Abstract
This paper is the first study on host-parasite relationship in wild Curimata incompta Vari, 1984 (Curimatidae) from Amazon river system, Northern Brazil. In 40 specimens examined from December 2012 to November 2013, 615,818 parasites were collected, such as Ichthyophthirius multifiliis, Piscinoodinium pilullare, Urocleidoides sp., Posthodiplostomum sp., Gorytocephalus elongorchis and Braga patagonica. The parasites’ component community had a low Brillouin diversity (0.16 ± 0.15), a low species richness (3.1 ± 0.7), a low evenness (0.09 ± 0.09) and a high dominance of Berger–Parker (0.96 ± 0.06). I. multifiliis was the dominant parasite species and it showed the highest prevalence and intensity in the host population. There was an aggregate dispersion of parasites, but the low parasitism did not affect the body condition of the host. The occurrence of parasites in C. incompta was due to their life habits and food behavior. This study, besides expanding the geographical distribution of G. elongorchis in Brazil, records the first occurrence of these six parasites in C. incompta.
Keywords: Ecology, Ectoparasites, Freshwater fish, Gills
Introduction
The Igarapé Fortaleza basin is an important tributary of the Amazonas river system in eastern Amazon region, in northern Brazil. It is in an estuarine coastal sector and is characterized by having extensive floodplains, which constitute physical systems of clogged rivers, drained by freshwater and connected to a main watercourse, influenced by high rainfalls and the tides of the Amazonas river. This tributary, eutrophized by urbanization, is widely used for refuge and food by many fish species (Gama and Halboth 2004; Tavares-Dias et al. 2013; Bittencourt et al. 2014), including Curimata spp. This study concerns the parasite fauna of freshwater fish, Curimata incompta Vari, 1984, a Curimatidae species widely distributed in South America and in the tropical region of Central America (Vari 2003; Lasso and Sánchez-Duarte 2011; Froese and Pauly 2014). Thus, this fish species is listed as “Least Concern” by IUCN.
Curimata incompta is a toothless characiform with a benthopelagic behavior and a detritivorous diet that consists mostly of algae and detritus. This fish lives in a lentic system, its spawning is total and usually occurs at the beginning of the flood season (Lasso and Sánchez-Duarte 2011; Froese and Pauly 2014). It has a relative importance in subsistence and for commercial fishery of the Amazon state. However, this fish has not been yet considered in studies on parasites’ fauna, but a few studies have been performed with other Curimata species.
In C. argentea gills of from the Arouca river (Trinidad), Molnar et al. (1974) described the monogenoidean Urocleidoides curimatae. In C. cyprinoides gills from western Amazon (Brazil), ergasilids Miracetyma etimaruya were described (Malta 1993). Domingues and Boeger (2005) reported monogenoideans Rhinoxenus guianensis in nasal cavities of C. cyprinoides from Iracoubo, Degrad Forian (French Guiana). For C. cyprinoides from Amazon river system (Brazil), infection by Ichthyophthirius multifiliis, Spironucleus sp., Urocleidoides sp., encysted metacercariae of Digenea and Polymorphus sp. was recorded (Tavares-Dias et al. 2013). For C. inormata from the Amazon river, in Pará State (Brazil), Azevedo and Matos (2009) described the Henneguya curimata.
Parasites can use intermediary and definite hosts through a trophic web, which allows them to infect the fish, but most parasite species shows a high specificity to their hosts. Thus, the knowledge of parasites infecting fishes is of particular interest regarding not only the host’s health, but also considering the relation between parasites and host within the aquatic environment (Bullard and Overstreet 2008; Takemoto et al. 2009; Tavares-Dias et al. 2013; Bittencourt et al. 2014). Parasites may cause alterations in population dynamics and in the behavior of their hosts, as well as influence the competition capacity in the predator–prey relation. Therefore, knowledge of the parasites’ fauna of wild fish constitutes a biodiversity assessment tool, allowing a greater understanding of host biology and its relation to parasites, which can be environmental indicators (Takemoto et al. 2009; Tavares-Dias et al. 2013; Shah et al. 2014). The present study investigated the host-parasite relationship in C. incompta from the Amazon river system (Brazil).
Materials and methods
Fish and collection area
From December 2012 to November 2013, 40 individuals of C. incompta (15.1 ± 1.0 cm and 48.9 ± 10.7 g) were collected in wetlands from Igarapé Fortaleza basin (00°00′56.3″S and 051°05′27.1″W), in a tributary river from the Amazon river system in the region of the municipality of Macapá, State of Amapá (Brazil), for parasitological analysis. All fish were collected with nets of different meshes. The pH (6.5 ± 0.2), water temperature (28.3 ± 0.3 °C) and dissolved oxygen levels (2.8 ± 0.3 mg/L) were determined using digital devices (YSI, USA) for each purpose.
Collection procedures and analysis of parasites
All fish were weighed (g) and measured for total length (cm), and then necropsied for parasitological analysis. For each individual, mouth, opercula, gills and gastrointestinal tract were examined in order to collect parasites (protozoans and metazoans). Gills were removed and analyzed with the aid of a microscope. To quantify metazoan parasites, each viscera was dissected separately and washed in running water. All materials retained on the 154 μm mesh were examined with a stereomicroscope. Parasites were then fixed, preserved and stained according to Eiras et al. (2006). Voucher parasites specimens were deposited at the Scientific and Technological Research Institute of the State of Amapá (IEPA), in the Scientific Collection Curation Office for the Fauna of Amapá (CCFA), under accession number IEPA 044-048-P.
Parasitological terminology used follows that described by Bush et al. (1997). Dispersion index (DI) and discrepancy index (D) were calculated using the Quantitative Parasitology 3.0 software to detect the distribution pattern of each infracommunity parasite species (Rózsa et al. 2000), with the prevalent species being >10 %. The DI significance for each infracommunity was tested using the td-statistics (Ludwig and Reynolds 1988). The Brillouin index (HB), evenness (E), Berger–Parker dominance index (d) and species richness (Magurran 2004) were calculated for parasites showing more than 10 % prevalence, in order to evaluate the community of parasites using the Diversity software (Pisces Conservation Ltd., UK), as well as the frequency of dominance (percentage of infracommunities in which a parasite species was numerically dominant) (Rohde et al. 1995; Magurran 2004).
Data on weight (g) and total length (cm) were used to calculate the relative condition factor (Kn) of hosts (Le-Cren 1951). The Pearson coefficient (r) was used to determine possible correlations of parasite intensity with length, weight, Kn, HB and species richness of examined hosts (Zar 2010).
Results
Of 40 C. incompta, 615,818 parasites were collected: two Protozoa, one Monogenoidea, one Digenea, one Acanthocephala and one Isopoda. However, higher infection levels were caused by I. multifiliis and Piscinodinum pilullare (Table 1), both parasites with dominance (Table 2). These parasites showed an aggregate dispersion (Table 2).
Table 1.
Parasites in Curimata incompta (n = 40) from the Amazon river system (Brazil)
| Parasites | P (%) | MI ± SD | MA ± SD | Range | TNP | SI |
|---|---|---|---|---|---|---|
| Ichthyophthirius multifiliis | 97.5 | 15,131.8 ± 12,524.5 | 14,753.5 ± 12,592.3 | 0–53,025 | 590,140 | Gills |
| Piscinodinum pilullare | 50.0 | 1030.7 ± 1122.4 | 515.3 ± 941.4 | 0–3762 | 20,613 | Gills |
| Urocleidoides sp. | 100 | 27.2 ± 33.2 | 27.2 ± 33.0 | 0–158 | 1088 | Gills |
| Posthodiplostomum sp. (metacercariae) | 60.0 | 165.4 ± 504.2 | 99.3 ± 395.8 | 0–2117 | 3970 | Gills |
| Gorytocephalus elongorchis | 5.0 | 2.0 ± 1.4 | 0.1 ± 0.5 | 0–3 | 4 | Intestine |
| Braga patagonica | 2.5 | 1.0 ± 0 | 0.03 ± 0.2 | 0–1 | 1 | Gills |
| Braga patagonica | 2.5 | 2.0 ± 0 | 0.05 ± 0.3 | 0–2 | 2 | Fins |
P prevalence, MI mean intensity, MA mean abundance, TNP total number of parasites, SI site of infection, SD standard deviation
Table 2.
Dispersion index (DI), d-statistic, discrepancy index (D) and frequency of dominance (FD) for main parasites of Curimata incompta (n = 40) from the Amazon river system (Brazil)
| Parasites | DI | d | D | FD (%) |
|---|---|---|---|---|
| Ichthyophthirius multifiliis | 2.111 | 4.06 | 0.275 | 0.958 |
| Piscinodinum pilullare | 4.264 | 9.46 | 0.623 | 0.033 |
| Urocleidoides sp. | 3.747 | 8.32 | 0.372 | 0.002 |
| Posthodiplostomum sp. | 3.498 | 7.75 | 0.600 | 0.006 |
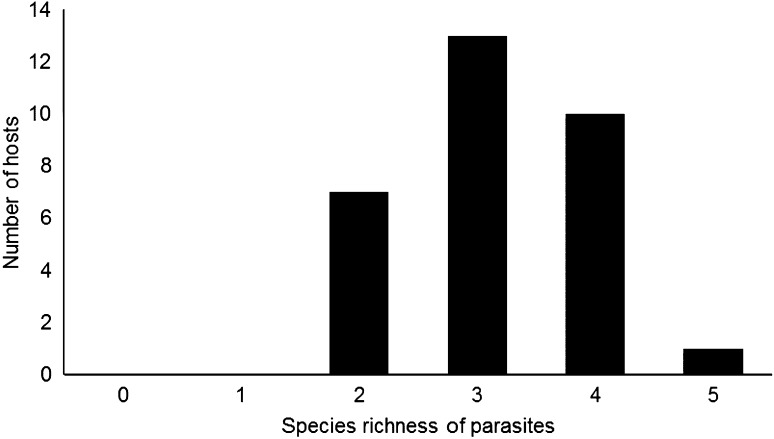
Parasites community showed a low mean diversity (HB = 0.16 ± 0.15), a low mean species richness (3.1 ± 0.7), a low evenness (E = 0.09 ± 0.09) and a high Berger–Parker dominance (d = 0.96 ± 0.06), with predominance of individuals parasitized by two to four species (Fig. 1).
Fig. 1.
Parasite species richness in Curimata incompcta (n = 40) from the Amazon river system (Brazil)
There was no correlation of length of the hosts with the richness of parasite species (rs = 0.27, p = 0.09) and the Brillouin diversity index (rs = 0.12, p = 0.45).
There was a weak positive correlation between the length (rs = 0.34, p = 0.032) and the abundance of Posthodiplostomum sp., but no correlation with weight (rs = 0.26, p = 0.10) was found. There was no correlation between length and the abundance of I. multifiliis (rs = −0.57, p = 0.73), P. pilullare (rs = 0.03, p = 0.84) and Urocleidoides sp. (rs = 0.14, p = 0.39), as well as between weight and the abundance of I. multifiliis (rs = −0.15, p = 0.34), P. pilullare (rs = −0.02, p = 0.92) and Urocleidoides sp. (rs = −0.02, p = 0.89).
For hosts, growth was a negative allometric (b = 2.32, r2 = 0.48), and body weight was positively related to total length (r = 0.70, p < 0.0001). This negative allometric growth indicates a greater increase in body weight than in body size. The relative condition factor (Kn = 1.00 ± 0.04) was not different (t = 0.0001, p = 1.00) of the Kn = 1.00 pattern, indicating good body conditions of the hosts.
Discussion
Curimata incompta was parasitized by I. multifiliis, P. pilullare, Urocleidoides sp., Posthodiplostomum sp., Gorytocephalus elongorchis and Braga patagonica, while C. cyprinoides, from the same hydrographic basin, was parasitized by I. multifiliis, Spironucleus sp., Urocleidoides sp., non-identified metacercariae Digenea and Polymorphus sp. (Tavares-Dias et al. 2013). However, in both studies, was similar dominance of I. multifiliis and overdispersion of the parasite species, but the diversity and species richness of parasites was higher for C. incompta. Differences in species richness and diversity of parasites may also be a result of the host’s individual responses to parasitism and transmission rates, among other factors (Takemoto et al. 2009; Tavares-Dias et al. 2013; Shah et al. 2014). Therefore, biotic factors such as the host immunity may have a differential effect on a fish-parasite environment system.
Cymothoids are parasites of fish, in both immature forms and adults, and they may cause damages in gill filaments, reducing the branchial surface area of the hosts (Rameshkumar and Ravichandran 2014). Only one individual of C. incompta had the gills and fins infested by B. patagonica, a cymothoid species common in fishes from the Amazon. Cymothoidae species tend to be associated with their hosts through their entire life, while species of other families are parasites only at their larval phase. As the pathogenicity of B. patagonica varies according to their location in the host, feeding behavior, strategy of attack and parasite size (Tavares-Dias et al. 2014). Thus, no clinical symptom was found. This is the first report for B. patagonica in C. incompta.
In C. incompta, the prevalence of I. multifiliis was similar to that reported for C. cyprinoides, while the intensity and abundance were higher in the hosts of this study. However, an infection by P. pilullare occurred only in C. incompta. Therefore, these results indicate a difference in response of both hosts regarding the opportunity of infection transmission by both protozoans, opportunist and common, mostly in environments with low oxygen levels (Tavares-Dias et al. 2013; Bittencourt et al. 2014). In addition, such protozoans species are directly transmitted from fish to fish, thus the proximity among hosts might be very important for a successful transmission. This is the first report of I. multifiliis and P. pilullare for C. incompta.
Infection by Urocleidoides sp. occurred in the gills of C. incompta, probably a new species of monogenoidean and similar to that reported for C. cyprinoides, a curimatid species also from Amazon river system. However, no Urocleidoides species had been described for Curimata spp. in Brazil (Cohen et al. 2013). Only U. curimatae has been known parasitizing the gills of C. argentea in Trinidad (Molnar et al. 1974) and R. guianensis parasitizing nasal cavities of C. cyprinoides in French Guyana (Domingues and Boeger 2005; Cohen et al. 2013). Therefore, this is the first record of Urocleidoides sp. in C. incompta. Infection levels of monogenoideans were higher in C. incompta compared to C. cyprinoides (Tavares-Dias et al. 2013), indicating a difference in exposition of these hosts to a same parasite species in a eutrophized environment.
In this study, metacercariae of Posthodiplostomum sp. were found on the gills of C. incompta, while for C. cyprinoides, the encysted metacercariae of Digenea, and were not identified. Posthodiplostomum sp. were reported in other wild fish species from the Amazon river system (Bittencourt et al. 2014). The genus Posthodiplostomum has a worldwide distribution due to its lack of host specificity, while most of the species has a restricted distribution. Thus, the metacercarial stage of this parasite can be found in various freshwater fish around the world. The life cycle of Posthodiplostomum species involves two intermediate hosts, a fish species and a snail species (metacercariae stage), and then a definitive host, a piscivorous bird (adult forms). Thus, the presence and abundance of this digenean depend on various abiotic (seasonality, temperature, pH, oxygen, etc.) and biotic (ecology, host size, age, etc.) factors related to these hosts and parasite (Bullard and Overstreet, 2008; Ritossa et al. 2013). Consequently, the infection levels by Posthodiplostomum sp. in C. incompta were higher than those observed for C. cyprinoides (Tavares-Dias et al. 2013). This was the first report of Posthodiplostomum sp. for C. incompcta.
Low infection levels by acanthocephalans G. elongorchis were found in C. incompta, while low Polymorphus sp. infection was reported for C. cyprinoides (Tavares-Dias et al. 2013). As both curimatid species are detritivorous, thus such infections by acanthocephalans were accidental. This difference of infection by different acanthocephalans reflects a non-habitat overlap for both hosts from the same locality. G. elongorchis is an endoparasite described parasitizing Loricariidae species, such as Hypostomus carinatus from Amazon river system (Thatcher 1979), H. cochliodon, H. regani and Loricaria sp. from Paraná river system (Lopes et al. 2011). Therefore, this is the first record of G. elongorchis for C. incompta, and suggest a wide distribution of this parasite in Brazil.
In conclusion, the parasites community in C. incompta composed by five taxa, it was characterized by low diversity, low species richness and low evenness, with a predominance of ectoparasite species. Because the hosts’ size was not a determining factor in parasite species richness and diversity, other factors may influence this parasitic fauna, and so they should be investigated in the near future. The reduced endoparasites community in C. incompta was due to its lower position in the food chain, serving as an intermediate host for other piscivorous fish.
References
- Azevedo C, Matos E. Fine structure of the myxosporean, Henneguya curimata n. sp., parasite of the Amazonian fish, Curimata inormata (Teleostei, Curimatidae) J Eukaryot Microbiol. 2009;49(3):197–200. doi: 10.1111/j.1550-7408.2002.tb00522.x. [DOI] [PubMed] [Google Scholar]
- Bittencourt LS, Pinheiro DA, Cárdenas MQ, Fernandes BMM, Tavares-Dias M. Parasites of native Cichlidae populations and invasive Oreochromis niloticus in tributary of Amazonas river (Brazil) Braz J Vet Parasitol. 2014;23(1):1–11. doi: 10.1590/s1984-29612014006. [DOI] [PubMed] [Google Scholar]
- Bullard SA, Overstreet RM. Digeneans as enemies of fishes. In: Eiras CE, Segner H, Wahli T, Kaporr BG, editors. Fish diseases. Jersey: Science Publishers; 2008. pp. 817–976. [Google Scholar]
- Bush AO, Lafferty KD, Lotz JM, Shostak W, et al. Parasitology meets ecology on its own terms: Margolis et al. revisited. J Parasitol. 1997;83(4):575–583. doi: 10.2307/3284227. [DOI] [PubMed] [Google Scholar]
- Cohen SC, Justo MCN, Kohn A. South American Monogenoidea parasites of fishes, amphibians and reptiles. Rio de Janeiro: Oficina de Livros; 2013. [Google Scholar]
- Domingues MV, Boeger WA. Neotropical Monogenoidea. 47. Phylogeny and coevolution of species of Rhinoxenus (Platyhelminthes, Monogenoidea, Dactylogyridae) and their Characiformes hosts (Teleostei, Ostariophysi) with description of four new species. Zoosystema. 2005;27:441–467. [Google Scholar]
- Eiras JC, Takemoto RM, Pavanelli GC. Métodos de estudo e técnicas laboratoriais em parasitologia de peixes. Maringá: Editora Eduem; 2006. [Google Scholar]
- Froese R, Pauly D (2014) Fish Base. World Wide Web electronic publication. www.fishbase.Org. Accessed Nov 2014
- Gama CS, Halboth DA. Ictiofauna das ressacas das bacias do Igarapé da Fortaleza e do Rio Curiaú. In: Takiyama LR, Silva AQ, editors. Diagnóstico de ressacas do estado do Amapá: bacias do Igarapé da Fortaleza e do Curiaú. Macapá: GEA/SETEC/IEPA; 2004. pp. 33–66. [Google Scholar]
- Lasso CA, Sánchez-Duarte P (2011) Los peces del delta del Orinoco. Diversidad, bioecología, uso y conservación. Fundación La Salle de Ciencias Naturales y Chevron C. A. Venezuela, Caracas
- Le-Cren ED. The length-weight relationship and seasonal cycle in gonadal weight and condition in the perch (Perca fluviatilis) J Anim Ecol. 1951;20(2):201–219. doi: 10.2307/1540. [DOI] [Google Scholar]
- Lopes MS, Fernandes BMM, Bastos OMP, Cohen SC, Kohn A. New hosts for two species of Acanthocephala of fishes from Paraná river, State of Paraná, Brazil. Rev Bras Zoociências. 2011;13(1–3):29–32. [Google Scholar]
- Ludwig JA, Reynolds JF. Statistical ecology: a primer on methods and computing. New York: Wiley-Interscience Pub; 1988. [Google Scholar]
- Magurran AE. Measuring biological diversity. Oxford: Blackwell Science; 2004. [Google Scholar]
- Malta JCO. Miracetyma etimaruya gen. et. sp. n. (Copepoda, Poecilostomatoida, Ergasilidae) from freshwater fishes of the Brazilian Amazon. Acta Amazon. 1993;23:49–57. doi: 10.1590/1809-43921993231057. [DOI] [Google Scholar]
- Molnar K, Hanek G, Fernardo CH. Ancyrocephalids (Monogenea) from freshwater fishes of Trinidad. J Parasitol. 1974;60:914–920. doi: 10.2307/3278511. [DOI] [PubMed] [Google Scholar]
- Rameshkumar G, Ravichandran S. Problems caused by isopod parasites in commercial fishes. J Parasit Dis. 2014;38(1):138–141. doi: 10.1007/s12639-012-0210-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritossa L, Flores VR, Viozzi G. Life cycle of a Posthodiplostomum species (Digenea: Diplostomidae) in Patagonia, Argentina. J Parasitol. 2013;99(5):777–780. doi: 10.1645/12-170.1. [DOI] [PubMed] [Google Scholar]
- Rohde K, Hayward C, Heap M. Aspects of the ecology of metazoan ectoparasites of marine fishes. Int J Parasitol. 1995;25(8):945–970. doi: 10.1016/0020-7519(95)00015-T. [DOI] [PubMed] [Google Scholar]
- Rózsa L, Reiczigel J, Majoros G. Quantifying parasites in samples of hosts. J Parasitol. 2000;86:228–232. doi: 10.1645/0022-3395(2000)086[0228:QPISOH]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Shah HB, Yousuf AR, Chrishti MZ, Shahnaz S, Ahmad F. Trophic status and helminth infracommunity of fish populations in Kashmir Himalayan lakes. J Helminthol. 2014;88:264–271. doi: 10.1017/S0022149X13000114. [DOI] [PubMed] [Google Scholar]
- Takemoto RM, Pavanelli GC, Lizama MAP, Lacerda ACF, Yamada FH, Ceschini TL, Bellay S. Diversity of parasites of fish the upper Paraná river floodplain, Brazil. Braz J Biol. 2009;69(2):691–705. doi: 10.1590/S1519-69842009000300023. [DOI] [PubMed] [Google Scholar]
- Tavares-Dias M, Neves LR, Pinheiro DA, Oliveira MSB, Marinho RGB. Parasites in Curimata cyprinoides (Characiformes: Curimatidae) from eastern Amazon, Brazil. Acta Sci Biol Sci. 2013;35(4):595–601. doi: 10.4025/actascibiolsci.v35i4.19649. [DOI] [Google Scholar]
- Tavares-Dias M, Araújo CSO, Barros MS, Viana GM. New hosts and distribution records of Braga patagonica, a parasite cymothoidae of fishes from the Amazon. Braz J Aquat Sci Technol. 2014;18(1):91–97. doi: 10.14210/bjast.v18n1.p91-97. [DOI] [Google Scholar]
- Thatcher VE. Uma nova espécie de Gorytocephalus Nickol e Thatcher, 1971 (Acanthocephala: Neoechinorhynchidae) do acari-bodo (Pisces: Loricariidae) da Amazônia, Brasil. Acta Amazon. 1979;9(1):199–202. [Google Scholar]
- Vari RP. Family Curimatidae (Toothless characiforms) In: Reis RE, Kullander SO, Ferraris CJJR, editors. Check list of the freshwater fishes of South and Central America. Porto Alegre: EDIPUCRS; 2003. pp. 51–74. [Google Scholar]
- Zar JH. Biostatistical analysis. 5. New Jersey: Prentice Hall; 2010. [Google Scholar]