Abstract
Calcinosis is one of the hallmark sequelae of juvenile dermatomyositis (JDM), and despite recent progress in the therapy of JDM, dystrophic calcification still occurs in approximately one-third of patients. This review discusses our current, albeit limited, understanding of risk factors for the development of calcinosis in JDM, as well as approaches to assessment, and current views on its pathogenesis. Anecdotal approaches to treating calcinosis associated with JDM, including both anti-inflammatory therapies and agents aimed at inhibiting the deposition of calcium hydroxyapatite, are reviewed. An improved understanding of the pathogenesis of calcinosis, the establishment of standardized measurement tools to assess calcinosis, and randomized controlled trials employing more sensitive outcome measures are needed to develop efficacious therapies for this often disabling complication.
Keywords: juvenile dermatomyositis, calcinosis, dystrophic calcification, treatment, pathogenesis
Introduction
Juvenile Dermatomyositis (JDM) is a rare childhood systemic autoimmune disease characterized by proximal muscle weakness and rashes due to chronic muscle and skin inflammation of unknown etiology [1]. Two rashes, Gottron’s papules and the heliotrope rash, are pathognomonic and assist in confirming the diagnosis. Evidence of myositis by muscle biopsy or electromyography (EMG) is also necessary to definitively establish the diagnosis [2]. The disease has a number of protean manifestations, but calcinosis, the abnormal deposition of insoluble calcium salts within the skin, subcutaneous tissue, myofascia, or muscle, is the sequela that is perhaps most characteristic for this illness, most troublesome, and least understood. This article updates a prior extensive review of calcinosis in JDM with current concepts on prevalence, risk factors, differential diagnosis, pathogenesis, and treatment [3].
Calcinosis occurs in up to 40% of patients with JDM, although current prevalence ranges from 10 – 70% [4–14]. This wide variation of the prevalence of calcinosis in JDM cohorts may depend on the length of follow-up and the treatment approaches utilized, among other factors, but there may be differences regionally and internationally in the frequency of calcinosis [8]. The calcification is dystrophic, which by definition occurs at sites of injured tissue with simultaneously generally normal serum calcium and phosphorous levels [15]. The sites most frequently affected are the elbows, knees, trunk, hands, feet, buttocks, and head, although it may occur virtually anywhere over the body [16]. The onset of calcinosis is most often 1 – 3 years after illness onset, but has been reported to occur from the time of illness onset to as long as 20 years later [7,17,18]. Compared to JDM, calcinosis in adult-onset dermatomyositis (DM) tends to occur later (7.8 years vs. 2.9 years), less frequently (20%), and with lesions occurring primarily on the extremities [16]. Prior to therapy with corticosteroids, when mortality exceeded 50%, the development of calcinosis was considered a good prognostic sign. Today, however, as our understanding of calcinosis associated with JDM evolves, it is instead deemed a marker of disease morbidity and possibly inadequate treatment.
Calcinosis Phenotypes
Calcinosis is pleomorphic and may present in multiple ways, including superficial plaques or nodules known as calcinosis circumscripta; larger nodular deposits that may extend to deeper tissue layers including muscle known as tumoral calcinosis or calcinosis universalis; collections along fascial planes of tendons or muscles; or an exoskeleton of calcium, an extensive hard calcium deposition over all surface areas which can lead to significant joint contractures and immobility. These four main phenotypes have been described, although there may be overlap and multiple subtypes may occur in individual patients [19]. In a prior series of children with JDM and calcinosis, 33% developed calcinosis circumscripta, 20% developed tumoral calcinosis, 16% developed calcinosis along fascial planes, 10% developed exoskeletal calcinosis, and 22% had a mixture of calcinosis subtypes [19]. Calcinosis is often painless, but may present with deep-seated pain and tenderness to palpation, with panniculitis on biopsy, or even with ulcerations [16]. These areas may be raised or erythematous, warm and tender, and can be confused for cellulitis. When calcium deposits breach the surface of the skin, these deposits may become a nidus for true infection, most commonly with staphylococcal and streptococcal organisms, but including mycobacteria and other species [20–22]. Areas of calcinosis may expand over time, spontaneously regress, or change subtype. Improvement may be more likely in patients with inactive disease and more superficial deposits.
Risk Factors for Calcinosis
Risk factors for calcinosis in patients with JDM are not well understood and information has been largely limited to retrospective series of patients, from which associations can be found but causality cannot be inferred. Calcinosis has had a long-standing association with delay to diagnosis and initiation of therapy, but also occurs in chronic, severe disease [7,23]. Patients with a chronic or polycyclic course, and therefore with longer duration of active disease, may be more likely to develop calcinosis despite adequate therapy [7,8,24]. The presence of cardiac involvement and the use of one or more immunosuppressive therapies (other than oral corticosteroids) have been associated with the development of calcinosis [25].
Calcinosis in myositis patients has been associated with anti-MJ autoantibodies that recognize the nuclear protein NXP-2/MORC3, and anti-MJ autoantibodies are present in up to 25% of children with JDM. In JDM patients with anti-MJ autoantibodies, calcinosis may be present in up to 54% [26–30]. Calcinosis has also been associated with the presence of PM-Scl autoantibodies [31]. Tumor necrosis factor-alpha (TNF-α), Interleukin-1 beta (IL-1β) and other pro-inflammatory cytokines have been found in the milk of calcium fluid examined from subjects [32]. Pro-inflammatory polymorphisms of the TNF-α-308A allele have been associated with increased risk of calcinosis in two separate cohorts, and IL-1α-889T is a protective allele [33,34]. Higher initial levels of serum creatine kinase and prolonged elevation of muscle enzymes have been associated with calcinosis as well [5,35]. An association with lipodystrophy and panniculitis may be seen due to the fact these complications are also associated with severe and prolonged disease [8,9,36]. A large international multicenter study found that calcinosis in JDM is associated with earlier year of onset, older age at onset, chronic polycyclic or chronic continuous illness course, and increased disease duration [8]. Analysis of a Danish cohort, including long-term follow-up, confirmed disease duration greater than 4 years to be a risk factor for calcinosis [9]. Another multicenter registry has described an association of calcinosis with African American race, even after adjustment for duration of disease and time to rheumatologic care [37]. Similarly, it was reported that African children with JDM have increased vasculitic disease and a high frequency of calcinosis, even with lower serum muscle enzyme levels [38]. In a JDM cohort from the UK, calcinosis was associated with younger age of disease onset [29].
Assessment/Diagnostics
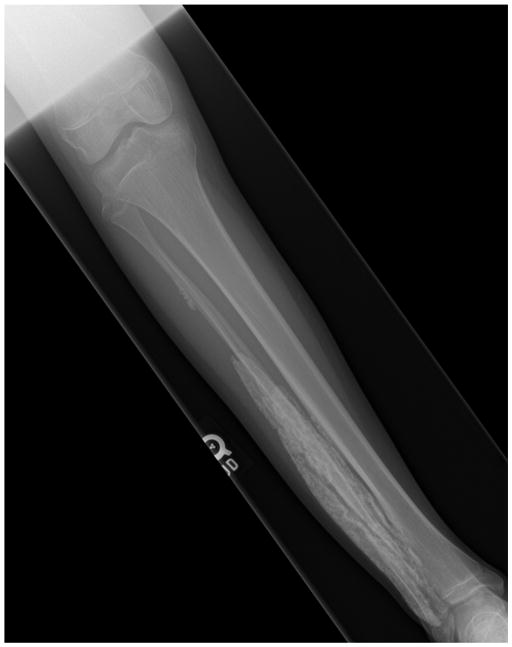
Conventional radiographs are generally sufficient for identifying calcinosis in the pediatric population and have been recommended as the initial imaging study of choice when there is clinical suspicion for calcinosis [39] (Figure 1). In a case series of 37 patients with autoimmune connective tissue disease and suspected calcinosis, including 17 cases of DM, of whom 7 were children, radiographs were able to detect calcinosis in all patients. The same series identified nodular lesions as the most common (84%), followed by sheet-like (32%), reticular (22%), amorphous (14%), and linear (5%) deposits. The presence of a reticular pattern has been suggested as predicting a more severe and chronic disease course in JDM patients [19].
Figure 1.
Plain radiograph demonstrating sheet-like calcification in the lower leg of a 13 year old girl with JDM.
In addition to plain radiography, other imaging modalities have been successfully utilized in assessing calcinosis in pediatric patients. Scintigraphy has identified soft-tissue calcinosis missed on radiographs, but may not be able to detect all lesions [40]. Thin-section computed tomography (CT) appears to be more sensitive for identifying early calcinosis lesions because of the sensitive bone-water interface [41], but is limited in utility due to associated radiation. Magnetic resonance imaging (MRI) can demonstrate calcified lesions as well as subcutaneous edema, which was found to be a precursor for radiographic calcinosis in one case series [42]. When lesions are superficial and without large echo shadows, ultrasound may be useful to monitor the surrounding edema and change in size of calcific deposits [Oberle, personal communication]. However, this method cannot assess the depth of these lesions or the presence of larger or deeper lesions.
Calcinosis is frequently one of many factors used in the assessment of myositis disease damage [43]. However, no standardized guidelines exist to grade or assess calcinosis. The development of sensitive assessment tools for calcinosis is required in order to assess the efficacy of therapeutic agents, as well as to identify those patients at greatest risk of poor functional outcomes. Such a tool should include clinical symptoms (pain scale), physical exam findings (location and size of lesions, associated erythema or other color change), imaging features (depth of lesions, effect on surrounding structures), and patient reported indicators (quality of life).
Differential Diagnosis
Calcinosis cutis can be associated with a number of conditions, including autoimmune connective tissue disorders, inherited disorders, cutaneous neoplasms, and following venipuncture (iatrogenic) or blunt trauma (Table 1). Aside from DM, scleroderma is the most common autoimmune disorder to present with calcinosis, with involvement reported in 25–40% of patients with limited cutaneous systemic scleroderma [16,45,46]. Calcinosis may develop at any time during the disease course and may predate the diagnosis of scleroderma [16], but typically occurs at least 10 years following diagnosis [15]. Lesions occur on the hands and feet, with a high predilection for fingertips and areas of microtrauma [16]. Raynaud’s and digital ulcers are risk factors for calcinosis in scleroderma, suggesting a role for vascular ischemia [46].
Table 1.
Differential Diagnosis for Calcinosis Cutis
| Autoimmune Connective Tissue Disorders | |
| Dermatomyositis | |
| Systemic Scleroderma | |
| Limited Scleroderma, CREST syndrome | |
| Systemic Lupus Erythematosus | |
| Lupus Panniculitis | |
| Mixed Connective Tissue Disease | |
| Overlap Connective Tissue Disease | |
| Sjogren’s Syndrome | |
| Rheumatoid Arthritis | |
| Polymyositis | |
| Pancreatic Panniculitis | |
| Porphyria Cutanea Tarda | |
| Inherited Disorders | Familial Tumoral Calcinosis/Hyperostosis-Hyperphosphatemia Syndrome |
| Ehlers-Danlos Syndrome | |
| Pseudoxanthoma Elasticum | |
| Werner Syndrome | |
| Fibrodysplasia Ossificans Progressiva | |
| Hypercalcemia | Chronic Renal Disease |
| Hypervitaminosis | |
| Hyperparathyroidism | |
| Milk alkali syndrome | |
| Post-Liver Transplant | |
| Neoplastic | Basal Cell Carcinoma |
| Pilomatricoma | |
| Trichilemmal cyst | |
| Iatrogenic | Vaccination, Venipuncture |
| Calcium infusion | |
|
Trauma Idiopathic |
Myositis ossificans circumscripta |
Calcinosis rarely occurs with systemic lupus erythematosus. The onset is generally after long-standing disease [16]. The presentation can vary greatly; however, patients generally are asymptomatic, with the calcifications incidentally found on radiographic studies [47]. Calcinosis may occur on the extremities, particularly around the joints, as well as the buttocks, and deep to active cutaneous lupus lesions [44]. The occurrence of calcinosis in other rheumatologic conditions has been reported, but is uncommon.
Familial tumoral calcinosis (FTC) is a rare autosomal recessive metabolic disorder characterized by the deposition of periarticular calcific masses either within the muscular or subcutaneous tissue in areas of repeated trauma. The hips, shoulders, elbows, and knees are most often involved [15]. Lesions vary in size and may enlarge, resulting in impairment of joint function. Lesions have been reported in young children, but tend to occur in healthy adolescents. Several genetic mutations related to the regulation of renal reabsorption of phosphate resulting in serum hyperphosphatemia have been identified in affected families; however, normal serum phosphate levels can occur in individuals with FTC [48].
Fibrodysplasia ossificans progressiva is a rare and severely debilitating, autosomal dominant disorder, resulting from mutations in activin receptor IA/activin-like kinase-2 (ACVRI/ALK2), which encodes a bone morphogenetic protein type I receptor, leading to progressive heterotopic ossification of skeletal muscle and soft connective tissues. Individuals appear normal at birth, aside from a characteristic malformation of the great toes, and over the first decade of life develop calcific deposits, initially in the proximal and cranial regions of the body followed by the appendicular and caudal areas. Affected regions become immobile with most individuals becoming wheelchair-bound by the third decade of life. The median age of survival is approximately 45 years [49].
Myositis ossificans circumscripta is a reactive process resulting in heterotopic ossification of muscle after an injury or repeated minor injuries [50]. The most common locations are the muscles of the thigh, buttocks, and upper arm. Lesions typically reach 4 to 10 centimeters in diameter. There can be resorption of the calcification over years, but these masses rarely resolve completely.
Pathogenesis
Local tissue trauma, active inflammation, and dysregulation of the proteins involved in calcium metabolism have all been suggested to play a role in the formation of calcinosis in patients with JDM [3]. Tissue-nonspecific alkaline phosphatase (TNAP) and it hydrolytic product inorganic pyrophosphate (PPi), a potent inhibitor of calcium hydroxyapatite formation, and other targets in this metabolic pathway, have been demonstrated to be important to several genetic disorders associated with vascular calcification, but a role for this pathway in JDM-associated calcinosis has not yet been determined [51]. Dystrophic calcification in JDM and other rheumatic diseases tends to occur at sites of repetitive use, localized trauma, or chronic mechanical stress [52]. Anecdotally, JDM patients with tissue injuries following minor trauma appear to develop calcinosis at the injured sites, particularly when the underlying myositis is still active. Examples include a JDM patient who developed calcinosis in the medial thigh with repetitive horseback riding, as well as along the humerus after being pulled when walking a dog; and a patient who developed a hematoma following a surgical muscle biopsy procedure and subsequent calcinosis at the same site [3]. Tissue injury and hematoma formation have been associated with other dystrophic calcification processes [15].
The calcinosis itself is also associated with inflammation. Several reports describe macrophages and pro-inflammatory cytokines, including IL-6, IL-1, TNF-α, soluble TNF receptors, neopterin, and IL-18, present in the milk of calcium fluid examined from patients with JDM [33,53,54]. Calcinosis has also been more frequently associated with the TNF- α-308A promoter polymorphism, which is associated with increased TNF-α production by peripheral blood mononuclear cells [34], and additional genetic and developmental risk factors are likely to be involved [36,55]. Also, new-onset calcinosis has been associated with subcutaneous edema in the same location, which is presumed to be inflammation, on a prior short tau inversion recovery (STIR) MRI exam [41].
The histopathology of surgically-removed specimens, often of longstanding duration, demonstrates chronic inflammatory cells encapsulating the mineral consisting apparently of a variety of cell types, including macrophages, giant cells, lymphocytes and eosinophils [33,56–58]. The calcium mineral itself may be a chemoattractant for macrophages and monocytes [59].
The mineral is calcium hydroxyapatite or carbonate apatite, from x-ray diffraction, infrared spectroscopy and X-ray micro-computed tomography studies [60,61]. The properties of the mineral in the calcinosis lesions are closest to that of enamel, and the mineral clearly differs from bone. The mineral appears to be deposited in fragments and becomes solid over time [61].
Several reports have demonstrated connective tissue and mineral-associated proteins in calcinosis lesions and JDM muscle tissue. Immunohistochemistry reveals a number of small integrin-binding ligand, N-linked glycoprotein (SIBLING) proteins, including osteocalcin, osteopontin (OPN), osteonectin, bone sialoprotein, and matrix-Gla protein (MGP), that promote and inhibit mineralization within the lesions, and osteoclasts at the periphery of the lesions that are secondarily infiltrating in an attempt to resolve the calcification [62]. The deposited calcium appears to be nucleated around collagen and elastic fibers [62]. MGP, a calcification inhibitor, is expressed at sites of muscle damage and by infiltrating macrophages in the muscle tissue of JDM patients, whereas only phosphorylated MGP is elevated in biopsies of JDM patients who developed calcinosis [63]. These proteins, likely up-regulated in expression by tissue macrophages, cytokines and tissue injury, and may serve as a nidus for nucleation of the mineralized calcium that would then promote crystal growth [64,65]. Some of these proteins may initiate or promote mineralization, including dentin matrix protein 1, and matrix extracellular phosphoglycoprotein (MEPE), whereas others, including osteopontin, decorin, albumin and fetuin-A, apparently inhibit hydroxyapatite crystal growth [55,66]. Osteopontin apparently not only inhibits mineral deposition, but also actively promotes its dissolution by inducing expression of carbonic anhydrase in monocytes and promoting acidification of the extracellular environment [67]. A balance of pro-mineralizing matrix proteins would favor growth of existing lesions and further deposition of new lesions.
Figure 1 provides a hypothesis of the pathogenesis of calcinosis in JDM, based on these limited data, providing a central role in the cascade for the associated inflammatory process [3].
Treatment
Multiple treatment strategies have been attempted to target calcinosis in JDM and other autoimmune diseases, including anti-inflammatory drugs, drugs that affect calcium metabolism, and mechanical modalities. (Table 2) However, no therapy has proven to be reproducibly efficacious, and evidence in the literature is limited to open-label case studies and case series [43,46,68].
Table 2.
Published treatment options for juvenile dermatomyositis.
| Medication | Dosing | Route | Mechanism of Action | JDM Publications |
|---|---|---|---|---|
| Anti-Inflammatory | ||||
| IVIG | 2 g/kg every 4 wks | IV | Uncertain, inhibits macrophages | Touimy et al., 2013 [64] |
| Infliximab | 3 mg/kg at 0, 2, 6 wks; then every 8 wks | IV | Inhibits TNF-α | Riley et al., 2008 [66] |
| Abatacept | 10 mg/kg at 0, 2, 4 wks; then every 4 wks | IV, SQ | Binds CD80/CD86 inhibiting T cell costimulatory signal | Arabshahi et al., 2012 [67] |
| Depomedrone, intra-lesional | 80 mg once | Local injection | Uncertain, facilitates dissolving and resorption of calcified materials | Al-Mayouf et al., 2010 [68] |
| Thalidomide | 50 mg/d (1.3 mg/kg/d) x 4 wks; then increased to 75 mg/d | PO | Inhibits TNF-α and IL-6, anti-angiogenesis | Miyamae et al., 2010 [69] |
| Rituximab | 750 – 1000 mg/m2/wk x 2 wks | IV | Binds CD20 on B cells | |
|
| ||||
| Calcium and phosphate modulators | ||||
| Diltiazem | 4–6 mg/kg/d | PO | Calcium channel blocker | Oliveri et al., 1996 [73] |
| Pamidronate | 1 mg/kg/d x 3 d; repeated every 3 mo | IV | Bisphosphonate: reduces bone resorption via reduced osteoclast activity, inhibits macrophages, deregulates expression of genes involved in phosphate homeostasis and mineralization | Slimani et al, 2010 [81] |
| 2 mg/kg/yr | IV | Marco Puche et al., 2010 [82] | ||
| Alendronate | 0.4 mg/kg/d | PO | Bisphosphonate | Ambler et al., 2005 [83] |
| Sodium Thiosulfate | 10–25% cream, daily | Topical | Uncertain, dissolves calcium deposits, chelates free calcium | Pagnini et al., 2014 [93] |
| 10 g, 3/wk x 2 wks; then 15 g, 2/wk x 3 mo | IV | Arabshahi et al., 2012 [67] | ||
| Aluminum Hydroxide | 1.8–2.4 g/d | PO | Decreases intestinal absorption of phosphate | Aihara et al., 1994 [94] Nakagawa et al., 1993 [95] Wang et al., 1988 [96] |
| Probenecid | 500 mg/d titrated up to 1000–1250 mg/d | PO | Uncertain: increases renal excretion of phosphate, decreases extracelullar ATP levels with resultant decrease in PPi | Nakamura et al., 2006 [97] Harel et al., 2001 [98] |
| Colchicine | 1–1.8 mg/d | PO | Uncertain, inhibits microtubule polymerization, inhibits IL-1β and inflammasome activation | Taborn et al., 1978 [99] |
|
| ||||
| Surgical Excision | N/A | Surgical | Direct removal of calcium deposits | Al-Mayouf et al., 2010 [68] Vitale et al., 2009 [102] Wu et al., 2008 [2008] |
Note: IVIG, intravenous immunoglobulin; g, gram; kg, kilogram; mg, milligram; d, day; wk, week; mo, month; yr, year; IV, intravenous; SQ, subcutaneous; PO, by mouth; TNF-a, Tumor Necrosis Factor alpha; IL, interleukin.
Anti-inflammatories
IVIG
Intravenous immunoglobulin (IVIG) has been used in rheumatology for a variety of anti-inflammatory indications, including the treatment of DM [69]. The potential mechanism in reducing calcinosis is uncertain, but may include the inhibition of activated macrophages [70]. Several case reports describe improvement in calcinosis in adults with DM and limited systemic scleroderma who had failed multiple other therapies, including warfarin, diltiazem, and extracorporeal shock wave lithotripsy [70–72]. The only case involving a child was a 10-year-old male with JDM who developed calcinosis universalis and had failed pamidronate, probenecid, cyclosporine, diltiazem, and alendronate. After 4 monthly infusions (2gm/kg/dose), he reported improved myalgias and muscle fatigue. Radiographs before and after treatment showed no new calcifications. Knee flexion contractures and limp resolved. At a 7 year follow up, sustained efficacy of IVIG was reported [73]. Conversely, Kalajian et al reported two adult patients with DM who showed no response to IVIG at the same dose and interval [74].
Infliximab
Given that TNF-α has been isolated from calcinosis lesions and the development of calcinosis has been associated with the TNF-α-308A promoter polymorphism, blockade of TNF-α may be of therapeutic benefit in treating calcinosis. This is supported by a series of 5 patients with JDM who had improvement in their manifestations of JDM including calcinosis after receiving infliximab at a dose of 3mg/kg at 0, 2, 6 weeks followed by every 8 weeks [75]. All 5 patients had improvement with joint contractures, calcinosis, and muscle weakness between 8 and 30 months of starting infliximab. In four patients, calcinosis was still present, but lesions were softer, painless, and less extensive [75]. A preliminary report in 30 JDM patients suggests improvement in calcinosis in 46% treated with infliximab [76].
Abatacept
A recent report describes the improvement of ulcerative skin lesions and calcinosis in a 14-year-old girl with JDM treated with abatacept, a monoclonal T-cell activation inhibitor, (10 mg/kg at weeks 0 and 2, and then monthly) and sodium thiosulfate IV and topical (10 g IV sodium thiosulfate administered 3 times weekly for 2 weeks, then 15 g twice weekly for the next 3 months; topical 3% increased to 10%). The patient’s calcinosis had previously failed to respond to tacrolimus, IVIG, cyclophosphamide, infliximab, colchicine, and alendronate. At 6 weeks analgesic use was decreased, and at 3 months pulse methylprednisilone was stopped and oral prednisone dosage was decreased by 30%. At 6 months she had significantly improvement in muscle strength and function, and her cutaneous ulcerations largely healed. Plain X-rays of her upper extremities confirmed lack of progression of calcinosis. However, concomitant use of both drugs makes it impossible to determine the role of abatacept in improvement of the patient’s calcinosis [77].
Thalidomide
Thalidomide inhibits the expression of TNF-α and IL-6 mRNA in monocytes; however, its use is often limited by toxicity concerns. Miyame et al reported improvement in the inflammatory calcinosis of a 14 yr old girl with JDM treated with 1.3mg/kg/day (50mg), increased to 75mg/day after 4 weeks. Her calcinosis had not progressed at 18-month follow-up, and a whole body PET- CT at 15 months post-treatment showed fewer hot spots around the subcutaneous lesions [54].
Rituximab
Rituximab, the anti-CD20 monoclonal antibody that eliminates peripheral B cells, has mixed results reported in adults with calcinosis associated with systemic scleroderma [78–80]. A 53-year-old woman with CREST and diffuse calcinosis affecting the knee, elbow, and thumb prone to frequent ulcerations and pain, was treated with Rituximab. At follow up 5 months later, lesions had not ulcerated, their sizes were stable, and no new lesions had developed. At one year follow up, knee and elbow lesions had significantly improved, and pain no longer required analgesics. She was given a second course of Rituximab in the hopes to further improve her condition. Six months after the second course, knee and elbow calcinosis lesions were significantly improved, the thumb lesion was unchanged, and there was no reported pain. At the most recent follow up, no new calcinosis had developed and her condition was stable [78]. To date, there are no reports regarding the impact of rituximab on calcinosis in DM or JDM. Our clinical experience is that of progression of calcinosis with rituximab therapy [Rider, unpublished observation,81].
Intra-lesional Steroids
A 10-year-old boy with JDM had resolution of calcinosis at his olecranon bursa with a local injection of 80 mg of depomedrone. The calcinosis had not recurred at 2 year follow-up. He had previously failed pamidronate and colchicine [82].
Colchicine
Hydroxyapatite crystals invoke an inflammatory response in joints and soft tissue, making colchicine a consideration when trying to treat calcinosis, particularly in light of its inhibition of IL-1β and the inflammasome [83]. Taborn et. al. reported two JDM patients with a good response. A 14-year-old girl in the midst of a disease flare with fever and acute calcinosis was started on 0.6 mg twice daily, and within days became afebrile and showed improved local inflammation. At one year follow up she was stable. A 13-year-old male also presented with disease flare, and had fever and generalized calcinosis universalis. He was treated with 0.65 mg three times daily, and was afebrile within 4 days. At one year follow up, he was clinically stable. These cases illustrate improvement with colchicine in the systemic inflammatory response attributed to hydroxyapatite [84]. Fuchs et. al. also had positive results in a patient with progressive systemic sclerosis (SSc), and a second with adult DM. There was significant regression in local inflammation and healing of skin ulcers within 2 months of treatment with 1 mg daily [85]. However, colchicine failures have been reported as well [86].
Treatments with minocycline, ceftriaxone, and salicylates have been tried with varying success [46,68].
Drugs that affect calcium or phosphorous metabolism
Calcium Channel Blockers
Diltiazem is the most widely studied calcium channel blocker used as medical therapy for calcinosis. It is hypothesized that by decreasing the intracellular calcium levels and decreasing the influx of calcium into the cells, the ability for calcinosis to form and for calcium to crystallize is reduced. An 8-year-old girl with JDM was treated with oral diltiazem (5 mg/kg/day) and oral pamidronate (4 mg/kg/day) with calcium and vitamin D supplementation. After 21 months on treatment she had dramatic regression of calcinosis, both clinically and radiographically [87]. A second case of a 3-year-old girl with JDM and multiple subcutaneous lesions and severe intraphalangeal calcinosis showed near resolution radiographically and clinically after 12 months of diltiazem (30 mg/day) [39]. A larger case series of adult patients with SSc-associated calcinosis showed only 3 of 12 SSc patients with improvement radiographically [88].
Bisphosphonates
This class of drugs is showing promising results in the treatment of calcinosis. Bisphosphonates are being used as therapy for disorders including vascular calcification, hypercalcemia and calcification disorders, such as Osteogenesis Imperfecta and Paget’s disease [89–91]. Bisphosphonates are known to inhibit calcium turnover and remodeling, but they also have effects on macrophages and inflammatory cytokines localized to calcinosis lesions [92–94], and also deregulate the expression of genes that are involved in phosphate homeostasis and mineralization [95]. A 14-year-old girl with JDM and associated calcinosis universalis who failed colchicine, had complete resolution of calcinosis after treatment with pamidronate (15 mg every 3 months for 1 year followed by 30 mg every 3 months for a second year), with no new calcifications at 5 year follow up [96]. Another report describes significant improvement in 3 patients with JDM-associated calcinosis with pamidronate, one having resolution, using the protocol established for osteogenesis imperfecta (1 mg/kg/day for 3 consecutive days each month) [97]. Alendronate therapy has also shown positive results with complete resolution of calcinosis in a 6-year-old boy with JDM who was unresponsive to diltiazem and probenecid [98]. The results for pamidronate and alendronate are better than those for etidronate, which have been largely negative [99–100]. Interestingly, two cases of DM (without calcinosis) have been reported in adults after administration of zoledronic acid [101–102]. The structural differences among these drugs may explain the difference in efficacy.
Sodium Thiosulfate
Among the effects of sodium thiosulfate are the ability to dissolve calcium deposits and chelate free calcium. There are numerous reports of the successful use of sodium thiosulfate in both topical and intravenous forms to treat various diseases of abnormal calcium deposition, including calcific uremic arteriopathy [103–107]. Only two reports describe the successful use of sodium thiosulfate to significantly improve calcinosis associated with JDM. The previously mentioned report used IV sodium thiosulfate in addition abatacept in a 14-year-old girl [77]. Most recently, a 4-year-old boy with JDM was reported to have improvement in calcinosis and cutaneous ulcerations using topical sodium thiosulfate (3%, followed by 10%) under occlusive dressings over 9 months [108].
Aluminum Hydroxide
Aluminum hydroxide is thought to decrease intestinal absorption of phosphate, which would potentially result in a reduction of calcium-phosphorus product in the serum, with a resultant decrease in calcium deposition in the tissue. Several case reports describe significant improvement in JDM-associated calcinosis with oral aluminum hydroxide therapy. One patient had complete resolution after 8 months of therapy, while two others showed significant improvement [109–111].
Probenecid
Probenecid is a known treatment for gout and has shown some efficacy in the treatment of calcinosis in two patients with JDM [112,113]. The mechanism is uncertain, but may be related to decreasing extracellular ATP levels with a resultant decrease in PPi, as well as increased renal excretion of phosphorus along with lowered systemic levels of phosphorus [114]. An important secondary effect to consider is the resultant decreased renal tubular secretion of methotrexate and non-steroidal anti-inflammatory drugs, which are commonly used to treat patients with JDM. An 11-year-old boy with refractory calcinosis from JDM had reduction in the size of lesions after 17 months of therapy [112]. A 9-year-old girl with JDM and extensive calcifications had resolution of calcification after 18 months of treatment [113].
Mechanical Therapy
Surgical Resection
Despite concerns about recurrence of lesion due to mechanical trauma at the operative site, surgical resection has been effective in treating calcinosis. Several successful surgical resections of calcinosis are reported in JDM with no or minimal recurrence of the lesions, although some reports have suggested the possibility for recurrence when underlying JDM disease activity is not well controlled [91,113,115,116]. Surgery is generally reserved for discrete lesions with problems of recurrent infection, severe pain, or functional impact.
Treatment with extracorporeal shock wave lithotripsy and carbon dioxide laser therapy have shown promise in a few cases and need further study [reviewed in 46,68].
Conclusion
In summary, dystrophic calcification as a sequelae of JDM is associated with prolonged or inadequately treated JDM disease activity, and has the potential to possibly be prevented through early, aggressive immunosuppressive therapy for JDM. Calcinosis is also frequently associated with not only active JDM, but a pro-inflammatory process surrounding the lesions. Early intervention with immunosuppressive agents, as well as drugs that alter calcium or phosphate metabolism may be helpful in prevention of further deposition based on anecdotal reports. The development of new assessment tools and outcome measures and studies involving randomized controlled trials are needed to develop evidence-based therapies for this complication. A better understanding of the pathogenesis of calcinosis should aid in improving its treatment.
Acknowledgments
This study was supported in part by the Intramural Research Program of the National Institute of Environmental Health Sciences, National Institutes of Health. We thank Drs. Olcay Jones and James Katz for helpful comments on the manuscript.
Contributor Information
Mark F. Hoeltzel, Email: mhoeltze@med.umich.edu, Assistant Professor, Pediatric Rheumatology, Mott Children’s Hospital, University of Michigan, 1500 E. Medical Center Dr. Ann Arbor, MI. 48109, Ph: 734-764-2224, FAX: 734-936-9090.
Edward J. Oberle, Email: eoberle.md@gmail.com, Instructor, Pediatric Rheumatology, Medical College of Wisconsin, Children’s Hospital of Wisconsin, 9000 W. Wisconsin Ave., Milwaukee, WI. 53151, Ph: 414-337-7060, FAX: 414-266-6695.
Angela Byun Robinson, Email: angela.robinson@uhhospitals.org, Assistant Professor, Pediatric Rheumatology, Rainbow Babies and Children’s Hospital/Case Medical Center, 11100 Euclid Ave. MS6008B, Cleveland, OH. 44106, Ph: 216-844-3645, FAX: 216-844-7587.
Arunima Agarwal, Email: arunima.agarwal@gmail.com, Fellow, Pediatric Rheumatology, Children’s Hospital of Los Angeles, 4650 W Sunset Blvd., Los Angeles, CA. 90027, Ph: 323-361-4178, FAX: 323-361-8030.
Lisa G. Rider, Email: riderl@mail.nih.gov, Deputy Chief, Environmental Autoimmunity Group, Program for Clinical Research, National Institute of Environmental Health Sciences, National Institutes of Health, Building 10, Rm 4-2352, 10 Center Drive, MSC 1301, Bethesda, MD. 20892-1301, Ph: 301-451-6272, FAX: 301-451-5588.
References
- 1.Rider LG, Lindsley C, Miller FW. Chapter 26: Juvenile dermatomyositis. In: Petty R, Laxer R, Wedderburn L, Lindsley C, editors. Textbook of Pediatric Rheumatology. 6. Saunders Elsevier; 2014. In press. [Google Scholar]
- 2.Bohan A, Peter JB. Polymyositis and dermatomyositis. N Engl J Med. 1975;292:344–7. 403–7. doi: 10.1056/NEJM197502132920706. [DOI] [PubMed] [Google Scholar]
- 3.Rider LG. Calcinosis in Juvenile Dermatomyositis: Pathogenesis and Current Therapies. Pediatr Rheumatol Online J. 2003;1:2. [Google Scholar]
- 4.Ramanan AV, Feldman BM. Clinical features and outcomes of juvenile dermatomyositis and other childhood onset myositis syndromes. Rheum Dis Clin North Am. 2002;28(4):833–58. doi: 10.1016/s0889-857x(02)00024-8. [DOI] [PubMed] [Google Scholar]
- 5.Fisler RE, Liang MG, Fuhlbrigge RC, et al. Aggressive management of juvenile dermatomyositis results in improved outcome and decreased incidence of calcinosis. J Am Acad Dermatol. 2002;47(4):505–11. doi: 10.1067/mjd.2002.122196. [DOI] [PubMed] [Google Scholar]
- 6.Sanner H, Gran JT, Sjaastad I, Flato B. Cumulative organ damage and prognostic factors in juvenile dermatomyositis: a cross-sectional study median 16. 8 years after symptom onset. Rheumatology. 2009;48:1541–7. doi: 10.1093/rheumatology/kep302. [DOI] [PubMed] [Google Scholar]
- 7.Sato JO, Sallum AM, Ferriani VP, et al. A Brazilian registry of juvenile dermatomyositis: onset features and classification of 189 cases. Clin Exp Rheumatol. 2009;27:1031–8. [PubMed] [Google Scholar]
- 8.Ravelli A, Trail L, Ferrari C, et al. Long-term outcome and prognostic factors of juvenile dermatomyositis: a multinational, multicenter study of 490 patients. Arthritis Care Res. 2010;62(1):63. doi: 10.1002/acr.20015. [DOI] [PubMed] [Google Scholar]
- 9.Mathiesen P, Hegaard H, Herlin T, et al. Long-term outcome in patients with juvenile dermatomyositis: a cross-sectional follow-up study. Scand J Rheumatol. 2012;41(1):50–8. doi: 10.3109/03009742.2011.608376. [DOI] [PubMed] [Google Scholar]
- 10.McCann LJ, Juggins AD, Maillard SM, et al. The Juvenile Dermatomyositis National Registry and Repository (UK and Ireland)-- clinical characteristics of children recruited within the first 5 yr. Rheumatology (Oxford) 2006;45(10):1255–60. doi: 10.1093/rheumatology/kel099. [DOI] [PubMed] [Google Scholar]
- 11*.Robinson AB, Hoeltzel MF, Wahezi DM, et al. Clinical characteristics of children with juvenile dermatomyositis: the Childhood Arthritis and Rheumatology Research Alliance Registry. Arth Care Res. 2014;66(3):404–10. doi: 10.1002/acr.22142. Description of a cross-sectional of an ongoing registry that collected a cohort of 384 patients with JDM across 55 pediatric rheumatology centers across the United States over 2 years. Includes demographic data, disease characteristics and functional and quality of life measures. Provides an important infrastructure for future clinical and translational research in this disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shah M, Mamyrova G, Targoff IN, et al. Childhood Myositis Heterogeneity Collaborative Study Group. The clinical phenotypes of the juvenile idiopathic inflammatory myopathies. Medicine (Baltimore) 2013;92(1):25–41. doi: 10.1097/MD.0b013e31827f264d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rider LG, Lachenbruch PA, Monroe JB, et al. IMACS Group. Damage extent and predictors in adult and juvenile dermatomyositis and polymyositis as determined with the myositis damage index. Arthritis Rheum. 2009;60(11):3425–35. doi: 10.1002/art.24904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clemente G, Piotto DG, Barbosa C, et al. High frequency of calcinosis in juvenile dermatomyositis: a risk factor study. Rev Bras Reumatol. 2012;52(4):549–53. [PubMed] [Google Scholar]
- 15.Walsh JS, Fairley JA. Calcifying disorders of the skin. J Am Acad Dermatol. 1995;33(5Pt 1):693–706. doi: 10.1016/0190-9622(95)91803-5. [DOI] [PubMed] [Google Scholar]
- 16**.Balin SJ, Wetter DA, Andersen LK, Davis MD. Calcinosis cutis occurring in association with autoimmune connective tissue disease: the Mayo Clinic experience with 78 patients, 1996–2009. Arch Dermatol. 2012;148(4):455–62. doi: 10.1001/archdermatol.2011.2052. Large series of calcinosis associated with a number of systemic autoimmune diseases, including description of the locations and subtypes of lesions. Also discusses treatment responses, finding diltiazem and surgical resection to be most successful in this series of patients. [DOI] [PubMed] [Google Scholar]
- 17.Guseinova D, Consolaro A, Trail L, et al. Comparison of clinical features and drug therapies among European and Latin American patients with juvenile dermatomyositis. Clin Exp Rheumatol. 2011;29(1):117–24. [PubMed] [Google Scholar]
- 18.Efthimiou P, Kukar M, Kagen LJ. Images in rheumatology. Severe adult-onset calcinosis in a patient with a history of juvenile dermatomyositis. J Rheumatol. 2010;37(1):194. doi: 10.3899/jrheum.090628. [DOI] [PubMed] [Google Scholar]
- 19.Blane CE, White SJ, Braunstein EM, Bowyer SL, Sullivan DB. Patterns of calcification in childhood dermatomyositis. Am J Roentgenol. 1984;142(2):397–400. doi: 10.2214/ajr.142.2.397. [DOI] [PubMed] [Google Scholar]
- 20.Douvoyiannis M, Litman N, Dulau A, Ilowite NT. Panniculitis, infection, and dermatomyositis: case and literature review. Clin Rheumatol. 2009;28(Suppl 1):S57–63. doi: 10.1007/s10067-009-1160-9. [DOI] [PubMed] [Google Scholar]
- 21.Marie I, Menard JF, Hachulla E, et al. Infectious complications in polymyositis and dermatomyositis: a series of 279 patients. Semin Arthritis Rheum. 2011;41(1):48–60. doi: 10.1016/j.semarthrit.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 22.Miyamae T, Mori M, Inamo Y, et al. Multi-center analysis of calcinosis in children with juvenile dermatomyositis. Ryumachi. 2003;43(3):538–43. [PubMed] [Google Scholar]
- 23.Pachman LM, Hayford JR, Chung A, et al. Juvenile dermatomyositis at diagnosis: clinical characteristics of 79 children. J Rheumatol. 1998;25(6):1198–204. [PubMed] [Google Scholar]
- 24.Mathiesen PR, Zak M, Herlin T, Nielsen SM. Clinical features and outcome in a Danish cohort of juvenile dermatomyositis patients. Clin Exp Rheumatol. 2010;28(5):782–9. [PubMed] [Google Scholar]
- 25.Sallum AM, Pivato FC, Doria-Filho U, et al. Risk factors associated with calcinosis of juvenile dermatomyositis. J Pediatr (Rio J) 2008;84(1):68–74. doi: 10.2223/JPED.1746. [DOI] [PubMed] [Google Scholar]
- 26*.Ceribelli A, Fredi M, Taraborelli M, et al. Anti-MJ/NXP-2 autoantibody specificity in a cohort of adult Italian patients with polymyositis/dermatomyositis. Arthritis Res Ther. 2012;14(2):R97. doi: 10.1186/ar3822. Italian adult DM/PM cohort that describes the prevalence of anti-MJ autoantibodies to be 20% in adult DM and 8% in adult PM. Anti-MJ autoantibodies are associated with calcinosis in 30% of patients, and also associated with younger age of onset, heliotrope rash (i.e., DM), and less frequent interstitial lung disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gunawardena H, Wedderburn LR, Chinoy H, et al. Juvenile Dermatomyositis Research Group, UK and Ireland. Autoantibodies to a 140-kd protein in juvenile dermatomyositis are associated with calcinosis. Arthritis Rheum. 2009;60(6):1807–14. doi: 10.1002/art.24547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sugiura K, Muro Y, Akiyama M. Autoantibodies to nuclear matrix protein 2/MJ in adult-onset dermatomyositis with severe calcinosis. J Am Acad Dermatol. 2012;67(4):e167–8. doi: 10.1016/j.jaad.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 29*.Tansley SL, Betteridge ZE, Shaddick G, et al. on behalf of the Juvenile Dermatomyositis Research Group. Calcinosis in juvenile dermatomyositis is influenced by both anti-NXP2 autoantibody status and age at disease onset. Rheumatology. 2014 Jul 1; doi: 10.1093/rheumatology/keu259. pii: keu259. Study from large UK JDM cohort that now defines younger age of disease onset and anti-NXP-2 autoantibodies in all age groups to be independently associated with the development of calcinosis in JDM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rider LG, Shah M, Mamyrova G, et al. Childhood Myositis Heterogeneity Collaborative Study Group. The myositis autoantibody phenotypes of the juvenile idiopathic inflammatory myopathies. Medicine. 2013;92(4):223–43. doi: 10.1097/MD.0b013e31829d08f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marguerie C, Bunn CC, Copier J, et al. The clinical and immunogenetic features of patients with autoantibodies to the nucleolar antigen PM-Scl. Medicine (Baltimore) 1992;71(6):327–36. doi: 10.1097/00005792-199211000-00001. [DOI] [PubMed] [Google Scholar]
- 32*.Shimizu M, Ueno K, Ishikawa S, et al. Role of activated macrophage and inflammatory cytokines in the development of calcinosis in juvenile dermatomyositis. Rheumatology. 2014;53(4):766–7. doi: 10.1093/rheumatology/ket360. Case report which finds many macrophages and some T lymphocytes in a surgically resected calcinosis lesion from a patients with JDM. Also detects large amounts of pro-inflammatory cytokines in draining calcinosis fluid. [DOI] [PubMed] [Google Scholar]
- 33.Pachman LM, Liotta-Davis MR, Hong DK, et al. TNFalpha-308A allele in juvenile dermatomyositis: association with increased production of tumor necrosis factor alpha, disease duration, and pathologic calcifications. Arthritis Rheum. 2000;43(10):2368–77. doi: 10.1002/1529-0131(200010)43:10<2368::AID-ANR26>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 34.Mamyrova G, O’Hanlon TP, Sillers L, et al. Childhood Myositis Heterogeneity Collaborative Study Group. Cytokine gene polymorphisms as risk and severity factors for juvenile dermatomyositis. Arthritis Rheum. 2008;58(12):3941–50. doi: 10.1002/art.24039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tabarki B, Ponsot G, Prieur AM, Tardieu M. Childhood dermatomyositis: clinical course of 36 patients treated with low doses of corticosteroids. Eur J Ped Neurol. 1998;2(4):205–11. doi: 10.1016/s1090-3798(98)80021-4. [DOI] [PubMed] [Google Scholar]
- 36.Bingham A, Mamyrova G, Rother KI, et al. Childhood Myositis Heterogeneity Study Group. Cytokine gene polymorphisms as risk and severity factors for juvenile dermatomyositis. Arthritis Rheum. 2008;58(12):3941–50. doi: 10.1002/art.24039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hoeltzel MF, Becker ML, Robinson AB, et al. Race is a risk factor for calcinosis in patients with JDM – early results from the CARRAnet Registry study. Pediatr Rheumatol Online J. 2012;10(Suppl 1):A65. [Google Scholar]
- 38*.Faller G, Mistry BJ, Tikly M. Juvenile dermatomyositis in South African children is characterised by frequent dystrophic calcification: a cross sectional study. Pediatr Rheumatol Online J. 2014;12(1):2. doi: 10.1186/1546-0096-12-2. In a small South African cohort of JDM patients, 29% presented with calcinosis at the time of diagnosis, but the cumulative frequency of calcinosis was quite high (71%). Calcinosis was strongly associated with vasculitic skin rashes, lower serum creatine kinase levels and Staphylococcus aureus skin infections. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39*.Shahi V, Wetter DA, Howe BM, et al. Plain radiography is effective for the detection of calcinosis cutis is occurring in association with autoimmune connective tissue disease. Br J Dermatol. 2014;170(5):1073–9. doi: 10.1111/bjd.12785. A study of plain radiography detected calcinosis is all patients examined; thus plain radiography is recommended for initial screening of calcinosis. Patients were primarily adults and had a number of different autoimmune diseases, including dermatomyositis, scleroderma, and overlap connective tissue disease. [DOI] [PubMed] [Google Scholar]
- 40.Bar-Sever Z, Mukamel M, Harel L, Hardoff R. Scintigraphic evaluation of calcinosis in juvenile dermatomyositis with Tc-9m MDP. Clin Nucl Med. 2000;25(12):1013–6. doi: 10.1097/00003072-200012000-00012. [DOI] [PubMed] [Google Scholar]
- 41.Agarwal V, Sachdev A, Dabra AK. Case 104: calcinosis in juvenile dermatomyositis. Radiology. 2007;242(1):307–11. doi: 10.1148/radiol.2421040423. [DOI] [PubMed] [Google Scholar]
- 42.Kimball AB, Summers RM, Turner M, et al. Magnetic resonance imaging detection of occult skin and subcutaneous abnormalities in juvenile dermatomyositis. Implications for diagnosis and therapy. Arthritis Rheum. 2000;43(8):1866–73. doi: 10.1002/1529-0131(200008)43:8<1866::AID-ANR24>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 43.Yassaee M, Fiorentino D, Okawa J, et al. Modification of the cutaneous dermatomyositis disease area and severity index, an outcome instrument. Br J Dermatol. 2010 Mar;162(3):669–73. doi: 10.1111/j.1365-2133.2009.09521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reiter N, El-Shabrawi L, Leinweber B, et al. Calcinosis cutis: Part I. Diagnostic pathway. J Am Acad Dermatol. 2011;65(1):1–12. doi: 10.1016/j.jaad.2010.08.038. [DOI] [PubMed] [Google Scholar]
- 45.Robertson LP, Marshall RW, HIckling P. Treatment of cutaneous calcinosis in limited systemic sclerosis with minocycline. Ann Rheum Dis. 2003;62(3):267–9. doi: 10.1136/ard.62.3.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chander S, Gordon P. Soft tissue and subcutaneous calcification in connective tissue diseases. Curr Opin Rheumatol. 2012;24(2):158–64. doi: 10.1097/BOR.0b013e32834ff5cd. [DOI] [PubMed] [Google Scholar]
- 47.Rothe MJ, Grant-Kels JM, Rothfield NF. Extensive calcinosis cutis with systemic lupus erythematosus. Arch Dermatol. 1990;126(8):1060–3. [PubMed] [Google Scholar]
- 48.Farrow EG, Imel EA, White KE. Hyperphosphatemic Familial Tumoral Calcinosis (FGF23, GALNT3, αKlotho) Best Pract Res Clin Rheumatol. 2011;25(5):735–47. doi: 10.1016/j.berh.2011.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pignolo RJ, Shore EM, Kaplan FS. Fibrodysplasia ossificans progressiva: diagnosis, management, and therapeutic horizons. Pediatr Endocrinol Rev. 2013;10(Suppl 2):437–48. [PMC free article] [PubMed] [Google Scholar]
- 50.McCarthy EF, Sundaram M. Heterotopic ossification: a review. Skeletal Radiol. 2005;34:609–19. doi: 10.1007/s00256-005-0958-z. [DOI] [PubMed] [Google Scholar]
- 51.Collins MT, Boehm M. It ANKH necessarily so. J Clin Endocrinol Metab. 2011;96(1):72–4. doi: 10.1210/jc.2010-2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Boulman N, Slobodin G, Rozenbaum M, Rosner I. Calcinosis in rheumatic diseases. Semin Arthritis Rheum. 2005;34(6):805–12. doi: 10.1016/j.semarthrit.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 53.Mukamel M, Horev G, Mimouni M. New insight into calcinosis of juvenile dermatomyositis: A study of composition and treatment. J Pediatr. 2001;138(5):763–6. doi: 10.1067/mpd.2001.112473. [DOI] [PubMed] [Google Scholar]
- 54.Miyamae T, Sano F, Ozawa R, et al. Efficacy of thalidomide in a girl with inflammatory calcinosis, a severe complication of juvenile dermatomyositis. Pediatr Rheumatol Online J. 2010;8(1):6. doi: 10.1186/1546-0096-8-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Marhaug G, Shah V, Shroff R, et al. Age-dependent inhibition of ectopic calcification: a possible role for fetuin-A and osteopontin in patients with juvenile dermatomyositis with calcinosis. Rheumatology (Oxford) 2008;47(7):1031–7. doi: 10.1093/rheumatology/ken136. [DOI] [PubMed] [Google Scholar]
- 56.Nielsen AO, Johnson E, Hentzer B, Kobayasi T. Dermatomyositis with calcinosis universalis: A histopathological and electron optic study. J Cutan Pathol. 1979;6(6):486–91. doi: 10.1111/j.1600-0560.1979.tb01175.x. [DOI] [PubMed] [Google Scholar]
- 57.Kawakami T, Nakamura C, Hasegawa H, et al. Ultrastructural study of calcinosis universalis with dermatomyositis. J Cutan Pathol. 1986;13(2):135–43. doi: 10.1111/j.1600-0560.1986.tb01514.x. [DOI] [PubMed] [Google Scholar]
- 58.Magid D, Fishman EK, Siegelman SS. Dermatomyositis with calcinosis cutis. Case report 317. Skeletal Radiol. 1985;14(2):126–31. doi: 10.1007/BF00349748. [DOI] [PubMed] [Google Scholar]
- 59.Olszak IT, Poznansky MC, Evans RH, et al. Extracellular calcium elicits a chemokinetic response from monocytes in vitro and in vivo. J Clin Invest. 2000;105(9):1299–305. doi: 10.1172/JCI9799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pachman LM, Veis A, Stock S, et al. Composition of calcifications in children with juvenile dermatomyositis: association with chronic cutaneous inflammation. Arthritis Rheum. 2006;54(10):3345–50. doi: 10.1002/art.22158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Eidelman N, Boyde A, Bushby AJ, et al. Microstructure and mineral composition of dystrophic calcification associated with the idiopathic inflammatory myopathies. Arthritis Res Ther. 2009;11(5):R159. doi: 10.1186/ar2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Urganus AL, Zhao YD, Pachman LM. Juvenile dermatomyositis calcifications selectively displayed markers of bone formation. Arthritis Rheum. 2009;61(4):501–8. doi: 10.1002/art.24391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.van Summeren MJH, Spliet WG, Royen-Kerkhof A, et al. Calcinosis in juvenile dermatomyositis: a possible role for the vitamin K-dependent protein matrix Gla protein. Rheumatology (Oxford) 2008;47(3):267–71. doi: 10.1093/rheumatology/kem360. [DOI] [PubMed] [Google Scholar]
- 64.Robey PG. Vertebrate mineralized matrix proteins: Structure and function. Connective Tissue Res. 1996;35(1–4):131–6. doi: 10.3109/03008209609029183. [DOI] [PubMed] [Google Scholar]
- 65.Boskey AL. Matrix proteins and mineralization: an overview. Connective Tissue Res. 1996;35(1–4):357–63. doi: 10.3109/03008209609029212. [DOI] [PubMed] [Google Scholar]
- 66.Pachman LM, Boskey AL. Clinical manifestations and pathogenesis of hydroxyapatite crystal deposition in juvenile dermatomyositis. Curr Rheumatol Rep. 2006;8(3):236–43. doi: 10.1007/s11926-996-0031-5. [DOI] [PubMed] [Google Scholar]
- 67.Steitz SA, Speer MY, McKee MD, et al. Osteopontin inhibits mineral deposition and promotes regression of ectopic calcification. Am J Pathol. 2002;161(6):2035–46. doi: 10.1016/S0002-9440(10)64482-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gutierrez A, Jr, Wetter DA. Calcinosis cutis in autoimmune connective tissue diseases. Dermatol Ther. 2012;25(2):195–206. doi: 10.1111/j.1529-8019.2012.01492.x. [DOI] [PubMed] [Google Scholar]
- 69.Dalakas MC. Images in clinical medicine. Calcifications in dermatomyositis. N Engl J Med. 1995;333(15):978. doi: 10.1056/NEJM199510123331506. [DOI] [PubMed] [Google Scholar]
- 70.Schanz S, Ulmer A, Fierlbeck G. Response of dystrophic calcification to intravenous immunoglobulin. Arch Dermatol. 2008;144(5):585–7. doi: 10.1001/archderm.144.5.585. [DOI] [PubMed] [Google Scholar]
- 71.Shahani L. Refractory calcinosis in a patient with dermatomyositis: response to intravenous immune globulin. BMJ Case Rep. 2012 doi: 10.1136/bcr-2012-006629. published online 20 Aug 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Peñate Y, Guillermo N, Melwani P, et al. Calcinosis cutis associated with amyopathic dermatomyositis: response to intravenous immunoglobulin. J Am Acad Dermatol. 2009;60(6):1076–7. doi: 10.1016/j.jaad.2008.09.051. [DOI] [PubMed] [Google Scholar]
- 73.Touimy M, Janani S, Rachidi W, et al. Calcinosis universalis complicating juvenile dermatomyositis: improvement after intravenous immunoglobulin therapy. Joint Bone Spine. 2013;80(1):108–9. doi: 10.1016/j.jbspin.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 74.Kalajian AH, Perryman JH, Callen JP. Intravenous immunoglobulin therapy for dystrophic calcinosis cutis: unreliable in our hands. Arch Dermatol. 2009;145(3):334. doi: 10.1001/archdermatol.2008.620. [DOI] [PubMed] [Google Scholar]
- 75.Riley P, McCann LJ, Maillard SM, et al. Effectiveness of infliximab in the treatment of refractory juvenile dermatomyositis with calcinosis. Rheumatology (Oxford) 2008;47(6):877–80. doi: 10.1093/rheumatology/ken074. [DOI] [PubMed] [Google Scholar]
- 76.Boulter EL, Beard L, Ryder C, Pilkington C. Effectiveness of Anti-Tumor Necrosis Factor-Agents in the Treatment of Refractory Juvenile Dermatomyositis. Arthritis and Rheum. 2011;63(suppl):S795. [Google Scholar]
- 77*.Arabshahi B, Silverman RA, Jones OY, Rider LG. Abatacept and sodium thiosulfate for treatment of recalcitrant juvenile dermatomyositis complicated by ulceration and calcinosis. J Pediatr. 2012;160(3):520–2. doi: 10.1016/j.jpeds.2011.11.057. Case report that describes improvement in severe calcinosis in a JDM patient following use of Abatacept in combination with topical and intravenous sodium thiosulfate therapy. Cutaneous ulcerations also healed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78*.Daoussis D, Antonopoulos I, Liossis SN, et al. Treatment of systemic sclerosis-associated calcinosis: a case report of rituximab-induced regression of CREST-related calcinosis and review of the literature. Semin Arthritis Rheum. 2012;41(6):822–9. doi: 10.1016/j.semarthrit.2011.11.007. Case report of improvement in calcinosis associated with systemic sclerosis following rituximab therapy, and provides a review of therapies for calcinosis. [DOI] [PubMed] [Google Scholar]
- 79.de Paula DR, Klem FB, Lorencetti PG, et al. Rituximab-induced regression of CREST-related calcinosis. Clin Rheumatol. 2013;32(2):281–3. doi: 10.1007/s10067-012-2124-z. [DOI] [PubMed] [Google Scholar]
- 80.Hurabielle C, Allanore Y, Kahan A, Avouac J. Flare of calcinosis despite rituximab therapy. Semin Arthritis Rheum. 2014 Apr 13; doi: 10.1016/j.semarthrit.2014.04.007. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 81.Oddis CV, Reed AM, Aggarwal R, et al. RIM Study Group. Rituximab in the treatment of refractory adult and juvenile dermatomyositis and adult polymyositis: a randomized, placebo-phase trial. Arthritis Rheum. 2013;65(2):314–24. doi: 10.1002/art.37754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Al-Mayouf SM, Alsonbul A, Alismail K. Localized calcinosis in juvenile dermatomyositis: successful treatment with intralesional corticosteroids injection. Int J Rheum Dis. 2010;13(3):e26–8. doi: 10.1111/j.1756-185X.2010.01483.x. [DOI] [PubMed] [Google Scholar]
- 83.Manukyan G, Petrek M, Tomankova T, et al. Colchicine modulates expression of pro-inflammatory genes in neutrophils from patients with familial Mediterranean fever and healthy subjects. J Biol Regul Homeost Agents. 2013;27(2):329–36. [PubMed] [Google Scholar]
- 84.Taborn J, Bole GG, Thompson GR. Colchicine suppression of local and systemic inflammation due to calcinosis universalis in chronic dermatomyositis. Ann Intern Med. 1978;8(5 Pt 1):648–9. doi: 10.7326/0003-4819-89-5-648. [DOI] [PubMed] [Google Scholar]
- 85.Fuchs D, Fruchter L, Fishel B, et al. Colchicine suppression of local inflammation due to calcinosis in dermatomyositis and progressive systemic sclerosis. Clin Rheumatol. 1986;5(4):527–30. [PubMed] [Google Scholar]
- 86.Terroso G, Bernardes M, Aleixo A, et al. Therapy of calcinosis universalis complicating adult dermatomyositis. Acta Reumatol Port. 2013;38(1):44–8. [PubMed] [Google Scholar]
- 87.Oliveri MB, Palermo R, Mautalen C, Hubscher O. Regression of calcinosis during diltiazem treatment in juvenile dermatomyositis. J Rheumatol. 1996;23(12):2152–5. [PubMed] [Google Scholar]
- 88.Vayssairat M, Hidouche D, Abdoucheli-Baudot N, Gaitz JP. Clinical significance of subcutaneous calcinosis in patients with systemic sclerosis. Does diltiazem induce its regression? Ann Rheum Dis. 1998;57(4):252–4. doi: 10.1136/ard.57.4.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Okamoto M, Yamanaka S, Yoshimoto W, Shigematsu T. Alendronate as an effective treatment for bone loss and vascular calcification in kidney transplant recipients. J Transplant. 2014;2014:269613. doi: 10.1155/2014/269613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Silverman SL. Bisphosphonate use in conditions other than osteoporosis. Ann N Y Acad Sci. 2011;1218:33–7. doi: 10.1111/j.1749-6632.2010.05769.x. [DOI] [PubMed] [Google Scholar]
- 91.Shoemaker LR. Expanding role of bisphosphonate therapy in children. J Pediatr. 1999;134(3):264–7. doi: 10.1016/s0022-3476(99)70447-6. [DOI] [PubMed] [Google Scholar]
- 92.Luckman SP, Coxon FP, Ebetino FH, et al. Heterocycle-containing bisphosphonates cause apoptosis and inhibit bone resorption by preventing protein prenylation: evidence from structure-activity relationships in J774 macrophages. J Bone Miner Res. 1998;13(11):1668–78. doi: 10.1359/jbmr.1998.13.11.1668. [DOI] [PubMed] [Google Scholar]
- 93.Van Gelder JM, Breuer E, Ornoy A, et al. Anticalcification and antiresorption effects of bisacylphosphonates. Bone. 1995;16(5):511–20. doi: 10.1016/8756-3282(95)00081-n. [DOI] [PubMed] [Google Scholar]
- 94.Ylitalo R. Bisphosphonates and atherosclerosis. Gen Pharmacol. 2002;35(6):287–96. doi: 10.1016/s0306-3623(01)00121-5. [DOI] [PubMed] [Google Scholar]
- 95.Apschner A, Huitema LF, Ponsioen B, et al. Zebrafish enpp1 mutants exhibit pathological mineralization, mimicking features of generalized arterial calcification of infancy (GACI) and pseudoxanthoma elasticum (PXE) Dis Model Mech. 2014;7(7):811–22. doi: 10.1242/dmm.015693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Slimani S, Abdessemed A, Haddouche A, Ladjouze-Rezig A. Complete resolution of universal calcinosis in a patient with juvenile dermatomyositis using pamidronate. Joint Bone Spine. 2010;77(1):70–2. doi: 10.1016/j.jbspin.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 97.Marco Puche A, Calvo Penades I, Lopez Montesinos B. Effectiveness of the treatment with intravenous pamidronate in calcinosis in juvenile dermatomyositis. Clin Exp Rheumatol. 2010;28(1):135–40. [PubMed] [Google Scholar]
- 98.Ambler GR, Chaitow J, Rogers M, et al. Rapid improvement of calcinosis in juvenile dermatomyositis with alendronate therapy. J Rheumatol. 2005;32(9):1837–9. [PubMed] [Google Scholar]
- 99.Van Gelder JM, Breuer E, Schlossman A, et al. In vitro and in vivo effects of tetrakisphosphonates on bone resorption, tumor osteolysis, ectopic calcification, and macrophages. J Pharmaceut Sci. 1997;86(3):283–9. doi: 10.1021/js960429h. [DOI] [PubMed] [Google Scholar]
- 100.Metzger AL, Singer FR, Bluestone R, Pearson CM. Failure of disodium etidronate in calcinosis due to dermatomyositis and scleroderma. N Engl J Med. 1974;291(24):1294–6. doi: 10.1056/NEJM197412122912408. [DOI] [PubMed] [Google Scholar]
- 101.Tong PL, Yu LL, Chan JJ. Drug-induced dermatomyositis after zoledronic acid. Australas J Dermatol. 2012;53(4):e73–5. doi: 10.1111/j.1440-0960.2011.00771.x. [DOI] [PubMed] [Google Scholar]
- 102.Apalla Z, Tzellos T, Lallas A, et al. Possible zoledronic acid-induced dermatomyositis. Clin Exp Dermatol. 2012;37(3):309–11. doi: 10.1111/j.1365-2230.2011.04198.x. [DOI] [PubMed] [Google Scholar]
- 103.Schlieper G, Brandenburg V, Kettler M, Floege J. Sodium thiosulfate in the treatment of calcific uremic arteriolopathy. Nat Rev Nephrol. 2009;5(9):539–43. doi: 10.1038/nrneph.2009.99. [DOI] [PubMed] [Google Scholar]
- 104.Raffaella C, Annapaola C, Tullio I, et al. Successful treatment of severe iatrogenic calcinosis cutis with intravenous sodium thiosulfate in a child affected by T-acute lymphoblastic leukemia. Pediatric Dermatol. 2009;26(3):311–5. doi: 10.1111/j.1525-1470.2008.00776.x. [DOI] [PubMed] [Google Scholar]
- 105.Wolf EK, Smite AC, Layman AE. Topical sodium thiosulfate therapy for leg ulcers with dystrophic calcification. Arch Dermatol. 2008;144:1560–2. doi: 10.1001/archderm.144.12.1560. [DOI] [PubMed] [Google Scholar]
- 106.Amin N, Gonzalez E, Leber M, et al. Successful treatment of calcific uremic arteriolopathy in a pediatric dialysis patient. Pediatric Nephrol. 2010;25(2):357–2. doi: 10.1007/s00467-009-1313-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ratsimbazafy V, Bahans C, Guigonis V. Dramatic diminution of a large calcification treated with topical sodium thiosulfate. Arthritis Rheum. 2012;64(11):3826. doi: 10.1002/art.34628. [DOI] [PubMed] [Google Scholar]
- 108.Pagnini L, Simonini G, Giani T, et al. Sodium thiosulfate for the treatment of calcinosis secondary to juvenile dermatomyositis. Clin Exp Rheumatol. 2014;32(3):408–9. [PubMed] [Google Scholar]
- 109.Aihara Y, Mori M, Ibe M, et al. A case of juvenile dermatomyositis with calcinosis universalis- remarkable improvement with aluminum hydroxide therapy. Ryumachi. 1994;34(5):879–84. [PubMed] [Google Scholar]
- 110.Nakagawa T, Takaiwa T. Calcinosis cutis in juvenile dermatomyositis responsive to aluminum hydroxide treatment. J Dermatol. 1993;20(9):558–60. doi: 10.1111/j.1346-8138.1993.tb01338.x. [DOI] [PubMed] [Google Scholar]
- 111.Wang WJ, Lo WL, Wong CK. Calcinosis cutis in juvenile dermatomyositis: remarkable response to aluminum hydroxide therapy. Arch Dermatol. 1988;124(11):1721–2. [PubMed] [Google Scholar]
- 112.Nakamura H, Kawakami A, Ida H, et al. Efficacy of probenecid for a patient with juvenile dermatomyositis complicated with calcinosis. J Rheumatol. 2006;33(8):1691–3. [PubMed] [Google Scholar]
- 113.Harel L, Harel G, Korenreich L, et al. Treatment of calcinosis in juvenile dermatomyositis with probenecid: the role of phosphorus metabolism in the development of calcifications. J Rheumatol. 2001;28(5):1129–32. [PubMed] [Google Scholar]
- 114.Costello JC, Rosenthal AK, Kurup IV, et al. Parallel regulation of extracellular ATP and inorganic pyrophosphate: roles of growth factors, transduction modulators, and ANK. Connect Tissue Res. 2011;52(2):139–46. doi: 10.3109/03008207.2010.491928. [DOI] [PubMed] [Google Scholar]
- 115.Vitale A, Delia G, La Torre F, et al. Massive gluteal calcinosis in a 10-year-old girl with juvenile dermatomyositis: successful surgical management. Plast Reconstr Surg. 2009;124(6):e456–8. doi: 10.1097/PRS.0b013e3181bcf703. [DOI] [PubMed] [Google Scholar]
- 116.Wu JJ, Metz BJ. Calcinosis cutis of juvenile dermatomyositis treated with incision and drainage. Dermatol Surg. 2008;34(4):575–7. doi: 10.1111/j.1524-4725.2007.34106.x. [DOI] [PubMed] [Google Scholar]