Abstract
Theileria annulata is common in tropical and subtropical regions especially in Iran and causes great economic losses in cattle industry. In Iran the epidemiological aspects of bovine theileriosis in different breeds of cattle is poorly understood. The aim of present study is comparison of the number of T. annulata carriers in the two major cattle breeds (Holstein–Friesian and Sistani) in Sistan of Iran by giemsa and polymerase chain reaction (PCR) methods. During winter 2013, 160 native cattle, from the two major breeds in Sistan, with the mean age of more than one year and without typical clinical symptoms of theileriosis were selected. At first, a thin layer smear was held from their ear sublime vein blood for Giemsa staining method. In order to do PCR assay, jugular vein blood sample of each cow was taken. The PCR employs primers specific for the 721-bp gene fragment encoding the 30-kDa major merozoite surface antigen of T. annulata. By PCR method, 38 (47.5 %) Holstein blood samples and 22 (27.5 %) Sistani blood samples had DNA of T. annulata and considered positive (The correlation was significant at values of P < 0.05). By checking 160 blood smears with light microscope and lens × 100, only 10 samples (6.25 %) were positive for T. annulata. Statistical comparison between PCR and smear method showed that the PCR method is more sensitive and accurate in comparison to Giemsa staining method to diagnose the asymptomatic carriers of T. annulata.
Keywords: Theileria annulata, Asymptomatic carriers, Sistani, Holstein, PCR
Introduction
Theileria annulata is the causative agent of Mediterranean or tropical Theileriosis (d’Oliveira et al. 1995). This parasite is an Apicomplexan blood protozoa transmitted mainly by vector ticks from the genus Hyalomma (Darghouth 2008) and causes an important economic loss on domestic animal production from the global perspective (Mehlhorn and Schein 1984; Schnittger et al. 2012).
Bovine tropical theileriosis is a disease affecting cattle and buffalo in Iran and many other countries covering North Africa, Southern Europe, and Asia (d’Oliveira et al. 1997; Hashemi-Fesharaki 1988). Exotic cattle (Bos taurus) is particularly susceptible with mortalities up to 40–80 % in some areas, whereas indigenous cattle (Bos indicus) generally suffers much lower mortalities (about 10 %) confined mainly to calves (Hashemi-Fesharaki 1988). Theileriosis infection in cattle is characterized by clinical signs like anorexia, emaciation, depressed rumination, lacrimation, corneal opacity, nasal discharge, diarrhea, terminal dyspnea and high fever (40–41.5 °C) (Kundave et al. 2013).
The native cattle breeds are more resistant to theileriosis comparable to the pure ones, and are affected by subclinical form of the disease (Hoghooghi-Rad et al. 2011). Bovine theileriosis is endemic in various parts of Iran although its mortality is variable, and possible factors such as animal breeds (Katzer et al. 1998), annual rainfall, and temperature are responsible for the occurrence of enzootic stability or instability (Darghouth et al. 1996; Mohammad Al-Saeed et al. 2010). The treated cows and the native ones are carriers for a long period and even until the end of life that within this period of time, only few number of erythrocytes are contaminated with the parasite which their observation and also demonstrating of their presence can hardly be done (Kirvar et al. 2000).
The precise determination of these hemoparasites is a necessity to find out their epidemiology and assortment. The methods traditionally used to discover and identify these organisms consist of microscopic examinations of thin blood smears (Heidarpour-Bami et al. 2009). Smear method is not a remarkable laboratory test to diagnose the carrier cows; also it is associated with technical problems (Shayan and Rahbari 2005). PCR has been the most preferred method for diagnosis of tropical theileriosis in epidemiological studies since this technique is more sensitive and specific than other conventional methods (d’Oliveira et al. 1995; Almeria et al. 2001; Martin-Sanchez et al. 1999).
The present study which has been accomplished in Iran for the first time was aimed to report the number of asymptomatic Holstein–Friesian and Sistani cattle breeds infected with T. annulata by molecular method in Sistan district of Sistan & Baluchistan province of Iran and comparison of the sensitivity of PCR method with the traditional giemsa staining method in diagnosis.
Materials and methods
Collection of samples
During winter 2013, 160 native cattle, 80 Holstein–Friesian and 80 Sistani breeds in Sistan, were selected with the mean age of more than one year and their characteristics such as age, sex, breed, physical examinations (including: enlargement of scapular and femoral lymph nodes, body temperature, existence of anemia) sampling area, date and the name of herdsman were registered in related papers. From each cattle a thin layer blood smear was prepared from its ear vein. Also 9 mL blood from the jugular vein of each cow was taken and collected in autoclaved tubes containing 1 ml of 3.2 % buffered citrate solution (Barker et al. 1992).
Microscopic examination
From each cattle a thin layer blood smear was prepared from its ear vein. The Giemsa staining procedure was done according to Kelly’s procedure (Kelly 1974). The slide was examined using light microscope and lens × 100. The presence of various shapes of Theileria in red blood cells confirmed the contamination.
DNA extraction and PCR
DNA was extracted from whole blood samples using DNA extraction kit (MBST, Iran) according to the manufacturer’s instructions. The isolated DNA from the blood samples was kept at −20 °C for the next purposes. One set of primers N516 (5′GTAACCTTTAAAAACGT 3′) and N517 (5′GTTACGAACATGGGTTT 3′) were used from the gene segment encoding the 30 kDa major merozoite antigen of T. annulata (Dumanli et al. 2005). The DNA was amplified by primers to obtain the desired sequence with the weight of 721 bp length. The PCR was performed in total volume of 50 µl including, 5 µl extracted DNA, 25 µl Taq DNA polymerase 2 × MasterMix (Pishgam Company, Iran), 50 pmol of each primer and the rest was distilled water, in automated thermocycler (eppendorf®, Germany) with the following program: an initial denaturation at 95 °C for 5 min and 35 cycles of denaturation step at 95 °C for 40 s, annealing step at 55 °C for 40 s, and the extension level at 72 °C for 40 s. This was followed by a final extension step at 72 °C for 8 min. The sample with confirmed microscopic Theileria piroplasm used as positive control. The PCR products were analyzed on 1 % agarose gel in 0.5 × TBE buffer and stained using ethidium bromide for 10 min and visualized in the UV illuminator (Fig. 1).
Fig. 1.
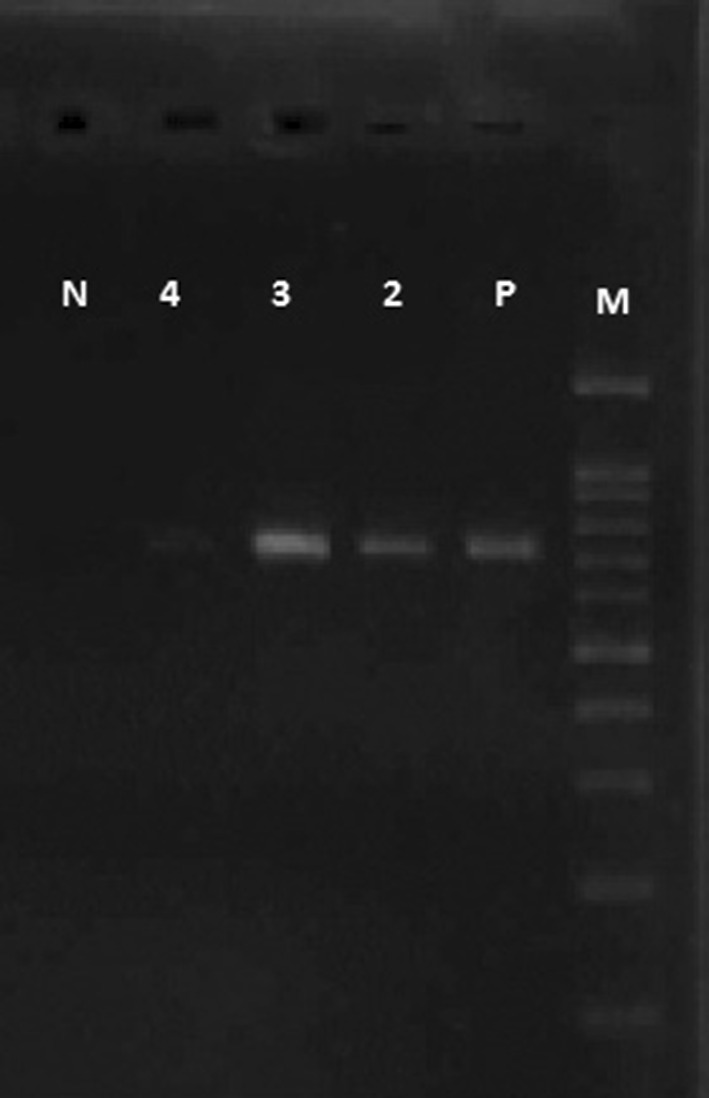
Lane 2, 3 and 4 PCR detection of 721-bp gene segment, M 100 bp Marker, P Positive control, N Negative control
Statistical analysis
For the analysis of contingency tables, we applied the Chi squared test. Data were transferred to SPSS 18.0 statistical software (IBM® PASW/SPSS Statistics 18.0), and differences were considered significant at values of P < 0.05. Also, the agreement value of PCR and microscopic examination methods was assessed with Kappa statistical test.
Results
Among total blood samples, from 80 samples of Sistani breed 22 (27.5 %) and from 80 samples of Holstein–Friesian 38 (47.5 %) were shown to be positive by PCR method. The Pearson’s Chi squared test showed that the observed difference between Sistani and Holstein breeds is statistically significant . By giemsa staining method of 160 blood samples, piroplasmic forms T. annulata were seen in 10 samples (6.25 %).Kappa test showed that there is Slight agreement between PCR and smear method. (Kappa = 0.136, 95 % Confidence Interval: 0.036–0.250). The sensitivity and specificity of smear method relative to PCR were 13 % (8/60) and 98 % (98/100) relatively (Table 2). The prevalence of Theileria in calves less than 1 years old was 14 cases (77.8 %) between 1 and 2 years old was 42 cases (47.2 %) and upper than 2 years old was 4 cases (7.5 %) and this observed difference is statistically significant . there weren’t actuarially significant differences about other parameters such as sex, presence of ticks, status of lymph nodes and mucosal membranes.
Table 2.
comparison between microscopy vs PCR examination
| PCR result | Positive | Negative | Total | ||
|---|---|---|---|---|---|
| Microscopic result | Positive | Negative | Positive | Negative | |
| Number | 8 | 52 | 2 | 98 | 160 |
| Percentage (%) | 5 | 32.5 | 1.25 | 61.25 | 100 |
The distribution of T. annulata prevalence based on PCR according to breed, sex, age, Tick presence and physical examinations presented in a table (Table 1).
Table 1.
The distribution of T. annulata prevalence based on PCR according to breed, sex, age, Tick presence and physical examinations
| Category | Level | Number tested | Positive prevalence (%) | 95 % confidence interval* |
|---|---|---|---|---|
| Breed | Holstein | 80 | 47.5 | 36–59 |
| Sistani | 80 | 27.5 | 18–39 | |
| Sex | Male | 72 | 44.4 | 33–57 |
| Female | 88 | 31.8 | 22–43 | |
| Age | Under 1 | 18 | 77.8 | 52–94 |
| Between 1 and 2 | 89 | 47.2 | 37–58 | |
| Upper 2 | 53 | 7.5 | 2–18 | |
| Tick presence | Yes | 31 | 51.6 | 33–70 |
| No | 89 | 34.1 | 39–60 | |
| Membranes | Normal | 127 | 35.4 | 27–44 |
| Pale | 33 | 45.5 | 28–64 | |
| Lymph node | Normal | 109 | 36.7 | 28–46 |
| Swelled | 51 | 39.2 | 26–54 |
* Confidence interval for positive prevalence calculated based on binomial distribution
Discussion
Management of livestock and control of tick-borne diseases play an important role to have a successful and healthy economy. One of the most important diseases in ruminants is infection with blood protozoan, Theileria, which causes large economic losses globally every year. Carriers such as treated, vaccinated or native cattle are the main sources of Theileria for ticks (Brown 1990; Neitz 1957), So early precise identification of these carriers is necessary for plotting control and prevention schemes. Also, this early diagnosis requires a simple and highly accurate method capable to recognize the animals with low parasitemia. Among some of these procedures, serological methods are employed in determining subclinical infections. False positive and negative results are commonly observed in serological tests due to cross-reaction, weakening in specific immune response as well as lack of determination of antibodies in carriers because of long term infection (Burridge et al. 1974; Gubbels et al. 2000; Leemans et al. 1999). The giemsa staining method of blood smears were shown false negative results under light microscope in our study, proved low sensitivity of this test and this may be due to artifacts, low parasitemia, destruction of piroplasmic forms in red blood cells because of hemolysis, thickness and dirtiness of slides (Hoghooghi-Rad et al. 2011). Of 160 blood samples, piroplasmic forms of T. annulata were seen in 10 samples (6.25 %). These results are similar to those, observed by some other researchers (d’Oliveira et al. 1995; Kirvar et al. 2000; Aktas et al. 2002; Azizi et al. 2008; Kirvar et al. 1998). Advances in molecular biology have enabled genotypic characterization, and have proved very useful for the identification and classification of many hemoparasites species (Heidarpour-Bami et al. 2009). Many researchers have emphasized that PCR assay is a good technique for discrimination of asymptomatic carriers of T. annulata than Giemsa staining method (Eamens et al. 2013; Heidarpour-Bami et al. 2009; Hoghooghi-Rad et al. 2011). These all have agreement with our achievements.
The results of present study showed the prevalence of infection in 27.5 % of Sistani (Bos indicus) and 47.5 % of Holstein (Bos taurus) cattle breeds. These results indicate resistance among the fleshy race, Sistani breed in comparison to the dairy, more sensitive breed, the Holstein-Friesia.
“Breed” is one of the important parameters that could fundamentally influence the prevalence and incidence of tropical theileriosis; As Spickett et al. (1989), Dolan (1989) and Sayin et al. (2003) have mentioned, pure breeds are more sensitive to the disease compared to cross × local and local breeds and this is in agreement with our results. In a study conducted by Nazifi et al. (2010) the blood samples of 24 indigenous and 26 Holstein cattle were obtained and Theileria infection was diagnosed based on hematological, biochemical and microbiological tests, clinical signs and epidemiological evidences; Iranian indigenous cattle had lower parasitemia rate, weaker response to T. annulata infection, milder clinical manifestations and significantly lower levels of acute phase proteins in comparison with Holstein and these results have conformity with our achievements. Glass et al. (2005) and Terada et al. (1995) also separately performed experiments between different cattle breeds infected to T. annulata and showed statistically significant differences among the pure and native breeds, as in our study. Ananda et al. (2009) mentioned high prevalence of T. annulata in cattles of 4–6 years of age that has disagreement with our results; We perceived that the mean age of PCR positive cattles were between 1 and 2 years. Also, high prevalence rate of young animals to T. annulata in comparison to adults is in aversion with findings of Kundave et al. (2013) who reported that young cows were more resistant than older cows. Antibodies to sporozoites, schizonts and piroplasms have been recorded in the colostrum of immune cows and the serum of their calves which protects the calves against theileriosis could be the reason for low prevalence in calves less than a year in our study (Morzaria et al. 1988). There weren’t statistically significant difference about sex and other parameters included in our research such as presence of ticks, status of lymph nodes and mucosal membranes, as Ananda et al. (2009) and Terada et al. (1995) had shown. Many researchers indicated in their publications that the PCR method is more sensitive and specific than other conventional methods like giemsa staining (d’Oliveira et al. 1995; Almeria et al. 2001; Martin-Sanchez et al. 1999; Noaman 2014). The present study results have agreement with their achievements (Table 2).
In conclusion, we revealed that the prevalence rate of infection to T. annulata is much lesser in Sistani (Bos taurus indicus) than Holstein–Friesian (Bos taurus taurus) breed. According to this fact that traditional maintenance of cattle is more prevalent in Sistan region comparable to industrialized breeding, so it could be advantageous to foster Sistani cattle breeds than Holstein–Friesian.
Acknowledgments
Conflict of interest
The authors declare that they have no conflict of interests.
References
- Aktas M, Dumanli N, Cetinkaya B, et al. Field evaluation of PCR in detecting Theileria annulata infection in cattle in eastern Turkey. Vet rec. 2002;150:548–549. doi: 10.1136/vr.150.17.548. [DOI] [PubMed] [Google Scholar]
- Almeria S, Castella J, Ferrer D, et al. Bovine piroplasms in Minorca (Balearic Islands, Spain): a comparison of PCR based and light microscopy detection. Vet Parasitol. 2001;99:249–259. doi: 10.1016/S0304-4017(01)00464-2. [DOI] [PubMed] [Google Scholar]
- Ananda K, D’Souza P, Puttalashmamma G. Prevalence of haemoprotozoan diseases in crossbred cattle in Bangalore North. Vet World. 2009;2(1):15–16. doi: 10.5455/vetworld.2009.15-16. [DOI] [Google Scholar]
- Azizi H, Shiran B, Dehkordi A, et al. Detection of Theileria annulata by PCR and its comparison with smear method in native carrier cows. Biotechnology. 2008;7(3):574–577. doi: 10.3923/biotech.2008.574.577. [DOI] [Google Scholar]
- Barker R, Banchongaksorn T, Courval J, et al. A simple method to detect Plasmodium falciparum directly from blood samples using the Polymerase chain reaction. J Trop Med Hyg. 1992;46(4):416–426. doi: 10.4269/ajtmh.1992.46.416. [DOI] [PubMed] [Google Scholar]
- Brown CGD. Control of tropical theileriosis (Theileria annulata infection of cattle) Parasitologia. 1990;32:23–31. [PubMed] [Google Scholar]
- Burridge M, Brown C, Kimber C. Theileria annulata: cross reactions between a cell culture schizont antigen and antigens of East African Theileria species in the indirect fluorescent antibody test. Exp Parasitol. 1974;35:374–379. doi: 10.1016/0014-4894(74)90043-5. [DOI] [PubMed] [Google Scholar]
- d’Oliveira C, van der Weide M, Habela M, et al. Detection of Theileria annulata in blood samples of carrier cattle by PCR. J Clin Microbiol. 1995;33(10):2665–2669. doi: 10.1128/jcm.33.10.2665-2669.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d’Oliveira C, van der Weide M, Jacquiet P, et al. Detection of Theileria annulata by PCR in ticks (Acari: Ixodidae) collected from cattle in Mauritania. Exp Appl Acarol. 1997;21:279–291. doi: 10.1023/A:1018455223462. [DOI] [PubMed] [Google Scholar]
- Darghouth M. Review on the experience with live attenuated vaccines against tropical theileriosis in Tunisia: considerations for the present and implications for the future. Vaccine. 2008;26:G4–G10. doi: 10.1016/j.vaccine.2008.09.065. [DOI] [PubMed] [Google Scholar]
- Darghouth M, Bouattour A, Ben-Milad L, et al. Epidemiology of tropical theileriosis (Theileria annulata infection of cattle) in an endemic region of Tunisia: characterization of endemicity states. Vet Parasitol. 1996;65:199–211. doi: 10.1016/S0304-4017(96)00974-0. [DOI] [PubMed] [Google Scholar]
- Dolan TT. Theileriosis: a comprehensive review. Rev Sci Tech Off Int Epizoot. 1989;8:11–36. doi: 10.20506/rst.8.1.398. [DOI] [PubMed] [Google Scholar]
- Dumanli N, Aktas M, Cetinkaya B, et al. Prevalence and distribution of tropical theileriosis in eastern Turkey. Vet Parasitol. 2005;127(1):9–15. doi: 10.1016/j.vetpar.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Eamens G, Bailey G, Jenkins C, et al. Significance of Theileria orientalis types in individual affected beef herds in New South Wales on clinical, smear and PCR findings. Vet Parasitol. 2013;196:96–105. doi: 10.1016/j.vetpar.2012.12.059. [DOI] [PubMed] [Google Scholar]
- Glass E, Preston P, Springbett A, et al. Bos taurus and Bos indicus (Sahiwal) calves respond differently to infection with theileria annulata and produce markedly different levels of acute phase proteins. Int J Parasitol. 2005;35(3):337–347. doi: 10.1016/j.ijpara.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Gubbels J, Katzer F, Hide G, et al. Generation of a mosaic pattern of diversity in the major merozoites piroplasm surface antigen of Theileria annulata. Mol Biochem Parasitol. 2000;110:23–32. doi: 10.1016/S0166-6851(00)00253-X. [DOI] [PubMed] [Google Scholar]
- Hashemi-Fesharaki R. Control of Theileria annulata in Iran. Parasitol Today. 1988;4(2):36–40. doi: 10.1016/0169-4758(88)90062-2. [DOI] [PubMed] [Google Scholar]
- Heidarpour-Bami M, Haddadzadeh H, Kazemi B, et al. Molecular identification of ovine Theileria species by a new PCR-RFLP method. Vet Parasitol. 2009;161:171–177. doi: 10.1016/j.vetpar.2009.01.035. [DOI] [PubMed] [Google Scholar]
- Hoghooghi-Rad N, Ghaemi P, Shayan P, et al. Detection of native carrier cattle infected with Theileria annulata by semi-nested PCR and smear method in Golestan province of Iran. World Appl Sci J. 2011;12(3):317–323. [Google Scholar]
- Katzer F, McKellar S, Kirvar E, et al. Polygenetic analysis of Theileria and Babesia equi in relation to the establishment of parasite populations within novel host species and the development of diagnostic tests. Mol Biochem Parasitol. 1998;95:33–44. doi: 10.1016/S0166-6851(98)00085-1. [DOI] [PubMed] [Google Scholar]
- Kelly WR. Veterinary Clinical Diagnosis. London: Bailliere Tindall; 1974. [Google Scholar]
- Kirvar E, Ilhan T, Katzer F, et al. Detection of Theileria lestoquardi (hirci) in ticks, sheep, goats using polymerase chain reaction. Ann NY Acad Sci. 1998;849:52–62. doi: 10.1111/j.1749-6632.1998.tb11033.x. [DOI] [PubMed] [Google Scholar]
- Kirvar E, Ilhan T, Katzer F, et al. Detection of Theileria annulata in cattle and vector ticks by PCR using the Tams1 gene sequences. Parasitology. 2000;120:245–254. doi: 10.1017/S0031182099005466. [DOI] [PubMed] [Google Scholar]
- Kundave VR, Patel AK, Patel PV, et al. Detection of theileriosis in cattle and buffaloes by polymerase chain reaction. J Parasit Dis. 2013 doi: 10.1007/s12639-013-0386-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leemans I, Brown D, Hooshmand-Rad P. Infectivity and cross immunity studies of Theileria lestoquardi and Theileria annulata in sheep and cattle I. In vivo response. Vet Parasitol. 1999;82:179–192. doi: 10.1016/S0304-4017(99)00013-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Sanchez J, Viseras J, Adroher FJ, et al. Nested polymerase chain reaction for detection of Theileria annulata and comparison with conventional diagnostic techniques: its use in epidemiology studies. Parasitol Res. 1999;85:243–245. doi: 10.1007/s004360050541. [DOI] [PubMed] [Google Scholar]
- Mehlhorn H, Schein E. The Piroplasms: life cycle and sexual stages. Adv Parasitol. 1984;23:37–103. doi: 10.1016/S0065-308X(08)60285-7. [DOI] [PubMed] [Google Scholar]
- Mohammad Al-Saeed AT, Omer LT, Abdo J, et al. Epidemiological studies on tropical theileriosis (Theileria annulata infection of cattle) in Kurdistan region, Iraq. Parasitol Res. 2010;106:403–407. doi: 10.1007/s00436-009-1675-7. [DOI] [PubMed] [Google Scholar]
- Morzaria SP, Musoke AJ, Latif AA. Recognition of Theileria parva antigens by field sera from Rusinga Islands. Kenya. Kenya Vet. 1988;12:8. [Google Scholar]
- Nazifi S, Razavi M, Reiszadeh M, et al. Evaluation of the resistance of indigenous Iranian cattle to Theileria annulata compared with Holstein cattle by measurement of acute phase proteins. Comp Clin Pathol. 2010;19:155–161. doi: 10.1007/s00580-009-0853-4. [DOI] [Google Scholar]
- Neitz WO. Theileriosis, gonderioses and cytauxzoonoses. Onderstepoort J Vet Res. 1957;27:275–430. [Google Scholar]
- Noaman V. Comparison of molecular and microscopic technique for detection of Theileria spp. in carrier cattle. J Parasit Dis. 2014;38:64–67. doi: 10.1007/s12639-012-0196-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayin F, Karaer Z, Dincer S, et al. Studies on the epidemiology of tropical theileriosis (Theileria annulata infection) in cattle in central Anatolia. Turkey. Trop Anim Health Prod. 2003;35(6):521–539. doi: 10.1023/A:1027348708038. [DOI] [PubMed] [Google Scholar]
- Schnittger L, Rodriguez A, Florin-Christensen A. Babesia, a world emerging. Infect Genet Evol. 2012;12:1788–1809. doi: 10.1016/j.meegid.2012.07.004. [DOI] [PubMed] [Google Scholar]
- Shayan P, Rahbari S. Simultaneous differentiation between Theileria spp. and Babesia spp. on stained blood smear using PCR. Parasitol Res. 2005;97(4):281–286. doi: 10.1007/s00436-005-1434-3. [DOI] [PubMed] [Google Scholar]
- Spickett AM, De Klerk D, Enslin CB, et al. Resistance of Nguni, Bonsmara and Hereford cattle to ticks in a Bushveld region of South Africa. Onderstepoort J Vet Res. 1989;56:245–250. [PubMed] [Google Scholar]
- Terada Y, Ishida M, Yamanaka H. Resistibility to Theileria sergenti infection in Holstein and Japanese Black cattle. J Vet Med Sci. 1995;57(6):1003–1006. doi: 10.1292/jvms.57.1003. [DOI] [PubMed] [Google Scholar]


