Abstract
Cannabis (Cannabis sativa, Cannabis indica) is the illicit drug most frequently abused by young men and women. The growing use of the drug has raised attention not only on the impact of direct exposure on the developing brain and behavior later in life, but also on potential cross-generational consequences. Our previous work demonstrated that adolescent exposure to Δ9-tetrahydrocannabinol (THC), the main psychoactive component of cannabis, affects reward-related behavior and striatal gene expression in male offspring that were unexposed to the drug during their own lifespan. The significant sex differences documented for most addiction and psychiatric disorders suggest that understanding the perturbation of the brain in the two sexes due to cannabis could provide insights about neuronal systems underpinning vulnerability to psychiatric illnesses. In the current study, we expanded our previous observations in males by analyzing the female brain for specific aberrations associated with cross-generational THC exposure. Based on the impact of adolescent development on subsequent adult behavioral pathology, we examined molecular patterns during both adolescence and adulthood. The results revealed a switch from the ventral striatum during adolescence to the dorsal striatum in adulthood in alterations of gene expression related to synaptic plasticity in both sexes. Females, however, exhibited stronger correlation patterns between genes and also showed locomotor disturbances not evident in males. Overall, the findings demonstrate cross-generational consequences of parental THC exposure in both male and female offspring.
Keywords: marijuana, cross-generational transmission, development, striatum, gender, synaptic plasticity
1. Introduction
Over the past decade there has been a significant shift in society regarding marijuana (Cannabis sativa, Cannabis indica) such that the current perceived lack of health risk of this drug has lead to more teens today smoking cannabis than cigarettes (Johnston et al., 2012). The growing acceptance in the Western world for the consumption of marijuana has important relevance particularly for women with far reaching implications. Firstly, more young women have increased their use of marijuana and this also extends to periods of pregnancy (SAMSHA, 2014). Secondly, the use of marijuana by women could possibly impact not only their immediate health, but also the health of their offspring, and such potential long-term consequences are still not known. It is clear that the biological differences between males and females influence the development of neuropsychiatric phenotypes (Figueira and Ouakinin, 2010; Savic and Engel, 2014; Shen et al., 2015), but the ways in which marijuana use causes long-term problems that affect the two sexes remain largely unexplored. As such, the inclusion of females in studies of cannabis is essential to decipher the neurobiological systems vulnerable in the different sexes to also ultimately guide targeted treatments in a sex-specific manner.
For decades researchers have debated whether the biological consequences of an individual’s life experiences are reprogrammed from parent to offspring. While some studies report no remarkable effects of such experiences, a growing body of literature has challenged these findings and demonstrated significant aberrations that influence disease risk through the germline from parent to child. (Bohacek and Mansuy, 2013; Szyf, 2015). By now, several cases of cross-generational (parent-child) transmission regarding drugs of abuse have been published, describing behavioral phenotypes and molecular disturbances in the offspring of parents that were exposed to drugs before mating, including cannabinoids (reviewed in (Szutorisz and Hurd, 2015; Vassoler and Sadri-Vakili, 2014; Yohn et al., 2015)). However, most of these studies did not address the impact of gender in the context of naturally occurring cannabinoids such as Δ9-tetrahydrocannabinol (THC), the main psychoactive component of marijuana (Byrnes et al., 2012; Vassoler et al., 2013).
Recently, observations from our previous studies have shown that exposure of adolescent rats to THC before mating (“germline exposure”) leads to cross-generational behavioral and gene regulation abnormalities in the striatum in the subsequent generation (“F1 offspring”), most likely achieved via impaired epigenetic regulatory processes (Szutorisz et al., 2014; Watson et al., 2015). Based on the observed striatal mRNA disturbances in F1 male progeny, along with the behavioral abnormalities identified in males, we set out to explore whether males and females are uniquely affected in this model. In the current study, we investigate potential sex-specific effects of cross-generational THC exposure on gene expression that could provide biological insights into neuropsychiatric vulnerability that frequently show sex differences in their incidence and course of the disorders.
2. Materials and Methods
2.1. Drugs
Δ9-THC (50 mg/ml in ethanol solution) was evaporated under nitrogen gas, dissolved in 0.9% NaCl (saline) containing 0.3% Tween 80 to a concentration of 0.75 mg/ml (Dinieri and Hurd, 2012). Control “vehicle” (VEH) solution was saline containing 0.3% Tween 80.
2.2. Animals and cross-generational THC paradigm
The cross-generational THC animal model has been described in detail previously (Szutorisz et al., 2014; Watson et al., 2015) and is illustrated in Fig. 1. Briefly, for the F0 breeding parents, 21-day old male and female Long-Evans rats were purchased from Charles River Laboratories, Inc. (Wilmington, MA) and housed in same sex groups. After one week of acclimation in the facility, the rats were administered THC (1.5 mg/kg intraperitoneally, every 3rd day from postnatal day 28-49) or VEH. After birth at ~PND2, mixed litters were established combining an approximately equal number (12-14) of pups from THC- and VEH-exposed parents with a balanced proportion of males and females in each litter. The litters were cross-fostered to drug-naïve surrogates, which were used as nursing mothers. F1 offspring were weaned at ~PND24 and groups of 3-4 animals were maintained without any drug treatment or testing on normal 12-h light/dark cycle with ad libitum access to food and water until adolescence (~PND35) or adulthood (~PND62). Animal care and handling were performed by technicians unfamiliar with parental treatment history. Animals were anaesthetized with CO2, decapitated, brains were frozen in isopentane, and stored at -80°C until subsequent experiments.
Figure 1.
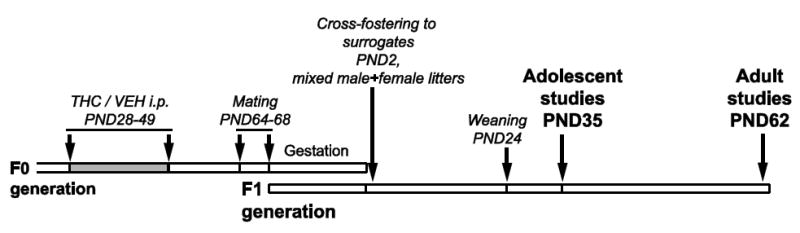
Schematic overview of the experimental approach used to study the cross-generational effects of parental germline THC exposure in F1 offspring. For details, see Materials and Methods.
2.3. Quantitative reverse transcription PCR (qRT-PCR) analyses
Striatal brain regions (dorsal and ventral striatum) were dissected from frozen adolescent and adult brains of rats with a 15-gauge sample punch on a cold plate based on landmarks according to commonly used atlases (Paxinos and Watson, 2007). RNA was prepared from bilateral tissue punches using the RNAqueous-Micro Kit (Thermo Fisher Scientific, Waltham, MA) and cDNA was obtained with a first-strand synthesis kit (Quanta Biosciences, Gaithersburg, MD). Quantitative real-time PCR was performed using the LightCycler480 Probes Master reagent (Roche, Basel, Switzerland) and the TaqMan PCR program in a LightCycler 480 instrument (Roche).
The following Taqman-based assays (Applied Biosystems, Foster City, CA) were used in triplicate PCR reactions: Cnr1, Rn00562880_m1; Grin1, Rn01436038_m1; Grin2A, Rn00561341_m1; Grin2B, Rn00680474_m1; Gria1, Rn00709588_m1; Gria2, Rn00568514_m1; Dlg4, Rn00571479_m1; Dlgap3, Rn00597609_m1; beta-2-microglobulin, Rn00560865_m1. Each gene of interest was run in duplex with a reference gene (beta-2-microglobulin), data were normalized via the ΔΔCT method (Livak and Schmittgen, 2001).
To directly compare qRT-PCR results in male and female rats from the same litter, results described for male subjects were re-analyzed from previously published data (Szutorisz et al., 2014) and combined with that from female littermates that derived from the same breeding cohort. Processing and qPCR analysis of all male and female brain samples were done simultaneously. Notably, the expression level of the reference gene was not different in males and females. In the adolescent male and female sample (N=5/treatment/sex), all subjects were derived from a different birth litter; in the adult sample (N=7-8/ treatment/sex) no more than two littermates per group were included in the experiment. No litter effects were found in the statistical analyses. None of the animals used for gene expression analyses underwent any behavioral testing or treatment, only handling.
2.4. Open field locomotor behavior testing
Rats were tested during the dark phase of the light/dark cycle in a standard squared plexiglass arena (40.6 cm × 40.6 cm; MED Associates Inc., St. Albans, VT), equipped with the Versamax activity monitor system (AccuScan Instruments, Inc., Colombus, OH). Locomotor activity was recorded for 60 minutes and the number of beam breaks were analyzed in 5 min time intervals. Test subjects were derived from the same breeding as the animals used for qRT-PCR experiments, each test group (N=10-12/treatment/sex) included animals from 5-7 birth litters and no litter effects were detected in the statistical analyses.
2.5. Statistical analyses
Outliers in the gene expression data were detected using Grubbs test. Treatment by gender interactions for single genes were tested using two-way analysis of variance models (ANOVAs). Comparisons of mRNA expression levels within each sex to assess direction of change in males and females were performed by Welch two-sample t-tests. Pearson correlations were calculated to assess and visualize the relationship between expression levels of genes using multivariate analyses in the JMP 12 software (SAS Institute, Cary, NC). Open field locomotor activity experiments were analyzed using ANOVAs with repeated measures using a multivariate model followed by post-hoc pairwise t-test comparisons when appropriate to address the effect of THC at specific time points.
3. Results
3.1. Cross-generational THC exposure leads to developmental gene expression abnormalities in the striatum of male and female offspring
Based on our initial findings (Szutorisz et al., 2014; Watson et al., 2015) and considering the central role of striatal circuitry in behaviors related to reward processing, motivation, emotion and motor activity (Everitt and Robbins, 2005; Girault, 2012; Koob and Volkow, 2010), we focused on studying gene expression profiles of the dorsal and ventral striatum (nucleus accumbens) in F1 subjects of parents with a prior history of adolescent THC or vehicle exposure. Different lines of evidence in our earlier work (Szutorisz et al., 2014; Watson et al., 2015) had already demonstrated alterations in the striatum as a consequence of germline THC exposure and the observed striatal changes pointed especially in the direction of components of the glutamatergic system and molecules relevant to synaptic plasticity.
To expand on the above work and address the impact of sex, here we characterized relevant gene expression profiles in the ventral and dorsal striatum of F1 male and female offspring (Table 1 and 2). We focused on genes encoding subunits of the CB1 receptor (Cnr1), the NMDA receptor (Grin1, Grin2A, Grin2B), and the AMPA receptor (Gria 1, Gria2). We also included two other important regulators of synaptic function: Dlg4 that encodes for postsynaptic density protein 95 (Psd-95) and Dlgap3 that generates the Sapap3 protein, which interacts with Psd-95. Both genes have been broadly implicated in neuropsychiatric phenotypes in humans and animals (de Bartolomeis et al., 2014; Hall et al., 2015; Rauch and Carlezon, 2013; Wan et al., 2014; Wang et al., 2014; Zuchner et al., 2009). mRNA levels were measured by quantitative reverse transcription PCR at two developmental time points, in adolescent (PND35) and young adult (PND62) F1 animals.
Table 1.
Alterations of ventral striatal mRNA levels in F1 offspring analyzed by ANOVAs in combined male and female data sets.
| Gene | Ventral Striatum - Adolescence | |||||
|---|---|---|---|---|---|---|
| % THC/VEH ± SEM | % Female/Male ± SEM | F ratio | Effect (p-value) | |||
| Treatment *Sex | Treatment | Sex | ||||
| Cnr1 | 110 ± 8.1 | 107 ± 8.2 | 2.07 | 0.040 | 0.391 | 0.515 |
| Grin1 | 91 ± 4.2 | 122 ± 7.1 | 4.28 | 0.012 | 0.376 | 0.065 |
| Grin2A | 108 ± 8.4 | 166 ± 6.4 | 14.22 | 0.062 | 0.357 | <0.0001 |
| Grin2B | 89 ± 4.0 | 129 ± 3.0 | 15.81 | 0.012 | 0.021 | <0.0001 |
| Gria1 | 90 ± 5.3 | 109 ± 5.2 | 2.41 | 0.069 | 0.175 | 0.255 |
| Gria2 | 111 ± 5.9 | 105 ± 7.4 | 1.26 | 0.140 | 0.313 | 0.598 |
| Dlg4 | 99 ± 9.8 | 185 ± 4.5 | 34.06 | 0.099 | 0.887 | <0.0001 |
| Dlgap3 | 105 ± 29.3 | 440 ±10.6 | 51.67 | 0.312 | 0.609 | <0.0001 |
| Gene | Ventral Striatum - Adulthood | |||||
| % THC/VEH ± SEM | % Female/Male ± SEM | F ratio | Effect (p-value) | |||
| Treatment *Sex | Treatment | Sex | ||||
| Cnr1 | 82 ± 8.0 | 97 ± 2.9 | 0.95 | 0.713 | 0.157 | 0.402 |
| Grin1 | 103 ± 4.5 | 107 ± 3.3 | 9.47 | 0.129 | 0.632 | <0.0001 |
| Grin2A | 80 ± 7.9 | 151 ± 3.5 | 14.88 | 0.586 | 0.022 | <0.0001 |
| Grin2B | 99 ± 3.4 | 132 ± 3.0 | 2.70 | 0.589 | 0.786 | 0.009 |
| Gria1 | 108 ± 4.6 | 112 ± 3.5 | 10.59 | 0.679 | 0.175 | <0.0001 |
| Gria2 | 103 ± 3.5 | 112 ± 3.4 | 12.01 | 0.816 | 0.482 | <0.0001 |
| Dlg4 | 103 ± 7.5 | 197 ± 2.3 | 58.92 | 0.864 | 0.248 | <0.0001 |
| Dlgap3 | 109 ± 10.2 | 436 ± 5.4 | 27.31 | 0.793 | 0.305 | <0.0001 |
N=10/treatment/sex in adolescent group; N=15-16/treatment/sex in adult group
Table 2.
Alterations of dorsal striatal mRNA levels in F1 offspring analyzed by ANOVAs in combined male and female data sets.
| Gene | Dorsal Striatum - Adolescence | |||||
|---|---|---|---|---|---|---|
| % THC/VEH ± SEM | % Female/Male ± SEM | F ratio | Effect (p-value) | |||
| Treatment *Sex | Treatment | Sex | ||||
| Cnr1 | 93 ± 3.0 | 113 ± 10.1 | 1.11 | 0.910 | 0.127 | 0.408 |
| Grin1 | 98 ± 3.6 | 123 ± 3.0 | 1.30 | 0.277 | 0.632 | 0.142 |
| Grin2A | 102 ± 7.5 | 59 ± 6.0 | 24.05 | 0.738 | 0.684 | <0.0001 |
| Grin2B | 86 ± 4.4 | 114 ± 3.4 | 17.43 | 0.165 | 0.004 | <0.0001 |
| Gria1 | 93 ± 3.1 | 128 ± 3.5 | 2.45 | 0.408 | 0.175 | 0.047 |
| Gria2 | 97 ± 3.7 | 123 ± 2.6 | 2.72 | 0.157 | 0.549 | 0.031 |
| Dlg4 | 96 ± 12.2 | 60 ± 2.9 | 144.40 | 0.177 | 0.258 | <0.0001 |
| Dlgap3 | 92 ± 25.8 | 186 ± 5.4 | 133.94 | 0.892 | 0.223 | <0.0001 |
| Gene | Dorsal Striatum - Adulthood | |||||
| % THC/VEH ± SEM | % Female/Male ± SEM | F ratio | Effect (p-value) | |||
| Treatment *Sex | Treatment | Sex | ||||
| Cnr1 | 87 ± 4.9 | 127 ± 4.2 | 6.29 | 0.909 | 0.039 | 0.001 |
| Grin1 | 88 ± 2.3 | 106 ± 2.7 | 4.32 | 0.701 | 0.003 | 0.192 |
| Grin2A | 82 ± 7.2 | 57 ± 3.8 | 35.39 | 0.570 | 0.001 | <0.0001 |
| Grin2B | 89 ± 2.8 | 99 ± 3.0 | 2.71 | 0.292 | 0.014 | 0.746 |
| Gria1 | 88 ± 2.8 | 105 ± 3.0 | 3.17 | 0.840 | 0.008 | 0.290 |
| Gria2 | 86 ± 2.6 | 100 ± 2.5 | 5.62 | 0.134 | 0.001 | 0.922 |
| Dlg4 | 91 ± 8.8 | 51 ± 2.7 | 103.33 | 0.677 | 0.016 | <0.0001 |
| Dlgap3 | 85 ± 5.0 | 147 ± 3.5 | 22.51 | 0.080 | 0.004 | <0.0001 |
N=10/treatment/sex in adolescent group; N=15-16/treatment/sex in adult group.
The data provided several interesting observations and are shown in Tables 1 and 2. In adolescence, analysis of the combined ventral striatal male and female datasets using two-way ANOVAs revealed interactions between treatment and sex for Cnr1 ((F(3,16)=2.07, p=0.04)), Grin1 ((F(3,16)=4.28, p=0.012)) and Grin2B ((F(3,16)=15.81, p=0.01)), as well as a main effect of sex for Grin2A (p<0.0001), Grin2B (p<0.0001) and Dlg4 (p<0.0001) and Dlgap3 (p<0.0001), indicating sex-specific effects of parental THC exposure on these genes (Table 1). These effects were primarily related to the fact that females tended to have higher expression levels than males (see Female/Male ration in Table 1) that was reduced by THC (Table 3). Some genes show remarkable sex-specific differences in their expression levels (e.g. Dlg4 and Dlgap3). In contrast to the ventral striatum, the pattern of expression in the adolescent dorsal striatum showed no significant treatment and sex interactions, but there was a main effect of drug for Grin2B and sex for Grin2A, Grin2B, Gria1, GriaB, Dlg4 and Dlgap3 (Table 2).
Table 3.
Direction of striatal mRNA expression changes in male and female offspring with parental THC exposure.
| Gene | Males | Females | ||||||
|---|---|---|---|---|---|---|---|---|
| Adolescence | Adulthood | Adolescence | Adulthood | |||||
| VS | DS | VS | DS | VS | DS | VS | DS | |
| Cnr1 | ↑ | ns | ns | ↓ | ns | ns | ns | ns |
| Grin1 | ↑ | ns | ns | ↓ | ↓ | ns | ns | ↓ |
| Grin2A | ↑↑ | ns | ↓ | ↓↓ | ns | ns | ↓ | ↓ |
| Grin2B | ns | ns | ns | ns | ↓ | ↓↓ | ns | ↓↓ |
| Gria1 | ns | ns | ns | ↓ | ↓ | ns | ns | ↓ |
| Gria2 | ↑ | ns | ns | ↓ | ns | ns | ns | ↓↓ |
| Dlg4 | ns | ns | ns | ↓ | ns | ns | ns | ↓ |
| Dlgap3 | ↑↑ | ↓↓ | ns | ns | ns | ns | ns | ↓↓ |
Arrowheads indicate direction of THC-related change (up- or down-regulated). One red arrow: p<0.05, two red arrows: p<0.01, black arrow: 0.05 p≤0.1, ns: non-significant. N =5/treatment/sex in adolescent group; N=7-8/treatment/sex in adult group. VS: ventral striatum, DS: dorsal striatum.
In the adult ventral striatum, a significant main effect of THC was detected for Grin2A (p=0.02), with parental THC exposure leading to decreased expression (Table 1). A main sex effect was also evident for Grin1, Grin2A, Grin2B, Gria1, Gria2, Dlg4 but no treatment and sex interactions were identified. A prominent feature of the adult dorsal striatal impairments noted for the genes studied was the impact of parental THC exposure (Table 2). Altered gene expression (reduced by THC) included Cnr1 (p=0.04), Grin1 (p=0.003), Grin2A (p=0.001), Grin2B (p=0.014), Gria1 (p=0.008), Gria2 (p=0.001), Dlg4 (p=0.02), and Dlgap3 (p=0.004). No significant interactions were detected between THC and sex, but the analysis indicated main sex effects for multiple genes [Cnr1 (p=0.0008), Grin2A (p<0.0001), Dlg4 (p<0.0001), and Dlgap3 (p<0.0001)] (Table 2). Females expressed either lower (Grin2A, Dlg4) or higher (Dlgap3) levels than males.
Considering the significant contributions of sex-related alterations, Table 3 was created to provide an overview regarding the directionality of THC-related change. The data clearly emphasizes an intriguing developmental shift from ventral to dorsal striatal abnormalities in males and females between adolescence and adulthood. Adolescent females already showed an “adult-like” pattern with less ventral striatal abnormalities detectable and a tendency for THC-related down-regulation compared with their male littermates. This was interesting considering that at the adolescent developmental time point of our analysis (PND35), female rats are typically more advanced in puberty than males (Schneider, 2008).
3.2 Sex-related profiles in the relationships between the mRNA levels of genes relevant to synaptic plasticity
The observed mRNA level abnormalities raised questions about potential functional associations between the various genes in the different striatal region of each sex. As such, we explored co-expression patterns between genes using pairwise correlation analyses in the combined dataset of THC and vehicle animals. We focused on adulthood given the fact that our adult cohort had a larger sample size (N=8 in each treatment group and sex) and that most of our previous work focused on identifying molecular and behavioral alterations in adult offspring with parental THC exposure (Szutorisz et al., 2014; Watson et al., 2015).
Figures 2A and B show that the strength and pattern of ventral striatal correlations were quite similar between the male and female offspring. In contrast, the analyses revealed a different profile between the sexes in the dorsal striatum (Fig. 2C and D), with a number of the associations being stronger in females. In particular, glutamate receptor subunit genes in the dorsal striatum, especially Grin2A, had greater positive correlations with other NMDA and AMPA receptor subunit genes in females than in males. In males the correlations of Grin2A with other genes ranged from r=0.15 (p=0.603) to 0.48 (p=0.477) whereas in females the correlations were stronger, ranging from 0.69 (p=0.005) to 0.78 (p=0.001). Intriguingly, relationships between the mRNA levels of Cnr1 and other genes were also highly significant in females (Cnr1-Grin1: r=0.61, p=0.016; Cnr1-Grin2B, r=0.64, p=0.01; Cnr1-Gria1, r=0.66, p=0.007; Cnr1-Gria2, r=0.67, p=0.006; Cnr1-Dlg4, r=0.73, p=0.002; Cnr1-Dlgap3, r=0.59, p=0.021) but not or less so in males.
Figure 2.
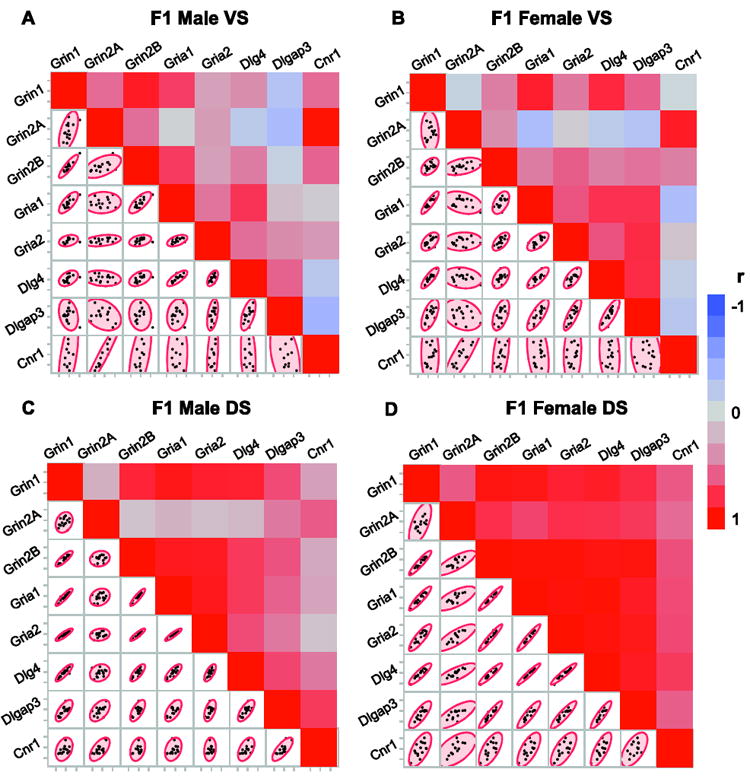
Relationships between the mRNA levels of synaptic regulators within striatal subregions and sexes. Correlation analysis of the mRNA levels measured by qRT-PCR in samples combining offspring with parental THC or VEH exposure. (A, C) Adult F1 males. (B, D) Adult F1 females. Colors in heat maps represent Pearson correlation coefficient (r) values. Scatter plots show the distribution of mRNA levels between animal subjects. N= 16 (8 males+8 females)/treatment group. VS, ventral striatum; DS, dorsal striatum.
3.3. Parental THC exposure alters the relationships between the mRNA levels of synaptic regulators in the dorsal striatum
In order to better assess the relationship of gene expression relevant to parental germline THC in each sex, we explored pairwise dorsal striatal mRNA level associations in adult offspring separately for the treatment groups (Fig. 3). Males failed to show a strong THC-related alteration in correlation patterns except for a few associations with the Dlg4 gene (for example, in animals with VEH history: Dlg4-Gria1: r=0.46, p=0.25; Dlg4-Gria2: r=0.24, p=0.57; and in animals with THC history: Dlg4-Gria1: r=0.82, p=0.023; Dlg4-Gria2: r=0.79, p=0.0.034). In females, a larger number of genes showed strong relationships in association with parental germline THC exposure, particularly for Dlgap3 and Cnr1 genes (Cnr1-Grin2A: r=0.87, p=0.039; Cnr1-Grin2B, r=0.72, p=0.009; Cnr1-Gria1, r=0.69, p=0.007; Cnr1-Gria2, r=0.74, p=0.006; Cnr1-Dlg4, r=0.79, p=0.002; Cnr1-Dlgap3, r=0.66, p=0.003). Altogether, these results suggest that cross-generational THC exposure enhances the association between the expression of genes related to synaptic plasticity to a greater extent in females than males.
Figure 3.
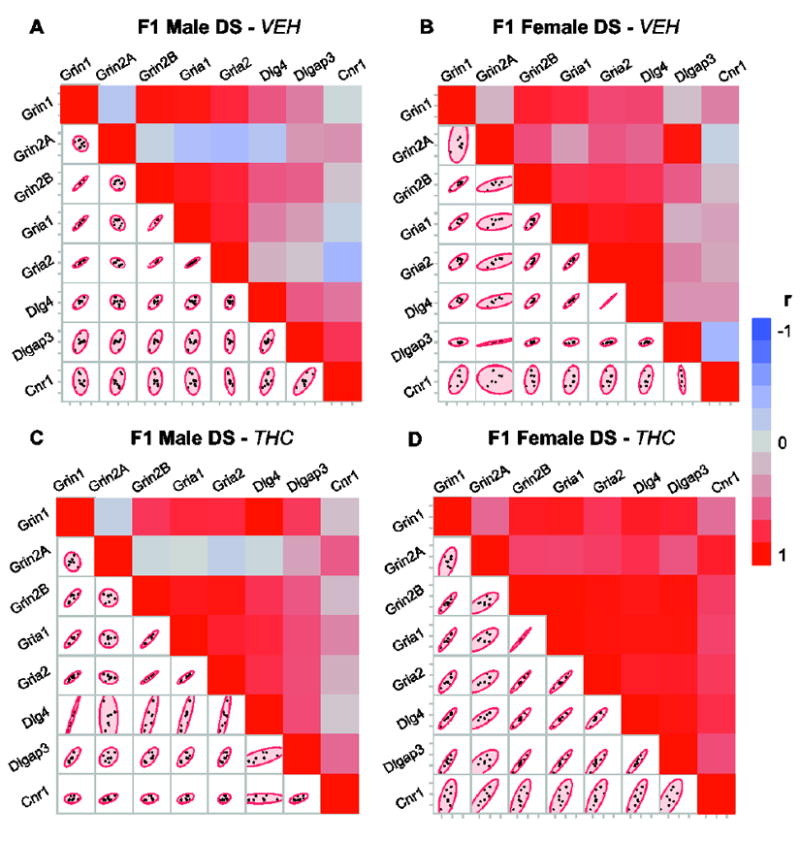
Parental THC exposure alters the relationships between the mRNA levels of synaptic regulators in the dorsal striatum. Correlation analysis of the mRNA levels measured by qRT-PCR. (A, C) Germline VEH- and THC-exposed adult F1 males, respectively. (B, D) Germline VEH- and THC-exposed adult F1 females, respectively. Colors in heat maps represent Pearson correlation coefficient (r) values. Scatter plots show the distribution of mRNA levels between animal subjects. N=8/ treatment/sex. DS, dorsal striatum.
3.4. Parental germline THC exposure leads to decreased locomotor activity in adult F1 females
The observation that the effects of cross-generational THC exposure were most prevalent in the dorsal striatum, together with our previously published data pointing to behavioral impairments (e.g. increased “compulsive-like” drug seeking and stereotypy, (Szutorisz et al., 2014)) are interesting given the strong role of the dorsal striatum in regulating motor behaviors. We therefore monitored the locomotor activity of adult male and female offspring in an open field test (Fig. 4). ANOVA analysis with repeated measures revealed a significant effect of parental THC history (F(1,18)=6.12, p=0.02). Post hoc pairwise t-test comparisons indicated decreased locomotor movements during the first 30 minutes (generally reflective of novelty exploration) of the test session (p<0.05) in females. No difference in locomotor activity was observed in males. In conclusion, the abnormal locomotor behavior associated with parental THC exposure in F1 females is consistent with the hypothesis that striatal molecular abnormalities in components of the glutamatergic system might relate to a motor behavioral functional outcome in adulthood.
Fig. 4.
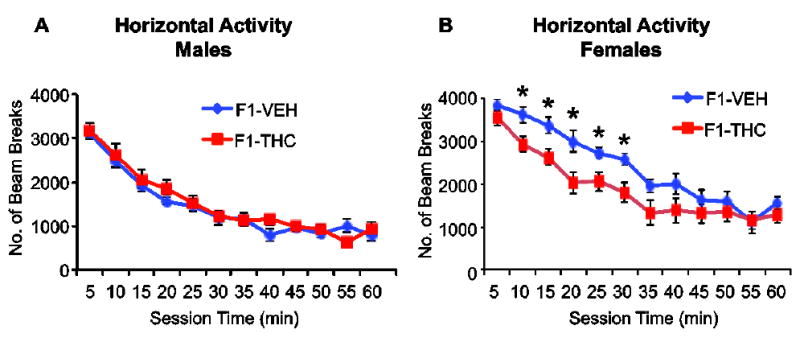
Parental germline THC exposure leads to decreased novelty-related locomotor activity in adult F1 females. Open field horizontal activity in adult F1 male (A) and female (B) offspring. N=10-12 animals/group. Values are expressed as a mean ± SEM. * indicates p< 0.05 vs control (F1-VEH) subjects.
4. Discussion
The results of our current study demonstrate that germline exposure to THC leads to significant gene expression abnormalities in both male and female F1 offspring, with different profiles noted in the magnitude of the disturbances between the sexes. Parental THC exposure was associated with altered mRNA expression of genes functionally implicated in the regulation of synaptic plasticity in the striatum, in line with the results of our previous work that only investigated male offspring (Szutorisz et al., 2014) or did not address sex-specific differences (Watson et al., 2015). Here, a key finding was the identification of alterations in the mRNA levels of a number of genes encoding regulators of glutamatergic synaptic plasticity in females and the relationships between different genes depending on sex. Moreover, the observed THC effects on the genes studied were more prevalent in the dorsal striatal subregion in adulthood in contrast to adolescence during which alterations were more evident in the ventral striatum.
A number of mRNA expression abnormalities seem to develop later in life (by early adulthood) in the dorsal striatum of both male and female offspring. This is interesting given that a number of neuropsychiatric disorders, including obsessive-compulsive disorder (OCD), also show a developmental progression and symptoms appear gradually over time, with clinical penetrance often evident in adulthood (Casey et al., 2015; Lenze and Wetherell, 2011; Schmitt et al., 2014). Indeed, a number of the genes currently investigated are relevant to some of these disorders. For example, genetic polymorphisms in the Dlgap3 gene, which encodes the Sapap3 protein that is a component of the postsynaptic density and interacts with Psd-95 physically and functionally, have been associated with a higher incidence of phenotypes within the OCD spectrum in humans (Bienvenu et al., 2009; Crane et al., 2011; Wu et al., 2013). Similarly, genetic deletion of the Dlgap3 gene in mice has been shown to result in a variety of OCD-like abnormalities such as extensive grooming, abnormal motor behavior, and anxiety (Welch et al., 2007). As a neurobiological consequence, loss of Dlgap3 expression has been reported to cause dorsal striatal impairments of glutamate receptor regulation and neurophysiological function (Wan et al., 2014; Wan et al., 2011). Dlg4/Psd-95 disturbances have also been linked with a variety of neuropsychiatric phenotypes such as schizophrenia, bipolar disorder and increased morphine sensitivity (de Bartolomeis et al., 2014; Dean et al., 2015; Hall et al., 2015; Kristiansen and Meador-Woodruff, 2005; Wang et al., 2014).
Intriguingly, our previous genome-wide study exploring epigenetic alterations (DNA methylation) in the striatum of F1 offspring with parental germline THC exposure identified Dlg4 as the most significant hub within a functional network (Watson et al., 2015). The genes identified in that network, including several glutamate receptor subunits and other molecules involved in the regulation of synaptic plasticity (e.g. Grin2A and Dlgap3), contained differentially methylated regions on the DNA level, emphasizing the long-term epigenetic disruptions in gene regulation due to parental THC exposure. Interestingly, the dynamic control of glutamate receptors (e.g. receptor trafficking) has been demonstrated to be regulated by DNA methylation (Sweatt, 2016).
The current study revealed several novel findings, one of which highlights that parental THC-related alterations are linked to sex-related changes in both the mRNA expression levels of a few individual genes and in co-expression patterns. The co-expression profiles shown in Figures 2 and 3 are particularly intriguing as co-regulation networks in recent years have been closely related to tissue-and cell type-specific biological functions and disturbances (Kopp et al., 2015; Monaco et al., 2015; Richiardi et al., 2015; Rotival and Petretto, 2014). Co-expression patterns in the brain are particularly of interest given the developmental concept of complex neuropsychiatric disorders, where it has been historically difficult to identify single causal molecular relationships (Gaiteri et al., 2014; Grennan et al., 2014). While the exact nature of the correlations between different genes and how germline THC exposure affects these relationships in subsequent progeny remains to be explored, our observations emphasize the importance of studying functional networks of genes in relation to cannabis exposure in the future. Moreover, although the current analysis focused on glutamatergic molecular abnormalities, future studies using genome-wide approaches are expected to provide greater insight about other neurotransmitter systems and neuronal components potentially impacted by parental germline THC exposure.
The finding that more significant abnormalities were identified in the dorsal striatum in adulthood is consistent with our previous report of impairment in dorsal (but not ventral) striatal long-term depression (LTD) as a functional neurophysiological change in synaptic plasticity in adult male offspring with parental THC exposure (Szutorisz et al., 2014). The mechanistic nature of the observed increased LTD remains to be explored in detail neurophysiologically. For example, which subtype of glutamate receptors are responsible for the observed effects and whether the synaptic plasticity is mediated by the endocannabinoid system needs to be investigated. The fact that parental THC exposure caused a highly significant strengthening of mRNA expression patterns between the Cnr1 gene (which encodes the CB1 receptor) and various glutamate receptor subunit genes (especially Grin2A), as well as Dlg4 and Dlgap3, is intriguing and suggests indeed an endocannabinoid link mechanistically that will be examined in subsequent neurophysiological studies.
It is unknown what particular behaviors may be causally linked to disturbances of striatal neurons expressing the molecular abnormalities currently detected in the adult dorsal striatum. It is clear that the parental history of THC did not induce a general gross impairment of motor behavior in either sex, but was quite specific to novelty reactivity in females. Within the dorsal striatum, the medial subregion is a crucial component of the prefrontal cortex-striatal circuit known to modulate novelty reactivity and integrate cognition and motor behaviors (Rinaldi et al., 2010). As such, specifically investigating the dorsomedial subregion of the striatum may provide some insight into the sex-specific differences observed in novelty seeking behavior.
The observations summarized above are in line with the concept of epigenetically inherited phenotypes. In-depth investigations will be needed to provide insights about gene regulatory mechanisms underlying the transmission of cannabis effects through the germline and how they relate to sex. The endocannabinoid system plays important roles not only in the development of the cells and physiological systems of the brain, but also in reproduction. It is known that both male and female reproductive tissues express CB receptors and endocannabinoid ligands, and that in males THC can disrupt gonadal functions (Banerjee et al., 2011; Bari et al., 2011). Studies on the impact of cannabinoids on epigenetic changes in male fertility have been conducted in Cnr1 null mutant mice that displayed abnormal histone retention in germ cells compared to wild type mice (Chioccarelli et al., 2010). In that study, Cnr1 expression was demonstrated to be necessary for spermiogenesis by controlling chromatin condensation, resulting in poor sperm quality. Adverse effects of cannabis exposure on the ovary of females have also been found to present a higher risk of infertility due to anovulation (Klonoff-Cohen et al., 2006). The effects of cannabis on the sperm and oocyte epigenome that could potentially lead to multigenerational transmission remain to be explored in different generations to demonstrate true transgenerational effects. Specifically, subsequent studies are required to assess how possible epigenetic processes (e.g. DNA methylation) are involved in the transmission of cannabinoid effects from parent to offspring. Overall, our findings demonstrate that germline THC exposure can impact offspring phenotype and neurobiology in both sexes and could possibly confer enhanced risk for the development of neuropsychiatric disorders that may manifest differentially in the two sexes.
5. Conclusions
Providing concrete insights about specific neurobiological disturbances in female and male offspring with parental THC exposure will have far-reaching impact for the neuroscience field given the critical role of the endocannabinoid system in early brain development, a time period now acknowledged to drive the course of psychiatric disorders expressed in adulthood. The results also emphasize the switch of ventral to dorsal striatal disturbances from adolescence to adulthood that may also have significant relevance to the age-dependent vulnerability seen with different neuropsychiatric disorders. Overall, our research highlights the important consideration that should be given for examining sex-specific effects not only related to events that occur during one’s own lifetime but even across generations.
Highlights.
Parental THC exposure causes striatal dysregulation in the expression of genes functionally related to synaptic plasticity in both male and female offspring.
Gene expression changes show a developmental shift between adolescence and adulthood.
Female offspring have stronger alterations in the mRNA co-expression patterns of various synaptic plasticity-related genes in the dorsal striatum.
Females with parental germline THC exposure exhibit decreased novelty seeking behavior, which is not observed in males.
Acknowledgments
We thank Yanhua Ren for discussion and Nayana D. Patel for technical assistance. Our research was supported by NIH grants DA030359 and DA033660.
Footnotes
Conflict of interest
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Banerjee A, Singh A, Srivastava P, Turner H, Krishna A. Effects of chronic bhang (cannabis) administration on the reproductive system of male mice. Birth defects research Part B Developmental and reproductive toxicology. 2011;92(3):195–205. doi: 10.1002/bdrb.20295. [DOI] [PubMed] [Google Scholar]
- Bari M, Battista N, Pirazzi V, Maccarrone M. The manifold actions of endocannabinoids on female and male reproductive events. Frontiers in bioscience. 2011;16:498–516. doi: 10.2741/3701. [DOI] [PubMed] [Google Scholar]
- Bienvenu OJ, Wang Y, Shugart YY, Welch JM, Grados MA, Fyer AJ, Rauch SL, McCracken JT, Rasmussen SA, Murphy DL, Cullen B, Valle D, Hoehn-Saric R, Greenberg BD, Pinto A, Knowles JA, Piacentini J, Pauls DL, Liang KY, Willour VL, Riddle M, Samuels JF, Feng G, Nestadt G. Sapap3 and pathological grooming in humans: Results from the OCD collaborative genetics study. American journal of medical genetics. Part B, Neuropsychiatric genetics : the official publication of the International Society of Psychiatric Genetics. 2009;150B(5):710–720. doi: 10.1002/ajmg.b.30897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohacek J, Mansuy IM. Epigenetic inheritance of disease and disease risk. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2013;38(1):220–236. doi: 10.1038/npp.2012.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrnes JJ, Johnson NL, Schenk ME, Byrnes EM. Cannabinoid exposure in adolescent female rats induces transgenerational effects on morphine conditioned place preference in male offspring. Journal of psychopharmacology. 2012;26(10):1348–1354. doi: 10.1177/0269881112443745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Glatt CE, Lee FS. Treating the Developing versus Developed Brain: Translating Preclinical Mouse and Human Studies. Neuron. 2015;86(6):1358–1368. doi: 10.1016/j.neuron.2015.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chioccarelli T, Cacciola G, Altucci L, Lewis SE, Simon L, Ricci G, Ledent C, Meccariello R, Fasano S, Pierantoni R, Cobellis G. Cannabinoid receptor 1 influences chromatin remodeling in mouse spermatids by affecting content of transition protein 2 mRNA and histone displacement. Endocrinology. 2010;151(10):5017–5029. doi: 10.1210/en.2010-0133. [DOI] [PubMed] [Google Scholar]
- Crane J, Fagerness J, Osiecki L, Gunnell B, Stewart SE, Pauls DL, Scharf JM Tourette Syndrome International Consortium for, G. Family-based genetic association study of DLGAP3 in Tourette Syndrome. American journal of medical genetics. Part B, Neuropsychiatric genetics : the official publication of the International Society of Psychiatric Genetics. 2011;156B(1):108–114. doi: 10.1002/ajmg.b.31134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bartolomeis A, Buonaguro EF, Iasevoli F, Tomasetti C. The emerging role of dopamine-glutamate interaction and of the postsynaptic density in bipolar disorder pathophysiology: Implications for treatment. Journal of psychopharmacology. 2014;28(6):505–526. doi: 10.1177/0269881114523864. [DOI] [PubMed] [Google Scholar]
- Dean B, Thomas N, Lai CY, Chen WJ, Scarr E. Changes in cholinergic and glutamatergic markers in the striatum from a sub-set of subjects with schizophrenia. Schizophrenia research. 2015 doi: 10.1016/j.schres.2015.10.028. [DOI] [PubMed] [Google Scholar]
- Dinieri JA, Hurd YL. Rat models of prenatal and adolescent cannabis exposure. Methods in molecular biology. 2012;829:231–242. doi: 10.1007/978-1-61779-458-2_14. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nature neuroscience. 2005;8(11):1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Figueira ML, Ouakinin S. Gender-related endocrinological dysfunction and mental disorders. Current opinion in psychiatry. 2010;23(4):369–372. doi: 10.1097/YCO.0b013e3283399b86. [DOI] [PubMed] [Google Scholar]
- Gaiteri C, Ding Y, French B, Tseng GC, Sibille E. Beyond modules and hubs: the potential of gene coexpression networks for investigating molecular mechanisms of complex brain disorders. Genes, brain, and behavior. 2014;13(1):13–24. doi: 10.1111/gbb.12106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girault JA. Integrating neurotransmission in striatal medium spiny neurons. Advances in experimental medicine and biology. 2012;970:407–429. doi: 10.1007/978-3-7091-0932-8_18. [DOI] [PubMed] [Google Scholar]
- Grennan KS, Chen C, Gershon ES, Liu C. Molecular network analysis enhances understanding of the biology of mental disorders. BioEssays : news and reviews in molecular cellular and developmental biology. 2014;36(6):606–616. doi: 10.1002/bies.201300147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J, Trent S, Thomas KL, O’Donovan MC, Owen MJ. Genetic risk for schizophrenia: convergence on synaptic pathways involved in plasticity. Biological psychiatry. 2015;77(1):52–58. doi: 10.1016/j.biopsych.2014.07.011. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future national results on adolescent drug use: Overview of key findings, 2011. Institute for Social Research, The University of Michigan; Ann Arbor: 2012. [Google Scholar]
- Klonoff-Cohen HS, Natarajan L, Chen RV. A prospective study of the effects of female and male marijuana use on in vitro fertilization (IVF) and gamete intrafallopian transfer (GIFT) outcomes. American journal of obstetrics and gynecology. 2006;194(2):369–376. doi: 10.1016/j.ajog.2005.08.020. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology : official publication of the American College of. Neuropsychopharmacology. 2010;35(1):217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp N, Climer S, Dougherty JD. Moving from capstones toward cornerstones: successes and challenges in applying systems biology to identify mechanisms of autism spectrum disorders. Frontiers in genetics. 2015;6:301. doi: 10.3389/fgene.2015.00301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristiansen LV, Meador-Woodruff JH. Abnormal striatal expression of transcripts encoding NMDA interacting PSD proteins in schizophrenia, bipolar disorder and major depression. Schizophrenia research. 2005;78(1):87–93. doi: 10.1016/j.schres.2005.06.012. [DOI] [PubMed] [Google Scholar]
- Lenze EJ, Wetherell JL. A lifespan view of anxiety disorders. Dialogues in clinical neuroscience. 2011;13(4):381–399. doi: 10.31887/DCNS.2011.13.4/elenze. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Monaco G, van Dam S, Casal Novo Ribeiro JL, Larbi A, de Magalhaes JP. A comparison of human and mouse gene co-expression networks reveals conservation and divergence at the tissue, pathway and disease levels. BMC evolutionary biology. 2015;15(1):259. doi: 10.1186/s12862-015-0534-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 6. Academic Press/Elsevier; Amsterdam ; Boston: 2007. [Google Scholar]
- Rauch SL, Carlezon WA., Jr Neuroscience. Illuminating the neural circuitry of compulsive behaviors. Science. 2013;340(6137):1174–1175. doi: 10.1126/science.1239652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richiardi J, Altmann A, Milazzo AC, Chang C, Chakravarty MM, Banaschewski T, Barker GJ, Bokde AL, Bromberg U, Buchel C, Conrod P, Fauth-Buhler M, Flor H, Frouin V, Gallinat J, Garavan H, Gowland P, Heinz A, Lemaitre H, Mann KF, Martinot JL, Nees F, Paus T, Pausova Z, Rietschel M, Robbins TW, Smolka MN, Spanagel R, Strohle A, Schumann G, Hawrylycz M, Poline JB, Greicius MD, consortium, I BRAIN NETWORKS. Correlated gene expression supports synchronous activity in brain networks. Science. 2015;348(6240):1241–1244. doi: 10.1126/science.1255905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaldi A, Romeo S, Agustin-Pavon C, Oliverio A, Mele A. Distinct patterns of Fos immunoreactivity in striatum and hippocampus induced by different kinds of novelty in mice. Neurobiology of learning and memory. 2010;94(3):373–381. doi: 10.1016/j.nlm.2010.08.004. [DOI] [PubMed] [Google Scholar]
- Rotival M, Petretto E. Leveraging gene co-expression networks to pinpoint the regulation of complex traits and disease, with a focus on cardiovascular traits. Briefings in functional genomics. 2014;13(1):66–78. doi: 10.1093/bfgp/elt030. [DOI] [PubMed] [Google Scholar]
- SAMSHA. Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings. Substance Abuse and Mental Health Services Administration Office of Applied Studies, HHS; Rockville, MD: 2014. Series Title: NSDUH Series H-48. [Google Scholar]
- Savic I, Engel J., Jr Structural and functional correlates of epileptogenesis - does gender matter? Neurobiology of disease. 2014;70:69–73. doi: 10.1016/j.nbd.2014.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt A, Malchow B, Hasan A, Falkai P. The impact of environmental factors in severe psychiatric disorders. Frontiers in neuroscience. 2014;8:19. doi: 10.3389/fnins.2014.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider M. Puberty as a highly vulnerable developmental period for the consequences of cannabis exposure. Addiction biology. 2008;13(2):253–263. doi: 10.1111/j.1369-1600.2008.00110.x. [DOI] [PubMed] [Google Scholar]
- Shen EY, Ahern TH, Cheung I, Straubhaar J, Dincer A, Houston I, de Vries GJ, Akbarian S, Forger NG. Epigenetics and sex differences in the brain: A genome-wide comparison of histone-3 lysine-4 trimethylation (H3K4me3) in male and female mice. Experimental neurology. 2015;268:21–29. doi: 10.1016/j.expneurol.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweatt JD. Dynamic DNA Methylation Controls Glutamate Receptor Trafficking and Synaptic Scaling. Journal of neurochemistry. 2016 doi: 10.1111/jnc.13564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szutorisz H, DiNieri JA, Sweet E, Egervari G, Michaelides M, Carter JM, Ren Y, Miller ML, Blitzer RD, Hurd YL. Parental THC exposure leads to compulsive heroin-seeking and altered striatal synaptic plasticity in the subsequent generation. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2014;39(6):1315–1323. doi: 10.1038/npp.2013.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szutorisz H, Hurd YL. Epigenetic Effects of Cannabis Exposure. Biological psychiatry. 2015 doi: 10.1016/j.biopsych.2015.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szyf M. Nongenetic inheritance and transgenerational epigenetics. Trends Mol Med. 2015;21(2):134–144. doi: 10.1016/j.molmed.2014.12.004. [DOI] [PubMed] [Google Scholar]
- Vassoler FM, Johnson NL, Byrnes EM. Female adolescent exposure to cannabinoids causes transgenerational effects on morphine sensitization in female offspring in the absence of in utero exposure. Journal of psychopharmacology. 2013;27(11):1015–1022. doi: 10.1177/0269881113503504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassoler FM, Sadri-Vakili G. Mechanisms of transgenerational inheritance of addictive-like behaviors. Neuroscience. 2014;264:198–206. doi: 10.1016/j.neuroscience.2013.07.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Y, Ade KK, Caffall Z, Ilcim Ozlu M, Eroglu C, Feng G, Calakos N. Circuit-selective striatal synaptic dysfunction in the Sapap3 knockout mouse model of obsessive-compulsive disorder. Biological psychiatry. 2014;75(8):623–630. doi: 10.1016/j.biopsych.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Y, Feng G, Calakos N. Sapap3 deletion causes mGluR5-dependent silencing of AMPAR synapses. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31(46):16685–16691. doi: 10.1523/JNEUROSCI.2533-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Yan P, Hui T, Zhang J. Epigenetic upregulation of PSD-95 contributes to the rewarding behavior by morphine conditioning. Eur J Pharmacol. 2014;732:123–129. doi: 10.1016/j.ejphar.2014.03.040. [DOI] [PubMed] [Google Scholar]
- Watson CT, Szutorisz H, Garg P, Martin Q, Landry JA, Sharp AJ, Hurd YL. Genome-Wide DNA Methylation Profiling Reveals Epigenetic Changes in the Rat Nucleus Accumbens Associated With Cross-Generational Effects of Adolescent THC Exposure. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2015;40(13):2993–3005. doi: 10.1038/npp.2015.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch JM, Lu J, Rodriguiz RM, Trotta NC, Peca J, Ding JD, Feliciano C, Chen M, Adams JP, Luo J, Dudek SM, Weinberg RJ, Calakos N, Wetsel WC, Feng G. Cortico-striatal synaptic defects and OCD-like behaviours in Sapap3-mutant mice. Nature. 2007;448(7156):894–900. doi: 10.1038/nature06104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu K, Hanna GL, Easter P, Kennedy JL, Rosenberg DR, Arnold PD. Glutamate system genes and brain volume alterations in pediatric obsessive-compulsive disorder: a preliminary study. Psychiatry research. 2013;211(3):214–220. doi: 10.1016/j.pscychresns.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yohn NL, Bartolomei MS, Blendy JA. Multigenerational and transgenerational inheritance of drug exposure: The effects of alcohol, opiates, cocaine, marijuana, and nicotine. Progress in biophysics and molecular biology. 2015;118(1-2):21–33. doi: 10.1016/j.pbiomolbio.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuchner S, Wendland JR, Ashley-Koch AE, Collins AL, Tran-Viet KN, Quinn K, Timpano KC, Cuccaro ML, Pericak-Vance MA, Steffens DC, Krishnan KR, Feng G, Murphy DL. Multiple rare SAPAP3 missense variants in trichotillomania and OCD. Molecular psychiatry. 2009;14(1):6–9. doi: 10.1038/mp.2008.83. [DOI] [PMC free article] [PubMed] [Google Scholar]


