Abstract
Toxoplasma gondii is one of the most common zoonotic parasitic diseases in human and warm-blooded animals worldwide. Birds are one of important intermediate hosts of T. gondii. The aim of this study is molecular detection of T. gondii in the house sparrow by LAMP and PCR methods in Tehran, Iran. A total 200 sparrows were captured in different regions of Tehran. DNA was extracted from tissue samples of each sparrow. LAMP and conventional PCR assays were carried out with a set of primers to detect the 529 bp fragment of T. gondii. LAMP and PCR were detected T. gondii from 17 (8.5 %) and 15 (7.5 %) of 200 sparrows respectively. These results indicated that sensitivity of LAMP was higher than conventional PCR. In our knowledge, this study is the first report of detection of T. gondii by LAMP method in bird hosts. Also, these findings provided an insight into epidemiological pattern of T. gondii infection in sparrow in Iran.
Keywords: Toxoplasma gondii, Sparrow, Loop-mediated isothermal amplification (LAMP), PCR, Iran
Introduction
Toxoplasma gondii is a widely prevalent protozoan parasite in human and warm-blooded animals worldwide (Tenter et al. 2000). Humans usually get infected through consuming drink or food contaminated with oocysts or by ingesting tissue cysts from undercooked meat. Felids, including domestic cats are definitive hosts in the life cycle of T. gondii. Cats become infected through eating infected tissues of intermediate hosts (Tenter et al. 2000). T. gondii infected a wide variety of warm-blooded animals including birds (Dubey 2002, 2010). Birds are one of important intermediate hosts of T. gondii, because infection in birds is one of the best indicators for environmental contamination with T. gondii oocysts due to their dietary habit. Moreover, infected birds are considered a good source of infection for human and cats (Dubey 2010). The house sparrow (Passer domesticus) is one of the common birds that easily adaptable to urban and rural areas. Recently, high molecular frequency of T. gondii infection in sparrow were reported from Iran (Khademvatan et al. 2013), China (Cong et al. 2013; Huang et al. 2012) and Brazil (Gondim et al. 2010; Vilela et al. 2011).
Loop-mediated isothermal amplification (LAMP) is a novel nucleic acid amplification method technology that amplifies DNA under isothermal condition with high sensitivity, specificity, and rapidity (Notomi et al. 2000). LAMP has been developed for molecular detection of several microorganisms [reviewed in (Mori et al. 2013)], including T. gondii (Kong et al. 2012; Lin et al. 2012; Zhang et al. 2009).
There is little information about molecular frequency of T. gondii in bird hosts in Iran (Asgari et al. 2009; Khademvatan et al. 2013; Zia-Ali et al. 2005). Hence, the aim of this study is determination of molecular prevalence of T. gondii in the house sparrow by LAMP and PCR methods in Tehran, center of Iran.
Materials and methods
Study area
Tehran province is located to the north of the central plateau of Iran. Tehran covers an area of about 12,981 square kilometres, and is located at 34–36.5° longitude north and 50–53° longitude east. Tehran has over 12 million inhabitants (17.5 % of Iran population) (2014). Tehran city has hot summers and moderate winters (ranging between 28 and 30 °C from mid-July to mid-September and around 1 °C from December to January) with an average annual rainfall of 200 mm (Farhadian 2011).
Sample collection and DNA extraction
A total 200 sparrows were captured (using traps) in different regions of Tehran from February to November 2013. Brain samples of each sparrow were homogenized and used for DNA extraction. Briefly, 50–70 mg of homogenized samples diluted with 800 μL of lysis buffer (50 mM Tris–HCL, pH8.0; 25 mM EDTA and 400 mM NaCl), 100 μL 10 % SDS (Biase et al. 2002), and 30 μL Proteinase K (20 μg/μL) (Fermentas, USA). After incubation at 55 °C for 3 h, proteins and undissolved debris were precipitated by adding 300 μL 6M NaCl, incubation at 4 °C for 15 min (Biase et al. 2002). Then, the samples were extracted with phenol–chloroform–isoamyl alcohol (25:24:1). DNA was precipitated by adding twice volume of 100 % ethanol and sodium acetate solution (3 M, pH 5.2), kept at −70 °C for 60 min, followed by centrifugation at 13,000×g for 5 min. Then the pellet washed twice with 70 % ethanol and resuspended in 100 μL of double-distilled water and stored at −20 °C until use.
Conventional PCR
Conventional PCR was performed using a pair of T. gondii-specific primers TOX4 (5′-CGCTGCAGGGAGGAAGACGAAAGTTG-3′) and TOX5 (5′-CGCTGCAG ACACAGTGCATCTGGATT-3′) as previously described by Homan et al. (2000), amplifying a region of 529 base pairs (bp) fragment and repeated 200–300 times in T. gondii genome (Homan et al. 2000). Amplification was performed with initial denaturation for 7 min at 94 °C, followed by 30 cycles for 35 s of 94 °C, annealing at 55 °C for 30 s, extension at 72 °C for 30 s and final extension at 72 °C for 10 min.
LAMP reaction
The LAMP assay was performed according to an earlier report with a set of four specific primers to detect the 200- to 300-fold repetitive 529 bp fragment of T. gondii (Kong et al. 2012; Lin et al. 2012). All samples were carried out in 25 μL reaction mixtures containing 40 pmol (each) of primers FIP and BIP, 5 pmol of primers B3 and F3, 1.4 mM of deoxynucleoside triphosphates (dNTP), 8 U of Bst DNA polymerase (New England Biolabs, USA), 2× reaction buffer (1.6 M betaine (Sigma-Aldrich), 40 mM of Tris–HCl (pH 8.8), 20 mM of KCl, 20 mM of (NH4)2SO4, 16 mM of MgSO4, and 0.2 % Tween 20) and 1 μL of template DNA. To determine the optimal time and temperature, the mixture was incubated at a range of temperatures at 60, 63, 65, 66, 67 and 68 °C for 50, 60, and 70 min. Subsequently, the mixture was incubated at 80 °C for 5 min to terminate of the reaction. For each reaction, a negative control (double distilled water) and positive control (T. gondii RH strain) were included.
The specificity of the T. gondii LAMP primers was examined using DNA derived from Neospora caninum, Leishmania major and Trichomonas vaginalis. A negative control without any template was included in each reaction. The detection limit of the LAMP and PCR was tested using serial tenfold dilutions of T. gondii DNA. The initial concentration of DNA for serial dilution was estimated using nanodrop. Furthermore, detection limit of LAMP and PCR were compared for detection of T. gondii DNA using samples of sparrows.
Analysis of the amplification products
The LAMP and PCR products were electrophoresed in a 2 % agarose gels stained with safe stain (CinaGen, Iran) and visualized under UV light. Visual inspection of the LAMP amplicons in the reaction tube was performed by adding 1 ml LAMP products to 10 μL of ethidium bromide stained solution (1 μg/ml) (Qiao et al. 2007).
Results
Detection of LAMP product
The LAMP successfully amplified Toxoplasma DNA under isothermal conditions. Optimum results were observed when the LAMP reaction was performed at 65 °C for 60 min. Agarose gel electrophoresis of the LAMP product showed ladder like patterns with multiple bands (Fig. 1). The direct visualization of LAMP amplicons using ethidium bromide stain was observed under UV transilluminator (302 nm) (Fig. 2a). A detectable yellow color pattern was observed in positive reaction and a red color pattern was shown in negative reaction (Fig. 2b).
Fig. 1.
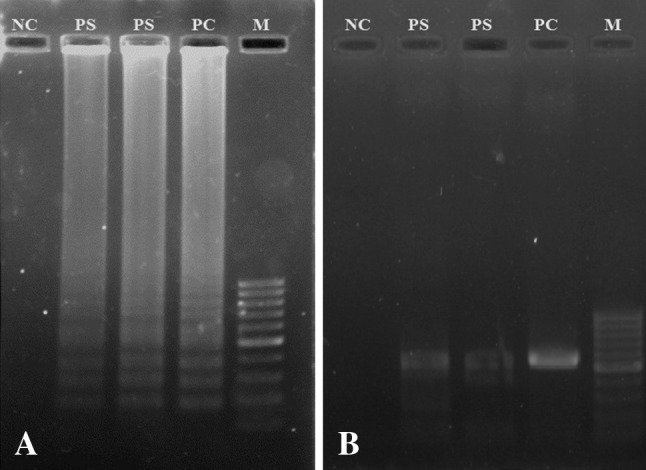
Agarose gel electrophoresis of LAMP (a) and PCR (b) products. Lane M, 100 bp DNA marker; PC positive control, PS positive sample. NC negative control
Fig. 2.
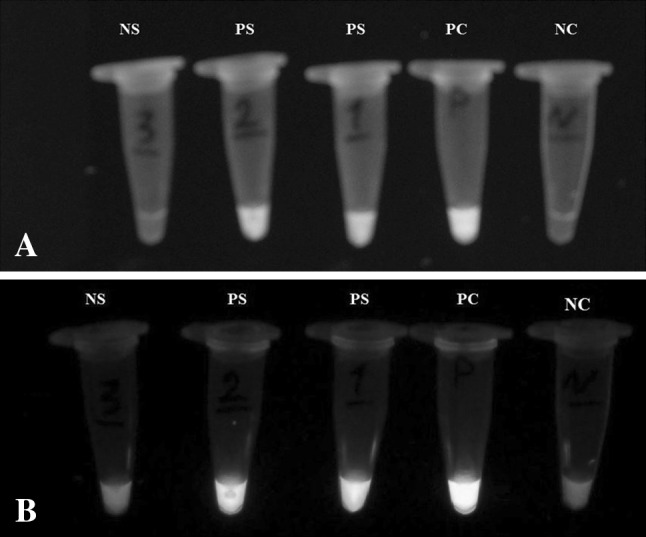
a Visual detection of LAMP under a UV transilluminator (302 nm) with black-with pattern (a), and color pattern (b). PC positive control, PS positive sample. NC negative control
The specificity of LAMP was tested using DNA of other parasites (N. caninum, L. major and T. vaginalis). As a result, the LAMP method was amplified only T. gondii DNA. Conventional PCR targeting the 529-bp repetitive element was also amplified only T. gondii DNA. The detection limit of LAMP was performed using serial dilutions of T. gondii DNA. The detection limits for the LAMP and PCR assays were 1 and 10 pg/μL respectively.
Among the 200 sparrow samples, 17 (8.5 %) and 15 (7.5 %) samples were positive by LAMP and conventional PCR, respectively (Figs. 1, 2). All of the PCR-positive samples were also positive by LAMP.
Discussion
In our study, the LAMP was successfully amplified Toxoplasma DNA at 65 °C for 60 min. We observed that the specificity of LAMP was similar to conventional PCR, while the LAMP and conventional PCR methods amplified only T. gondii DNA and no cross-reaction with non-targeted DNA from other parasites were found. The results showed that the detection limit of LAMP was higher than conventional PCR. While in a serial dilution of T. gondii DNA, the detection limits of the LAMP and PCR assays were 1 and 10 pg/μL respectively. In clinical samples, T. gondii was detected from 8.5 to 7.5 % of 200 sparrows by LAMP and conventional PCR, respectively. To our knowledge, this study is the first report of detection of T. gondii by LAMP method in bird hosts. Several reports were confirmed the higher sensitivity of LAMP for detection of T. gondii than conventional PCR targeting 529 bp fragment of T. gondii genome (Fallahi et al. 2014; Kong et al. 2012; Lin et al. 2012; Zhang et al. 2009) or other specific targeting of T. gondii DNA regions (Fallahi et al. 2014; Hu et al. 2012; Kong et al. 2012; Krasteva et al. 2009; Lau et al. 2010; Lin et al. 2012; Sotiriadou and Karanis 2008; Zhang et al. 2009). Until now, LAMP method was used for detection of T. gondii in pig (Lili et al. 2014; Lin et al. 2012; Qu et al. 2013; Wang et al. 2013; Zhang et al. 2009), sheep (Lin et al. 2012), mice (Hu et al. 2012; Kong et al. 2012; Krasteva et al. 2009), and human (Fallahi et al. 2014; Lau et al. 2010). LAMP was also used for detection of T. gondii oocysts in water samples (Gallas-Lindemann et al. 2013; Koloren and Demirel 2013; Sotiriadou and Karanis 2008).
There are some reports on molecular frequency of T. gondii in bird hosts in Iran. In this regard, (Khademvatan et al. 2013) could detect T. gondii DNA from 15.5 % of 103 free-ranging chickens (Gallus domesticus), 6.9 % of 43 pigeons (Columba livia), 12.8 % of 39 starlings (Sturnus vulgaris), and 26.5 % of 64 sparrows (Passer domesticus) by PCR technique in Khuzestan province (southwest of Iran) (Khademvatan et al. 2013). Zia-Ali et al. (2005) detected T. gondii DNA from six out of 46 free-ranging chickens (13.4 %) and one out of 13 ducks (7.7 %) in Mazandaran province (northern Iran) (Zia-Ali et al. 2005). Asgari et al. (2009) detected anti-Toxoplasma antibodies in 58 out of 231 free-ranging chickens (25.1 %) in Fars province (Southern Iran). Then, 29 seropositive tissue samples were selected for molecular detection of T. gondii by PCR. Out of 29 seropositive tissue samples, T. gondii DNA was detected in 27 livers, 25 brains, and 16 heart samples (Asgari et al. 2009). Sarkari et al. (2014) detected anti-Toxoplasma antibodies in 89.8 % of 54 turkeys and T. gondii DNA in 61.6 % of seropositive turkeys in Fars province (Sarkari et al. 2014). Recently, Tavalla et al. (2013) isolated T. gondii DNA in 8.6 % (13/150) of soil samples in Tehran (Tavalla et al. 2013). Interestingly, this result is very similar to our report with 8.5 % infection in sparrows, and confirms that birds are one of the best indicators for environmental contamination of T. gondii oocysts.
Anti-Toxoplasma antibodies were also detected a range between 2.7 and 86 % among stray cats in different parts of Iran (Derakhshan and Mousavi 2014; Hooshyar et al. 2007; Mosallanejad et al. 2011; Raeghi and Sedeghi 2011; Sharif et al. 2009; Tehrani-sharif et al. 2014). Meat born toxoplasmosis has been considered a major source of T. gondii infection (Kijlstra and Jongert 2009). People in some parts of Iran consume the sparrow meat traditionally. Hence, consumption of sparrow meat may be an important source of T. gondii infection in cats and human in Iran.
Molecular frequency of T. gondii in sparrows was also reported in Brazil (Gondim et al. 2010; Vilela et al. 2011) and China (Cong et al. 2013; Huang et al. 2012). In this regard, Gondim et al. (2010) detected anti-Toxoplasma antibodies in 1.02 % (3/293) and T. gondii DNA from 17.5 % (7/40) of sparrows in the northeast of Brazil (Gondim et al. 2010). Vilela et al. (2011) reported 60.3 % (90/51) T. gondii seropositivity among sparrows in region of Pernambuco, Brazil. They also detected T. gondii DNA from four sparrows by Nested-PCR (Vilela et al. 2011). Cong et al. (2013) detected anti-Toxoplasma antibodies among 12.46 % (39/313) of house sparrows in northwestern China. Moreover, T. gondii DNA was detected in 11 seropositive birds (Cong et al. 2013). In another study in China, T. gondii DNA was detected in four out of 178 wild birds (including 98 pheasants and 80 sparrows) (Huang et al. 2012). In a previous study, T. gondii antibodies were detected in 12.3 % of 227 house sparrows (Passer domesticus) and 4.9 % of 41 tree sparrows (Passer montanus) in Poland and the Czech Republic (Literák et al. 1997). T. gondii was also isolated in 0.5–40 % of sparrows from different regions of Europe [reviewed by (Dubey 2002)]. Experimental infection of house sparrows by oocysts of T. gondii showed a peak of antibody production at 7 weeks post-infection and reduction of the antibody titers at 12 weeks post-infection. These results demonstrated that sparrows do not produce a significant antibody in response to T. gondii (Literák et al. 1999).
Taken together with our results and other reports about high sensitivity and specificity of LAMP, this method can be used as an alternative assay for detection of toxoplasmosis. The prevalence of T. gondii infection in ground-foraging birds (like sparrows) has epidemiological significance, because indicating environmental contamination with T. gondii oocysts. Moreover, infected birds are potential reservoirs for T. gondii transmission to human and cats. Therefore, the results of the present study provided an insight into epidemiological pattern of T. gondii infection in house sparrows in Iran.
Acknowledgments
This work was financially supported by Tarbiat Modares. The authors would like to thank Dr. Pirestani and Dr. Sadraei for their kind assistance.
Conflict of interest
The authors have no conflicts of interest.
References
- Asgari Q, Motazedian M, Esmaeelzadeh B, Kalantari M, Hatam G. The prevalence of toxoplasma infection among free-ranging chickens in Southern Iran using IFA and nested-PCR. Iran J Parasitol. 2009;4(4):29–36. [Google Scholar]
- Biase FH, Franco MM, Goulart LR, Antunes RC. Protocol for extraction of genomic DNA from swine solid tissues. Genet Mol Biol. 2002;25(3):313–315. doi: 10.1590/S1415-47572002000300011. [DOI] [Google Scholar]
- Cong W, et al. Prevalence and genetic characterization of Toxoplasma gondii in house sparrows (Passer domesticus) in Lanzhou. China. Korean J Parasitol. 2013;51(3):363–367. doi: 10.3347/kjp.2013.51.3.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derakhshan M, Mousavi M. Serological survey of antibodies to Toxoplasma gondii in cats, goats, and sheep in Kerman. Iran. Comp Clin Pathol. 2014;23(2):267–268. doi: 10.1007/s00580-012-1605-4. [DOI] [Google Scholar]
- Dubey J. A review of toxoplasmosis in wild birds. Vet Parasitol. 2002;106(2):121–153. doi: 10.1016/S0304-4017(02)00034-1. [DOI] [PubMed] [Google Scholar]
- Dubey J. Toxoplasma gondii infections in chickens (Gallus domesticus): prevalence, clinical disease, diagnosis and public health significance. Zoonoses Public Health. 2010;57(1):60–73. doi: 10.1111/j.1863-2378.2009.01274.x. [DOI] [PubMed] [Google Scholar]
- Fallahi S, Seyyed Tabaei SJ, Pournia Y, Zebardast N, Kazemi B. Comparison of loop-mediated isothermal amplification (LAMP) and nested-PCR assay targeting the RE and B1 gene for detection of Toxoplasma gondii in blood samples of children with leukaemia. Diag Microbiol Infect Dis. 2014;79(3):347–354. doi: 10.1016/j.diagmicrobio.2014.02.014. [DOI] [PubMed] [Google Scholar]
- Farhadian AH. Environment, geography & geology of Tehran. J Appl Environ Biol Sci. 2011;1(9):366–369. [Google Scholar]
- Gallas-Lindemann C, Sotiriadou I, Mahmoodi MR, Karanis P. Detection of Toxoplasma gondii oocysts in different water resources by loop mediated isothermal amplification (LAMP) Acta Trop. 2013;125(2):231–236. doi: 10.1016/j.actatropica.2012.10.007. [DOI] [PubMed] [Google Scholar]
- Gondim LS, et al. Toxoplasma gondii and Neospora caninum in sparrows (Passer domesticus) in the Northeast of Brazil. Vet Parasitol. 2010;168(1):121–124. doi: 10.1016/j.vetpar.2009.09.055. [DOI] [PubMed] [Google Scholar]
- Homan W, Vercammen M, De Braekeleer J, Verschueren H. Identification of a 200-to 300-fold repetitive 529 bp DNA fragment in Toxoplasma gondii, and its use for diagnostic and quantitative PCR. Int J Parasitol. 2000;30(1):69–75. doi: 10.1016/S0020-7519(99)00170-8. [DOI] [PubMed] [Google Scholar]
- Hooshyar H, Rostamkhani P, Talari S, Arbabi M. Toxoplasma gondii infection in stray cats. Iran J Parasitol. 2007;2(1):18–22. [Google Scholar]
- Hu X, Pan C-W, Li Y-F, Wang H, Tan F. Urine sample used for detection of toxoplasma gondii infection by loop-mediated isothermal amplification (LAMP) Folia Parasitol. 2012;59(1):21–26. doi: 10.14411/fp.2012.004. [DOI] [PubMed] [Google Scholar]
- Huang S-Y, et al. First report of genotyping of Toxoplasma gondii isolates from wild birds in China. J Parasitol. 2012;98(3):681–682. doi: 10.1645/GE-3038.1. [DOI] [PubMed] [Google Scholar]
- Khademvatan S, Saki J, Yousefi E, Abdizadeh R. Detection and genotyping of Toxoplasma gondii strains isolated from birds in the southwest of Iran. Br Poult Sci. 2013;54(1):76–80. doi: 10.1080/00071668.2013.763899. [DOI] [PubMed] [Google Scholar]
- Kijlstra A, Jongert E. Toxoplasma-safe meat: close to reality? Trends Parasitol. 2009;25(1):18–22. doi: 10.1016/j.pt.2008.09.008. [DOI] [PubMed] [Google Scholar]
- Koloren Z, Demirel E. Detection of Toxoplasma gondii in Turkish river and drinking water samples by different PCR and LAMP methods. CLEAN–Soil, Air, Water. 2013;41(10):963–968. [Google Scholar]
- Kong Q-M, et al. Loop-mediated isothermal amplification (LAMP): early detection of Toxoplasma gondii infection in mice. Parasit Vectors. 2012 doi: 10.1186/1756-3305-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasteva D, Toubiana M, Hartati S, Kusumawati A, Dubremetz J, Widada J. Development of loop-mediated isothermal amplification (LAMP) as a diagnostic tool of toxoplasmosis. Vet Parasitol. 2009;162(3):327–331. doi: 10.1016/j.vetpar.2009.03.014. [DOI] [PubMed] [Google Scholar]
- Lau YL, Meganathan P, Sonaimuthu P, Thiruvengadam G, Nissapatorn V, Chen Y. Specific, sensitive, and rapid diagnosis of active toxoplasmosis by a loop-mediated isothermal amplification method using blood samples from patients. J Clin Microbiol. 2010;48(10):3698–3702. doi: 10.1128/JCM.00462-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lili C, Cheng R, Lin Y, Shuxian Y, Xinhua Y. Establishment and application of a loop-mediated isothermal amplification method for simple, specific, sensitive and rapid detection of Toxoplasma gondii. J Vet Med Sci. 2014;76(1):9–14. doi: 10.1292/jvms.13-0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Z, Zhang Y, Zhang H, Zhou Y, Cao J, Zhou J. Comparison of loop-mediated isothermal amplification (LAMP) and real-time PCR method targeting a 529-#bp repeat element for diagnosis of toxoplasmosis. Vet Parasitol. 2012;185(2):296–300. doi: 10.1016/j.vetpar.2011.10.016. [DOI] [PubMed] [Google Scholar]
- Literák I, Pinowski J, Anger M, Juřicová Z, Kyu-Hwang H, Romanowski J. Toxoplasma gondii antibodies in house sparrows (Passer domesticus) and tree sparrows (P. montanus) Avian Pathol. 1997;26(4):823–827. doi: 10.1080/03079459708419255. [DOI] [PubMed] [Google Scholar]
- Literák I, Sedlák K, Juricova Z, Pavlásek I. Experimental toxoplasmosis in house sparrows (Passer domesticus) Avian Pathol. 1999;28(4):363–368. doi: 10.1080/03079459994623. [DOI] [PubMed] [Google Scholar]
- Mori Y, Kanda H, Notomi T. Loop-mediated isothermal amplification (LAMP): recent progress in research and development. J Infect Chemother. 2013;19(3):404–411. doi: 10.1007/s10156-013-0590-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosallanejad B, Avizeh R, Razi Jalali M, Pourmehdi M. A study on seroprevalence and coproantigen detection of Toxoplasma gondii in companion cats in Ahvaz area, southwestern Iran. Iran J Vet Res. 2011;12(2):139–144. [Google Scholar]
- Notomi T, et al. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000;28(12):e63. doi: 10.1093/nar/28.12.e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao Y-M, et al. Loop-mediated isothermal amplification for rapid detection of Bacillus anthracis spores. Biotechnol Lett. 2007;29(12):1939–1946. doi: 10.1007/s10529-007-9472-9. [DOI] [PubMed] [Google Scholar]
- Qu D, et al. Development of reverse transcription loop-mediated isothermal amplification (RT-LAMP) as a diagnostic tool of Toxoplasma gondii in pork. Vet Parasitol. 2013;192(1):98–103. doi: 10.1016/j.vetpar.2012.10.010. [DOI] [PubMed] [Google Scholar]
- Raeghi S, Sedeghi S. Prevalence of Toxoplasma gondii antibodies in cats in Urmia, northwest of Iran. J Anim Plant Sci. 2011;21(2):132–134. [Google Scholar]
- Sarkari B, et al. Molecular and serological evaluation of Toxoplasma gondii infection in reared turkeys in Fars province. Iran. Jundishapur J Microbiol. 2014 doi: 10.5812/jjm.11598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharif M, Daryani A, Nasrolahei M, Ziapour SP. Prevalence of Toxoplasma gondii antibodies in stray cats in Sari, northern Iran. Trop Anim Health Prod. 2009;41(2):183–187. doi: 10.1007/s11250-008-9173-y. [DOI] [PubMed] [Google Scholar]
- Sotiriadou I, Karanis P. Evaluation of loop-mediated isothermal amplification for detection of Toxoplasma gondii in water samples and comparative findings by polymerase chain reaction and immunofluorescence test (IFT) Diagn Microbiol Infect Dis. 2008;62(4):357–365. doi: 10.1016/j.diagmicrobio.2008.07.009. [DOI] [PubMed] [Google Scholar]
- Tavalla M, et al. Genotyping of Toxoplasma gondii isolates from soil samples in Tehran, Iran. Iran J Parasitol. 2013;8(2):227. [PMC free article] [PubMed] [Google Scholar]
- Tehran Province (2014) In: http://fa.wikipediaorg/wiki
- Tehrani-sharif M, Jahan S, Alavi SM, Khodami M. Seroprevalence of Toxoplasma gondii antibodies of stray cats in Garmsar, Iran. J Parasit Dis. 2014 doi: 10.1007/s12639-013-0349-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenter AM, Heckeroth AR, Weiss LM. Toxoplasma gondii: from animals to humans. Int J Parasitol. 2000;30(12):1217–1258. doi: 10.1016/S0020-7519(00)00124-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilela SM, et al. Sparrows (Passer domesticus L.) as intermediary hosts of Toxoplasma gondii in poultry farms from the “agreste” region of Pernambuco, Brazil. Pesq Vet Bras. 2011;31(2):169–172. doi: 10.1590/S0100-736X2011000200013. [DOI] [Google Scholar]
- Wang Y, Wang G, Zhang D, Yin H, Wang M. Detection of acute toxoplasmosis in pigs using loop-mediated isothermal amplification and quantitative PCR. Korean J Parasitol. 2013;51(5):573–577. doi: 10.3347/kjp.2013.51.5.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, et al. Toxoplasma gondii: sensitive and rapid detection of infection by loop-mediated isothermal amplification (LAMP) method. Exp Parasitol. 2009;122(1):47–50. doi: 10.1016/j.exppara.2009.01.012. [DOI] [PubMed] [Google Scholar]
- Zia-Ali N, et al. Molecular characterization of Toxoplasma gondii from bird hosts. Iran J Public Health. 2005;34(3):27–30. [Google Scholar]


