Abstract
Neoadjuvant therapy has been established as an effective therapeutic approach for patients with locally advanced breast cancer. Similar outcomes between neoadjuvant and adjuvant chemotherapy have been demonstrated in several trials. Nevertheless, neoadjuvant therapy has some advantages over adjuvant therapy, including tumour downstaging, in vivo assessment of therapeutic efficacy, reduced treatment durations, and the need to enrol fewer patients for clinical trials to reach their preplanned objectives. The number of neoadjuvant trials in patients with breast cancer has increased substantially in the past 5 years, particularly in the context of HER2-positive disease. Substantial improvements in the pathological complete response rate to anti-HER2 therapy, a proposed surrogate end point for long-term clinical benefit, have been observed with neoadjuvant dual-agent HER2 blockade. Thus, it was hypothesized that this approach would provide additional survival benefits over standard-of-care therapy with the anti-HER2 antibody trastuzumab in the adjuvant setting. Emerging data, however, are calling this notion into question. We discuss potential reasons why results of neoadjuvant trials of targeted therapies have not been mirrored in the adjuvant setting, and other than inherent differences in clinical-trial designs and statistical power, we consider how the biology of the disease, patient characteristics, and drug administration and schedule might influence the results.
Preoperative, or ‘neoadjuvant’, therapy is a treatment option for patients with early stage breast cancer and is the standard of care for patients with locally advanced breast cancer1. Neoadjuvant therapy has advantages over adjuvant therapy, given that preoperative therapy often results in downstaging of both the primary tumour and axillary disease, enables in vivo assessment of tumour biology, and represents the ideal scenario for studying predictive biomarkers and intermediate end points that might predict long-term clinical outcomes2, 3. In addition, the neoadjuvant approach offers opportunities for response-guided therapeutic strategies, whereby therapeutic regimens can be adjusted when tumour tissue is available for response monitoring4.
Neoadjuvant chemotherapy, compared to conventional adjuvant therapy, does not seem to improve the overall survival of patients with breast cancer5, 6. Indeed, randomized controlled trials have demonstrated similar outcomes, in terms of disease-free survival (DFS) and overall survival, between neoadjuvant and adjuvant systemic chemotherapy or endocrine therapy in patients with breast cancer5-7. Nevertheless, the increasing rates of pathological complete response (pCR) to neoadjuvant therapy have had a marked effect on locoregional-treatment considerations, as neoadjuvant chemotherapy can provide increased opportunities to perform breast-conserving surgery in patients with locally advanced breast cancer8. Patient preferences, surgeons’ recommendations, and the possible failure to achieve tumour control through breast-conserving surgery (in circumstances such as a predicted insufficient response to chemotherapy, or a patient not being a suitable candidate for breast-conserving surgery) contribute to the choice of the timing for systemic therapy9, 10.
The number of trials investigating neoadjuvant therapy for breast cancer increased substantially over the past decade, particularly in the context of HER2-positive disease, a subtype that is associated with a poor prognosis if not treated with anti-HER2 agents11, 12. HER2-positive breast cancers account for 15–20% of all invasive breast cancers13. The state-of-the-art treatment for HER2-positive breast cancer consists of trastuzumab-based therapy, which has been shown to improve the DFS and overall survival of patients with early stage and metastatic HER2-positive breast cancer, compared with chemotherapy alone12, 14-16. In the neoadjuvant setting, the addition of trastuzumab to standard chemotherapy results in an increase in the pCR rate compared with neoadjuvant chemotherapy alone17, 18. Likewise, the use of trastuzumab, in addition to standard chemotherapy, is associated with confirmed long-term improvements in DFS and overall survival in large adjuvant studies15, 16, 19.
Other novel anti-HER2 therapeutic strategies have been approved or are under investigation for the treatment of patients with HER2-positive breast cancer20-23. Notably, a pertuzumab-based neoadjuvant treatment regimen, also comprising trastuzumab and standard chemotherapy, was approved by the FDA for patients with locally advanced, inflammatory, or early stage HER2-positive breast cancers greater than 2 cm in diameter and/or with axillary-lymph-node involvement24, 25.
The benefit of neoadjuvant dual-agent HER2 blockade in patients with HER2-positive breast cancer has been supported by both preclinical and clinical studies. For instance, in preclinical studies, compelling evidence indicates that dual-agent HER2 blockade with trastuzumab and the small-molecule HER1/2-tyrosine-kinase inhibitor lapatinib has better efficacy than trastuzumab because of nonoverlapping mechanisms of action and synergic interaction between these agents26, 27. In the setting of HER2-positive breast cancer, four phase III neoadjuvant trials testing trastuzumab plus another anti-HER2 agent versus trastuzumab alone have shown dramatic increases in pCR rates, a proposed surrogate end point for long-term clinical benefit: NeoSPHERE (Neoadjuvant Study of Pertuzumab and Herceptin in an Early Regimen Evaluation)24, NeoALTTO (Neoadjuvant Lapatinib and/or Trastuzumab Treatment Optimisation)28, CHER-LOB (Chemotherapy, Herceptin and Lapatinib in Operable Breast Cancer)29, and LPT 109096 (preoperative lapatinib and trastuzumab, separately and combined prior to neoadjuvant chemotherapy)30. Two of these neoadjuvant trials, NeoSPHERE24, and NeoALTTO28, have adjuvant counterparts: APHINITY (Adjuvant Pertuzumab and Herceptin in Initial Therapy; NCT01358877)31 and ALTTO (Adjuvant Lapatinib and/or Trastuzumab Treatment Optimisation; NCT00490139)32, respectively.
On the basis of the statistically significant improvements in pCR rates observed with neoadjuvant dual-agent HER2 blockade, and the association between pCR and long-term outcomes of other neoadjuvant treatments, it was hypothesized that this approach would also provide additional survival benefits over standard-of-care therapy (comprising single-agent HER2 blockade with trastuzumab) in the adjuvant setting; however, data emerging from the ALTTO trial since 2014 have called this notion into question28,32. A similar situation has also been observed with the incorporation of the anti-VEGF antibody bevacizumab into adjuvant therapy (chemotherapy with an anthracycline or taxane, or both) for triple-negative breast cancer (TNBC) in the BEATRICE trial33 or in unselected patients in the E5103 study34 (chemotherapy with an anthracycline and taxane)— this treatment approach had previously been associated with an increase rate of pCR in the neoadjuvant setting (although have not translated into improved survival outcomes)35,36, 37.
In this Review, we address the potential reasons why changes in pCR rates following neoadjuvant therapy might not equate with changes in DFS of the same magnitude when the same treatment is applied in the adjuvant setting, or even improvements in long-term outcomes in the neoadjuvant setting. We discuss the hypothesis that, in addition to inherent differences in the designs, statistical power, and use of surrogate markers between neoadjuvant and adjuvant trials, the biology of the disease and the characteristics of the drugs might also influence the translation of increased pCR rates into improvements in DFS and overall survival in the adjuvant setting. Furthermore, the ongoing studies that could shed additional light on this topic are reviewed, and we suggest additional approaches that might help realize the potential use of pCR rates after neoadjuvant therapy to infer the actual effect of systemic therapies on the survival of patients with breast cancer.
Comparing NeoALTTO with ALTTO
Treatment efficacy
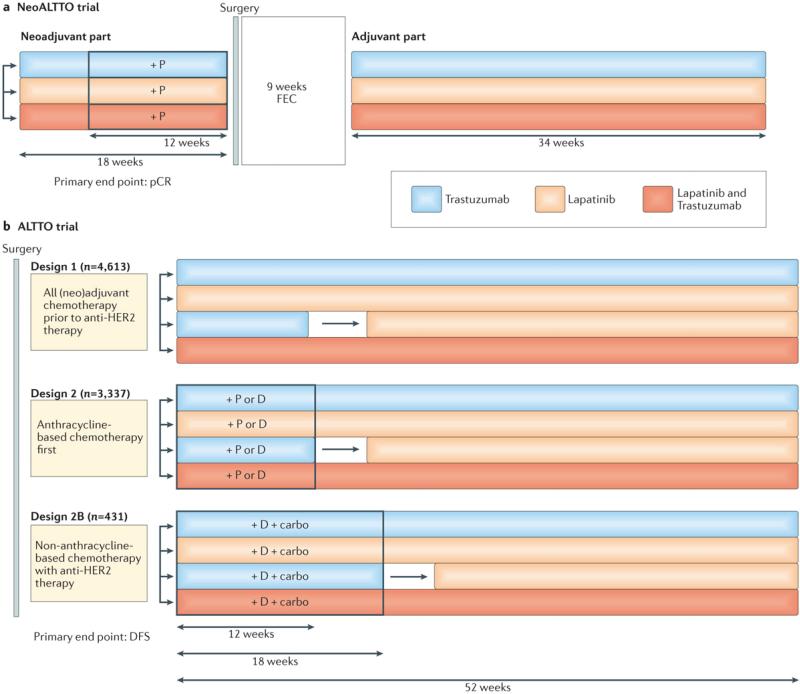
In NeoALTTO and ALTTO28, 32, investigators compared dual-agent HER2 blockade consisting of trastuzumab plus lapatinib to either lapatinib or trastuzumab alone, in the neoadjuvant and adjuvant settings, respectively, with the aim of improving the outcomes of patients with HER2-positive breast cancer. In NeoALTTO, the trastuzumab plus lapatinib cohort were treated with both drugs concomitantly, whereas in the ALTTO trial, these drugs were used either concomitantly or sequentially (that is, trastuzumab together with or followed by lapatinib) in different patient subgroups (FIG. 1). Of note, the lapatinib alone arm of the ALTTO trial was closed in 2011 on the recommendation of an Independent Data Monitoring Committee (IDMC), after the first planned interim analysis. The IDMC reported that the comparison of lapatinib alone versus trastuzumab alone crossed the futility boundary, indicating that the lapatinib alone arm was unlikely to meet the prespecified criteria to demonstrate non-inferiority to trastuzumab alone, with respect to DFS28, 32.
Figure 1. Design of the ALTTO and NeoALTTO clinical trials.
The efficacy of lapatinib monotherapy, standard-of-care trastuzumab monotherapy, and dual HER2 blockade with lapatinib and trastuzumab for patients with HER2-positive breast cancer was compared in the adjuvant and neoadjuvant settings in the ALTTO and NeoALTTO, respectively. In both trials, patients also received chemotherapy according to varying regimens, as indicated. a | In the NeoALLTO trial, patients continued to be treated with the same anti-HER2 agent(s) that they received during the neoadjuvant period. b | The ALTTO trial included an extra treatment arm to determine the efficacy of trastuzumab (for 12 weeks in design 2 and for 18 weeks in design 2B), followed by lapatinib (for 34 and 28 weeks, respectively) after a 6-week ‘wash out’ period. Anti-HER2 therapy was initiated after completing all chemotherapy (design 1), concurrently with a taxane after chemotherapy with an anthracycline (design 2), or concomitant with platinum-containing, non-anthracycline chemotherapy (design 2B). *The lapatinib-only arm of the ALTTO trial was closed in August 2011 for futility. Carbo, carboplatin (AUC 6); D, docetaxel (75–100 mg/m2 every 3 weeks); P, paclitaxel (80 mg/m2 weekly); FEC, 5-fluorouracil (500 mg/m2), epirubicin (100 mg/m2), and cyclophosphamide (500 mg/m2) given on the same day every 3 weeks.
Despite being performed in patients with the same disease subtype, the NeoALTTO and ALTTO trials had fundamentally different results. The primary objective of the NeoALTTO trial was met, with a substantial increase in the pCR rate with dual-agent HER2 blockade compared with trastuzumab alone (51.3% versus 29.5%, P = 0·0001)28. By contrast, the primary end point of the ALTTO trial, was not met; that is, no statistically significant DFS benefit for adjuvant dual-agent HER2 blockade was observed at a median follow up for 4.5 years (HR 0.84 for the comparison of concomitant trastuzumab and lapatinib versus trastuzumab alone, P = 0.048 — the protocol modification require P ≤0.025 for significance)32.
Of note, however, survival outcomes were not superior for the dual-agent HER2 blockade group versus the trastuzumab alone cohort overall in the NeoALTTO trial (event-free survival (EFS): HR 0.78, 95% CI 0.47–1.28, P = 0.33; overall survival: HR 0.62, 95% CI 0.30–1.25, P = 0.19)38. In a landmark analysis (an approached used to compare time-to-event outcomes between groups, determined during study follow up, which includes controls for guarantee time bias39) performed at 30 weeks after randomization, the NeoALTTO investigators demonstrated that 3-year EFS (HR 0.38, 95% CI 0.22–0.63, P = 0.0003) and 3-year overall survival (HR 0.35, 95% CI 0.15–0.70, P = 0.005) were significantly improved in women who achieved pCR compared with those who did not38. These improvements were observed for all of the patients with a pCR, as a whole (that is, when the treatment administered was not considered), as well as for the hormone-receptor-negative group, overall (3-year EFS: HR 0.34, 95% CI 0.17–0.63, P = 0.001; 3-year overall survival: HR 0.29, 95% CI 0.11–0.62, P = 0.003)38. Caution should be exercised when interpreting these data, however, because patients who were hormone-receptor negative were more likely to be included in the landmark analysis than those who were hormone-receptor positive38; moreover, the hormone-receptor negative patients were also more likely to achieve pCR and to have an disease-progression event during follow up38. In addition, although achievement of a pCR was associated with improved 3-year EFS and overall survival in the hormone-receptor negative group, and not in hormone-receptor positive group, the test for interaction was not significant (Pinteraction = 0.34)38.
Disparities between the trials
A number of potential factors might explain the disparities between the results, with regard to the primary end points, of NeoALTTO and ALTTO clinical trials. These factors are discussed the following sections.
Patient/disease characteristics
Differences in the characteristics of the patients with HER2-positive breast cancer enrolled in the NeoALTTO and ALTTO trials can be recognized (TABLE 1). Notably, in the ALTTO clinical trial, patients had low risk of recurrence, on the basis that at least ~85% of patients had tumours ≤5 cm in diameter (compared with a maximum of <65% of the NeoALTTO cohorts), and 40% had axillary-lymph-node-negative disease28, 32. These discrepancies in clinicopathological data (that is, smaller tumour sizes and less axillary-lymph-node involvement) suggest that patients in the ALTTO trial had less-aggressive disease than those in NeoALTTO. These characteristics might explain the low frequency of DFS events reported and, therefore, might underlie the failure to demonstrate a statistically significant difference in this end point between the treatment groups32.
Table 1.
Clinicopathological characteristics of the NeoALTTO and ALTTO trials
| Characteristic | NeoALTTO (n = 455) | ALTTO (n = 8,381) |
|---|---|---|
| Primary end point | Breast pCR | DFS |
| Approach to dual HER2 blockade | Lapatinib plus trastuzumab | Lapatinib plus trastuzumab, or trastuzumab then lapatinib |
| Menopausal status | NA | Postmenopausal or male: 56–57% (0–0.25% male) Premenopausal: 43–44% |
| Tumour size* | ≤ 2cm: 1.3–2.6% >2cm and ≤5: 44.3–61.8% >5 cm: 35.5–53% Missing or NA: 0–1.3% |
≤ 2cm: 41% >2cm and ≤5: 44–45% >5 cm: 5–6% Missing or NA: 9–10% |
| Axillary-lymph-node status* | N0–1: 83.8–84.6% ≥N2, Nx, or missing: 15.4–16.2% |
N0: 40% N1–3: 29–30% ≥N4: 22% Missing or NA: 8–9% |
| ER status* | ER negative: 48.1–49.7% ER positive: 50.3–51.9% |
ER negative: 42–43% ER positive: 57–58% |
Ranges represent the different values across treatment arms.
DFS, disease-free survival; ER, oestrogen receptor; N, regional lymph node stage; NA, not available; Nx, regional lymph nodes could not be assessed (for example, previously removed); pCR, pathological complete response.
Pathological features that include tumour grade, and the expression levels of oestrogen receptors (ERs), progesterone receptors (PR), and markers of cell proliferation (that is, the Ki67 index) seem to be associated with different responses to neoadjuvant therapies: patients harbouring tumours with a high proliferative index and/or of a high grade have a higher likelihood of achieving a pCR40, 41. For example, in neoadjuvant trials of anti-HER2 agents, patients with ER-negative and HER2-positive (ER−/HER2+) breast cancer have been found to have remarkably different pathological outcomes compared with those with ER+/HER2+ disease24, 28, 42. Indeed, in the NeoALTTO trial28, the pCR rate was higher in patients with ER−/HER2+ tumours, compared with those with ER+/HER2+ disease, in all treatment groups (dual HER2 blockade, 61.3% versus 41.6%; single-agent HER2 blockade with trastuzumab, 36.5% versus 22.7%). In the landmark analysis performed by the NeoALTTO trial investigators, the hazard ratios for EFS in the patients who achieved a pCR versus those without a pCR were significant in the hormone-receptor-negative cohort (HR 0.34, 95% CI 0.17–0.63, P = 0.001), but not in the hormone-receptor-positive cohort (HR 0.50, 0.18–1.13, P = 0.13). These findings support the notion that these subgroups comprise different disease entities within the HER2-positive breast-cancer subtype. This information could potentially be useful in the stratification of patients who are likely to derive additional benefit from the administration of dual-agent HER2 blockade versus trastuzumab monotherapy in future clinical trials. In the NeoALTTO trial, however, no statistically significant difference between the treatment groups was demonstrated when EFS and overall survival were analysed by hormone-receptor status. Notably, no statistically significant differences between the effects of therapy on the outcomes of patient with these disease subtypes have been demonstrated in postoperative setting, including the ALTTO trial15, 16, 32. Nevertheless, NeoALTTO comprised a higher proportion of patients with ER-negative disease than ALTTO (>48% versus <43%), which could potentially have contributed to the differences in the outcomes of these trials, with regard to the primary end points.
Treatment regimens and schedules
Treatment-related issues might be relevant to understanding the possible reasons for the disparity between the results of both trials. In particular, the timing of anthracycline administration in the NeoALTTO and ALTTO, and the administration schedule of lapatinib deserve to be mentioned. In the NeoALTTO trial28, anthracycline-based chemotherapy was administered after surgery — not as part of the preoperative treatment. In the ALTTO trial32, anthracycline-based chemotherapy was incorporated as part of design number 1 (anti-HER2 therapy after all chemotherapy) and design 2 (concomitant anti-HER2 therapy and taxane chemotherapy after anthracycline chemotherapy), although design 2B (simultaneous anti-HER2 and platinum–docetaxel doublet chemotherapy) was planned not to include anthracycline administration. Whether achievement of a pCR would have translated into a 3-year EFS and overall survival benefit in the NeoALTTO trial38 if no anthracycline-based chemotherapy had been given after surgery remains an open question.
With regard to the lapatinib schedule, preclinical and early clinical evidence indicate that intermittent administration of lapatinib is associated with better efficacy than standard continuous dosing43, 44. Thus, the use of the standard schedule of lapatinib in the ALTTO and NeoALTTO trials, in addition to the consistent reports of increased adverse event rates and discontinuation or interruption of treatment occurring in the lapatinib-containing arms38, could potentially indicate that the efficacy of lapatinib might not have been optimal in these studies.
Primary end points
The designs and primary end points of both clinical trials were different. The primary end point of the NeoALTTO trial28 — a significant increase in the rate of pCR in the breast (not including the axilla) between the dual-agent HER2 blockade and trastuzumab monotherapy cohorts — was met. In the ALTTO trial32, however, the primary end point was DFS, which was not met as the treatment cohorts shared similar DFS rates at a median follow up of 4.5 years: 86% to 88%.
Relevant concerns over the discrepancies between the neoadjuvant and adjuvant trials of dual-agent HER2 blockade, with regard to achievement of the primary end points, relate to statistical power. The investigators of the ALTTO trial planned to perform the primary statistical analysis when either 850 DFS events required for the comparison of lapatinib plus trastuzumab versus trastuzumab had occurred (for 80% power to detect a HR of 0.80 using a two-sided alpha error of 0.0167), or a median follow up of 4.5 years was reached.28, 32 Although the ALTTO trial involved more than 8,300 patients, the first analysis was based on 555 DFS events — that is, 30% less than the planned total of 850 events required for 80% power — at a median follow up of 4.49 years (range: 1 day to 6.4 years)28, 32. Notably, the time-driven analysis was used in favour of the event-driven analysis to obtain earlier results. The fact that this trial did not demonstrate the superiority of dual-agent anti-HER2 therapy was probably because of the time-driven primary end point used. Thus, a more-mature analysis (after a greater number of events) should be explored and might provide different results.
As described, in the ALTTO trial28, 32, the DFS HR for the combination of anti-HER2 agents versus trastuzumab alone was 0.84 (97.5% CI 0.70–1.02), with a two-sided P value of 0.048; this result was not significant owing to the splitting of the type I error rate in the original design between the comparisons with the sequential and concurrent approaches to dual-agent HER2 blockade28, 32. In other words, the investigators added another comparison, to establish the non-inferiority of trastuzumab followed by lapatinib versus trastuzumab alone45. Thus, if the ALTTO trial had randomly assigned patients to receive anti-HER2 therapy with only trastuzumab or concomitant trastuzumab plus lapatinib, the P value obtained for this comparison would have been considered statistically significant and the trial positive for its primary end point.
Another important point to highlight is that the overall-survival data for patients in the ALTTO trial was not inferior to that of women in the HERA trial (also known as the Herceptin Adjuvant trial, which compared treatment with trastuzumab for 1 and 2 years with observation after standard neoadjuvant chemotherapy or adjuvant chemotherapy, or both)15, 46. Performing an accurate head-to-head comparison between these trials is not feasible; bearing in mind the caveats of indirect comparison, however, the HRs for overall survival reported for dual-agent HER2 blockade versus trastuzumab arms in the ALTTO trial are similar to those for the comparison between trastuzumab and observation (after standard neoadjuvant and/or adjuvant chemotherapy) in the HERA trial: HRs of 0.80 and 0.91 for trastuzumab plus lapatinib and trastuzumab followed by lapatinib, respectively, versus trastuzumab in the ALTTO trial;32 HR 0.76 for trastuzumab-based therapy versus observation in the HERA trial15, 46.
The absence of a requirement for a lymph-node complete response in the definition of pCR used in the NeoALTTO trial is another potential reason why the primary outcome of the NeoALTTO was met, whereas that of the ALTTO trial was not (see ‘Insights from other neoadjuvant trials’ section), and further exploratory analysis should shed some light on this possibility. According the FDA criteria, absence of disease at both the primary-tumour site and axillary lymph nodes seems to be the optimal definition of a pCR when considering accelerated approval of a treatment. Indeed, evidence suggests that a response in both primary breast tumour and axillary lymph nodes is associated prolonged survival; hence, definitions of pCR that include response in only the primary breast tumour, as used in the NeoALTTO trial, might not correlate as closely with long-term outcomes.
Of note, in the NeoALTTO trial, the secondary EFS end point was more analogous to primary end point of the ALTTO trial, DFS, and this did not differ for the dual-agent HER2 versus the monotherapy cohorts overall. Of note, the small sample size limited the power to reveal differences in EFS in the neoadjuvant setting, and the fact that the effects of the adjuvant parts of NeoALTTO treatment schedules could not considered in the pCR assessment, but are relevant to EFS, is an important concern. For example, patients without pCR might benefit from subsequent anthracycline treatment, whereas patients who already have pCR might not. Fundamentally, therefore, the differing results between NeoALTTO and ALTTO might be a consequence of being able to stratify those patients with a pCR in the neoadjuvant setting, and report significantly improved long-term outcomes for these patients, despite no overall improvement in the outcomes of the entire treatment cohort. In the adjuvant setting, stratifying patients on the basis of an end point associated with residual disease in not possible at present. If such an end point could be assessed in the adjuvant setting (for example, using assays for detecting minimal residual disease such as circulating tumour DNA (ctDNA) or circulating tumour cells), the findings of ALTTO and NeoALTTO might have been similar — that is, if patients who had no residual tumour cells after adjuvant therapy could be identified, these patients would probably have better long-term outcomes (that is, DFS and/or overall survival) than those with residual cells. Thus, stating that those patients with a pCR in the NeoALTTO trial have better long-term outcomes is important, but this ignores the patients who do not achieve a pCR and the fact that the magnitude of the effect of dual-agent HER2 blockade was insufficient to make this approach superior for the overall population. Currently, one cannot predict the patients who will have a pCR to neoadjuvant therapy, and, therefore, the long-term outcomes of the entire cohorts are critically important for clinical decision-making. Hence, from this perspective, the situation seems to be analogous in both neoadjuvant and adjuvant trials (that is, NeoALTTO and ALTTO trials): dual-agent HER2 blockade has no significant effect on overall survival, for the overall ‘intention-to-treat’ population. Only with regard to the primary end points is NeoALTTO considered a success while ALTTO is not.
pCR as a surrogate for survival
Varying definitions and correlations
The achievement of a pCR, albeit strongly correlated with EFS and overall survival after neoadjuvant chemotherapy, is known to be an imperfect surrogate for prediction of the survival of patients with breast cancer. Furthermore, the pCR end point can also vary in its definition35, 47, 48. Indeed, a universally accepted definition for pCR is not available at present, and differences in the definitions of pCR used in neoadjuvant trials have rendered the reporting and interpretation of data challenging. For the purpose of designing trials, the FDA48 recognizes the following two definitions of pCR to neoadjuvant systemic therapy, based on haematoxylin and eosin (H&E) evaluation of the complete resected breast specimen and all sampled regional lymph nodes: either the absence of residual invasive cancer (classified as ypT0/Tis ypN0 according to the current American Joint Committee on Cancer (AJCC) staging system); or, the absence of residual invasive and in situ cancer (AJCC stage ypT0 ypN0).
In addition, previous studies have addressed clinical and biological factors associated with a pCR to neoadjuvant chemotherapy in patients with breast cancer. In these studies, investigators demonstrated that a high tumour grade and/or ER-negative disease, as well as combined trastuzumab treatment and chemotherapy for HER2-positive disease as factors associated with a greater likelihood of achieving a pCR17, 24, 28, 40, 49-52. In fact, the frequency and prognostic value of pCR was shown to vary among breast cancer intrinsic subtypes47, 53. Specifically, for tumour subtypes characterized by slow proliferation (such as, luminal A-like breast cancer), pCR is not associated with prognosis, whereas for subtypes comprising highly proliferating tumours (such as TNBC), pCR can be used to accurately discriminate between patients with a good or a poor prognosis54. The findings of NeoALTTO28, as well as those of other trials29, 55 that used dual-agent HER2 blockade in the context of HER2-positive breast cancer, indicate that ER-positive disease and ER-negative disease discriminate two different subgroups of patients with HER2-positive breast cancer. As patients with ER-negative tumours had higher rates of pCR than those with ER-positive disease, the new generation of neoadjuvant trials should study these two patient populations independently.
Indeed, the validity of using pCR as a surrogate end point for survival in patients with breast cancer is an issue that has been raised in various discussions of the NeoALTTO and ALTTO trials 28, 32, 47, 56, 57. An FDA-led meta-analysis58, in which data from 12 international trials and a total of 11,955 patients were assessed, has often been cited in this context. This study demonstrated unequivocal associations between achievement of a pCR and long-term survival benefits. For example, in patients with HER2-positive tumours, achievement of a pCR was strongly associated with EFS (HR 0.39, 95% CI 0.31–0.50) and overall survival (HR 0.34, 95% CI 0.24–0.47)58. The study found no clear relationship, however, between increases in the pCR rate and the HRs for EFS and overall survival at the level of the entire trial cohorts, and thus failed to establish the pCR rate as a surrogate end point for improved EFS and overall survival58. The Prentice criterion59 for surrogate end points essentially requires the surrogate variable to capture any relationship between the treatment and the true end point. To meet this criterion, statistical hypothesis testing is required to establish that the treatment has no residual effect on the true end point after adjusting for the surrogate measure59-64; pCR rate did not satisfy this criterion in the context of the FDA meta-analysis58. The evidence that a pCR to treatment is predictive of a good outcome in patients with breast cancer is, nevertheless, unequivocal. Thus, the key question is, in a neoadjuvant trial, what magnitude of difference in pCR rates between two treatment arms and across different breast cancer subtypes would translate into statistically significant improvements in survival in the overall trial population?
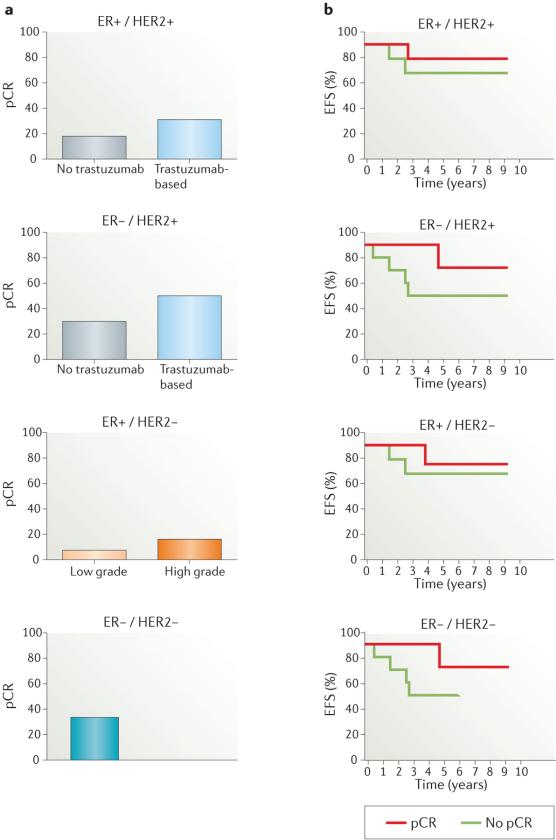
As discussed, pCR rates for patients with the different clinical subtypes of breast cancer vary, and are lower in those with ER+/HER2– breast cancer than in women with HER2-positive or ER–/HER2– disease subtypes (FIG. 2a). Furthermore, the strongest associations between pCR and long-term outcome have been reported for aggressive breast cancer subtypes (that is, ER–/HER2– and ER–/HER2+; FIG. 2b)58. In the FDA-led meta-analysis58, most of the studies examined enrolled patients with biologically heterogeneous breast-cancer subtypes; an exception to this pattern was the disease-specific NOAH trial51, in which trastuzumab plus chemotherapy or chemotherapy alone were administered only to patients with HER2-positive breast cancer (64% ER negative and 36% ER positive for both treatment arms). In the NOAH trial51, EFS was strongly associated with pCR, particularly in patients given trastuzumab: the hazard ratio for EFS between the patients with pCR who received trastuzumab versus those with a pCR who did not receive this agent was 0.29 (95% CI 0.11–0.78, P = 0.0135). This finding suggests that the relationship between pCR and long-term outcomes might also depend on the specific therapy used.
Figure 2. Pathological complete response (pCR) and outcomes.
a | The graphs demonstrate the proportion of patients who achieve a pCR to neoadjuvant therapy, stratified according to breast-cancer subtype. For patients with HER2-positive primary tumours (both oestrogen receptor (ER)-positive and ER-negative), pCR data for the trastuzumab-based and no trastuzumab treatment arms are shown, based on a pooled analysis by Cortazar et al.58 In the ER+/HER2− group, pCR is shown for both low-grade and high-grade tumours. In the ER−/HER2− group, pCR data is shown without dichotomization. b | Illustrative Kaplan–Meier curves showing the association between pCR and event-free survival (EFS), stratified according to breast-cancer subtype.
Likewise, the updated data from the NeoALTTO trial38 indicate that, although no differences in the secondary end points — EFS and overall survival — were observed between the treatment arms, patients who experienced a pCR had significantly better outcomes than those patients without a pCR. This relationship (that, is, achieving a pCR) held true for EFS and overall survival in the entire cohort of patients and the subset of patients with hormone-receptor-negative disease, and pCR was also independently associated with EFS, but not overall survival, in the patients treated with dual-agent HER2 therapy38; in the other treatment groups, pCR was not associated with EFS or overall survival38. However, it should be emphasized that this trial was not powered to detect statistically significant differences in EFS or overall survival. Thus, evidence suggests that if a new therapeutic option produces an increase in the frequency of pCR compared with standard therapy alone, the novel treatment is likely to result in long-term survival benefits (EFS and overall survival), particularly if treatment is focused on well-defined breast-cancer subtypes that are known respond better to neoadjuvant therapy (such as ER–/HER2– and ER–/HER2+ disease). Nevertheless, adequately powered trials are required to confirm this holds true for dual-agent HER2 blockade.
Insights from other neoadjuvant trials
In addition to NeoALTTO28, other neoadjuvant studies have investigated the addition of lapatinib to trastuzumab for the treatment of patients with HER2-positive breast cancer42, 55, 65. The Cancer and Leukemia Group B 40601 trial (CALGB 40601)42 quantified the pCR rates of patients who received weekly paclitaxel and either trastuzumab plus lapatinib or trastuzumab alone; the pCR rate was modestly higher with combined dual-agent HER2 blockade, but this difference did not reach statistical significance: 51% (95% CI 42–60) versus 40% (95% CI 32–49), P = 0.11). The authors also analysed the results according to gene-expression-based intrinsic molecular subtypes, and gene sequence and copy-number abnormalities in primary tumours and residual disease; these studies revealed that pCR rates were higher among the patients with a specific molecular disease subtype defined as ‘HER2-enriched’42, 66. In addition, the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-41 trial55 and European Organisation for Research and Treatment of Cancer (EORTC) 10054 trial65 randomly assigned patients to receive neoadjuvant chemotherapy with either trastuzumab, lapatinib, or the combination of both anti-HER2 agents. The dual-agent HER2-targeted therapy was associated with numerically higher, but not statistically significant, pCR rates than single-agent HER2-directed therapy in both trials55,65.
Thus, unlike the NeoALTTO findings28, the results of the CALGB 40601, NSABP B-41, and the EORTC 10054 trials did not demonstrate a statistically significant increase in pCR rates for the trastuzumab plus lapatinib combination42, 55, 65. Of note, the NSABP B-41 treatment regimen was more similar to that used in the ALTTO study than the NeoALTTO treatment approach28, 32; however, the difference in the characteristics of the study populations might also, at least partially, explain the discordance between the results of these trials, as well as the NeoSPHERE and NeoALTTO trials24, 28,55, 65. In particular, the negative results of both the CALGB 40601 and the NSABP B-41 trials might also be at least partially explained by the inclusion of a higher proportions of women with ER-positive disease (in general with lower frequency of pCR than patients with ER-negative tumours) than in the NeoALTTO and NeoSPHERE trials24, 28, 55, 65. Of note, in the CALGB 40601 trial42, patients with ER-negative tumours had higher pCR rates after dual-agent versus single-agent HER2 blockade (77% versus 55%), but the differences were modest in those with ER-positive tumours (42% versus 39%); however, the improvement with the addition of lapatinib to trastuzumab was not statistically significant in either group.
In the CALGB 40601, NSABP-B41, EORTC 10054 trials42, 55, 65, pCR was defined as breast pCR only. Similarly, the primary end point of the NeoALTTO trial, as reported in 201228, was the rate of pCRs defined as the absence of only invasive tumour cells in the breast at the time of surgery (ypT0). Additionally, however, the rate of locoregional total pCR, as defined by no invasive cancer in the breast and no pathological involvement of the axillary lymph nodes (ypT0/Tis ypN0), was a secondary end point of NeoALTTO. This secondary end point was included in NeoALTTO protocol amendment 3 (published on 17th May 2013), and was introduced to achieve consistency with recommendations from the FDA38 — the FDA suggests that either ypT0/Tis ypN0 or ypT0 ypN0 should be used as the primary definition of pCR in neoadjuvant trials58. The absence of a requirement of lymph-node complete response in the NeoALTTO trial might constitute another reason why differences in the primary end points were observed between the NeoALTTO and ALTTO trials. Further exploratory analysis of these factors is warranted.
By contrast, the lack of efficacy with lapatinib monotherapy has been consistently demonstrated in the neoadjuvant trials that compared anti-HER2 monotherapy with dual-agent HER2 blockade, as well as in the ALTTO trial in the adjuvant setting28, 32, 42, 55, 65, 67. Moreover, akin to the NeoALTTO trial, the results of the Geparquinto trial67 indicated a significantly lower pCR rate with lapatinib versus trastuzumab monotherapy, when added to a 24-week chemotherapy regimen comprising an anthracycline and taxane. The CALGB 40601 trial42 data also showed a trend towards a lower pCR rate with 16 weeks of lapatinib versus trastuzumab therapy, as did data from the EORTC 10054 study65. In the NSABP B-41 trial55, investigators also demonstrated no difference in pCR rates between the lapatinib and trastuzumab monotherapy arms, but a higher pCR rates for the dual-agent HER2 blockage.
Using pCR to identify new biomarkers
At present, we cannot limit dual-agent HER2 treatment to only those patients who are likely to benefit (over standard therapy) in either the neoadjuvant or the neoadjuvant setting. Thus, when treating the entire population of HER2-positive patients (assuming we cannot select those who will achieve a pCR beforehand) only a small fraction (20%) might gain additional benefit from dual-agent therapy, compared with the standard of care, but the larger fraction of HER2-positive patients will not and will be exposed to the excess biological and fiscal toxicity of dual-agent HER2 blockade. Further combined analysis of the pCR data and tumour molecular characteristics might enable those patients who will benefit from dual-agent (over the single-agent) HER2-targeted therapy to be identified, so that future trials can be stratified by predictive biomarkers — ultimately making the harm to benefit ratio of the dual-agent approach more favourable. Deciphering which patients are likely to achieve a pCR is, therefore, a thought-provoking topic; theoretically, the same features that make patients ‘responders’ in the neoadjuvant setting might also have the same implications in the adjuvant setting.
Studies have been conducted to discover biomarkers indicative of response to dual-agent therapy using NeoALTTO tumour-tissue biopsy samples68, 69. For example, baseline biopsy samples were screened for mutations in the gene encoding the phosphatidylinositol 3-kinase (PI3K) catalytic subunit (PIK3CA)68. Patients treated with a dual-agent HER2 therapy who had a wild-type PIK3CA status had a total pCR rate of 53.1% versus 28.6% in patients with tumours that harboured PIK3CA mutations (P = 0.012). This finding has already enabled stratification of patients for treatment to be incorporated into the design of some clinical trials. For instance, in the NeoPHOEBE trial (NCT01816594)70, patients with breast cancer have been molecularly stratified to determine whether any benefit of the addition of a PI3K inhibitor to neoadjuvant trastuzumab and paclitaxel is dependent on PIK3CA-mutation status.
High levels of tumour-infiltrating lymphocytes (TILs) TILs have been consistently linked to a more-favourable prognosis in patients with early stage triple-negative and HER2-positive breast cancers71-73. Thus, investigators have also analysed whether the presence of TILs at diagnosis was associated with achievement of a pCR and/or EFS in patients treated in the NeoALTTO trial69. They found that the presence of TILs at diagnosis was an independent prognostic marker for a pCR — TILs comprising >5% of the tumour were associated with higher pCR rates, independent of treatment group, (adjusted odds ratio 2.60, 95% CI 1.26–5.39, P = 0.01)69. EFS was also related to TIL levels: every 1% increase in the proportion of TIL was associated with a 3% decrease in the event rate (HR 0.97, 95% CI 0.95–0.99, P = 0.002) across all treatment groups69. These results suggest that enhancing antitumour immune responses is important to improving survival outcomes.
Other challenges in (neo)adjuvant trials
The design of neoadjuvant and adjuvant clinical trials in patients with early stage breast cancer can influence the tumour responses and thus patients’ prognoses. Neoadjuvant and adjuvant clinical trials, however, have important differences (TABLE 2): compared with adjuvant clinical trials, neoadjuvant trials have smaller sample sizes; the patients enrolled are sometimes have shorter durations of drug exposure and follow up, resulting in less long-term safety information being obtained; and the primary end point usually is pCR, rather than outcomes related directly to disease progression and/or survival. In addition, some of the greatest benefits of using the neoadjuvant approach are possibility of tumour downstaging (local control), and the ability to assess the clinical and radiological responses of the primary breast tumour and axillary lymph nodes in vivo3 — which can vary from mild responses (or even progression) to complete responses (that is, pCRs). Neoadjuvant trials are important, however, not only for local control of disease, but also to accelerate drug development57.
Table 2.
Comparison of main characteristics of neoadjuvant and adjuvant trials
| Parameter | Neoadjuvant trials | Adjuvant trials |
|---|---|---|
| Definition | Treatment given before surgery | Treatment given after surgery |
| Sample size | Smaller | Larger |
| End points | Response rates and duration (pCR, EFS) | Relapse and survival (DFS, overall survival) |
| Time taken to complete | Months | Years |
| Costs | Lower | Higher |
| Advantages | Tumour downstaging—that is, converting a previously unresectable, locally advanced breast cancer to an operable tumour Enables early assessment of response to treatment Response-guided therapeutic planning is possible Enables comparison of the characteristics of pretreatment and post-treatment tumour samples (in those without a pCR), which might facilitate biomarker discovery, prognostication, patient stratification, and assessment of responses to therapy |
Designed to increase the chance of long-term survival Quality-of-life questionnaires can be used to assess the long-term effects of treatment in multiple domains |
| Disadvantages | Local tumour control might be delayed Complicates pathological assessments and staging In some circumstances, duration of drug exposure is shorter, which might reduce its effectiveness Less long-term safety information is available Potential for selection of resistant clones owing to relatively high disease burden at the time of systemic treatment |
Risk of exposing a large number of patients to treatment that is more toxic and/or no more (or even less) effective than the current standard of care Precludes assessment of response to systemic treatment and, thus, does not enable response-guided treatment planning |
DFS, disease-free survival; EFS, event-free survival; pCR, pathological complete response.
Adjuvant trials have become less sustainable because they take a long time to complete, are costly, and require enrolment of a large number of patients57. Neoadjuvant trials could potentially overcome many of these challenges if the short-term results (pCR) are able to predict long-term outcomes, such as EFS and overall survival. At present, the literature includes evidence that cancer biology, tumour heterogeneity, and mechanisms of resistance are other variables, in addition to the inherent differences in clinical-trial designs and statistical power, that might have an important role in explaining the discrepancies between neoadjuvant and adjuvant trials in breast cancer; however, no definitive answers are available, and preclinical and clinical validation is required. Furthermore, comparing the results of neoadjuvant trials that used the imperfect primary end point of pCR (and indeed, demonstrated no effect on EFS or overall survival) and adjuvant trials with traditional survival end points has obvious caveats. We nevertheless discuss the potential biological reasons to explain the differences in the results of neoadjuvant and adjuvant studies that use essentially the same therapeutic regimens.
Capturing the dynamics of resistance
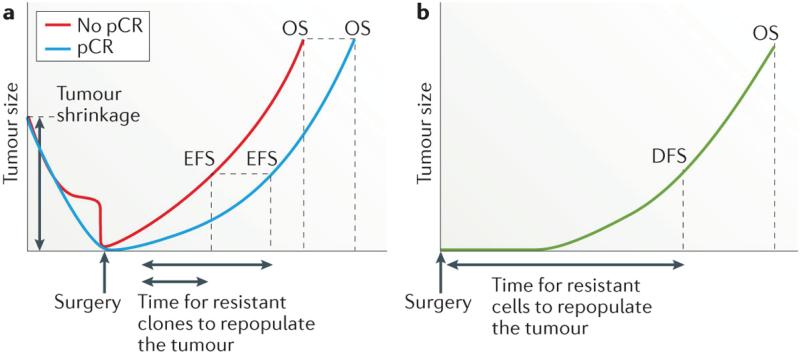
Given the differences in the treatment duration and tumour burden between neoadjuvant and adjuvant trials of the same drugs, capturing the dynamics of resistance might be useful in predicting therapeutic resistance and the dynamics of tumour repopulation by a resistant subclone. Treatment resistance due to resistant clones that either pre-existed prior to treatment or were acquired over the course of therapy is probably the key contributor to the lack of long-term survival improvement in both neoadjuvant and adjuvant settings. Potentially, the presence of a higher tumour burden at the time of systemic treatment in the neoadjuvant setting versus the adjuvant setting could increase the probability that therapy will select for more-aggressive clones that, if left behind after surgery, repopulate the tumour more quickly. Thus, one could be hypothesize that patients without a pCR might have worse outcomes with neoadjuvant versus adjuvant treatment because, presumably, the level of intrinsic resistance is already higher in these individuals. This possibility might also provide an explanation for the lack of EFS and overall survival benefit observed when considering the entire dual-agent HER2 treatment cohort, despite the improved pCR rate — that is, the patients without a pCR might have had worse outcomes after neoadjuvant therapy (rapid disease progression owing to selection of resistant clones), thus diluting the pCR benefit in the overall treatment cohort.
Conceivably, the early benefit of novel therapies in patients who achieve a pCR versus those who do not achieve a pCR, as observed in neoadjuvant trials, might be diminished in the long term as resistant clones repopulate the tumour and eventually lead to disease recurrence in selected cases (FIG. 3). The Luria-Delbruck models74 of bacterial resistance have been adapted to study pre-existing drug resistance in several cancer types, including chronic myeloid leukaemia (CML)75, 76, lung cancer77, and colorectal cancer78. The theoretical predictions imply that the probability of pre-existing resistance increases with tumour size at diagnosis and the mutation rate79-81. Diaz et al.78, performed mathematical calculations based on the dynamics of KRAS mutations in ctDNA over the course of anti-EGFR therapy in 28 patients with colorectal cancer. Their results suggest that resistance mutations in KRAS and other genes were highly likely to be present in subclonal tumour-cell populations prior to treatment initiation78. This hypothesis is in line with existing experimental evidence for other targeted agents76, 82-84. The authors concluded that resistance is a fait accompli, and the time to recurrence is thus the interval required for the resistance clones to repopulate the tumour78.
Figure 3. The influence of therapeutic response on survival.
a | In the neoadjuvant setting, achievement of a pathological complete response (pCR) is hypothesized to result in prolonged both event-free survival (EFS) and overall survival (OS), compared with an incomplete response, because fewer drug-resistant clones are present and, therefore, even fewer are likely to be left behind after completion of surgery and systemic treatments; thus repopulation of the tumour and/or the development of clinically detectable metastases is delayed or might never happen, and OS is prolonged in the population of patients with a pCR. The early benefit on EFS associated with achieving a pCR to neoadjuvant therapy, compared with an incomplete response, might be diminished in the long-term, however, as resistant clones repopulate the tumour and eventually lead to clinical disease recurrence and, in some cases, death; that is, attaining a pCR might improve OS, but the proportion of patients who are cured might not be significantly different. b | In the adjuvant setting, the time taken for any residual resistant cells to repopulate the tumour is reflected in the duration of disease-free survival (DFS) and OS. In all scenarios, the probability of pre-existing resistance, and thus the chance of residual cells being left after all treatment is completed, increases with tumour size at diagnosis and might be at least partially related to the breast cancer subtype. It should be noted, for example, that triple-negative breast cancers tend to be larger and to have more-rapid growth than other breast cancer subtypes;116 therefore, the risk of relapse might be higher, and the time to relapse shorter, for this subtype compared with others. Ultimately, the benefit of complete tumour shrinkage in patients with a pCR, in comparison with the outcomes of patients who receive only adjuvant therapy, might be minimal — assuming that almost all tumour cells are removed when R0 resection is achieved and that, in the adjuvant setting, the small number of residual cells are less likely to be treatment resistant.
One can also posit, therefore, that if the effectiveness of analogous neoadjuvant and adjuvant therapy are the same, the extent of tumour-cell killing/removal would predominantly determine recurrence and survival outcomes. Thus, the degree and characteristics of any micrometastatic residual disease that remains after neoadjuvant or adjuvant therapy might ultimately negate any overall benefit expected based on increased pCR rates or DFS rates with novel therapies, respectively. In this context, identifying micrometastatic residual disease after neoadjuvant and also after adjuvant therapies might indicate targetable biomarkers (which can be different from those present in the primary breast tumour) that are associated with therapeutic resistance and create opportunities for therapeutic interventions before the development of clinical metastasis85. The non-invasive monitoring of micrometastatic residual disease through liquid biospies (that is, ctDNA) might be used as a surrogate or complementary tool for response-guided therapy, and could lead to the optimization of response assessment in the neoadjuvant and adjuvant settings85-87. In patients with nonmetastatic breast cancer, liquid biopsies offer the potential to capture and monitor minimal residual disease following curative resection, preceding the development of clinical or radiological recurrence, and represent a tool to facilitate assessments of tumour dormancy85-92.
Tumour heterogeneity and drug resistance
The development of massively parallel sequencing technologies and digital genomic analyses has provided preliminary evidence of breast-cancer heterogeneity. Tumour heterogeneity has been documented to be both spatial93-96 and temporal93, 97, 98, and might explain why not all patients who receive a given therapeutic approach benefit from it, or why some patients have only transient responses. How tumour heterogeneity affects clinical outcomes is a subject for ongoing research86,99, 100,101,96, 102.
Using laser-capture microdissection and in situ single-cell analysis, investigators have demonstrated that HER2 amplification and PIK3CA mutations are distributed heterogeneously in breast tumours96,103. This spatiotemporal intratumour heterogeneity could interfere with responses to neoadjuvant and adjuvant treatments. For instance, when tumour samples collected from patients with HER2-positive breast cancer prior to and after neoadjuvant therapy were compared, the frequency of cells containing HER2 amplification was reduced and samples were enriched for cells containing PIK3CA mutations96. This observation might go some way to explaining the suboptimal efficacy of anti-HER2 therapy in the post-surgery period after neoadjuvant therapy (that is, using EFS and overall survival as end points), as proportionally fewer cells containing HER2 amplification might be present in some patients after surgery. By contrast, the enrichment of PIK3CA-mutant cells by neoadjuvant therapy could potentially increase the chance of these resistant cells being left behind after surgery, compared with the use of adjuvant systemic therapy only, thus negatively affecting the outcomes of neoadjuvant therapy. Of note, however, in some breast cancers with a heterogeneous HER2 amplification pattern (that is, HER2-amplified and HER2-non amplified components in the same tumour), the HER2-amplification might not be truncal in the tumour (that is, is not present in all cancer cells of a tumour), and other genetic alterations could be driving the HER2-negative cells of those tumours103.
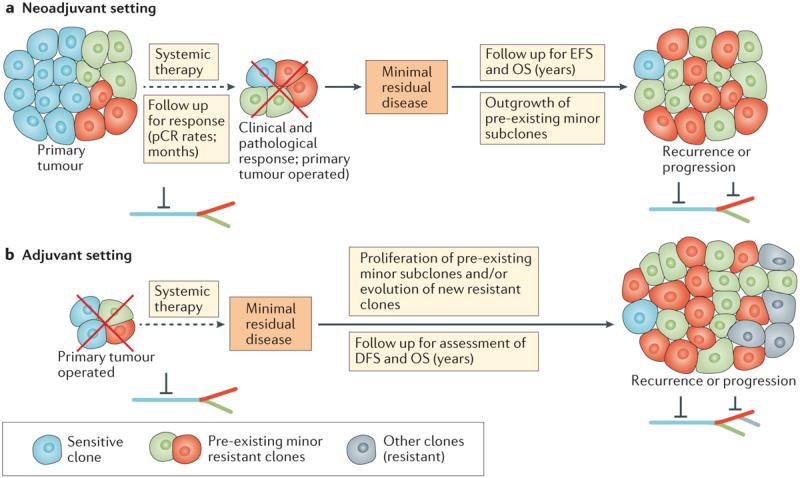
Cancer seems to follow Darwinian rules of evolution93, 104, 105. The trunk–branch model for tumour heterogeneity suggests that the trunk (clonal events, present in all tumour cells, at all tumour regions or sites) and branch (subclonal events, present in a limited number of cells, potentially at distinct sites of a tumour) phylogeny of primary tumours could provide hints for deciphering therapeutic resistance (FIG. 4). Ideally, targeting actionable truncal genetic alterations, which are less susceptible to sampling bias and are present ubiquitously, should represent the best therapeutic strategy106. If a tumour has multiple branches, however, monotherapy could represent a specific selective pressure that promotes the outgrowth of resistant clones over time. The branches should also be targeted because subclones that harbour a resistant event can be present. Thus, in addition to targeting truncal events, targeting subclones (the branches) and their interrelationships should be another strategy to prevent clonal outgrowth and resistance.
Figure 4. Tumour heterogeneity and drug resistance in the neoadjuvant and adjuvant breast cancer settings.
a | The efficacy of neoadjuvant treatment targeting ‘trunk’ (clonal) mutations or aberrations that are expressed by most or all of the tumour cells might nevertheless be limited by the overgrowth of spatially distinct distributed subclones with intrinsic resistance mechanisms (green and red cells), leading to primary drug resistance. This outgrowth of resistant subclones might underlie incomplete responses, or eventual relapse in those with a complete response. b | In the course of adjuvant therapy, the treatment duration and follow-up time are longer, creating a niche for the development of secondary (acquired) resistance to therapy, and/or outgrowth of pre-existing minor resistant subclones that were left behind after surgery and could persist despite systemic therapy. Contemporary data suggest, however, that the latter process is likely to predominate — most resist clones that underlie recurrence are detectable prior to treatment and, therefore, eventual acquired resistance is probably a consequence of low-level intrinsic resistance. The trunk–branch model for tumour heterogeneity is shown: the trunk of the tree bears ubiquitous genomic alterations; acquired subclonal mutations that are present only in a subset of the tumour cells compose the branches. Red inhibitory symbols represent pharmacological blockade. DFS, disease-free survival; EFS, event-free survival; OS, overall survival; pCR, pathological complete response
Neoadjuvant therapy seems to interfere with the genetic diversity of the tumour-cell population and, therefore, poses challenges for the clinical management of patients and for the development of systemic therapeutics — as the molecular characteristics of tumours might change under the selective pressure of treatment107. Evidence indicates that post-treatment tumours have different biological characteristics compared with primary tumours99-101,96, 102. Indeed, residual tumours after neoadjuvant treatment are likely to have a different, and frequently less-favourable, characteristics and composition than those of the diagnostic sample108, 109. At present, however, no data exist regarding the influence of tumour evolution on the achievement of a pCR and the subsequent translation of such responses into overall survival benefits. Such data might shed some light on the biological heterogeneity of breast tumours and support the need for retesting the HER2 status of the surgical sample after neoadjuvant therapy, in order to accurately determine the appropriate use of further targeted therapy, in suitable patients.
At present, firm evidence on the levels to which heterogeneity affects the clinical management of patients with breast cancer is lacking, and to what extent this issue might explain the discrepancies between the results of neoadjuvant and adjuvant trials in breast cancer remains unknown. Potential reasons for why pCR rates increased with dual-agent HER2 blockade, whereas DFS and overall survival in the adjuvant trials (as well as EFS and overall survival in the neoadjuvant trials) did not, might be manifold. First, if the higher tumour burden present during neoadjuvant therapy increases the risk that treatment will result in selection of resistant clones, the outcomes with dual-agent HER2 blockade should have been better in the adjuvant setting — wherein most of the tumour is removed, reducing the probability of resistant clones remaining and also the tumour heterogeneity); however, evidence from NeoALTTO and ALTTO have shown the opposite. Second, the properties of any minimal residual disease in the post-surgery setting could contravene the potential benefits of a therapy that is known to result in deeper responses (increase pCR rates) in the neoadjuvant setting.
The use of adaptive trial designs, in which genetic information for the tumours of patients with breast cancer is used to guide therapeutic approaches, should be envisioned. Trials incorporating this approach should be adapted to encompass low-frequency somatic events that are associated with therapeutic resistance and further morbidity.
Conclusions
Neoadjuvant studies have provided insights into the association between pCR and long-term outcomes in patients with breast cancer, particularly for those with HER2-positive disease. The neoadjuvant setting seems to be a different scenario compared with the adjuvant setting. Indeed, therapeutic benefits detected in the neoadjuvant setting — as defined by increases in pCR — have not, however, been translated into significant survival increases when the same modern targeted therapies are used in the adjuvant setting. In general, however, neoadjuvant therapies do not seem to improve the overall survival of unselected patients with breast cancers, when compared with conventional postoperative adjuvant therapy5, 6. The increasing rates of pCR following neoadjuvant therapy have, nevertheless, had a clinically significant effect on locoregional treatment considerations.
At present, the data describing long-term outcomes of patients after anti-HER2 based neoadjuvant therapy remain limited110. The burden of residual disease after neoadjuvant chemotherapy has been associated with long-term prognosis111, 112. In the neoadjuvant clinical trials, empirical definitions of pCR have been used, with the purpose of utilizing pCR as a surrogate for long-term outcomes. The NeoALTTO trial suggested better outcomes in those with a pCR, independent of treatment, but in the overall cohorts, the increased response rates with dual-agent HER2 blockade did not correlate with EFS or overall survival28. Indeed, evidence regarding the association between pCR and EFS for new therapies in breast cancer remains limited58,57.
The design of future clinical trials in the neoadjuvant setting should incorporate several features to overcome the lack of evidence as to whether increased pCR rates translate into better long-term outcomes: standardization of the definition of pCR across clinical trials; biomarker-based stratification of patients who are most likely to benefit from a specific regimen; and investigation of whether agents brought from the metastatic setting to the neoadjuvant setting would be active and induce pCRs, and the time span of these events (that is, do they occur within the duration of typical neoadjuvant therapy or require a longer period).
In fact, neoadjuvant trials to test specific agents based on biomarker signature of HER2-positive breast cancers have already been initiated113, 114. For example, the I-SPY 2 trial114 is a neoadjuvant dynamic adaptive-design phase II trial that is enrolling patients with high-risk breast cancer. In this trial, tumour biomarker signatures are being determined in order to match experimental drug regimens to the patients who would most benefit from them. The results of this study will likely shed some light on the predictive and prognostic values of pCR, while also providing information on tumour biology and the simultaneous administration of novel therapeutic agents. A phase III registration trial (I-SPY 3) of neratinib (a small-molecule inhibitor of HER2) plus standard neoadjuvant therapy is planned, based on the identification of HER2-positive hormone-receptor-negative group in the phase II study who might benefit from this therapy114.
In addition to lapatinib plus trastuzumab, dual-agent HER blockade with pertuzumab and trastuzumab is being explored in the adjuvant APHINITY trial31, and whether the positive results for this approach observed in the NeoSPHERE trial (which also reported doubling of the pCR rate for dual-agent HER2 blockade as compared to single-agent HER2 inhibition with trastuzumab) will be translated to the adjuvant setting is unknown. In addition, whether a subgroup of patients with HER2-positve breast cancers who would be sufficiently treated with trastuzumab as the only anti-HER2 therapy (as opposed to dual-agent HER2 blockade) can be identified remains to be determined.
The new wave of neoadjuvant targeted therapy trials has resulted in a paradigm shift in the treatment of breast cancer; however, the limitations of the current approaches, as outlined herein, should to be recognized. Intratumour heterogeneity and mathematical modelling might have thought-provoking roles in addressing such challenges. In the neoadjuvant and adjuvant settings, biomarkers that predict response and resistance, and that can capture the molecular characteristics of micrometastatic disease are urgently needed115. Further developments of massively parallel sequencing technologies should provide more opportunities to characterize the genetics of micrometastatic disease and help realize the potential of precision medicine.
Key points.
- Neoadjuvant therapy is the standard of care for patients with locally advanced breast cancer and can improve operability of breast cancer
- For patients with HER2-positive breast cancer, the long-term outcomes of neoadjuvant treatment with the anti-HER2 agent trastuzumab should be considered equivalent to those of adjuvant therapy with this drug
- Despite the success of some neoadjuvant trials of dual-agent HER2 therapy for HER2-positive breast cancer, additional overall survival benefits of this approach over single-agent trastuzumab have not been documented in adjuvant trials
- Differences in clinical trial designs, patient characteristics, breast cancer biology, and the sequence and schedule of drug administration might have influenced the results of neoadjuvant trials and the contrasting results seen in adjuvant trials
- Prospective neoadjuvant trials with treatment informed by biomarkers and administration of matched experimental drug regimens should address the biological heterogeneity observed within breast-cancer subtypes
Review criteria.
A review of the biomedical English-language literature was conducted using the PubMed and MEDLINE databases. Articles published before October 2015 were included. The search terms used included “breast cancer”, “neoadjuvant therapy”, “adjuvant therapy”, “targeted therapy”, “NeoALTTO”, and “ALTTO”. Relevant abstracts of studies presented at the ASCO and ESMO annual meetings, and San Antonio Breast Cancer Symposium were also included.
Biography
Leticia De Mattos-Arruda, MD, is a medical oncologist and translational investigator at the Vall d'Hebron Institute of Oncology and University Hospital Vall d'Hebron, Barcelona, Spain, and visiting investigator in the Department of Pathology at the Memorial Sloan-Kettering Cancer Center, New York, NY, USA. Her main areas of interest are clinical and translational research in breast and brain cancers, investigating intratumour genetic heterogeneity and liquid biopsies (cell-free tumour DNA), and how therapeutic responses are modulated. She is a member of ASCO and the European Society of Medical Oncology (ESMO), and an active member of the ESMO Young Oncologist Committee. She is currently President of the Flims Alumni Club Steering Committee, an advisory board of the European Cancer Organisation (ECCO).
Ronglai Shen, PhD, is a biostatistician at the Memorial Sloan Kettering Cancer Center. Her research interest lies in developing statistical and computational methods for the analysis of data from high-throughput genomic analyses, with a focus on modelling molecular heterogeneity in cancer. She has developed the iCluster algorithm, a multiplatform clustering method for cancer-subtype discovery. iCluster has been used extensively in the The Cancer Genome Atlas (TCGA) project of the National Cancer Institute (NCI)–National Human Genome Research Institute (NHGRI) for integrated subtype analysis of genomic, epigenomic, transcriptomic, and proteomic profiling data. She is particularly interested in using the tools for the discovery of novel subtypes of breast cancer and lung cancer that are biologically and clinically relevant. She also co-developed the FACETS algorithm for estimating tumour purity, ploidy, allele-specific copy number, and clonal heterogeneity analysis of whole-genome, whole-exome, and targeted-capture DNA sequencing of tumour samples. She is interested in evaluating clonal heterogeneity within an individual patient's tumour as a predictor for treatment response and disease progression.
Jorge S. Reis-Filho, MD, PhD, FRCPath, is a member and attending pathologist at the Department of Pathology, and an affiliate member of the Human Oncology and Pathogenesis Program at the Memorial Sloan Kettering Cancer Center. Dr Reis-Filho has published over 370 peer-reviewed papers on pathology and genetics. His research interests include the development of a predictive classification system and the identification of novel therapeutic targets for breast cancers through a combination of traditional histopathology, massively parallel sequencing and functional genomics, and the characterization of the influence of intratumour genetic heterogeneity on breast cancer evolution and therapeutic response.
Javier Cortés, MD, PhD, received his degree in medicine and surgery from the Universidad Autónoma of Madrid, Spain, in 1996. He continued his studies at the University of Navarra, in Navarra, Spain, specializing in medical oncology. Since 2003, Dr Cortés has worked in the Department of Medical Oncology at the University Hospital Vall d'Hebron, where he has been Head of the Breast Cancer and Melanoma Units and coordinator of the Medical Oncology Training Programme for oncology residents. From September 2015, he has been the Head of the Breast Cancer Programme at Ramon y Cajal University Hospital in Madrid, and an investigator at the Vall d'Hebron Institute of Oncology. Dr Cortés has authored more than 140 publications, focusing on breast tumours and new drugs, and has given more than 300 communications at different conferences. He is an active member of the Spanish, European and American Societies of Medical Oncology (SEOM, ESMO, ASCO), and is a member of the Scientific Committee of ESMO.
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology: Breast Cancer. Version I. NCCN; 2016. [January 2, 2016]. website. http://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. [Google Scholar]
- 2.Schott AF, Hayes DF. Defining the benefits of neoadjuvant chemotherapy for breast cancer. J Clin Oncol. 2012;30:1747–9. doi: 10.1200/JCO.2011.41.3161. [DOI] [PubMed] [Google Scholar]
- 3.Untch M, Konecny GE, Paepke S, von Minckwitz G. Current and future role of neoadjuvant therapy for breast cancer. Breast. 2014 doi: 10.1016/j.breast.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 4.von Minckwitz G, et al. Response-guided neoadjuvant chemotherapy for breast cancer. J Clin Oncol. 2013;31:3623–30. doi: 10.1200/JCO.2012.45.0940. [DOI] [PubMed] [Google Scholar]
- 5.Fisher B, et al. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol. 1998;16:2672–85. doi: 10.1200/JCO.1998.16.8.2672. [DOI] [PubMed] [Google Scholar]
- 6.van der Hage JA, et al. Preoperative chemotherapy in primary operable breast cancer: results from the European Organization for Research and Treatment of Cancer trial 10902. J Clin Oncol. 2001;19:4224–37. doi: 10.1200/JCO.2001.19.22.4224. [DOI] [PubMed] [Google Scholar]
- 7.Mauri D, Pavlidis N, Ioannidis JP. Neoadjuvant versus adjuvant systemic treatment in breast cancer: a meta-analysis. J Natl Cancer Inst. 2005;97:188–94. doi: 10.1093/jnci/dji021. [DOI] [PubMed] [Google Scholar]
- 8.Chen AM, et al. Breast conservation after neoadjuvant chemotherapy: the MD Anderson cancer center experience. J Clin Oncol. 2004;22:2303–12. doi: 10.1200/JCO.2004.09.062. [DOI] [PubMed] [Google Scholar]
- 9.Morrow M, et al. Surgeon recommendations and receipt of mastectomy for treatment of breast cancer. JAMA. 2009;302:1551–6. doi: 10.1001/jama.2009.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.King TA, Morrow M. Surgical issues in patients with breast cancer receiving neoadjuvant chemotherapy. Nat Rev Clin Oncol. 2015;12:335–43. doi: 10.1038/nrclinonc.2015.63. [DOI] [PubMed] [Google Scholar]
- 11.De Mattos-Arruda L, Cortes J. Advances in first-line treatment for patients with HER-2+ metastatic breast cancer. Oncologist. 2012;17:631–44. doi: 10.1634/theoncologist.2011-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dawood S, Broglio K, Buzdar AU, Hortobagyi GN, Giordano SH. Prognosis of women with metastatic breast cancer by HER2 status and trastuzumab treatment: an institutional-based review. J Clin Oncol. 2010;28:92–8. doi: 10.1200/JCO.2008.19.9844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Slamon DJ, et al. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–82. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 14.Slamon DJ, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–92. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 15.Piccart-Gebhart MJ, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–72. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 16.Romond EH, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–84. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 17.Buzdar AU, et al. Significantly higher pathologic complete remission rate after neoadjuvant therapy with trastuzumab, paclitaxel, and epirubicin chemotherapy: results of a randomized trial in human epidermal growth factor receptor 2-positive operable breast cancer. J Clin Oncol. 2005;23:3676–85. doi: 10.1200/JCO.2005.07.032. [DOI] [PubMed] [Google Scholar]
- 18.Buzdar AU, et al. Neoadjuvant therapy with paclitaxel followed by 5-fluorouracil, epirubicin, and cyclophosphamide chemotherapy and concurrent trastuzumab in human epidermal growth factor receptor 2-positive operable breast cancer: an update of the initial randomized study population and data of additional patients treated with the same regimen. Clin Cancer Res. 2007;13:228–33. doi: 10.1158/1078-0432.CCR-06-1345. [DOI] [PubMed] [Google Scholar]
- 19.Slamon D, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365:1273–83. doi: 10.1056/NEJMoa0910383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geyer CE, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006;355:2733–43. doi: 10.1056/NEJMoa064320. [DOI] [PubMed] [Google Scholar]
- 21.Swain SM, et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA study): overall survival results from a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2013;14:461–71. doi: 10.1016/S1470-2045(13)70130-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Verma S, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367:1783–91. doi: 10.1056/NEJMoa1209124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Mattos-Arruda L, Cortes J. Use of pertuzumab for the treatment of HER2-positive metastatic breast cancer. Adv Ther. 2013;30:645–58. doi: 10.1007/s12325-013-0043-2. [DOI] [PubMed] [Google Scholar]
- 24.Gianni L, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13:25–32. doi: 10.1016/S1470-2045(11)70336-9. [DOI] [PubMed] [Google Scholar]
- 25.Schneeweiss A, et al. Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: a randomized phase II cardiac safety study (TRYPHAENA). Ann Oncol. 2013;24:2278–84. doi: 10.1093/annonc/mdt182. [DOI] [PubMed] [Google Scholar]
- 26.Scaltriti M, et al. Lapatinib, a HER2 tyrosine kinase inhibitor, induces stabilization and accumulation of HER2 and potentiates trastuzumab-dependent cell cytotoxicity. Oncogene. 2009;28:803–14. doi: 10.1038/onc.2008.432. [DOI] [PubMed] [Google Scholar]
- 27.Xia W, et al. Combining lapatinib (GW572016), a small molecule inhibitor of ErbB1 and ErbB2 tyrosine kinases, with therapeutic anti-ErbB2 antibodies enhances apoptosis of ErbB2-overexpressing breast cancer cells. Oncogene. 2005;24:6213–21. doi: 10.1038/sj.onc.1208774. [DOI] [PubMed] [Google Scholar]
- 28.Baselga J, et al. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): a randomised, open-label, multicentre, phase 3 trial. Lancet. 2012;379:633–40. doi: 10.1016/S0140-6736(11)61847-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guarneri V, et al. Preoperative chemotherapy plus trastuzumab, lapatinib, or both in human epidermal growth factor receptor 2-positive operable breast cancer: results of the randomized phase II CHER-LOB study. J Clin Oncol. 2012;30:1989–95. doi: 10.1200/JCO.2011.39.0823. [DOI] [PubMed] [Google Scholar]
- 30.Holmes FA, et al. Correlation of molecular effects and pathologic complete response to preoperative lapatinib and trastuzumab, separately and combined prior to neoadjuvant breast cancer chemotherapy. J Clin Oncol. 2011;29(suppl) 2011. abstr 506. [Google Scholar]
- 31.Zardavas D, Fouad TM, Piccart M. Optimal adjuvant treatment for patients with HER2-positive breast cancer in 2015. Breast. 2015;24(Suppl 2):S143–8. doi: 10.1016/j.breast.2015.07.034. [DOI] [PubMed] [Google Scholar]
- 32.Piccart-Gebhart M, et al. Adjuvant Lapatinib and Trastuzumab for Early Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer: Results From the Randomized Phase III Adjuvant Lapatinib and/or Trastuzumab Treatment Optimization Trial. J Clin Oncol. 2015 doi: 10.1200/JCO.2015.62.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cameron D, et al. Adjuvant bevacizumab-containing therapy in triple-negative breast cancer (BEATRICE): primary results of a randomised, phase 3 trial. Lancet Oncol. 2013;14:933–42. doi: 10.1016/S1470-2045(13)70335-8. [DOI] [PubMed] [Google Scholar]
- 34.Miller K, O'Neill A, Dang C. Bevacizumab (Bv) in the adjuvant treatment of HER2-negative breast cancer: final results from Eastern Cooperative Oncology Group E5103. J Clin Oncol. 2014;32(5 suppl) abstr 500. [Google Scholar]
- 35.von Minckwitz G, et al. Neoadjuvant chemotherapy and bevacizumab for HER2-negative breast cancer. N Engl J Med. 2012;366:299–309. doi: 10.1056/NEJMoa1111065. [DOI] [PubMed] [Google Scholar]
- 36.Bear HD, et al. Bevacizumab added to neoadjuvant chemotherapy for breast cancer. N Engl J Med. 2012;366:310–20. doi: 10.1056/NEJMoa1111097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.von Minckwitz G, et al. Survival after neoadjuvant chemotherapy with or without bevacizumab or everolimus for HER2-negative primary breast cancer (GBG 44-GeparQuinto)dagger. Ann Oncol. 2014;25:2363–72. doi: 10.1093/annonc/mdu455. [DOI] [PubMed] [Google Scholar]
- 38.de Azambuja E, et al. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): survival outcomes of a randomised, open-label, multicentre, phase 3 trial and their association with pathological complete response. Lancet Oncol. 2014;15:1137–46. doi: 10.1016/S1470-2045(14)70320-1. [DOI] [PubMed] [Google Scholar]
- 39.Giobbie-Hurder A, Gelber RD, Regan MM. Challenges of guarantee-time bias. J Clin Oncol. 2013;31:2963–9. doi: 10.1200/JCO.2013.49.5283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ring AE, Smith IE, Ashley S, Fulford LG, Lakhani SR. Oestrogen receptor status, pathological complete response and prognosis in patients receiving neoadjuvant chemotherapy for early breast cancer. Br J Cancer. 2004;91:2012–7. doi: 10.1038/sj.bjc.6602235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kurozumi S, et al. ER, PgR, Ki67, p27(Kip1), and histological grade as predictors of pathological complete response in patients with HER2-positive breast cancer receiving neoadjuvant chemotherapy using taxanes followed by fluorouracil, epirubicin, and cyclophosphamide concomitant with trastuzumab. BMC Cancer. 2015;15:622. doi: 10.1186/s12885-015-1641-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carey LA, et al. Clinical and translational results of CALGB 40601: A neoadjuvant phase III trial of weekly paclitaxel and trastuzumab with or without lapatinib for HER2-positive breast cancer. J Clin Oncol. 2013;31(suppl) 2013. abstr 500. [Google Scholar]
- 43.Amin DN, et al. Resiliency and vulnerability in the HER2-HER3 tumorigenic driver. Sci Transl Med. 2010;2:16ra7. doi: 10.1126/scitranslmed.3000389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chien AJ, et al. Phase I dose-escalation study of 5-day intermittent oral lapatinib therapy in patients with human epidermal growth factor receptor 2-overexpressing breast cancer. J Clin Oncol. 2014;32:1472–9. doi: 10.1200/JCO.2013.52.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.White J, DeMichele A. Neoadjuvant therapy for breast cancer: controversies in clinical trial design and standard of care. Am Soc Clin Oncol Educ Book. 2015;35:e17–23. doi: 10.14694/EdBook_AM.2015.35.e17. [DOI] [PubMed] [Google Scholar]
- 46.Goldhirsch A, et al. 2 years versus 1 year of adjuvant trastuzumab for HER2-positive breast cancer (HERA): an open-label, randomised controlled trial. Lancet. 2013;382:1021–8. doi: 10.1016/S0140-6736(13)61094-6. [DOI] [PubMed] [Google Scholar]
- 47.von Minckwitz G, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012;30:1796–804. doi: 10.1200/JCO.2011.38.8595. [DOI] [PubMed] [Google Scholar]
- 48.Guidance for Industry . Pathological Complete Response in Neoadjuvant Treatment of High-Risk Early-Stage Breast Cancer: Use as an Endpoint to Support Accelerated Approval. U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER); 2014. http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm305501.pdf. [Google Scholar]
- 49.von Minckwitz G, et al. Doxorubicin with cyclophosphamide followed by docetaxel every 21 days compared with doxorubicin and docetaxel every 14 days as preoperative treatment in operable breast cancer: the GEPARDUO study of the German Breast Group. J Clin Oncol. 2005;23:2676–85. doi: 10.1200/JCO.2005.05.078. [DOI] [PubMed] [Google Scholar]
- 50.Untch M, et al. Neoadjuvant treatment with trastuzumab in HER2-positive breast cancer: results from the GeparQuattro study. J Clin Oncol. 2010;28:2024–31. doi: 10.1200/JCO.2009.23.8451. [DOI] [PubMed] [Google Scholar]
- 51.Gianni L, et al. Neoadjuvant and adjuvant trastuzumab in patients with HER2-positive locally advanced breast cancer (NOAH): follow-up of a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet Oncol. 2014;15:640–7. doi: 10.1016/S1470-2045(14)70080-4. [DOI] [PubMed] [Google Scholar]
- 52.Untch M, et al. Intensive dose-dense compared with conventionally scheduled preoperative chemotherapy for high-risk primary breast cancer. J Clin Oncol. 2009;27:2938–45. doi: 10.1200/JCO.2008.20.3133. [DOI] [PubMed] [Google Scholar]
- 53.Esposito A, Criscitiello C, Curigliano G. Highlights from the 14(th) St Gallen International Breast Cancer Conference 2015 in Vienna: Dealing with classification, prognostication, and prediction refinement to personalize the treatment of patients with early breast cancer. Ecancermedicalscience. 2015;9:518. doi: 10.3332/ecancer.2015.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liedtke C, et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol. 2008;26:1275–81. doi: 10.1200/JCO.2007.14.4147. [DOI] [PubMed] [Google Scholar]
- 55.Robidoux A, et al. Lapatinib as a component of neoadjuvant therapy for HER2-positive operable breast cancer (NSABP protocol B-41): an open-label, randomised phase 3 trial. Lancet Oncol. 2013;14:1183–92. doi: 10.1016/S1470-2045(13)70411-X. [DOI] [PubMed] [Google Scholar]
- 56.Berruti A, et al. Pathologic complete response as a potential surrogate for the clinical outcome in patients with breast cancer after neoadjuvant therapy: a meta-regression of 29 randomized prospective studies. J Clin Oncol. 2014;32:3883–91. doi: 10.1200/JCO.2014.55.2836. [DOI] [PubMed] [Google Scholar]
- 57.Berry DA, Hudis CA. Neoadjuvant Therapy in Breast Cancer as a Basis for Drug Approval. JAMA Oncol. 2015;1:875–6. doi: 10.1001/jamaoncol.2015.1293. [DOI] [PubMed] [Google Scholar]
- 58.Cortazar P, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384:164–72. doi: 10.1016/S0140-6736(13)62422-8. [DOI] [PubMed] [Google Scholar]
- 59.Prentice RL. Surrogate endpoints in clinical trials: definition and operational criteria. Stat Med. 1989;8:431–40. doi: 10.1002/sim.4780080407. [DOI] [PubMed] [Google Scholar]
- 60.Freedman LS, Graubard BI, Schatzkin A. Statistical validation of intermediate endpoints for chronic diseases. Stat Med. 1992;11:167–78. doi: 10.1002/sim.4780110204. [DOI] [PubMed] [Google Scholar]
- 61.Fleming TR, Prentice RL, Pepe MS, Glidden D. Surrogate and auxiliary endpoints in clinical trials, with potential applications in cancer and AIDS research. Stat Med. 1994;13:955–68. doi: 10.1002/sim.4780130906. [DOI] [PubMed] [Google Scholar]
- 62.Buyse M, Molenberghs G, Burzykowski T, Renard D, Geys H. The validation of surrogate endpoints in meta-analyses of randomized experiments. Biostatistics. 2000;1:49–67. doi: 10.1093/biostatistics/1.1.49. [DOI] [PubMed] [Google Scholar]
- 63.Venkatraman ES, Begg CB. Properties of a nonparametric test for early comparison of treatments in clinical trials in the presence of surrogate endpoints. Biometrics. 1999;55:1171–6. doi: 10.1111/j.0006-341x.1999.01171.x. [DOI] [PubMed] [Google Scholar]
- 64.Li Y, Taylor JM. Predicting treatment effects using biomarker data in a meta-analysis of clinical trials. Stat Med. 2010;29:1875–89. doi: 10.1002/sim.3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bonnefoi H, et al. Neoadjuvant treatment with docetaxel plus lapatinib, trastuzumab, or both followed by an anthracycline-based chemotherapy in HER2-positive breast cancer: results of the randomised phase II EORTC 10054 study. Ann Oncol. 2015;26:325–32. doi: 10.1093/annonc/mdu551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Parker JS, et al. Supervised risk predictor of breast cancer based on intrinsic subtypes. J Clin Oncol. 2009;27:1160–7. doi: 10.1200/JCO.2008.18.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Untch M, et al. Lapatinib versus trastuzumab in combination with neoadjuvant anthracycline-taxane-based chemotherapy (GeparQuinto, GBG 44): a randomised phase 3 trial. Lancet Oncol. 2012;13:135–44. doi: 10.1016/S1470-2045(11)70397-7. [DOI] [PubMed] [Google Scholar]
- 68.Majewski IJ, et al. PIK3CA mutations are associated with decreased benefit to neoadjuvant human epidermal growth factor receptor 2-targeted therapies in breast cancer. J Clin Oncol. 2015;33:1334–9. doi: 10.1200/JCO.2014.55.2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Salgado R, et al. Tumor-Infiltrating Lymphocytes and Associations With Pathological Complete Response and Event-Free Survival in HER2-Positive Early-Stage Breast Cancer Treated With Lapatinib and Trastuzumab: A Secondary Analysis of the NeoALTTO Trial. JAMA Oncol. 2015;1:448–54. doi: 10.1001/jamaoncol.2015.0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zardavas D, Phillips WA, Loi S. PIK3CA mutations in breast cancer: reconciling findings from preclinical and clinical data. Breast Cancer Res. 2014;16:201. doi: 10.1186/bcr3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ali HR, et al. Association between CD8+ T-cell infiltration and breast cancer survival in 12,439 patients. Ann Oncol. 2014;25:1536–43. doi: 10.1093/annonc/mdu191. [DOI] [PubMed] [Google Scholar]
- 72.Salgado R, et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol. 2015;26:259–71. doi: 10.1093/annonc/mdu450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Savas P, et al. Clinical relevance of host immunity in breast cancer: from TILs to the clinic. Nat Rev Clin Oncol. 2015 doi: 10.1038/nrclinonc.2015.215. [DOI] [PubMed] [Google Scholar]
- 74.Luria SE, Delbruck M. Mutations of Bacteria from Virus Sensitivity to Virus Resistance. Genetics. 1943;28:491–511. doi: 10.1093/genetics/28.6.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Michor F, et al. Dynamics of chronic myeloid leukaemia. Nature. 2005;435:1267–70. doi: 10.1038/nature03669. [DOI] [PubMed] [Google Scholar]
- 76.Leder K, et al. Fitness conferred by BCR-ABL kinase domain mutations determines the risk of pre-existing resistance in chronic myeloid leukemia. PLoS One. 2011;6:e27682. doi: 10.1371/journal.pone.0027682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Foo J, Chmielecki J, Pao W, Michor F. Effects of pharmacokinetic processes and varied dosing schedules on the dynamics of acquired resistance to erlotinib in EGFR-mutant lung cancer. J Thorac Oncol. 2012;7:1583–93. doi: 10.1097/JTO.0b013e31826146ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Diaz LA, Jr., et al. The molecular evolution of acquired resistance to targeted EGFR blockade in colorectal cancers. Nature. 2012;486:537–40. doi: 10.1038/nature11219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Iwasa Y, Nowak MA, Michor F. Evolution of resistance during clonal expansion. Genetics. 2006;172:2557–66. doi: 10.1534/genetics.105.049791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Durrett R, Moseley S. Evolution of resistance and progression to disease during clonal expansion of cancer. Theor Popul Biol. 2010;77:42–8. doi: 10.1016/j.tpb.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 81.Komarova NL, Wodarz D. Drug resistance in cancer: principles of emergence and prevention. Proc Natl Acad Sci U S A. 2005;102:9714–9. doi: 10.1073/pnas.0501870102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Montagut C, et al. Identification of a mutation in the extracellular domain of the Epidermal Growth Factor Receptor conferring cetuximab resistance in colorectal cancer. Nat Med. 2012;18:221–3. doi: 10.1038/nm.2609. [DOI] [PubMed] [Google Scholar]
- 83.Maheswaran S, et al. Detection of mutations in EGFR in circulating lung-cancer cells. N Engl J Med. 2008;359:366–77. doi: 10.1056/NEJMoa0800668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Turke AB, et al. Preexistence and clonal selection of MET amplification in EGFR mutant NSCLC. Cancer Cell. 2010;17:77–88. doi: 10.1016/j.ccr.2009.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Garcia-Murillas I, et al. Mutation tracking in circulating tumor DNA predicts relapse in early breast cancer. Sci Transl Med. 2015;7:302ra133. doi: 10.1126/scitranslmed.aab0021. [DOI] [PubMed] [Google Scholar]
- 86.Olsson E, et al. Serial monitoring of circulating tumor DNA in patients with primary breast cancer for detection of occult metastatic disease. EMBO Mol Med. 2015 doi: 10.15252/emmm.201404913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.De Mattos-Arruda L, et al. Capturing intra-tumor genetic heterogeneity by de novo mutation profiling of circulating cell-free tumor DNA: a proof-of-principle. Ann Oncol. 2014;25:1729–35. doi: 10.1093/annonc/mdu239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.De Mattos-Arruda L, et al. Circulating tumour cells and cell-free DNA as tools for managing breast cancer. Nat Rev Clin Oncol. 2013;10:377–89. doi: 10.1038/nrclinonc.2013.80. [DOI] [PubMed] [Google Scholar]
- 89.Crowley E, Di Nicolantonio F, Loupakis F, Bardelli A. Liquid biopsy: monitoring cancer-genetics in the blood. Nat Rev Clin Oncol. 2013;10:472–84. doi: 10.1038/nrclinonc.2013.110. [DOI] [PubMed] [Google Scholar]
- 90.Bidard FC, Weigelt B, Reis-Filho JS. Going with the flow: from circulating tumor cells to DNA. Sci Transl Med. 2013;5:207ps14. doi: 10.1126/scitranslmed.3006305. [DOI] [PubMed] [Google Scholar]
- 91.Chen Z, et al. Analysis of cancer mutation signatures in blood by a novel ultra-sensitive assay: monitoring of therapy or recurrence in non-metastatic breast cancer. PLoS One. 2009;4:e7220. doi: 10.1371/journal.pone.0007220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Beaver JA, et al. Detection of cancer DNA in plasma of patients with early-stage breast cancer. Clin Cancer Res. 2014;20:2643–50. doi: 10.1158/1078-0432.CCR-13-2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gerlinger M, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med. 2012;366:883–92. doi: 10.1056/NEJMoa1113205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Geyer FC, et al. Molecular analysis reveals a genetic basis for the phenotypic diversity of metaplastic breast carcinomas. J Pathol. 2010;220:562–73. doi: 10.1002/path.2675. [DOI] [PubMed] [Google Scholar]
- 95.Navin N, et al. Tumour evolution inferred by single-cell sequencing. Nature. 2011;472:90–4. doi: 10.1038/nature09807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Janiszewska M, et al. In situ single-cell analysis identifies heterogeneity for PIK3CA mutation and HER2 amplification in HER2-positive breast cancer. Nat Genet. 2015 doi: 10.1038/ng.3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shah SP, et al. Mutational evolution in a lobular breast tumour profiled at single nucleotide resolution. Nature. 2009;461:809–13. doi: 10.1038/nature08489. [DOI] [PubMed] [Google Scholar]
- 98.Ding L, et al. Genome remodelling in a basal-like breast cancer metastasis and xenograft. Nature. 2010;464:999–1005. doi: 10.1038/nature08989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gonzalez-Angulo AM, et al. PI3K pathway mutations and PTEN levels in primary and metastatic breast cancer. Mol Cancer Ther. 2011;10:1093–101. doi: 10.1158/1535-7163.MCT-10-1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nik-Zainal S, et al. The life history of 21 breast cancers. Cell. 2012;149:994–1007. doi: 10.1016/j.cell.2012.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yates LR, et al. Subclonal diversification of primary breast cancer revealed by multiregion sequencing. Nat Med. 2015;21:751–9. doi: 10.1038/nm.3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Niikura N, et al. Changes in Tumor Expression of HER2 and Hormone Receptors Status after Neoadjuvant Chemotherapy in 21,755 Patients from the Japanese Breast Cancer Registry. Ann Oncol. 2015 doi: 10.1093/annonc/mdv611. [DOI] [PubMed] [Google Scholar]
- 103.Ng CK, et al. Intra-tumor genetic heterogeneity and alternative driver genetic alterations in breast cancers with heterogeneous HER2 gene amplification. Genome Biol. 2015;16:107. doi: 10.1186/s13059-015-0657-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Burrell RA, Swanton C. Tumour heterogeneity and the evolution of polyclonal drug resistance. Mol Oncol. 2014;8:1095–1111. doi: 10.1016/j.molonc.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yap TA, Gerlinger M, Futreal PA, Pusztai L, Swanton C. Intratumor heterogeneity: seeing the wood for the trees. Sci Transl Med. 2012;4:127ps10. doi: 10.1126/scitranslmed.3003854. [DOI] [PubMed] [Google Scholar]
- 106.Fisher R, Pusztai L, Swanton C. Cancer heterogeneity: implications for targeted therapeutics. Br J Cancer. 2013;108:479–85. doi: 10.1038/bjc.2012.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Alizadeh AA, et al. Toward understanding and exploiting tumor heterogeneity. Nat Med. 2015;21:846–53. doi: 10.1038/nm.3915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Merlo LM, Pepper JW, Reid BJ, Maley CC. Cancer as an evolutionary and ecological process. Nat Rev Cancer. 2006;6:924–35. doi: 10.1038/nrc2013. [DOI] [PubMed] [Google Scholar]
- 109.Almendro V, et al. Inference of tumor evolution during chemotherapy by computational modeling and in situ analysis of genetic and phenotypic cellular diversity. Cell Rep. 2014;6:514–27. doi: 10.1016/j.celrep.2013.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mayer EL, et al. Long-term follow-up after preoperative trastuzumab and chemotherapy for HER2-overexpressing breast cancer. Clin Breast Cancer. 2015;15:24–30. doi: 10.1016/j.clbc.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 111.Rouzier R, et al. Incidence and prognostic significance of complete axillary downstaging after primary chemotherapy in breast cancer patients with T1 to T3 tumors and cytologically proven axillary metastatic lymph nodes. J Clin Oncol. 2002;20:1304–10. doi: 10.1200/JCO.2002.20.5.1304. [DOI] [PubMed] [Google Scholar]
- 112.Symmans WF, et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol. 2007;25:4414–22. doi: 10.1200/JCO.2007.10.6823. [DOI] [PubMed] [Google Scholar]
- 113.De Mattos-Arruda L, Rodon J. Pilot studies for personalized cancer medicine: focusing on the patient for treatment selection. Oncologist. 2013;18:1180–8. doi: 10.1634/theoncologist.2013-0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Park JW, Liu MC. Neratinib plus standard neoadjuvant therapy for high-risk breast cancer: Efficacy results from the I-SPY 2 trial. 2014 AACR Annual Meeting. Abstract CT227. 2014 [Google Scholar]
- 115.Sahin O, et al. Biomarker-guided sequential targeted therapies to overcome therapy resistance in rapidly evolving highly aggressive mammary tumors. Cell Res. 2014;24:542–59. doi: 10.1038/cr.2014.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med. 2010;363:1938–48. doi: 10.1056/NEJMra1001389. [DOI] [PubMed] [Google Scholar]