Abstract
Orofacial pain conditions including temporomandibular joint disorder and migraine are characterized by peripheral and central sensitization of trigeminal nociceptive neurons. Although calcitonin gene-related peptide (CGRP) is implicated in the development of central sensitization, the pathway by which elevated spinal cord CGRP levels promote peripheral sensitization of primary trigeminal nociceptive neurons is not well understood. The goal of this study was to investigate the role of CGRP in promoting bidirectional signaling within the trigeminal system to mediate sensitization of primary trigeminal ganglion nociceptive neurons. Adult male Sprague Dawley rats were injected in the upper spinal cord with CGRP or co-injected with the receptor antagonist CGRP8-37 or KT 5720, an inhibitor of protein kinase A (PKA). Nocifensive head withdrawal response to mechanical stimulation of trigeminal nerves was investigated using von Frey filaments. Expression of PKA, GFAP, and Iba1 in the spinal cord and P-ERK in the trigeminal ganglion was studied using immunohistochemistry. Some animals were co-injected intracisternally with CGRP and Fast Blue dye and trigeminal ganglion imaged using fluorescent microscopy. Intracisternal CGRP increased nocifensive responses to mechanical stimulation when compared to control levels. Co-injection of CGRP8-37 or KT 5720 with CGRP inhibited the nocifensive response. CGRP stimulated expression of PKA and GFAP in the spinal cord, and P-ERK in trigeminal ganglion neurons. Seven days post injection, Fast Blue was observed in trigeminal ganglion neurons and satellite glial cells. Our results demonstrate that elevated levels of CGRP in the upper spinal cord promote sensitization of primary trigeminal nociceptive neurons via a mechanism that involves activation of PKA centrally and P-ERK in trigeminal ganglion neurons. Our findings provide evidence of bidirectional signaling within the trigeminal system that can facilitate increased neuron-glia communication within the trigeminal ganglion associated with peripheral sensitization.
Keywords: calcitonin gene-related peptide, nociception, neuronal sensitization, protein kinase A, trigeminal ganglion, glia
Peripheral and central sensitization of trigeminal nociceptive neurons is associated with the pathology of prevalent and debilitating orofacial pain conditions including migraine and temporomandibular joint disorder (TMD). Migraine is a neurological disorder characterized by a severe headache, photophobia, phonophobia, and nausea that can persist up to 72 hours (Goadsby, 2005, Olesen et al., 2009). Similarly, TMD is a chronic painful condition that is characterized by persistent pain in the muscles and temporomandibular joint (TMJ) associated with mastication (Poveda Roda et al., 2007, Greenspan et al., 2011, Ohrbach et al., 2011, Furquim et al., 2015). The pathological pain and inflammation associated with migraine and TMD involves activation of trigeminal ganglion nerves, which provide sensory innervation of the head and face and relay nociceptive signals to the upper cervical spinal cord (Ballegaard et al., 2008, Bevilaqua Grossi et al., 2009, Dahan et al., 2015). Following peripheral activation of trigeminal nerves in response to tissue injury, the neuropeptide calcitonin gene-related peptide (CGRP) and other inflammatory mediators facilitate excitation of second order neurons and glial cells involved in the initiation and maintenance of central sensitization and persistent pain (Seybold, 2009, Sessle, 2011). Elevated CGRP levels in the spinal cord are implicated in the development of central sensitization by mediating changes in the expression of ion channels, receptors, and inflammatory genes in second order neurons and glial cells including astrocytes and microglia. Activation of astrocytes and microglia results in a prolonged inflammatory response that helps to sustain central sensitization and promotes a pathological pain state (Xie, 2008, Davies et al., 2010, Ikeda et al., 2012).
CGRP is involved in the initiation and maintenance of central sensitization via activation of CGRP receptors that are localized on secondary neurons and glial cells within the spinal cord (Moreno et al., 2002, Marvizon et al., 2007, Lennerz et al., 2008). The CGRP receptor complex is comprised of three separate proteins: a G-protein-coupled receptor (GPCR), calcitonin receptor-like receptor (CLR), and an accessory protein, receptor activity-modifying protein 1 (RAMP1) that confers ligand-binding specificity (Benemei et al., 2007, Russell et al., 2014). Activation of CGRP receptors in neurons and glial cells causes an increase in intracellular levels of the secondary messenger cAMP that binds to and stimulates activation of protein kinase A (PKA). The signaling protein PKA induces expression of pro-inflammatory genes such as cytokines that are involved in sustaining a sensitized state of second order neurons (Staud, 2015). Elevated PKA levels in the cytosol are correlated with sensitization and activation of nociceptive neurons and glial cells via modulation of receptor expression and ion channel activity (Sun et al., 2004, Seybold, 2009). CGRP is also known to cause activation of the mitogen activated protein (MAP) kinases including p38, c-Jun kinase (JNK), and extracellular regulated kinase (ERK) in trigeminal neurons and glia (Thalakoti et al., 2007, Cady et al., 2011), that facilitate inflammation and nociception in the spinal cord (Ji et al., 2009). Similar to PKA, increased expression of these signaling proteins leads to a prolonged state of sensitization via modulation of ion channels, receptors, and transcription factors.
In acute models of pain, peripheral sensitization, which results from the interaction of nociceptors with inflammatory substances released when tissue is damaged or inflamed (Sessle, 2011), has been shown to promote cellular changes that mediate central sensitization of second order neurons involved in pain transmission to the thalamus (Dodick and Silberstein, 2006, Sessle, 2011, Bernstein and Burstein, 2012). However, in prolonged pain states, central sensitization is maintained in the absence of evidence of peripheral tissue damage. In our study, we wanted to determine if elevated levels of CGRP in upper cervical spinal cord could promote bidirectional signaling and thus mediate peripheral sensitization of primary trigeminal neurons to mechanical stimulation. Findings from this study demonstrate that CGRP promotes sensitization of primary trigeminal nociceptive neurons via a mechanism involving PKA activation centrally, and is associated with increased levels of P-ERK and increased neuron-satellite glial cell coupling in the trigeminal ganglion. Furthermore, our results provide evidence of bidirectional signaling within the trigeminal system that may help explain how trigeminal nociceptive neurons become sensitized, as reported in chronic orofacial pain conditions, in the absence of any physical trauma.
EXPERIMENTAL PROCEDURES
Animals
Sixty-seven Sprague-Dawley rats were used for this study. All animal studies were performed in accordance with the protocols approved by the Missouri State University Institutional Animal Care and Use Committee and were in agreement with guidelines set forth in the National Institutes of Health and the Animal Welfare Act of 2007. An effort was made to reduce the number of animals used in the study as well as to minimize suffering. Adult, male Sprague-Dawley rats (350-500 g) were obtained from Charles River Laboratories Inc. (Wilmington, MA) or purchased from Missouri State University (internal breeding colonies). All animals were housed in clean, plastic standard rat cages (VWR, West Chester, PA) in an animal holding room on a 12-hour light/dark cycle starting at 7 A.M. with ambient temperature maintained from 22-24 °C and access to food and water ad libitum. Animals were acclimated to the environment for a minimum of 1 week upon arrival prior to use.
Reagents
Stock solutions of CGRP or CGRP8-37 (American Peptide Company, Sunnyvale, CA) were prepared at a concentration of 1 mM in 0.9% saline solution (Fisher-Scientific, Fair Lawn, NJ). The PKA inhibitor KT 5720 (Tocris, Bristol, UK) was prepared at a stock concentration of 1 mM in DMSO (Sigma-Aldrich, St. Louis, MO). On the day of the experiment, an aliquot of 1 mM CGRP was thawed and diluted in 0.9% sterile saline to a concentration of 1 μM either alone or in solution with one of the two inhibitors. The inhibitors CGRP8-37 and KT 5720 were prepared in 0.9% saline solution with CGRP at concentrations of 5 μM and 500 nM, respectively. The retrograde labeling dye Fast Blue (Polysciences Inc., Warrington, PA) was diluted to a concentration of 4% in sterile phosphate buffered saline (PBS) containing 1 μM CGRP.
Behavioral Testing
All behavioral procedures were conducted between the hours of 7 A.M. and 11 A.M. Behavioral assessments were performed essentially as described in previously published studies in our laboratory using the Durham Animal Holder (Ugo Basile, Varese Lakes, Italy) (Garrett et al., 2012, Cady et al., 2014, Hawkins et al., 2015) on a total of 48 animals. Prior to testing, the rats were acclimated by guiding them into the holding device and secured in the holder for 5 minutes using a plastic blockade inserted behind the hindpaws. To minimize false responses during von Frey filament testing, a pipette tip was used to touch the animal’s head and face to acclimate the rats to having the cutaneous tissue over the masseter muscle touched with a filament. This was done for the three consecutive days prior to testing with von Frey filaments. During this acclimation period, if a rat appeared to be unwilling to go into the device or was continuously moving and shifting within the device, the animal was removed from the study.
Following acclimations, nocifensive thresholds were determined in response to a series of calibrated von Frey filaments (North Coast Medical, Inc., Gilroy, CA; 60, 100, 180 grams) applied to the cutaneous tissue over the masseter muscle. A positive response was recorded when an animal visibly withdrew its head from a filament prior to it bending, while pressure was being applied. Each filament was applied 5 times on each side of the face, and the data are reported as the median number of responses obtained from 5 applications of each specific calibrated filament ± the interquartile range. The 100 g force was used for subsequent studies since the average number of positive head withdrawal responses to this force was less than 1 out of 5 for both right and left masseter muscles under basal conditions.
Once baseline values were established, the animals were anesthetized by inhalation of 5% isoflurane. Animals were then injected intracisternally using a 26 ½ gauge needle (Becton Dickinson, Franklin Lakes, NJ) and a 50 μL Hamilton syringe (Hamilton Company, Reno, NV) at the midline between the occipital bone and the first cervical vertebrae (C1) with 20 μl of CGRP (1 μM) either alone, or co-injected with CGRP8-37 (5 μM) or KT 5720 (500 nM), a selective signaling inhibitor of PKA. Control animals were injected with saline alone or received no injection (naïve). Mechanical testing for nocifensive reactions at 2 h and days 1, 2, and 3 post injections was done using the same method as baseline values.
Immunohistochemistry
Twenty-three animals were used for immunohistochemical studies. To correlate behavioral responses to cellular changes in protein levels within the spinal cord, injections were performed as described above with CGRP alone or co-injected with KT 5720. The upper spinal cord (6 mm posterior to the obex) was removed at 2 h and at days 2 and 3 following injection and incubated in 4% paraformaldehyde at 4 °C for approximately 24 h. Tissues were then placed in 12.5% sucrose at 4 °C for approximately 1 h and then incubated in 25% sucrose for a minimum of 8 h. Following cryopreservation, tissues were stored at −20 °C. Tissues were embedded in Optimal Cutting Temperature compound (OCT; Sakura Finetek, Torrance, CA) and transverse sections 14 μm in thickness were taken between 4 and 5 mm caudal to the obex of the upper spinal cord, using a cryostat set at −24 °C. Sections from control and treated animals were placed on Superfrost Plus slides (Fisher Scientific, Pittsburg, PA) with the caudal side of the spinal cord in contact with the glass and stored at −20 °C. To determine changes in levels of the active form of ERK (P-ERK) in trigeminal ganglia, both ganglion were removed 2 h after intracisternal injection of CGRP and prepared for immunohistochemistry as described for the spinal cord tissue.
Sections were blocked and permeabilized in a solution of 0.1% Triton X-100 in 5% donkey serum (Jackson ImmunoResearch Laboratories, West Grove, PA) for 20 min at room temperature. Following thorough rinsing in PBS, sections were incubated with primary antibodies for proteins of interest (Table 1) for either 3 h at room temperature or overnight in a humidified chamber at 4 °C. Sections were next incubated in solutions of secondary antibodies (Table 1) for one hour at room temperature, then mounted in Vectashield medium (H-1200) containing 4’,6-diamidino-2-phenylindole (DAPI; Vector Laboratories, Burlingame, CA) to stain cell nuclei in preparation of viewing using fluorescent microscopy. As a control, some slides were incubated with only secondary antibodies to confirm specificity. Images were taken using a Zeiss Axiocam mRm camera mounted on a Zeiss Imager Z2 fluorescent microscope with an Apotome. Image acquisition was performed using Zeiss Zen 2012 software (Thornwood, NY).
Table 1.
Antibodies and Incubation Conditions Used for Immunohistochemistry.
| Protein | Dilution | Company | Incubation Time |
Incubation Temperature |
|---|---|---|---|---|
| GFAP | 1:5,000 | Abcam | 3 hours | 20-22 °C |
| Iba1 | 1:500 | Abcam | 3 hours | 20-22 °C |
| NeuN | 1:1,000 | Millipore | 3 hours | 20-22 °C |
| P-ERK | 1:500 | Bioworld | Overnight | 20-22 °C |
| PKA | 1:500 | Abcam | 3 hours | 4 °C |
| Alexa 488 | 1:200 | Life Technologies |
1 hour | 20-22 °C |
| Alexa 567 | 1:200 | Life Technologies |
1 hour | 20-22 °C |
| Alexa 647 | 1:200 | Life Technologies |
1 hour | 20-22 °C |
Retrograde labeling
In initial studies, the fluorescent dye Fast Blue was injected into the intracisternal space between the occipital bone and C1 and its localization in the trigeminal ganglion observed using fluorescent microscopy. To directly investigate if CGRP could promote bidirectional signaling from terminals of primary sensory nerves fibers localized in the spinal cord, 50 μL of Fast Blue and 1 μM CGRP diluted in PBS were co-injected, while some animals were only injected with Fast Blue. Seven days later, the animals in both groups were euthanized via asphyxiation with carbon dioxide followed by decapitation. Trigeminal ganglia were removed and prepared for fluorescent microscopy as for tissue immunostaining. Briefly, longitudinal sections (14 μm) were prepared using a cryostat and mounted on Superfrost Plus slides with the caudal side in contact with the glass. Tissues were rehydrated with PBS and then mounted in Vectashield medium without DAPI to localize fluorescent staining from retrograde transport of the dye from the spinal cord.
Statistical analysis
Statistical analysis was performed essentially as described in previous published studies from our laboratory (Cady et al., 2014, Hawkins et al., 2015, Hawkins and Durham, 2016). For the mechanical stimulation studies, the data are reported as the median number of withdrawal responses ± the interquartile range to 100 g of force at each condition and time point. Subsequent analysis was then performed on data with n = 6 or greater for each experimental condition using a Friedman’s ANOVA to test for general statistical significance between time points for each group, followed by a Wilcoxon test to find changes within groups from basal, and a Kruskal Wallis followed by a Mann-Whitney U test for differences between groups at each time-point. Statistical significance was set at P < 0.05. For analysis of the immunohistological images of the spinal cord (n = at least 3 independent experiments per condition), relative levels of the proteins of interest were analyzed using NIH image J software. Fluorescent intensity was measured in ten rectangular regions in laminas I-III in the medullary horn. To normalize intensity measurements within each image, background intensity values were obtained from five non-overlapping regions in either the acellular area of the outer lamina as determined by DAPI, and average values subtracted from region of interest staining intensity values. All data are presented as mean fold-change from the average naïve value ± S.E.M. All immunohistochemical data were normally distributed as determined by a Shapiro-Wilk test. Analysis was performed for each separate time point using a one-way ANOVA with a Tukey’s post-hoc. For quantification of P-ERK expression in the trigeminal ganglion, the number of neuronal cells exhibiting nuclear localization of P-ERK was divided by the total number of visible neuronal nuclei as identified by NeuN staining in the overlapping V1/V2 region and distinct V3 region. Results are reported as the average percent ± S.E.M of neurons with P-ERK nuclear staining. Statistical significance was set at P < 0.05.
RESULTS
CGRP Mediates Nociceptive Response in Trigeminal Ganglion Neurons
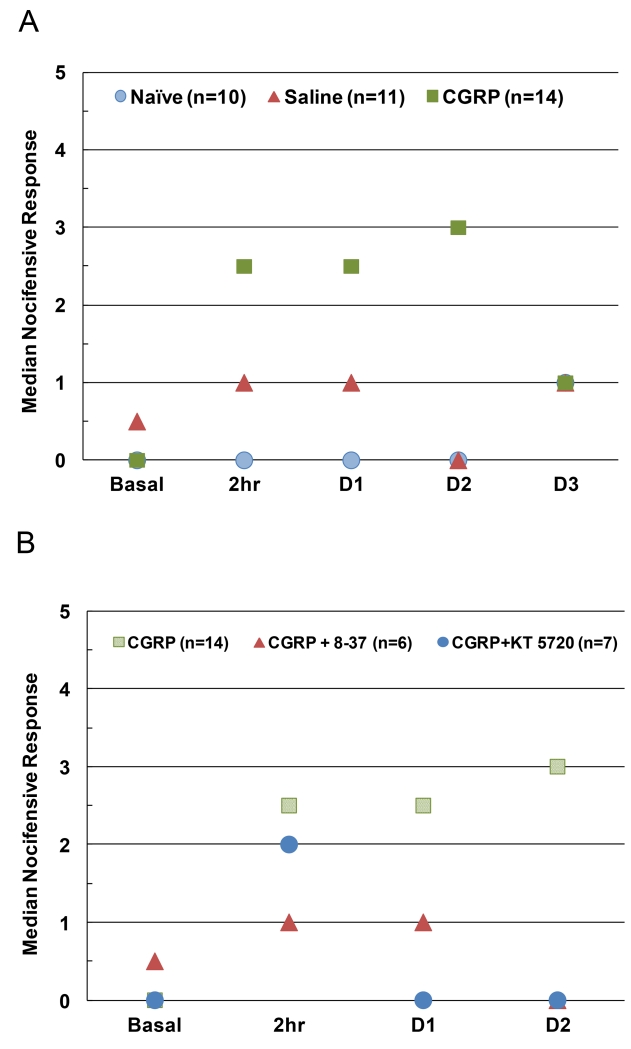
To evaluate the effect of increased CGRP levels in the upper spinal cord on primary nociceptor sensitivity, nocifensive responses were examined in response to mechanical stimulation of the cutaneous region over the masseter muscle. The number of withdrawals from the 100 g filament by animals was tested basally, 2 h post-injection, and on days 1, 2, and 3 post-injection (Fig. 1A). A Friedman test on all behavioral data indicated a significant difference between time-points for CGRP-injected animals (Χ2=15.921, n = 14, P ≤ 0.01), but not saline or naïve animals. Subsequent pairwise analysis showed no significant difference in basal responses between conditions. In contrast, significantly increased nocifensive responses were observed at 2 h (2.5 ± 3, Z = −2.9, n = 14, P ≤ 0.01) and on days 1 (2.5 ± 3, Z = −2.9, n = 14, P ≤ 0.05) and 2 (3.0 ± 4, Z = −3.0, n = 14, P ≤ 0.01) in animals injected with 1 μM CGRP when compared to basal values (0.0 ± 1). Nocifensive responses from animals injected with CGRP were also significantly different from saline controls at 2 h (0.5 ± 1.25, U = 32.5, nCGRP = 14, nsaline = 11, P ≤ 0.05) and on day 1 (1.0 ± 1.25, U = 35.0, n = 11, P ≤ 0.05) and day 2 post-injection (0.0 ± 2, U = 24.0, n = 11, P ≤ 0.01). Nocifensive responses in CGRP animals were also significantly higher than naïve control levels at 2 h (0.0 ± 1, U = 20.0, nCGRP = 14, nnaive = 10, P ≤ 0.01) and on day 1 (0.0 ± 1, U = 24.0, nCGRP = 14, nnaive = 10, P ≤ 0.01) and day 2 post-injection (0.0 ± 1, U = 27.0, nCGRP = 14, nnaive = 10, P ≤ 0.05). However, CGRP-induced responses were again similar to naïve and saline control values 3 days after injection.
Figure 1.
A. Intracisternal injection of CGRP in upper cervical spinal cord increased nociceptive responses to mechanical stimulation of trigeminal neurons. The median number of nocifensive head withdrawals to the 100 g filament in naïve animals compared to animals basally and at 2 h, 1 day, 2 days, or 3 days post intracisternal injection of saline or CGRP is shown. B. The median number of nocifensive withdrawal responses to the 100 g filament was decreased in a time-dependent manner by inhibiting CGRP or PKA activity. Animals were injected intracisternally with CGRP or co-injected with CGRP and the truncated CGRP receptor antagonist peptide CGRP8-37 (CGRP + 8-37) or the selective PKA inhibitor KT 5720 (CGRP + KT 5720).
PKA Is Involved in CGRP-Mediated Nociception
To investigate the signaling pathways involved in CGRP-mediated increases in nociception, animals were co-injected with a truncated version of CGRP (CGRP8-37) that is a known competitive CGRP receptor inhibitor or KT 5720, a selective inhibitor of the downstream signaling enzyme PKA. A Friedman test on behavioral data from animals receiving CGRP and CGRP8-37 showed no significance between time-points. Responses from animals that received CGRP8-37 in conjunction with CGRP were not significant from saline or naïve controls at 2 h, or days 1 and 2 post-injection. Nocifensive responses from these animals also failed to reach significance from basal levels at 2 h, or days 1 or 2 following injections.
A Friedman test on behavioral data from animals receiving CGRP and KT 5720 indicated a significant difference between time-points (Χ2= 13.289, n = 7, P 0.01). Animals co-injected with CGRP and the PKA inhibitor KT 5720 exhibited significant increases in nocifensive responses to the 100 g filament at 2 h post-injection (2.0 ± 3.75) as compared to naïve control groups (U = 8.0, nnaive = 10, nKT5720 = 7, P ≤ 0.01), saline control groups (U)= 16.0, nsaline = 11, nKT5720 = 7, P ≤ 0.05), and basal readings (0.50 ± 1, Z = −2.2, n = 7, P ≤ 0.05) (Fig. 1B). In contrast, co-injected animals did not exhibit significantly increased nocifensive responses on day 1 (0.0 ± 2) or day 2 post-injection (0.0 ± 1) as compared to saline and naïve groups, as well as basal levels. Animals that received CGRP and KT 5720 reacted significantly less to the 100 g filament than those receiving CGRP alone at 1 d (U = 16.0, nCGRP = 14, nKT5720 = 7, P ≤ 0.5) and 2 d (U = 13.5, nCGRP = 14, nKT5720 = 7, P ≤ 0.05).
Expression of PKA and GFAP but Not Iba1 Were Increased in Response to CGRP
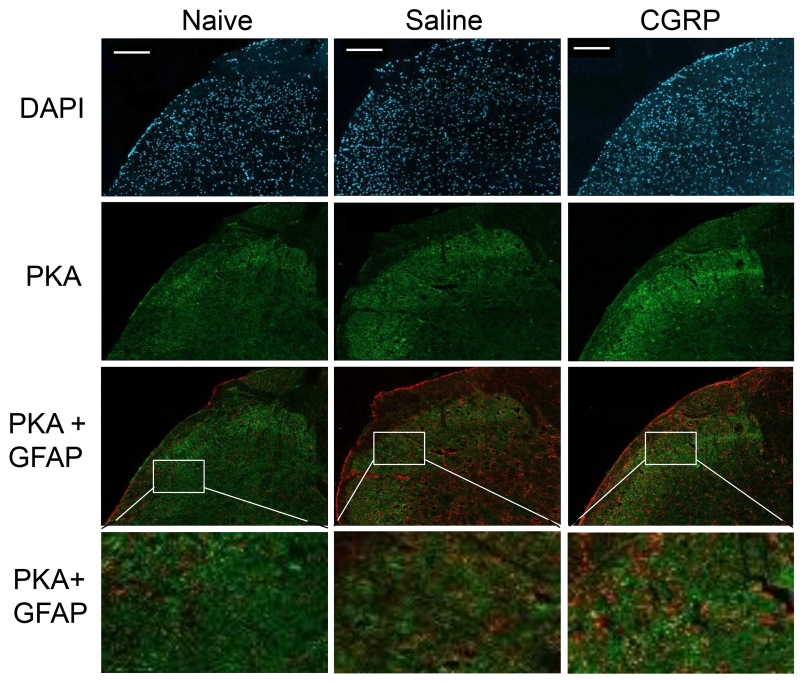
To identify the cell types influenced by CGRP, the expression of PKA, GFAP, and Iba1 were investigated using immunohistochemistry of upper spinal cord tissue. In naïve animals, low levels of the active form of PKA were detected in the upper spinal cord tissue (1.00 ± 0.06, n = 3; Fig. 2). General statistical significance was detected at 2 h (F(2, 6) = 14.4, P ≤ 0.01, η2 = 0.827) and 2 d (F(2, 6) = 17.3, P ≤ 0.05, η2 = 0.733) post-treatment. These changes were no longer statistically significant by 3 d post-treatment. The level of PKA staining was not significantly different from naïve controls in animals injected with sterile saline alone at any time point. At 2 h, CGRP-injected animals exhibited an increase in PKA immunostaining intensity (2.43 ± 0.11 fold, n = 3) in comparison to levels in naïve (P ≤ 0.01) and saline animals (P ≤ 0.01). PKA levels were still significantly elevated at 2 days post-injection (2.43 ± 0.15, n = 3) as compared to both naïve (P ≤ 0.05) and saline (P ≤ 0.05) animals. After 3 days, PKA levels in CGRP-injected animals were no longer significantly elevated when compared to naïve and saline control levels. Based on co-staining results, CGRP-mediated increase of PKA was observed in NeuN (neuronal nuclei marker) and GFAP stained cells, but not with Iba1. Thus, the stimulating effect of PKA involves changes in neurons and astrocytes.
Figure 2.
CGRP injection increased expression of PKA in upper spinal cord compared to naïve and saline control. Representative images of sections from the upper spinal cord obtained from naïve (left), and saline treated (center) or CGRP treated (right) animals 2 days post injection are shown. All cell nuclei are identified by DAPI staining (top panel). The same sections were also stained for PKA or co-stained for PKA and the astrocyte biomarker GFAP. Enlarged images of the region of the medullary horn are shown. Scale bars = 200 μm.
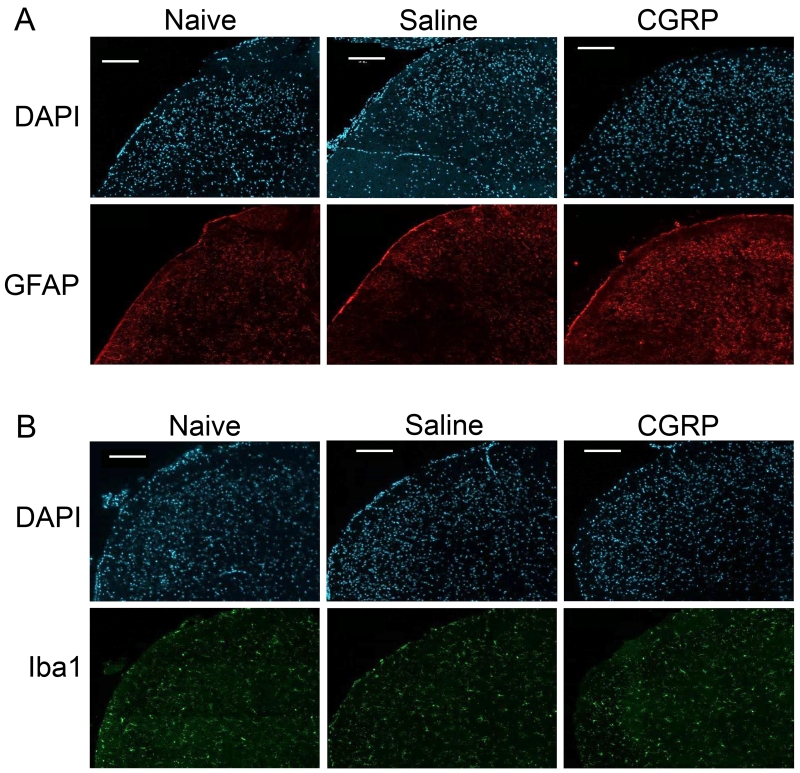
Levels of GFAP were compared between treatment conditions to evaluate changes in astrocyte activity in the spinal cord (Fig. 3A). Low levels of glial fibrillary acidic protein (GFAP) were observed in upper spinal cord tissues of naïve animals (1.00 ± 0.07, n = 3). Initial testing indicated a significant difference in GFAP levels in tissues taken 2 hours (F(2, 6) = 7.1, P ≤ 0.05, η2 = 0.702) 2 days (F(2, 6) = 17.3, P ≤ 0.01, η2 = 0.852) and 3 days post-injection (F(2, 6) = 9.3, P ≤ 0.05, η2 = 0.755). Post-hoc analysis showed that injection of sterile saline alone was not sufficient to elicit a change in expression of GFAP in astrocytes in the upper spinal cord at any time point as compared to naïve levels. Animals injected with CGRP were significantly different from naïve levels 2 h post-injection (1.88 ± 0.13, P ≤ 0.05), but did not show significant changes compared with saline controls at 2 h. In contrast, levels of GFAP in animals injected with CGRP were significantly higher from both naïve (P ≤ 0.01, n = 3) and saline controls (P ≤ 0.01, n = 3) 2 days after injection (3.03 ± 0.17). Three days post injection, spinal cord tissue from rats injected with CGRP still showed significantly different GFAP levels to naïve (1.93 ± 0.20, P ≤ 0.05) and saline (P ≤ 0.05) tissues.
Figure 3.
Intracisternal injection of CGRP increased expression of GFAP and transiently elevated Iba1 levels. Representative images of sections from the medullary horn of upper spinal cords obtained from naïve (left), and saline (center) or CGRP treated (right) animals 2 days post CGRP administration are shown. All cell nuclei identified by staining with DAPI are shown in the top panels, while immunostaining of the same tissue sections for GFAP (A) or Iba1 (B) are seen in the lower panels. Scale bars = 200 μm.
Relative levels of Iba1, a marker for active microglia, were assessed in the spinal cord to evaluate whether there were changes in microglia activity in response to CGRP (Fig. 3B). Statistically significant differences were not detected at any time point, indicating that neurons and astrocytes are primarily responsible for changes of sensitivity in the medullary horn.
Expression of P-ERK in Trigeminal Ganglion
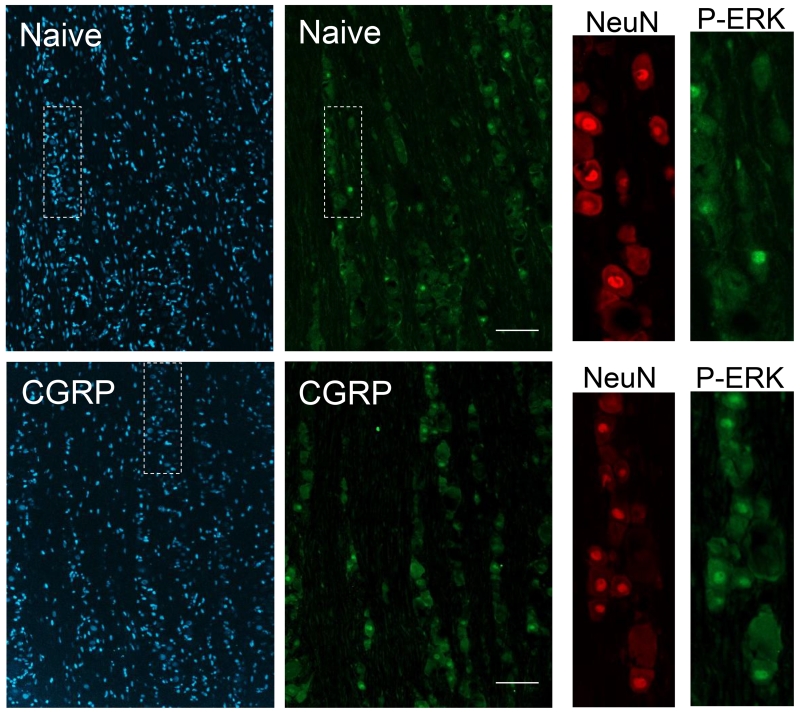
Having shown that elevated levels of CGRP in the upper spinal cord led to an increased sensitivity of V3 trigeminal neurons that provide sensory innervation of the TMJ tissues, immunohistochemistry was utilized to study changes in P-ERK levels in primary neurons. While P-ERK immunostaining was observed primarily in the cytosol of neuronal cell bodies in naïve animals, the active form was more localized in the nuclei of trigeminal neurons in animals 2 h post CGRP injection (Fig. 4). P-ERK expression in neuronal nuclei was confirmed through tissue morphology and its colocalization with the NeuN expression. Nuclear localization of P-ERK expression was evaluated as a percentage of the total neuronal nuclei present in an image, normalized to number of visible neurons identified by DAPI staining. The staining pattern in the V3 region of naïve tissues (n = 3) showed an average of 65.3 ± 2.8 visible neuronal nuclei, with an average of 33.7 ± 0.72, or 51.9 ± 3.2%, of these expressing nuclear P-ERK. Animals injected with saline at 2 h post-injection (n = 3) contained an average of 64.3 ± 3.4 neurons present in pictures taken of the V3 region of the trigeminal ganglion, with 31.7 ± 2.3, or 49.2 ± 2.9%, of those neurons exhibiting nuclear P-ERK. Animals injected with CGRP at 2 h post-injection (n = 3) had an average of 57.7 ± 7.6 neurons present in pictures taken of the V3 region of the trigeminal ganglion. On average, 47.7 ± 5.6 of those neurons showed nuclear localization of P-ERK, making up 83.0 ± 1.4% of the total neurons per image. There was a statistically significant difference in nuclear P-ERK levels between groups (F(2, 6) = 39.8, P ≤ 0.001, η2 = 0.930). The difference in nuclear P-ERK between naïve and saline tissues was not significant. The percentage of nuclear P-ERK in the V3 region of CGRP tissues was significantly higher than that in naïve tissues (P 0.001) and saline tissues (P ≤ 0.001). In the V1/V2 region of the ganglion, naïve tissues (n = 3) had an average of 77.0 ± 14.6 neuronal nuclei visible in each tissue, with an average of 23.3 ± 5.25 of those neurons exhibiting nuclear P-ERK expression, comprising 30.2 ± 2.2% of the total neurons. Ganglia from animals that had been injected with saline alone (n = 3) had an average of 68.3 ± 7.3 neuronal nuclei visible in each tissue, with an average of 31.3 ± 4.8 neurons, or 45.9 ± 4.6% of total neurons exhibiting nuclear P-ERK expression. Tissues from animals injected with CGRP at 2 h post-injection (n = 3) were evaluated as having an average of 65.0 ± 7.7 neurons present in pictures taken of the V1V2 region of the trigeminal ganglion. On average, 52.3 ± 8.9 of those neurons showed nuclear localization of P-ERK, making up 79.1 ± 4.0% of the total neurons per tissue. A one-way ANOVA showed a statistically significant difference of nuclear P-ERK levels between groups (F(2, 6) = 34.3, P ≤ 0.001, η2 = 0.920). A Tukey post-hoc showed no significant difference in nuclear P-ERK between naïve and saline tissues. However, the percentage of nuclear P-ERK in the V1V2 region of CGRP tissues was significantly higher than that in naïve (P ≤ 0.001) or saline tissues (P ≤ 0.01).
Figure 4.
Intracisternal CGRP injection increased expression of P-ERK in trigeminal ganglion neurons. Representative images of sections from the V1/V2 region of trigeminal ganglia obtained from naïve and CGRP treated animals are shown. All cell nuclei are identified by the nuclear dye DAPI (left panel), while the same tissue sections that were positive for P-ERK are seen in the second panel. Enlarged images of the region of the ganglion containing numerous neuronal cell bodies (white box) stained for neuronal protein NeuN (third panel) and the same region co-stained for P-ERK (far right panel) are shown. Scale bars = 100 μm.
Evidence of Bidirectional Signaling within Trigeminal System
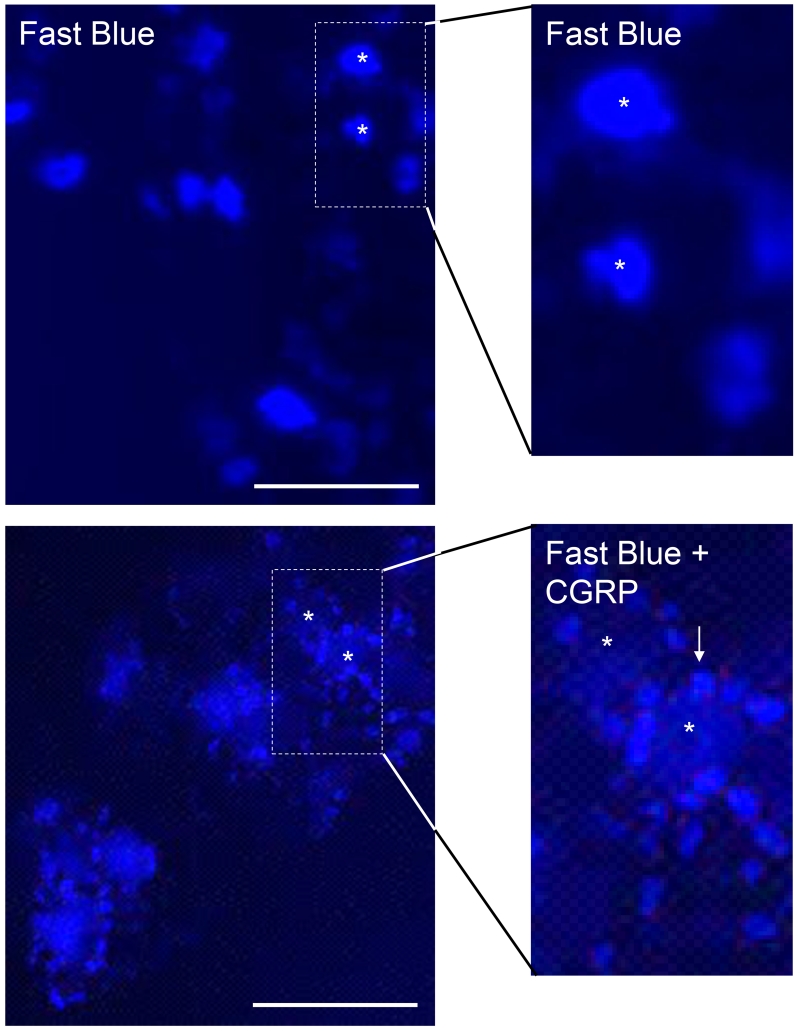
The retrograde fluorescent tracer dye Fast Blue was observed in neuronal cell bodies located in the V3 region of the trigeminal ganglion seven days after dye injection into the upper spinal cord in unstimulated animals (Fig. 5). To test whether elevated CGRP levels in the spinal cord could promote increased neuron-glial signaling in the trigeminal ganglion, animals were co-injected with Fast Blue and 1 μM CGRP. Seven days post injection, the Fast Blue was observed in the cell body of trigeminal neurons and surrounding satellite glial cells throughout all three branches of the ganglion. In contrast, Fast Blue was not detected in Schwann cells, which are the other prevalent glial cell type present within the ganglion.
Figure 5.
Evidence of bidirectional signaling within the trigeminal system from the upper spinal cord to trigeminal ganglion cells and increased neuron-glia coupling in response to CGRP. The fluorescent dye Fast Blue was localized primarily in the cell body of neurons 7 days after dye injection in unstimulated animals but the dye was seen in both neuronal cell bodies and satellite glial cells in animals co-injected with dye and CGRP. A white asterisk was used to indicate the cell body of several neurons, while arrows identify satellite glial cells containing the dye. Scale bars = 100 μm.
DISCUSSION
Elevated levels of CGRP are implicated in the development and maintenance of central sensitization and an enhanced pain state characterized by hyperalgesia and allodynia in diseases involving trigeminal nerves including TMD and migraine (Sun et al., 2003, Sun et al., 2004, Seybold, 2009, Sessle, 2011). However, the mechanisms by which elevated levels of CGRP in the upper spinal cord promote peripheral sensitization of primary nociceptive trigeminal neurons that provide sensory innervation to tissues in the head and face is not well understood. We found that administration of CGRP in the upper spinal cord resulted in a significant increase in the number of nocifensive head withdrawals in response to mechanical stimulation of the trigeminal nerve. This finding is in agreement with data from human studies in which both TMD and migraine patients report increased sensitivity to mechanical pressure applied to the head and face during an attack (Burstein et al., 2015, Dahan et al., 2015, Furquim et al., 2015). Under normal physiological conditions, the increase in pain sensitivity could provide a protective mechanism to minimize further damage to the tissue. As shown in our study, the enhanced nocifensive response was seen as early as 2 h post administration and this sensitized state was maintained for at least 48 h with resolution by 72 h post intracisternal CGRP injection. This finding is in agreement with the time course reported by migraine patients during an acute attack in which the severe pain and enhanced sensitivity is rarely sustained past 72 hours (Olesen et al., 2009). Our finding that elevated levels of CGRP within the upper spinal cord can lower the activation threshold of trigeminal primary sensory neurons to mechanical stimulation provide evidence to help explain the association of CGRP and the enhanced pain states reported by migraine and TMD patients.
The physiological and cellular effects of CGRP are mediated via activation of the CGRP receptor, which is expressed on primary trigeminal neurons that synapse in the outer lamina of the spinal cord and the associated glial cells, astrocytes, and microglia (Wang et al., 2010, Hansen et al., 2016). To demonstrate the specificity of the CGRP-mediated response, we co-administered CGRP with the truncated CGRP molecule (CGRP8-37), which acts as a competitive inhibitor of the CGRP receptor (Chiba et al., 1989, Edvinsson et al., 2007). We found that CGRP8-37 could inhibit the stimulatory effect of CGRP on nociception at each of the time points. This finding provides evidence that blocking the CGRP receptor within the spinal cord is sufficient to suppress the initiation and maintenance of peripheral sensitization of trigeminal nociceptive neurons. Our result is in agreement with other studies that reported intrathecal administration of a CGRP receptor antagonist can block mechanically evoked nocifensive responses in tissues innervated by dorsal root ganglion sensory neurons (Sun et al., 2004, Adwanikar et al., 2007, Tzabazis et al., 2007, Hansen et al., 2016). Following binding of CGRP to its G-protein-coupled receptor, there is an increase in adenylate cyclase activity within those cells leading to an increase in the intracellular level of the secondary messenger cAMP (Brain and Cox, 2006). Elevated levels of cAMP then cause an increase in expression of the active form of the signaling kinase PKA via binding to an allosteric site in the protein. In contrast to blocking the CGRP receptor, we found that co-injection of the PKA inhibitor (KT 5720) with CGRP did not inhibit trigeminal sensitization to mechanical stimulation at the 2 h time point. However, blocking PKA activation did suppress mechanical sensitivity 24 and 48 h post CGRP injection. We can only speculate that the difference in temporal response may be due to CGRP eliciting an immediate increase in nuclear P-ERK in primary neurons, as shown in our study, independent of PKA activity in the central nervous system. The maintenance of the sensitization, however, is likely mediated by central PKA expression at least partially in astrocytes since CGRP stimulated GFAP expression, which is used as a biomarker of activated astrocytes. Taken together, our data support the notion that the initial increase in neuronal sensitivity in trigeminal nociceptors is due to cellular changes within the primary neurons while the more sustained sensitized state is attributable, at least in part, to activation of glial cells.
Data from our behavioral studies provide evidence of the involvement of PKA activation in mediating the downstream stimulatory effects of CGRP. To determine if elevated CGRP levels in the spinal cord could stimulate increased expression of the active form of PKA in neurons and glial cells within the medullary horn, we used immunohistochemistry to directly study changes in PKA levels. The rationale for investigating PKA is further supported by evidence from other studies that PKA activation is associated with development of central sensitization (Levy and Strassman, 2002, Hu et al., 2003, Kohno et al., 2008). Based on colocalization of PKA with the proteins NeuN and GFAP, we found that PKA levels were significantly increased in cell bodies of second order neurons and astrocytes in response to CGRP at 2 hours post injection when compared to levels in naïve and saline treated animals. PKA levels remained significantly elevated at 48 and 72 h post injection. This finding is suggestive that, although a CGRP-mediated enhancement in nociceptive sensitivity had resolved by 72 h, the neurons and glia within the upper spinal cord retained a level of cellular sensitization. Our finding is in agreement with other studies that have shown that elevated levels of PKA within the lower spinal cord are involved in the initiation and maintenance of central sensitization (Aley and Levine, 1999, Hu et al., 2003, Hucho and Levine, 2007). The modulatory effects of PKA are thought to involve activation of pathways and transcription factors that regulate the expression and activity level of ion channels and receptors in nociceptive neurons and increase expression of pro-inflammatory molecules in both neurons and glial cells (Seybold, 2009). For example, elevated PKA levels in the spinal cord are implicated in the development of central sensitization by enhancing the activity of glutamate receptors that are expressed on second order nociceptive neurons (Aley and Levine, 1999, Hucho and Levine, 2007, Latremoliere and Woolf, 2009). Furthermore, activation of PKA intracellular signaling pathways have been shown to promote the initiation and prolonged state of sensitization and persistent pain via ion channel phosphorylation (Fitzgerald et al., 1999, Bhave et al., 2002, Han et al., 2005), and inducing pro-inflammatory cytokine genes containing CRE regulatory promoter sequences (Kawasaki et al., 2004). Further evidence for an important role of PKA in mediating nociception was provided by results demonstrating that blocking PKA signaling inhibits inflammation-induced hyperalgesic behaviors (Malmberg et al., 1997, Aley and Levine, 1999). In sum, our results provide evidence to further support the notion that PKA signaling plays a central role in mediating the stimulatory effects of CGRP, and thus is likely to be an important signaling pathway in promoting central sensitization associated with TMD and migraine.
Sensitization of nociceptive neurons associated with the development of prolonged pain states is known to involve activation of spinal cord glial cells (Wieseler-Frank et al., 2004, Ren and Dubner, 2008, Gosselin et al., 2010). In support of this notion, we detected elevated immunoreactive levels of GFAP, which is a protein implicated in astrocyte activation, of CGRP injected animals. The observed increase in GFAP occurred at the 2 hour time point with levels greatest after 48 hours post injection, and remained significantly elevated even at 72 hours, a finding similar to our PKA results. In contrast, CGRP did not cause an increase in the expression of Iba1 in microglia when compared to control levels at any of the time points. Astrocytes can promote and sustain sensitization of peripheral and central neurons through the release of cytokines and other inflammatory molecules by increasing neuron-glial cell interactions in the spinal cord (Miller et al., 2009). Based on our findings, the stimulatory effects in response to intracisternal administration of CGRP appears to be mediated primarily by astrocytes with minimal contribution from microglial cells.
To investigate a possible mechanism by which elevated CGRP levels in the spinal cord could lead to our observed increase in nocifensive head withdrawal response mediated by primary trigeminal nociceptive neurons, we determined changes in the level of the MAP kinase ERK in trigeminal neurons. Intracisternal CGRP caused a significant large increase in the nuclear localization of active, phosphorylated form of ERK (P-ERK) in the cell bodies of trigeminal neurons throughout the entire ganglion 2 hours post injection. In contrast in naïve control ganglion, P-ERK was mostly localized in the cytosol of neurons. Elevated P-ERK levels are reported to mediate a sensitized state of primary nociceptive neurons via modulating expression and activation levels of ion channels and membrane receptors (Cheng and Ji, 2008, Takeda et al., 2009). The importance of MAP kinases in the development of peripheral sensitization and an enhanced level of nociceptor sensitivity is supported by findings that selective inhibition of MAP kinase activity can suppress nociceptive cellular events (Milligan et al., 2003, Tsuda et al., 2004, Ji et al., 2009). Results from our study provide evidence that CGRP induces cellular changes of trigeminal ganglion neurons that correlate with the development of peripheral sensitization of primary nociceptive neurons. These data are in agreement with a previous study from our laboratory that demonstrated that nicotine, which promotes central sensitization by promoting an increase in neuron-glial signaling and cytokine production within the upper spinal cord, caused an significant elevation in P-ERK levels in primary trigeminal nociceptive neurons (Hawkins et al., 2015). Taken together, our findings provide evidence to support the notion of bidirectional signaling within the trigeminal system such that central sensitization can induce changes in trigeminal nociceptive neurons.
To directly demonstrate that CGRP can promote retrograde signal transduction from the spinal cord to neuronal cell bodies located in the trigeminal ganglion, the retrograde dye Fast Blue was co-injected with CGRP in the upper spinal cord. Fast Blue is a fluorescent dye most commonly used as a retrograde neuronal tracer since it has been shown to be effectively transported retrogradely over long distances in various animal models (Casatti et al., 1999, Bossowska et al., 2009, Ivanusic, 2009). While we detected Fast Blue in the cell bodies of neurons throughout all regions of the trigeminal ganglion seven days post intracisternal injection in unstimulated animals, we observed the dye in both neuronal cell bodies and associated satellite glial cells in response to intracisternal CGRP. To our knowledge, these data for the first time provide direct evidence of bidirectional signaling from the cerebrospinal fluid to neuronal cell bodies within the trigeminal ganglion and coupling to satellite glial cells. The movement of the dye from neuronal cell bodies to the satellite glial cells likely involved the formation of gap junctions between these two cells. This type of neuron-glia coupling within the trigeminal ganglion has been observed following peripheral inflammation or in response to inflammation and nerve injury (Cherkas et al., 2004, Vit et al., 2008, Durham and Garrett, 2010, Villa et al., 2010). Importantly, increased signaling between neurons and glia with the ganglion is associated with development and maintenance of peripheral sensitization of nociceptive neurons. Our results support the idea that elevated levels of CGRP within the spinal cord can facilitate bidirectional signaling and sensitization of primary trigeminal neurons by mediating increased neuron-glial cell coupling in the trigeminal ganglion.
In summary, findings from this study demonstrate that CGRP promotes peripheral sensitization of primary trigeminal nociceptive neurons to mechanical stimulation via a mechanism involving CGRP induction of PKA activity in neurons and glia and upregulation of GFAP in astrocytes in the upper spinal cord. Elevated levels of CGRP increased neuronal expression of P-ERK and promoted neuron-satellite glial cell coupling in the trigeminal ganglion. Thus, our results provide evidence to support the notion that CGRP-mediated central sensitization leads to an increase in trigeminal nociceptor sensitivity. Furthermore, we speculate that central to peripheral signaling as observed in our study may help to explain how peripheral nociceptors become sensitized, as reported in chronic orofacial pain conditions, even in the absence of any physical trauma or signs of inflammation.
Intrathecal CGRP promotes sensitization of primary trigeminal nociceptive neurons
Stimulatory effects of CGRP are mediated by PKA and involve astrocyte activation
CGRP-dependent behavioral changes associate with increased P-ERK levels in ganglion
Elevated CGRP levels in spinal cord promote neuron-glia communication in ganglion
Our results provide direct evidence of bidirectional signaling within trigeminal system
ACKNOWLEDGEMENTS
We would like to thank Jennifer Cashler and Angela Goerndt for their assistance with the animals. This work was supported by the National Institutes of Health [DE024629].
Abbreviations
- CGRP
calcitonin gene-related peptide
- TMJ
temporomandibular joint
- PKA
protein kinase A
- PBS
phosphate buffered saline
- P-ERK
phosphorylated extracellular signal-regulated kinase
- GFAP
glial fibrillary acidic protein
- SEM
standard error of the mean
- Iba1
ionized calcium-binding adapter molecule 1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: The authors of this paper do not have any conflicts of interest to report.
REFERENCES
- Adwanikar H, Ji G, Li W, Doods H, Willis WD, Neugebauer V. Spinal CGRP1 receptors contribute to supraspinally organized pain behavior and pain-related sensitization of amygdala neurons. Pain. 2007;132:53–66. doi: 10.1016/j.pain.2007.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aley KO, Levine JD. Role of protein kinase A in the maintenance of inflammatory pain. J Neurosci. 1999;19:2181–2186. doi: 10.1523/JNEUROSCI.19-06-02181.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballegaard V, Thede-Schmidt-Hansen P, Svensson P, Jensen R. Are headache and temporomandibular disorders related? A blinded study. Cephalalgia: an international journal of headache. 2008;28:832–841. doi: 10.1111/j.1468-2982.2008.01597.x. [DOI] [PubMed] [Google Scholar]
- Benemei S, Nicoletti P, Capone JA, Geppetti P. Pain pharmacology in migraine: focus on CGRP and CGRP receptors. Neurol Sci. 2007;28(Suppl 2):S89–93. doi: 10.1007/s10072-007-0757-5. [DOI] [PubMed] [Google Scholar]
- Bernstein C, Burstein R. Sensitization of the trigeminovascular pathway: perspective and implications to migraine pathophysiology. Journal of clinical neurology. 2012;8:89–99. doi: 10.3988/jcn.2012.8.2.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevilaqua Grossi D, Lipton RB, Bigal ME. Temporomandibular disorders and migraine chronification. Curr Pain Headache Rep. 2009;13:314–318. doi: 10.1007/s11916-009-0050-9. [DOI] [PubMed] [Google Scholar]
- Bhave G, Zhu W, Wang H, Brasier DJ, Oxford GS, Gereau RWt. cAMP-dependent protein kinase regulates desensitization of the capsaicin receptor (VR1) by direct phosphorylation. Neuron. 2002;35:721–731. doi: 10.1016/s0896-6273(02)00802-4. [DOI] [PubMed] [Google Scholar]
- Bossowska A, Crayton R, Radziszewski P, Kmiec Z, Majewski M. Distribution and neurochemical characterization of sensory dorsal root ganglia neurons supplying porcine urinary bladder. J Physiol Pharmacol. 2009;60(Suppl 4):77–81. [PubMed] [Google Scholar]
- Brain SD, Cox HM. Neuropeptides and their receptors: innovative science providing novel therapeutic targets. Br J Pharmacol. 2006;147(Suppl 1):S202–211. doi: 10.1038/sj.bjp.0706461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burstein R, Noseda R, Borsook D. Migraine: multiple processes, complex pathophysiology. J Neurosci. 2015;35:6619–6629. doi: 10.1523/JNEUROSCI.0373-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cady RJ, Denson JE, Sullivan LQ, Durham PL. Dual orexin receptor antagonist 12 inhibits expression of proteins in neurons and glia implicated in peripheral and central sensitization. Neuroscience. 2014;269:79–92. doi: 10.1016/j.neuroscience.2014.03.043. [DOI] [PubMed] [Google Scholar]
- Cady RJ, Glenn JR, Smith KM, Durham PL. Calcitonin gene-related peptide promotes cellular changes in trigeminal neurons and glia implicated in peripheral and central sensitization. Mol Pain. 2011;7:94. doi: 10.1186/1744-8069-7-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casatti CA, Frigo L, Bauer JA. Origin of sensory and autonomic innervation of the rat temporomandibular joint: a retrograde axonal tracing study with the fluorescent dye fast blue. J Dent Res. 1999;78:776–783. doi: 10.1177/00220345990780031001. [DOI] [PubMed] [Google Scholar]
- Cheng JK, Ji RR. Intracellular signaling in primary sensory neurons and persistent pain. Neurochem Res. 2008;33:1970–1978. doi: 10.1007/s11064-008-9711-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherkas PS, Huang TY, Pannicke T, Tal M, Reichenbach A, Hanani M. The effects of axotomy on neurons and satellite glial cells in mouse trigeminal ganglion. Pain. 2004;110:290–298. doi: 10.1016/j.pain.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Chiba T, Yamaguchi A, Yamatani T, Nakamura A, Morishita T, Inui T, Fukase M, Noda T, Fujita T. Calcitonin gene-related peptide receptor antagonist human CGRP-(8-37) Am J Physiol. 1989;256:E331–335. doi: 10.1152/ajpendo.1989.256.2.E331. [DOI] [PubMed] [Google Scholar]
- Dahan H, Shir Y, Velly A, Allison P. Specific and number of comorbidities are associated with increased levels of temporomandibular pain intensity and duration. J Headache Pain. 2015;16:528. doi: 10.1186/s10194-015-0528-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies AJ, Kim YH, Oh SB. Painful Neuron-Microglia Interactions in the Trigeminal Sensory System. TOPAINJ. 2010:14–28. [Google Scholar]
- Dodick D, Silberstein S. Central sensitization theory of migraine: clinical implications. Headache. 2006;46(Suppl 4):S182–191. doi: 10.1111/j.1526-4610.2006.00602.x. [DOI] [PubMed] [Google Scholar]
- Durham PL, Garrett FG. Emerging importance of neuron-satellite glia interactions within trigeminal ganglia in craniofacial pain. TOPAINJ. 2010;3:3–13. [Google Scholar]
- Edvinsson L, Nilsson E, Jansen-Olesen I. Inhibitory effect of BIBN4096BS, CGRP(8-37), a CGRP antibody and an RNA-Spiegelmer on CGRP induced vasodilatation in the perfused and non-perfused rat middle cerebral artery. Br J Pharmacol. 2007;150:633–640. doi: 10.1038/sj.bjp.0707134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald EM, Okuse K, Wood JN, Dolphin AC, Moss SJ. cAMP-dependent phosphorylation of the tetrodotoxin-resistant voltage-dependent sodium channel SNS. J Physiol. 1999;516(Pt 2):433–446. doi: 10.1111/j.1469-7793.1999.0433v.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furquim BD, Flamengui LM, Conti PC. TMD and chronic pain: a current view. Dental Press J Orthod. 2015;20:127–133. doi: 10.1590/2176-9451.20.1.127-133.sar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett FG, Hawkins JL, Overmyer AE, Hayden JB, Durham PL. Validation of a novel rat-holding device for studying heat- and mechanical-evoked trigeminal nocifensive behavioral responses. J Orofac Pain. 2012;26:337–344. [PMC free article] [PubMed] [Google Scholar]
- Goadsby PJ. Migraine pathophysiology. Headache. 2005;45(Suppl 1):S14–24. doi: 10.1111/j.1526-4610.2005.4501003.x. [DOI] [PubMed] [Google Scholar]
- Gosselin RD, Suter MR, Ji RR, Decosterd I. Glial cells and chronic pain. Neuroscientist. 2010;16:519–531. doi: 10.1177/1073858409360822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenspan JD, Slade GD, Bair E, Dubner R, Fillingim RB, Ohrbach R, Knott C, Mulkey F, Rothwell R, Maixner W. Pain sensitivity risk factors for chronic TMD: descriptive data and empirically identified domains from the OPPERA case control study. J Pain. 2011;12:T61–74. doi: 10.1016/j.jpain.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han JS, Li W, Neugebauer V. Critical role of calcitonin gene-related peptide 1 receptors in the amygdala in synaptic plasticity and pain behavior. J Neurosci. 2005;25:10717–10728. doi: 10.1523/JNEUROSCI.4112-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen RR, Vacca V, Pitcher T, Clark AK, Malcangio M. Role of extracellular calcitonin gene-related peptide in spinal cord mechanisms of cancer-induced bone pain. Pain. 2016;157:666–676. doi: 10.1097/j.pain.0000000000000416. [DOI] [PubMed] [Google Scholar]
- Hawkins JL, Denson JE, Miley DR, Durham PL. Nicotine stimulates expression of proteins implicated in peripheral and central sensitization. Neuroscience. 2015;290:115–125. doi: 10.1016/j.neuroscience.2015.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins JL, Durham PL. Prolonged Jaw Opening Promotes Nociception and Enhanced Cytokine Expression. Journal of oral & facial pain and headache. 2016;30:34–41. doi: 10.11607/ofph.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu HJ, Glauner KS, Gereau RWt. ERK integrates PKA and PKC signaling in superficial dorsal horn neurons. I. Modulation of A-type K+ currents. J Neurophysiol. 2003;90:1671–1679. doi: 10.1152/jn.00340.2003. [DOI] [PubMed] [Google Scholar]
- Hucho T, Levine JD. Signaling pathways in sensitization: toward a nociceptor cell biology. Neuron. 2007;55:365–376. doi: 10.1016/j.neuron.2007.07.008. [DOI] [PubMed] [Google Scholar]
- Ikeda H, Kiritoshi T, Murase K. Contribution of microglia and astrocytes to the central sensitization, inflammatory and neuropathic pain in the juvenile rat. Mol Pain. 2012;8:43. doi: 10.1186/1744-8069-8-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanusic JJ. Size, neurochemistry, and segmental distribution of sensory neurons innervating the rat tibia. J Comp Neurol. 2009;517:276–283. doi: 10.1002/cne.22160. [DOI] [PubMed] [Google Scholar]
- Ji RR, Gereau RWt, Malcangio M, Strichartz GR. MAP kinase and pain. Brain Res Rev. 2009;60:135–148. doi: 10.1016/j.brainresrev.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki Y, Kohno T, Zhuang ZY, Brenner GJ, Wang H, Van Der Meer C, Befort K, Woolf CJ, Ji RR. Ionotropic and metabotropic receptors, protein kinase A, protein kinase C, and Src contribute to C-fiber-induced ERK activation and cAMP response element-binding protein phosphorylation in dorsal horn neurons, leading to central sensitization. J Neurosci. 2004;24:8310–8321. doi: 10.1523/JNEUROSCI.2396-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohno T, Wang H, Amaya F, Brenner GJ, Cheng JK, Ji RR, Woolf CJ. Bradykinin enhances AMPA and NMDA receptor activity in spinal cord dorsal horn neurons by activating multiple kinases to produce pain hypersensitivity. J Neurosci. 2008;28:4533–4540. doi: 10.1523/JNEUROSCI.5349-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain. 2009;10:895–926. doi: 10.1016/j.jpain.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennerz JK, Ruhle V, Ceppa EP, Neuhuber WL, Bunnett NW, Grady EF, Messlinger K. Calcitonin receptor-like receptor (CLR), receptor activity-modifying protein 1 (RAMP1), and calcitonin gene-related peptide (CGRP) immunoreactivity in the rat trigeminovascular system: differences between peripheral and central CGRP receptor distribution. J Comp Neurol. 2008;507:1277–1299. doi: 10.1002/cne.21607. [DOI] [PubMed] [Google Scholar]
- Levy D, Strassman AM. Distinct sensitizing effects of the cAMP-PKA second messenger cascade on rat dural mechanonociceptors. J Physiol. 2002;538:483–493. doi: 10.1113/jphysiol.2001.013175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmberg AB, Brandon EP, Idzerda RL, Liu H, McKnight GS, Basbaum AI. Diminished inflammation and nociceptive pain with preservation of neuropathic pain in mice with a targeted mutation of the type I regulatory subunit of cAMP-dependent protein kinase. J Neurosci. 1997;17:7462–7470. doi: 10.1523/JNEUROSCI.17-19-07462.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvizon JC, Perez OA, Song B, Chen W, Bunnett NW, Grady EF, Todd AJ. Calcitonin receptor-like receptor and receptor activity modifying protein 1 in the rat dorsal horn: localization in glutamatergic presynaptic terminals containing opioids and adrenergic alpha2C receptors. Neuroscience. 2007;148:250–265. doi: 10.1016/j.neuroscience.2007.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RJ, Jung H, Bhangoo SK, White FA. Cytokine and chemokine regulation of sensory neuron function. Handbook of experimental pharmacology. 2009:417–449. doi: 10.1007/978-3-540-79090-7_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan ED, Twining C, Chacur M, Biedenkapp J, O’Connor K, Poole S, Tracey K, Martin D, Maier SF, Watkins LR. Spinal glia and proinflammatory cytokines mediate mirror-image neuropathic pain in rats. J Neurosci. 2003;23:1026–1040. doi: 10.1523/JNEUROSCI.23-03-01026.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno MJ, Terron JA, Stanimirovic DB, Doods H, Hamel E. Characterization of calcitonin gene-related peptide (CGRP) receptors and their receptor-activity-modifying proteins (RAMPs) in human brain microvascular and astroglial cells in culture. Neuropharmacology. 2002;42:270–280. doi: 10.1016/s0028-3908(01)00176-9. [DOI] [PubMed] [Google Scholar]
- Ohrbach R, Fillingim RB, Mulkey F, Gonzalez Y, Gordon S, Gremillion H, Lim PF, Ribeiro-Dasilva M, Greenspan JD, Knott C, Maixner W, Slade G. Clinical findings and pain symptoms as potential risk factors for chronic TMD: descriptive data and empirically identified domains from the OPPERA case-control study. J Pain. 2011;12:T27–45. doi: 10.1016/j.jpain.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olesen J, Burstein R, Ashina M, Tfelt-Hansen P. Origin of pain in migraine: evidence for peripheral sensitisation. The Lancet Neurology. 2009;8:679–690. doi: 10.1016/S1474-4422(09)70090-0. [DOI] [PubMed] [Google Scholar]
- Poveda Roda R, Bagan JV, Diaz Fernandez JM, Hernandez Bazan S, Jimenez Soriano Y. Review of temporomandibular joint pathology. Part I: classification, epidemiology and risk factors. Medicina oral, patologia oral y cirugia bucal. 2007;12:E292–298. [PubMed] [Google Scholar]
- Ren K, Dubner R. Neuron-glia crosstalk gets serious: role in pain hypersensitivity. Curr Opin Anaesthesiol. 2008;21:570–579. doi: 10.1097/ACO.0b013e32830edbdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell FA, King R, Smillie SJ, Kodji X, Brain SD. Calcitonin gene-related peptide: physiology and pathophysiology. Physiol Rev. 2014;94:1099–1142. doi: 10.1152/physrev.00034.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sessle BJ. Peripheral and central mechanisms of orofacial inflammatory pain. Int Rev Neurobiol. 2011;97:179–206. doi: 10.1016/B978-0-12-385198-7.00007-2. [DOI] [PubMed] [Google Scholar]
- Seybold VS. The role of peptides in central sensitization. Handbook of experimental pharmacology. 2009:451–491. doi: 10.1007/978-3-540-79090-7_13. [DOI] [PubMed] [Google Scholar]
- Staud R. Cytokine and immune system abnormalities in fibromyalgia and other central sensitivity syndromes. Curr Rheumatol Rev. 2015;11:109–115. doi: 10.2174/1573397111666150619094819. [DOI] [PubMed] [Google Scholar]
- Sun RQ, Lawand NB, Willis WD. The role of calcitonin gene-related peptide (CGRP) in the generation and maintenance of mechanical allodynia and hyperalgesia in rats after intradermal injection of capsaicin. Pain. 2003;104:201–208. doi: 10.1016/s0304-3959(03)00008-3. [DOI] [PubMed] [Google Scholar]
- Sun RQ, Tu YJ, Lawand NB, Yan JY, Lin Q, Willis WD. Calcitonin gene-related peptide receptor activation produces PKA- and PKC-dependent mechanical hyperalgesia and central sensitization. J Neurophysiol. 2004;92:2859–2866. doi: 10.1152/jn.00339.2004. [DOI] [PubMed] [Google Scholar]
- Takeda M, Takahashi M, Matsumoto S. Contribution of the activation of satellite glia in sensory ganglia to pathological pain. Neurosci Biobehav Rev. 2009;33:784–792. doi: 10.1016/j.neubiorev.2008.12.005. [DOI] [PubMed] [Google Scholar]
- Thalakoti S, Patil VV, Damodaram S, Vause CV, Langford LE, Freeman SE, Durham PL. Neuron-glia signaling in trigeminal ganglion: implications for migraine pathology. Headache. 2007;47:1008–1023. doi: 10.1111/j.1526-4610.2007.00854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda M, Mizokoshi A, Shigemoto-Mogami Y, Koizumi S, Inoue K. Activation of p38 mitogen-activated protein kinase in spinal hyperactive microglia contributes to pain hypersensitivity following peripheral nerve injury. Glia. 2004;45:89–95. doi: 10.1002/glia.10308. [DOI] [PubMed] [Google Scholar]
- Tzabazis AZ, Pirc G, Votta-Velis E, Wilson SP, Laurito CE, Yeomans DC. Antihyperalgesic effect of a recombinant herpes virus encoding antisense for calcitonin gene-related peptide. Anesthesiology. 2007;106:1196–1203. doi: 10.1097/01.anes.0000267603.32634.03. [DOI] [PubMed] [Google Scholar]
- Villa G, Ceruti S, Zanardelli M, Magni G, Jasmin L, Ohara PT, Abbracchio MP. Temporomandibular joint inflammation activates glial and immune cells in both the trigeminal ganglia and in the spinal trigeminal nucleus. Mol Pain. 2010;6:89. doi: 10.1186/1744-8069-6-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vit JP, Ohara PT, Bhargava A, Kelley K, Jasmin L. Silencing the Kir4.1 potassium channel subunit in satellite glial cells of the rat trigeminal ganglion results in pain-like behavior in the absence of nerve injury. J Neurosci. 2008;28:4161–4171. doi: 10.1523/JNEUROSCI.5053-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Ma W, Chabot JG, Quirion R. Calcitonin gene-related peptide as a regulator of neuronal CaMKII-CREB, microglial p38-NFkappaB and astroglial ERK-Stat1/3 cascades mediating the development of tolerance to morphine-induced analgesia. Pain. 2010;151:194–205. doi: 10.1016/j.pain.2010.07.006. [DOI] [PubMed] [Google Scholar]
- Wieseler-Frank J, Maier SF, Watkins LR. Glial activation and pathological pain. Neurochem Int. 2004;45:389–395. doi: 10.1016/j.neuint.2003.09.009. [DOI] [PubMed] [Google Scholar]
- Xie YF. Glial involvement in trigeminal central sensitization. Acta Pharmacol Sin. 2008;29:641–645. doi: 10.1111/j.1745-7254.2008.00801.x. [DOI] [PubMed] [Google Scholar]