Abstract
Background
The purpose of this study was to investigate the impact of surgical intervention on detection of circulating tumor cells (CTCs) in patients with squamous cell carcinoma of the head and neck (SCCHN.)
Methods
We utilized a negative depletion technique to identify cytokeratin (CK)-positive CTCs. The numbers of CTCs immediately before and after surgical resection were compared.
Results
Seventy-six blood samples from 38 patients with SCCHN were examined. Seventy-nine percent of the patients had CTCs detected before and after surgery. A total of 7.89% had no CTCs before surgery, yet had CTCs identified after surgery. Overall, 60.5% of patients had an increased number of CTCs/mL after surgery with a mean increase of 6.63-fold. A statistically significant increase in CTCs was seen after surgery (p = .02).
Conclusion
The timing of sample collection in patients with SCCHN who have surgical intervention can potentially impact the number of CTCs identified.
Keywords: circulating tumor cell, squamous cell carcinoma, surgery, immunomagnetic separation
INTRODUCTION
Cancer of the upper aerodigestive system is estimated to have 59,340 cases in the United States in 2015 and comprise 3.5% of all cancers.1 Squamous cell carcinoma of the head and neck (SCCHN) makes up approximately 95% of these tumors. Despite medical advances, the overall 5-year survival rate of approximately 50% has not changed significantly over the last several decades.2–4 Studies in other solid cancers, such as breast,5 prostate,6 lung,7,8 and colon cancers,9 have shown that the presence of circulating tumor cells (CTCs) that disseminates from the primary tumor has prognostic significance, and this dissemination may be an important step in hematogenous metastasis. We have demonstrated that CTCs can be detected in the peripheral blood of patients with SCCHN and the presence of CTCs correlated with reduced short-term disease-free survival.10 Before our studies, Partridge et al11 used a similar negative depletion technology and found that detection of disseminated tumor cells in patients with SCCHN correlated with a higher risk of local/distant recurrence as well as reduced survival.11 In inoperable SCCHN, the presence of CTCs has been linked to regional lymph nodal staging of 2b or higher.12 To date, the presence of lymph node metastasis in SCCHN is the most predictive prognostic factor that impacts survival.13,14
Beyond basic enumeration of CTCs in a patient's blood, it has also been reported that CTCs may appear in the blood stream because of mechanical manipulation of the tumor during surgical intervention,15–18 fine-needle aspirations,19 during colonoscopy,20 and endorectal ultrasound.21 The release of these cells is a potential concern on multiple levels, the least of which is influencing traditional CTC counts. As far as we know, CTC counts before and after open surgical resection in the operating room has not been performed in SCCHN. Our previously published SCCHN studies have used blood samples taken after surgical intervention.
The head and neck region is a highly vascularized tissue. A released cell, such as a CTC, potentially could enter circulation after physical manipulation during surgery, through lymphatics or veins that return blood to the superior vena cava to the right atrium and subsequently the ventricle of the heart. From the right side of the heart, this blood circulates to the lungs for oxygenation through a capillary network and then returns to the heart for pumping into arterial circulation where distal end organs again contain a capillary network before entering venous circulation and returning to the heart. A common assumption of CTC is that they are relatively large compared to normal peripheral blood cells; several methodologies to identify CTCs are based on size alone.22,23 With head and neck surgery, the first small vasculature that such a proposed released cell would encounter would be the capillary network in the lungs. It should be noted, however, as we have reported previously, that the relative size of the putative CTCs in our previous studies are similar in size to normal peripheral blood cells. Such cells may continue to repeatedly circulate around the body.
MATERIALS AND METHODS
This study was approved by the Institutional Review Board. Informed consent was obtained from patients who were undergoing surgical intervention at the Arthur G. James Cancer Hospital and Solove Research Institute and Comprehensive Cancer Center at The Ohio State University. Inclusion criteria were adults >18 years with histologically confirmed SCCHN undergoing an open surgical excision of the primary tumor. Exclusion criteria included patients with known metastases and any patient who received a blood transfusion intraoperatively. Peripheral venous blood samples were procured from 38 patients (total of 76 samples.) Five to 10 mL were collected in green-top BD Vacutainer tubes immediately before and after surgery while in the operating room. Each venous sample was collected from an extremity (arm or leg) where the intravenous fluids were not being administered and the initial 2 to 3 mL was drawn off and discarded to prevent contamination. A fresh syringe was then used to obtain the sample. The samples were stored at 4°C and were processed within 24 hours. Operators were completely blinded to the time point of the samples as well as the clinical and correlative information during the sample processing and CTC enumeration steps. The CTC enrichment process has been previously discussed in detail.24 Briefly, the red blood cells were lysed using a lysis buffer and the remaining leukocytes were immunomagnetically labeled using anti-CD45 tetrameric antibody complex and dextran-coated magnetic nanoparticles (cat #18259; Stem Cell Tech, Vancouver, BC). The magnetically labeled cells were then passed through our optimized immunomagnetic separation system, to deplete a majority of the leukocytes. An aliquot of the enriched sample containing the CTCs was used for CTC enumeration. Cell suspension containing CTCs was stored in 10% neutral buffered formalin until further processing. Cells were cytospun onto a microscopic slide using a Shandon Cytospin instrument at 1800 rpm for 5 minutes. The cytospun slides were hydrated in phosphate-buffered saline before staining with anti-cytokeratin fluorescein isothiocyanate (FITC) antibody (1:10, Miltenyi Biotech, clone:CK3-6H5, 130-080-101) targeting cytokeratins 8, 18, and 19 for 30 minutes at 37°C before mounting with mounting media containing 4′,6′-diamidino-2-phenylindole (DAPI; Vector Laboratories, H-1500). Slides were observed under a Nikon 80i epifluorescent microscope equipped with band pass filters for diamidino-phenylindole (DAPI) and FITC emission, and cells that were positive for both FITC and DAPI and had high nuclear to cytoplasmic ratios were counted as CTCs. Images of the slides were taken using a Zeiss LSM510 confocal microscope. The number of CTCs per mL of peripheral blood was calculated. Blood samples were drawn from healthy volunteers or patients with benign conditions and processed in the same manner as a clinical sample. Statistical analyses were performed using GraphPad Prism version 6 (San Diego, CA). The D'Agostino and Pearson omnibus normality test was performed to test if the CTC counts before or after surgery were normally distributed. Wilcoxon matched-pair sign rank test was used to determine if there was a statistically significant difference between CTC numbers before and after surgery. Any p value ≤ .05 was considered statistically significant.
RESULTS
A total of 38 patients with SCCHN (mean age, 64.2 years; range, 51–83 years) who all underwent open surgical excision in the operating room, had their venous blood examined for CTCs. Men comprised 63% and women comprised 37% of the patients. The sites were 62% in the oral cavity, 15% in the oropharynx, and 23% in the larynx. Overall staging included: 14% stage 1; 20% stage 2; 26% stage 3; and 40% stage 4. Figures 1 and 2 display examples of enriched patient samples from a patient in which no change in putative CTC concentration was observed, and a second patient in which a large increase in CTC concentration was observed. Overall, as shown in Table 1, CTCs were detected in 79.0% of patients before and after surgery. It was noted that 7.89% of patients had detected CTCs after surgical intervention when none were detected in the before sample. An additional 10.5% of patients had CTCs detected in sample before surgery, but not in the after surgery sample. The D'Agostino & Pearson omnibus normality test showed that the data were not normally distributed (p value < .0001 for both presurgery and postsurgery). Because the data were not normally distributed, a nonparametric Wilcoxon matched-pair sign rank test was performed to test for statistical significance. There was a significant difference in CTC counts using matched pairs analysis as a result of surgical intervention (2-tailed p value = .0224). In particular, CTCs measured soon after surgery were significantly higher than CTCs measured before surgery (1-tailed p value = .0112). There was no correlation to age, sex, or overall stage of cancer. There was no correlation between CTCs and positive margins, disruption of tumor or aerodigestive tract, or length of surgery. It was found that 60.5% of patients had an increased number of CTCs after surgical intervention (see Figure 3). The range of CTCs in the peripheral blood before/after surgical intervention from oral cavity, oropharyngeal, and laryngeal sites is shown in Figure 4. None of the healthy control blood samples had similar, brightly stained, cytokeratin-positive cells, or cells with any morphology resembling CTCs.
FIGURE 1.
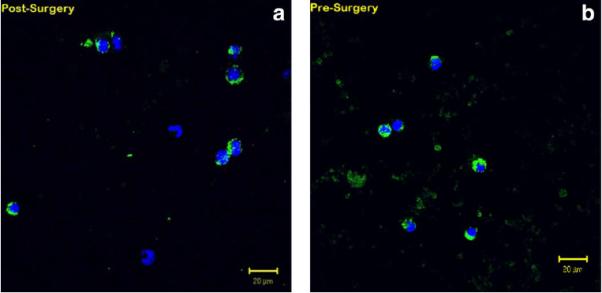
Representative patient with squamous cell carcinoma of the head and neck (SCCHN) sample with no change in circulating tumor cells/mL peripheral blood post-surgery (a) compared to pre-surgery (b), as determined by dual color immunofluorescent staining: green = cytokeratin; blue = diamidino-phenylindole.
FIGURE 2.
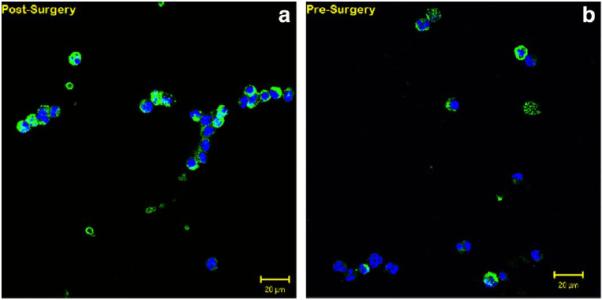
Representative patient with squamous cell carcinoma of the head and neck (SCCHN) sample demonstrating a 2.5-fold change in circulating tumor cells/mL peripheral blood post-surgery (a) compared to pre-surgery (b), as determined by dual color immunofluorescent staining: green = cytokeratin; blue = diamidino-phenylindole.
TABLE 1.
Correlation between circulating tumor cell detection and the time of distal venous blood draw (presurgery or postsurgery) in 38 patients undergoing surgical intervention for squamous cell carcinoma of the head and neck.
| Before surgical intervention | After surgical intervention | Overall frequency |
|---|---|---|
| Negative | Negative | 1/38 (2.6%) |
| Negative | Positive | 3/38 (7.89%) |
| Positive | Negative | 4/38 (10.5%) |
| Positive | Positive | 30 (79.0%) |
| 34/38 (89.5%) positive | 33/38 (86.8%) positive |
FIGURE 3.
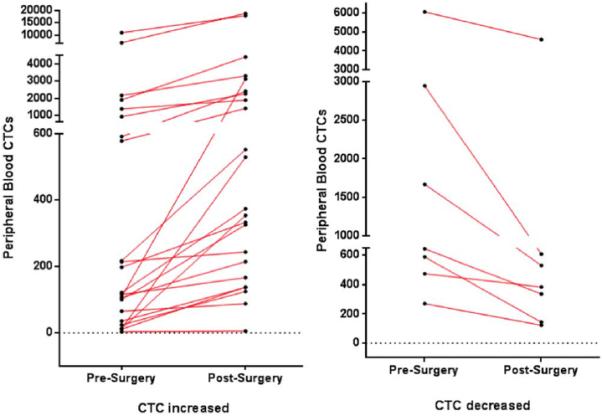
Ladder plots displaying the number and degree of circulating tumor cell increase (left) and decrease (right) in pre-surgical and post-surgical specimens. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com]
FIGURE 4.
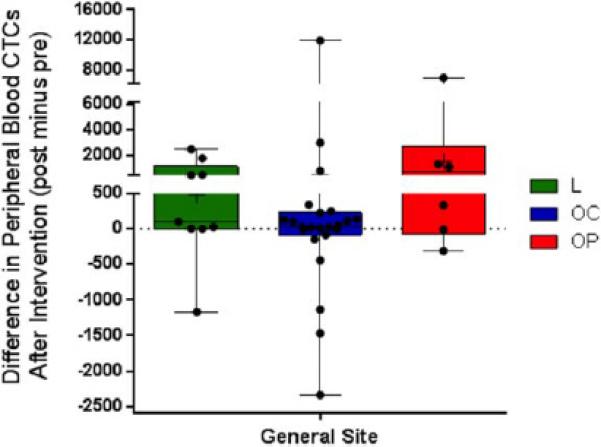
Changes in circulating tumor cells (CTCs)/mL of peripheral blood before and after surgical intervention by general site of tumor (L = larynx; OC = oral cavity; OP, oropharynx). Each data point, minimum and maximum, is shown for these sites. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com]
DISCUSSION
There has been limited data on the impact of an intervention on SCCHN with regard to CTCs. Ramani et al25 looked at the presence of cytokeratin (CK)-19 by reverse transcriptase-polymerase chain reaction before and after incisional biopsy of oral cavity squamous cell carcinoma. They found that, in the 10 patients studied, there was no dissemination of cancer cells after incisional biopsy in these patients.25 A prior study by Kusukawa et al26 demonstrated the detection of CK-19 reverse transcriptase-polymerase chain reaction in this same tumor site, and reported that dissemination of cancer cells into circulation does occur after an incisional biopsy. Although both of these studies looked at the presence of mRNA alone, neither had visual conformation of CTCs. Studies by Ohtake et al27 have shown that an incision has caused increased lymph node metastasis in dimethylbenz[a]anthracene-induced tongue cancer in the hamster model; the incidence of regional lymph node metastasis increased by 65.9% after repeated incisions of the lesion. It is not clear what the clinical relevance of such findings is at this time.
It has been suggested that CTCs may be destroyed in the microvascular capillary networks by the host immune surveillance system, including macrophages and natural killer cells.28 We have identified CTCs in the blood from the distal extremity venous system with patients with SCCHN undergoing open surgical resection +/− cervical lymphadenectomy, meaning that these CTCs have gone through a minimum of 2 capillary networks (likely circulating numerous times) before detection. The open surgical procedures ranged from approximately 90 minutes to 6 hours duration. As shown in Figures 1 and 2, clumps of CTCs are seen postsurgery, but these aggregates likely would form intravascularly after passing through capillary circulation. The mechanisms by which this happens is not yet elucidated, but the CTCs in SCCHN are likely of similar size to circulating leukocytes. Some have reported that CTCs may be only transiently detectable, and that it is a dynamic and periodic event.29 There are several unanswered questions with regard to timing of blood sample collection when any tumor-related intervention is performed, the location of venous blood sample collection with regard to the tumor location, and what clinical relevance exists. Each solid cancer should be individually investigated as the specific protocols of sample collection with invasive procedures may be critical and influence CTC detection. This is important as most published studies with CTCs in all solid cancers have not addressed this issue in the literature. Even within a specific type of cancer, like SCCHN, the tumor location may also influence identification of CTCs, as shown in Figure 4. Another important concept to consider with respect to the concentration of these rare cells is the intravascular fluid balance in patients under general anesthesia during these surgical procedures. There is always an estimated blood loss that occurs. None of the patients in this study received any blood transfusion; however, all surgical patients had a positive fluid balance, indicating they received additional intravenous fluid (directly into a distal vein, unrelated to the location of the blood sample taken for this study), which increases blood volume several fold more than the estimated blood loss, resulting in hemodilution. Because of dilution of the blood, the concentration of CTCs/mL blood may be underestimated.
We have previously demonstrated some of the cells we are reporting as putative CTC in this study are also epithelial cell adhesion molecule negative, cytokeratin and vimentin positive, and can be further positive for epidermal growth factor receptor or CD44.30 At this point, it is unclear what, if any, clinical significance can be associated with these changes in concentrations of putative CTC after surgery. Epithelial to mesenchymal transition may occur as cancer cells acquire a more aggressive migratory phenotype.31–34 It is possible that these surgically released CTCs are inherently different than CTCs that enter the intravascular space naturally. None of the 7.89% of patients who had CTCs detected postoperatively, not preoperatively, developed locoregional disease recurrence or distant disease recurrence. Further characterization of these CTCs identified before and after surgery, with multimarker analysis in combination with long-term clinical follow-up may yield a greater understanding of the role of surgically released CTCs in SCCHN.
CONCLUSIONS
A statistically significant increase in the CTC counts immediately after surgical intervention, while still in the operating room, was seen in patients with SCCHN. The timing of blood sample collection for such solid cancers that undergo surgical intervention, such as SCCHN, can potentially impact the number of CTCs identified. Although a prognostic blood test for CTCs could have important treatment and surveillance implications, the viability and clinical significance of potentially surgically released CTCs in SCCHN is still not known; it is possible that these cells have a different phenotype than CTCs that enter the intravascular space by other mechanisms.
Acknowledgments
Contract grant sponsor: This work was supported by the following agencies: the National Science Foundation (BES-0124897); the National Cancer Institute (R01 CA97391-01A1); the State of Ohio Third Frontier Program (ODOD 26140000:TECH 07-001); the National Cancer Institute CCC Core Grant (P30 CA16058), and the National Science Foundation (EEC 0425626).
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Gath HJ, Brakenhoff RH. Minimal residual disease in head and neck cancer. Cancer Metastasis Rev. 1999;18:109–126. doi: 10.1023/a:1006268621730. [DOI] [PubMed] [Google Scholar]
- 3.Cohen EE, Lingen MW, Vokes EE. The expanding role of systemic therapy in head and neck cancer. J Clin Oncol. 2004;22:1743–1752. doi: 10.1200/JCO.2004.06.147. [DOI] [PubMed] [Google Scholar]
- 4.Cooper JS, Pajak TF, Forastiere AA, et al. Postoperative concurrent radio-therapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med. 2004;350:1937–1944. doi: 10.1056/NEJMoa032646. [DOI] [PubMed] [Google Scholar]
- 5.Braun S, Vogl FD, Naume B, et al. A pooled analysis of bone marrow micrometastasis in breast cancer. N Engl J Med. 2005;353:793–802. doi: 10.1056/NEJMoa050434. [DOI] [PubMed] [Google Scholar]
- 6.Köllermann J, Weikert S, Schostak M, et al. Prognostic significance of disseminated tumor cells in the bone marrow of prostate cancer patients treated with neoadjuvant hormone treatment. J Clin Oncol. 2008;26:4928–4933. doi: 10.1200/JCO.2007.15.0441. [DOI] [PubMed] [Google Scholar]
- 7.Kasimir-Bauer S, Schleucher N, Weber R, Neumann R, Seeber S. Evaluation of different markers in non-small cell lung cancer: prognostic value of clinical staging, tumour cell detection and tumour marker analysis for tumour progression and overall survival. Oncol Rep. 2003;10:475–482. [PubMed] [Google Scholar]
- 8.Pantel K, Izbicki J, Passlick B, et al. Frequency and prognostic significance of isolated tumour cells in bone marrow of patients with non-small-cell lung cancer without overt metastases. Lancet. 1996;347:649–653. doi: 10.1016/s0140-6736(96)91203-9. [DOI] [PubMed] [Google Scholar]
- 9.Leinung S, Würl P, Schönfelder A, Weiss CL, Röder I, Schönfelder M. Detection of cytokeratin-positive cells in bone marrow in breast cancer and colorectal carcinoma in comparison with other factors of prognosis. J Hematother Stem Cell Res. 2000;9:905–911. doi: 10.1089/152581600750062354. [DOI] [PubMed] [Google Scholar]
- 10.Jatana KR, Balasubramanian P, Lang JC, et al. Significance of circulating tumor cells in patients with squamous cell carcinoma of the head and neck: initial results. Arch Otolaryngol Head Neck Surg. 2010;136:1274–1279. doi: 10.1001/archoto.2010.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Partridge M, Brakenhoff R, Phillips E, et al. Detection of rare disseminated tumor cells identifies head and neck cancer patients at risk of treatment failure. Clin Cancer Res. 2003;9:5287–5294. [PubMed] [Google Scholar]
- 12.Hristozova T, Konschak R, Stromberger C, et al. The presence of circulating tumor cells (CTCs) correlates with lymph node metastasis in nonresectable squamous cell carcinoma of the head and neck region (SCCHN). Ann Oncol. 2011;22:1878–1885. doi: 10.1093/annonc/mdr130. [DOI] [PubMed] [Google Scholar]
- 13.Jones AS, Roland NJ, Field JK, Phillips DE. The level of cervical lymph node metastases: their prognostic relevance and relationship with head and neck squamous carcinoma primary sites. Clin Otolaryngol Allied Sci. 1994;19:63–69. doi: 10.1111/j.1365-2273.1994.tb01150.x. [DOI] [PubMed] [Google Scholar]
- 14.Leemans CR, Tiwari R, Nauta JJ, van der Waal I, Snow GB. Regional lymph node involvement and its significance in the development of distant metastases in head and neck carcinoma. Cancer. 1993;71:452–456. doi: 10.1002/1097-0142(19930115)71:2<452::aid-cncr2820710228>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 15.Sawabata N, Okumura M, Utsumi T, et al. Circulating tumor cells in peripheral blood caused by surgical manipulation of non-small-cell lung cancer: pilot study using an immunocytology method. Gen Thorac Cardiovasc Surg. 2007;55:189–192. doi: 10.1007/s11748-007-0101-2. [DOI] [PubMed] [Google Scholar]
- 16.Healy WL, Pfeifer BA, Kurtz SR, et al. Evaluation of autologous shed blood for autotransfusion after orthopaedic surgery. Clin Orthop Relat Res. 1994;299:53–59. [PubMed] [Google Scholar]
- 17.Hansen E, Wolff N, Knuechel R, Ruschoff J, Hofstaedter F, Taeger K. Tumor cells in blood shed from the surgical field. Arch Surg. 1995;130:387–393. doi: 10.1001/archsurg.1995.01430040049007. [DOI] [PubMed] [Google Scholar]
- 18.Papavasiliou P, Fisher T, Kuhn J, Nemunaitis J, Lamont J. Circulating tumor cells in patients undergoing surgery for hepatic metastases from colorectal cancer. Proc (Bayl Univ Med Cent) 2010;23:11–14. doi: 10.1080/08998280.2010.11928572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu XC, Chow LW. Fine needle aspiration may shed breast cells into peripheral blood as determined by RT-PCR. Oncology. 2000;59:217–222. doi: 10.1159/000012164. [DOI] [PubMed] [Google Scholar]
- 20.Koch M, Kienle P, Sauer P, et al. Hematogenous tumor cell dissemination during colonoscopy for colorectal cancer. Surg Endosc. 2004;18:587–591. doi: 10.1007/s00464-003-9066-0. [DOI] [PubMed] [Google Scholar]
- 21.Koch M, Antolovic D, Kienle P, et al. Increased detection rate and potential prognostic impact of disseminated tumor cells in patients undergoing endorectal ultrasound for rectal cancer. Int J Colorectal Dis. 2007;22:359–365. doi: 10.1007/s00384-006-0152-3. [DOI] [PubMed] [Google Scholar]
- 22.Vona G, Sabile A, Louha M, et al. Isolation by size of epithelial tumor cells: a new method for the immunomorphological and molecular characterization of circulating tumor cells. Am J Pathol. 2000;156:57–63. doi: 10.1016/S0002-9440(10)64706-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Giorgi V, Pinzani P, Salvianti F, et al. Application of a filtration- and isolation-by-size technique for the detection of circulating tumor cells in cutaneous melanoma. J Invest Dermatol. 2010;130:2440–2447. doi: 10.1038/jid.2010.141. [DOI] [PubMed] [Google Scholar]
- 24.Yang L, Lang JC, Balasubramanian P, et al. Optimization of an enrichment process for circulating tumor cells from the blood of head and neck cancer patients through depletion of normal cells. Biotechnol Bioeng. 2009;102:521–534. doi: 10.1002/bit.22066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramani P, Thomas G, Ahmed S. Use of Rt-PCR in detecting disseminated cancer cells after incisional biopsy among oral squamous cell carcinoma patients. J Cancer Res Ther. 2005;1:92–97. doi: 10.4103/0973-1482.16708. [DOI] [PubMed] [Google Scholar]
- 26.Kusukawa J, Suefuji Y, Ryu F, Noguchi R, Iwamoto O, Kameyama T. Dissemination of cancer cells into circulation occurs by incisional biopsy of oral squamous cell carcinoma. J Oral Pathol Med. 2000;29:303–307. doi: 10.1034/j.1600-0714.2000.290703.x. [DOI] [PubMed] [Google Scholar]
- 27.Ohtake K, Shingaki S, Nakajima T. Effects of incision and irradiation on regional lymph node metastasis in carcinoma of the hamster tongue. Oral Surg Oral Med Oral Pathol. 1990;70:62–69. doi: 10.1016/0030-4220(90)90180-z. [DOI] [PubMed] [Google Scholar]
- 28.Hanna N. The role of natural killer cells in the control of tumor growth and metastasis. Biochim Biophys Acta. 1985;780:213–226. doi: 10.1016/0304-419x(85)90004-6. [DOI] [PubMed] [Google Scholar]
- 29.Romsdahl MD, Chu EW, Hume R, Smith RR. The time of metastasis and release of circulating tumor cells as determined in an experimental system. Cancer. 1961;14:883–888. doi: 10.1002/1097-0142(199007/08)14:4<883::aid-cncr2820140426>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 30.Balasubramanian P, Lang JC, Jatana KR, et al. Multiparameter analysis, including EMT markers, on negatively enriched blood samples from patients with squamous cell carcinoma of the head and neck. PLoS One. 2012;7:e42048. doi: 10.1371/journal.pone.0042048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berx G, Rasp e E, Christofori G, Thiery JP, Sleeman JP. Pre-EMTing metastasis? Recapitulation of morphogenetic processes in cancer. Clin Exp Metastasis. 2007;24:587–597. doi: 10.1007/s10585-007-9114-6. [DOI] [PubMed] [Google Scholar]
- 32.Chaffer CL, Thompson EW, Williams ED. Mesenchymal to epithelial transition in development and disease. Cells Tissues Organs. 2007;185:7–19. doi: 10.1159/000101298. [DOI] [PubMed] [Google Scholar]
- 33.Mani SA, Guo W, Liao MJ, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sarriö D, Rodriguez-Pinilla SM, Hardisson D, Cano A, Moreno-Bueno G, Palacios J. Epithelial-mesenchymal transition in breast cancer relates to the basal-like phenotype. Cancer Res. 2008;68:989–997. doi: 10.1158/0008-5472.CAN-07-2017. [DOI] [PubMed] [Google Scholar]


