Abstract
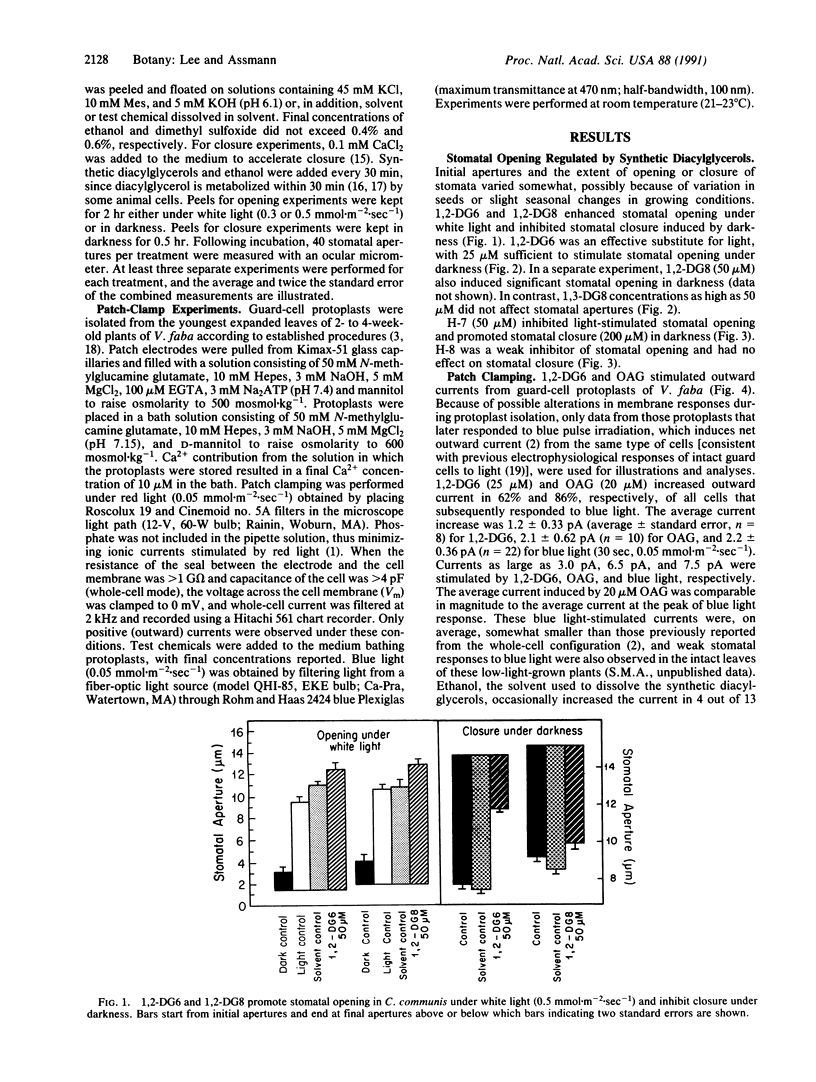
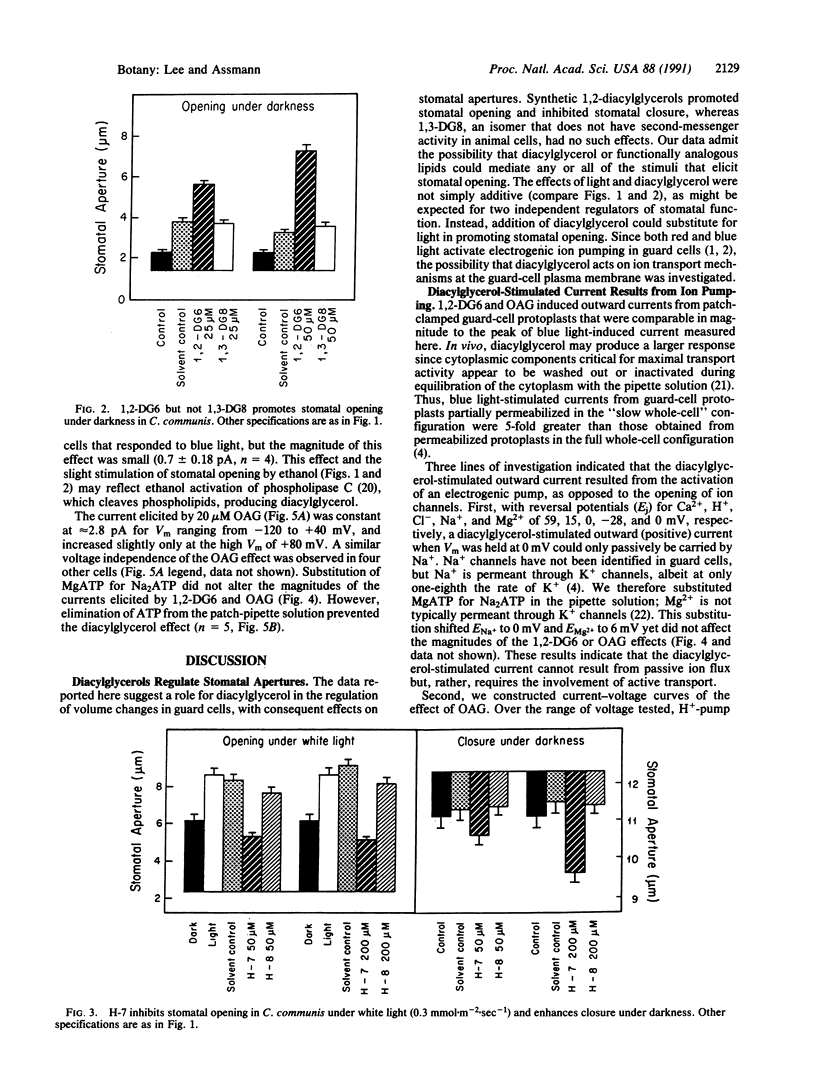
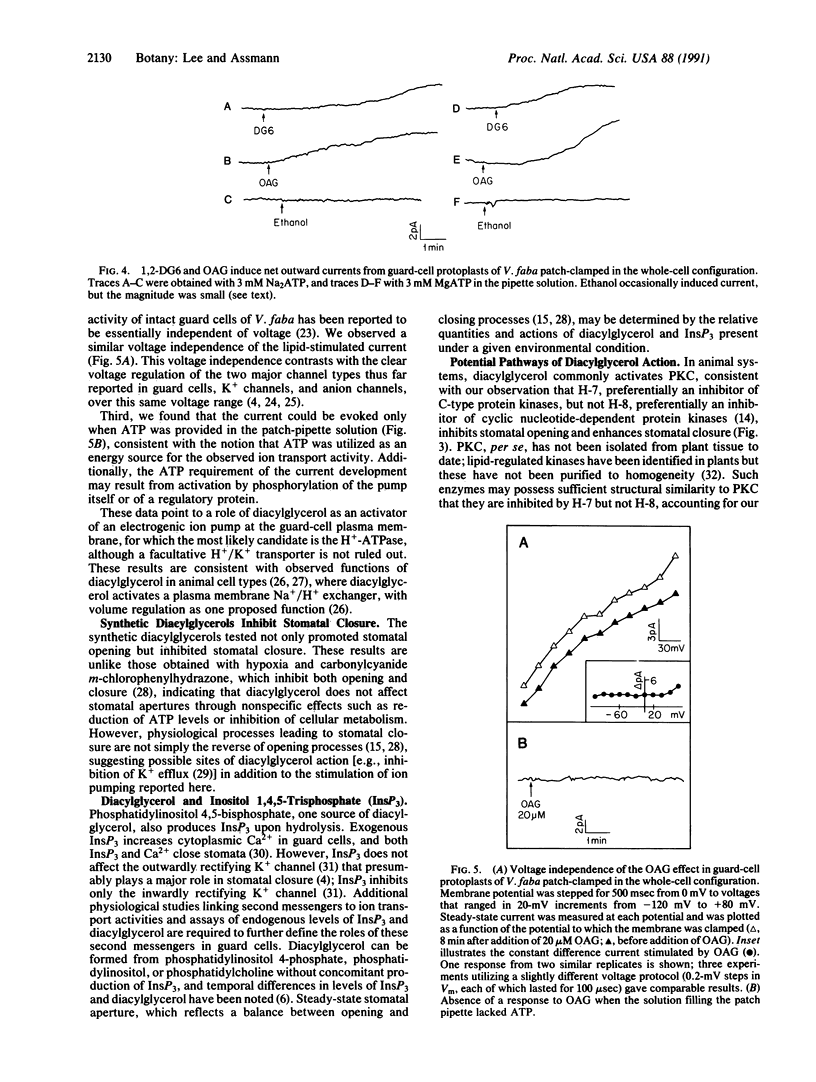
Stomatal guard cells in leaves regulate the apertures of microscopic pores through which photosynthetic gas exchange and water vapor loss occur. Environmental signals, including light, high humidity, and low CO2 concentrations, open stomata by increasing the volume of guard cells. Activation of a plasma membrane H+ pump initiates K+ and Cl- influx, accompanied by malate synthesis, resulting in osmotic water flow into the guard cells, a bowing apart of the guard-cell pair, and consequent stomatal opening. Physiological and electrophysiological techniques were employed to investigate the possibility that a second-messenger lipid, 1,2-diacylglycerol, is involved in the transduction of opening stimuli. The synthetic diacylglycerols 1,2-dihexanoylglycerol and 1,2-dioctanoylglycerol enhanced light-induced stomatal opening in Commelina communis and induced stomatal opening under darkness, whereas an isomer with no known second-messenger role, 1,3-dioctanoylglycerol, did not affect stomatal responses. 1-(5-Isoquinolinylsulfonyl)-2-methylpiperazine (H-7), an inhibitor of protein kinase C, the enzyme typically activated by 1,2-diacylglycerol in animal cells, inhibited light-stimulated stomatal opening and enhanced dark-induced stomatal closure. N-[(2-Methylamino)ethyl]-5-isoquinolinesulfonamide (H-8), which inhibits cyclic nucleotide-dependent protein kinases preferentially over lipid-dependent protein kinases such as protein kinase C, had little effect on stomatal apertures. Whole-cell patch clamping of guard-cell protoplasts of Vicia faba revealed that 1,2-dihexanoylglycerol and 1-oleoyl-2-acetylglycerol activated an ATP-dependent, voltage-independent current, suggesting activation of an electrogenic ion pump such as the H+ pump. Diacylglycerol or functionally similar lipids may act through protein phosphorylation to provide the intracellular signals that mediate H+-ATPase activation and stomatal opening in response to light or other opening stimuli.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blatt M. R., Thiel G., Trentham D. R. Reversible inactivation of K+ channels of Vicia stomatal guard cells following the photolysis of caged inositol 1,4,5-trisphosphate. Nature. 1990 Aug 23;346(6286):766–769. doi: 10.1038/346766a0. [DOI] [PubMed] [Google Scholar]
- Colby K. A., Blaustein M. P. Inhibition of voltage-gated K channels in synaptosomes by sn-1,2-dioctanoylglycerol, an activator of protein kinase C. J Neurosci. 1988 Dec;8(12):4685–4692. doi: 10.1523/JNEUROSCI.08-12-04685.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einspahr K. J., Peeler T. C., Thompson G. A., Jr Rapid changes in polyphosphoinositide metabolism associated with the response of Dunaliella salina to hypoosmotic shock. J Biol Chem. 1988 Apr 25;263(12):5775–5779. [PubMed] [Google Scholar]
- Exton J. H. Mechanisms of action of calcium-mobilizing agonists: some variations on a young theme. FASEB J. 1988 Aug;2(11):2670–2676. doi: 10.1096/fasebj.2.11.2456243. [DOI] [PubMed] [Google Scholar]
- Gilroy S., Read N. D., Trewavas A. J. Elevation of cytoplasmic calcium by caged calcium or caged inositol triphosphate initiates stomatal closure. Nature. 1990 Aug 23;346(6286):769–771. doi: 10.1038/346769a0. [DOI] [PubMed] [Google Scholar]
- Grinstein S., Cohen S., Goetz J. D., Rothstein A. Osmotic and phorbol ester-induced activation of Na+/H+ exchange: possible role of protein phosphorylation in lymphocyte volume regulation. J Cell Biol. 1985 Jul;101(1):269–276. doi: 10.1083/jcb.101.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidaka H., Inagaki M., Kawamoto S., Sasaki Y. Isoquinolinesulfonamides, novel and potent inhibitors of cyclic nucleotide dependent protein kinase and protein kinase C. Biochemistry. 1984 Oct 9;23(21):5036–5041. doi: 10.1021/bi00316a032. [DOI] [PubMed] [Google Scholar]
- Hoek J. B., Thomas A. P., Rubin R., Rubin E. Ethanol-induced mobilization of calcium by activation of phosphoinositide-specific phospholipase C in intact hepatocytes. J Biol Chem. 1987 Jan 15;262(2):682–691. [PubMed] [Google Scholar]
- Kaibuchi K., Takai Y., Sawamura M., Hoshijima M., Fujikura T., Nishizuka Y. Synergistic functions of protein phosphorylation and calcium mobilization in platelet activation. J Biol Chem. 1983 Jun 10;258(11):6701–6704. [PubMed] [Google Scholar]
- Kruse T., Tallman G., Zeiger E. Isolation of Guard Cell Protoplasts from Mechanically Prepared Epidermis of Vicia faba Leaves. Plant Physiol. 1989 Aug;90(4):1382–1386. doi: 10.1104/pp.90.4.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapetina E. G., Reep B., Ganong B. R., Bell R. M. Exogenous sn-1,2-diacylglycerols containing saturated fatty acids function as bioregulators of protein kinase C in human platelets. J Biol Chem. 1985 Feb 10;260(3):1358–1361. [PubMed] [Google Scholar]
- Lawton M. A., Yamamoto R. T., Hanks S. K., Lamb C. J. Molecular cloning of plant transcripts encoding protein kinase homologs. Proc Natl Acad Sci U S A. 1989 May;86(9):3140–3144. doi: 10.1073/pnas.86.9.3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitan I. B. Phosphorylation of ion channels. J Membr Biol. 1985;87(3):177–190. doi: 10.1007/BF01871217. [DOI] [PubMed] [Google Scholar]
- Martiny-Baron G., Scherer G. F. Phospholipid-stimulated protein kinase in plants. J Biol Chem. 1989 Oct 25;264(30):18052–18059. [PubMed] [Google Scholar]
- May W. S., Lapetina E. G., Cuatrecasas P. Intracellular activation of protein kinase C and regulation of the surface transferrin receptor by diacylglycerol is a spontaneously reversible process that is associated with rapid formation of phosphatidic acid. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1281–1284. doi: 10.1073/pnas.83.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moolenaar W. H., Tertoolen L. G., de Laat S. W. Phorbol ester and diacylglycerol mimic growth factors in raising cytoplasmic pH. Nature. 1984 Nov 22;312(5992):371–374. doi: 10.1038/312371a0. [DOI] [PubMed] [Google Scholar]
- Morse M. J., Crain R. C., Satter R. L. Light-stimulated inositolphospholipid turnover in Samanea saman leaf pulvini. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7075–7078. doi: 10.1073/pnas.84.20.7075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y. Studies and perspectives of protein kinase C. Science. 1986 Jul 18;233(4761):305–312. doi: 10.1126/science.3014651. [DOI] [PubMed] [Google Scholar]
- Nomura H., Ase K., Sekiguchi K., Kikkawa U., Nishizuka Y., Nakano Y., Satoh T. Stereospecificity of diacylglycerol for stimulus-response coupling in platelets. Biochem Biophys Res Commun. 1986 Nov 14;140(3):1143–1151. doi: 10.1016/0006-291x(86)90754-0. [DOI] [PubMed] [Google Scholar]
- Oishi K., Raynor R. L., Charp P. A., Kuo J. F. Regulation of protein kinase C by lysophospholipids. Potential role in signal transduction. J Biol Chem. 1988 May 15;263(14):6865–6871. [PubMed] [Google Scholar]
- Palmgren M. G., Sommarin M. Lysophosphatidylcholine stimulates ATP dependent proton accumulation in isolated oat root plasma membrane vesicles. Plant Physiol. 1989 Jul;90(3):1009–1014. doi: 10.1104/pp.90.3.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder J. I. K+ transport properties of K+ channels in the plasma membrane of Vicia faba guard cells. J Gen Physiol. 1988 Nov;92(5):667–683. doi: 10.1085/jgp.92.5.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz A., Ilan N., Grantz D. A. Calcium Effects on Stomatal Movement in Commelina communis L. : Use of EGTA to Modulate Stomatal Response to Light, KCl and CO(2). Plant Physiol. 1988 Jul;87(3):583–587. doi: 10.1104/pp.87.3.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano E. E., Zeiger E., Hagiwara S. Red light stimulates an electrogenic proton pump in Vicia guard cell protoplasts. Proc Natl Acad Sci U S A. 1988 Jan;85(2):436–440. doi: 10.1073/pnas.85.2.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short T. W., Briggs W. R. Characterization of a Rapid, Blue Light-Mediated Change in Detectable Phosphorylation of a Plasma Membrane Protein from Etiolated Pea (Pisum sativum L.) Seedlings. Plant Physiol. 1990 Jan;92(1):179–185. doi: 10.1104/pp.92.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]


