Abstract
A case of sarcoptic mange caused by Trixacarus caviae in a conventional guinea pig breeding colony is reported. The infestation was reported in a large colony of guinea pigs during the month of July, 2013 affecting 30 breeder guinea pigs. Severely infested animals were treated individually with subcutaneous injection of ivermectin 1 % w/v (Neomec®) at the rate of 400 µg/kg body weight 10 days apart. Three doses of ivermectin were sufficient to eliminate the parasite which tested negative after 30 days of the first treatment. The entire colony was given preventive dose of ivermectin spray (2 mg/ml solution) following the same schedule. Strict hygienic measures were followed. New hair growth in the severely affected animals was evidenced on 30th day of treatment.
Keywords: Guinea pig, Ivermectin, Trixacarus caviae
Introduction
Ectoparasites are a cause of concern to laboratory animal colonies that are maintained for research, as they cause severe annoyance to the host, affecting colony production, besides transmitting zoonotic diseases. The most commonly occurring ectoparasites of guinea pigs are: mite (Trixacarus caviae and Chirodiscoides caviae) and chewing lice (Gliricola porcelli and Gyropus ovalis). In comparision to Chirodiscoides caviae, which causes asymptomatic infestation (Harikrishnan et al. 2009), chewing lice Gliricola porcelli and Gyropus ovalis infestation causes mild to moderate hair loss, and crusting of the skin (Mircean et al. 2009), while T. caviae infestation causes severe itching, hair loss, erythema and flaking skin (Singh et al. 2013). Besides, Demodex caviae is also reported to cause diffuse alopecia, erythema, papules and crusts with moderate pruritus in guinea pigs, though rarely (Mircean et al. 2009). Persons in contact with T. caviae infested guinea pigs might develop papular dermatitis and pruritus and hence T. caviae infestation is considered as a potential occupational and public health hazard. T. caviae is reported from different parts of the world (Honda et al. 2011; Mederle and Indre 2009) including India (Singh et al. 2013). The avermectin group of drugs e.g. ivermectin (Eshar and Bdolah-Abram 2012), selamectin (Honda et al. 2011; Eshar and Bdolah-Abram 2012), doramectin (Singh et al. 2013) etc. are most commonly used to treat T. caviae infestation in guinea pigs. However, other drugs such as Amitraz (Mederle and Indre 2009), lime sulfur (Ackerman 1987) are also reported to control the infestation successfully. In most of the reported cases, it is either from a pet house, or small colony of animals, where the treatment of individual animal and control approach is much easier as compared to a large breeding colony. In a large conventional breeding colony, like ours, where an effective microbiological barrier system is not available to contain the spread of the disease, such infestation necessitates to adopt a comprehensive preventive, curative and eradication plan in the entire colony. The treatment and control of T. caviae infestation in a large conventional guinea pig breeding colony using ivermectin is reported.
Case history
At the time of infestation, the Laboratory Animal Division of Pasteur Institute of India, Coonoor maintained a random bred conventionally reared guinea pig breeding colony (120 male and 480 female breeders) at Coonoor, located in Nilgiris hill in South India as per the guidelines of committee for the purpose of control and supervision of experiments on animal (CPCSEA). The animals were maintained in groups (polygamous, one Male, four Females) in polypropylene cages of approximately 65 cm (L) × 50 cm (B) × 30 cm (H) dimensions. They were provided with ad libitum supply of commercial pellet feed, germinated Bengal gram and germ free drinking water supplemented with ascorbic acid (1 g/l).
The facility also houses a random bred Swiss albino mice colony, with proper physical separation from the guinea pig colony. Personnel working in the laboratory animal facility are provided with the requirements for maintaining proper hygiene and cleanliness viz. wash basin, toilet, bathroom etc. along with protective clothing. A routine health monitoring practice is in place for both the personnel working in the facility and the animals housed.
In July 2013, a total of six cages, each cage containing one male and four female breeder guinea pigs were reported to be infested with prominent clinical symptoms. The affected animals showed symptoms like alopecia of the ventral abdomen, severe scaling in and around their mouth, genital organs, ear pinna and limbs (Figs. 1, 2). New born pups in these breeding cages were also affected.
Fig. 1.
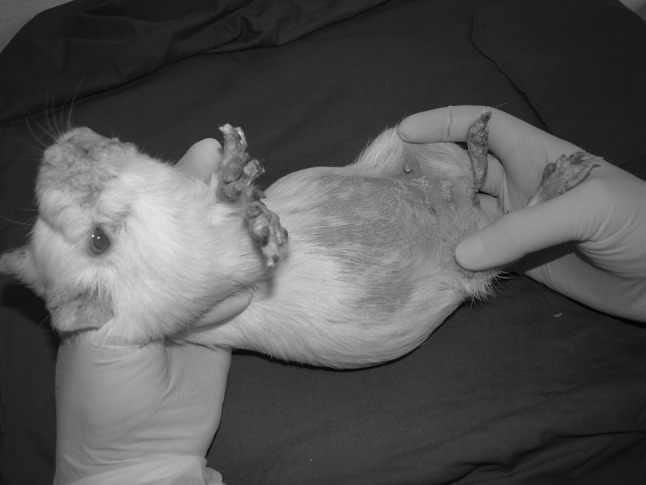
An adult female guinea pig infested with T. caviae. Alopecia of ventral abdomen, severe scaling in fore and hind limbs, around the nostrils and mouth were prominent symptoms
Fig. 2.
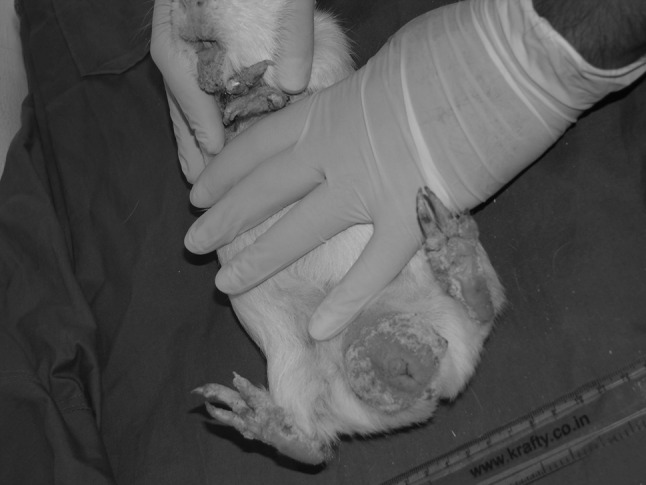
Lesions in adult male. Severe scaling in all the limbs, around mouth and nostril, and scrotum was predominant
Laboratory tests and diagnosis
Deep skin scrapings from the affected areas were collected and treated with 10 % w/v KOH solution. The prepared slides were examined thoroughly in a low power microscope. The mites were identified based on the morphological characteristics attributable to T. caviae as per available literature.
Trixacarus caviae is not difficult to differentiate from similar mites like Sarcoptes scabiei or Notoedres cati. Fuentealba and Hanna (1996) have described the differentiating features which include the size of the females (larger in S. scabiei than T. caviae), location of the anus (dorsal in male T. caviae and both sexes of N. cati, ventral in female T. caviae and both sexes of S. scabiei), and surface specialization (simple setae and sharp dorsal spines in T. caviae, and dorsal cones and spines in S. scabiei).
Skin scraping from clinically affected animals revealed numerous mites (adult and eggs) consistent with T. caviae. Figure 3 shows an adult female mite under ×40 magnification.
Fig. 3.
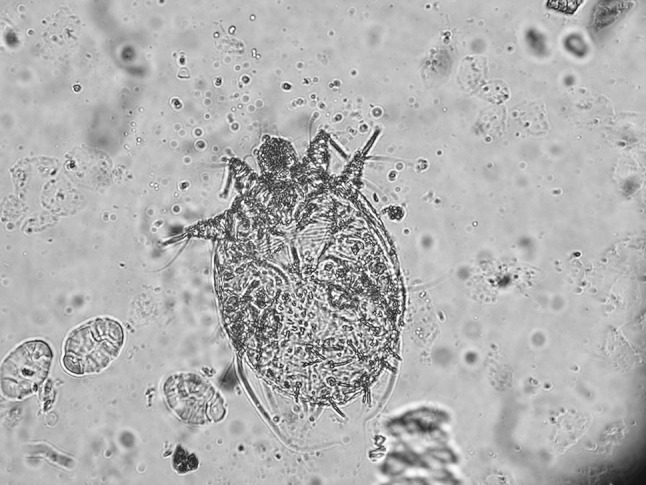
Trixacarus caviae adult female under ×40 magnification
Treatment
Guinea pigs showing severe clinical symptoms were injected subcutaneously with Neomec® (ivermectin Injection IP 1 % w/v, Intas Pharaceuticals Ltd, Ahmedabad, India) @ 400 µg/kg body weight 10 days apart on three occasions. This was based on most frequently used ivermectin treatment regimens in guinea pigs. Considering the life cycle of the parasite, and the fact that ivermectin is not ovicidal, a single dose is not sufficient to destroy the newly hatched mites (Méabed 2013). Hence a repeated treatment regimen on 2–3 occasions at suitable interval of 7–14 days is used to ensure complete cure. Skin scrapings taken at 10 days interval were also examined simultaneously. On the 30th day of treatment the skin scraping samples were completely free from the mite. The crust tissues shedding was almost complete and new hair growth was evidenced. The treated animals did not reveal any short term or long term side effects. Ivermectin is a relatively safe drug compared to some other insecticidal preparations; however, the possible side effect with injectable ivermectin includes dermal inflammation or necrosis, which might be due to propylene glycol, a commonly used diluent in ivermectin preparations (Mandigers et al. 1993).
Ivermectin is a member of the avermectin group of drugs and is the most frequently used ecto- endoparasticidal drug of choice. It has been reported successful in treating different mange mite infestations including T. caviae in guinea pigs (Mandigers et al. 1993; Eshar and Bdolah-Abram 2012). Other closely related avermectin drugs, viz., selamectin and doramectin have also been reported to be used to treat T. caviae infestations. Singh et al. (2013) reported intramuscular use of doramectin for two occasions 1 week apart, at the rate of 400 µg/kg body weight to control the infestation. Eshar and Bdolah-Abram (2012) compared the efficacy, safety and convenience of selamectin versus ivermectin for the treatment of T. caviae in pet guinea pigs. The comparison was made for topical selamectin (15 mg/kg) as a single dose and subcutaneous injection of ivermectin (400 µg/kg) 10 days apart on four occasions. Although there was no significant difference in treatment outcomes, they opined that a one-time topical treatment with selamectin would be preferred by the pet owners than an injection series. Honda et al. (2011) also reported 13.6–18.75 mg/head of selamectin for clinical management of such infestation. The pharmacokinetics of ivermectin varies widely in different animal species, breed, body weight, dose, route of administration etc. The half-life of ivermectin in different animal species can be found in FAO Monograph 41-3 (see ref FAO). There is no information available on the half-life of ivermectin in guinea pigs. However, McKellar et al. (1992) demonstrated that after subcutaneous, oral and topical administration of ivermectin @500 μg/kg, the mean plasma concentration of 0.7 ± 0.3 ng/ml could be detected after 72 h, only after subcutaneous administration.
As the number of animals clinically infested was manageable for individual treatment, they were treated individually. However, if more numbers of animals are infested in a colony, it might not be possible to afford individual treatment. In such cases spray or dip with ivermectin in similar concentration might be useful. Oral ivermectin treatment was also reported to be effective and promising in ordinary sarcoptic mange infestation in human (Méabed 2013).
The transmission is via direct contact or from the infested utensil and/or animal handlers. The general conception is that the mite is also present in many clinically healthy animals and the disease flares up if the animals are exposed to stress due to adverse environment, other illness, poor husbandry conditions and poor nutrition (Mederle and Indre 2009). This may justify that some of the animals in our colony might have been exposed to certain unintentional and unpredictable stress condition which resulted in the clinical disease. Guinea pigs require regular supply of ascorbic acid and it is ensured by incorporating ascorbic acid in feed or drinking water. It is not well documented if ascorbic acid has any role to play in the pharmacokinetics or pharmacodynamics of ivermectin.
The role played by the innate, antibody dependant and independent pathways of the host defence system on susceptibility and resistance of human to sarcoptic mite infestation are now being explored. The genetic predisposition for susceptibility or resistance to Sarcoptes scabiei infection in humans is hypothesized to correlate with the dominance of an IgE-driven Th2 response in severe disease or an interferon-γ-dominated Th1 response that promotes parasite control (Walton 2010). Molecular studies also suggests that a family of multiple scabies mite homologues of the group 3 serine protease allergens have been identified in S. scabiei, the products of all but one of these genes are predicted to be catalytically inactive due to mutations at a critical triad of amino acids at the active site. The possibility that these genes for inactivated proteases have been conserved because they mediate a novel host defence evasion strategy that the mite has evolved as an adaptation to parasitism of the epidermis has also been explored (Holt et al. 2003).
Control measures
Although T. caviae is known to be host specific, transient infestation in human is reported (Kummel et al. 1980). Another species of this genus, T. diversus is reported to occur in brown rat (Rattus norvegicus) (Izdebska and Rolbiecki 2013). Routine health monitoring of our mice colony did not reveal any symptoms suspected to be of sarcoptid mite infestation.
It is possible that some guinea pigs are asymptomatic carrier of this parasite, and develop clinical disease only when they are stressed by poor nutrition, cold temperature and other illness (Mederle and Indre 2009). Further, the colony of guinea pig was maintained in conventional way. Hence, there is every possibility that outbreaks of any infectious disease in some animals of the colony will spread to the other susceptible animals, and it calls for a preventive action or treatment for the entire colony. Attempts were therefore made to screen some random skin scraping of some apparently healthy guinea pigs from the colony, and the parasite was not detected. However, it is not advisable to ignore carrier animals or further spread of infestation in such a large conventional colony. Therefore, to ensure that any asymptomatic but infested animals are not overlooked, a preventive spray of ivermectin solution (2 mg/ml) was given to the entire colony at the rate of 1–2 burst in each side of the animals, following the same schedule as in case of clinically symptomatic animals. Such preventive spray of ivermectin in other conventional rodent colony for treating ectoparasite infestation is not uncommon (Harikrishnan et al. 2009).
The animal cages were dipped in hot water (82 °C), washed with water containing teepol (50 ml/50 l), rinsed, dried and autoclaved. Racks were steam washed and animal shelters were mopped with 1:20 diluted Lysol. Such hygienic measures would ensure to prevent further spread of infestation to other susceptible animals and to eradicate the pathogens from the colony.
Conclusion
Guinea pigs infested with sarcoptiform mite T. caviae are generally asymptomatic. However, guinea pigs exposed to stress due to adverse environmental conditions including animal husbandry, poor nutrition and other illness may develop severe alopecia, intense pruritus, scales, crusts and hyperkeratosis. An outbreak of T. caviae infestation in a large conventionally reared random bred guinea pig colony was confirmed by demonstration of the parasite in the skin scrapings of affected animals. Severely affected animals were treated individually with subcutaneous injection of ivermectin @ 400 µg/kg body weight 10 days apart on three occasions. All the animals recovered, as evidenced by shedding of skin scales and crusts, and new hair growth on 30th day of treatment. In the infested conventional colony, where a comprehensive and effective barrier system does not exist, it calls for adoption of preventive, curative and eradication measures. Though no parasite could be detected in clinically healthy guinea pigs in the colony, to prevent further spread of infestation a preventive spray of ivermectin solution (2 mg/ml) was used to treat the entire colony following the same schedule along with adoption of strict hygienic measures.
Acknowledgments
The author thankfully acknowledges the Director, Pasteur institute of India, Coonoor and Dr K N Venkataramana, Assistant director for providing infrastructure to carry out the work, Dr S Sahu for valuable inputs in correcting the manuscript, Mr N M Ramakrishnan and all the staff members of Laboratory Animal Division for their technical support, Dr S. Islam and Dr N N Barman, CVSc, Khanapara, Assam for their valuable guidance in diagnosis and treatment of the case.
References
- Ackerman L. Trixacarus caviae infestation in a guinea pig. Can Vet J. 1987;28:613. [PMC free article] [PubMed] [Google Scholar]
- Eshar D, Bdolah-Abram T. Comparison of efficacy, safety, and convenience of selamectin versus ivermectin for treatment of Trixacarus caviae mange in pet guinea pigs (Cavia porcellus) J Am Vet Med Assoc. 2012;241(8):1056–1058. doi: 10.2460/javma.241.8.1056. [DOI] [PubMed] [Google Scholar]
- FAO monograph 41-3, Residues of some veterinary drugs in foods and animals. Available at: ftp:ftp.//fao.org/ag/agn/jecfa/vetdrug/41-3-ivermectin.pdf or http://www.fao.org/food/food-safety-quality/scientific-advice/jecfa/jecfa-vetdrugs/en/
- Fuentealba C, Hanna P. Mange induced by Trixacarus caviae in a guinea pig. Can Vet J. 1996;37:749–750. [PMC free article] [PubMed] [Google Scholar]
- Harikrishnan VS, Ranaraj VR, Fernandez AC. Incidence of Chirodiscoides caviae in laboratory rats-screening, identification and treatment. Scand J Lab Anim Sci. 2009;36(2):147–153. [Google Scholar]
- Holt DC, Fischer K, Allen GE, Wilson D, Wilson P, Slade R, Currie BJ, Walton SF, Kemp DJ. Mechanisms for a novel immune evasion strategy in the scabies mite Sarcoptes Scabiei: a multigene family of inactivated serine proteases. J Invest Dermatol. 2003;121(6):1419–1424. doi: 10.1046/j.1523-1747.2003.12621.x. [DOI] [PubMed] [Google Scholar]
- Honda M, Namikawa K, Hirata H, Neo S, Marou T, Lynch J, Chida A, Morita T. An outbreak of Trixacarus caviae infestation in guinea pigs at an animal petting facility and an evaluation of the safety and suitable dose of selamectin treatment. J Parasitol. 2011;97(4):731–734. doi: 10.1645/GE-2725.1. [DOI] [PubMed] [Google Scholar]
- Izdebska JN, Rolbiecki L. Sarcoptic mites (Acari, Sarcoptidae) parasitizing the brown rat Rattus norvegicus (Berkenhout 1769) (Rodentia, Muridae), with a new data for the fauna of Poland. Ann Parasitol. 2013;59(3):125–128. [PubMed] [Google Scholar]
- Kummel BA, Estes SA, Arlian LG. Trixacarus caviae infestation of guinea pigs. J Am Vet Med Assoc. 1980;177:903–908. [PubMed] [Google Scholar]
- Mandigers PJ, van der Hage MH, Westerhof I, Dorrestein GM. A field study of the efficacy of ivermectin in propylene glycol in the treatment of mange in guinea pigs [in Dutch] Tijdschr Diergeneeskd. 1993;118:42–46. [PubMed] [Google Scholar]
- McKellar QA, Midgley DM, Galbraith EA, Scott EW, Bradley A. Clinical and pharmacological properties of ivermectin in rabbits and guinea pigs. Vet Rec. 1992;130(4):71–73. doi: 10.1136/vr.130.4.71. [DOI] [PubMed] [Google Scholar]
- Méabed EMH. Oral ivermectin as a therapeutic agent in patients with scabies. Rawal Med J. 2013;38:127–130. [Google Scholar]
- Mederle N, Indre D. Trixacarus caviae infestation in guinea pigs case report. Lucrări Ştiinłifice Med Vet XLII. 2009;1:101–104. [Google Scholar]
- Mircean V, Titilincu A, Băgut T, Dumitrache M. Research on the etiology of skin diseases in laboratory animals. Bull UASVM, Vet Med. 2009;66(2):112–118. [Google Scholar]
- Singh SK, Dimri U, Ahmed QS, Sayedda K, Singh KV. Efficacy of doramectin in Trixacarus caviae infestation in guinea pig (Cavia porcellus) J Parasit Dis. 2013;37(1):148–150. doi: 10.1007/s12639-012-0155-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton SF. The immunology of susceptibility and resistance to scabies. Parasite Immunol. 2010;32:532–540. doi: 10.1111/j.1365-3024.2010.01218.x. [DOI] [PubMed] [Google Scholar]


