Abstract
The present study was aimed to evaluate the lethal effects of Zataria multiflora Boiss (Lamiaceae) methanolic extract against Echinococcus granulosus protoscoleces. Protoscoleces were aseptically aspirated from sheep livers having hydatid cysts. Various concentrations of the essential oil (2.5–20 mg/mL) were used for 10–60 min. Viability of protoscoleces was confirmed using eosin exclusion test (0.1 % eosin staining). Obtained results showed that Z. multiflora extract at the concentration of 20 mg/mL after 10 min of exposure killed 100 % protoscoleces. The mean of mortality rate of protoscoleces after 20 min of exposure to the concentration of 10 mg/mL was also 100 %. Lower concentrations of Z. multiflora extract provoked a delayed protoscolicidal activity. The findings indicated potential of Z. multiflora methanolic extract as a natural source for the producing of new scolicidal agent for use in hydatid cyst surgery.
Keywords: Cystic echinococcosis, Zataria multiflora Boiss, Scolicidal, Protoscoleces
Introduction
Hydatid cyst (cystic echinococcosis, CE), caused by the larval stage of the dog tapeworm Echinococcus granulosus, remains a major public health problem on several continents and is reemerging in several countries (WHO 1996). For many years, surgery was the only option for treatment of hydatid cyst. Moreover, chemotherapy with benzimidazole compounds (albendazole and mebendazole) and, later, treatment by PAIR (cyst puncture, aspiration, injection of chemicals, and re-aspiration) were subsequently introduced, however, and these treatments have increasingly supplemented or even replaced surgery (Eckert and Deplazes 2004). During surgery to reduce the risk of intraoperative spillage of the cyst contents (protoscoleces) and subsequently recurrence of CE and secondary infection, which is observed in nearly 10 % of the postoperative cases, the use of effective scolicidal agents are obligatory (Brunetti et al. 2010). Nowadays, there are several scolicidal agents including hypertonic saline, silver-nitrate, cetrimide, and ethanol which have been used for inactivation of the cyst contents. However, these scolicidal agents are associated with various side effects such as sclerosane colangititis (biliary tract fibrosis), liver necrosis and methaemoglobinaemia (Mahmoudvand et al. 2014a). Thus, enormous efforts have been made to reach new scolicidal agents especially from natural resources with low side effects and more efficacies for hydatid cyst surgery.
Medicinal plants have been used for medical purposes since the beginning of human history and are the basis of modern medicine due to having the high availability, high efficacy, and low side effects (Cosa et al. 2006). Zataria multiflora Boiss (Lamiaceae) commonly grows in Iran, Afghanistan and Pakistan. Z. multiflora called “Avishane Shirazi” in Persian is used as a flavor agent (spice) in a variety of foodstuffs in Iran (Mahboubi and Ghazian Bidgoli 2010). Previous studies have shown immunostimulant, pain-relieving, antinociceptive, anti-inflammatory, antioxidant, antibacterial, antiviral and antifungal effects of Z. multiflora (Sharififar et al. 2007; Saei-Dehkordi et al. 2010). This investigation was aimed to evaluate the lethal effects of Z. multiflora methanolic extract against hydatid cyst protoscoleces.
Experimental section
Plant materials
The leaves of Z. multiflora were collected from rural regions of Kerman district (Kerman province, Iran) in September 2014. The plant materials were identified by a botanist at the Botany Department of Shahid Bahonar University, Kerman, Iran. A voucher specimen of the plant materials was deposited at the Herbarium of Department of Pharmacognosy of School of Pharmacy, Kerman University of Medical Science, Iran (KF1375).
Preparing methanolic extract
Air dried plant materials (100 g) were separately extracted by percolation method with 80 % methanol successively for 72 h in room temperature. The extracts were passed through filter paper (Whatman No. 3, Sigma, Germany) to remove plant debris. The extracts were finally concentrated in vacuum at 50 °C using a rotary evaporator (Heidolph, Germany) and stored at −20 °C, until testing (Mahmoudvand et al. 2014b).
Collection of protoscoleces
The protoscoleces of hydatid cysts were collected from the naturally infected livers of sheep and goats slaughtered at Kerman abattoir, southeastern Iran and carried to the Parasitology Laboratory at the, Kerman University of Medical Sciences, Iran. The hydatid fluid aspirated by a 50 mL syringe and aseptically transferred into a flask was left to set for 30 min for protoscoleces to settle down. The supernatant was discarded and the protoscoleces were washed two times with PBS (pH 7.2) solution. The number of protoscoleces per mL was adjusted as 2 × 103 protoscoleces in 0.9 % NaCl solution with at least 90 % viability rate. The viability of the protoscoleces was confirmed by their flame cell motility and impermeability to 0.1 % eosin solution (Sigma Aldrich, St Louis, MO, USA) under a light microscope.
Effect on protoscoleces
To determine scolicidal effects of Z. multiflora extract against protoscoleces of hydatid cysts, various concentrations of the extract (2.5–20 mg/mL) were used for 10, 20, 30, and 60 min at first, 0.5 mL of the protoscoleces (2 × 103/mL) solution was placed in test tubes. Then 0.5 mL of various concentrations of the essential oil was added to each test tube. The contents of the tubes were gently mixed and then incubated at 37 °C for 10, 20, 30, and 60 min. At the end of each incubation time, the upper phase was carefully removed so as not to interrupt the protoscoleces. 50 µL of 0.1 % eosin stain was then added to the remaining settled protoscoleces and mixed gently. The upper portion of the solution was discarded after 10 min of incubation. The remaining pellet of protoscoleces was then smeared on a glass slide, covered with a cover glass and examined under a light microscope. The percentages of dead protoscoleces were determined by counting 300 protoscoleces. We also used normal saline and hypertonic saline 20 % as negative and positive control group (Mahmoudvand et al. 2014c).
Viability test
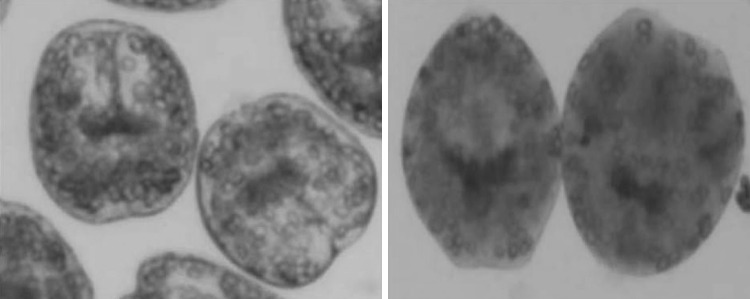
To determine the viability of protoscoleces of hydatid cysts eosin exclusion test was used (Smyth and Barrett 1998). Eosin solution with a concentration of 0.1 % (1 g of eosin powder in 1000 mL distilled water) was used. After exposure to the stain, live protoscoleces remained colorless and displayed characteristic muscular movements and flame cell activity; while dead protoscoleces absorbed eosin and colored red (Fig. 1).
Fig. 1.
Live (left) and dead (right) protoscoleces of hydatid cysts after exposure with 0.1 % eosin
Statistical analysis
All the tests were performed in triplicate. Data analysis was carried out by using SPSS statistical package (version 17.0) (SPSS Inc., Chicago, IL, USA). Differences between test and control groups were analyzed by t test. In addition, P < 0.05 was considered statistically significant.
Results
Table 1 indicates scolicidal effects of Z. multiflora methanolic extract at various concentrations following different exposure times. Obtained findings revealed that Z. multiflora extract at the concentration of 20 mg/mL after 10 min of exposure killed 100 % protoscoleces. Similarly, the mean of mortality rate of protoscoleces after 20 min of exposure to the concentration of 10 mg/mL was 100 %. Furthermore, lower concentrations of Z. multiflora extract provoked a delayed protoscolicidal effects; so that, at the concentration of 5 mg/mL killed 15.6, 51.3, 92.6, and 100 % of the protoscoleces and at the concentration of 2.5 mg/mL killed 3.3, 26, 58.3, and 86.6 % of the protoscoleces after 10, 20, 30, and 60 min of incubation, respectively. The mortality rate of protoscoleces in the negative and positive control was 5.3 and 100 % after 60 and 10 min exposure, respectively. These results also demonstrated that Z. multiflora extract at all of concentrations had significant (p < 0.05) scolicidal effects compared with the control group.
Table 1.
Protoscolicidal effects of Zataria multilfolira extract against protoscoleces of hydatid cyst at various concentrations following various exposure times. Data are expressed as the mean ± SD (n = 3)
| Concentration (mg/mL) | Exposure time (min) | Mean of mortality rate (%) |
|---|---|---|
| 20* | 10 | 100 ± 0.0 |
| 20 | 100 ± 0.0 | |
| 30 | 100 ± 0.0 | |
| 60 | 100 ± 0.0 | |
| 10* | 10 | 57.6 ± 5.5 |
| 20 | 100 ± 0.0 | |
| 30 | 100 ± | |
| 60 | 0.0 | |
| 5* | 10 | 15.6 ± 2.5 |
| 20 | 51.3 ± 4.5 | |
| 30 | 92.6 ± 6.5 | |
| 60 | 100 ± 0.0 | |
| 2.5* | 10 | 3.3 ± 0.5 |
| 20 | 26.0 ± 2.5 | |
| 30 | 58.3 ± 4.0 | |
| 60 | 86.6± 5.5 | |
| Normal saline | 10 | 1.6 ± 0.5 |
| 20 | 2.6 ± 0.5 | |
| 30 | 3.2 ± 0.5 | |
| 60 | 5.3 ± 0.5 | |
| 20 % Hypertonic saline* | 10 | 100 ± 0.0 |
| 20 | 100 ± 0.0 | |
| 30 | 100 ± 0.0 | |
| 60 | 100 ± 0.0 |
* Difference is significant (p < 0.05) compared with control group
Discussion
Natural products, such as plants extract, either as pure compounds or as standardized extracts, due to having low toxicity, low cost, high efficacy, and high availability provide unlimited opportunities for new drug discoveries because of the unmatched availability of chemical diversity (Mahmoudvand et al. 2014d). This study was aimed to evaluate the protoscolicidal effects of Z. multiflora methanolic extract on an in vitro model. Results showed that Z. multiflora extract at the concentrations of 20 and 10 mg/mL after 10 and 20 min of exposure killed 100 % protoscoleces.
Nowadays, an ideal scolicidal agent explained by its potency at lower doses, high efficacy in a shorter time of exposure, stability in the presence of cystic fluid, scolicidal ability inside a cyst, lower toxicity, higher availability, and ability for rapid preparation (WHO 1996). However, existing scolicidal agents are associated with serious adverse effects and their efficacy is controversial (Mahmoudvand et al. 2014e). Our findings revealed that Z. multiflora extract had remarkable scolicidal activity which is comparable with the existing scolicidal agents such as 20 % hypertonic saline (15 min), 20 % silver nitrate (20 min), 0.5–1 % cetrimide (10 min), H2O2 3 % (15 min), and 95 % ethyl alcohol (15 min). Thus, findings of present investigation supported the idea that Z. multiflora extract could be a natural source for the production of a new scolicidal agent for use in hydatid cyst surgery. Main mechanisms of scolicidal effects of Z. multiflora are not clear and further studies are needed to elucidate these mechanisms. However, it has been previously proven that Z. multiflora, due to having higher content phenolic compounds especially thymol and carvacrol, acts on the cell membrane microorganisms and causes damage and depletion of the contents of the cells (Moshayedi et al. 2012). Moreover, Kavoosi et al. reported that Z. multiflora essential oil, thymol, and carvacrol significantly reduced activities of nitric oxide and H2O2 production as well as NO synthase and NADH oxidase in LPS-stimulated murine. Thus, exact mechanisms of scolicidal activity (Kavoosi et al. 2012).
In the regard to toxicity effects of Z. multiflora, Hosseinzadeh et al. reported that LD50 of the infusion and maceration Z. multiflora extract was 3.85 and 3.47 g/kg, respectively (Hosseinzadeh et al. 2000). In addition, Malekinejad et al. showed Z. multiflora had no toxicity on Chinook salmon (Oncorhynchus tshawytscha) embryo (CHSE-214) cells (Malekinejad et al. 2012). According to a toxicity classification, the methanolic extract of Z. multiflora had no significant toxicity and is safe for mammalian cells (Loomis 1968).
Conclusion
Findings of the present study demonstrated that Z. multiflora extract might be a natural source for the production of new scolicidal agents to reduce the risk of protoscoleces spillage during hydatid cyst surgery. However, further studies will be needed to confirm these results by checking the extract in a clinical setting as a new scolicidal agent.
Acknowledgments
We would like to thank Mr. Mirbadie and Mr. Sadooghian for preparation of protoscoleces.
Conflict of interests
The author declares that there is no conflict of interests in this study.
References
- Brunetti E, Kern P, Vuitton DA. Writing panel for the WHO-IWGE expert consensus for the diagnosis and treatment of cystic and alveolar echinococcosis in humans. Acta Trop. 2010;114(1):1–16. doi: 10.1016/j.actatropica.2009.11.001. [DOI] [PubMed] [Google Scholar]
- Cosa P, Vlietinck AJ, Berghe DV, Mae L. Anti-infective potential of natural products: how to develop a stronger in vitro ‘proof-of-concept’. J Ethnopharmacol. 2006;106:290–302. doi: 10.1016/j.jep.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Eckert J, Deplazes P. Biological, epidemiological, and clinical aspects of echinococcosis, a zoonosis of increasing concern. Clin Microbiol Rev. 2004;17:107–135. doi: 10.1128/CMR.17.1.107-135.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseinzadeh H, Ramezani M, Salmani G. Antinociceptive, anti-inflammatory and acute toxicity effects of Zataria multiflora Boiss extracts in mice and rats. J Ethnopharmacol. 2000;73(3):379–385. doi: 10.1016/S0378-8741(00)00238-5. [DOI] [PubMed] [Google Scholar]
- Kavoosi G, Teixeira da Silva JA, Saharkhiz MJ. Inhibitory effects of Z. multiflora essential oil and its main components on nitric oxide and hydrogen peroxide production in lipopolysaccharide-stimulated macrophages. J Pharm Pharmacol. 2012;64:1492–1500. doi: 10.1111/j.2042-7158.2012.01510.x. [DOI] [PubMed] [Google Scholar]
- Loomis TA. Essential of toxicology. Philadelphia: Lea and Febige; 1968. [Google Scholar]
- Mahboubi M, Ghazian Bidgoli F. Antistaphylococcal activity of Zataria multiflora essential oil and its synergy with vancomycin. Phytomedicine. 2010;17:548–550. doi: 10.1016/j.phymed.2009.11.004. [DOI] [PubMed] [Google Scholar]
- Mahmoudvand H, Fasihi Harandi M, Shakibaie M, Aflatoonian MR, Makki MS, Jahanbakhsh S. Scolicidal effects of biogenic selenium nanoparticles against protoscolices of hydatid cysts. Int J Surg. 2014;12(5):399–403. doi: 10.1016/j.ijsu.2014.03.017. [DOI] [PubMed] [Google Scholar]
- Mahmoudvand H, Sepahvand A, Jahanbakhsh S, Ezatpour B, Ayatollahi Mousavi SA. Evaluation of antifungal activities of the essential oil and various extracts of Nigella sativa and its main component, thymoquinone against pathogenic dermatophyte strains. J Mycol Med. 2014;24(4):155–161. doi: 10.1016/j.mycmed.2014.06.048. [DOI] [PubMed] [Google Scholar]
- Mahmoudvand H, Sharififar F, Saedi Dezaki E, Ezatpour B, Jahanbakhsh S, Fasihi Harandi M. Protoscolecidal effect of Berberis vulgaris root extract and its main compound, berberine in cystic echinococcosis. Iran J Parasitol. 2014;9(4):26–34. [PMC free article] [PubMed] [Google Scholar]
- Mahmoudvand H, Tavakoli R, Sharififar F, Minaie K, Ezatpour B, Jahanbakhsh S, Sharifi I. Leishmanicidal and cytotoxic activities of Nigella sativa and its active principle, thymoquinone. Pharm Biol. 2014;4:1–6. doi: 10.3109/13880209.2014.957784. [DOI] [PubMed] [Google Scholar]
- Mahmoudvand H, Dezaki ES, Kheirandish F, Ezatpour B, Jahanbakhsh S, Harandi MF. Scolicidal effects of black cumin seed (Nigella sativa) essential oil on hydatid cysts. Korean J Parasitol. 2014;52(6):653–659. doi: 10.3347/kjp.2014.52.6.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malekinejad H, Bazargani-Gilani B, Tukmechi A, Ebrahimi H. A cytotoxicity and comparative antibacterial study on the effect of Zataria multiflora Boiss, Trachyspermum copticum essential oils, and Enrofloxacin on Aeromonas hydrophila. Avicenna J Phytomed. 2012;2(4):188–195. [PMC free article] [PubMed] [Google Scholar]
- Moshayedi SH, Shahraz F, Schaffner DW, Khanlarkhani A, Shojaee-Aliabadi S, Shahnia M, Khaksar R. In vitro control of Enterococcus faecalis by Zatariamultilfolira Boiss, Origanum vulgare L and Mentha pulegium essential oils. J Food Saf. 2012;4:1745–4565. [Google Scholar]
- Saei-Dehkordi SS, Tajik H, Moradi M, Khalighi-Sigaroodi F. Chemical composition of essential oils in Zataria multiflora Boiss. From different parts of Iran and their radical scavenging and antimicrobial activity. Food Chem Toxicol. 2010;48:1562–1567. doi: 10.1016/j.fct.2010.03.025. [DOI] [PubMed] [Google Scholar]
- Sharififar F, Moshafi MH, Mansouri SH, Khodashenas M, Khoshnoodi M. In vitro evaluation of antibacterial and antioxidant activities of the essential oil and methanol extract of endemic Zataria multiflora Boiss. Food Control. 2007;18:800–805. doi: 10.1016/j.foodcont.2006.04.002. [DOI] [Google Scholar]
- Smyth JD, Barrett NJ. Procedures for testing the viability of human hydatid cysts following surgical removal, especially after chemotherapy. Trans R Soc Trop Med Hyg. 1998;74:649–652. doi: 10.1016/0035-9203(80)90157-1. [DOI] [PubMed] [Google Scholar]
- World Health Organization Informal working group on echinococcosis. Bull WHO. 1996;74:231–242. [PMC free article] [PubMed] [Google Scholar]