced’ anhedonia; Pizzagalli, 2014).
Dispositional negativity predicts a multitude of practically important outcomes, from satisfaction and wealth to marital stability and disease. Increased dispositional negativity is associated with lower levels of educational attainment (Damian, Su, Shanahan, Trautwein, & Roberts, 2015; Hengartner, Kawohl, Haker, Rossler, & Ajdacic-Gross, 2016) and occupational success (Heineck, 2011; Hengartner, Kawohl, et al., 2016; Ng et al., 2005; Shanahan, Bauldry, Roberts, Macmillan, & Russo, 2014; Soldz & Vaillant, 1999; Sutin, Costa, R., & Eaton, 2009; Uysal & Pohlmeier, 2011; Viinikainen, Kokko, Pulkkinen, & Pehkonen, 2010). In a nationally representative sample of 81,000 high school students, individuals who were one standard-deviation above the mean lost the equivalent of half an academic year in educational attainment and $3,628 in annual income by the time they reached mid-life compared to those one standard-deviation below the mean (in 2014 dollars; Damian et al., 2015).
Individuals with elevated dispositional negativity report reduced satisfaction with their lives (Dyrenforth, Kashy, Donnellan, & Lucas, 2010; Soto & Luhmann, 2013), jobs (Wayne, Musisca, & Fleeson, 2004), friends (R. E. Wilson, Harris, & Vazire, 2015), and spouses (Dyrenforth et al., 2010; Solomon & Jackson, 2014). In fact, elevated levels of dispositional negativity in adolescence have been shown to predict lower levels of job satisfaction 50 years later (Staw, Bell, & Clausen, 1986) and lower levels of psychological well-bring 36 years later (Abbott et al., 2008). Heightened dispositional negativity is also a strong predictor of future loneliness (Pressman et al., 2005; Stokes, 1985) and divorce (Karney & Bradbury, 1995; Kurdek, 1993). In one particularly compelling example, 278 married couples were longitudinally assessed between 1936 and 1981 (Kelly & Conley, 1987). Of these, 50 divorced. Self- and acquaintance-ratings of husbands’ and wives’ dispositional negativity at the initial assessment were among the strongest prenuptial predictors of divorce across the 45-year follow-up period. Similar results have been reported for spouses with pre-existing anxiety disorders—for example, the odds of getting divorced among patients with a pre-marital diagnosis of generalized anxiety disorder is 1.7-fold greater than psychiatrically healthy controls (Kessler, Walters, & Forthofer, 1998). More frequent marital dissolution is, in turn, associated with reduced reproductive success. In a prospective study of more than 1,500 Finnish adults, a one standard-deviation increase in dispositional negativity decreased the odds of having a second and third child by 11% and 15%, respectively, largely due to the higher frequency of marital dissolution and divorce (Jokela et al., 2009).
From the perspective of physical health, dispositional negativity is associated with sleep problems (Hintsanen et al., 2014), metabolic syndrome (Phillips et al., 2010), elevated cholesterol levels (Hengartner, Kawohl, et al., 2016), and a wide variety of other physical diseases and subjective health complaints (e.g., coronary heart disease; Deary, Weiss, & Batty, 2010; Gale et al., 2016; Iacovino, Bogdan, & Oltmanns, 2016; Jokela, Pulkki-Raback, et al., 2014; Lahey, 2009; Mund & Neyer, in press). Among nearly 7,000 older adults (mean age = 68.4 years) followed as part of the Health and Retirement study, a one standard-deviation increase in dispositional negativity prospectively increased the odds of being diagnosed with a heart condition by 24%, lung disease by 29%, and hypertension by 37% during the four-year follow-up period (Weston, Hill, & Jackson, 2015). Increased morbidity partially reflects dispositional negativity’s association with unhealthy behaviors, such as chronic tobacco and substance use (Gale et al., 2016; Hakulinen, Hintsanen, et al., 2015; Hengartner, Kawohl, et al., 2016; Kotov et al., 2010; Leventhal et al., 2012; Malouff et al., 2007; Soldz & Vaillant, 1999), and likely contributes to premature mortality among individuals with a more negative disposition (Chapman, Fiscella, Kawachi, & Duberstein, 2010; Jackson, Connolly, Garrison, Leveille, & Connolly, 2015; Terracciano, Lockenhoff, Zonderman, Ferrucci, & Costa, 2008; R. S. Wilson et al., 2005).
Dispositional negativity is also a key risk factor for anxiety disorders, depression, and substance abuse (Clauss & Blackford, 2012; Conway, Craske, Zinbarg, & Mineka, 2016; Grav, Stordal, Romild, & Hellzen, 2012; Hakulinen, Elovainio, et al., 2015; Hengartner, Kawohl, et al., 2016; Kendler & Gardner, 2014; Soldz & Vaillant, 1999; Watson & Naragon-Gainey, 2014; S. Wilson, Vaidyanathan, Miller, McGue, & Iacono, 2014) —psychiatric disorders that are highly prevalent, debilitating, and often challenging to treat (Bystritsky, 2006; Collins et al., 2011; DiLuca & Olesen, 2014; Griebel & Holmes, 2013; Insel, 2012; Kessler, Petukhova, Sampson, Zaslavsky, & Wittchen, 2012; Whiteford et al., 2013). The magnitude of these associations is substantial: a meta-analysis incorporating 175 studies reported that the mean Cohen’s d across mood, anxiety, and substance use disorders was 1.65, ranging from d ≅ 2 for dysthmia and anxiety disorders to d = .77 for alcohol use disorder (Kotov et al., 2010). Elevated dispositional negativity is among the strongest prospective predictors of future internalizing disorders (see also D. A. Clark, Durbin, Hicks, Iacono, & McGue, in press; k = 46 studies; mean Cohen’s d = .63; Ormel et al., 2013). For example, data from the Zurich Cohort Study (n = 591) indicates that a one standard-deviation increase in dispositional negativity at the time of the baseline assessment in 1988 increased the odds of developing a major depressive episode by 41% and an anxiety disorder by 32% during the twenty-year follow-up period (Hengartner, Ajdacic-Gross, Wyss, Angst, & Rossler, 2016). These relations are evident after eliminating overlapping item content (Uliaszek et al., 2009). They also appear to be strengthened by exposure to stress (Kopala-Sibley et al., in press; Kopala-Sibley et al., 2016; Vinkers et al., 2014), suggesting that high levels of dispositional negativity represent a diathesis (Monroe & Simons, 1991) for the internalizing spectrum of disorders (i.e., anxiety and depression). Among patients with a history of internalizing disorders, higher levels of dispositional negativity are associated with a greater number of co-morbid diagnoses (Hengartner, Kawohl, et al., 2016) and a more pessimistic prognosis (Berlanga, Heinze, Torres, Apiquian, & Cabellero, 1999; Duggan, Lee, & Murray, 1990; Faravelli, Ambonetti, Pallanti, & Pazzagli, 1986; Hirschfeld, Klerman, Andreasen, Clayton, & Keller, 1986; Kendler, Neale, Kessler, & Heath, 1993; Ormel, Oldehinkel, & Vollebergh, 2004; Quilty et al., 2008; Scott, Williams, Brittlebank, & Ferrier, 1995; Weissman, Prusoff, & Klerman, 1978). For example, Steunenberg and colleagues found that individuals with above-median levels of dispositional negativity were 2.8-times more likely to relapse or experience a new depressive episode across a six-year follow-up period (Steunenberg, Beekman, Deeg, & Kerkhof, 2010). Among parents, higher levels of dispositional negativity are also associated with elevated clinician and teacher ratings of internalizing symptoms in their offspring (Ellenbogen & Hodgins, 2004). Determining the biological and psychological mechanisms underlying this intergenerational transmission of psychopathology remains an important challenge for future research.
Given this panoply of adverse, often co-morbid outcomes, dispositional negativity imposes a tremendous burden on healthcare providers and the global economy (Goodwin, Hoven, Lyons, & Stein, 2002; ten Have, Oldehinkel, Vollebergh, & Ormel, 2005). A recent Dutch study estimated that each individual in the upper-quartile of the dispositional negativity distribution is associated with $6,362 in excess costs each year, largely due to increased use of health services and loss of productivity (in 2015 dollars; Cuijpers et al., 2010). In a population the size of the United States, this would translate to $388 billion annually or nearly one-third of federal discretionary spending.
Trait-State Links Inferred from Self-Report and Behavior
Despite its profound significance for health and wealth, the processes linking individual differences in dispositional negativity (trait) to heightened negative affect (state) have only recently started to come into focus. As shown schematically in Figure 1, self-report data reported over the past several decades suggest that three inter-related processes explain most of the heightened negative affect characteristic of individuals with a more negative disposition:
Increased stressor reactivity: Dispositionally negative individuals report elevated negative affect in response to a range of stressors, including negative life events, daily hassles, interpersonal conflicts, and aversive laboratory challenges.
Increased negative affect in the absence of clear stressors: Dispositionally negative individuals frequently report exaggerated apprehension and distress in relaxed and familiar settings, when potential stressors are remote, diffuse, or altogether absent. This pervasive, context-independent negative affect has been described as a ‘tonic’ or ‘endogenous’ effect of temperament, given the absence of a clear external source of distress (Gross, Sutton, & Ketelaar, 1998; Watson & Clark, 1984).
Increased stressor exposure and generation: Individuals with a more negative disposition tend to act in ways that increase the likelihood of experiencing hassles, conflict, and rejection, particularly during times of heightened stress. Increased exposure to stressors, in turn, promotes more frequent, intense, or persistent negative affect.
Increased stressor reactivity
Individuals with a more negative disposition are emotionally volatile and tend to over-react to novelty, threat, and other stressors. In one particularly compelling example, Hengartner and colleagues assessed reactions to an ‘active shooter’ incident that recently occurred on the campus of a Swiss university (Hengartner, van der Linden, Bohleber, & von Wyl, 2016). During the incident, an alarm sounded continuously while more than 100 heavily armed police officers secured the site. Three hours later, the ‘all clear’ signal was given, and students and staff were allowed to leave their shelters. On-line surveys revealed that individuals with a more negative disposition retrospectively (6–26 days later) reported experiencing elevated levels of fear, worry, and terror during the incident.
Experience-sampling studies show that individuals with high levels of dispositional negativity also tend to report elevated levels of negative affect in response to more mundane hassles and interpersonal conflicts in the home, school, and workplace (Bolger & Schilling, 1991; Gable et al., 2000; Komulainen et al., 2014; Leger, Charles, Turiano, & Almeida, in press; Mroczek & Almeida, 2004; Suls & Martin, 2005; Tan et al., 2012; Zautra et al., 2005). Heightened reactivity to everyday stressors, in turn, predicts the onset of future internalizing symptoms and episodes, characterizes patients with acute anxiety disorders and depression, and remits with pharmacological therapy (Farmer & Kashdan, 2015; Tan et al., 2012; van Winkel et al., 2015; Wichers et al., 2009).
In the laboratory, individuals with a more negative disposition report elevated distress in response to standardized aversive challenges (e.g., amputation film clips; Gross et al., 1998; Matthews et al., 2009), suggesting that heightened emotional reactivity is not an artifact of systematic response biases, mnemonic distortions, or differences in stressor exposure. These self-report data are consistent with evidence that dispositionally negative children, adults, and monkeys show exaggerated behavioral (e.g., avoidance, crying, inhibition), psychophysiological (e.g., startle, skin conductance), and neuroendocrine (e.g., cortisol) reactions to novelty and potential threat (Brooker et al., in press; Buss et al., 2003a; Hengartner, van der Linden, et al., 2016; Kagan, Snidman, Kahn, & Towsley, 2007; Norris, Larsen, & Cacioppo, 2007; Oler et al., 2016; Schmidt & Fox, 1998; Shackman et al., 2013; Vaidyanathan, Patrick, & Cuthbert, 2009).
Taken together, these observations indicate that dispositional negativity represents a diathesis that serves to enhance the likelihood, intensity, or duration of negative affect elicited by a range of common stressors. Heightened stressor reactivity also appears to causally contribute to the development and recurrence of pathological anxiety and depression.
Increased negative affect in the absence of clear stressors
Dispositionally negative individuals often report heightened negative affect in the absence of clear and imminent stressors (Watson & Clark, 1984). In controlled laboratory settings, dispositionally negative adolescents and adults report more intense or frequent negative thoughts and feelings at ‘baseline,’ while viewing emotionally-neutral control stimuli or simply relaxing (Craske et al., 2009; Glue, Wilson, Coupland, Ball, & Nutt, 1995; Gross et al., 1998; Larsen & Ketelaar, 1989, 1991; Watson & Clark, 1984). Likewise, children with a more negative disposition show elevated heart rate at ‘baseline’ (Reznick et al., 1986). In their daily lives, dispositionally negative adults report elevated negative affect in comfortable, familiar settings, such as their home (Suls & Martin, 2005). In a seminal study, Bolger and Schilling (1991) leveraged 6 weeks of daily reports collected from more than 300 individuals to show that individuals with a more negative disposition report elevated distress in daily life. Next, they used statistical decomposition techniques (i.e., hierarchical linear modeling) to show that nearly 60% of this effect reflects ‘tonic’ differences in distress, in settings where their subjects did not report a clear concurrent source of stress, more than double the variance attributable to either stressor reactivity or stressor exposure.
Heightened negative affect in the absence of clear, exogenous stressors may reflect dispositionally negative individuals’ tendency to experience spill-over of negative affect across sequential moments, contexts, or days (Houben et al., 2015; Judge, Simon, Hurst, & Kelley, 2014; Koval & Kuppens, 2012; Suls & Martin, 2005). Data from several large U.S. studies (ns > 1,000) show that individuals with heightened levels of dispositional negativity tend to carry negative affect from work to home and vice versa (Horwitz, Luong, & Charles, 2008; Wayne et al., 2004). Spill-over and emotional inertia has also been observed in patients with internalizing disorders (Houben et al., 2015; Newman & Fisher, 2013; Peeters, Nicolson, Berkhof, Delespaul, & deVries, 2003). Among patients, spill-over and inertia predict the severity of symptoms (Brose, Schmiedek, Koval, & Kuppens, 2015; Houben et al., 2015; Koval, Kuppens, Allen, & Sheeber, 2012; Newman & Fisher, 2013), foreshadow future episodes (van de Leemput et al., 2014), improves with treatment (Newman & Fisher, 2013), and predict treatment response (Newman & Fisher, 2013). This may reflect maladaptive emotion regulation. Individuals with a more negative disposition are prone to worrying about the future and ruminating about the past (Grupe & Nitschke, 2013; Nolan, Roberts, & Gotlib, 1998; Wupperman & Neumann, 2006) and these maldadaptive coping strategies tend to promote pervasive negative affect in otherwise quiescent settings (Barlow et al., 2013; Nolen-Hoeksema, Wisco, & Lyubomirsky, 2008).
In short, several decades of self-report research demonstrates that persistently elevated, context-independent negative affect is a key feature of dispositional negativity. More recently acquired experience-sampling data suggest that spill-over and inertia of negative affect play a role in the development and recurrence of internalizing disorders.
Increased stressor exposure and generation
Like the anxiety disorders (American Psychiatric Association, 2013), dispositional negativity is thought to be associated with heightened avoidance of punishment and potential threat (Barlow et al., 2013; Cavanagh & Shackman, 2015; Gray & McNaughton, 2000; Grupe & Nitschke, 2013; Hengartner, van der Linden, et al., 2016). Yet there is also compelling evidence that dispositionally negative individuals are more frequently exposed to hassles, stressors, and more severe kinds of adversity. In fact, converging lines of prospective-longitudinal (Carter, Garber, Ciesla, & Cole, 2006; Fergusson & Horwood, 1987; Headey & Wearing, 1989; Kercher, Rapee, & Schniering, 2009; Lakdawalla & Hankin, 2008; Ludtke et al., 2011; Magnus, Diener, Fujita, & Pavot, 1993; Ormel & Wohlfarth, 1991; Specht, Egloff, & Schmukle, 2011; van Os, Park, & Jones, 2001; Vollrath, 2000; Wetter & Hankin, 2009; Whittington & Huppert, 1998), behavioral-genetic (Billig, Hershberger, Iacono, & McGue, 1996; Kandler, Bleidorn, Riemann, Angleitner, & Spinath, 2012; Middeldorp, Cath, Beem, Willemsen, & Boomsma, 2008; Power et al., 2013; Saudino, Pedersen, Lichtenstein, McClearn, & Plomin, 1997), and daily-diary data (Berry, Willingham, & Thayer, 2000; Bolger & Schilling, 1991; Bolger & Zuckerman, 1995; J. P. David, Green, Martin, & Suls, 1997; Gunthert, Cohen, & Armeli, 1999; Hankin, 2010; Hankin, Fraley, & Abela, 2005; Leger et al., in press; Marco & Suls, 1993; Suls, Green, & Hillis, 1998) acquired from more than 50,000 individuals in the U.S. and abroad, some followed for as long as 16 years, demonstrate that adolescents and adults with high levels of dispositional negativity report more frequent personal difficulties and conflicts, particularly those of an interpersonal nature. Similar effects have been reported in children and adults with internalizing disorders (J. L. Allen & Rapee, 2009; Farmer & Kashdan, 2015; Hoehn-Saric, McLeod, Funderburk, & Kowalski, 2004; Kendler & Karkowski-Shuman, 1997; Kendler, Karkowski, & Prescott, 1999; Liu & Alloy, 2010; Liu, Kraines, Massing-Schaffer, & Alloy, 2014).
Increased stressor exposure has been observed using subjective ratings and more objective experimenter ratings of stressor intensity (Gleason, Powers, & Oltmanns, 2012; Iacovino et al., 2016; Jeronimus et al., 2014). For example, data from the Virginia Twin Registry (n > 7,000) indicates that dispositional negativity strongly predicts job loss, marital problems, and conflicts with family and co-workers, but is unrelated to random misfortunes (e.g., robbery; Kendler, Gardner, & Prescott, 2003). Analyses of nearly 1,000 datasets from the Dunedin longitudinal sample show that individuals with a more negative disposition at age 18 report progressively higher levels of conflict and abuse in romantic relationships across young adulthood (Robins et al., 2002). Using digital audio recorders, Mehl and colleagues showed that individuals with higher levels of dispositional negativity were more likely to argue in their daily lives (Mehl, Gosling, & Pennebaker, 2006). Leveraging survey data collected from 900 AmeriCorps volunteers pseudo-randomly assigned to 100 teams, Klein and colleagues (2014) demonstrated that levels of dispositional negativity at the time of employment strongly influenced the formation of spontaneous social networks 10 months later. Individuals with a more negative disposition tended to become central to what they termed ‘adversarial networks’ (i.e., are disliked by many teammates) and to show low centrality in both friendship and advice networks (Klein, Lim, Saltz, & Mayer, 2004). Among children, elevated levels of dispositional negativity are prospectively associated with peer rejection, social exclusion, victimization, and reduced friendship quality (Coplan, Arbeau, & Armer, 2007; Gazelle, 2008; Gazelle & Ladd, 2003; Ladd, Kochenderfer-Ladd, Eggum, Kochel, & McConnell, 2011; K. Rubin, Bowker, & Gazelle, 2010; K. H. Rubin, Wojslawowicz, Rose-Krasnor, Booth-LaForce, & Burgess, 2006; Strauss, Frame, & Forehand, 1987). In short, there is compelling evidence that elevated levels of dispositional negativity are associated with increased exposure to a range of psycho-social stressors across the lifespan.
Other work suggests that dispositionally negative individuals play an instrumental role in generating stressors; that they tend to act in ways that increase the likelihood or chronicity of negative life events (e.g., divorce, financial difficulties) and daily hassles (e.g., interpersonal conflict, social rejection). In adulthood, their friends report more frequent conflict and heightened irritation (Berry et al., 2000), their romantic partners report reduced relationship security (Neyer & Voigt, 2004), their spouses report reduced marital and sexual satisfaction (Dyrenforth et al., 2010; Malouff et al., 2010; Solomon & Jackson, 2014; Watson, Hubbard, & Wiese, 2000; Watson & Humrichouse, 2006), and their offspring report more frequent parent-child conflict (Hutteman, Bleidorn, Kereste, et al., 2014). Likewise, the parents of children with a more negative disposition describe their relationship with their offspring as challenging and emotionally exhausting (Shamir-Essakow, Ungerer, Rapee, & Safier, 2004). Similar effects have been reported for the parents of children with anxiety disorders (Lebowitz, Scharfstein, & Jones, 2014; Lebowitz et al., 2013).
These kinds of informant reports are complemented by laboratory studies showing that randomly assigned social partners judge dispositionally negative adults to be moody, uncomfortable, and negative (Creed & Funder, 1998). This negativity begets negativity and random partners tend to respond with elevated levels of criticism, contempt, and hostility (Creed & Funder, 1998) and to judge the interaction more negatively (Heerey & Kring, 2007). Likewise, dispositionally negative children tend to evoke more negative reactions from unfamiliar peers (Stewart & Rubin, 1995; O. L. Walker, Degnan, Fox, & Henderson, in press).
Heightened interpersonal stress and social rejection may stem from dispositionally negative individuals’ tendency to express lower levels of warmth and empathy; to be less responsive and disclosing to relationship partners; to overreact and escalate negative affect during conflicts (e.g., angry venting, hostile or aggressive confrontation); and to engage in toxic interpersonal behaviors (i.e., criticism, contempt, and sarcasm), particularly during periods of heightened stress (Ackerman & Corretti, 2015; L. A. Clark, Kochanska, & Ready, 2000; Connor-Smith & Flachsbart, 2007; de Haan, Dekovic, & Prinzie, 2012; Donnellan, Conger, & Bryant, 2004; Ellenbogen & Hodgins, 2004; Kendler & Karkowski-Shuman, 1997; Kochanska, Clark, & Goldman, 1997; McNulty, 2008; Neyer & Asendorpf, 2001; Prinzie et al., 2009; Romero-Canyas, Downey, Berenson, Ayduk, & J., 2010; Vater & Schröder-Abé, 2015; Wang, Repetti, & Campos, 2011)4. Likewise, individuals with anxiety disorders are prone to more frequent and intense conflict with their partners and spouses (Johnson, Cohen, Kasen, & Brook, 2004; Metz, Majdandzic, & Bogels, in press).
Studies of patients with social phobia in semi-structured ‘getting acquainted’ tasks suggests that these and other maladaptive expressive behaviors elicit negative affect in others which, in turn, promotes discord, alienation, and rejection (Alden & Taylor, 2004; Plasencia, Alden, & Taylor, 2011). Similar results have been found in more naturalistic observational and experience-sampling studies (Pasch, Bradbury, & Davila, 1997; Zaider, Heimberg, & Iida, 2010). Interventions targeting these maladaptive behaviors reduce conflict and rejection, indicating a causal role (Snyder & Halford, 2012; Taylor & Alden, 2011). In sum, individuals with a more negative disposition and patients with internalizing disorders actively, if unintentionally, shape their environment in ways that generate stress. Increased exposure to hassles, conflict, and other, more severe psycho-social stressors (e.g., divorce), in turn, tends to promote more intense or pervasive negative affect.
The Psychophysiology and Neurobiology of Dispositional Negativity
The self-report and behavioral data that we have reviewed suggest that the link between dispositional negativity and heightened levels of momentary negative affect reflects a combination of increased stressor reactivity, tonic or ‘endogenous’ increases in negative affect, and increased stressor exposure (Figure 1). As described in more detail below, brain imaging, neuropsychological, and more mechanistic kinds of data gleaned from animal models strongly corroborate the link binding dispositional negativity to heightened stressor reactivity. But biological data raise the possibility that seemingly ‘tonic’ increases in negative affect may actually reflect increased reactivity5 to stressors that are mild, remote, uncertain, or diffuse (Figure 2). This is likely to be exacerbated by stress-induced sensitization of brain regions, such as the amygdala, that play a key role in assembling states of fear and anxiety6. While the neurobiological mechanisms underlying increased stressor generation and exposure remain largely opaque, the existing neurobiological record suggests that the tendency to behave in ways that promote interpersonal conflict and evoke social rejection may reflect variation in the function of circuits that underlie the appraisal of emotionally-salient social cues.
Increased reactivity to aversive challenges in the laboratory
Decades ago, Gordon Allport suggested that “traits are cortical [or] subcortical…dispositions having the capacity to gate or guide specific phasic reactions” (1966). To this day, most neurobiologically grounded models of dispositional negativity remain rooted in the idea that temperament and personality reflect differences in the magnitude or likelihood of reactions to punctate, trait-relevant challenges (e.g., conflict, criticism, punishment, danger; Eysenck, 1967; Goldsmith et al., 1987; Kagan, Reznick, & Snidman, 1988; Reiss, 1997; Spielberger, 1966; Zuckerman, 1976)7.
Consistent with this perspective, there is clear evidence that humans and monkeys with a more negative disposition show heightened reactions to threat-related cues in a number of brain regions, including the amygdala, anterior hippocampus, anterior insula, bed nucleus of the stria terminalis (BST), mid-cingulate cortex, orbitofrontal cortex (OFC), and periaqueductal gray (PAG) (Avery et al., 2016; Calder et al., 2011; Cavanagh & Shackman, 2015; A. S. Fox & Kalin, 2014; A. S. Fox, Oler, Shackman, et al., 2015; A. S. Fox, Oler, Tromp, et al., 2015; Shackman & Fox, in press; Shackman et al., 2011).
Here, we focus on the most intensively scrutinized of these regions, the amygdala. The amygdala is a heterogeneous collection of nuclei buried beneath the temporal lobe (Freese & Amaral, 2009; Yilmazer-Hanke, 2012). As shown in Figure 3, the amygdala is poised to assemble a broad spectrum of emotional reactions via projections to the downstream regions that directly mediate the behavioral (e.g., passive and active avoidance), peripheral physiological (e.g., cardiovascular and neuroendocrine activity, startle), and cognitive (e.g., vigilance) components of momentary negative affect (M. Davis & Whalen, 2001; Freese & Amaral, 2009). Lesion, imaging, and electrophysiological evidence demonstrate that the amygdala can trigger shifts of attention to threat-relevant social cues (e.g., eyes) and that reentrant projections from the basolateral (BL) nucleus of the amygdala to the visual cortex and superior colliculus play a crucial role in prioritizing the processing of threat-relevant cues (Shackman, Kaplan, et al., in press). The amygdala is also poised to promote non-specific states of vigilance via projections to ascending neurochemical systems (i.e., acetylcholine, dopamine, norepinephrine) in the basal forebrain and brainstem that can modulate the responsiveness of sensory cortex to incoming information (Arnsten, 2009, 2015; M. Davis & Whalen, 2001; Freese & Amaral, 2009).
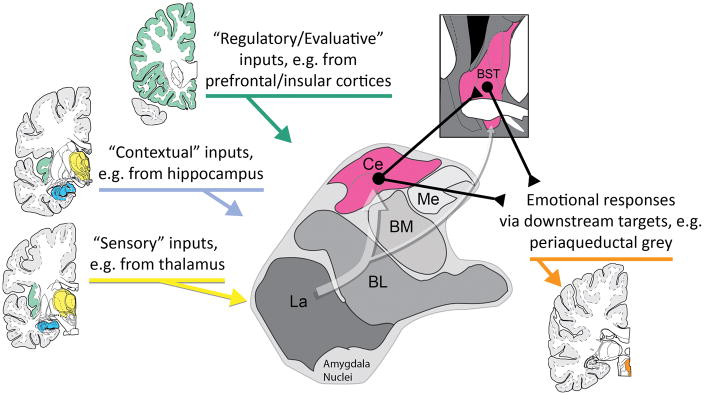
Figure 3. Central extended amygdala circuitry.
Simplified schematic of key inputs and outputs to the central extended amygdala (magenta) in humans and other primates. The central amygdala encompasses the central nucleus of the amygdala (Ce) and neighboring bed nucleus of the stria terminalis (BST). As shown by the translucent white arrow at the center of the figure, most sensory (yellow), contextual (blue), and regulatory (green) inputs to the central extended amygdala are indirect (i.e., poly-synaptic), and first pass through adjacent amygdala nuclei before arriving at the Ce. In primates, projections linking the Ce to the BST are predominantly unidirectional (Ce → BST). The Ce and BST are poised to orchestrate or trigger momentary negative affect via projections to downstream effector regions (orange). Portions of this figure were adapted with permission from the atlas of Mai and colleagues (Mai, Paxinos, & Voss, 2007). Abbreviations: Basolateral (BL), Basomedial (BM), Central (Ce), Lateral (La), and Medial (Me) nuclei of the amygdala; Bed nucleus of the stria terminalis (BST).
Imaging studies show that dispositionally negative individuals show increased or prolonged activation in the dorsal amygdala in response to novelty or potential threat (Blackford, Avery, Shelton, & Zald, 2009; Calder et al., 2011; Fonzo et al., 2015; A. S. Fox & Kalin, 2014; Schuyler et al., 2012; Stein, Simmons, Feinstein, & Paulus, 2007) (Figures 4a–b). This is particularly evident following periods of acute stress (Everaerd, Klumpers, van Wingen, Tendolkar, & Fernandez, 2015). Amygdala reactivity also tends to habituate more slowly in adults and youth with a more negative disposition (Blackford, Allen, Cowan, & Avery, 2013; Blackford, Avery, Cowan, Shelton, & Zald, 2011; Hare et al., 2008).
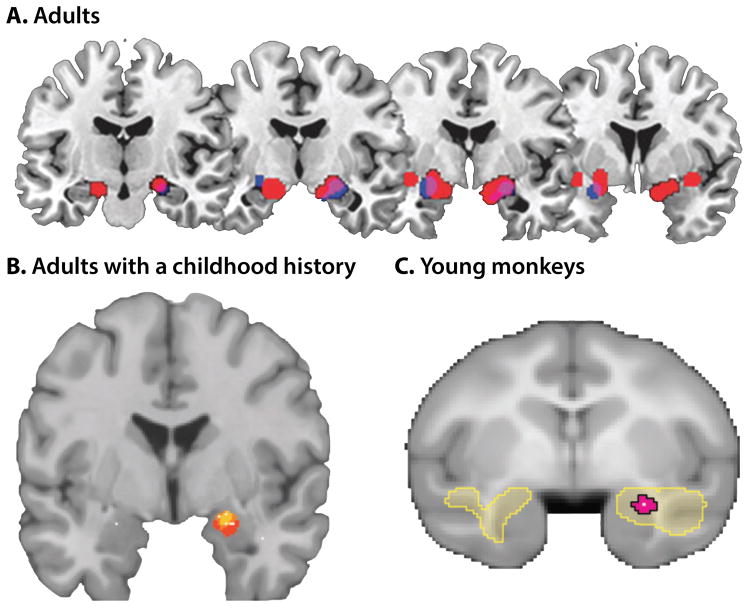
Figure 4. The dorsal amygdala is more reactive to acute threat-related cues in dispositionally negative individuals.
A. Adults with elevated dispositional negativity. Meta-analysis of six published imaging studies reveals consistently elevated activation bilaterally in the vicinity of the dorsal amygdala (Calder et al., 2011). Significant relations with dispositional negativity (trait) are shown in blue; significant relations with momentary negative affect (state) are depicted in red; and the overlap is shown in purple. B. Adults with a childhood history of elevated dispositional negativity. Meta-analysis of seven published imaging studies reveals consistently elevated activation in the right dorsal amygdala (A. S. Fox, Oler, Tromp, et al., 2015). Six of eight amygdala peaks overlapped (yellow) in the dorsal amygdala; four of the peaks extended into the region shown in red. C. Young monkeys. Using high-resolution 18-fluorodeoxyglucose-positron emission tomography (FDG-PET) acquired from 238 young rhesus monkeys, Oler and colleagues (2010) demonstrated that threat-related activity in the right Ce (i.e., dorsal amygdala) predicts stable individual differences in dispositional negativity. Figure depicts regions identified by a voxelwise regression analysis (yellow; p < .05, whole-brain corrected). The peak voxel and corresponding 95% spatial confidence interval are depicted in white and magenta, respectively. Portions of this figure were adapted with permission from (Calder et al., 2011; A. S. Fox & Kalin, 2014; A. S. Fox, Oler, Tromp, et al., 2015).
Large-scale (n = 238–592) positron emission tomography (PET) studies in juvenile monkeys show that threat-related metabolic activity in the dorsal amygdala, in the region of the central (Ce) nucleus (Figure 4c), is stable over time and context (i.e., trait-like), heritable, and associated with heightened behavioral and neuroendocrine reactions to threat (A. S. Fox & Kalin, 2014; A. S. Fox, Oler, Shackman, et al., 2015; A. S. Fox et al., 2012; Oler et al., 2010; Shackman et al., 2013). For example, Fox and colleagues reported that metabolic activity in the Ce during prolonged exposure to an unfamiliar human intruder’s profile (Figure 2b) showed an intra-class correlation (ICC) of 0.64 across three occasions over a 1.1 year span, similar to the concurrent re-test stability of dispositional negativity in young monkeys (ICC = 0.72; A. S. Fox et al., 2012) and the 5-year stability of dispositional negativity in humans (partial R = .60; n = 56,735; Hakulinen, Elovainio, et al., 2015). Other work in nonhuman primates demonstrates that elevated amygdala activity is a core substrate for different presentations of dispositional negativity (Figure 5). Like humans, monkeys express dispositional negativity in different ways. Some individuals characteristically respond to potential threat with high levels of the stress-sensitive hormone cortisol and middling levels of behavioral inhibition, whereas others show the reverse profile. What all of these individuals share is heightened threat-related activity in the Ce (Shackman et al., 2013). Collectively, these observations show that individual differences in dispositional negativity partially reflect trait-like variation in the function of the amygdala.
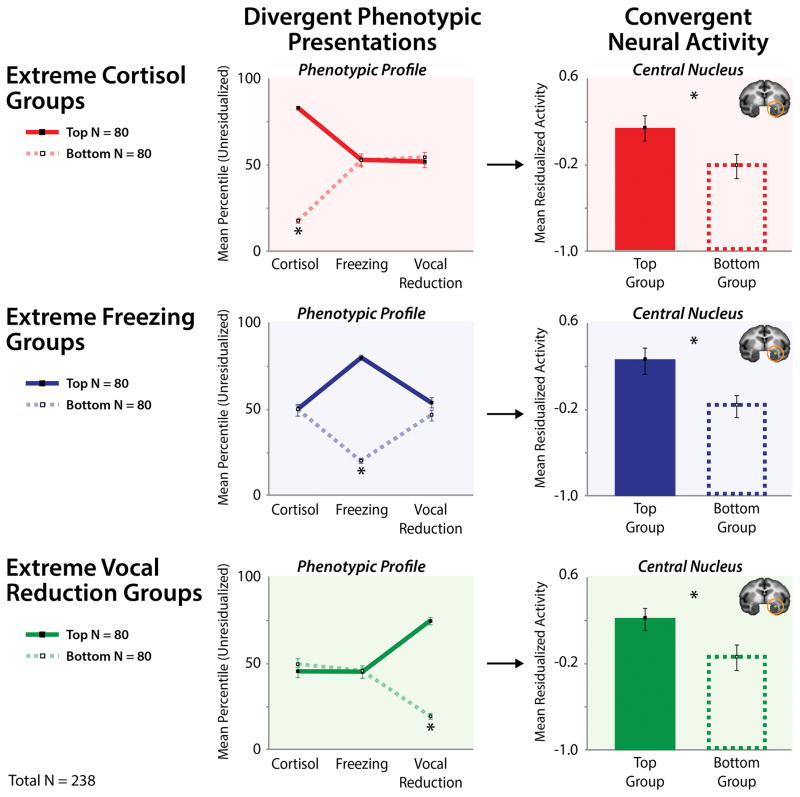
Figure 5. Elevated amygdala activity is a shared substrate for different phenotypic presentations of dispositional negativity.
Shackman and colleagues (2013) used a well-established monkey model of childhood dispositional negativity and high-resolution FDG-PET to demonstrate that individuals with different presentations of the negative phenotype show increased activity in the central (Ce) nucleus of the amygdala (orange ring). Divergent phenotypic presentations: To illustrate this, phenotypic profiles are plotted for groups (N = 80/group; Total N = 238) selected to be extreme on a particular dimension of the phenotype (Top tercile: solid lines; Bottom tercile: broken lines). The panels on the left illustrate how this procedure sorts individuals into groups with divergent presentations of dispositional negativity. Convergent neural activity: To illustrate the consistency of Ce activity across divergent presentations, mean neural activity for the extreme groups (± SEM) is shown on the right. Individuals with high levels of cortisol, freezing, or vocal reductions (and intermediate levels of the other two responses on average) evinced greater metabolic activity in the Ce compared with those with low levels (ps < .05). This figure was adapted with permission from (Shackman et al., 2013).
The amygdala’s contribution to dispositional negativity appears to be causal. In monkeys and rodents, selective lesions of the amygdala, particularly the Ce, markedly reduce the expression of fear and anxiety elicited by a broad spectrum of learned and innate threats (Calhoon & Tye, 2015; J. S. Choi & Kim, 2010; Izquierdo, Suda, & Murray, 2005; Janak & Tye, 2015; Kalin et al., in press; Kalin, Shelton, & Davidson, 2004; Mason, Capitanio, Machado, Mendoza, & Amaral, 2006; Tovote, Fadok, & Luthi, 2015). These experimental findings in animals are consistent with observations made in humans with circumscribed amygdala damage (Adolphs, in press; Feinstein, Adolphs, Damasio, & Tranel, 2011; Klumpers, Morgan, Terburg, Stein, & van Honk, in press). Patient SM, who has near-complete bilateral destruction of the amygdala, shows a profound lack of negative affect when exposed to frightening movies, haunted houses, tarantulas, snakes, and even real-world assaults (Feinstein et al., 2011)8. Importantly, she also reports abnormally low levels of dispositional negativity on standardized psychometric measures (Feinstein et al., 2011), consistent with informal clinician ratings of temperament (Tranel, Gullickson, Koch, & Adolphs, 2006).
Other work suggests that elevated amygdala reactivity contributes to the development of pathological anxiety and depression. Activity in the amygdala co-varies with changes in threat-elicited peripheral physiology (e.g., startle potentiation, skin conductance) and self-reported arousal (Cheng, Knight, Smith, & Helmstetter, 2006; Cheng, Richards, & Helmstetter, 2007; Knight, Nguyen, & Bandettini, 2005; Kragel & LaBar, 2015; LaBar, Gatenby, Gore, LeDoux, & Phelps, 1998; van Well, Visser, Scholte, & Kindt, 2012; Wood, Ver Hoef, & Knight, 2014). Amygdala reactivity is amplified by exposure to the same kinds of stressors and psychological pathogens that can precipitate acute mental illness, including combat and childhood maltreatment (Dannlowski et al., 2012; Seo, Tsou, Ansell, Potenza, & Sinha, 2014; Swartz, Williamson, & Hariri, 2015; van Wingen, Geuze, Vermetten, & Fernandez, 2011), and predicts the development of internalizing symptoms following exposure to stress or trauma (Admon et al., 2009; McLaughlin et al., 2014; Swartz, Knodt, Radtke, & Hariri, 2015). Amygdala reactivity is elevated in children and adults diagnosed with internalizing disorders (Etkin & Wager, 2007; Hamilton et al., 2012; Thomas et al., 2001) and is reduced by clinically effective treatments for anxiety and depression (Arce, Simmons, Lovero, Stein, & Paulus, 2008; Brown et al., 2015; Felmingham et al., 2007; Furmark et al., 2002; Harmer, Mackay, Reid, Cowen, & Goodwin, 2006; Paulus, Feinstein, Castillo, Simmons, & Stein, 2005; Phan et al., 2013; Sheline et al., 2001; Windischberger et al., 2010).
In sum, converging lines of epidemiological, physiological, and mechanistic evidence indicate that dispositionally negative individuals’ exaggerated reactivity to threat and other aversive challenges partially reflects larger or longer-lasting responses in the dorsal amygdala. Like dispositional negativity, individual differences in amygdala reactivity are trait-like and elevated levels of reactivity predict the future development of internalizing symptoms among individuals exposed to stress.
Trait-like individual differences in reactivity are discernible at ‘rest’
Although most neurobiological research has focused on reactivity to acute stressors and threat-related cues—aversive cues, faces, images, films and so on—individual differences in threat-reactivity can also be discerned in the brain’s spontaneous or ‘resting’ activity. For example, monkeys with elevated metabolic activity in the amygdala at ‘baseline’—in their home-cage with a familiar cage-mate—show increased freezing and elevated levels of cortisol when threat is encountered in other contexts (A. S. Fox, Shelton, Oakes, Davidson, & Kalin, 2008). Likewise, humans with higher levels of dispositional negativity show heightened activity in the amygdala at rest, as indexed by 18-fluorodeoxyglucose PET (FDG-PET) or perfusion functional magnetic resonance imaging (fMRI; Abercrombie et al., 1998; Canli et al., 2006; Kaczkurkin et al., in press).
Monkeys, children, and adults with a more negative disposition also show greater electroencephalographic (EEG) activity over the right compared to the left prefrontal cortex (PFC) at ‘rest’ (Figure 6a) (Buss et al., 2003b; Davidson, Jackson, & Kalin, 2000; N. A. Fox, Henderson, Marshall, Nichols, & Ghera, 2005; Kalin, Larson, Shelton, & Davidson, 1998; Oler et al., 2016; Shackman, McMenamin, Maxwell, Greischar, & Davidson, 2009; Wacker et al., 2010). Like dispositional negativity and amygdala reactivity, individual differences in resting prefrontal EEG asymmetry first emerge early in life and are relatively stable over time, heritable, and predict the intensity of negative affect elicited by aversive laboratory challenges (Buss et al., 2003b; Davidson et al., 2000; N. A. Fox et al., 2005; Kalin et al., 1998; Smit, Posthuma, Boomsma, & De Geus, 2007; Tomarken, Davidson, Wheeler, & Kinney, 1992; Towers & Allen, 2009; Wheeler, Davidson, & Tomarken, 1993).
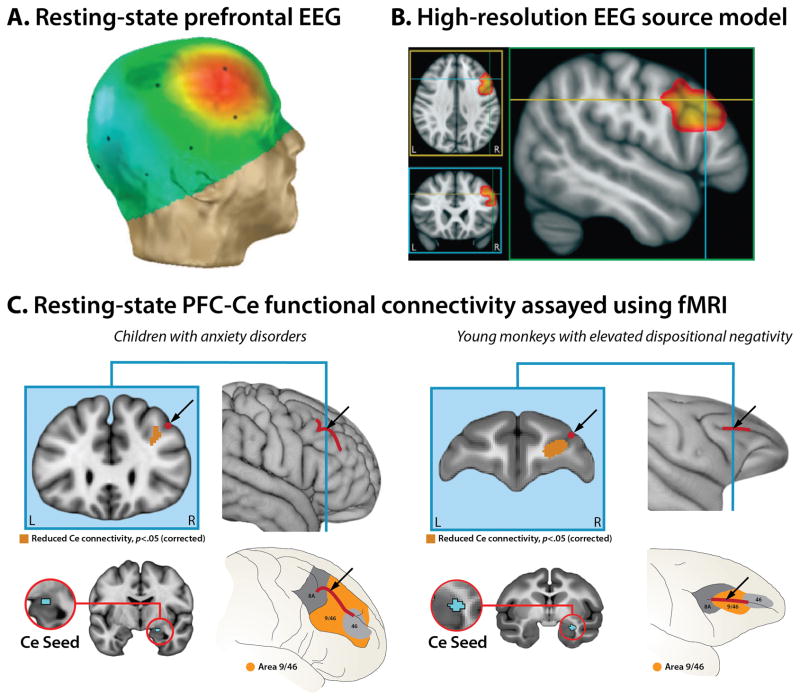
Figure 6. Individuals with a more negative disposition show altered resting-state activity and functional connectivity in the right dorsolateral prefrontal cortex (PFC).
A. Resting-state prefrontal EEG. Monkeys, children, and adults with a more negative disposition show greater resting-state activity on the scalp overlying the right compared to the left dorsolateral PFC. Figure depicts typical EEG scalp topography. B. High-resolution EEG source model. Shackman and colleagues (2009) used 128-channel EEG recordings and distributed source modeling techniques to provide evidence that this scalp-recorded asymmetry reflects increased activity in the right dorsolateral PFC (yellow-orange cluster). C. Resting-state functional connectivity between the dorsolateral PFC and the Ce assayed using fMRI. Birn and colleagues (2014) demonstrated that children with anxiety disorders (left) and young monkeys with elevated levels of dispositional negativity (right) both show reduced functional connectivity between the Ce (cyan region in the red rings) and right dorsolateral PFC (black arrows). Pediatric data were collected while patients were quietly resting. Nonhuman primate data were collected under sedation, eliminating potential individual differences in scanner-elicited apprehension. Portions of this figure were adapted with permission from (Birn et al., 2014; Nusslock et al., 2011; Shackman et al., 2009).
Like amygdala reactivity, individual differences in prefrontal EEG asymmetry also confer increased risk for the development of internalizing disorders. This asymmetric pattern of ‘resting’ activity prospectively predicts the first-onset of mood disorders (Nusslock et al., 2011), is exaggerated in patients with internalizing disorders (Thibodeau et al., 2006), and is normalized by anxiolytic drugs (Davidson, Kalin, & Shelton, 1993; Davidson, Kalin, & Shelton, 1992). Furthermore, neurofeedback interventions targeting this pattern of scalp electrical activity cause lasting reductions in stress reactivity (J. J. Allen, Harmon-Jones, & Cavender, 2001). Along with the pharmacological evidence, this suggests that the neural circuit or circuits responsible for generating this marker make a causal contribution to individual differences in dispositional negativity. Recent efforts to pinpoint the source of the scalp-recorded EEG asymmetry have highlighted the importance of the dorsolateral prefrontal cortex (dlPFC; Shackman et al., 2009) (Figure 6b), consistent with this region’s role in regulating momentary negative affect (Buhle et al., 2014).
Other work by our group suggests that the dlPFC and Ce form a coherent, evolutionarily-conserved functional circuit. Reduced functional connectivity between the two regions is associated with pathological anxiety in children and heightened dispositional negativity in monkeys (Birn et al., 2014) (Figure 6c). Selective lesions of the amygdala, including the Ce, are associated with chronically reduced metabolism in the dlPFC (Machado, Snyder, Cherry, Lavenex, & Amaral, 2008), reinforcing the idea that these regions represent an integrated functional circuit. A key challenge for future studies will be to use mechanistic techniques to clarify the functional architecture and causal contribution of this circuit to extreme anxiety.
In sum, individuals with a more negative disposition, who are prone to hyper-react to potential threat, show altered activity in the amygdala and dlPFC in the absence of explicit threat. These observations suggest that variation in the basal activity and functional connectivity of these regions represents a diathesis for heightened negative affect, given an appropriate trait-relevant challenge. More broadly, they indicate that reactive features of dispositional negativity can be discerned in the spontaneous, on-going activity of the brain, even under sedation (Birn et al., 2014).
Altered ‘resting’ activity—States or traits, reactive or tonic differences?
At present, it remains unclear whether alterations in ‘resting’ activity reflect heightened reactivity to the experimental setting, which often entails a mixture of acute and diffuse threats; ‘tonic’ differences in negative affect (Gross et al., 1998; Watson & Clark, 1984); or more likely some combination of the two processes. It is clear that most neurophysiological assays are intrusive and can elicit substantial negative affect. FDG-PET requires the injection of a radiotracer. EEG and PET studies in monkeys entail unexpected separation from cage-mates and manual restraint. In humans, resting EEG procedures have been shown to increase negative affect (Blackhart, Kline, Donohue, LaRowe, & Joiner, 2002). MRI data collection requires subjects to lie motionless in a spatially-confined, often dark tube while being bombarded by loud noise for tens of minutes. Not surprisingly, MRI procedures have been shown to elicit feelings ranging from mild apprehension to severe panic, to increase cortisol levels, and to activate the sympathetic nervous system in children and adults (Eatough, Shirtcliff, Hanson, & Pollak, 2009; Lueken, Muehlhan, Evens, Wittchen, & Kirschbaum, 2012; Melendez & McCrank, 1993; Muehlhan et al., 2013; Törnqvist, Månsson, Larsson, & Hallström, 2006; Tyc, Fairclough, Fletcher, Leigh, & Mulhern, 1995). Furthermore, MRI-induced negative affect is amplified in individuals with a more negative disposition (Harris, Cumming, & Menzies, 2004; Harris, Robinson, & Menzies, 2001), even with prior acclimation in a mock scanner (Shechner et al., 2013). Psychometric analyses (i.e., latent state-trait or generalizability-theory models) indicate that resting-state prefrontal EEG asymmetry reflects the joint contribution of traits and states, in about equal measure (Coan, Allen, & McKnight, 2006; Hagemann, Hewig, Seifert, Naumann, & Bartussek, 2005; Hagemann, Naumann, Thayer, & Bartussek, 2002; Tomarken et al., 1992). But as yet, the relative contribution of traits and states to PET and fMRI measures of brain function remains unknown, making this another important avenue for future research.
‘Tonic’ increases in self-reported negative affect may reflect heightened reactivity to uncertain or diffuse threat
Questionnaire data collected in the laboratory and in the field indicate that dispositionally negative individuals often report heightened negative affect in the absence of clear and immediate external stressors. Although this may reflect a ‘tonic’ or direct effect of temperament on mood (Watson & Clark, 1984), a wealth of psychophysiological and behavioral evidence suggests that persistent negative affect may reflect increased reactivity to stressors that are mild, uncertain, remote, or diffuse (D. M. Clark, 2001; M. Davis et al., 2010; Grupe & Nitschke, 2013).
For example, children, adolescents, and adults with a more negative disposition show exaggerated psychophysiological responses (e.g., startle, skin conductance) and report heightened negative affect during periods of explicit safety (i.e., CS−, inter-cue interval, CS+ paired with a safety cue) embedded within instructed and associative fear learning paradigms—that is, during the interstitial periods before and after the randomized presentation of genuine threat (CS+) (Baas, van Ooijen, Goudriaan, & Kenemans, 2008; Barker, Reeb-Sutherland, & Fox, 2014, in press; Chan & Lovibond, 1996; Craske et al., 2009; Gazendam et al., 2015; Gazendam, Kamphuis, & Kindt, 2013; Grillon, 2002; Grillon & Ameli, 2001; Haaker et al., 2015; Jovanovic et al., 2014; Reeb-Sutherland et al., 2009; Schmidt & Fox, 1998). Conceptually similar effects have been found in monkeys (Shackman, Fox, et al., in press; Shiba et al., 2014) and in patients with anxiety disorders (Duits et al., 2015). In fact, a comprehensive recent meta-analysis incorporating data from more than 2,000 individuals showed that patients consistently respond more strongly than controls to safety cues (CS−: Cohen’s d = .30, p < .001), whereas the two groups do not consistently differ in their response to acute threat (CS+: Cohen’s d = .07, p = .41) (Duits et al., 2015).
Research using more naturalistic challenges in children also highlights the importance of contextually inappropriate negative affect. For example, dispositionally negative children show elevated psychophysiological defensive responses to neutral faces (Waters, Neumann, Henry, Craske, & Ornitz, 2008). Relative to typical children, two-year-olds with an extremely negative disposition show only modestly elevated negative affect in response to high-threat challenges (e.g., approaching robotic spider: Typical Children: ~32% time; Negative Children: ~45% time), whereas group differences are dramatically larger during interspersed low-threat challenges (e.g., puppet show: Typical Children: ~28%; Negative Children: ~65% time; Buss, 2011). Moreover, contextually inappropriate negative affect in the laboratory at age 2 predicts elevated parent- and teacher-reports of anxiety in preschool and kindergarten and heightened wariness around unfamiliar peers at age 5 (Buss, 2011; Buss et al., 2013).
Among adults, indiscriminate or contextually inappropriate negative affect has also been associated with increased avoidance of the aversive learning context. Grillon (2002), for example, showed that subjects who were unable to correctly report the shock-cue contingency following the training phase of a simple differential fear conditioning paradigm (CS+ cue paired with noxious electric shock compared to an unpaired CS− cue) were nearly four times more likely to fail to return for a follow-up session (n = 125, risk ratio = 3.80, p < .005). Furthermore, well-established anxiolytic compounds, like ethyl alcohol and benzodiazepines, have been shown to dampen persistent negative affect elicited by uncertain threat in a dose-dependent manner, while sparing phasic reactions to cues associated with clear and imminent danger (Bradford, Shapiro, & Curtin, 2013; Glue et al., 1995; Grillon et al., 2006; Hefner & Curtin, 2012; Hefner, Jaber, Grant, & Curtin, 2009; Hefner, Moberg, Hachiya, & Curtin, 2013; Moberg & Curtin, 2009).
This body of psychophysiological and behavioral research motivates the hypothesis that seemingly endogenous increases in negative affect, as described in the self-report literature, may reflect heightened sensitivity to weak, distal, or uncertain stressors, rather than a fixed or ‘tonic’ consequence of temperament. At a more granular level, this may reflect difficulties discriminating threat from safety, overgeneralization of threat to perceptually similar safety cues (i.e., a broader tuning of threat-detection mechanisms and higher tolerance of false alarms; cf. Nettle & Bateson, 2012; Pollak & Kistler, 2002), or problems using or learning to use safety-related information to regulate momentary negative affect, leading to mood spillover or affective inertia (Davidson, Fox, & Kalin, 2007; Davidson et al., 2000; Grupe & Nitschke, 2013; Kheirbek, Klemenhagen, Sahay, & Hen, 2012; Lissek, 2012).
Mechanistic work in rodents (Calhoon & Tye, 2015; M. Davis et al., 2010; Gungor & Paré, in press; Tovote et al., 2015) suggests that defensive responses to diffuse, uncertain threats (Figure 2a) are organized by the central extended amygdala, an anatomical concept encompassing the Ce and the lateral division of BST (magenta regions in Figure 3) (Alheid & Heimer, 1988; A. S. Fox, Oler, Tromp, et al., 2015; Shackman & Fox, in press; Yilmazer-Hanke, 2012). Like humans with high levels of dispositional negativity, some rodents show poor discrimination of cues associated with danger and safety (i.e., elevated fear and anxiety in response to CS−; often termed ‘over-generalization’; Lissek, 2012) and heightened defensive responses during sustained exposure to diffusely threatening contexts (e.g., the cage paired with cued fear learning or the elevated plus-maze) (Duvarci, Bauer, & Paré, 2009). Selective lesions of the BST reduce these maladaptive emotional responses, while sparing more adaptive, phasic responses to cues signaling imminent danger (CS+) (Duvarci et al., 2009). Anatomically, both the Ce and BST are poised to orchestrate key features of sustained negative affect—including alterations in arousal, behavioral inhibition, and neuroendocrine activation—via dense mono- and poly-synaptic (e.g., via the medial division of the Ce) projections to brainstem and subcortical effector regions (M. Davis et al., 2010; M. Davis & Whalen, 2001; A. S. Fox, Oler, Tromp, et al., 2015; Freese & Amaral, 2009).
Recent imaging work in humans and monkeys demonstrates that individuals with a more negative disposition show heightened activity in the BST during prolonged periods of diffuse or uncertain threat (Figures 2c and 7a, b) (A. S. Fox et al., 2008; Shackman, Fox, et al., in press; Somerville, Whalen, & Kelley, 2010), with parallel effects reported for patients with anxiety disorders (Munsterkotter et al., in press; Straube, Mentzel, & Miltner, 2007; Yassa, Hazlett, Stark, & Hoehn-Saric, 2012). Work in nonhuman primates shows that threat-related metabolic activity in the BST is heritable and genetically correlated with individual differences in dispositional negativity, suggesting that it contributes to the inter-generational transmission of this dispositional phenotype (A. S. Fox, Oler, Shackman, et al., 2015) (Figure 7b). In humans and monkeys, BST activity and functional connectivity co-vary with self-reported negative affect, freezing, skin conductance, cardiovascular activity, and cortisol elicited by uncertain or diffuse threat (Alvarez et al., 2015; Banihashemi, Sheu, Midei, & Gianaros, 2015; Jahn et al., 2010; Kalin, Shelton, Fox, Oakes, & Davidson, 2005; McMenamin, Langeslag, Sirbu, Padmala, & Pessoa, 2014; Somerville et al., 2013), consistent with a causal contribution to momentary negative affect. At present, the consequences of selective BST lesions have yet to be unexplored in humans or nonhuman primates.
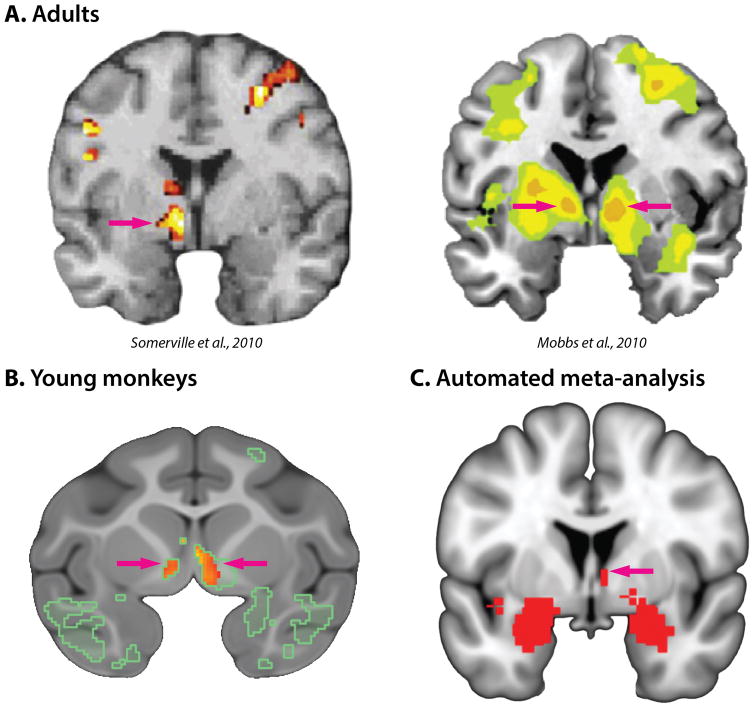
Figure 7. Individuals with a more negative disposition show heightened activity in the bed nucleus of the stria terminalis (BST) during periods of diffuse or uncertain threat.
Clusters in the vicinity of the BST are indicated by magenta arrows. A. Adults. Recent human fMRI studies reveal increased activation in the BST in response to uncertain threat (Mobbs et al., 2010; Somerville et al., 2010). B. Young monkeys. Using high-resolution FDG-PET acquired from 592 young rhesus macaques, Fox and colleagues demonstrated that activity in the right BST is heritable and mediates heritable individual differences in dispositional negativity (i.e., BST activity and dispositional negativity are ‘genetically correlated;’ A. S. Fox, Oler, Shackman, et al., 2015). Regions where activity predicted dispositional negativity are outlined in green. C. Automated meta-analysis. An automated Neurosynth (Yarkoni, Poldrack, Nichols, Van Essen, & Wager, 2011) meta-analysis of 312 brain imaging studies featuring the term ‘anxiety’ revealed several significant regions (red; Z > 6.0 and FDR q < .05, whole-brain corrected), including the BST. Portions of this figure were adapted with permission from (A. S. Fox, Oler, Shackman, et al., 2015; Mobbs et al., 2010; Somerville et al., 2010).
BST activity is often described as a ‘sustained’ response to uncertain threat. Yet, mechanistic work in rodents suggests that BST engagement could begin quite early, between 4 and 60 s following the onset of threat-related cues (M. Davis et al., 2010). Consistent with this, a number of human imaging studies have found transient activation in the BST in response to punctate threats (Figure 7a), such as a 4-sec video clip of an approaching tarantula (J. M. Choi, Padmala, & Pessoa, 2012; Grupe, Oathes, & Nitschke, 2013; Klumpers et al., 2015; Mobbs et al., 2010). Likewise, a recent large-scale imaging study (n = 168) reported phasic activation of the BST in response to 4-s shock-predictive cues (Klumpers et al., 2015). In fact, imaging studies of fear and anxiety consistently reveal activation in the central extended amygdala—including the BST and the dorsal amygdala in the region of the Ce—across a range of populations, paradigms, and time-scales (Figure 7c). This and other recent work in humans and rodents highlights the importance of these two closely related regions across a broad spectrum of aversive challenges (Shackman & Fox, in press)9.
Individual differences in the function of the central extended amygdala may reflect altered communication with the orbitofrontal cortex (OFC) (cf. “Regulatory/Evaluative” inputs in Figure 3). Large-scale imaging studies in monkeys (n = 592) reveal that threat-related metabolic activity in the posterior OFC/anterior insula is heritable and, like the BST, genetically correlated with individual differences in dispositional negativity (A. S. Fox, Oler, Shackman, et al., 2015). Aspiration lesions of the OFC markedly reduce passive avoidance of potential threat (i.e., freezing) (Kalin, Shelton, & Davidson, 2007; Rudebeck, Saunders, Prescott, Chau, & Murray, 2013) and this appears to be mediated by downstream changes in BST metabolism (A. S. Fox et al., 2010). Reduced BST activity has also been found in humans with OFC damage (Motzkin, Philippi, Oler, et al., 2015), suggesting that this circuit is conserved across primate species. Interestingly, viral vector manipulations that increase metabolic activity in the Ce—the other major component of the central extended amygdala—are associated with elevated metabolic activity in the OFC, increased functional connectivity between the Ce and OFC, and heightened signs of fear and anxiety during prolonged exposure to threat (Kalin et al., in press). Conversely, Ce lesions are associated with reduced metabolic activity in the OFC (Machado et al., 2008). In other words, perturbations targeting one region (e.g., OFC or Ce damage) propagate to the others (e.g., reduced BST or OFC metabolism) and damage to either the Ce or OFC reduces, but does not abolish, defensive responses to threat.
Collectively, these imaging and mechanistic findings suggest that the extended amygdala and OFC form a functionally integrated circuit that plays a crucial role in detecting and organizing persistent responses to uncertain threat10. Much remains unknown about this circuit, including the necessity of the primate BST to persistent negative affect, the differential contributions of its three constituents (Ce, BST, OFC), the nature of their interactions with one another and other brain regions associated with dispositional negativity (e.g., dlPFC, PAG), and the relevance of this circuit to persistent, contextually inappropriate negative affect in the real world.
‘Tonic’ increases in self-reported negative affect may reflect stress-induced sensitization
Self-report data indicate that individuals with a more negative disposition tend to carry negative affect from stressful to less stressful contexts (e.g., work to home) and to behave in ways that promote interpersonal conflict during times of heightened stress (Suls & Martin, 2005; Wang et al., 2011)4. Recent work in humans suggests that the amygdala could contribute to the spill-over of negative mood via stress-induced sensitization, consistent with models derived from animal research (Rosen & Schulkin, 1998). In particular, there is evidence that acute stressors (e.g., threat-of-shock, aversive film clips) potentiate defensive reactions (i.e., startle) to threat-related facial expressions (Grillon & Charney, 2011), cause persistent increases in spontaneous amygdala activity (Cousijn et al., 2010), and potentiate amygdala reactivity to threat-related faces (Pichon, Miendlarzewska, Eryilmaz, & Vuilleumier, 2015; van Marle, Hermans, Qin, & Fernandez, 2009). Acute stressors produce even longer-lasting changes (i.e., minutes to hours) in amygdala functional connectivity (Vaisvaser et al., 2013; van Marle, Hermans, Qin, & Fernandez, 2010). Furthermore, these neurobiological spill-over effects are exaggerated in individuals with a more negative disposition. In particular, a recent large-scale imaging study (n = 120) showed that dispositionally negative individuals exhibit potentiated activation to threat-related faces following acute stressor exposure (Everaerd et al., 2015).
Persistent amygdala sensitization could promote negative affect either directly, by potentiating emotional reactions to mild threat (Grillon & Charney, 2011), or indirectly, by increasing the likelihood that attention will be allocated to threat-related cues in the surrounding environment. Circuits centered on the amygdala play a central role in allocating attentional resources to threat and a rapidly growing body of longitudinal (Perez-Edgar, Bar-Haim, et al., 2010; Perez-Edgar et al., 2011; White et al., in press) and clinical-intervention studies (Linetzky et al., 2015; MacLeod & Clarke, 2015) indicate that attentional biases to threat causally contribute to the development and maintenance of extreme anxiety and distress (Shackman, Kaplan, et al., in press). For example, computer-based interventions targeting attentional biases to threat have been shown to reduce self-reported negative affect, behavioral signs of anxiety, and intrusive thoughts elicited by aversive challenges, such as public speaking tasks (Dennis & O’Toole, 2014; MacLeod & Mathews, 2012). Likewise, psychological manipulations that potentiate amygdala reactivity (e.g., exposure to loud, unpredictable trains of auditory stimuli) also enhance attentional biases to threat (Herry et al., 2007).
Collectively, these observations indicate that exposure to stress alters the activity and connectivity of the amygdala in ways that could promote contextually inappropriate negative affect in individuals with a negative disposition. A key challenge for the future will be to understand whether this reflects sensitization of circuits involved in orchestrating states of negative affect or a downstream consequence of changes in circuits involved in detecting and attending to threat.
Increased stressor exposure and generation may reflect maladaptive information processing and choices
Self-report data indicate that individuals with a more negative disposition tend to behave in ways that increase the likelihood of hassles, social conflict, inter-personal rejection, and other stressors (e.g., unemployment, divorce)4. At present, the neurobiological mechanisms underlying these kinds of recursive Temperament—Environment—Affect relations have received little attention and remain poorly understood. Nonetheless, it is known that the amygdala and other brain regions involved in dispositional negativity can influence information processing and bias decision-making in ways that could promote stress. Imaging studies provide evidence that amygdala activation is associated with reduced trust and is sensitive to potential threat and betrayal during simple economic bargaining games (Baumgartner, Heinrichs, Vonlanthen, Fischbacher, & Fehr, 2008; Bhatt, Lohrenz, Camerer, & Montague, 2012; Gospic et al., 2011; Li, Xiao, Houser, & Montague, 2009). Conversely, amygdala damage is associated with heightened, even pathological levels of approach and trust (Adolphs, Tranel, & Damasio, 1998; Feinstein et al., 2011; Kennedy, Glascher, Tyszka, & Adolphs, 2009; van Honk, Eisenegger, Terburg, Stein, & Morgan, 2013). Patient studies suggest that the amygdala also plays a role in inhibiting approach in the face of potentially aversive outcomes (i.e., monetary loss aversion; De Martino, Camerer, & Adolphs, 2010). Imaging studies have linked the amygdala to reactive aggression and punishment (Coccaro, McCloskey, Fitzgerald, & Phan, 2007; Treadway et al., 2014), consistent with limited mechanistic work in rodents (Nelson & Trainor, 2007). These observations raise the possibility that stable individual differences in amygdala function contribute to dispositionally negative individuals’ tendency to engage in behaviors—including reduced self-disclosure, heightened inhibition and avoidance, escalation during conflict—that promote social discord and rejection. Via its role in pessimism (Sharot, Riccardi, Raio, & Phelps, 2007) and loss aversion, it is plausible that the amygdala contributes, albeit in a more distal way, to the development or maintenance of other stressors (e.g., lost opportunities for financial or social success, difficulties escaping or improving adverse situations and relationships, avoidance of medical professionals and other potential sources of ‘bad news’). While speculative, the development of more ecologically valid tasks (Jarcho et al., 2013; Somerville, Heatherton, & Kelley, 2006), in combination with longitudinal imaging studies, makes these hypotheses increasingly tractable.
An Integrative Perspective on the Processes Linking Dispositional Negativity to Momentary Emotional Experience and Behavior
The past two decades have witnessed the emergence of powerful tools for assaying emotion and brain function and a number of important insights into the pathways linking stable individual differences in temperament to momentary emotional experience, behavior, and peripheral physiology. These observations provide an integrative framework for understanding the role of dispositional negativity in the development of a broad spectrum of adverse outcomes.
First, there is clear evidence that dispositionally negative individuals respond more strongly to explicit stressors and aversive challenges in the laboratory and in their daily lives. Variation in threat-reactivity reflects stable individual differences in the sensitivity and functional connectivity of several brain regions, including the dorsal amygdala and dlPFC, that have been implicated in the etiology and maintenance of internalizing disorders and substance abuse (Figures 3–6) (Chase et al., 2011; Etkin & Wager, 2007; Hamilton et al., 2012; Kuhn & Gallinat, 2011; McLaughlin et al., 2014; Swartz, Knodt, et al., 2015; Tang et al., 2012; Thibodeau et al., 2006; R. A. Wise & Koob, 2014). These differences manifest as heightened activation in response to punctate challenges, but they are also evident in on-going brain activity, in the absence of explicit tasks or mood manipulations (Figure 6). At present, it remains unclear whether temperament-related variation in resting-state activity and functional connectivity reflects increased reactivity to the measurement context, which is often novel or mildly aversive; tonic differences in neurophysiology; or more likely, some combination of these two pathways.
Second, although increased threat-reactivity is important, individuals with a more negative disposition often report elevated levels of negative affect in contexts where stressors are uncertain, psychologically diffuse, or temporally remote. Mechanistic work in rodents and imaging research in humans and monkeys suggests that persistent, contextually indiscriminate negative affect reflects alterations in a neural circuit encompassing the extended amygdala and OFC (Figures 3 and 7). Other work indicates that persistent changes in amygdala reactivity and functional connectivity following exposure to acute stress could contribute to the spill-over of negative mood across contexts.
Third, individuals with a more negative disposition tend to behave in ways that evoke or intensify interpersonal conflict and social rejection, increasing the likelihood that they will experience momentary negative affect. Although the neurobiological mechanisms underlying stressor generation have received scant attention, the existing evidence highlights the potential importance of an amygdala-centered circuit responsible for evaluating the trustworthiness and approachability of other individuals.
Of these three pathways, the tendency to experience sustained levels of heightened negative affect in response to diffuse, uncertain, or remote threat appears to be most central to daily function (Bolger & Schilling, 1991). The vast majority of negative affect experienced by dispositionally negative individuals in their daily lives appears to be indiscriminate and cannot be attributed to clear and immediate stressors. In the laboratory, heightened negative affect in explicitly or implicitly safe contexts is generally more discriminative of dispositional negativity and pathological anxiety than that elicited by overt threat (M. Davis et al., 2010; Duits et al., 2015; Watson & Clark, 1984). This tendency is amplified by exposure to early adversity (e.g., abuse, neglect, loss of caregiver; Wolitzky-Taylor et al., 2014) and it predicts the first onset of anxiety disorders in adolescents (Craske et al., 2012) and the intensification of internalizing symptoms in children and adults (Barker, Reeb-Sutherland, Degnan, et al., in press; Lenaert et al., 2014).
This pervasive, context-insensitive emotional bias likely reinforces other components of the negative phenotype, such as elevated avoidance and hyper-vigilance, and may serve to promote the expression of deleterious behaviors that elicit social rejection (Barlow et al., 2013; Carter et al., 2006; Cramer et al., 2012a, 2012b; Grupe & Nitschke, 2013; Jeronimus et al., 2014; Nettle & Bateson, 2012). This reciprocal cycle of maladaptive social transactions may help to explain why dispositionally negative individuals experience lower levels of occupational, financial, and marital success and are more likely to develop internalizing disorders.
A Roadmap to Future Challenges
The data that we have reviewed provide important new insights into the psychological and neurobiological mechanisms that support individual differences in dispositional negativity and the processes that link this disposition to more transient states of negative affect. Yet, it is clear that our understanding remains far from complete. Throughout the review, we highlighted a number of specific conceptual challenges for future research in this area. Here, we outline some broader questions for the field and offer some methodological strategies for beginning to address them11.
How does the brain represent and respond to different kinds of threat?
A major challenge for future research is to understand how the central extended amygdala, OFC, and other key brain regions represent and respond to different kinds of threat (Figure 2). Threats differ along several key dimensions—including their certainty, imminence (i.e., physical distance or temporal latency), diffuseness (i.e., cues versus contexts), and duration (D.C. Blanchard, Griebel, & Blanchard, 2001; D. C. Blanchard & Pearson, in press; R. J. Blanchard, Blanchard, & Hori, 1989; M. Davis et al., 2010; Fanselow, 1989, 1994; Fanselow & Lester, 1988). There is compelling evidence that these dimensions are psychiatrically important (Craske et al., 2012; M. Davis et al., 2010; Duits et al., 2015; Shackman, Stockbridge, LeMay, & Fox, in press) and pharmacologically dissociable (D. C. Blanchard, Griebel, & Blanchard, 2003; Bradford et al., 2013; M. Davis et al., 2010; Kalin & Shelton, 1989). Yet, we know remarkably little about how the human brain represents and differentially responds to them. Although some important advances have been made (Mobbs et al., 2010; Somerville et al., 2013), progress has been slowed by the use of paradigms and assays that confound these dimensions (e.g., if vs. when threat will occur; brief-cues vs. prolonged-contexts).
Drawing strong inferences about the neural systems supporting phasic and sustained responses to different dimensions of threat requires the use of well-matched tasks, both in humans (Luck, 2005; Shackman et al., 2006) and in animals (Hammack, Todd, Kocho-Schellenberg, & Bouton, 2015). Conditions must be equated for motor requirements and perceptual characteristics, including paired reinforcers (e.g., shocks, aversive images). Investigators should be cautious when comparing neural activity across conditions that markedly differ in duration or number of trials (i.e., in the precision of the estimated activation), as in paradigms where long blocks of uncertain threat are compared to more punctate aversive challenges. Parametric manipulations of threat probability (if threat will occur), imminence (when or where it will occur), and duration (as in Bradford et al., 2013; Mobbs et al., 2010) would be particularly useful. The use of dynamic parametric tasks (e.g., where threat imminence or probability is smoothly and continuously varied) would also afford powerful new opportunities for understanding the kinds of uncertainty most relevant to fear and anxiety (Bach & Dolan, 2012; de Berker et al., 2016) and for identifying circuits involved in triggering behavioral and physiological ‘phase transitions’ (e.g., from vigilance to behavioral inhibition to active defense; Mobbs et al., 2015; Mobbs & Kim, 2015). Putative double dissociations (e.g., regions sensitive to certain versus uncertain threat) need to be rigorously assessed by testing the appropriate Region × Condition interaction (as in Somerville et al., 2010). Absent that, strong claims of anatomical dissociation are unwarranted. Likewise, concluding that a particular brain region is ‘not involved’ in a complex, multidimensional psychological function, like ‘fear,’ based on a null statistical test or a single assay is unwarranted.
Emotional disorders are defined and diagnosed on the basis of symptoms (i.e., feelings) and human imaging studies will be particularly valuable for understanding the neural mechanisms underlying the subjective experience of different kinds of threat-elicited negative affect (e.g., Chang, Gianaros, Manuck, Krishnan, & Wager, 2015; Satpute et al., 2013; Somerville et al., 2013), something that cannot be assessed in animal models (Anderson & Adolphs, 2014; LeDoux, 2015). To this end, it is will be critical for human studies to verify the presence of target emotions separately for each task or condition (Shackman et al., 2006) and examine relations with on-going neural activity. For correlational techniques, such as fMRI and EEG, trial-by-trial relations between neural signals and emotional experience provide the strongest and most direct link between the brain and self-reported emotion (Atlas, Bolger, Lindquist, & Wager, 2010; Lim, Padmala, & Pessoa, 2009). Multi-voxel classifier approaches, in which machine learning techniques are used to identify patterns of activation predictive of subjective emotional states, are also likely to be fruitful (Chang et al., 2015; Krishnan et al., 2016; Wager et al., 2013; Woo et al., 2014).
Which brain circuits underlie individual differences in dispositional negativity?
It is widely believed that dispositional negativity, like other psychologically and psychiatrically relevant processes, reflects the coordinated activity of distributed brain circuits (Shackman, Fox, & Seminowicz, 2015). Here, we reviewed recent work highlighting the importance of five functional circuits: (a) Projections from the Ce and BST to the subcortical and brainstem regions that proximally mediate key somatomotor and neuroendocrine features of momentary negative affect (Figure 3), (b) projections from the Ce to neurotransmitter systems in the basal forebrain and brainstem that modulate sensory processing and promote vigilance (Figure 3), (c) projections from the BL to the visual cortex and superior colliculus that play a role in re-directing or amplifying the amount of attention allocated to threat-relevant cues, (d) a poly-synaptic circuit encompassing the Ce and dlPFC that is associated with differences in dispositional negativity in monkeys and anxiety disorders in children (Figure 6c), and (e) a circuit encompassing the central extended amygdala and OFC that co-varies with dispositional negativity and supports heightened reactivity to a broad spectrum of aversive challenges (Figures 4, 5, and 7), including contexts associated with diffuse or temporally remote threat. Naturally, other circuits are likely to prove important. More broadly, our understanding of the neural circuits underlying dispositional negativity and momentary negative affect remains in its infancy (Calhoon & Tye, 2015; Janak & Tye, 2015; Okon-Singer, Hendler, Pessoa, & Shackman, 2015; Shackman et al., 2015; Tovote et al., 2015). Overcoming this important barrier requires that we accelerate the transition from localization strategies to network-based approaches (Anticevic et al., 2013; Fornito, Zalesky, & Breakspear, 2015; McMenamin et al., 2014; Petersen & Sporns, 2015; Servaas et al., 2014; Turk-Browne, 2013).
It will also be important to determine the specificity of these circuits to different components of dispositional negativity (e.g., anxious distress vs. irritable distress)2 and assess whether they are equally relevant to all disorders on the internalizing spectrum (e.g., generalized anxiety disorder vs. major depression). The existing evidentiary record suggests that many of the brain regions that we have highlighted likely contribute to multiple components and disorders, a one-to-many mapping (Shackman et al., 2015). The amygdala, for example, has been linked to anxiety disorders (Etkin & Wager, 2007), depression (Hamilton et al., 2012), substance abuse (Chase et al., 2011; Kuhn & Gallinat, 2011; Tang et al., 2012), psychopathy (Koenigs, Baskin-Sommers, Zeier, & Newman, 2011), and autism (Nacewicz et al., 2006).
What mechanisms underlie individual differences in dispositional negativity?
Much of the data that we have reviewed comes from brain imaging studies. Aside from unresolved questions about the origins and significance of the measured signals (Logothetis, 2008), the most important limitation of these techniques is that they cannot address causation. A crucial challenge for future studies is to develop a mechanistic understanding of the neural circuits that underlie dispositional negativity and control the expression of negative affect. Addressing these fundamental questions mandates coordinated research efforts in humans and nonhuman animal models. This could be achieved by combining mechanistic approaches (e.g., viral vector, chemogenetic, or optogenetic techniques) in animals with the same whole-brain imaging strategies routinely used in humans, enabling the development of bidirectional translational models (Borsook, Becerra, & Hargreaves, 2006; Casey et al., 2013; Desai et al., 2011; Ferenczi et al., 2016; A. S. Fox et al., 2010). The enhanced precision afforded by optogentic and chemogenetic techniques would also open the door to identifying the specific molecules, cells, and sub-regions of the central extended amygdala that mediate effects detected in imaging studies (cf. Ferenczi et al., 2016). Nonhuman primate models are likely to be particularly useful for modeling and understanding the neurobiology of dispositional negativity because monkeys and humans share similar genes and brains (Freese & Amaral, 2009; Gibbs et al., 2007; Preuss, 2007), which endow the two species with a common repertoire of complex socio-emotional responses to potential threat and enable the use of similar behavioral assays (A. S. Fox & Kalin, 2014; Kaiser & Feng, 2015; Oler et al., 2016).
In humans, imaging approaches can be applied to patients with circumscribed brain damage (Adolphs, 2016; Motzkin, Philippi, Oler, et al., 2015; Motzkin, Philippi, Wolf, Baskaya, & Koenigs, 2014, 2015; Spunt et al., 2015). Alternatively, fMRI or EEG can be combined with noninvasive perturbation techniques (Bestmann & Feredoes, 2013; Reinhart & Woodman, 2014), neurofeedback (deBettencourt, Cohen, Lee, Norman, & Turk-Browne, 2015; Greer, Trujillo, Glover, & Knutson, 2014; Stoeckel et al., 2014), pharmacological manipulations (Duff et al., 2015; Paulus et al., 2005), or cognitive-behavioral interventions (Britton et al., 2015; Schnyer et al., 2015). Prospective longitudinal imaging studies represent another fruitful approach to identifying candidate mechanisms, especially in relation to the development of internalizing disorders and other kinds of adverse outcomes (Admon, Milad, & Hendler, 2013; Burghy et al., 2012; Herringa et al., 2013; McLaughlin et al., 2014; Swartz, Williamson, et al., 2015). From a broader perspective, human studies will be crucial for identifying the features of animal models that are conserved across species and, hence, most relevant to developing improved interventions for human suffering (Birn et al., 2014). They also afford an opportunity to develop objective biomarkers of psychiatric disease or disease-risk (Borsook et al., 2006; Borsook, Hargreaves, Bountra, & Porreca, 2014; J. Davis et al., 2015; R. G. Wise & Preston, 2010) and for generating novel hypotheses that can be mechanistically assessed in animal models (‘reverse translation;’ Ferenczi et al., 2016; Janak & Tye, 2015).
What is the relevance of individual differences in brain function for negative affect in daily life?
Biological studies of dispositional negativity and negative affect in humans rely on a limited number of well-controlled, but highly artificial manipulations—fearful and angry faces, aversive sounds and images, electric shocks, and so on—collected under unnatural conditions. Most of these manipulations are only mildly threatening, when compared to the kinds of stressors regularly encountered in daily life or those routinely used in animal models (LeDoux, 2015; Levenson, in press; Shackman et al., 2006), and are subject to self-selection biases, precluding the study of individuals with more extreme dispositions (Scherer, 1986). Consequently, the real-world significance of neural systems identified in the laboratory, including the five circuits that we have highlighted, remains unclear.
Given the limitations of ambulatory measures of brain activity—there is no ‘fMRI helmet’ as yet—addressing these fundamental questions requires integrating assays of brain function and behavior acquired in the scanner with measures of negative affect and motivated behavior assessed under naturalistic conditions in the laboratory (e.g., during semi-structured interactions; Creed & Funder, 1998; Laidlaw, Foulsham, Kuhn, & Kingstone, 2011; Perez-Edgar, McDermott, et al., 2010; Pfeiffer, Vogeley, & Schilbach, 2013) or in the field. Recent work combining fMRI with intensive experience-sampling techniques underscores the value of this approach for identifying the neural systems associated with naturalistic variation in mood and behavior, a central goal of psychology, psychiatry, and the behavioral neurosciences (Berkman & Falk, 2013; Forbes et al., 2009; Heller et al., in press; Lopez, Hofmann, Wagner, Kelley, & Heatherton, 2014; S. J. Wilson, Smyth, & MacLean, 2014). To our knowledge, this approach has yet to be applied to the study of dispositional negativity, although on-going work by our group has begun to do so.
The development of robust mobile eye trackers, the emergence of commercial software for automated analyses of facial expressions (Olderbak, Hildebrandt, Pinkpank, Sommer, & Wilhelm, 2014), and the widespread dissemination of smart-phone and other kinds of ‘wearable’ technology afford additional opportunities for objectively, efficiently, and unobtrusively quantifying social attention and context, negative affect, and motivated behavior in situ (Gosling & Mason, 2015; Onnela & Rauch, 2016; Sano et al., 2015; Wrzus & Mehl, 2015). Combining these measures with laboratory assays of brain function would open the door to discovering the neural systems underlying persistent, contextually inappropriate negative affect and pathology-promoting behaviors (e.g., social withdrawal, avoidance, and hyper-vigilance) in the real world, close to clinically relevant end-points. This approach promises a depth of understanding that cannot be achieved using either animal models or isolated measures of brain function. Even in the absence of biological measures, these new tools promise important clues about the dynamics of negative affect in daily life (e.g., spill-over of mood across sequential contexts and assessments) and the social factors and coping behaviors that help govern the momentary expression of dispositional negativity.
Conclusions
Dispositional negativity has enormous consequences for global health, wealth, and happiness. Self-report data suggest that three key pathways—increased stressor reactivity, tonic increases in negative affect, and increased stressor exposure—explain most of the heightened negative affect characteristic of individuals with a more negative disposition. Of these three pathways, indiscriminate negative affect appears to be the most central to daily life and most relevant to the development and maintenance of anxiety disorders and depression. Mechanistic and imaging studies in humans, monkeys, and rodents suggest that sustained, indiscriminate negative affect reflects variation in the activity and connectivity of several key brain regions, including the central extended amygdala and OFC. These observations provide an integrative framework for understanding the cascading network of psychological and biological processes that bind emotional traits to emotional states and emotional disorders and, ultimately, for guiding the development of more effective prevention and treatment strategies.
Acknowledgments
Authors acknowledge assistance from L. Friedman, L. Pessoa, and J. Smith and constructive feedback from M. Barstead, J. Curtin, M. Gamer, D. Grupe, E. Lemay, K. Rubin, J. Stern, three anonymous reviewers, and the Editor. This work was supported by the University of California, University of Maryland, National Institute of Drug Abuse (DA040717), National Institute of Mental Health (MH107444), and a National Science Foundation Graduate Research Fellowship.
Footnotes
‘Emotion’ is a fuzzy, contentious category that conventionally includes valenced processes (e.g., action tendencies, attention, motivated learning, overt behavior, subjective feelings, and alterations in peripheral physiology) that are triggered by specific external or internal antecedents, such as actual or remembered threat in the case of fear (Anderson & Adolphs, 2014; Beck, 2015; Gendron & Barrett, 2009; Izard, 2010; Lapate & Shackman, in press).
Our review incorporates evidence derived from 41 more narrowly focused meta-analyses and systematic reviews (Avery, Clauss, & Blackford, 2016; Bastiaansen et al., 2014; Buhle et al., 2014; Calder, Ewbank, & Passamonti, 2011; Cavanagh & Shackman, 2015; Chase, Eickhoff, Laird, & Hogarth, 2011; Clauss & Blackford, 2012; Connor-Smith & Flachsbart, 2007; Duits et al., 2015; Etkin & Wager, 2007; A. S. Fox & Kalin, 2014; A. S. Fox, Oler, Tromp, Fudge, & Kalin, 2015; Hakulinen, Elovainio, et al., 2015; Hakulinen, Hintsanen, et al., 2015; Hamilton et al., 2012; Houben, Van Den Noortgate, & Kuppens, 2015; Jokela, Pulkki-Raback, Elovainio, & Kivimaki, 2014; Karney & Bradbury, 1995; Kotov, Gamez, Schmidt, & Watson, 2010; Kuhn & Gallinat, 2011; Lahey, 2009; Linetzky, Pergamin-Hight, Pine, & Bar-Haim, 2015; Malouff, Thorsteinsson, Rooke, & Schutte, 2007; Malouff, Thorsteinsson, Schutte, Bhullar, & Rooke, 2010; Matthews, Deary, & Whiteman, 2009; Ng, Eby, Sorensen, & Feldman, 2005; Ormel et al., 2013; Polderman et al., in press; Prinzie, Stams, Dekovic, Reijntjes, & Belsky, 2009; Roberts & DelVecchio, 2000; Roberts, Walton, & Viechtbauer, 2006; Shackman et al., 2011; Steel, Schmidt, & Shultz, 2008; Tang, Fellows, Small, & Dagher, 2012; Thibodeau, Jorgensen, & Kim, 2006; Turkheimer, Pettersson, & Horn, 2014; Vukasovic & Bratko, 2015; Wacker, Chavanon, & Stemmler, 2010; Watson & Clark, 1984; Watson & Naragon-Gainey, 2014).
While there are potential benefits to focusing on narrowly defined phenotypic traits (Block, 1995, 2010; Clifford, Lemery-Chalfant, & Goldsmith, 2015; K. H. Rubin & Asendorpf, 1993), adopting a narrow perspective substantially reduces the size and scope of the relevant evidentiary record. At present, very little is known about some facets of dispositional negativity—including anger, guilt, and shame—contribute to adverse outcomes (Kopala-Sibley et al., in press), making this an important challenge for future research. From a public health perspective, focusing on broad phenotypes that confer elevated risk for a range of adverse outcomes maximizes the opportunity to develop broad-spectrum interventions (Barlow, Ellard, Sauer-Zavala, Bullis, & Carl, 2014; Chronis-Tuscano et al., 2015; Moffitt et al., 2013).
Drawing on a unique body of anthropological research conducted in subject’s own homes, Wang and colleagues provide a vivid description of this negative interpersonal style: “Ed Anderson…scored high on…[dispositional negativity] and reported high job stress. He is married to Rhoda and together they have three young daughters…On Day 1 of videotaping, Ed returned home and was immediately very engaged with his family members…However, he appeared somewhat irritated during these interactions with Rhoda and his daughters, and often sighed, rubbed his head in annoyance, and used a mildly sarcastic tone. At the start of dinner, Ed learned from an apologetic Rhoda that the fish she had made would not be ready for another 20 minutes. Although Ed said “OK, I’ll eat it later,” he made several references to the dinner that showed a critical undertone…Several minutes into the meal, Ed silently left the table and began loudly crunching on chips. Rhoda continued to be apologetic about the delay with the fish, but Ed told her “I’m not even really hungry”…Although Ed continued to inquire when the fish would be ready, he ultimately rejected the fish when it came out of the oven. After dinner, Ed continued to show irritation (e.g., raised eyebrows, annoyed looks, sighs, sarcastic tone)” (Wang et al., 2011, p. 451).
This is similar to what others have conceptualized as a reduced threshold for responding (Davidson, 1998; Reznick et al., 1986).
A growing number of researchers draw a sharp distinction between states of ‘fear’ and ‘anxiety’ (e.g., Barlow, 2000; D. C. Blanchard & Pearson, in press; M. Davis, Walker, Miles, & Grillon, 2010; LeDoux, 2015). Yet lay people, scholars in other areas, the American Psychiatric Association’s Diagnostic and Statistical Manual (American Psychiatric Association, 2013), and even domain experts—at least in unguarded moments—often use these terms interchangeably or inconsistently. As one psychiatrist noted almost 40 years ago, “The word ‘anxiety’ has become confused. It has so many meanings in so many languages, that…it has come to be a synonym for the generic term ‘fear’” (Gaylin, 1979, p. 18). Other commentators have emphasized the difficulty of drawing sharp operational boundaries between the terms (Perusini & Fanselow, 2015). To avoid misunderstanding, we recommend that researchers eschew these problematic redefinitions of everyday language (Shackman & Fox, in press).
The revised Reinforcement Sensitivity Theory is one notable exception (Corr, 2008; Gray & McNaughton, 2000; Reuter, Cooper, Smillie, Markett, & Montag, 2015).
Over the past two decades, “She has been held up at knife point and at gun point, she was once physically accosted by a woman twice her size, she was nearly killed in an act of domestic violence, and on more than one occasion she has been explicitly threatened with death...What stands out most is that, in many of these situations, SM’s life was in danger, yet her behavior lacked any sense of desperation or urgency.” (Feinstein et al., 2011, p. 307).
Inspired by the seminal work of Michael Davis, David Walker, and their colleagues (M. Davis, 1998, 2006; M. Davis, Walker, & Lee, 1997; M. Davis et al., 2010; Grillon, 2008; D. L. Walker & Davis, 2008; D. L. Walker, Miles, & Davis, 2009; D. L. Walker, Toufexis, & Davis, 2003), it is widely thought that phasic and sustained responses to threat reflect dissociable circuits centered on the Ce and the BST, respectively. Indeed, a version of this influential hypothesis has been incorporated into the National Institute of Mental Health (NIMH) Research Domain Criteria (RDoC) as Acute Threat (‘Fear’) and Potential Threat (‘Anxiety’) (cf. https://www.nimh.nih.gov/research-priorities/rdoc/constructs/potential-threat-anxiety.shtml; https://www.nimh.nih.gov/research-priorities/rdoc/negative-valence-systems-workshop-proceedings.shtml; Kozak & Cuthbert, 2016). But as described in greater detail elsewhere (Calhoon & Tye, 2015; Gungor & Paré, in press; Janak & Tye, 2015; Shackman & Fox, in press; Tovote et al., 2015), more recent observations in rodents, monkeys, and humans make it clear that the central extended amygdala forms a tightly interconnected functional unit and that claims of strict functional segregation are no longer tenable.
Other mechanisms, including overgeneralization of threat to perceptually-similar safety cues or contexts (Camp et al., 2012; Ciocchi et al., 2010; Ghosh & Chattarji, 2015; Kheirbek et al., 2012; Laufer, Israeli, & Paz, in press; Lissek et al., 2014; Shaban et al., 2006) and deficient filtering of threat-related information from working memory (Stout, Shackman, Johnson, & Larson, 2014; Stout, Shackman, & Larson, 2013), are also likely to be important. In fact, given the central role of working memory in goal-directed cognition (e.g., attention, imagination, memory retrieval), emotion regulation (e.g., worry), and behavior, aberrant gating of threat-related information from working memory storage may represent a key source of sustained apprehension and negative affect in humans (Mobbs, Hagan, Dalgleish, Silston, & Prevost, 2015; Stout et al., 2013).
For more general recommendations about best practices, see (Button et al., 2013a, 2013b; Chalmers et al., 2014; S. P. David et al., 2013; Howells, Sena, & Macleod, 2014; Ioannidis, Greenland, et al., 2014; Ioannidis, Munafo, Fusar-Poli, Nosek, & David, 2014; Lueken et al., 2016; Poldrack et al., 2016; Stelzer, Lohmann, Mueller, Buschmann, & Turner, 2014; Steward & Balice-Gordon, 2014).
Authors declare no conflicts of interest.
References
- Abbott RA, Croudace TJ, Ploubidis GB, Kuh D, Richards M, Huppert FA. The relationship between early personality and midlife psychological well-being: evidence from a UK birth cohort study. Social Psychiatry and Psychiatric Epidemiology. 2008;43:679–687. doi: 10.1007/s00127-008-0355-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abercrombie HC, Schaefer SM, Larson CL, Oakes TR, Lindgren KA, Holden JE, … Davidson RJ. Metabolic rate in the right amygdala predicts negative affect in depressed patients. Neuroreport. 1998;9:3301–3307. doi: 10.1097/00001756-199810050-00028. [DOI] [PubMed] [Google Scholar]
- Ackerman RA, Corretti CA. Pathological personality traits and intimacy processes within roommate relationships. European Journal of Personality. 2015;29:152–172. [Google Scholar]
- Admon R, Lubin G, Stern O, Rosenberg K, Sela L, Ben-Ami H, Hendler T. Human vulnerability to stress depends on amygdala’s predisposition and hippocampal plasticity. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:14120–14125. doi: 10.1073/pnas.0903183106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Admon R, Milad MR, Hendler T. A causal model of post-traumatic stress disorder: disentangling predisposed from acquired neural abnormalities. Trends Cogn Sci. 2013;17:337–347. doi: 10.1016/j.tics.2013.05.005. [DOI] [PubMed] [Google Scholar]
- Adolphs R. Human lesion studies in the 21st century. Neuron. 2016;90:1151–1153. doi: 10.1016/j.neuron.2016.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolphs R. Consequences of developmental bilateral amygdala lesions in humans. In: Amaral DG, Adolphs R, editors. Living without an amygdala. NY: Guilford Press; in press. [Google Scholar]
- Adolphs R, Tranel D, Damasio AR. The human amygdala in social judgment. Nature. 1998;393:470–474. doi: 10.1038/30982. [DOI] [PubMed] [Google Scholar]
- Alden LE, Taylor CT. Interpersonal processes in social phobia. Clinical Psychology Review. 2004;24:857–882. doi: 10.1016/j.cpr.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Aldinger M, Stopsack M, Ulrich I, Appel K, Reinelt E, Wolff S, … Barnow S. Neuroticism developmental courses--implications for depression, anxiety and everyday emotional experience; a prospective study from adolescence to young adulthood. BMC Psychiatry. 2014:14. doi: 10.1186/s12888-014-0210-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alheid GF, Heimer L. New perspectives in basal forebrain organization of special relevance for neuropsychiatric disorders: the striatopallidal, amygdaloid, and corticopetal components of substantia innominata. Neuroscience. 1988;27:1–39. doi: 10.1016/0306-4522(88)90217-5. [DOI] [PubMed] [Google Scholar]
- Alisch RS, Chopra P, Fox AS, Chen K, White AT, Roseboom PH, … Kalin NH. Differentially methylated plasticity genes in the amygdala of young primates are linked to anxious temperament, an at risk phenotype for anxiety and depressive disorders. Journal of Neuroscience. 2014;34:15548–15556. doi: 10.1523/JNEUROSCI.3338-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JJ, Harmon-Jones E, Cavender JH. Manipulation of frontal EEG asymmetry through biofeedback alters self-reported emotional responses and facial EMG. Psychophysiology. 2001;38:685–693. [PubMed] [Google Scholar]
- Allen JL, Rapee RM. Are reported differences in life events for anxious children and controls due to comorbid disorders? Journal of Anxiety Disorders. 2009;23:511–518. doi: 10.1016/j.janxdis.2008.10.005. [DOI] [PubMed] [Google Scholar]
- Alvarez RP, Chen G, Bodurka J, Kaplan R, Grillon C. Phasic and sustained fear in humans elicits distinct patterns of brain activity. Neuroimage. 2011;55:389–400. doi: 10.1016/j.neuroimage.2010.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez RP, Kirlic N, Misaki M, Bodurka J, Rhudy JL, Paulus MP, Drevets WC. Increased anterior insula activity in anxious individuals is linked to diminished perceived control. Transl Psychiatry. 2015;5:e591. doi: 10.1038/tp.2015.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5 2013. [Google Scholar]
- Anderson DJ, Adolphs R. A framework for studying emotions across species. Cell. 2014;157:187–200. doi: 10.1016/j.cell.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anticevic A, Cole MW, Repovs G, Savic A, Driesen NR, Yang G, … Krystal JH. Connectivity, pharmacology, and computation: toward a mechanistic understanding of neural system dysfunction in schizophrenia. Front Psychiatry. 2013;4:169. doi: 10.3389/fpsyt.2013.00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arce E, Simmons AN, Lovero KL, Stein MB, Paulus MP. Escitalopram effects on insula and amygdala BOLD activation during emotional processing. Psychopharmacology (Berl) 2008;196(4):661–672. doi: 10.1007/s00213-007-1004-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF. Stress signalling pathways that impair prefrontal cortex structure and function. Nature Reviews Neuroscience. 2009;10:410–422. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF. Stress weakens prefrontal networks: molecular insults to higher cognition. Nature Neuroscience. 2015;18:1376–1385. doi: 10.1038/nn.4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atlas LY, Bolger N, Lindquist MA, Wager TD. Brain mediators of predictive cue effects on perceived pain. Journal of Neuroscience. 2010;30:12964–12977. doi: 10.1523/JNEUROSCI.0057-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery SN, Clauss JA, Blackford JU. The human BNST: Functional role in anxiety and addiction. Neuropsychopharmacology. 2016;41:126–141. doi: 10.1038/npp.2015.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas JM, van Ooijen L, Goudriaan A, Kenemans JL. Failure to condition to a cue is associated with sustained contextual fear. Acta Psychologica. 2008;127:581–592. doi: 10.1016/j.actpsy.2007.09.009. [DOI] [PubMed] [Google Scholar]
- Bach DR, Dolan RJ. Knowing how much you don’t know: a neural organization of uncertainty estimates. Nature Reviews Neuroscience. 2012;13:572–586. doi: 10.1038/nrn3289. [DOI] [PubMed] [Google Scholar]
- Banihashemi L, Sheu LK, Midei AJ, Gianaros PJ. Childhood physical abuse predicts stressor-evoked activity within central visceral control regions. Soc Cogn Affect Neurosci. 2015;10:474–485. doi: 10.1093/scan/nsu073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker TV, Reeb-Sutherland B, Degnan KA, Walker OL, Chronis-Tuscano A, Henderson HA, … Fox NA. Contextual startle responses moderate the relation between behavioral inhibition and anxiety in middle childhood. Psychophysiology. doi: 10.1111/psyp.12517. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker TV, Reeb-Sutherland BC, Fox NA. Individual differences in fear potentiated startle in behaviorally inhibited children. Developmental Psychobiology. 2014;56(1):133–141. doi: 10.1002/dev.21096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker TV, Reeb-Sutherland BC, Fox NA. Individual differences in fear potentiated startle in behaviorally inhibited children. Developmental Psychobiology. doi: 10.1002/dev.21096. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow DH. Unraveling the mysteries of anxiety and its disorders from the perspective of emotion theory. American Psychologist. 2000;55:1247–1263. doi: 10.1037//0003-066x.55.11.1247. [DOI] [PubMed] [Google Scholar]
- Barlow DH, Ellard KK, Sauer-Zavala S, Bullis JR, Carl JR. The origins of neuroticism. Perspectives on Psychological Science. 2014;9:481–496. doi: 10.1177/1745691614544528. [DOI] [PubMed] [Google Scholar]
- Barlow DH, Sauer-Zavala S, Carl JR, Bullis JR, Ellard KK. The nature, diagnosis, and treatment of neuroticism: Back to the future. Clinical Psychological Science. 2013;2 [Google Scholar]
- Bastiaansen JA, Servaas MN, Marsman JB, Ormel J, Nolte IM, Riese H, Aleman A. Filling the gap: Relationship between the serotonin-transporter-linked polymorphic region and amygdala activation. Psychol Sci. 2014;25:2058–2066. doi: 10.1177/0956797614548877. [DOI] [PubMed] [Google Scholar]
- Baumgartner T, Heinrichs M, Vonlanthen A, Fischbacher U, Fehr E. Oxytocin shapes the neural circuitry of trust and trust adaptation in humans. Neuron. 2008;58:639–650. doi: 10.1016/j.neuron.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Beck J. Hard feelings: Science’s struggle to define emotions. The Atlantic 2015 Feb 24; [Google Scholar]
- Bennett K, Manassis K, Duda S, Bagnell A, Bernstein GA, Garland EJ, … Wilansky P. Preventing child and adolescent anxiety disorders: Overview of systematic reviews. Depression and Anxiety. 2015;32:909–918. doi: 10.1002/da.22400. [DOI] [PubMed] [Google Scholar]
- Berkman ET, Falk EB. Beyond brain mapping: Using neural measures to predict real-world outcomes. Curr Dir Psychol Sci. 2013;22:45–50. doi: 10.1177/0963721412469394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlanga C, Heinze G, Torres M, Apiquian R, Cabellero A. Personality and clinical predictors of recurrence in depression. Psychiatric Services. 1999;50:376–380. doi: 10.1176/ps.50.3.376. [DOI] [PubMed] [Google Scholar]
- Berry DS, Willingham JK, Thayer CA. Affect and personality as predictors of conflict and closeness in young adults’ friendships. Journal of Research in Personality. 2000;34:84–107. [Google Scholar]
- Bestmann S, Feredoes E. Combined neurostimulation and neuroimaging in cognitive neuroscience: past, present, and future. Annals of the New York Academy of Sciences. 2013;1296:11–30. doi: 10.1111/nyas.12110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt MA, Lohrenz T, Camerer CF, Montague PR. Distinct contributions of the amygdala and parahippocampal gyrus to suspicion in a repeated bargaining game. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:8728–8733. doi: 10.1073/pnas.1200738109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billig JP, Hershberger SL, Iacono WG, McGue M. Life events and personality in late adolescence: Genetic and environmental relations. Behavior Genetics. 1996;26:543–554. doi: 10.1007/BF02361227. [DOI] [PubMed] [Google Scholar]
- Birn RM, Shackman AJ, Oler JA, Williams LE, McFarlin DR, Rogers GM, … Kalin NH. Evolutionarily conserved dysfunction of prefrontal-amygdalar connectivity in early-life anxiety. Molecular Psychiatry. 2014;19:915–922. doi: 10.1038/mp.2014.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackford JU, Allen AH, Cowan RL, Avery SN. Amygdala and hippocampus fail to habituate to faces in individuals with an inhibited temperament. Soc Cogn Affect Neurosci. 2013;8:143–150. doi: 10.1093/scan/nsr078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackford JU, Avery SN, Cowan RL, Shelton RC, Zald DH. Sustained amygdala response to both novel and newly familiar faces characterizes inhibited temperament. Soc Cogn Affect Neurosci. 2011;6:621–629. doi: 10.1093/scan/nsq073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackford JU, Avery SN, Shelton RC, Zald DH. Amygdala temporal dynamics: temperamental differences in the timing of amygdala response to familiar and novel faces. BMC Neurosci. 2009;10:145. doi: 10.1186/1471-2202-10-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackhart GC, Kline JP, Donohue KF, LaRowe SD, Joiner TE. Affective responses to EEG preparation and their link to resting anterior EEG asymmetry. Personality and Individual Differences. 2002;32:167–174. [Google Scholar]
- Blanchard DC, Griebel G, Blanchard RJ. Mouse defensive behaviors: pharmacological and behavioral assays for anxiety and panic. Neuroscience and Biobehavioral Reviews. 2001;25:205–218. doi: 10.1016/s0149-7634(01)00009-4. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Griebel G, Blanchard RJ. The mouse defense test battery: Pharmacological and behavioral assays for anxiety and panic. European Journal of Pharmacology. 2003;463:97–116. doi: 10.1016/s0014-2999(03)01276-7. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Pearson BL. Emotion as an evolutionary adaptive pattern: The roles of context and cognition. In: Fox AS, Lapate RC, Shackman AJ, Davidson RJ, editors. The nature of emotion. Fundamental questions. 2. New York: Oxford University Press; in press. [Google Scholar]
- Blanchard RJ, Blanchard DC, Hori K. Ethoexperimental approaches to the study of defensive behavior. In: Blanchard RJ, Brain PF, Blanchard DC, Parmigiani S, editors. Ethoexperimental approaches to the study of behavior. Dordrecht: Kluwer; 1989. pp. 114–136. [Google Scholar]
- Block J. A contrarian view of the five-factor approach to personality description. Psychological Bulletin. 1995;117:187–215. doi: 10.1037/0033-2909.117.2.187. [DOI] [PubMed] [Google Scholar]
- Block J. The five-factor framing of personality and beyond: Some ruminations. Psychological Inquiry. 2010;21:2–25. [Google Scholar]
- Bogdan R, Pagliaccio D, Baranger DA, Hariri AR. Genetic moderation of stress effects on corticolimbic circuitry. Neuropsychopharmacology. 2016;41:275–296. doi: 10.1038/npp.2015.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boissy A. Fear and fearfulness in animals. Quarterly Review of Biology. 1995;70:165–191. doi: 10.1086/418981. [DOI] [PubMed] [Google Scholar]
- Bolger N, Schilling EA. Personality and the problems of everyday life: the role of neuroticism in exposure and reactivity to daily stressors. Journal of Personality. 1991;59:355–386. doi: 10.1111/j.1467-6494.1991.tb00253.x. [DOI] [PubMed] [Google Scholar]
- Bolger N, Zuckerman A. A framework for studying personality in the stress process. Journal of Personality and Social Psychology. 1995;69:890–902. doi: 10.1037//0022-3514.69.5.890. [DOI] [PubMed] [Google Scholar]
- Borsook D, Becerra L, Hargreaves R. A role for fMRI in optimizing CNS drug development. Nature Reviews Drug Discovery. 2006;5:411–424. doi: 10.1038/nrd2027. [DOI] [PubMed] [Google Scholar]
- Borsook D, Hargreaves R, Bountra C, Porreca F. Lost but making progress — Where will new analgesic drugs come from? Sci Transl Med. 2014;6:249sr243. doi: 10.1126/scitranslmed.3008320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford DE, Shapiro BL, Curtin JJ. How bad could it be? Alcohol dampens stress responses to threat of uncertain intensity. Psychol Sci. 2013;24:2541–2549. doi: 10.1177/0956797613499923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton JC, Suway JG, Clementi MA, Fox NA, Pine DS, Bar-Haim Y. Neural changes with attention bias modification for anxiety: a randomized trial. Soc Cogn Affect Neurosci. 2015;10:913–920. doi: 10.1093/scan/nsu141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooker RJ, Buss KA, Lemery-Chalfant K, Aksan NJ, DR, Goldsmith HH. The development of stranger fear in infancy and toddlerhood: Normative development, individual differences, antecedents, and outcomes. Developmental Science. doi: 10.1111/desc.12058. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brose A, Schmiedek F, Koval P, Kuppens P. Emotional inertia contributes to depressive symptoms beyond perseverative thinking. Cogn Emot. 2015;29(3):527–538. doi: 10.1080/02699931.2014.916252. [DOI] [PubMed] [Google Scholar]
- Brown GG, Ostrowitzki S, Stein MB, von Kienlin M, Liu TT, Simmons A, … Paulus M. Temporal profile of brain response to alprazolam in patients with generalized anxiety disorder. Psychiatry Research. 2015;233:394–401. doi: 10.1016/j.pscychresns.2015.06.016. [DOI] [PubMed] [Google Scholar]
- Buchel C, Morris J, Dolan RJ, Friston KJ. Brain systems mediating aversive conditioning: an event-related fMRI study. Neuron. 1998;20:947–957. doi: 10.1016/s0896-6273(00)80476-6. [DOI] [PubMed] [Google Scholar]
- Buckholtz JW, Callicott JH, Kolachana B, Hariri AR, Goldberg TE, Genderson M, … Meyer-Lindenberg A. Genetic variation in MAOA modulates ventromedial prefrontal circuitry mediating individual differences in human personality. Molecular Psychiatry. 2008;13:313–324. doi: 10.1038/sj.mp.4002020. [DOI] [PubMed] [Google Scholar]
- Buhle JT, Silvers JA, Wager TD, Lopez R, Onyemekwu C, Kober H, … Ochsner KN. Cognitive reappraisal of emotion: a meta-analysis of human neuroimaging studies. Cerebral Cortex. 2014;24:2981–2990. doi: 10.1093/cercor/bht154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burghy CA, Stodola DE, Ruttle PL, Molloy EK, Armstrong JM, Oler JA, … Birn RM. Developmental pathways to amygdala-prefrontal function and internalizing symptoms in adolescence. Nature Neuroscience. 2012;15:1736–1741. doi: 10.1038/nn.3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss KA. Which fearful toddlers should we worry about? Context, fear regulation, and anxiety risk. Developmental Psychology. 2011;47:804–819. doi: 10.1037/a0023227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss KA, Davis EL, Kiel EJ, Brooker RJ, Beekman C, Early MC. Dysregulated fear predicts social wariness and social anxiety symptoms during kindergarten. Journal of Clinical Child and Adolescent Psychology. 2013;42:603–616. doi: 10.1080/15374416.2013.769170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss KA, Schumacher JR, Dolski I, Kalin NH, Goldsmith HH, Davidson RJ. Right frontal brain activity, cortisol, and withdrawal behavior in 6-month-old infants. Behavioral Neuroscience. 2003a;117:11–20. doi: 10.1037//0735-7044.117.1.11. [DOI] [PubMed] [Google Scholar]
- Buss KA, Schumacher JRM, Dolski I, Kalin NH, Goldsmith HH, Davidson RJ. Right frontal brain activity, cortisol, and withdrawal behavior in 6-month-old infants. Behavioral Neuroscience. 2003b;117:11–20. doi: 10.1037//0735-7044.117.1.11. [DOI] [PubMed] [Google Scholar]
- Button KS, Ioannidis JP, Mokrysz C, Nosek BA, Flint J, Robinson ES, Munafo MR. Confidence and precision increase with high statistical power. Nature Reviews Neuroscience. 2013a;14:585–586. doi: 10.1038/nrn3475-c4. [DOI] [PubMed] [Google Scholar]
- Button KS, Ioannidis JP, Mokrysz C, Nosek BA, Flint J, Robinson ES, Munafo MR. Power failure: why small sample size undermines the reliability of neuroscience. Nature Reviews Neuroscience. 2013b;14:365–376. doi: 10.1038/nrn3475. [DOI] [PubMed] [Google Scholar]
- Bystritsky A. Treatment-resistant anxiety disorders. Molecular Psychiatry. 2006;11:805–814. doi: 10.1038/sj.mp.4001852. [DOI] [PubMed] [Google Scholar]
- Calder AJ, Ewbank MP, Passamonti L. Personality influences the neural responses to viewing facial expressions of emotion. Philosophical Transactions of the Royal Society B: Biological Sciences. 2011;366:1684–1701. doi: 10.1098/rstb.2010.0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoon GG, Tye KM. Resolving the neural circuits of anxiety. Nature Neuroscience. 2015;18:1394–1404. doi: 10.1038/nn.4101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camp MC, Macpherson KP, Lederle L, Graybeal C, Gaburro S, Debrouse LM, … Holmes A. Genetic strain differences in learned fear inhibition associated with variation in neuroendocrine, autonomic, and amygdala dendritic phenotypes. Neuropsychopharmacology. 2012;37(6):1534–1547. doi: 10.1038/npp.2011.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canli T, Qiu M, Omura K, Congdon E, Haas BW, Amin Z, … Lesch KP. Neural correlates of epigenesis. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(43):16033–16038. doi: 10.1073/pnas.0601674103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter JS, Garber J, Ciesla JA, Cole DA. Modeling relations between hassles and internalizing and externalizing symptoms in adolescents: a four-year prospective study. Journal of Abnormal Psychology. 2006;115:428–442. doi: 10.1037/0021-843X.115.3.428. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Craddock N, Cuthbert BN, Hyman SE, Lee FS, Ressler KJ. DSM-5 and RDoC: progress in psychiatry research? Nature Reviews Neuroscience. 2013;14:810–814. doi: 10.1038/nrn3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Roberts BW, Shiner RL. Personality development: stability and change. Annual Review of Psychology. 2005;56:453–484. doi: 10.1146/annurev.psych.55.090902.141913. [DOI] [PubMed] [Google Scholar]
- Cavanagh JF, Shackman AJ. Frontal midline theta reflects anxiety and cognitive control: Meta-analytic evidence. Journal of Physiology, Paris. 2015;109:3–15. doi: 10.1016/j.jphysparis.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalmers I, Bracken MB, Djulbegovic B, Garattini S, Grant J, Gulmezoglu AM, … Oliver S. How to increase value and reduce waste when research priorities are set. Lancet. 2014;383:156–165. doi: 10.1016/S0140-6736(13)62229-1. [DOI] [PubMed] [Google Scholar]
- Chan CK, Lovibond PF. Expectancy bias in trait anxiety. Journal of Abnormal Psychology. 1996;105:637–647. doi: 10.1037//0021-843x.105.4.637. [DOI] [PubMed] [Google Scholar]
- Chang LJ, Gianaros PJ, Manuck SB, Krishnan A, Wager TD. A sensitive and specific neural signature for picture-induced negative affect. PLoS Biol. 2015;13:e1002180. doi: 10.1371/journal.pbio.1002180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman BP, Fiscella K, Kawachi I, Duberstein PR. Personality, socioeconomic status, and all-cause mortality in the United States. American Journal of Epidemiology. 2010;171:83–92. doi: 10.1093/aje/kwp323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase HW, Eickhoff SB, Laird AR, Hogarth L. The neural basis of drug stimulus processing and craving: an activation likelihood estimation meta-analysis. Biological Psychiatry. 2011;70:785–793. doi: 10.1016/j.biopsych.2011.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng DT, Knight DC, Smith CN, Helmstetter FJ. Human amygdala activity during the expression of fear responses. Behavioral Neuroscience. 2006;120:1187–1195. doi: 10.1037/0735-7044.120.5.1187. [DOI] [PubMed] [Google Scholar]
- Cheng DT, Richards J, Helmstetter FJ. Activity in the human amygdala corresponds to early, rather than late period autonomic responses to a signal for shock. Learning & Memory. 2007;14:485–490. doi: 10.1101/lm.632007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JM, Padmala S, Pessoa L. Impact of state anxiety on the interaction between threat monitoring and cognition. Neuroimage. 2012;59:1912–1923. doi: 10.1016/j.neuroimage.2011.08.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JS, Kim JJ. Amygdala regulates risk of predation in rats foraging in a dynamic fear environment. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:21773–21777. doi: 10.1073/pnas.1010079108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian BT, Fox AS, Oler JA, Vandehey NT, Murali D, Rogers J, … Kalin NH. Serotonin transporter binding and genotype in the nonhuman primate brain using [C-11]DASB PET. Neuroimage. 2009;47:1230–1236. doi: 10.1016/j.neuroimage.2009.05.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chronis-Tuscano A, Rubin KH, O’Brien KA, Coplan RJ, Thomas SR, Dougherty LR, … Wimsatt M. Preliminary evaluation of a multimodal early intervention program for behaviorally inhibited preschoolers. Journal of Consulting and Clinical Psychology. 2015;83:534–540. doi: 10.1037/a0039043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciocchi S, Herry C, Grenier F, Wolff SB, Letzkus JJ, Vlachos I, … Luthi A. Encoding of conditioned fear in central amygdala inhibitory circuits. Nature. 2010;468:277–282. doi: 10.1038/nature09559. [DOI] [PubMed] [Google Scholar]
- Clark DA, Durbin CE, Hicks BM, Iacono WG, McGue M. Personality in the age of Iindustry: Structure, heritability, and correlates of personality in middle childhood from the perspective of parents, teachers, and children. Journal of Research in Personality. doi: 10.1016/j.jrp.2016.06.013. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark DM. A cognitive perspective on social phobia. In: Crozier WR, Alden LE, editors. International handbook of social anxiety. NY: Wiley; 2001. pp. 405–430. [Google Scholar]
- Clark LA, Kochanska G, Ready R. Mothers’ personality and its interaction with child temperament as predictors of parenting behavior. Journal of Personality and Social Psychology. 2000;79:274–285. doi: 10.1037//0022-3514.79.2.274. [DOI] [PubMed] [Google Scholar]
- Clark LA, Watson D. Temperament: An organizing paradigm for trait psychology. In: John OP, Robins RW, Pervin LA, editors. Handbook of personality: Theory and research. 3. NY: Guilford; 2008. pp. 265–286. [Google Scholar]
- Clauss JA, Blackford JU. Behavioral inhibition and risk for developing social anxiety disorder: a meta-analytic study. Journal of the American Academy of Child and Adolescent Psychiatry. 2012;51:1066–1075. doi: 10.1016/j.jaac.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifford S, Lemery-Chalfant K, Goldsmith HH. The unique and shared genetic and environmental contributions to fear, anger, and sadness in childhood. Child Development. 2015;86:1538–1556. doi: 10.1111/cdev.12394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coan JA, Allen JJB, McKnight PE. A capability model of individual differences in frontal EEG asymmetry. Biological Psychology. 2006;72:198–207. doi: 10.1016/j.biopsycho.2005.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccaro EF, McCloskey MS, Fitzgerald DA, Phan KL. Amygdala and orbitofrontal reactivity to social threat in individuals with impulsive aggression. Biological Psychiatry. 2007;62:168–178. doi: 10.1016/j.biopsych.2006.08.024. [DOI] [PubMed] [Google Scholar]
- Collins PY, Patel V, Joestl SS, March D, Insel TR, Daar AS, … Stein DJ. Grand challenges in global mental health. Nature. 2011;475:27–30. doi: 10.1038/475027a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly JJ, Kavanagh EJ, Viswesvaran C. The convergent validity between self and observer ratings of personality: A meta-analytic review. International Journal of Selection and Assessment. 2007;15:110–117. [Google Scholar]
- Connor-Smith JK, Flachsbart C. Relations between personality and coping: a meta-analysis. Journal of Personality and Social Psychology. 2007;93:1080–1107. doi: 10.1037/0022-3514.93.6.1080. [DOI] [PubMed] [Google Scholar]
- Conway CC, Craske MG, Zinbarg RE, Mineka S. Pathological personality traits and naturalistic course of internalizing disorders among high-risk young adults. Depression and Anxiety. 2016;33:84–93. doi: 10.1002/da.22404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coplan R, Arbeau K, Armer M. Don’t fret, be supportive! Maternal characteristics linking child shyness to psychosocial and school adjustment in kindergarten. Journal of Abnormal Child Psychology. 2007;36:359–371. doi: 10.1007/s10802-007-9183-7. [DOI] [PubMed] [Google Scholar]
- Corr PJ, editor. The reinforcement sensitivity theory of personality. NY: Cambridge University Press; 2008. [Google Scholar]
- Cousijn H, Rijpkema M, Qin S, van Marle HJ, Franke B, Hermans EJ, … Fernandez G. Acute stress modulates genotype effects on amygdala processing in humans. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:9867–9872. doi: 10.1073/pnas.1003514107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer AOJ, van der Sluis S, Noordhof A, Wichers M, Geschwind N, Aggen SH, … Borsboom D. Dimensions of normal personality as networks in search of equilibrium: You can’t like parties if you don’t like people. European Journal of Personality. 2012a;26:414–431. [Google Scholar]
- Cramer AOJ, van der Sluis S, Noordhof A, Wichers M, Geschwind N, Aggen SH, … Borsboom D. Measurable like temperature or mereological like flocking? On the nature of personality traits. European Journal of Personality. 2012b;26:451–459. [Google Scholar]
- Craske MG, Waters AM, Nazarian M, Mineka S, Zinbarg RE, Griffith JW, … Ornitz EM. Does neuroticism in adolescents moderate contextual and explicit threat cue modulation of the startle reflex? Biological Psychiatry. 2009;65:220–226. doi: 10.1016/j.biopsych.2008.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craske MG, Wolitzky-Taylor KB, Mineka S, Zinbarg R, Waters AM, Vrshek-Schallhorn S, … Ornitz E. Elevated responding to safe conditions as a specific risk factor for anxiety versus depressive disorders: evidence from a longitudinal investigation. Journal of Abnormal Psychology. 2012;121(2):315–324. doi: 10.1037/a0025738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creed AT, Funder DC. Social anxiety: from the inside and outside. Personality and Individual Differences. 1998;25:19–33. [Google Scholar]
- Cuijpers P, Smit F, Penninx BW, de Graaf R, ten Have M, Beekman AT. Economic costs of neuroticism: a population-based study. Archives of General Psychiatry. 2010;67:1086–1093. doi: 10.1001/archgenpsychiatry.2010.130. [DOI] [PubMed] [Google Scholar]
- Damian RI, Su R, Shanahan M, Trautwein U, Roberts BW. Can personality traits and intelligence compensate for background disadvantage? Predicting status attainment in adulthood. Journal of Personality and Social Psychology. 2015;109:473–489. doi: 10.1037/pspp0000024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannlowski U, Stuhrmann A, Beutelmann V, Zwanzger P, Lenzen T, Grotegerd D, … Kugel H. Limbic scars: long-term consequences of childhood maltreatment revealed by functional and structural magnetic resonance imaging. Biological Psychiatry. 2012;71:286–293. doi: 10.1016/j.biopsych.2011.10.021. [DOI] [PubMed] [Google Scholar]
- David JP, Green PJ, Martin R, Suls J. Differential roles of neuroticism, extraversion, and event desirability for mood in daily life: an integrative model of top-down and bottom-up influences. Journal of Personality and Social Psychology. 1997;73:149–159. doi: 10.1037//0022-3514.73.1.149. [DOI] [PubMed] [Google Scholar]
- David SP, Ware JJ, Chu IM, Loftus PD, Fusar-Poli P, Radua J, … Ioannidis JP. Potential reporting bias in fMRI studies of the brain. PLoS ONE. 2013;8:e70104. doi: 10.1371/journal.pone.0070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson RJ. Affective style and affective disorders: Perspectives from affective neuroscience. Cognition & Emotion. 1998;12:307–330. [Google Scholar]
- Davidson RJ, Fox AS, Kalin NH. Neural bases of emotion regulation in nonhuman primates and humans. In: Gross JJ, editor. Handbook of emotion regulation. NY: Guilford Press; 2007. pp. 47–68. [Google Scholar]
- Davidson RJ, Jackson DC, Kalin NH. Emotion, plasticity, context, and regulation: Perspectives from affective neuroscience. Psychological Bulletin. 2000;126:890–909. doi: 10.1037/0033-2909.126.6.890. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Kalin NH, Shelton S. Lateralized response to diazepam predicts temperamental style in rhesus monkeys. Behavioral Neuroscience. 1993;107:1106–1110. doi: 10.1037//0735-7044.107.6.1106. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Kalin NH, Shelton SE. Lateralized effects of diazepam on frontal brain electrical asymmetries in rhesus monkeys. Biological Psychiatry. 1992;32:438–451. doi: 10.1016/0006-3223(92)90131-i. [DOI] [PubMed] [Google Scholar]
- Davis J, Maes M, Andreazza A, McGrath JJ, Tye SJ, Berk M. Towards a classification of biomarkers of neuropsychiatric disease: from encompass to compass. Molecular Psychiatry. 2015;20(2):152–153. doi: 10.1038/mp.2014.139. [DOI] [PubMed] [Google Scholar]
- Davis M. Are different parts of the extended amygdala involved in fear versus anxiety? Biological Psychiatry. 1998;44:1239–1247. doi: 10.1016/s0006-3223(98)00288-1. [DOI] [PubMed] [Google Scholar]
- Davis M. Neural systems involved in fear and anxiety measured with fear-potentiated startle. American Psychologist. 2006;61:741–756. doi: 10.1037/0003-066X.61.8.741. [DOI] [PubMed] [Google Scholar]
- Davis M, Walker DL, Lee Y. Roles of the amygdala and bed nucleus of the stria terminalis in fear and anxiety measured with the acoustic startle reflex. Possible relevance to PTSD. Annals of the New York Academy of Sciences. 1997;821:305–331. doi: 10.1111/j.1749-6632.1997.tb48289.x. [DOI] [PubMed] [Google Scholar]
- Davis M, Walker DL, Miles L, Grillon C. Phasic vs sustained fear in rats and humans: Role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology. 2010;35:105–135. doi: 10.1038/npp.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Whalen PJ. The amygdala: vigilance and emotion. Molecular Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- de Berker AO, Rutledge RB, Mathys C, Marshall L, Cross GF, Dolan RJ, Bestmann S. Computations of uncertainty mediate acute stress responses in humans. Nat Commun. 2016;7:10996. doi: 10.1038/ncomms10996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bolle M, De Fruyt F, McCrae RR, Lockenhoff CE, Costa PT, Jr, Aguilar-Vafaie ME, … Terracciano A. The emergence of sex differences in personality traits in early adolescence: A cross-sectional, cross-cultural study. Journal of Personality and Social Psychology. 2015;108:171–185. doi: 10.1037/a0038497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Haan AD, Dekovic M, Prinzie P. Longitudinal impact of parental and adolescent personality on parenting. Journal of Personality and Social Psychology. 2012;102:189–199. doi: 10.1037/a0025254. [DOI] [PubMed] [Google Scholar]
- De Martino B, Camerer CF, Adolphs R. Amygdala damage eliminates monetary loss aversion. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:3788–3792. doi: 10.1073/pnas.0910230107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deary IJ, Weiss A, Batty GD. Intelligence and personality as predictors of illness and death: How researchers in differential psychology and chronic disease epidemiology are collaborating to understand and address health inequalities. Psychological Science in the Public Interest. 2010;11:53–79. doi: 10.1177/1529100610387081. [DOI] [PubMed] [Google Scholar]
- deBettencourt MT, Cohen JD, Lee RF, Norman KA, Turk-Browne NB. Closed-loop training of attention with real-time brain imaging. Nature Neuroscience. 2015;18:470–475. doi: 10.1038/nn.3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis TA, O’Toole L. Mental health on the go: Effects of a gamified attention bias modification mobile application in trait anxious adults. Clin Psychol Sci. 2014;2:576–590. doi: 10.1177/2167702614522228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai M, Kahn I, Knoblich U, Bernstein J, Atallah H, Yang A, … Boyden ES. Mapping brain networks in awake mice using combined optical neural control and fMRI. Journal of Neurophysiology. 2011;105:1393–1405. doi: 10.1152/jn.00828.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiLuca M, Olesen J. The cost of brain diseases: a burden or a challenge? Neuron. 2014;82:1205–1208. doi: 10.1016/j.neuron.2014.05.044. [DOI] [PubMed] [Google Scholar]
- Donnellan MB, Conger RD, Bryant CM. The Big Five and enduring marriages. Journal of Research in Personality. 2004;38:481–504. [Google Scholar]
- Duckworth AL, Allred KM. Temperament in the classroom. In: Shiner RL, Zentner M, editors. Handbook of temperament. NY: Guilford; 2012. pp. 627–644. [Google Scholar]
- Duff EP, Vennart W, Wise RG, Howard MA, Harris RE, Lee M, … Smith SM. Learning to identify CNS drug action and efficacy using multistudy fMRI data. Sci Transl Med. 2015;7:274ra216. doi: 10.1126/scitranslmed.3008438. [DOI] [PubMed] [Google Scholar]
- Duggan CF, Lee AS, Murray RM. Does personality predict long-term outcome in depression? British Journal of Psychiatry. 1990;157:19–24. doi: 10.1192/bjp.157.1.19. [DOI] [PubMed] [Google Scholar]
- Duits P, Cath DC, Lissek S, Hox JJ, Hamm AO, Engelhard IM, … Baas JM. Updated meta-analysis of classical fear conditioning in the anxiety disorders. Depression and Anxiety. 2015;32:239–253. doi: 10.1002/da.22353. [DOI] [PubMed] [Google Scholar]
- Duvarci S, Bauer EP, Paré D. The bed nucleus of the stria terminalis mediates inter-individual variations in anxiety and fear. Journal of Neuroscience. 2009;29:10357–10361. doi: 10.1523/JNEUROSCI.2119-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyrenforth PS, Kashy DA, Donnellan MB, Lucas RE. Predicting relationship and life satisfaction from personality in nationally representative samples from three countries: The relative importance of actor, partner, and similarity effects. Journal of Personality and Social Psychology. 2010;99:690–702. doi: 10.1037/a0020385. [DOI] [PubMed] [Google Scholar]
- Eatough EM, Shirtcliff EA, Hanson JL, Pollak SD. Hormonal reactivity to MRI scanning in adolescents. Psychoneuroendocrinology. 2009;34:1242–1246. doi: 10.1016/j.psyneuen.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellenbogen MA, Hodgins S. The impact of high neuroticism in parents on children’s psychosocial functioning in a population at high risk for major affective disorder: a family-environmental pathway of intergenerational risk. Development and Psychopathology. 2004;16:113–136. doi: 10.1017/s0954579404044438. [DOI] [PubMed] [Google Scholar]
- Epstein S. Trait theory as personality theory: Can a part be as great as the whole? Psychological Inquiry. 1994;5:120–122. [Google Scholar]
- Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. American Journal of Psychiatry. 2007;164:1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everaerd D, Klumpers F, van Wingen G, Tendolkar I, Fernandez G. Association between neuroticism and amygdala responsivity emerges under stressful conditions. Neuroimage. 2015;112:218–224. doi: 10.1016/j.neuroimage.2015.03.014. [DOI] [PubMed] [Google Scholar]
- Eysenck HJ. The biological basis of personality. Springfield, IL: Charles C. Thomas; 1967. [Google Scholar]
- Eysenck HJ. Cicero and the state-trait theory of anxiety: Another case of delayed recognition. American Psychologist. 1983;38:114–115. [Google Scholar]
- Fanselow MS. The adaptive function of conditioned defensive behavior: An ecological approach to Pavlovian stimulus substitution theory. In: Blanchard RJ, Brain PF, Blanchard DC, Parmigiani S, editors. Ethoexperimental approaches to the study of behavior. Boston: Kluver; 1989. pp. 151–166. [Google Scholar]
- Fanselow MS. Neural organization of the defensive behavior system responsible for fear. Psychonomic Bulletin & Review. 1994;1:429–438. doi: 10.3758/BF03210947. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, Lester LS. A functional behavioristic approach to aversively motivated behavior: Predatory imminence as a determinant of the topography of defensive behavior. In: Bolles RC, Beecher MD, editors. Evolution and learning. Hillsdale, NJ: Erlbaum; 1988. pp. 185–211. [Google Scholar]
- Faravelli C, Ambonetti A, Pallanti S, Pazzagli A. Depressive relapses and incomplete recovery from index episode. American Journal of Psychiatry. 1986;7:888–891. doi: 10.1176/ajp.143.7.888. [DOI] [PubMed] [Google Scholar]
- Farmer AS, Kashdan TB. Stress sensitivity and stress generation in social anxiety disorder: a temporal process approach. Journal of Abnormal Psychology. 2015;124:102–114. doi: 10.1037/abn0000036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinstein JS, Adolphs R, Damasio A, Tranel D. The human amygdala and the induction and experience of fear. Current Biology. 2011;21:1–5. doi: 10.1016/j.cub.2010.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felmingham K, Kemp A, Williams L, Das P, Hughes G, Peduto A, Bryant R. Changes in anterior cingulate and amygdala after cognitive behavior therapy of posttraumatic stress disorder. Psychol Sci. 2007;18:127–129. doi: 10.1111/j.1467-9280.2007.01860.x. [DOI] [PubMed] [Google Scholar]
- Ferenczi EA, Zalocusky KA, Liston C, Grosenick L, Warden MR, Amatya D, … Deisseroth K. Prefrontal cortical regulation of brainwide circuit dynamics and reward-related behavior. Science. 2016;351:aac9698. doi: 10.1126/science.aac9698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson E, Heckman JJ, Corr PJ. Personality and economics: Overview and proposed framework. Personality and Individual Differences. 2011;51:201–209. [Google Scholar]
- Fergusson DM, Horwood LJ. Vulnerability to life events exposure. Psychological Medicine. 1987;17:739–749. doi: 10.1017/s0033291700025976. [DOI] [PubMed] [Google Scholar]
- Fleeson W. Toward a structure- and process-integrated view of personality: Traits as density distributions of states. Journal of Personality & Social Psychology. 2001;80:1011–1027. [PubMed] [Google Scholar]
- Fonzo GA, Ramsawh HJ, Flagan TM, Sullivan SG, Letamendi A, Simmons AN, … Stein MB. Common and disorder-specific neural responses to emotional faces in generalised anxiety, social anxiety and panic disorders. British Journal of Psychiatry. 2015;206:206–215. doi: 10.1192/bjp.bp.114.149880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Hariri AR, Martin SL, Silk JS, Moyles DL, Fisher PM, … Dahl RE. Altered striatal activation predicting real-world positive affect in adolescent major depressive disorder. American Journal of Psychiatry. 2009;166:64–73. doi: 10.1176/appi.ajp.2008.07081336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornito A, Zalesky A, Breakspear M. The connectomics of brain disorders. Nature Rev Neurosci. 2015;16:159–172. doi: 10.1038/nrn3901. [DOI] [PubMed] [Google Scholar]
- Fox AS, Kalin NH. A translational neuroscience approach to understanding the development of social anxiety disorder and its pathophysiology. American Journal of Psychiatry. 2014;171:1162–1173. doi: 10.1176/appi.ajp.2014.14040449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox AS, Oler JA, Shackman AJ, Shelton SE, Raveendran M, McKay DR, … Kalin NH. Intergenerational neural mediators of early-life anxious temperament. Proceedings of the National Acadademy of Sciences USA. 2015;112:9118–9122. doi: 10.1073/pnas.1508593112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox AS, Oler JA, Shelton SE, Nanda SA, Davidson RJ, Roseboom PH, Kalin NH. Central amygdala nucleus (Ce) gene expression linked to increased trait-like Ce metabolism and anxious temperament in young primates. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:18108–18113. doi: 10.1073/pnas.1206723109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox AS, Oler JA, Tromp DP, Fudge JL, Kalin NH. Extending the amygdala in theories of threat processing. Trends in Neurosciences. 2015;38:319–329. doi: 10.1016/j.tins.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox AS, Shelton SE, Oakes TR, Converse AK, Davidson RJ, Kalin NH. Orbitofrontal cortex lesions alter anxiety-related activity in the primate bed nucleus of stria terminalis. Journal of Neuroscience. 2010;30:7023–7027. doi: 10.1523/JNEUROSCI.5952-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox AS, Shelton SE, Oakes TR, Davidson RJ, Kalin NH. Trait-like brain activity during adolescence predicts anxious temperament in primates. PLoS ONE. 2008;3:e2570. doi: 10.1371/journal.pone.0002570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox NA, Henderson HA, Marshall PJ, Nichols KE, Ghera MM. Behavioral inhibition: linking biology and behavior within a developmental framework. Annual Review of Psychology. 2005;56:235–262. doi: 10.1146/annurev.psych.55.090902.141532. [DOI] [PubMed] [Google Scholar]
- Fraley RC, Roberts BW. Patterns of continuity: a dynamic model for conceptualizing the stability of individual differences in psychological constructs across the life course. Psychological Review. 2005;112:60–74. doi: 10.1037/0033-295X.112.1.60. [DOI] [PubMed] [Google Scholar]
- Freese JL, Amaral DG. Neuroanatomy of the primate amygdala. In: Whalen PJ, Phelps EA, editors. The human amygdala. NY: Guilford; 2009. pp. 3–42. [Google Scholar]
- Funder DC. Explaining traits. Psychological Inquiry. 1994;5:125–127. [Google Scholar]
- Furmark T, Tillfors M, Marteinsdottir I, Fischer H, Pissiota A, Langstrom B, Fredrikson M. Common changes in cerebral blood flow in patients with social phobia treated with citalopram or cognitive-behavioral therapy. Archives of General Psychiatry. 2002;59:425–433. doi: 10.1001/archpsyc.59.5.425. [DOI] [PubMed] [Google Scholar]
- Gable SL, Reis HT, Elliot AJ. Behavioral activation and inhibition in everyday life. Journal of Personality and Social Psychology. 2000;78:1135–1149. doi: 10.1037//0022-3514.78.6.1135. [DOI] [PubMed] [Google Scholar]
- Gale CR, Hagenaars SP, Davies G, Hill WD, Liewald DC, Cullen B, … Harris SE. Pleiotropy between neuroticism and physical and mental health: findings from 108–038 men and women in UK Biobank. Transl Psychiatry. 2016;6:e791. doi: 10.1038/tp.2016.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaylin W. Feelings: Our vital signs. NY: Harper & Row; 1979. [Google Scholar]
- Gazelle H. Behavioral profiles of anxious solitary children and heterogeneity in peer relations. Developmental Psychology. 2008;44:1604–1624. doi: 10.1037/a0013303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazelle H, Ladd GW. Anxious solitude and peer exclusion: A diathesis-stress model of internalizing trajectories in childhood. Child Development. 2003;74:257–278. doi: 10.1111/1467-8624.00534. [DOI] [PubMed] [Google Scholar]
- Gazendam FJ, Kamphuis JH, Eigenhuis A, Huizenga HMH, Soeter M, Bos MGN, … Kindt M. Personality predicts individual variation in fear learning: A multilevel growth modeling approach. Clinical Psychological Science. 2015;3:175–188. [Google Scholar]
- Gazendam FJ, Kamphuis JH, Kindt M. Deficient safety learning characterizes high trait anxious individuals. Biological Psychology. 2013;92(2):342–352. doi: 10.1016/j.biopsycho.2012.11.006. [DOI] [PubMed] [Google Scholar]
- Gendron M, Barrett LF. Reconstructing the past: A century of ideas about emotion in psychology. Emotion Review. 2009;1:316–339. doi: 10.1177/1754073909338877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Chattarji S. Neuronal encoding of the switch from specific to generalized fear. Nature Neuroscience. 2015;18(1):112–120. doi: 10.1038/nn.3888. [DOI] [PubMed] [Google Scholar]
- Gibbs RA, Rogers J, Katze MG, Bumgarner R, Weinstock GM, Mardis ER, … Zwieg AS. Evolutionary and biomedical insights from the rhesus macaque genome. Science. 2007;316:222–234. doi: 10.1126/science.1139247. [DOI] [PubMed] [Google Scholar]
- Gleason ME, Powers AD, Oltmanns TF. The enduring impact of borderline personality pathology: risk for threatening life events in later middle-age. Journal of Abnormal Psychology. 2012;121:447–457. doi: 10.1037/a0025564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glue P, Wilson S, Coupland N, Ball D, Nutt D. The relationship between benzodiazepine receptor sensitivity and neuroticism. Journal of Anxiety Disorders. 1995;9:33–45. [Google Scholar]
- Goldsmith HH, Buss AH, Plomin R, Rothbart MK, Thomas A, Chess S, … McCall RB. Roundtable: what is temperament? Four approaches. Child Development. 1987;58:505–529. [PubMed] [Google Scholar]
- Goodwin RD, Hoven CW, Lyons JS, Stein MB. Mental health service utilization in the United States. The role of personality factors. Social Psychiatry and Psychiatric Epidemiology. 2002;37:561–566. doi: 10.1007/s00127-002-0563-6. [DOI] [PubMed] [Google Scholar]
- Gosling SD. Personality in non-human animals. Social and Personality Psychology Compass. 2008;2:985–1001. [Google Scholar]
- Gosling SD, Mason W. Internet research in psychology. Annual Review of Psychology. 2015;66:877–902. doi: 10.1146/annurev-psych-010814-015321. [DOI] [PubMed] [Google Scholar]
- Gospic K, Mohlin E, Fransson P, Petrovic P, Johannesson M, Ingvar M. Limbic justice--amygdala involvement in immediate rejection in the Ultimatum Game. PLoS Biol. 2011;9:e1001054. doi: 10.1371/journal.pbio.1001054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grav S, Stordal E, Romild UK, Hellzen O. The relationship among neuroticism, extraversion, and depression in the HUNT Study: in relation to age and gender. Issues in Mental Health Nursing. 2012;33:777–785. doi: 10.3109/01612840.2012.713082. [DOI] [PubMed] [Google Scholar]
- Gray JA, McNaughton N. The neuropsychology of anxiety. 2. NY: Oxford University Press; 2000. [Google Scholar]
- Greer SM, Trujillo AJ, Glover GH, Knutson B. Control of nucleus accumbens activity with neurofeedback. Neuroimage. 2014;96:237–244. doi: 10.1016/j.neuroimage.2014.03.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griebel G, Holmes A. 50 years of hurdles and hope in anxiolytic drug discovery. Nature Reviews Drug Discovery. 2013;12:667–687. doi: 10.1038/nrd4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C. Associative learning deficits increase symptoms of anxiety in humans. Biological Psychiatry. 2002;51:851–858. doi: 10.1016/s0006-3223(01)01370-1. [DOI] [PubMed] [Google Scholar]
- Grillon C. Models and mechanisms of anxiety: evidence from startle studies. Psychopharmacology. 2008;199:421–437. doi: 10.1007/s00213-007-1019-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, Ameli R. Conditioned inhibition of fear-potentiated startle and skin conductance in humans. Psychophysiology. 2001;38(5):807–815. [PubMed] [Google Scholar]
- Grillon C, Baas JM, Pine DS, Lissek S, Lawley M, Ellis V, Levine J. The benzodiazepine alprazolam dissociates contextual fear from cued fear in humans as assessed by fear-potentiated startle. Biological Psychiatry. 2006;60(7):760–766. doi: 10.1016/j.biopsych.2005.11.027. [DOI] [PubMed] [Google Scholar]
- Grillon C, Charney DR. In the face of fear: anxiety sensitizes defensive responses to fearful faces. Psychophysiology. 2011;48:1745–1752. doi: 10.1111/j.1469-8986.2011.01268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, Pellowski M, Merikangas KR, Davis M. Darkness facilitates the acoustic startle reflex in humans. Biological Psychiatry. 1997;42:453–460. doi: 10.1016/S0006-3223(96)00466-0. [DOI] [PubMed] [Google Scholar]
- Gross JJ, Sutton SK, Ketelaar T. Relations between affect and personality: Support for the affect-level and affective reactivity views. Personality and Social Psychology Bulletin. 1998;24:279–288. [Google Scholar]
- Grupe DW, Nitschke JB. Uncertainty and anticipation in anxiety: an integrated neurobiological and psychological perspective. Nature Reviews Neuroscience. 2013;14:488–501. doi: 10.1038/nrn3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grupe DW, Oathes DJ, Nitschke JB. Dissecting the anticipation of aversion reveals dissociable neural networks. Cerebral Cortex. 2013;23:1874–1883. doi: 10.1093/cercor/bhs175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gungor NZ, Paré D. Functional heterogeneity in the bed nucleus of the stria terminalis. Journal of Neuroscience. doi: 10.1523/JNEUROSCI.0856-16.2016. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunthert KC, Cohen LH, Armeli S. The role of neuroticism in daily stress and coping. Journal of Personality and Social Psychology. 1999;77:1087–1100. doi: 10.1037//0022-3514.77.5.1087. [DOI] [PubMed] [Google Scholar]
- Haaker J, Lonsdorf TB, Schumann D, Menz M, Brassen S, Bunzeck N, … Kalisch R. Deficient inhibitory processing in trait anxiety: Evidence from context-dependent fear learning, extinction recall and renewal. Biological Psychology. 2015;111:65–72. doi: 10.1016/j.biopsycho.2015.07.010. [DOI] [PubMed] [Google Scholar]
- Hagemann D, Hewig J, Seifert J, Naumann E, Bartussek D. The latent state-trait structure of resting EEG asymmetry: replication and extension. Psychophysiology. 2005;42:740–752. doi: 10.1111/j.1469-8986.2005.00367.x. [DOI] [PubMed] [Google Scholar]
- Hagemann D, Naumann E, Thayer JF, Bartussek D. Does resting electroencephalograph asymmetry reflect a trait? An application of latent state-trait theory. Journal of Personality and Social Psychology. 2002;82:619–641. [PubMed] [Google Scholar]
- Hakulinen C, Elovainio M, Pulkki-Raback L, Virtanen M, Kivimaki M, Jokela M. Personality and depressive symptoms: Individual participant meta-analysis of 10 cohort studies. Depression and Anxiety. 2015;32:461–470. doi: 10.1002/da.22376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakulinen C, Hintsanen M, Munafo MR, Virtanen M, Kivimaki M, Batty GD, Jokela M. Personality and smoking: individual-participant meta-analysis of nine cohort studies. Addiction. 2015;110:1844–1852. doi: 10.1111/add.13079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton JP, Etkin A, Furman DJ, Lemus MG, Johnson RF, Gotlib IH. Functional neuroimaging of major depressive disorder: a meta-analysis and new integration of base line activation and neural response data. American Journal of Psychiatry. 2012;169:693–703. doi: 10.1176/appi.ajp.2012.11071105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammack SE, Todd TP, Kocho-Schellenberg M, Bouton ME. Role of the bed nucleus of the stria terminalis in the acquisition of contextual fear at long or short context-shock intervals. Behavioral Neuroscience. 2015;129:673–678. doi: 10.1037/bne0000088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankin BL. Personality and depressive symptoms: Stress generation and cognitive vulnerabilities to depression in a prospective daily diary study. J Soc Clin Psychol. 2010;29:369–401. doi: 10.1521/jscp.2010.29.4.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankin BL, Fraley RC, Abela JR. Daily depression and cognitions about stress: evidence for a traitlike depressogenic cognitive style and the prediction of depressive symptoms in a prospective daily diary study. Journal of Personality and Social Psychology. 2005;88:673–685. doi: 10.1037/0022-3514.88.4.673. [DOI] [PubMed] [Google Scholar]
- Hare TA, Tottenham N, Galvan A, Voss HU, Glover GH, Casey BJ. Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biological Psychiatry. 2008;63:927–934. doi: 10.1016/j.biopsych.2008.03.015015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmer CJ, Mackay CE, Reid CB, Cowen PJ, Goodwin GM. Antidepressant drug treatment modifies the neural processing of nonconscious threat cues. Biological Psychiatry. 2006;59:816–820. doi: 10.1016/j.biopsych.2005.10.015. [DOI] [PubMed] [Google Scholar]
- Harris LM, Cumming SR, Menzies RG. Predicting anxiety in magnetic resonance imaging scans. International Journal of Behavioral Medicine. 2004;11:1–7. doi: 10.1207/s15327558ijbm1101_1. [DOI] [PubMed] [Google Scholar]
- Harris LM, Robinson J, Menzies RG. Predictors of panic symptoms during magnetic resonance imaging scans. International Journal of Behavioral Medicine. 2001;8:80–87. doi: 10.1207/s15327558ijbm1101_1. [DOI] [PubMed] [Google Scholar]
- Headey B, Wearing A. Personality, life events, and subjective well-being: Toward a dynamic equilibrium model. Journal of Personality and Social Psychology. 1989;57:731–739. [Google Scholar]
- Heerey EA, Kring AM. Interpersonal consequences of social anxiety. Journal of Abnormal Psychology. 2007;116:125–134. doi: 10.1037/0021-843X.116.1.125. [DOI] [PubMed] [Google Scholar]
- Hefner KR, Curtin JJ. Alcohol stress response dampening: selective reduction of anxiety in the face of uncertain threat. J Psychopharmacol. 2012;26(2):232–244. doi: 10.1177/0269881111416691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hefner KR, Jaber JN, Grant AM, Curtin JJ. Alcohol intoxication: Selective reduction of anxiety in the face of uncertain threat. Psychophysiology. 2009;46:S64. [Google Scholar]
- Hefner KR, Moberg CA, Hachiya LY, Curtin JJ. Alcohol stress response dampening during imminent versus distal, uncertain threat. Journal of Abnormal Psychology. 2013;122(3):756–769. doi: 10.1037/a0033407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heineck G. Does it pay to be nice? Personality and earnings in the United Kingdom. Industrial and Labor Relations Review. 2011;64:1020–1038. [Google Scholar]
- Heller AS, Fox AS, Wing E, Mayer K, Vack NJ, Davidson RJ. Affective neurodynamics predict prolonged real-world emotional responses. Journal of Neuroscience. 35:10503–10509. doi: 10.1523/JNEUROSCI.0569-15.2015. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hengartner MP, Ajdacic-Gross V, Wyss C, Angst J, Rossler W. Relationship between personality and psychopathology in a longitudinal community study: a test of the predisposition model. Psychological Medicine. 2016;46:1693–1705. doi: 10.1017/S0033291716000210. [DOI] [PubMed] [Google Scholar]
- Hengartner MP, Kawohl W, Haker H, Rossler W, Ajdacic-Gross V. Big Five personality traits may inform public health policy and preventive medicine: Evidence from a cross-sectional and a prospective longitudinal epidemiologic study in a Swiss community. Journal of Psychosomatic Research. 2016;84:44–51. doi: 10.1016/j.jpsychores.2016.03.012. [DOI] [PubMed] [Google Scholar]
- Hengartner MP, van der Linden D, Bohleber L, von Wyl A. Big Five Personality Traits and the General Factor of Personality as Moderators of Stress and Coping Reactions Following an Emergency Alarm on a Swiss University Campus. Stress Health. 2016 doi: 10.1002/smi.2671. [DOI] [PubMed] [Google Scholar]
- Herringa RJ, Birn RM, Ruttle PL, Burghy CA, Stodola DE, Davidson RJ, Essex MJ. Childhood maltreatment is associated with altered fear circuitry and increased internalizing symptoms by late adolescence. Proceedings of the National Academy of Sciences of the United States of America. 2013 doi: 10.1073/pnas.1310766110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herry C, Bach DR, Esposito F, Di Salle F, Perrig WJ, Scheffler K, … Seifritz E. Processing of temporal unpredictability in human and animal amygdala. Journal of Neuroscience. 2007;27:5958–5966. doi: 10.1523/JNEUROSCI.5218-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hintsanen M, Puttonen S, Smith K, Tornroos M, Jokela M, Pulkki-Raback L, … Keltikangas-Jarvinen L. Five-factor personality traits and sleep: evidence from two population-based cohort studies. Health Psychology. 2014;33:1214–1223. doi: 10.1037/hea0000105. [DOI] [PubMed] [Google Scholar]
- Hirschfeld RM, Klerman GL, Andreasen NC, Clayton PJ, Keller MB. Psycho-social predictors of chronicity in depressed patients. British Journal of Psychiatry. 1986;148:648–654. doi: 10.1192/bjp.148.6.648. [DOI] [PubMed] [Google Scholar]
- Hoehn-Saric R, McLeod DR, Funderburk F, Kowalski P. Somatic symptoms and physiologic responses in generalized anxiety disorder and panic disorder: an ambulatory monitor study. Archives of General Psychiatry. 2004;61(9):913–921. doi: 10.1001/archpsyc.61.9.913. [DOI] [PubMed] [Google Scholar]
- Horwitz BN, Luong G, Charles ST. Neuroticism and extraversion share genetic and environmental effects with negative and positive mood spillover in a nationally representative sample. Personality & Individual Differences. 2008;45:636–642. doi: 10.1016/j.paid.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houben M, Van Den Noortgate W, Kuppens P. The relation between short-term emotion dynamics and psychological well-being: A meta-analysis. Psychological Bulletin. 2015 doi: 10.1037/a0038822. [DOI] [PubMed] [Google Scholar]
- Howells DW, Sena ES, Macleod MR. Bringing rigour to translational medicine. Nature Reviews Neurology. 2014;10:37–43. doi: 10.1038/nrneurol.2013.232. [DOI] [PubMed] [Google Scholar]
- Hutteman R, Bleidorn W, Kereste G, Brkovic I, Butkovic A, Denissen JJA. Reciprocal associations between parenting challenges and parents’ personality development in young and middle adulthood. European Journal of Personality. 2014;28:168–179. [Google Scholar]
- Hutteman R, Bleidorn W, Kerestes G, Brkovic I, Butkovic A, Denissen JJA. Reciprocal associations between parenting challenges and parents’ personality development in young and middle adulthood. European Journal of Personality. 2014;28:168–179. [Google Scholar]
- Iacovino JM, Bogdan R, Oltmanns TF. Personality predicts health declines through stressful life events during late mid-life. Journal of Personality. 2016;84:536–546. doi: 10.1111/jopy.12179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR. Next-generation treatments for mental disorders. Sci Transl Med. 2012;4:155ps119. doi: 10.1126/scitranslmed.3004873. [DOI] [PubMed] [Google Scholar]
- Ioannidis J, Greenland S, Hlatky M, Khoury M, Macleod M, Moher D, … Tibshirani R. Increasing value and reducing waste in research design, conduct, and analysis. Lancet. 2014;383:166–175. doi: 10.1016/S0140-6736(13)62227-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioannidis J, Munafo M, Fusar-Poli P, Nosek B, David S. Publication and other reporting biases in cognitive sciences: detection, prevalence, and prevention. Trends in Cognitive Sciences. 2014;18:235–241. doi: 10.1016/j.tics.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izard CE. The many meanings/aspects of emotion: Definitions, functions, activation, and regulation. Emotion Review. 2010;2:363–370. [Google Scholar]
- Izquierdo A, Suda RK, Murray EA. Comparison of the effects of bilateral orbital prefrontal cortex lesions and amygdala lesions on emotional responses in rhesus monkeys. Journal of Neuroscience. 2005;25(37):8534–8542. doi: 10.1523/JNEUROSCI.1232-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson JJ, Connolly JJ, Garrison SM, Leveille MM, Connolly SL. Your friends know how long you will live: a 75-year study of peer-rated personality traits. Psychol Sci. 2015;26:335–340. doi: 10.1177/0956797614561800. [DOI] [PubMed] [Google Scholar]
- Jacobs N, van Os J, Derom C, Thiery E, Delespaul P, Wichers M. Neuroticism explained? From a non-informative vulnerability marker to informative person-context interactions in the realm of daily life. British Journal of Clinical Psychology. 2011;50:19–32. doi: 10.1348/014466510X491397. [DOI] [PubMed] [Google Scholar]
- Jahn AL, Fox AS, Abercrombie HC, Shelton SE, Oakes TR, Davidson RJ, Kalin NH. Subgenual prefrontal cortex activity predicts individual differences in hypothalamic-pituitary-adrenal activity across different contexts. Biological Psychiatry. 2010;67:175–181. doi: 10.1016/j.biopsych.2009.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janak PH, Tye KM. From circuits to behaviour in the amygdala. Nature. 2015;517:284–292. doi: 10.1038/nature14188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarcho JM, Leibenluft E, Walker OL, Fox NA, Pine DS, Nelson EE. Neuroimaging studies of pediatric social anxiety: paradigms, pitfalls and a new direction for investigating the neural mechanisms. Biol Mood Anxiety Disord. 2013;3:14. doi: 10.1186/2045-5380-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeronimus BF, Riese H, Sanderman R, Ormel J. Mutual reinforcement between neuroticism and life experiences: a five-wave, 16-year study to test reciprocal causation. Journal of Personality and Social Psychology. 2014;107:751–764. doi: 10.1037/a0037009. [DOI] [PubMed] [Google Scholar]
- Johnson JG, Cohen P, Kasen S, Brook JS. Paternal psychiatric symptoms and maladaptive paternal behavior in the home during the child rearing years. Journal of Child and Family Studies. 2004;13:421–437. [Google Scholar]
- Jokela M, Hakulinen C, Singh-Manoux A, Kivimaki M. Personality change associated with chronic diseases: pooled analysis of four prospective cohort studies. Psychological Medicine. 2014;44:2629–2640. doi: 10.1017/S0033291714000257. [DOI] [PubMed] [Google Scholar]
- Jokela M, Kivimaki M, Elovainio M, Keltikangas-Jarvinen L. Personality and having children: a two-way relationship. J Pers Soc Psychol. 2009;96:218–230. doi: 10.1037/a0014058. [DOI] [PubMed] [Google Scholar]
- Jokela M, Pulkki-Raback L, Elovainio M, Kivimaki M. Personality traits as risk factors for stroke and coronary heart disease mortality: pooled analysis of three cohort studies. Journal of Behavioral Medicine. 2014;37(5):881–889. doi: 10.1007/s10865-013-9548-z. [DOI] [PubMed] [Google Scholar]
- Jovanovic T, Nylocks KM, Gamwell KL, Smith A, Davis TA, Norrholm SD, Bradley B. Development of fear acquisition and extinction in children: effects of age and anxiety. Neurobiology of Learning and Memory. 2014;113:135–142. doi: 10.1016/j.nlm.2013.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judge TA, Simon LS, Hurst C, Kelley K. What I experienced yesterday is who I am today: Relationship of work motivations and behaviors to within-individual variation in the five-factor model of personality. Journal of Applied Psychology. 2014;99:199–221. doi: 10.1037/a0034485. [DOI] [PubMed] [Google Scholar]
- Kaczkurkin AN, Moore TM, Ruparel K, Ciric R, Calkins ME, Shinohara RT, … Sattherthwaite TD. Elevated amygdala perfusion mediates developmental sex differences in trait anxiety. Biological Psychiatry. doi: 10.1016/j.biopsych.2016.04.021. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan J, Reznick JS, Snidman N. Biological bases of childhood shyness. Science. 1988;240:167–171. doi: 10.1126/science.3353713. [DOI] [PubMed] [Google Scholar]
- Kagan J, Snidman N, Kahn V, Towsley S. The preservation of two infant temperaments into adolescence. Monographs of the Society for Research in Child Development. 2007;72:1–75. doi: 10.1111/j.1540-5834.2007.00436.x. [DOI] [PubMed] [Google Scholar]
- Kaiser T, Feng G. Modeling psychiatric disorders for developing effective treatments. Nature Medicine. 2015;21:979–988. doi: 10.1038/nm.3935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin NH, Fox AS, Kovner R, Riedel MK, Fekete EM, Roseboom PH, … Oler JA. Overexpressing corticotropin-releasing hormone in the primate amygdala increases anxious temperament and alters its neural circuit. Biological Psychiatry. doi: 10.1016/j.biopsych.2016.01.010. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin NH, Larson C, Shelton SE, Davidson RJ. Asymmetric frontal brain activity, cortisol, and behavior associated with fearful temperament in rhesus monkeys. Behavioral Neuroscience. 1998;112:286–292. doi: 10.1037//0735-7044.112.2.286. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE. Defensive behaviors in infant rhesus monkeys: environmental cues and neurochemical regulation. Science. 1989;243:1718–1721. doi: 10.1126/science.2564702. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Davidson RJ. The role of the central nucleus of the amygdala in mediating fear and anxiety in the primate. Journal of Neuroscience. 2004;24:5506–5515. doi: 10.1523/JNEUROSCI.0292-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Davidson RJ. Role of the primate orbitofrontal cortex in mediating anxious temperament. Biological Psychiatry. 2007;62:1134–1139. doi: 10.1016/j.biopsych.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Davidson RJ, Lynn DE. A new method for aversive Pavlovian conditioning of heart rate in rhesus monkeys. Physiology and Behavior. 1996;60:1043–1046. doi: 10.1016/0031-9384(96)00145-x. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Fox AS, Oakes TR, Davidson RJ. Brain regions associated with the expression and contextual regulation of anxiety in primates. Biological Psychiatry. 2005;58:796–804. doi: 10.1016/j.biopsych.2005.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandler C, Bleidorn W, Riemann R, Angleitner A, Spinath FM. Life events as environmental states and genetic traits and the role of personality: A longitudinal twin study. Behavior Genetics. 2012;42:57–72. doi: 10.1007/s10519-011-9491-0. [DOI] [PubMed] [Google Scholar]
- Karney BR, Bradbury TN. The longitudinal course of marital quality and stability: a review of theory, method, and research. Psychological Bulletin. 1995;118:3–34. doi: 10.1037/0033-2909.118.1.3. [DOI] [PubMed] [Google Scholar]
- Kelly EL, Conley JJ. Personality and compatibility: A prospective analysis of marital stability and marital satisfaction. Journal of Personality and Social Psychology. 1987;52:27–40. doi: 10.1037//0022-3514.52.1.27. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Gardner CO. Sex differences in the pathways to major depression: a study of opposite-sex twin pairs. American Journal of Psychiatry. 2014;171:426–435. doi: 10.1176/appi.ajp.2013.13101375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Gardner CO, Prescott CA. Personality and the experience of environmental adversity. Psychological Medicine. 2003;33:1193–1202. doi: 10.1017/s0033291703008298. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Karkowski-Shuman L. Stressful life events and genetic liability to major depression: genetic control of exposure to the environment? Psychological Medicine. 1997;27:539–547. doi: 10.1017/s0033291797004716. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Karkowski LM, Prescott CA. Causal relationship between stressful life events and the onset of major depression. American Journal of Psychiatry. 1999;156:837–841. doi: 10.1176/ajp.156.6.837. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Neale MC, Kessler RC, Heath AC. A longitudinal twin study of personality and major depression in women. Archives of General Psychiatry. 1993;50:853–862. doi: 10.1001/archpsyc.1993.01820230023002. [DOI] [PubMed] [Google Scholar]
- Kennedy DP, Glascher J, Tyszka JM, Adolphs R. Personal space regulation by the human amygdala. Nature Neuroscience. 2009;12:1226–1227. doi: 10.1038/nn.2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kercher AJ, Rapee RM, Schniering CA. Neuroticism, life events and negative thoughts in the development of depression in adolescent girls. Journal of Abnormal Child Psychology. 2009;37:903–915. doi: 10.1007/s10802-009-9325-1. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Petukhova M, Sampson NA, Zaslavsky AM, Wittchen HU. Twelve-month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. Int J Methods Psychiatr Res. 2012;21:169–184. doi: 10.1002/mpr.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Walters EE, Forthofer MS. The social consequences of psychiatric disorders, III: Probability of marital stability. American Journal of Psychiatry. 1998;155:1092–1096. doi: 10.1176/ajp.155.8.1092. [DOI] [PubMed] [Google Scholar]
- Kheirbek MA, Klemenhagen KC, Sahay A, Hen R. Neurogenesis and generalization: a new approach to stratify and treat anxiety disorders. Nature Neuroscience. 2012;15(12):1613–1620. doi: 10.1038/nn.3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein KJ, Lim BC, Saltz JL, Mayer DM. How do we get there? An examination of the antecedants of centrality in team networks. Academy of Management Journal. 2004;47:952–963. [Google Scholar]
- Klumpers F, Kroes MC, Heitland I, Everaerd D, Akkermans SE, Oosting RS, … Baas JM. Dorsomedial prefrontal cortex mediates the impact of serotonin transporter linked polymorphic region genotype on anticipatory threat reactions. Biological Psychiatry. 2015;78:582–589. doi: 10.1016/j.biopsych.2014.07.034. [DOI] [PubMed] [Google Scholar]
- Klumpers F, Morgan B, Terburg D, Stein DJ, van Honk J. Impaired acquisition of classically conditioned fear-potentiated startle reflexes in humans with focal bilateral basolateral amygdala damage. Social Cognitive and Affective Neuroscience. :nsu164. doi: 10.1093/scan/nsu164. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight DC, Nguyen HT, Bandettini PA. The role of the human amygdala in the production of conditioned fear responses. Neuroimage. 2005;26:1193–1200. doi: 10.1016/j.neuroimage.2005.03.020. [DOI] [PubMed] [Google Scholar]
- Kochanska G, Clark LA, Goldman MS. Implications of mothers’ personality for their parenting and their young children’s developmental outcomes. Journal of Personality. 1997;65:387–420. doi: 10.1111/j.1467-6494.1997.tb00959.x. [DOI] [PubMed] [Google Scholar]
- Koenigs M, Baskin-Sommers A, Zeier J, Newman JP. Investigating the neural correlates of psychopathy: a critical review. Molecular Psychiatry. 2011;16:792–799. doi: 10.1038/mp.2010.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komulainen E, Meskanen K, Lipsanen J, Lahti JM, Jylha P, Melartin T, … Ekelund J. The effect of personality on daily life emotional processes. PLoS ONE. 2014;9:e110907. doi: 10.1371/journal.pone.0110907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopala-Sibley DC, Danzig AP, Kotov R, Bromet EJ, Carlson GA, Olino TM, … Klein DN. Negative emotionality and its facets moderate the effects of exposure to hurricane Sandy on children’s postdisaster depression and anxiety symptoms. Journal of Abnormal Psychology. doi: 10.1037/abn0000152. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopala-Sibley DC, Kotov R, Bromet EJ, Carlson GA, Danzig AP, Black SR, Klein DN. Personality diatheses and Hurricane Sandy: effects on post-disaster depression. Psychological Medicine. 2016;46:865–875. doi: 10.1017/S0033291715002378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotov R, Gamez W, Schmidt F, Watson D. Linking “big” personality traits to anxiety, depressive, and substance use disorders: a meta-analysis. Psychological Bulletin. 2010;136:768–821. doi: 10.1037/a0020327. [DOI] [PubMed] [Google Scholar]
- Koval P, Kuppens P. Changing emotion dynamics: individual differences in the effect of anticipatory social stress on emotional inertia. Emotion. 2012;12(2):256–267. doi: 10.1037/a0024756. [DOI] [PubMed] [Google Scholar]
- Koval P, Kuppens P, Allen NB, Sheeber L. Getting stuck in depression: the roles of rumination and emotional inertia. Cogn Emot. 2012;26(8):1412–1427. doi: 10.1080/02699931.2012.667392. [DOI] [PubMed] [Google Scholar]
- Kozak MJ, Cuthbert BN. The NIMH research domain criteria initiative: Background, issues, and pragmatics. Psychophysiology. 2016;53:286–297. doi: 10.1111/psyp.12518. [DOI] [PubMed] [Google Scholar]
- Kragel PA, LaBar KS. Multivariate neural biomarkers of emotional states are categorically distinct. Soc Cogn Affect Neurosci. 2015;10:1437–1448. doi: 10.1093/scan/nsv032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan A, Woo CW, Chang LJ, Ruzic L, Gu X, Lopez-Sola M, … Wager TD. Somatic and vicarious pain are represented by dissociable multivariate brain patterns. Elife. 2016:5. doi: 10.7554/eLife.15166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn S, Gallinat J. Common biology of craving across legal and illegal drugs - a quantitative meta-analysis of cue-reactivity brain response. European Journal of Neuroscience. 2011;33:1318–1326. doi: 10.1111/j.1460-9568.2010.07590.x. [DOI] [PubMed] [Google Scholar]
- Kurdek LA. Predicting marital dissolution: A 5-year prospective longitudinal study of newlywed couples. Journal of Personality & Social Psychology. 1993;64:221–242. [Google Scholar]
- LaBar KS, Gatenby JC, Gore JC, LeDoux JE, Phelps EA. Human amygdala activation during conditioned fear acquisition and extinction: a mixed-trial fMRI study. Neuron. 1998;20:937–945. doi: 10.1016/s0896-6273(00)80475-4. [DOI] [PubMed] [Google Scholar]
- Laceulle OM, Nederhof E, Karreman A, Ormel J, Van Aken MAG. Stressful events and temperament change during early and middle adolescence: The Trails study. European Journal of Personality. 2011;26:276–284. [Google Scholar]
- Ladd GW, Kochenderfer-Ladd B, Eggum ND, Kochel KP, McConnell EM. Characterizing and comparing the friendships of anxious-solitary and unsociable preadolescents. Child Development. 2011;82:1434–1453. doi: 10.1111/j.1467-8624.2011.01632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahey BB. Public health significance of neuroticism. American Psychologist. 2009;64:241–256. doi: 10.1037/a0015309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laidlaw KE, Foulsham T, Kuhn G, Kingstone A. Potential social interactions are important to social attention. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:5548–5553. doi: 10.1073/pnas.1017022108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakdawalla Z, Hankin BL. Personality as a prospective vulnerability to dysphoric symptoms among college students: Proposed mechanisms. J Psychopathol Behav Assess. 2008;30:121–131. [Google Scholar]
- Lake RI, Eaves LJ, Maes HH, Heath AC, Martin NG. Further evidence against the environmental transmission of individual differences in neuroticism from a collaborative study of 45,850 twins and relatives on two continents. Behavior Genetics. 2000;30:223–233. doi: 10.1023/a:1001918408984. [DOI] [PubMed] [Google Scholar]
- Lapate RC, Shackman AJ. Afterword: What is an emotion? In: Fox AS, Lapate RC, Shackman AJ, Davidson RJ, editors. The nature of emotion: Fundamental questions. 2. New York: Oxford University Press; in press. [Google Scholar]
- Larsen RJ, Ketelaar T. Extraversion, neuroticism and susceptibility to positive and negative mood induction procedures. Personality & Individual Differences. 1989;10:1221–1228. [Google Scholar]
- Larsen RJ, Ketelaar T. Personality and susceptibility to positive and negative emotional states. Journal of Personality and Social Psychology. 1991;61:132–140. doi: 10.1037//0022-3514.61.1.132. [DOI] [PubMed] [Google Scholar]
- Laufer O, Israeli D, Paz R. Behavioral and neural mechanisms of overgeneralization in anxiety. Current Biology. doi: 10.1016/j.cub.2016.01.023. in press. [DOI] [PubMed] [Google Scholar]
- Lebowitz ER, Scharfstein LA, Jones J. Comparing family accommodation in pediatric obsessive-compulsive disorder, anxiety disorders, and nonanxious children. Depression and Anxiety. 2014;31:1018–1025. doi: 10.1002/da.22251. [DOI] [PubMed] [Google Scholar]
- Lebowitz ER, Woolston J, Bar-Haim Y, Calvocoressi L, Dauser C, Warnick E, … Leckman JF. Family accommodation in pediatric anxiety disorders. Depression and Anxiety. 2013;30:47–54. doi: 10.1002/da.21998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE. Anxious. Using the brain to understand and treat fear and anxiety. NY: Viking; 2015. [Google Scholar]
- Leger KA, Charles ST, Turiano NA, Almeida DM. Personality and stressor-related affect. Journal of Personality & Social Psychology. doi: 10.1037/pspp0000083. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenaert B, Boddez Y, Griffith JW, Vervliet B, Schruers K, Hermans D. Aversive learning and generalization predict subclinical levels of anxiety: a six-month longitudinal study. Journal of Anxiety Disorders. 2014;28(8):747–753. doi: 10.1016/j.janxdis.2014.09.006. [DOI] [PubMed] [Google Scholar]
- Levenson RW. What is the added value of studying the brain for understanding emotion? In: Fox AS, Lapate RC, Shackman AJ, Davidson RJ, editors. The nature of emotion. Fundamental questions. 2. NY: Oxford University Press; in press. [Google Scholar]
- Leventhal AM, Japuntich SJ, Piper ME, Jorenby DE, Schlam TR, Baker TB. Isolating the role of psychological dysfunction in smoking cessation: relations of personality and psychopathology to attaining cessation milestones. Psychol Addict Behav. 2012;26:838–849. doi: 10.1037/a0028449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Xiao E, Houser D, Montague PR. Neural responses to sanction threats in two-party economic exchange. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:16835–16840. doi: 10.1073/pnas.0908855106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman L, Liu H, Huggins AA, Katz AC, Zvolensky MJ, Shankman SA. Comparing the validity of informant and self-reports of personality using laboratory indices of emotional responding as criterion variables. Psychophysiology. doi: 10.1111/psyp.12680. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim SL, Padmala S, Pessoa L. Segregating the significant from the mundane on a moment-to-moment basis via direct and indirect amygdala contributions. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:16841–16846. doi: 10.1073/pnas.0904551106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linetzky M, Pergamin-Hight L, Pine DS, Bar-Haim Y. Quantitative evaluation of the clinical efficacy of attention bias modification treatment for anxiety disorders. Depression and Anxiety. 2015;32:383–391. doi: 10.1002/da.22344. [DOI] [PubMed] [Google Scholar]
- Lissek S. Toward an account of clinical anxiety predicated on basic, neurally mapped mechanisms of Pavlovian fear-learning: the case for conditioned overgeneralization. Depression and Anxiety. 2012;29:257–263. doi: 10.1002/da.21922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissek S, Bradford DE, Alvarez RP, Burton P, Espensen-Sturges T, Reynolds RC, Grillon C. Neural substrates of classically conditioned fear-generalization in humans: a parametric fMRI study. Soc Cogn Affect Neurosci. 2014;9:1134–1142. doi: 10.1093/scan/nst096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RT, Alloy LB. Stress generation in depression: A systematic review of the empirical literature and recommendations for future study. Clinical Psychology Review. 2010;30:582–593. doi: 10.1016/j.cpr.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RT, Kraines MA, Massing-Schaffer M, Alloy LB. Rejection sensitivity and depression: mediation by stress generation. Psychiatry. 2014;77(1):86–97. doi: 10.1521/psyc.2014.77.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis NK. What we can do and what we cannot do with fMRI. Nature. 2008;453:869–878. doi: 10.1038/nature06976. [DOI] [PubMed] [Google Scholar]
- Lopez RB, Hofmann W, Wagner DD, Kelley WM, Heatherton TF. Neural predictors of giving in to temptation in daily life. Psychol Sci. 2014;25(7):1337–1344. doi: 10.1177/0956797614531492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luck SJ. Ten simple rules for designing ERP experiments. In: Handy TC, editor. Event-related potentials: A methods handbook. Cambridge, MA: MIT Press; 2005. pp. 17–32. [Google Scholar]
- Ludtke O, Roberts BW, Trautwein U, Nagy G. A random walk down university avenue: life paths, life events, and personality trait change at the transition to university life. Journal of Personality and Social Psychology. 2011;101:620–637. doi: 10.1037/a0023743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lueken U, Muehlhan M, Evens R, Wittchen HU, Kirschbaum C. Within and between session changes in subjective and neuroendocrine stress parameters during magnetic resonance imaging: A controlled scanner training study. Psychoneuroendocrinology. 2012;37:1299–1308. doi: 10.1016/j.psyneuen.2012.01.003. [DOI] [PubMed] [Google Scholar]
- Lueken U, Zierhut KC, Hahn T, Straube B, Kircher T, Reif A, … Domschke K. Neurobiological markers predicting treatment response in anxiety disorders: A systematic review and implications for clinical application. Neuroscience and Biobehavioral Reviews. 2016;66:143–162. doi: 10.1016/j.neubiorev.2016.04.005. [DOI] [PubMed] [Google Scholar]
- Luyten L, Casteels C, Vansteenwegen D, van Kuyck K, Koole M, Van Laere K, Nuttin B. Micro-positron emission tomography imaging of rat brain metabolism during expression of contextual conditioning. Journal of Neuroscience. 2012;32:254–263. doi: 10.1523/JNEUROSCI.3701-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado CJ, Snyder AZ, Cherry SR, Lavenex P, Amaral DG. Effects of neonatal amygdala or hippocampus lesions on resting brain metabolism in the macaque monkey: a microPET imaging study. Neuroimage. 2008;39:832–846. doi: 10.1016/j.neuroimage.2007.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod C, Clarke PJF. The attentional bias modification approach to anxiety intervention. Clinical Psychological Science. 2015;3:58–78. [Google Scholar]
- MacLeod C, Mathews A. Cognitive bias modification approaches to anxiety. Annu Rev Clin Psychol. 2012;8:189–217. doi: 10.1146/annurev-clinpsy-032511-143052. [DOI] [PubMed] [Google Scholar]
- Magnus K, Diener E, Fujita F, Pavot W. Extraversion and neuroticism as predictors of objective life events: A longitudinal analysis. Journal of Personality and Social Psychology. 1993;65:1046–1053. doi: 10.1037//0022-3514.65.5.1046. [DOI] [PubMed] [Google Scholar]
- Mai JK, Paxinos G, Voss T. Atlas of the human brain. 3. San Diego, CA: Academic Press; 2007. [Google Scholar]
- Malouff JM, Thorsteinsson EB, Rooke SE, Schutte NS. Alcohol involvement and the Five-Factor model of personality: a meta-analysis. Journal of Drug Education. 2007;37:277–294. doi: 10.2190/DE.37.3.d. [DOI] [PubMed] [Google Scholar]
- Malouff JM, Thorsteinsson EB, Schutte NS, Bhullar N, Rooke SE. The five-factor model of personality and relationship satisfaction of intimate partners: A meta-analysis. Journal of Research in Personality. 2010;44:124–127. [Google Scholar]
- Marco CA, Suls J. Daily stress and the trajectory of mood: spillover, response assimilation, contrast, and chronic negative affectivity. Journal of Personality and Social Psychology. 1993;64:1053–1063. doi: 10.1037//0022-3514.64.6.1053. [DOI] [PubMed] [Google Scholar]
- Markon KE, Krueger RF, Watson D. Delineating the structure of normal and abnormal personality: an integrative hierarchical approach. Journal of Personality and Social Psychology. 2005;88:139–157. doi: 10.1037/0022-3514.88.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason WA, Capitanio JP, Machado CJ, Mendoza SP, Amaral DG. Amygdalectomy and responsiveness to novelty in rhesus monkeys (Macaca mulatta): generality and individual consistency of effects. Emotion. 2006;6:73–81. doi: 10.1037/1528-3542.6.1.73. [DOI] [PubMed] [Google Scholar]
- Matthews G, Deary IJ, Whiteman MC. Stable traits and transient states. In: Matthews G, Deary IJ, Whiteman MC, editors. Personality traits. 3. NY: Cambridge University Press; 2009. pp. 85–120. [Google Scholar]
- McCrae RR, Costa PT. Adding liebe und arbeit: The full five-factor model and well-being. Personality and Social Psychology Bulletin. 1991;17:227–232. [Google Scholar]
- McCrae RR, Costa PT., Jr Validation of the five-factor model of personality across instruments and observers. Journal of Personality and Social Psychology. 1987;52:81–90. doi: 10.1037//0022-3514.52.1.81. [DOI] [PubMed] [Google Scholar]
- McLaughlin KA, Busso DS, Duys A, Green JG, Alves S, Way M, Sheridan MA. Amygdala response to negative stimuli predicts PTSD symptom onset following a terrorist attack. Depression and Anxiety. 2014;31:834–842. doi: 10.1002/da.22284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMenamin BW, Langeslag SJ, Sirbu M, Padmala S, Pessoa L. Network organization unfolds over time during periods of anxious anticipation. Journal of Neuroscience. 2014;34:11261–11273. doi: 10.1523/JNEUROSCI.1579-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNulty JK. Neuroticism and interpersonal negativity: the independent contributions of perceptions and behaviors. Personality and Social Psychology Bulletin. 2008;34:1439–1450. doi: 10.1177/0146167208322558. [DOI] [PubMed] [Google Scholar]
- Mehl MR, Gosling SD, Pennebaker JW. Personality in its natural habitat: manifestations and implicit folk theories of personality in daily life. Journal of Personality and Social Psychology. 2006;90:862–877. doi: 10.1037/0022-3514.90.5.862. [DOI] [PubMed] [Google Scholar]
- Melendez JC, McCrank E. Anxiety-related reactions associated with magnetic resonance imaging examinations. Journal of the American Medical Association. 1993;270:745–747. doi: 10.1001/jama.1993.03510060091039. [DOI] [PubMed] [Google Scholar]
- Metz M, Majdandzic M, Bogels S. Concurrent and predictive associations between infants’ and toddlers’ fearful temperament, coparenting, and parental anxiety disorders. Journal of Clinical Child and Adolescent Psychology. doi: 10.1080/15374416.2015.1121823. in press. [DOI] [PubMed] [Google Scholar]
- Middeldorp CM, Cath DC, Beem AL, Willemsen G, Boomsma DI. Life events, anxious depression and personality: a prospective and genetic study. Psychological Medicine. 2008;38(11):1557–1565. doi: 10.1017/S0033291708002985. [DOI] [PubMed] [Google Scholar]
- Mihalopoulos C, Vos T, Rapee RM, Pirkis J, Chatterton ML, Lee YC, Carter R. The population cost-effectiveness of a parenting intervention designed to prevent anxiety disorders in children. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2015;56:1026–1033. doi: 10.1111/jcpp.12438. [DOI] [PubMed] [Google Scholar]
- Miles L, Davis M, Walker D. Phasic and sustained fear are pharmacologically dissociable in rats. Neuropsychopharmacology. 2011;36:1563–1574. doi: 10.1038/npp.2011.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobbs D, Hagan CC, Dalgleish T, Silston B, Prevost C. The ecology of human fear: survival optimization and the nervous system. Front Neurosci. 2015;9:55. doi: 10.3389/fnins.2015.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobbs D, Kim JJ. Neuroethological studies of fear, anxiety, and risky decision-making in rodents and humans. Current Opinion in Behavioral Sciences. 2015;5:8–15. doi: 10.1016/j.cobeha.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobbs D, Yu R, Rowe JB, Eich H, FeldmanHall O, Dalgleish T. Neural activity associated with monitoring the oscillating threat value of a tarantula. Proceedings of the National Acadademy of Sciences USA. 2010;107:20582–20586. doi: 10.1073/pnas.1009076107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moberg CA, Curtin JJ. Alcohol selectively reduces anxiety but not fear: Startle response during unpredictable vs. predictable threat. J Abnormal Psychol. 2009;118:335–347. doi: 10.1037/a0015636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffitt TE, Poulton R, Caspi A. Lifelong impact of early self-control. American Scientist. 2013;101:352–359. [Google Scholar]
- Monroe SM, Simons AD. Diathesis-stress theories in the context of life stress research: implications for the depressive disorders. Psychological Bulletin. 1991;110(3):406–425. doi: 10.1037/0033-2909.110.3.406. [DOI] [PubMed] [Google Scholar]
- Motzkin JC, Philippi CL, Oler JA, Kalin NH, Baskaya MK, Koenigs M. Ventromedial prefrontal cortex damage alters resting blood flow to the bed nucleus of stria terminalis. Cortex. 2015;64:281–288. doi: 10.1016/j.cortex.2014.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motzkin JC, Philippi CL, Wolf RC, Baskaya MK, Koenigs M. Ventromedial prefrontal cortex lesions alter neural and physiological correlates of anticipation. Journal of Neuroscience. 2014;34(31):10430–10437. doi: 10.1523/JNEUROSCI.1446-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motzkin JC, Philippi CL, Wolf RC, Baskaya MK, Koenigs M. Ventromedial prefrontal cortex is critical for the regulation of amygdala activity in humans. Biological Psychiatry. 2015;77(3):276–284. doi: 10.1016/j.biopsych.2014.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mroczek DK, Almeida DM. The effect of daily stress, personality, and age on daily negative affect. Journal of Personality. 2004;72:355–378. doi: 10.1111/j.0022-3506.2004.00265.x. [DOI] [PubMed] [Google Scholar]
- Muehlhan M, Lueken U, Siegert J, Wittchen HU, Smolka MN, Kirschbaum C. Enhanced sympathetic arousal in response to FMRI scanning correlates with task induced activations and deactivations. PLoS ONE. 2013;8:e72576. doi: 10.1371/journal.pone.0072576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mund M, Neyer FJ. The winding paths of the lonesome cowboy: Evidence for mutual influences between personality, subjective health, and loneliness. J Personality. doi: 10.1111/jopy.12188. in press. [DOI] [PubMed] [Google Scholar]
- Munsterkotter AL, Notzon S, Redlich R, Grotegerd D, Dohm K, Arolt V, … Dannlowski U. Spider or no spider? Neural correlates of sustained and phasic fear in spider phobia. Depression and Anxiety. doi: 10.1002/da.22382. in press. [DOI] [PubMed] [Google Scholar]
- Nacewicz BM, Dalton KM, Johnstone T, Long MT, McAuliff EM, Oakes TR, … Davidson RJ. Amygdala Volume and Nonverbal Social Impairment in Adolescent and Adult Males With Autism. Archives of General Psychiatry. 2006;63:1417–1428. doi: 10.1001/archpsyc.63.12.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson RJ, Trainor BC. Neural mechanisms of aggression. Nature Reviews Neuroscience. 2007;8:536–546. doi: 10.1038/nrn2174. [DOI] [PubMed] [Google Scholar]
- Nettle D, Bateson M. The evolutionary origins of mood and its disorders. Current Biology. 2012;22:R712–721. doi: 10.1016/j.cub.2012.06.020. [DOI] [PubMed] [Google Scholar]
- Newman MG, Fisher AJ. Mediated moderation in combined cognitive behavioral therapy versus component treatments for generalized anxiety disorder. Journal of Consulting and Clinical Psychology. 2013;81:405–414. doi: 10.1037/a0031690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neyer FJ, Asendorpf JB. Personality-relationship transaction in young adulthood. Journal of Personality and Social Psychology. 2001;81:1190–1204. [PubMed] [Google Scholar]
- Neyer FJ, Voigt D. Personality and social network effects on romantic relationships: A dyadic approach. European Journal of Personality. 2004;18:279–299. [Google Scholar]
- Ng TWH, Eby LT, Sorensen KL, Feldman DC. Predictors of objective and subjective career success. A meta-analysis. Personnel Psychology. 2005;58:367–408. [Google Scholar]
- Nolan SA, Roberts JE, Gotlib IH. Neuroticism and ruminative response style as predictors of change in depressive symptomatology. Cognitive Therapy and Research. 1998;22:445–455. [Google Scholar]
- Nolen-Hoeksema S, Wisco BE, Lyubomirsky S. Rethinking rumination. Perspectives on Psychological Science. 2008;3:400–424. doi: 10.1111/j.1745-6924.2008.00088.x. [DOI] [PubMed] [Google Scholar]
- Norris CJ, Larsen JT, Cacioppo JT. Neuroticism is associated with larger and more prolonged electrodermal responses to emotionally evocative pictures. Psychophysiology. 2007;44:823–826. doi: 10.1111/j.1469-8986.2007.00551.x. [DOI] [PubMed] [Google Scholar]
- Nusslock R, Shackman AJ, Harmon-Jones E, Alloy LB, Coan JA, Abramson LY. Cognitive vulnerability and frontal brain asymmetry: common predictors of first prospective depressive episode. Journal of Abnormal Psychology. 2011;120(2):497–503. doi: 10.1037/a0022940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okbay A, Baselmans BM, De Neve JE, Turley P, Nivard MG, Fontana MA, … Cesarini D. Genetic variants associated with subjective well-being, depressive symptoms, and neuroticism identified through genome-wide analyses. Nature Genetics. doi: 10.1038/ng.3552. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okon-Singer H, Hendler T, Pessoa L, Shackman AJ. The neurobiology of emotion-cognition interactions: Fundamental questions and strategies for future research. Frontiers in Human Neuroscience. 2015;9 doi: 10.3389/fnhum.2015.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olderbak S, Hildebrandt A, Pinkpank T, Sommer W, Wilhelm O. Psychometric challenges and proposed solutions when scoring facial emotion expression codes. Behav Res Methods. 2014;46:992–1006. doi: 10.3758/s13428-013-0421-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oler JA, Fox AS, Shackman AJ, Kalin NH. The central nucleus of the amygdala is a critical substrate for individual differences in anxiety. In: Amaral DG, Adolphs R, editors. Living without an amygdala. NY: Guilford; 2016. [Google Scholar]
- Oler JA, Fox AS, Shelton SE, Christian BT, Murali D, Oakes TR, … Kalin NH. Serotonin transporter availability in the amygdala and bed nucleus of the stria terminalis predicts anxious temperament and brain glucose metabolic activity. Journal of Neuroscience. 2009;29:9961–9966. doi: 10.1523/JNEUROSCI.0795-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oler JA, Fox AS, Shelton SE, Rogers J, Dyer TD, Davidson RJ, … Kalin NH. Amygdalar and hippocampal substrates of anxious temperament differ in their heritability. Nature. 2010;466:864–868. doi: 10.1038/nature09282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onnela JP, Rauch SL. Harnessing smartphone-based digital phenotyping to enhance behavioral and mental health. Neuropsychopharmacology. 2016;41:1691–1696. doi: 10.1038/npp.2016.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ormel J, Jeronimus BF, Kotov R, Riese H, Bos EH, Hankin B, … Oldehinkel AJ. Neuroticism and common mental disorders: meaning and utility of a complex relationship. Clinical Psychology Review. 2013;33:686–697. doi: 10.1016/j.cpr.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ormel J, Oldehinkel AJ, Vollebergh W. Vulnerability before, during, and after a major depressive episode: a 3-wave population-based study. Archives of General Psychiatry. 2004;61:990–996. doi: 10.1001/archpsyc.61.10.990. [DOI] [PubMed] [Google Scholar]
- Ormel J, Wohlfarth T. How neuroticism, long-term difficulties, and life situation change influence psychological distress: A longitudinal model. Journal of Personality and Social Psychology. 1991;60:744–755. doi: 10.1037//0022-3514.60.5.744. [DOI] [PubMed] [Google Scholar]
- Parker PD, Ludtke O, Trautwein U, Roberts BW. Personality and relationship quality during the transition from high school to early adulthood. Journal of Personality. 2012;80:1061–1089. doi: 10.1111/j.1467-6494.2012.00766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasch LA, Bradbury TN, Davila J. Gender, negative affectivity, and observed social support behavior in marital interaction. Personal Relationships. 1997;4:361–378. [Google Scholar]
- Paulus MP, Feinstein JS, Castillo G, Simmons AN, Stein MB. Dose-dependent decrease of activation in bilateral amygdala and insula by lorazepam during emotion processing. Archives of General Psychiatry. 2005;62:282–288. doi: 10.1001/archpsyc.62.3.282. [DOI] [PubMed] [Google Scholar]
- Peeters F, Nicolson NA, Berkhof J, Delespaul P, deVries M. Effects of daily events on mood states in major depressive disorder. Journal of Abnormal Psychology. 2003;112(2):203–211. doi: 10.1037/0021-843x.112.2.203. [DOI] [PubMed] [Google Scholar]
- Perez-Edgar K, Bar-Haim Y, McDermott JM, Chronis-Tuscano A, Pine DS, Fox NA. Attention biases to threat and behavioral inhibition in early childhood shape adolescent social withdrawal. Emotion. 2010;10:349–357. doi: 10.1037/a0018486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Edgar K, McDermott JN, Korelitz K, Degnan KA, Curby TW, Pine DS, Fox NA. Patterns of sustained attention in infancy shape the developmental trajectory of social behavior from toddlerhood through adolescence. Developmental Psychology. 2010;46:1723–1730. doi: 10.1037/a0021064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Edgar K, Reeb-Sutherland BC, McDermott JM, White LK, Henderson HA, Degnan KA, … Fox NA. Attention biases to threat link behavioral inhibition to social withdrawal over time in very young children. Journal of Abnormal Child Psychology. 2011;39(6):885–895. doi: 10.1007/s10802-011-9495-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perusini JN, Fanselow MS. Neurobehavioral perspectives on the distinction between fear and anxiety. Learning and Memory. 2015;22:417–425. doi: 10.1101/lm.039180.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen SE, Sporns O. Brain networks and cognitive architectures. Neuron. 2015;88:207–219. doi: 10.1016/j.neuron.2015.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer UJ, Vogeley K, Schilbach L. From gaze cueing to dual eye-tracking: novel approaches to investigate the neural correlates of gaze in social interaction. Neuroscience and Biobehavioral Reviews. 2013;37:2516–2528. doi: 10.1016/j.neubiorev.2013.07.017. [DOI] [PubMed] [Google Scholar]
- Phan KL, Coccaro EF, Angstadt M, Kreger KJ, Mayberg HS, Liberzon I, Stein MB. Corticolimbic brain reactivity to social signals of threat before and after sertraline treatment in generalized social phobia. Biological Psychiatry. 2013;73(4):329–336. doi: 10.1016/j.biopsych.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips AC, Batty GD, Weiss A, Deary I, Gale CR, Thomas GN, Carroll D. Neuroticism, cognitive ability, and the metabolic syndrome: The Vietnam Experience Study. Journal of Psychosomatic Research. 2010;69(2):193–201. doi: 10.1016/j.jpsychores.2010.01.016. [DOI] [PubMed] [Google Scholar]
- Pichon S, Miendlarzewska EA, Eryilmaz H, Vuilleumier P. Cumulative activation during positive and negative events and state anxiety predicts subsequent inertia of amygdala reactivity. Soc Cogn Affect Neurosci. 2015;10:180–190. doi: 10.1093/scan/nsu044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA. Depression, stress, and anhedonia: toward a synthesis and integrated model. Annu Rev Clin Psychol. 2014;10:393–423. doi: 10.1146/annurev-clinpsy-050212-185606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plasencia ML, Alden LE, Taylor CT. Differential effects of safety behaviour subtypes in social anxiety disorder. Behaviour Research and Therapy. 2011;49:665–675. doi: 10.1016/j.brat.2011.07.005. [DOI] [PubMed] [Google Scholar]
- Polderman TJ, Benyamin B, de Leeuw CA, Sullivan PF, van Bochoven A, Visscher PM, Posthuma D. Meta-analysis of the heritability of human traits based on fifty years of twin studies. Nature Genetics. doi: 10.1038/ng.3285. in press. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Baker CI, Durnez J, Gorgolewski KJ, Matthews PM, Munafo M, … Yarkoni T. Scanning the horizon: Future challenges for neuroimaging research. bioRxiv. 2016 doi: 10.1038/nrn.2016.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak SD, Kistler DJ. Early experience is associated with the development of categorical representations for facial expressions of emotion. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:9072–9076. doi: 10.1073/pnas.142165999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power RA, Pluess M. Heritability estimates of the Big Five personality traits based on common genetic variants. Transl Psychiatry. 2015;5:e604. doi: 10.1038/tp.2015.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power RA, Wingenbach T, Cohen-Woods S, Uher R, Ng MY, Butler AW, … McGuffin P. Estimating the heritability of reporting stressful life events captured by common genetic variants. Psychological Medicine. 2013;43:1965–1971. doi: 10.1017/S0033291712002589. [DOI] [PubMed] [Google Scholar]
- Pressman SD, Cohen S, Miller GE, Barkin A, Rabin BS, Treanor JJ. Loneliness, social network size, and immune response to influenza vaccination in college freshmen. Health Psychology. 2005;24:297–306. doi: 10.1037/0278-6133.24.3.297. [DOI] [PubMed] [Google Scholar]
- Preuss TM. Primate brain evolution in phylogenetic context. In: Kaas JH, Preuss TM, editors. Evolution of Nervous Sytems. Vol. 4. NY: Elsevier; 2007. pp. 3–34. [Google Scholar]
- Prinzie P, Stams GJJM, Dekovic M, Reijntjes AHA, Belsky J. The relations between parents? Big Five personality factors and parenting: A meta-analytic review. Journal of Personality and Social Psychology. 2009;97:351–362. doi: 10.1037/a0015823. [DOI] [PubMed] [Google Scholar]
- Quilty LC, De Fruyt F, Rolland JP, Kennedy SH, Rouillon PF, Bagby RM. Dimensional personality traits and treatment outcome in patients with major depressive disorder. Journal of Affective Disorders. 2008;108:241–250. doi: 10.1016/j.jad.2007.10.022. [DOI] [PubMed] [Google Scholar]
- Reeb-Sutherland BC, Helfinstein SM, Degnan KA, Perez-Edgar K, Henderson HA, Lissek S, … Fox NA. Startle response in behaviorally inhibited adolescents with a lifetime occurrence of anxiety disorders. J Am Acad Child Adolesc Psychiatry. 2009;48:610–617. doi: 10.1097/CHI.0b013e31819f70fb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhart RM, Woodman GF. Causal control of medial-frontal cortex governs electrophysiological and behavioral indices of performance monitoring and learning. Journal of Neuroscience. 2014;34(12):4214–4227. doi: 10.1523/JNEUROSCI.5421-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss S. Trait anxiety: it’s not what you think it is. Journal of Anxiety Disorders. 1997;11:201–214. doi: 10.1016/s0887-6185(97)00006-6. [DOI] [PubMed] [Google Scholar]
- Reuter M, Cooper AJ, Smillie LD, Markett S, Montag C. A new measure for the revised reinforcement sensitivity theory: psychometric criteria and genetic validation. Front Syst Neurosci. 2015;9:38. doi: 10.3389/fnsys.2015.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reznick JS, Kagan J, Snidman N, Gersten M, Baak K, Rosenberg A. Inhibited and uninhibited children: A follow-up study. Child Development. 1986;57:660–680. [Google Scholar]
- Roberts BW, Caspi A, Moffitt TE. Work experiences and personality development in young adulthood. Journal of Personality and Social Psychology. 2003;84:582–593. [PubMed] [Google Scholar]
- Roberts BW, DelVecchio WF. The rank-order consistency of personality traits from childhood to old age: a quantitative review of longitudinal studies. Psychological Bulletin. 2000;126:3–25. doi: 10.1037/0033-2909.126.1.3. [DOI] [PubMed] [Google Scholar]
- Roberts BW, Kuncel NR, Shiner R, Caspi A, Goldberg LR. The power of personality. The comparative validity of personality traits, socioeconomic status, and cognitive ability for predicting important life outcomes. Perspect Psychol Sci. 2007;2:313–345. doi: 10.1111/j.1745-6916.2007.00047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts BW, Mroczek D. Personality trait change in adulthood. Curr Dir Psychol Sci. 2008;17:31–35. doi: 10.1111/j.1467-8721.2008.00543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts BW, Walton KE, Viechtbauer W. Patterns of mean-level change in personality traits across the life course: a meta-analysis of longitudinal studies. Psychological Bulletin. 2006;132:1–25. doi: 10.1037/0033-2909.132.1.1. [DOI] [PubMed] [Google Scholar]
- Robins RW, Caspi A, Moffitt TE. It’s not just who you’re with, it’s who you are: Personality and relationship experiences across multiple relationships. Journal of Personality. 2002;70:925–964. doi: 10.1111/1467-6494.05028. [DOI] [PubMed] [Google Scholar]
- Rogers J, Raveendran M, Fawcett GL, Fox AS, Shelton SE, Oler JA, … Kalin NH. CRHR1 genotypes, neural circuits and the diathesis for anxiety and depression. Molecular Psychiatry. 2013;18:700–707. doi: 10.1038/mp.2012.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Canyas R, Downey G, Berenson K, Ayduk O, JK Rejection sensitivity and the rejection-hostility link in romantic relationships. Journal of Personality. 2010;78:119–148. doi: 10.1111/j.1467-6494.2009.00611.x. [DOI] [PubMed] [Google Scholar]
- Roseboom PH, Nanda SA, Fox AS, Oler JA, Shackman AJ, Shelton SE, … Kalin NH. Neuropeptide Y receptor gene expression in the primate amygdala predicts anxious temperament and brain metabolism. Biological Psychiatry. 2014;76:850–857. doi: 10.1016/j.biopsych.2013.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen JB, Schulkin J. From normal fear to pathological anxiety. Psychological Review. 1998;105:325–350. doi: 10.1037/0033-295x.105.2.325. [DOI] [PubMed] [Google Scholar]
- Rubin K, Bowker J, Gazelle H. Social withdrawal in childhood and adolescence: Peer relationships and social competence. In: Rubin KH, Coplan RJ, editors. The development of shyness and social withdrawal. NY: Guilford; 2010. pp. 131–154. [Google Scholar]
- Rubin KH, Asendorpf J. Social withdrawal, inhibition, and shyness in childhood: Conceptual and definitional issues. In: Rubin KH, Asendorpf JB, editors. Social withdrawal, inhibition and shyness in children. Hillsdale, NJ: Erlbaum; 1993. pp. 3–17. [Google Scholar]
- Rubin KH, Wojslawowicz JC, Rose-Krasnor L, Booth-LaForce C, Burgess KB. The best friendships of shy/withdrawn children: Prevalence, stability, and relationship quality. Journal of Abnormal Child Psychology. 2006;34:143–157. doi: 10.1007/s10802-005-9017-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudebeck PH, Saunders RC, Prescott AT, Chau LS, Murray EA. Prefrontal mechanisms of behavioral flexibility, emotion regulation and value updating. Nature Neuroscience. 2013;16:1140–1145. doi: 10.1038/nn.3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano A, Phillips AJ, Yu AZ, McHill AW, Taylor S, Jaques N, … Picard RW. Recognizing academic performance, sleep quality, stress level, and mental health using personality traits, wearable sensors and mobile phones. Paper presented at the 12th International IEEE Conference on Wearable and Implantable Body Sensor Networks; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satpute AB, Wager TD, Cohen-Adad J, Bianciardi M, Choi JK, Buhle JT, … Barrett LF. Identification of discrete functional subregions of the human periaqueductal gray. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:17101–17106. doi: 10.1073/pnas.1306095110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saudino KJ, Pedersen NL, Lichtenstein P, McClearn GE, Plomin R. Can personality explain genetic influences on life events? Journal of Personality and Social Psychology. 1997;72:196–206. doi: 10.1037//0022-3514.72.1.196. [DOI] [PubMed] [Google Scholar]
- Scherer KR. Studying emotion empirically: Issues and a paradigm for research. In: Scherer KR, Wallbott HG, Summerfield AB, editors. Experiencing emotion: A cross-cultural study. NY: Cambridge University Press; 1986. pp. 1–27. [Google Scholar]
- Schmidt LA, Fox NA. Fear-potentiated startle responses in temperamentally different human infants. Developmental Psychobiology. 1998;32:113–120. doi: 10.1002/(sici)1098-2302(199803)32:2<113::aid-dev4>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Schmitt DP, Realo A, Voracek M, Allik J. Why can’t a man be more like a woman? Sex differences in Big Five personality traits across 55 cultures. Journal of Personality and Social Psychology. 2008;94:168–182. doi: 10.1037/0022-3514.94.1.168. [DOI] [PubMed] [Google Scholar]
- Schnyer DM, Beevers CG, deBettencourt MT, Sherman SM, Cohen JD, Norman KA, Turk-Browne NB. Neurocognitive therapeutics: from concept to application in the treatment of negative attention bias. Biol Mood Anxiety Disord. 2015;5:1. doi: 10.1186/s13587-015-0016-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuyler BS, Kral TR, Jacquart J, Burghy CA, Weng HY, Perlman DM, … Davidson RJ. Temporal dynamics of emotional responding: amygdala recovery predicts emotional traits. Soc Cogn Affect Neurosci. 2012;9:176–181. doi: 10.1093/scan/nss131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J, Williams JM, Brittlebank A, Ferrier IN. The relationship between premorbid neuroticism, cognitive dysfunction and persistence of depression: a 1-year follow-up. Journal of Affective Disorders. 1995;33:167–172. doi: 10.1016/0165-0327(94)00085-n. [DOI] [PubMed] [Google Scholar]
- Seo D, Tsou KA, Ansell EB, Potenza MN, Sinha R. Cumulative adversity sensitizes neural response to acute stress: association with health symptoms. Neuropsychopharmacology. 2014;39:670–680. doi: 10.1038/npp.2013.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servaas MN, Geerligs L, Renken RJ, Marsman JB, Ormel J, Riese H, Aleman A. Connectomics and neuroticism: An altered functional network organization. Neuropsychopharmacology. 2014;40:296–304. doi: 10.1038/npp.2014.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaban H, Humeau Y, Herry C, Cassasus G, Shigemoto R, Ciocchi S, … Luthi A. Generalization of amygdala LTP and conditioned fear in the absence of presynaptic inhibition. Nature Neuroscience. 2006;9(8):1028–1035. doi: 10.1038/nn1732. [DOI] [PubMed] [Google Scholar]
- Shackman AJ, Fox AS. Contributions of the central extended amygdala to fear and anxiety. Journal of Neuroscience. doi: 10.1523/JNEUROSCI.0982-16.2016. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman AJ, Fox AS, Oler JA, Shelton SE, Davidson RJ, Kalin NH. Neural mechanisms underlying heterogeneity in the presentation of anxious temperament. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:6145–6150. doi: 10.1073/pnas.1214364110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman AJ, Fox AS, Oler JA, Shelton SE, Oakes TR, Davidson RJ, Kalin NH. Heightened extended amygdala metabolism following threat characterizes the early phenotypic risk to develop anxiety-related psychopathology. Molecular Psychiatry. doi: 10.1038/mp.2016.132. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman AJ, Fox AS, Seminowicz DA. The cognitive-emotional brain: Opportunities and challenges for understanding neuropsychiatric disorders. Behavioral and Brain Sciences. 2015;38:e86. doi: 10.1017/S0140525X14001010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman AJ, Kaplan CM, Stockbridge MD, Tillman RM, Tromp DPM, Fox AS, Gamer M. The neurobiology of anxiety and attentional biases to threat: Implications for understanding anxiety disorders in adults and youth. Journal of Experimental Psychopathology. doi: 10.5127/jep.054015. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman AJ, McMenamin BW, Maxwell JS, Greischar LL, Davidson RJ. Right dorsolateral prefrontal cortical activity and behavioral inhibition. Psychological Science. 2009;20:1500–1506. doi: 10.1111/j.1467-9280.2009.02476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ, Davidson RJ. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nature Reviews Neuroscience. 2011;12:154–167. doi: 10.1038/nrn2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman AJ, Sarinopoulos I, Maxwell JS, Pizzagalli DA, Lavric A, Davidson RJ. Anxiety selectively disrupts visuospatial working memory. Emotion. 2006;6:40–61. doi: 10.1037/1528-3542.6.1.40. [DOI] [PubMed] [Google Scholar]
- Shackman AJ, Stockbridge MD, LeMay EP, Fox AS. The psychological and neurobiological bases of dispositional negativity. In: Fox AS, Lapate RC, Shackman AJ, Davidson RJ, editors. The nature of emotion. Fundamental questions. 2. NY: Oxford University Press; in press. [Google Scholar]
- Shamir-Essakow G, Ungerer J, Rapee R, Safier R. Caregiving representations of mothers of behaviorally inhibited and uninhibited preschool children. Developmental Psychology. 2004;40:899–910. doi: 10.1037/0012-1649.40.6.899. [DOI] [PubMed] [Google Scholar]
- Shanahan MJ, Bauldry S, Roberts BW, Macmillan R, Russo R. Personality and the reproduction of social class. Social Forces. 2014;93:209–240. [Google Scholar]
- Sharot T, Riccardi AM, Raio CM, Phelps EA. Neural mechanisms mediating optimism bias. Nature. 2007;450:102–105. doi: 10.1038/nature06280. [DOI] [PubMed] [Google Scholar]
- Shechner T, Wakschlag N, Britton JC, Jarcho J, Ernst M, Pine DS. Empirical examination of the potential adverse psychological effects associated with pediatric FMRI scanning. Journal of Child and Adolescent Psychopharmacology. 2013;23:357–362. doi: 10.1089/cap.2012.0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Barch DM, Donnelly JM, Ollinger JM, Snyder AZ, Mintun MA. Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: an fMRI study. Biological Psychiatry. 2001;50(9):651–658. doi: 10.1016/s0006-3223(01)01263-x. [DOI] [PubMed] [Google Scholar]
- Shiba Y, Santangelo AM, Braesicke K, Agustin-Pavon C, Cockcroft G, Haggard M, Roberts AC. Individual differences in behavioral and cardiovascular reactivity to emotive stimuli and their relationship to cognitive flexibility in a primate model of trait anxiety. Front Behav Neurosci. 2014;8:137. doi: 10.3389/fnbeh.2014.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiner RL. Personality as lasting individual differences in emotions. In: Fox AS, Lapate RC, Shackman AJ, Davidson RJ, editors. The nature of emotion. Fundamental questions. 2. NY: Oxford University Press; in press-a. [Google Scholar]
- Shiner RL. What develops in emotional development? In: Fox AS, Lapate RC, Shackman AJ, Davidson RJ, editors. The nature of emotion. Fundamental questions. 2. NY: Oxford University Press; in press-b. [Google Scholar]
- Shiner RL, Buss KA, McClowry S, Putnam S, Saudino K, Zentner M. What is temperament now? Assessing progress in temperament research on the twenty-fifth anniversary of Goldsmith et al. (1987) Child Development Perspectives. 2012;6:436–444. [Google Scholar]
- Smit DJA, Posthuma D, Boomsma DI, De Geus EJ. The relation between frontal EEG asymmetry and the risk for anxiety and depression. Biological Psychology. 2007;74(1):26–33. doi: 10.1016/j.biopsycho.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Smith DJ, Escott-Price V, Davies G, Bailey MES, Conde LC, Ward J, … O’Donovan M. Genome-wide analysis of over 106, 000 individuals identifies 9 neuroticism-associated loci. bioRxiv. 2015 doi: 10.1038/mp.2016.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder DK, Halford WK. Evidence-based couple therapy: current status and future directions. Journal of Family Therapy. 2012;34:229–249. [Google Scholar]
- Soldz S, Vaillant GE. The big five personaliy traits and the life course: A 45-year longitudinal study. Journal of Research in Personality. 1999;33:208–232. [Google Scholar]
- Solomon BC, Jackson JJ. Why do personality traits predict divorce? Multiple pathways through satisfaction. Journal of Personality and Social Psychology. 2014;106:978–996. doi: 10.1037/a0036190. [DOI] [PubMed] [Google Scholar]
- Somerville LH, Heatherton TF, Kelley WM. Anterior cingulate cortex responds differentially to expectancy violation and social rejection. Nature Neuroscience. 2006;9:1007–1008. doi: 10.1038/nn1728. [DOI] [PubMed] [Google Scholar]
- Somerville LH, Wagner DD, Wig GS, Moran JM, Whalen PJ, Kelley WM. Interactions between transient and sustained neural signals support the generation and regulation of anxious emotion. Cerebral Cortex. 2013;23:49–60. doi: 10.1093/cercor/bhr373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Whalen PJ, Kelley WM. Human bed nucleus of the stria terminalis indexes hypervigilant threat monitoring. Biological Psychiatry. 2010;68:416–424. doi: 10.1016/j.biopsych.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soskin DP, Carl JR, Alpert J, Fava M. Antidepressant effects on emotional temperament: toward a biobehavioral research paradigm for major depressive disorder. CNS Neurosci Ther. 2012;18:441–451. doi: 10.1111/j.1755-5949.2012.00318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto CJ, John OP. Traits in transition: the structure of parent-reported personality traits from early childhood to early adulthood. Journal of Personality. 2014;82:182–199. doi: 10.1111/jopy.12044. [DOI] [PubMed] [Google Scholar]
- Soto CJ, John OP. The next Big Five Inventory (BFI-2): Developing and assessing a hierarchical model with 15 Facets to enhance bandwidth, fidelity, and predictive power. Journal of Personality and Social Psychology. doi: 10.1037/pspp0000096. in press. [DOI] [PubMed] [Google Scholar]
- Soto CJ, John OP, Gosling SD, Potter J. Age differences in personality traits from 10 to 65: Big Five domains and facets in a large cross-sectional sample. Journal of Personality and Social Psychology. 2011;100:330–348. doi: 10.1037/a0021717. [DOI] [PubMed] [Google Scholar]
- Soto CJ, Luhmann M. Who can buy happiness? Personality traits moderate the effects of stable income differences and income fluctuations on life satisfaction. Social Psychological and Personality Science. 2013;4:46–53. [Google Scholar]
- Specht J, Egloff B, Schmukle SC. Stability and change of personality across the life course: the impact of age and major life events on mean-level and rank-order stability of the Big Five. Journal of Personality and Social Psychology. 2011;101:862–882. doi: 10.1037/a0024950. [DOI] [PubMed] [Google Scholar]
- Spielberger CD. Theory and research on anxiety. In: Spielberger CD, editor. Anxiety and behavior. NY: Academic Press; 1966. pp. 3–22. [Google Scholar]
- Spunt RP, Elison JT, Dufour N, Hurlemann R, Saxe R, Adolphs R. Amygdala lesions do not compromise the cortical network for false-belief reasoning. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:4827–4832. doi: 10.1073/pnas.1422679112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staw BM, Bell NE, Clausen JA. The dispositional approach to job attitudes: A lifetime longitudinal test. Administrative Science Quarterly. 1986;31:56–77. [Google Scholar]
- Steel P, Schmidt J, Shultz J. Refining the relationship between personality and subjective well-being. Psychological Bulletin. 2008;134:138–161. doi: 10.1037/0033-2909.134.1.138. [DOI] [PubMed] [Google Scholar]
- Stein MB, Simmons AN, Feinstein JS, Paulus MP. Increased amygdala and insula activation during emotion processing in anxiety-prone subjects. American Journal of Psychiatry. 2007;164:318–327. doi: 10.1176/ajp.2007.164.2.318. [DOI] [PubMed] [Google Scholar]
- Stelzer J, Lohmann G, Mueller K, Buschmann T, Turner R. Deficient approaches to human neuroimaging. Front Hum Neurosci. 2014;8:462. doi: 10.3389/fnhum.2014.00462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steunenberg B, Beekman AT, Deeg DJ, Kerkhof AJ. Personality predicts recurrence of late-life depression. Journal of Affective Disorders. 2010;123:164–172. doi: 10.1016/j.jad.2009.08.002. [DOI] [PubMed] [Google Scholar]
- Steward O, Balice-Gordon R. Rigor or mortis: best practices for preclinical research in neuroscience. Neuron. 2014;84:572–581. doi: 10.1016/j.neuron.2014.10.042. [DOI] [PubMed] [Google Scholar]
- Stewart SL, Rubin KH. The social problem-solving skills of anxious-withdrawan children. Development and Psychopathology. 1995;7:323–336. [Google Scholar]
- Stoeckel LE, Garrison KA, Ghosh S, Wighton P, Hanlon CA, Gilman JM, … Evins AE. Optimizing real time fMRI neurofeedback for therapeutic discovery and development. Neuroimage Clin. 2014;5:245–255. doi: 10.1016/j.nicl.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes JJ. The relation of social netowork and individual difference variables to loneliness. Journal of Personality and Social Psychology. 1985;48:981–990. [Google Scholar]
- Stout DM, Shackman AJ, Johnson JS, Larson CL. Worry is associated with impaired gating of threat from working memory. Emotion. 2014;15:6–11. doi: 10.1037/emo0000015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout DM, Shackman AJ, Larson CL. Failure to filter: Anxious individuals show inefficient gating of threat from working memory. Frontiers in Human Neuroscience. 2013;7 doi: 10.3389/fnhum.2013.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straube T, Mentzel HJ, Miltner WHR. Waiting for spiders: Brain activation during anticipatory anxiety in spider phobics. Neuroimage. 2007;37:1427–1436. doi: 10.1016/j.neuroimage.2007.06.023. [DOI] [PubMed] [Google Scholar]
- Strauss CC, Frame CL, Forehand R. Psychosocial impairment associated with anxiety in children. J Clin Child Psychol. 1987;16:235–239. [Google Scholar]
- Suls J, Green PJ, Hillis S. Emotional reactivity to everyday problems, affective inertia, and neuroticism. Personality and Social Psychology Bulletin. 1998;24:127–136. [Google Scholar]
- Suls J, Martin R. The daily life of the garden-variety neurotic: Reactivity, stressor exposure, mood spillover, and maladaptive coping. Journal of Personality. 2005;73:1485–1509. doi: 10.1111/j.1467-6494.2005.00356.x. [DOI] [PubMed] [Google Scholar]
- Sutin A, Costa R, RM, Eaton W. Personality and career success: Concurrent and longitudinal relations. European Journal of Personality. 2009;23:71–84. doi: 10.1002/per.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz JR, Knodt AR, Radtke SR, Hariri AR. A neural biomarker of psychological vulnerability to future life stress. Neuron. 2015;85(3):505–511. doi: 10.1016/j.neuron.2014.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz JR, Williamson DE, Hariri AR. Developmental change in amygdala reactivity during adolescence: effects of family history of depression and stressful life events. American Journal of Psychiatry. 2015;172(3):276–283. doi: 10.1176/appi.ajp.2014.14020195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan PZ, Forbes EE, Dahl RE, Ryan ND, Siegle GJ, Ladouceur CD, Silk JS. Emotional reactivity and regulation in anxious and nonanxious youth: a cell-phone ecological momentary assessment study. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2012;53:197–206. doi: 10.1111/j.1469-7610.2011.02469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang DW, Fellows LK, Small DM, Dagher A. Food and drug cues activate similar brain regions: a meta-analysis of functional MRI studies. Physiology and Behavior. 2012;106:317–324. doi: 10.1016/j.physbeh.2012.03.009. [DOI] [PubMed] [Google Scholar]
- Taylor CT, Alden LE. To see ourselves as others see us: an experimental integration of the intra and interpersonal consequences of self-protection in social anxiety disorder. Journal of Abnormal Psychology. 2011;120(1):129–141. doi: 10.1037/a0022127. [DOI] [PubMed] [Google Scholar]
- ten Have M, Oldehinkel A, Vollebergh W, Ormel J. Does neuroticism explain variations in care service use for mental health problems in the general population? Results from the Netherlands Mental Health Survey and Incidence Study (NEMESIS) Social Psychiatry and Psychiatric Epidemiology. 2005;40:425–431. doi: 10.1007/s00127-005-0916-z. [DOI] [PubMed] [Google Scholar]
- Terracciano A, Lockenhoff CE, Zonderman AB, Ferrucci L, Costa PT., Jr Personality predictors of longevity: activity, emotional stability, and conscientiousness. Psychosomatic Medicine. 2008;70:621–627. doi: 10.1097/PSY.0b013e31817b9371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibodeau R, Jorgensen RS, Kim S. Depression, anxiety, and resting frontal EEG asymmetry: a meta-analytic review. Journal of Abnormal Psychology. 2006;115:715–729. doi: 10.1037/0021-843X.115.4.715. [DOI] [PubMed] [Google Scholar]
- Thomas KM, Drevets WC, Dahl RE, Ryan ND, Birmaher B, Eccard CH, … Casey BJ. Amygdala response to fearful faces in anxious and depressed children. Archives of General Psychiatry. 2001;58:1057–1063. doi: 10.1001/archpsyc.58.11.1057. [DOI] [PubMed] [Google Scholar]
- Tomarken AJ, Davidson RJ, Wheeler RE, Kinney L. Psychometric properties of resting anterior EEG asymmetry: Temporal stability and internal consistency. Psychophysiology. 1992;29:576–592. doi: 10.1111/j.1469-8986.1992.tb02034.x. [DOI] [PubMed] [Google Scholar]
- Törnqvist E, Månsson Å, Larsson EM, Hallström I. It’s like being in another world–patients’ lived experience of magnetic resonance imaging. Journal of Clinical Nursing. 2006;15:954–961. doi: 10.1111/j.1365-2702.2006.01499.x. [DOI] [PubMed] [Google Scholar]
- Tovote P, Fadok JP, Luthi A. Neuronal circuits for fear and anxiety. Nature Reviews Neuroscience. 2015;16:317–331. doi: 10.1038/nrn3945. [DOI] [PubMed] [Google Scholar]
- Towers DN, Allen JJB. A better estimate of the internal consistency reliability of frontal EEG asymmetry scores. Psychophysiology. 2009;46:132–142. doi: 10.1111/j.1469-8986.2008.00759.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tranel D, Gullickson G, Koch M, Adolphs R. Altered experience of emotion following bilateral amygdala damage. Cognitive Neuropsychiatry. 2006;11:219–232. doi: 10.1080/13546800444000281. [DOI] [PubMed] [Google Scholar]
- Treadway MT, Buckholtz JW, Martin JW, Jan K, Asplund CL, Ginther MR, … Marois R. Corticolimbic gating of emotion-driven punishment. Nature Neuroscience. 2014;17:1270–1275. doi: 10.1038/nn.3781. [DOI] [PubMed] [Google Scholar]
- Turk-Browne NB. Functional interactions as big data in the human brain. Science. 2013;342:580–584. doi: 10.1126/science.1238409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkheimer E, Pettersson E, Horn EE. A phenotypic null hypothesis for the genetics of personality. Annual Review of Psychology. 2014;65:515–540. doi: 10.1146/annurev-psych-113011-143752. [DOI] [PubMed] [Google Scholar]
- Turner CA, Clinton SM, Thompson RC, Watson SJ, Jr, Akil H. Fibroblast growth factor-2 (FGF2) augmentation early in life alters hippocampal development and rescues the anxiety phenotype in vulnerable animals. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:8021–8025. doi: 10.1073/pnas.1103732108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyc VL, Fairclough D, Fletcher B, Leigh L, Mulhern RK. Children’s distress during magnetic resonance imaging procedures. Children’s Health Care. 1995;24:5–19. doi: 10.1207/s15326888chc2401_2. [DOI] [PubMed] [Google Scholar]
- Tye KM, Prakash R, Kim SY, Fenno LE, Grosenick L, Zarabi H, … Deisseroth K. Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature. 2011;471:358–362. doi: 10.1038/nature09820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uliaszek AA, Hauner KK, Zinbarg RE, Craske MG, Mineka S, Griffith JW, Rose RD. An examination of content overlap and disorder-specific predictions in the associations of neuroticism with anxiety and depression. J Res Pers. 2009;43:785–794. doi: 10.1016/j.jrp.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uysal SD, Pohlmeier W. Unemployment duration and personality. Journal of Economic Psychology. 2011;32:980–992. [Google Scholar]
- Vaidyanathan U, Patrick CJ, Cuthbert BN. Linking dimensional models of internalizing psychopathology to neurobiological systems: affect-modulated startle as an indicator of fear and distress disorders and affiliated traits. Psychological Bulletin. 2009;135:909–942. doi: 10.1037/a0017222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaisvaser S, Lin T, Admon R, Podlipsky I, Greenman Y, Stern N, … Hendler T. Neural traces of stress: cortisol related sustained enhancement of amygdala-hippocampal functional connectivity. Front Hum Neurosci. 2013;7:313. doi: 10.3389/fnhum.2013.00313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Leemput IA, Wichers M, Cramer AO, Borsboom D, Tuerlinckx F, Kuppens P, … Scheffer M. Critical slowing down as early warning for the onset and termination of depression. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(1):87–92. doi: 10.1073/pnas.1312114110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg SM, de Moor MH, McGue M, Pettersson E, Terracciano A, Verweij KJ, … Boomsma DI. Harmonization of neuroticism and extraversion phenotypes across inventories and cohorts in the Genetics of Personality Consortium: an application of item response theory. Behav Genet. 2014;44(4):295–313. doi: 10.1007/s10519-014-9654-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Honk J, Eisenegger C, Terburg D, Stein DJ, Morgan B. Generous economic investments after basolateral amygdala damage. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:2506–2510. doi: 10.1073/pnas.1217316110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Marle HJ, Hermans EJ, Qin S, Fernandez G. From specificity to sensitivity: how acute stress affects amygdala processing of biologically salient stimuli. Biological Psychiatry. 2009;66:649–655. doi: 10.1016/j.biopsych.2009.05.014. [DOI] [PubMed] [Google Scholar]
- van Marle HJ, Hermans EJ, Qin S, Fernandez G. Enhanced resting-state connectivity of amygdala in the immediate aftermath of acute psychological stress. Neuroimage. 2010;53:348–354. doi: 10.1016/j.neuroimage.2010.05.070. [DOI] [PubMed] [Google Scholar]
- van Os J, Park SB, Jones PB. Neuroticism, life events and mental health: evidence for person-environment correlation. British Journal of Psychiatry Supplement. 2001;40:s72–77. doi: 10.1192/bjp.178.40.s72. [DOI] [PubMed] [Google Scholar]
- van Well S, Visser RM, Scholte HS, Kindt M. Neural substrates of individual differences in human fear learning: evidence from concurrent fMRI, fear-potentiated startle, and US-expectancy data. Cogn Affect Behav Neurosci. 2012;12:499–512. doi: 10.3758/s13415-012-0089-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wingen GA, Geuze E, Vermetten E, Fernandez G. Perceived threat predicts the neural sequelae of combat stress. Molecular Psychiatry. 2011;16(6):664–671. doi: 10.1038/mp.2010.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Winkel M, Nicolson NA, Wichers M, Viechtbauer W, Myin-Germeys I, Peeters F. Daily life stress reactivity in remitted versus non-remitted depressed individuals. Eur Psychiatry. 2015;30(4):441–447. doi: 10.1016/j.eurpsy.2015.02.011. [DOI] [PubMed] [Google Scholar]
- Vater A, Schröder-Abé M. Explaining the link between personality and relationship satisfaction: Emotion regulation and interpersonal behavior in conflict discussions. European Journal of Personality. 2015;29:201–215. [Google Scholar]
- Viinikainen J, Kokko K, Pulkkinen L, Pehkonen J. Personality and labour market income: Evidence from longitudinal data. Labour. 2010;24:201–220. [Google Scholar]
- Vinkers CH, Joels M, Milaneschi Y, Kahn RS, Penninx BW, Boks MP. Stress exposure across the life span cumulatively increases depression risk and is moderated by neuroticism. Depression and Anxiety. 2014;31:737–745. doi: 10.1002/da.22262. [DOI] [PubMed] [Google Scholar]
- Vollrath M. Personality and hassles among university students: A three-year longitudinal study. European Journal of Personality. 2000;14:199–215. [Google Scholar]
- Vukasovic T, Bratko D. Heritability of personality: A meta-analysis of behavior genetic studies. Psychological Bulletin. 2015;141:769–785. doi: 10.1037/bul0000017. [DOI] [PubMed] [Google Scholar]
- Wacker J, Chavanon ML, Stemmler G. Resting EEG signatures of agentic extraversion: New results and meta-analytic integration. Journal of Research in Personality. 2010;44:167–179. [Google Scholar]
- Wager TD, Atlas LY, Lindquist MA, Roy M, Woo CW, Kross E. An fMRI-based neurologic signature of physical pain. New England Journal of Medicine. 2013;368(15):1388–1397. doi: 10.1056/NEJMoa1204471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DL, Davis M. Double dissociation between the involvement of the bed nucleus of the stria terminalis and the central nucleus of the amygdala in startle increases produced by conditioned versus unconditioned fear. Journal of Neuroscience. 1997;17:9375–9383. doi: 10.1523/JNEUROSCI.17-23-09375.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DL, Davis M. Role of the extended amygdala in short-duration versus sustained fear: a tribute to Dr. Lennart Heimer. Brain Struct Funct. 2008;213:29–42. doi: 10.1007/s00429-008-0183-3. [DOI] [PubMed] [Google Scholar]
- Walker DL, Miles LA, Davis M. Selective participation of the bed nucleus of the stria terminalis and CRF in sustained anxiety-like versus phasic fear-like responses. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2009;33:1291–1308. doi: 10.1016/j.pnpbp.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DL, Toufexis DJ, Davis M. Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. European Journal of Pharmacology. 2003;563:199–216. doi: 10.1016/s0014-2999(03)01282-2. [DOI] [PubMed] [Google Scholar]
- Walker OL, Degnan KA, Fox NA, Henderson HA. Early social fear in relation to play with an unfamiliar peer: Actor and partner effects. Developmental Psychology. doi: 10.1037/a0039735. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SW, Repetti RL, Campos B. Job stress and family social behavior: the moderating role of neuroticism. Journal of Occupational Health Psychology. 2011;16:441–456. doi: 10.1037/a0025100. [DOI] [PubMed] [Google Scholar]
- Waters AM, Neumann DL, Henry J, Craske MG, Ornitz EM. Baseline and affective startle modulation by angry and neutral faces in 4–8-year-old anxious and non-anxious children. Biological Psychology. 2008;78:10–19. doi: 10.1016/j.biopsycho.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA. Negative affectivity: the disposition to experience aversive emotional states. Psychological Bulletin. 1984;96:465–490. [PubMed] [Google Scholar]
- Watson D, Clark LA. On traits and temperament: general and specific factors of emotional experience and their relation to the five-factor model. Journal of Personality. 1992;60(2):441–476. doi: 10.1111/j.1467-6494.1992.tb00980.x. [DOI] [PubMed] [Google Scholar]
- Watson D, Hubbard B, Wiese D. General traits of personality and affectivity as predictors of satisfaction in intimate relationships: Evidence from self- and partner-ratings. Journal of Personality. 2000;68:413–449. doi: 10.1111/1467-6494.00102. [DOI] [PubMed] [Google Scholar]
- Watson D, Humrichouse J. Personality development in emerging adulthood: Integrating evidence from self-ratings and spouse ratings. Journal of Personality & Social Psychology. 2006;91:959–974. doi: 10.1037/0022-3514.91.5.959. [DOI] [PubMed] [Google Scholar]
- Watson D, Naragon-Gainey K. Personality, emotions, and the emotional disorders. Clinical Psychological Science. 2014;2:422–442. doi: 10.1177/2167702614536162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayne JH, Musisca N, Fleeson W. Considering the role of personality in the work–family experience: Relationships of the big five to work–family conflict and facilitation. Journal of Vocational Behavior. 2004;64:108–130. [Google Scholar]
- Weissman MM, Prusoff BA, Klerman GL. Personality and the prediction of long-term outcome of depression. American Journal of Psychiatry. 1978;135:797–800. doi: 10.1176/ajp.135.7.797. [DOI] [PubMed] [Google Scholar]
- Weston SJ, Hill PL, Jackson JJ. Personality traits predict the onset of disease. Social Psychological and Personality Science. 2015;6:309–317. [Google Scholar]
- Wetter EK, Hankin BL. Mediational pathways through which positive and negative emotionality contribute to anhedonic symptoms of depression: a prospective study of adolescents. Journal of Abnormal Child Psychology. 2009;37:507–520. doi: 10.1007/s10802-009-9299-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler RE, Davidson RJ, Tomarken AJ. Frontal brain asymmetry and emotional reactivity: A biological substrate of affective style. Psychophysiology. 1993;30:82–89. doi: 10.1111/j.1469-8986.1993.tb03207.x. [DOI] [PubMed] [Google Scholar]
- White LK, Degnan KA, Henderson HA, Pérez-Edgar KA, Walker OL, Shechner T, … Fox NA. Developmental relations between behavioral inhibition, anxiety, and attention biases to threat and positive information. Child Development. doi: 10.1111/cdev.12696. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteford HA, Degenhardt L, Rehm J, Baxter AJ, Ferrari AJ, Erskine HE, … Vos T. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet. 2013;382:1575–1586. doi: 10.1016/S0140-6736(13)61611-6. [DOI] [PubMed] [Google Scholar]
- Whittington JE, Huppert FA. Neuroticism, psychiatric symptoms and life events. Personality and Individual Differences. 1998;24:97–107. [Google Scholar]
- Wichers MC, Barge-Schaapveld DQ, Nicolson NA, Peeters F, de Vries M, Mengelers R, van Os J. Reduced stress-sensitivity or increased reward experience: the psychological mechanism of response to antidepressant medication. Neuropsychopharmacology. 2009;34(4):923–931. doi: 10.1038/npp.2008.66. [DOI] [PubMed] [Google Scholar]
- Widiger TA. Neuroticism. In: Leary MR, Hoyle RH, editors. Handbook of individual differences in social behavior. NY: Guilford; 2009. pp. 129–146. [Google Scholar]
- Wilson RE, Harris KM, Vazire S. Personality and friendship satisfaction in daily life: Do everyday social interactions account for individual differences in friendship satisfaction. European Journal of Personality. 2015;29:173–186. [Google Scholar]
- Wilson RS, Krueger KR, Gu L, Bienias JL, Mendes de Leon CF, Evans DA. Neuroticism, extraversion, and mortality in a defined population of older persons. Psychosomatic Medicine. 2005;67:841–845. doi: 10.1097/01.psy.0000190615.20656.83. [DOI] [PubMed] [Google Scholar]
- Wilson S, Vaidyanathan U, Miller MB, McGue M, Iacono WG. Premorbid risk factors for major depressive disorder: are they associated with early onset and recurrent course? Development and Psychopathology. 2014;26(4 Pt 2):1477–1493. doi: 10.1017/S0954579414001151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SJ, Smyth JM, MacLean RR. Integrating ecological momentary assessment and functional brain imaging methods: new avenues for studying and treating tobacco dependence. Nicotine Tob Res. 2014;16(Suppl 2):S102–110. doi: 10.1093/ntr/ntt129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windischberger C, Lanzenberger R, Holik A, Spindelegger C, Stein P, Moser U, … Kasper S. Area-specific modulation of neural activation comparing escitalopram and citalopram revealed by pharmaco-fMRI: a randomized cross-over study. Neuroimage. 2010;49:1161–1170. doi: 10.1016/j.neuroimage.2009.10.013. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Noble PL, Davis M. Modulation of fear-potentiated startle and vocalizations in juvenile rhesus monkeys by morphine, diazepam, and buspirone. Biological Psychiatry. 2007;61:389–395. doi: 10.1016/j.biopsych.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Wise RA, Koob GF. The development and maintenance of drug addiction. Neuropsychopharmacology. 2014;39(2):254–262. doi: 10.1038/npp.2013.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RG, Preston C. What is the value of human FMRI in CNS drug development? Drug Discovery Today. 2010;15:973–980. doi: 10.1016/j.drudis.2010.08.016. [DOI] [PubMed] [Google Scholar]
- Wolitzky-Taylor K, Vrshek-Schallhorn S, Waters AM, Mineka S, Zinbarg R, Ornitz E, … Craske MG. Adversity in early and mid-adolescence is associated with elevated startle responses to safety cues in late adolescence. Clin Psychol Sci. 2014;2:202–213. doi: 10.1177/2167702613495840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo CW, Koban L, Kross E, Lindquist MA, Banich MT, Ruzic L, … Wager TD. Separate neural representations for physical pain and social rejection. Nat Commun. 2014;5:5380. doi: 10.1038/ncomms6380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood KH, Ver Hoef LW, Knight DC. The amygdala mediates the emotional modulation of threat-elicited skin conductance response. Emotion. 2014;14:693–700. doi: 10.1037/a0036636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrzus C, Mehl MR. Lab and/or field? Measuring personality processes and their social consequences. European Journal of Personality. 2015;29:250–271. [Google Scholar]
- Wupperman P, Neumann CS. Depressive symptoms as a function of sex-role, rumination, and neuroticism. Personality and Individual Differences. 2006;40:189–201. [Google Scholar]
- Yarkoni T, Poldrack RA, Nichols TE, Van Essen DC, Wager TD. Large-scale automated synthesis of human functional neuroimaging data. Nat Methods. 2011;8:665–670. doi: 10.1038/nmeth.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Hazlett RL, Stark CE, Hoehn-Saric R. Functional MRI of the amygdala and bed nucleus of the stria terminalis during conditions of uncertainty in generalized anxiety disorder. Journal of Psychiatric Research. 2012;46:1045–1052. doi: 10.1016/j.jpsychires.2012.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmazer-Hanke DM. Amygdala. In: Mai JK, Paxinos G, editors. The human nervous system. San Diego: Academic Press; 2012. pp. 759–834. [Google Scholar]
- Zaider TI, Heimberg RG, Iida M. Anxiety disorders and intimate relationships: a study of daily processes in couples. Journal of Abnormal Psychology. 2010;119:163–173. doi: 10.1037/a0018473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zautra AJ, Affleck GG, Tennen H, Reich JW, Davis MC. Dynamic approaches to emotions and stress in everyday life: Bolger and Zuckerman reloaded with positive as well as negative affects. Journal of Personality. 2005;73:1511–1538. doi: 10.1111/j.0022-3506.2005.00357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman M. General and situation-specific traits and states: new approaches to assessment of anxiety and other constructs. In: Zuckerman M, Spielberger CD, editors. Emotions and anxiety: new concepts, methods and applications. Hillsdale, NJ: Lawrence Erlbaum; 1976. pp. 133–174. [Google Scholar]