Abstract
The prevalence of gastrointestinal helminths of local chickens in Nsukka region of Southeastern Nigeria was studied using 125 free range local birds purchased from four communities in Nsukka zone namely, Obollo-afor, Orba, Nsukka urban and Owerre Eze-orba. The birds were sacrificed humanely and their oesophagus, crop, proventriculus, gizzard, small intestine and caecum examined for the presence of gastrointestinal helminths. Worms when present were isolated and identified using standard parasitological procedures. The study identified four species of cestodes namely Raillietina echinobothridia, R. tetragona, R. cesticillus and Choanotaenia infundibulum and two species of nematodes namely, Ascaridia galli and Heterakis gallinarum. Results obtained showed 96.8 % prevalence of gastrointestinal helminth parasites in the birds with cestodes being the more prevalent class (70.4 %). Raillietina spp was the most prevalent cestode encountered and A. galli the most prevalent nematode. Prevalence rates of infections recorded 14.4 % for nematode species, 26.4 % for cestodes and 56 % for mixed infections of nematodes and cestodes. It was concluded that local chickens are common in the area and could serve as a potential source of helminth infections to intensively managed birds in the study area.
Keywords: Cestodes, Nematodes, Gastrointestinal parasites, Prevalence, Local chicken, Nigeria
Introduction
The poultry industry occupies an important position in the provision of animal protein (meat and egg) to man and generally plays a vital role in the national economy as a revenue provider (Obiora 1992). Given the protein demand and supply situation in developing countries such as Nigeria, with demand greatly surpassing supply, the poultry industry is one of the pillars on which the future of improved protein supply rests. In most areas of the world including Nigeria, poultry production is very much separated into intensive and free range management systems. The commercial poultry sector has poultry farms that may or may not be mechanized but are usually large scale and deals with improved/exotic breeds of poultry with an intensive system of management. The free range poultry is on the other hand un-mechanized and in most cases, the birds are left to roam from place to place scavenging during the daytime to obtain their feed, majority of which are insects and seeds.
The local chickens are reared free range in the tropics (Perimin et al. 1997) with little or no supplementary feeding and veterinary care (Fakae et al. 1991). Besides being an important source of income and a cheap source of protein to the village/rural people, the free range poultry is an integral part of village life, and has an important social value (Ikpi and Akiniwumi 1981; Dessie and Ogle 2001). Obioha et al. (1983) noted that the meat of local chicken is much preferred locally than those of the exotic breeds. Parasitism ranks high among factors that threaten local chicken production (Adene and Dipeolu 1975; Minga et al. 1989; Ojok 1993), and usually leads to lowered productivity. Helminth parasitic infections, though less obvious than those of viral, protozoal and bacteria have been shown to cause major economic losses in free range poultry due to their very high prevalence. Studies in Nigeria and other countries of Africa have shown that the prevalence of gastrointestinal parasitic infestation in village chicken flocks is close to 100 %, and individual birds may harbor more than one parasite type (Permin et al. 2002; Phiri et al. 2007).
Given that the prevalence of helminth parasites vary considerably from one geographic region to another (Oliveira-Sequeira et al. 2002), it is therefore, necessary for periodic surveillance of the prevalence of these parasites within a given locality for successful formulation and implementation of an effective worm control strategy. Available literature on the prevalence of GI helminth infection of local chicken in Enugu state is limited to works done by Fakae and Nwalusi (2001), and Nnadi and George (2010). This study was therefore designed to identify and determine the prevalence of gastrointestinal helminths affecting free range chickens from different localities in the Nsukka region of Enugu state. It is expected that data obtained from this study will update the existing ones and assist in making objective decisions in formulating control strategies.
Materials and methods
Animals
A total of 125 free range local chickens comprising of both sexes and aged 7–9 months were used for the study. They were bought from four communities in Nsukka area of Enugu state, Southeastern Nigeria namely, Obollo-afor, Orba, Nsukka urban and Owerre Eze-orba. Twenty-five chickens were purchased from each community except Nsukka where 50 chickens were bought. Not more than five chickens were bought from a particular seller. The local chickens were taken to and housed in the Department of Veterinary Parasitology and Entomology animal house in covered hand woven baskets until when used for the study.
Worm identification
The chickens were humanely slaughtered by cervical dislocation and their gastrointestinal tracts dissected out. These were separated into oesophagus, crop, proventriculus, gizzard, small intestine and caecum and their contents emptied into their respectively labeled beaker. The contents were washed into a petri dish and examined under a stereomicroscope and their worms recovered as described by Hansen and Perry (1994). The recovered worms were preserved in bottles containing 10 % formal-saline solution. The recovered worms were identified under light microscope by observation of their distinctive morphological features as described by Soulsby (1982). The worms from each community were individually counted after identification.
Handling of experimental animals
Ethical clearance and valid approval were obtained from the University of Nigeria, Nsukka Ethics Committee for Medical and Scientific Research (MSR) before the commencement of the experiment.
Statistical analysis
Data obtained from the prevalence study were analyzed using descriptive statistics and the results summarized as percentages. Students’ T test was used to analyse the difference between the prevalence of cestodes and nematodes. One way analysis of variance (ANOVA) was used to analyse the data generated from worm counts and variant mean separated by the Duncan’s multiple range test. Probabilities (P) of 0.05 or less were considered significant.
Results
The overall prevalence of gastrointestinal helminths in the study area as shown in Table 1 was 96.8 % (121/125). The results as presented in Table 1 also showed that all the birds from Obollo-Afor and Owerri-Ezoba were infected with one or more GI helminth parasite while 24 out of 25 (96 %) and 47 out of 50 (94 %) birds from Orba and Nsukka respectively were infected. Also in Table 1, the prevalence rates of chicken with single cestode and nematode infections were 68.6 and 16.5 % respectively while 14.9 % of the chicken had mixed cestode and nematode infections. There was no significant (P > 0.05) difference in the mean nematode and cestode counts of the chickens from the various communities sampled as shown in Table 2.
Table 1.
Prevalence of nematode and cestode helminths in local chickens from various communities in Nsukka zone of Enugu state
| Communities | Number of chicken sampled | Prevalence of helminths (%) | Prevalence of cestodes only (%) | Prevalence of nematodes only (%) | Prevalence of mixed infection (%) |
|---|---|---|---|---|---|
| Orba | 25 | 24 (96.0) | 17 (70.9) | 4 (16.6) | 3 (12.5) |
| Obollo-afor | 25 | 25 (100) | 13 (52.0) | 6 (24.0) | 6 (24) |
| Owerre eze-orba | 25 | 25 (100) | 15 (60.0) | 4 (16.0) | 6 (24) |
| Nsukka urban | 50 | 47 (94.0) | 38 (80.8) | 6 (12.8) | 3 (6.4) |
| Overall | 125 | 121 (96.8) | 83 (68.6) | 20 (16.5) | 18 (14.9) |
Table 2.
Mean worm counts of helminthes found in free range chickens from Nsukka region of Enugu state
| Helminth spp/communities | Nematodes | Cestodes | ||
|---|---|---|---|---|
| A. galli | H. gallinarum | Raillietina spp | C. infundibulum | |
| Orba | 12.40 ± 3.10 | 6.7 ± 2.30 | 39.00 ± 17.40 | 1.30 ± 0.91 |
| Obollo-afor | 15.00 ± 6.00 | 5.1 ± 1.20 | 26.00 ± 15.10 | 0.25 ± 0.11 |
| Owerre eze-orba | 12.00 ± 3.00 | 10.3 ± 2.80 | 38.00 ± 11.40 | 1.24 ± 0.22 |
| Nsukka urban | 11.00 ± 4.30 | 10.7 ± 4.12 | 36.00 ± 13.90 | 3.40 ± 1.31 |
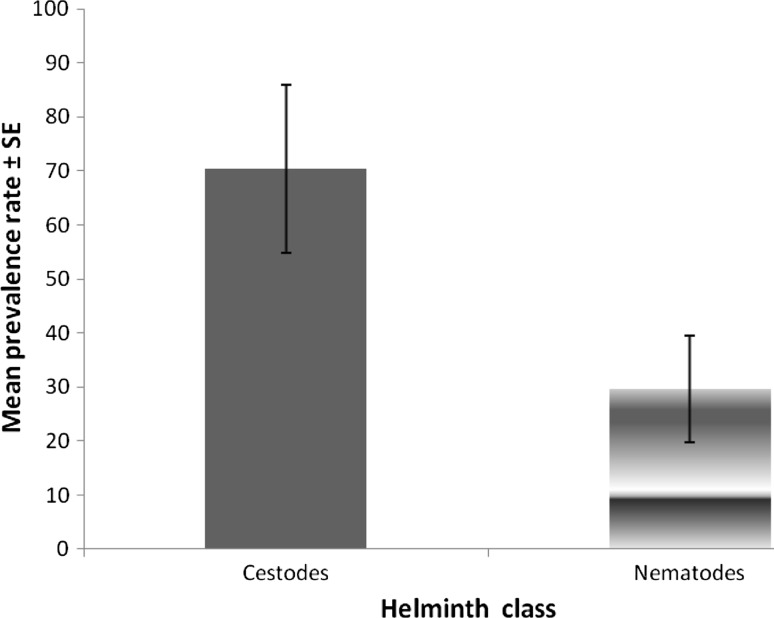
Four cestode species namely, Raillietina echinobothridia (64.5 %), R. tetragona (49.6 %), R. cesticillus (4.9 %), C. infundibulum (9.9 %) and two nematode species namely, A. galli (22.3 %) and H. gallinarum (12.4 %) were identified in the study as shown in Table 3. The prevalence rate of cestode parasites in the chickens was 70.4 % while nematodes had a prevalence of 29.6 % as shown in Fig. 1. Analysis by Independent sample T test showed that the difference was significant (P = 0.03).
Table 3.
Prevalence of cestodes and nematodes in examined in free range local chickens from from Nsukka region of Enugu state
| Helminth species | Number of chicken sampled | Prevalence (%) |
|---|---|---|
| Raillietina echinobothridia | 125 | 78 (64.5) |
| Raillietina tetragona | 125 | 60 (49.9) |
| Raillietina cesticillus | 125 | 6 (4.9) |
| Choanotaenia infundibulum | 125 | 12 (9.9) |
| Heterakis gallinarum | 125 | 15 (12.4) |
| Ascaridia galli | 125 | 27 (22.3) |
Fig. 1.
Mean prevalence rates of helminths in free range local chicken in Nsukka region of Enugu state
Discussion
The results of this study showed that free range local chickens in Nsukka area of Enugu state are commonly infected with a variety of gastro-intestinal helminth parasites. The results also showed that the prevalence and mean worm counts recorded in the various communities were comparable. According to the study, the overall prevalence of free range local chicken with one or more GI helminth parasite species was 96.8 % which is comparable with 96.3 % recorded by Fakae and Nwalusi (2001) but much higher than 35.5 % by Nnadi and George (2010) in the same study area. The prevalence is also a little higher than 90.2 % recorded by Matur et al. (2010) in Abuja, North Central Nigeria, 87.8 % by Yoriyo et al. (2008) in Bauchi, North East Nigeria and 81.5 % by Junaidu et al. (2014) in Kaduna, North West Nigeria. The high prevalence observed in this study among the local chickens is believed to be associated with the free range nature of the local birds which roam from place to place in search of food by scavenging on superficial layer of the soil which contains various arthropods and earthworms that serve as the intermediate and paratenic hosts for most helminthes of poultry (Soulsby 1982). The birds used in this study were mostly raised traditionally under extensive management with little or no supplementary feeding and without veterinary care. The occurrence of helminth infection among the chicken at such a high level is an indication of high availability of infective stages of the worms in the study area and conditions necessary for the survival of the preparasitic stages.
Four cestode species namely, Raillietina echinobothridia, R. tetragona, R. cesticillus and Choanotaenia infundibulum and two nematode species, Ascaridia galli and Heterakis gallinarum were encountered in this study following worm identification and count. R. echinobothridia had the highest prevalence (64.5 %) followed closely by R. tetragona (49.9 %). This agrees with the report of Yoriyo et al. (2008) who recorded prevalence rates of 42.0 and 38.5 % for R. echinobothridia and R. tetragona respectively. It is also in agreement with 63.7 and 56.5 % respectively reported for the two species by Hussen et al. (2012) in Ethiopia. Other workers have also reported R. echinobothridia as the most prevalent helminths among local chickens (Amin-Babjee et al. 1997; Eshetu et al. 2001; Rahman et al. 2009). Conversely, Fakae and Nwalusi (2001) and Junaidu et al. (2014) identified R. tetragona as the most prevalent GI helminth of local chickens in their studies. Nnadi and George (2010) identified a prevalence of 16.13 % for Raillietina spp while Ngongeh et al. 2012 did not identify any cestode in their studies on parasites of local chickens in Nsukka and Umuahia areas of South eastern Nigeria respectively. It is believed that the low prevalence rate of cestodes observed by Nnadi and George (2010) and its absence in the report of Ngongeh et al. (2012) could be because these researchers based their prevalence on identification and counts of helminths ova in faeces rather than actual identification and counts of worms in their predilection sites as is the case with the present study and other studies mentioned above. It is also possible that the flotation medium (saturated salt solution) used by these researchers in their studies was not sensitive in identifying cestode eggs. Floatation media with higher specific gravities such as saturated solutions of magnesium sulphate or sodium nitrate are known to yield higher numbers of cestode eggs and therefore prove to be more sensitive (Sloss et al. 1994).
The results of the present study identified A. galli as the most prevalent nematode and third most prevalent helminth species in the local chickens with a prevalence rate of 22.3 % followed by H. gallinarum (12.4 %) which ranked 4th in the overall prevalence. This finding agrees in part with several studies (Fakae and Nwalusi 2001; Nnadi and George 2010; Matur et al. 2010; Ngongeh et al. 2012) in Nigeria which identified A. galli as the most prevalent nematode in chickens but disagrees with them on the basis of being the most prevalent helminth species. Rahman et al. (2009) observed that infection rates in nematodes depend on many factors namely, rainfall pattern, soil type, locality and the types of food given to the chickens which vary from place to place. The variations in these factors may explain the differences observed between the prevalence of nematode infections by various researchers.
The majority of the species identified in this study have been reported as potentially pathogenic for poultry, inducing ulcerations and nodule formations and varying degrees of enteritis leading to diarrhoea, anorexia, depression, emaciation and death if untreated (Soulsby 1982). Also, such parasitized free-range birds can be sources of infections to more intensively managed poultry through contaminated equipments and animal handlers. In addition, the finding of H. gallinarum in this study is particularly significant due to its association with Histomonas meleagridis, the causal agent of blackhead of poultry especially in domesticated turkey (Soulsby 1982); as such these birds could act as reservoirs of the disease. Therefore, the results obtained from this work points alarmingly to the possible economic losses that may arise from these high levels of infection among free range local chickens.
Based on the results of our study, we conclude that helminth infections are very common among free-range local chicken in Nsukka area of Southeastern Nigeria with their consequent effects on productivity. We therefore, recommend that an epidemiologically structured worm control programme be put in place to minimize worm infestation and its effects in order to maximize the potentials of local chickens and backyard poultry.
Acknowledgments
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Adene DF, Dipeolu OO. Survey of blood and ectoparasites of domestic fowls in Ibadan, western state of Nigeria. Bull Anim Health Prod Afr. 1975;23:333–335. [Google Scholar]
- Amin-Babjee SM, Lee CC, Mahmood AA. Prevalence of cestodes and nematodes in different age groups of village chickens. Malays Vet J. 1997;9:61–65. [Google Scholar]
- Dessie T, Ogle B. Village poultry production systems in the central highlands of Ethiopia. Trop Anim Health Prod. 2001;33:521–537. doi: 10.1023/A:1012740832558. [DOI] [PubMed] [Google Scholar]
- Eshetu Y, Mulualem E, Ibrahim H, Berhanu A, Aberra K. Study of gastrointestinal helminthes of scavenging chicken in four districts of Amhara Region, Ethiopia. Rev Sci Tech. 2001;20:791–796. doi: 10.20506/rst.20.3.1310. [DOI] [PubMed] [Google Scholar]
- Fakae BB, Nwalusi CU (2001) Rainy season helminths infections in the domestic fowl (Gallus gallus domesticus) in eastern Nigeria. Book of proceedings 25th Annual NSAP conference Umudike, 19–23 March 2001, pp 292–295
- Fakae BB, Umeorizu JM, Orajaka LSE. Gastro-intestinal helminth infection of the domestic fowl (Gallus gallus domesticus) during the dry season in eastern Nigerian. J Afr Zool. 1991;105:503–508. [Google Scholar]
- Hansen J, Perry B (1994) The epidemiology, diagnosis and control of helminth parasites of ruminants. ILRAD, Nairobi. Xt. p 171
- Hussen H, Chaka H, Deneke Y, Bitew M. Gastrointestinal helminthes are highly prevalent in scavenging chickens of selected districts of eastern Shewa zone Ethiopia. Pak J Biol Sci. 2012;15(6):284–289. doi: 10.3923/pjbs.2012.284.289. [DOI] [PubMed] [Google Scholar]
- Ikpi A, Akiniwumi J. The future of the poultry industry in Nigeria. World Poult Sci J. 1981;37(1):39–43. doi: 10.1079/WPS19810005. [DOI] [Google Scholar]
- Junaidu HI, Luka SA, Mijinyawa A. Prevalence of gastrointestinal helminth parasites of the domestic fowl (Gallus-gallus domesticus) slaughtered in Giwa market, Giwa local government, area, Kaduna state, Nigeria. J Nat Sci Res. 2014;4(19):120–125. [Google Scholar]
- Matur BM, Dawam NN, Malann YD. Gastrointestinal helminth parasites of local and exotic chickens slaughtered in Gwagwalada, Abuja (FCT), Nigeria. NY Sci J. 2010;3(5):96–99. [Google Scholar]
- Minga UM, Katule AM, Maeda T, Musasa J (1989) Potential and problems of the traditional chicken industry in Tanzania. Proceedings of the 7th Tanzanian Veterinary Scientific Conference, Arusha, vol 3–5, pp 207–215
- Ngongeh LA, Onyeabor A, Erumaka IG. Prevalence of gastrointestinal parasites in free-ranged and intensively reared chickens in Umuahia area in south eastern Nigeria. J Sustain Agric Environ. 2012;13:125–136. [Google Scholar]
- Nnadi PA, George SO (2010) A cross sectional survey on parasites of chickens in selected villages in the sub-humid zones of South-eastern Nigeria. J Parasitol Res 2010:1–6 [DOI] [PMC free article] [PubMed]
- Obioha FC, Nwosu CC, Gown F, Etim DE, Obanu ZA, Ihemelandu E, Onuora GI. Comparative meat yield and anthropometric indices of the Nigerian Chicken and exotic strain. World Rev Anim Prod. 1983;9:59–64. [Google Scholar]
- Obiora FC. A guide to poultry Production in the Tropics. 1. Enugu: Acena Publishers; 1992. [Google Scholar]
- Ojok L. Diseases as important factor affecting increased poultry production in Uganda. Der Tropenland Wirt, Zeitschrift fur die Landwirtschaft in den Tropen und Subtropen. 1993;94:37–44. [Google Scholar]
- Oliveira-Sequeira TC, Amarante AF, Ferrari TB, Nunes LC. Prevalence of intestinal parasites in dogs from Sao Paulo state. Braz Vet Parasitol. 2002;103:19–27. doi: 10.1016/S0304-4017(01)00575-1. [DOI] [PubMed] [Google Scholar]
- Perimin A, Magwisha H, Kassuku AA, Nansen P, Bisgaard M, Frandsen F, Gibbons L. A cross-sectional study of helminthes in rural scavenging poultry in Tanzania in relation to season and climate. J Helminthol. 1997;71:233–240. doi: 10.1017/S0022149X00015972. [DOI] [PubMed] [Google Scholar]
- Permin A, Esmann CH, Hove T, Mukaratirwa S. Ecto-, endo- and haemoparasites in free-range chickens in the Goronmonzi district in Zimbabwe. Prev Vet Med. 2002;54:213–224. doi: 10.1016/S0167-5877(02)00024-7. [DOI] [PubMed] [Google Scholar]
- Phiri IK, Phiri AM, Ziela M, Chota A, Masuka M, Monrad J. Prevalence and distribution and distribution of gastrointestinal helminths and their effects on weight gain in free range chicken in Central Zambia. Trop Anim Health Prod. 2007;39:309–315. doi: 10.1007/s11250-007-9021-5. [DOI] [PubMed] [Google Scholar]
- Rahman AW, Salim H, Ghause MS. Helminthic parasites of scavenging chickens (Gallus domesticus) from villages in Penang Island, Malaysia. Trop Life Sci Res. 2009;20(1):1–6. [Google Scholar]
- Sloss MW, Kemp RL, Zajac AM. Veterinary clinical parasitology. 6. Iowa: Iowa State University Press; 1994. [Google Scholar]
- Soulsby EJ. Helminth, arthropods and protozoa of domesticated animals. 7. London: Bailliere Tindall; 1982. [Google Scholar]
- Yoriyo KL, Adang J, Fabiyi P, Adamu SU. Helminth parasites of local chicken in Bauchi state Nigeria. Sci World J. 2008;3(2):35–37. [Google Scholar]