Abstract
A total of 2089 faecal samples (956 buffaloes and 1133 cattle) were collected from 21 districts of five major agro-climatic zones of Punjab during April, 2013–May, 2014. An overall prevalence rate of 32.17 % (672/2089) was recorded for coccidiosis in dairy animals with a significantly higher (P < 0.01) infection rate in buffaloes (35.46 %) in comparison to cattle (29.39 %). Quantitative analysis revealed oocyst per gram of faeces ranging from 200 to 10,000 (1083.72 ± 227.20) and 100 to 2400 (748.72 ± 81.38), in cattle and buffalo populations, respectively. The results of multivariate analysis showed that prevalence of coccidiosis in cattle population was associated with various risk factors viz. districts (P = 0.000; OR 2.749; 95 % CI 1.238–6.107), agro-climatic zones (P = 0.004; OR 2.707; 95 % CI 1.707–4.293) and seasons (P = 0.000; OR 1.106; 95 % CI 0.811–1.509). Similarly, in buffalo population an association with districts (P = 0.000; OR 2.163; 95 % CI 0.702–6.668), agro-climatic zones (P = 0.000; OR 1.237; 95 % CI 0.673–2.277) and seasons (P = 0.100; OR 1.382; 95 % CI 1.003–1.903) was recorded. The findings of the current study would provide a basis for evolving effective control strategy for the management of coccidiosis in dairy animals of the region.
Keywords: Coccidiosis, Dairy animals, Prevalence, Punjab, Risk factors
Introduction
Coccidiosis, an important stress induced enteric protozoan parasitic disease affecting several animal species viz. cattle, sheep, goats, buffaloes, rabbits and poultry worldwide (Daugschies and Najdrowski 2005) is caused by microscopic apicomplexan organisms called coccidia. The disease is present in clinical form mostly in younger animals; however, adults may also be affected severely, at times. In comparison to the clinical entity, inapparent or sub-clinical coccidiosis is most frequently encountered and may account for >95 % of the losses. The clinical form of disease is mainly seen in calves and yearlings with symptoms of bloody diarrhoea, weight loss, poor and retarded growth and bodily condition and sometimes mortality. It has been estimated that annual losses due to coccidiosis in cattle and buffaloes approximate $400 million/year (Matjila and Penzhorn 2002).
More than 20 Eimeria spp. have been described in dairy animals, most of which are considered non-pathogenic and mixed infections are generally encountered (Daugschies and Najdrowski 2005). Eimeria bovis and E. zuernii, are particularly more pathogenic to calves and young animals while E. alabamensis has been reported to cause clinical disease in older animals. Further, E. auburnensis and E. ellipsoidalis have also been occasionally observed to cause diarrhoea in adult bovines (Soulsby 1982).
The prevalence of coccidiosis in cattle and buffaloes has been well reported from different parts of world (Yu et al. 2011; Dong et al. 2012) including India (Manya et al. 2008; Laha et al. 2013) but information regarding prevalence of coccidiosis in cattle and buffalo populations from Punjab state seems to be sporadic (Haque et al. 2011; Jyoti et al. 2011; Singh et al. 2012a, b). Moreover, no attempt has been made to determine the species composition of coccidian infection in Punjab. Hence the present study was undertaken to determine the species composition (morphotypes) of coccidian species affecting dairy animals along with the prevalence and assessment of associated risk factors.
Materials and methods
Study area
The state of Punjab extends from latitudes 29°30′N to 32°32′N and longitudes 73°55′E to 76°50′E. It covers a geographical area of 50,362 km2 and lies between altitudes 180 and 300 m above sea level. The climate is hot and dry in summers and winters are cool with some frosts.
Collection of samples
A total of 2089 faecal samples of dairy animals (956 buffaloes and 1133 cattle) from different agro-climatic zones of Punjab state, India were collected directly from the rectum during April, 2013–May, 2014. The samples were placed in sterile polythene bags, properly labeled, kept in a cool transport box and brought to the laboratory for further examination.
Coprological examination
The faecal samples were first subjected to standard qualitative faecal sample examination by using direct smear method and floatation technique for detection of coccidian oocysts. The coccidian parasites were identified on basis of the morphological features of oocysts as described by Soulsby (1982). For quantitative faecal sample examination, standard McMaster’s technique was used to calculate the oocysts per gram (OPG) of faeces (Soulsby 1982).
Sporulation of oocysts
Faecal samples positive for coccidian oocysts were mixed with 2.5 % potassium dichromate in petri dishes and kept in an incubator at a temperature ranging between 25 and 27 °C for sporulation. The dimensions of the oocysts were recorded and species were identified according to their size and morphological characteristics (shape, thickness of the oocyst wall, presence/absence of micropyle, micropylar cap, etc.).
Statistical analysis
All data analyses were performed by using statistical software program (SPSS for Windows, Version 19.0, USA). Association between the prevalence of coccidian infection and various factors was carried out by Chi square (χ2 test). Variables with significant association at P < 0.05 (two-sided) were subjected to the multivariate logistic regression model. The results were each expressed as P value and odds ratio (OR) with a 95 % confidence interval (CI 95 %).
Results
Of the total 2089 faecal samples examined by qualitative faecal examination, 672 were found to be positive for coccidian oocysts, with a prevalence rate of 32.17 %. A significantly higher (P < 0.01) infection rate was recorded in buffaloes (35.46 %) as compared to cattle (29.39 %). Further, quantitative analysis revealed OPG range from 200 to 10,000 (1083.72 ± 227.20) and 100 to 2400 (748.72 ± 81.38), in cattle and buffalo population, respectively.
Prevalence of coccidiosis in different districts of Punjab
A significant variation (P < 0.01) in prevalence of coccidiosis was recorded among the dairy animals of various districts of Punjab state. Highest infection rate was recorded in Ludhiana (56.69 %) and Fatehgarh Sahib (58.82 %) in cattle and buffalo populations, respectively (Table 1). On animal level, districts were found to be associated with coccidiosis in cattle (β = 1.530) and buffalo (β = 2.629) population of Punjab (Table 2). Further, higher infection rates were recorded from districts with higher annual rainfall.
Table 1.
Prevalence of coccidiosis in dairy animals of various districts of Punjab
| Districts | No. of samples (cattle) | No. of samples (buffalo) | ||
|---|---|---|---|---|
| Examined | Positive (%) | Examined | Positive (%) | |
| Amritsar | 49 | 14 (28.57) | 29 | 10 (34.48) |
| Barnala | 31 | 10 (32.26) | 19 | 4 (21.05) |
| Bathinda | 28 | 1 (3.57) | 31 | 10 (32.26) |
| Faridkot | 84 | 19 (22.62) | 37 | 17 (45.94) |
| Fatehgarh Sahib | 57 | 19 (32.20) | 51 | 30 (58.82) |
| Fazilka | 16 | 0 | 11 | 1 (9.09) |
| Ferozpur | 27 | 4 (14.81) | 24 | 4 (16.67) |
| Gurdaspur | 33 | 9 (27.27) | 10 | 3 (30.0) |
| Hoshiarpur | 151 | 31 (20.53) | 62 | 22 (35.48) |
| Jalandhar | 29 | 4 (13.79) | 25 | 10 (40.0) |
| Kapurthala | 16 | 3 (18.75) | 12 | 4 (33.33) |
| Ludhiana | 224 | 127 (56.69) | 328 | 120 (36.58) |
| Mansa | 26 | 0 | 16 | 4 (25.0) |
| Moga | 36 | 12 (33.33) | 30 | 13 (43.33) |
| Mohali | 57 | 14 (24.56) | 15 | 8 (53.33) |
| Muktsar | 32 | 9 (28.12) | 20 | 6 (30.0) |
| Patiala | 79 | 14 (17.72) | 78 | 12 (15.38) |
| Ropar | 13 | 0 | 28 | 10 (35.71) |
| Sangrur | 45 | 6 (13.33) | 17 | 3(17.65) |
| SBS Nagar | 71 | 32 (44.44) | 89 | 40 (44.94) |
| Tarn Taran | 29 | 5 (17.24) | 24 | 8 (33.33) |
| χ2 value | – | 162.898** | – | 49.215** |
| Total | 1133 | 333 (29.39) | 956 | 339 (35.46) |
** P < 0.01
Table 2.
Final logistic regression model for factors associated with the prevalence of coccidiosis in dairy animals on animal level
| Animal | Variable | Regression coefficient (β) | Standard error (SE) | P value | Odds ratio | Confidence interval (95 %) |
|---|---|---|---|---|---|---|
| Cattle | Season | −0.314 | 0.085 | 0.000 | 1.106 | 0.811–1.509 |
| Zone | −0.357 | 0.123 | 0.004 | 2.707 | 1.707–4.293 | |
| District | 1.530 | 0.241 | 0.000 | 2.749 | 1.238–6.107 | |
| Buffalo | Season | −0.156 | 0.094 | 0.100 | 1.382 | 1.003–1.903 |
| Zone | −0.682 | 0.179 | 0.000 | 1.237 | 0.673–2.277 | |
| District | 2.629 | 0.267 | 0.000 | 2.163 | 0.702–6.668 |
Prevalence of coccidiosis in different agro-climatic zones
A significant variation (P < 0.01) was recorded in prevalence of coccidiosis in cattle population from different agro-climatic zones whereas, in buffaloes the variation was non-significant (Table 3). It was observed that prevalence was higher in wet and humid zones whereas, it was lower in hot and dry zones. On animal level, agro-climatic zones were found to be associated with prevalence of coccidiosis in cattle (β = −0.357) and buffalo (β = −0.682) populations (Table 2). A negative value of regression coefficient (β) was recorded between the prevalence of coccidiosis and various agro-climatic zones, when we moved from the zone receiving maximum annual rainfall (submountain undulating zone) towards the one with least annual rainfall (western zone).
Table 3.
Prevalence of coccidiosis in dairy animals of Punjab
| Variables | Number of samples (cattle) | Number of samples (buffalo) | |||
|---|---|---|---|---|---|
| Examined | Positive (%) | Examined | Positive (%) | ||
| Season | |||||
| Summer | 342 | 101 (29.53) | 331 | 123 (37.16) | |
| Rainy | 336 | 107 (31.85) | 278 | 112 (40.29) | |
| Winter | 455 | 125 (27.47) | 347 | 104 (29.97) | |
| χ2 value | – | 1.785 | – | 7.817* | |
| Agro-climatic zone | |||||
| Sub-mountain undulating | 184 | 40 (21.73) | 72 | 25 (34.72) | |
| Undulating plain | 141 | 46 (32.62) | 132 | 58 (43.93) | |
| Central plain | 483 | 186 (38.51) | 547 | 194 (35.47) | |
| Western plain | 127 | 23 (18.11) | 72 | 22 (30.56) | |
| Western | 198 | 38 (19.19) | 133 | 40 (30.08) | |
| χ2 value | – | 42.965** | – | 6.606 | |
| Total | 1133 | 333 (29.39) | 956 | 339 (35.46) | |
* P < 0.05; ** P < 0.01
Seasonal dynamics of coccidiosis in dairy animals
The highest infection rate was recorded in monsoon with a non-significant variation among seasons in cattle population whereas, in buffaloes the variation was significant (P < 0.05) (Table 3). On animal level, seasons were found to be significantly associated with prevalence of coccidiosis in cattle (β = −0.314) and buffalo (β = −0.156) populations (Table 2). The negative β values recorded between the prevalence of coccidiosis and various seasons (summer followed by monsoon and winter) indicated a decreased prevalence of coccidiosis with decrease in ambient temperature (Table 2).
Identification of morphotypes
The microscopic examination of the sporulated oocysts revealed the presence 10 morphotypes of Eimeria species in Punjab state, India namely E. zuernii, E. bovis, E. subspherica, E. cylindrica, E. illinoisensis, E. brasiliensis, E. canadensis, E. ellipsoidalis, E. auburnensis and E. alabamensis on the basis of their size and morphological characters (Fig. 1). Regarding morphological dimension of the oocysts, the size ranged from 11 to 27 µm in width and 11 to 39 µm in height with the most commonly encountered shape as elliptical followed by spherical and oval. Both single and double walled oocysts were found, with or without micropyle depending upon the species.
Fig. 1.
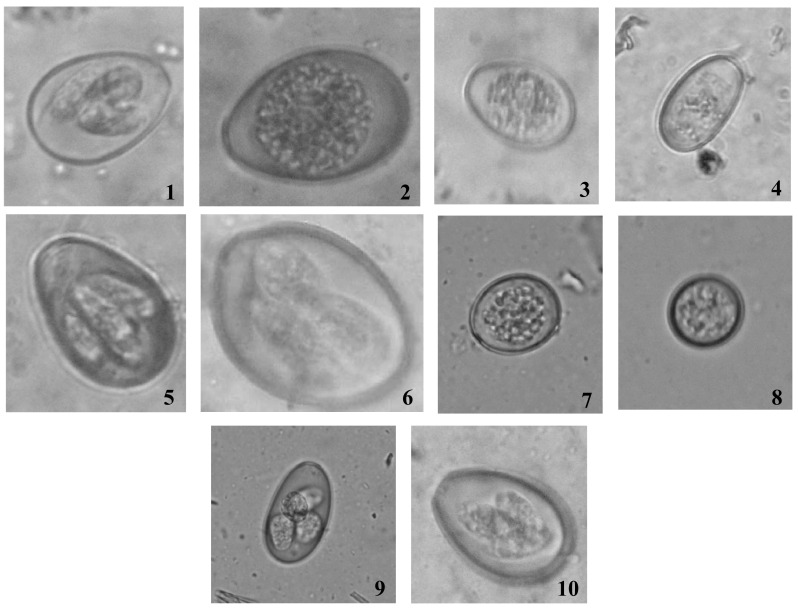
Various morphotypes of Eimeria reported from dairy animals of Punjab 1 = E. illinoisensis; 2 = E. canadensis; 3 = E. ellipsoidalis; 4 = E. cylindrica; 5 = E. auburnensis; 6 = E. brasiliensis; 7 = E. zuernii; 8 = E. subspherica; 9 = E. alabamensis; 10 = E. bovis
Discussion
Coccidiosis is prevalent in dairy animals worldwide affecting young calves particularly, where it results in heavy mortality, if untreated. The adult animals generally suffer from sub-clinical form of disease and act as a source of infection to young and/or newly added stock. A variable prevalence rate of coccidiosis in adult dairy animals has been reported by several workers worldwide, including India (Muraleedharan 2005; Manya et al. 2008; Yu et al. 2011; Dong et al. 2012; Gupta et al. 2012; Laha et al. 2013). Recent studies from Punjab state reported a prevalence rate of 3.77 % (Singh et al. 2012a) and 0.95 % (Singh et al. 2012b) from cattle and buffalo populations, respectively. However, a comparatively higher prevalence rates have also been on the record (Haque et al. 2011).
The present study revealed 35.46 % buffaloes and 29.39 % cattle positive for coccidian oocysts with an overall prevalence of 32.17 %. The findings of the present study are in close agreement with that of Yu et al. (2011) who reported 34.94 % positivity for coccidian infections. Further, a high prevalence rate of 75.0 % has been reported in adult buffaloes from Turkey (Nalbantoglu et al. 2008) who also mentioned about the presence of novel Isospora spp. oocyst in buffaloes. Similarly, Jyoti et al. (2011) from the same region reported 44.18 % prevalence of coccidia in cow calves which is comparatively higher than present study as young animals are immunologically naive and initially unable to prevent parasite establishment and reproduction and adaptive immunity to coccidian infection is more effective in adults (Stewart and Penzhorn 2004). However, the variations regarding prevalence in various studies can most likely be attributed to the differences in agro-ecology, management, sample size, time of sampling and husbandry practices of the study animals in different countries.
In the present study, highest infection rates were encountered in rainy season, followed by summer and least in winters. These findings are in agreement to that reported by Manya et al. (2008) who suggested that higher humidity and temperature in rainy season and post-monsoon season were conducive for optimum sporulation of the oocysts. Further, various workers, have suggested that reduced immune-tolerance in rainy season may also be responsible for the higher incidence of coccidiosis during monsoon (Muraleedharan 2005). High prevalence of coccidian infection in central plain zone and undulating plain zone may be attributed to the fact that these parts receive maximum rainfall in Punjab especially in monsoon, leading to favorable conditions in terms of temperature and moisture for growth and perpetuation of coccidia. Lower infection rates in western plain zone and western zone may be due to the fact that these areas are drier and hot which leads to the desiccation of the oocysts and hence make the conditions unfavourable for the growth and development of coccidia.
Higher prevalence rate of coccidiosis in buffaloes similar to current study had been reported in the past (Manya et al. 2008; Gupta et al. 2011) and may be due to warm and wet environmental conditions favoured by buffaloes which also proves conducive for the development of pre-parasitic free living stages of these parasites (Muraleedharan 2005). Further, the feeding habits and habitats of buffaloes and non adoption of prophylactic measures against coccidiosis which is never a part of regular deworming for calves in field conditions contribute heavily towards the high prevalence rates of coccidiosis in buffaloes (Bilal et al. 2009).
In the current study, 10 morphotypes of coccidia viz. E. zuernii, E. bovis, E. subspherica, E. cylindrica, E. illinoisensis, E. brasiliensis, E. canadensis, E. ellipsoidalis, E. auburnensis and E. alabamensis were recorded. Around 17 species of coccidian species had been reported from dairy animals around the world (Nalbantoglu et al. 2008). Similarly, 4–9 morphotypes of Eimeria have been reported from cattle and buffaloes from India (Manya et al. 2008). Past reports indicate E. zuernii, E. bovis, E. albamensis, E. ellipsoidalis as the most common species which are in congruent with the results of present study.
Acknowledgments
The authors are thankful to The Director of Research-cum-Dean, Postgraduate Studies, GADVASU, Ludhiana for providing facilities to carry out the research work.
Conflict of interest
We declare that we have no conflict of interest.
References
- Bilal MQ, Hameed A, Ahmad T. Prevalence of gastrointestinal parasites in buffalo and cow calves in rural areas of Toba Tek Singh, Pakistan. J Anim Plant Sci. 2009;19:67–70. [Google Scholar]
- Daugschies A, Najdrowski M. Eimeriosis in cattle: current understanding. J Vet Med. 2005;52:417–427. doi: 10.1111/j.1439-0450.2005.00894.x. [DOI] [PubMed] [Google Scholar]
- Dong H, Qiping Z, Hongyu H, Lianlian J, Shunhai Z, Ting LI, Kong C, Huang B. Prevalence of coccidial infection in dairy cattle in shanghai, China. J Parasitol. 2012;5:963–966. doi: 10.1645/GE-2966.1. [DOI] [PubMed] [Google Scholar]
- Gupta A, Dixit AK, Dixit P. Incidence of gastrointestinal parasites in dairy animals in Jabalpur. Vet Pract. 2011;12:251–252. [Google Scholar]
- Gupta A, Dixit AK, Dixit P, Mahajan C. Prevalence of gastrointestinal parasites in cattle and buffaloes in and around Jabalpur, Madhya Pradesh. J Vet Parasitol. 2012;26:186–188. [Google Scholar]
- Haque M, Jyothi, Singh NK, Juyal PD, Singh H, Singh R, Rath SS. Incidence of gastrointestinal parasites in dairy animals of western plains of Punjab. J Vet Parasitol. 2011;25:168–170. [Google Scholar]
- Jyothi, Singh NK, Juyal PD, Haque M, Rath SS. Epidemiology and transmission factors of gastrointestinal parasites in cow calves in different agro-climatic zones of Punjab. J Vet Parasitol. 2011;25:46–49. [Google Scholar]
- Laha R, Das M, Goswami A. Gastrointestinal parasitic infections in organized cattle farms of Meghalaya. Vet World. 2013;6:109–112. doi: 10.5455/vetworld.2013.109-112. [DOI] [Google Scholar]
- Manya P, Sinha SRP, Sinha S, Verma SB, Sharma SK, Mandal KG. Prevalence of bovine coccidiosis at Patna. J Vet Parasitol. 2008;22:73–76. [Google Scholar]
- Matjila PT, Penzhorn BL. Occurrence and diversity of bovine coccidia at three localities in South Africa. Vet Parasitol. 2002;104:93–102. doi: 10.1016/S0304-4017(01)00605-7. [DOI] [PubMed] [Google Scholar]
- Muraleedharan K. Prevalence of gastroinestinal parasites of livestock in a central dry zone of Karnataka. J Vet Parasitol. 2005;19:31–33. [Google Scholar]
- Nalbantoglu S, Sari B, Cicek H, Karaer Z. Prevalence of coccidian species in the water buffalo (Bubalus bubalis) in the province of Afyon, Turkey. Acta Vet Brno. 2008;77:111–116. doi: 10.2754/avb200877010111. [DOI] [Google Scholar]
- Singh NK, Singh H, Haque M, Jyothi, Rath SS. Prevalence of parasitic infections in cattle of Ludhiana district, Punjab. J Parasit Dis. 2012;36:256–259. doi: 10.1007/s12639-012-0119-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh NK, Singh H, Haque M, Jyothi, Rath SS. Prevalence of parasitic infections in buffaloes in and around Ludhiana district, Punjab, India: a Preliminary Study. J Buffalo Sci. 2012;1:113–115. [Google Scholar]
- Soulsby EJL. Helminths, arthropods and protozoa of domesticated animals. 7. London: ELBS Bailliere Tindall; 1982. [Google Scholar]
- Stewart CG, Penzhorn BL. Infectious diseases of livestock. 2. Cape Town: Oxford University Press; 2004. [Google Scholar]
- Yu SK, Gao M, Huang N, Jia YQ, Lin Q. Prevalence of coccidial infection in cattle in Shaanxi Province, Northwestern China. J Anim Vet Adv. 2011;10:2716–2719. [Google Scholar]


