Abstract
Nematode worms are among the most ubiquitous organisms on earth. They include free-living forms as well as parasites of plants, insects, humans and other animals. Recently, there has been an explosion of interest in nematode biology, including the area of nematode ultrastructure. Nematodes are round with a body cavity. They have one way guts with a mouth at one end and an anus at the other. They have a pseudocoelom that is lined on one side with mesoderm and on the other side with endoderm. It appears that the cuticle is a very complex and evolutionarily plastic feature with important functions involving protection, body movement and maintaining shape. They only have longitudinal muscles so; they seem to thrash back and forth. While nematodes have digestive, reproductive, nervous and excretory systems, they do not have discrete circulatory or respiratory systems. Nematodes use chemosensory and mechanosensory neurons embedded in the cuticle to orient and respond to a wide range of environmental stimuli. Adults are made up of roughly 1000 somatic cells and hundreds of those cells are typically associated with the reproductive systems. Nematodes ultrastructure seeks to provide studies which enable their use as models for diverse biological processes including; human diseases, immunity, host-parasitic interactions and the expression of phylogenomics. The latter has, however, not been brought into a single inclusive entity. Consequently, in the current review we tried to provide a comprehensive approach to the current knowledge available for nematodes ultrastructures.
Keywords: Nematodes, Ultrastructure, Electron microscope
Introduction
Nematodes have been in focus since the 1990s when the free-living bacteriovore Caenorhabditis elegansbecame was the first multicellular organism to have a fully sequenced genome (Aboobaker and Blaxter 2000). This provided an important potential for exploring other nematode species, but it was an additional decade before it became accessible for the parasitic nematode Brugia malayi (Aguinaldo et al. 1997). They dwell in marine, freshwater and terrestrial surroundings. Some of them are useful in soil mineralization while, others cause plant diseases (Du four et al. 2003).
Mutually with arthropods, nematodes are included in the most diverse taxa in the animal kingdom with a reasonable global estimate of more than 1 million species (Scheffers et al. 2012). Nematodes were categorized with a variable assembly of animals gathered according to their generally worm-like exterior, uncomplicated construction of an internal body cavity called a pseudocoelom, the deficiency of cilia and a well-defined head (Storer et al. 1979; Buchsbaum et al. 1987; Aguinaldo et al. 1997). These nematodes, variously known as Aschelminths or Pseudocoelomata, have resulted from simplification from a more composite body design of more than one group of ancestral organisms (Wallace et al. 1996).
Nematodes in the animal tree of life are actually related to the arthropods and priapulids in a newly recognized clade of molting organisms, the Ecdysozoa (Telford et al. 2008). In helminthes, all morphological aspects must be studied under light microscope (LM), because these structures are very important in the context of their systematic classification (González and Hamann 2010). Even so, early observations by LM described nematodes as a tube within a tube, lacking eyes, appendages, true segmentation and comprised of nearly 1000 somatic cells in their fully developed stage. Nematodes use chemosensory and mechanosensory neurons embedded in the cuticle to orient and respond to a wide range of environmental stimuli (Neher and Powers 2005). However, owing to the limitations of LM, the introduction of electron microscopy has expanded the elucidation of nematodes to a broad range of small nematodes and revealed the presence of numerous nematode organelles that have never been distinguished by the LM (Bird and Bird 1991). In studying nematodes organization, the value of the electron microscope lies in its capacity to provide three-dimensional descriptions with high magnification that allow recognition of the spatial associations among nematodes structures. It could be used to split groups that are morphologically identical when examined under LM, validate species and display variations between populations or races (Gibbons 1986). Even though frequent literatures reported that nematodes have a conserved morphology, in fact, the contrary is accurate that species of the phylum Nemata are truly morphologically inconsistent. Consequently, this review represents an attempt of evaluating the organization of nematodes soft-tissues in order to relate their ultrastructures to their functional specialization, behavior in the host micro-environment and immunocytochemical characterization.
Body wall of nematodes
The nematode body wall is composed of a cuticle and a single layer of longitudinal muscle cells (Fig. 1). These are separated by a thin sheet of hypodermis (epidermis) that expands medially at intervals around the circumference to form the hypodermal cords (Gaugler and Bilgrami 2004).
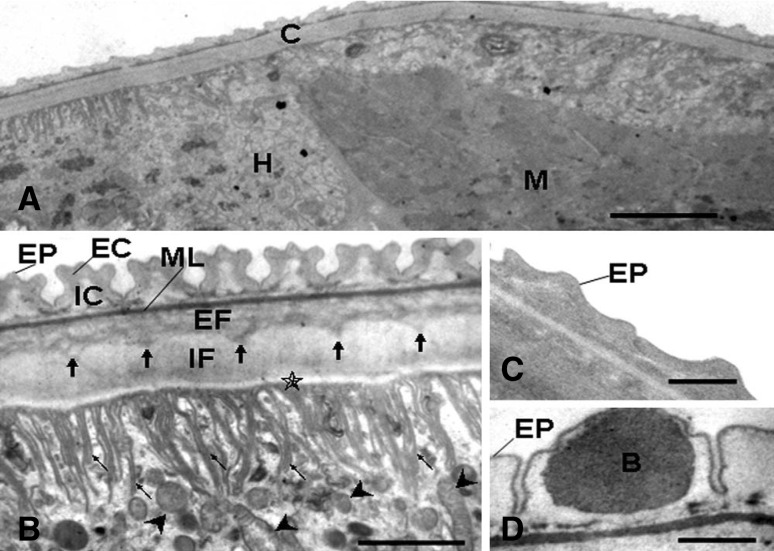
Fig. 1.
a–d Wuchereria bancrofti analysis by TEM. a Hypodermal cytoplasm (H) with cellular organelles including endoplasmic reticulum (ER), Golgi apparatus (GA) and mitochondria (head arrow). The muscular layer (M) underlined by lamellae basal (BM) (bar .6 mm). b Transverse section showing cuticle (C), hypodermal cytoplasm (H) with nucleous (N) and glycogen particles (G) (bar 1 mm). c Section showing the cuticle (C), basal layer (asterisk) with membranous projections (thin arrow) oriented toward the hypodermis (H), glycogen particles (G) (bar 1 mm). d Section showing the cuticle (C) with epicuticle (EP), external cortical layer (EC), inner cortical layer (IC), median layer (ML), external fibrous layer (EF), inner fibrous layer (IF), basal layer (asterisk) with membranous projections (thin arrow) emanating towards the hypodermis (H) and muscular layer (M) surrounded by lamellae basal (BM) and endoplasmatic reticulum (ER) (bar 1 mm) (Oliveira-Menezes et al. 2010)
Cuticle
There has been substantial current concern in the biochemistry, immunology and molecular biology of the cuticle of nematodes since its function as an interface connecting nematodes and their host, acting to protect the animal from the external environment and allowing it to remain homeostatic inside. The elongated, unsegmented nematode body is covered by a thick cuticle which is flexible outer covering composed primarily of protein with trace amounts of lipid and carbohydrates. It lines the buccal cavity, esophagus, anus, cloaca, excretory pore and vagina. This cuticle is covered by a carbohydrate rich surface coat epicuticle (5–20 nm) which is important in evasive immune mechanisms. The cuticle is formed of three regions: cortical zone, median zone and basal zone (Fig. 2). The cortical zone contains highly resistant protein (cuticulin) stabilized by dityrosine cross links. In the middle zone, even by EM, little structures could be distinguished. However, one can recognize 2–3 fibrous layers in the basal zone; the first layer runs at an angle of about 75° to the longitudinal axis of the worm while the second layer runs at an angle of 135° to the first layer forming a lattice like arrangement. Notably, these fibrous layers are not extensible but allow longitudinal stretching by modifying the angels and thus represent chief mechanisms of the hydrostatic skeleton of nematodes (Fetterer and Rhoads 1993).
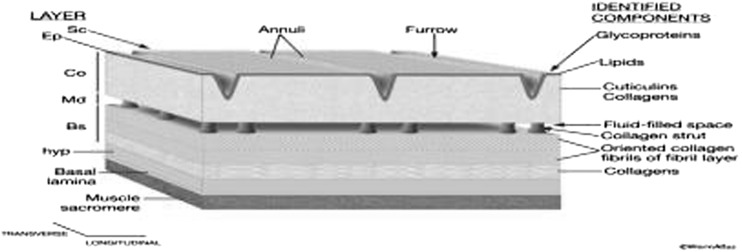
Fig. 2.
Schematic diagram showing layers of the adult cuticle and identified molecular components in each. Sc Surface coat, Ep epicuticle, Co cortical zone, Md medial zone, Bs basal zone (Blaxter and Robertson 1998)
With aging, the thickness of the adult cuticle increases due to expansion of the basal zone (Herndon et al. 2002).The buccal cavity, pharynx, rectum, excretory duct and pore are chief openings of the nematode that are also cuticle-lined. Though, on the contrary to body cuticle, cuticles that line the openings do not appear to be made of layers. It is thought that these cuticles are secreted by underlying cells that are generally epithelial. Regions of distinctive patterning are seen in the excretory duct and pore cuticles. However, the pharyngeal cuticle provides the most striking example as it contains as a minimum four inconsistent types of structures: bridging cuticle, flaps, grinder, sieve, and channels (Fig. 3). These may serve to strengthen the pore and to keep it open (Wright and Thomson 1981).
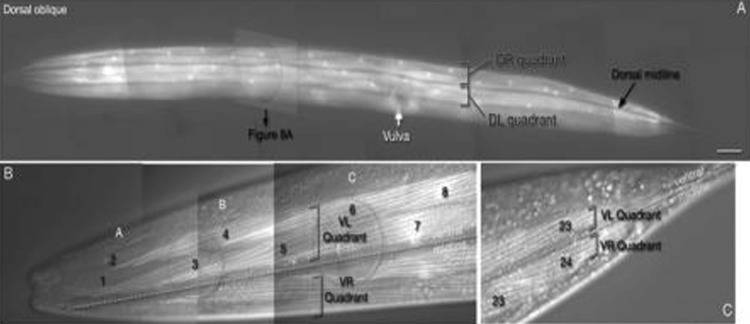
Fig. 3.

Cuticular structures of the pharynx. DIC image of an adult nematode head (lateral view) showing the compartments of the pharynx (black labels) and the various structures found in lumen cuticle lining (white labels). The narrow gap between the two bulbs is the isthmus, and is the location of the nerve ring in the surrounding somatic tissue (Altun and Hall 2012)
The cuticle may be smooth or ornamented with rings, longitudinal striations, or spikes, or it may have well-developed wing-like structures called lateral alae, when present they extend along the entire length of the body. Cervical alae are found on the anterior part of the body while caudal alae are on the tail end of some males (Gardner 2001). A model of variability in the cuticular complex includes the cuticular arêtes found in species of the order Strongylida: Trichostrongyloidea which represent exocuticular alteration into a series of cuticular ridges. The cuticular ridges are supported by skeletal rods termed the synlophe. In these forms, the ridges extending along the body of the nematode preserve their location in the intestine of their hosts. Other forms have an exocuticle with indented ridges, bumps, or may be very smooth. Some groups have large cuticularized suckers just anterior to the cloaca that are bounded by sensory papillae that permit the male to locate the female in the intestine of the host (Gardner 2001). The phylogenetic interpretation of the nematode cuticle ultrastructure is reviewed within the framework of recent DNA-sequence data. Elements of the cuticle seem to have arisen independently several times within the Nematoda and thus are highly homoplasious (e.g. the cortical or basal radial striae, spiral fiber layers and a fluid matrix with struts) (Decraemer et al. 2003). Up till now, the ultrastructures of the external body-cuticle are intractable. However, in a most astonishing display, the cuticle of the nematode may be able to oppose digestion in the hostile stomachs of the vertebrate hosts. On the other hand, nematodes may be very fragile, they remain integral only within the osmotically balanced tissues of the host (members of the order Filaroidea), and they may burst if swept to tissues with less salts. This depends on the nematode species and its life-history attributes (Gardner 2001).
Epicuticle (hypodermis)
The outer most layer of the cuticle is the epicuticle which is 6–10 nm thickness and consists of 2 dark lamellae separated by a darker inter space. Despite its similarity to cell membrane, it is not derived from the outer hypodermal layer (Mehlhorn 2008).
In adult worms, it is usually syncytial and the nuclei lie in 4 thickened portions (hypodermal cords). The hypodermal cords run longitudinally and divide the somatic musculature into 4 quadrants. In most species, the ventral and dorsal cords contain longitudinal nerve trunks where the lateral cords contain the lateral canals of the excretory system. The epicuticle contains mitochondria and endoplasmic reticulum especially at the regions of the cords and it is responsible for secretion of the cuticle (Bird and Bird 1991). The bacillary bands are modifications of the lateral (or medial) hypodermal cells in some nematode species. In which non-syncytial hypodermal cells appear to have a glandular specialization, directed outwards to secrete material towards a cuticular pore (bacillary pore), perhaps involved in osmoregulation. In some species, cells containing neurosecretory granules project ciliated endings into the bacillary band (gland cell), permitting granule release towards the pseuocoelom in response to sensory input to those cilia (Herndon et al. 2010).
Muscle layer
Mechanically, the nematode body wall consists of multilayered cuticle and a longitudinal strap-like spindle of muscle cells (Fig. 4). Nematodes muscle is unlike any other muscle in that each sarcomere is perpendicularly connected to the site of force application, the cuticle. The sarcomere is formed of thin and thick layers of myofilaments extending between two cylindrical bodies, like the z-lines of vertebrate muscles (Gaugler and Bilgrami 2004). Nematodes contains 2 type of muscles, somatic related to the body structure and specialized muscles related to certain parts of the body for particular purpose (Bird and Bird 1991). There are two main types of muscle fibers architecture in nematodes, platymyarian and coelomyarian (Burr and Gans 1987). The musculature is striated and each muscle cell consists of individualized contractile and non- contractile portions. The platymyarian muscle cell (Fig. 5) is rather ovoid in cross section and contains its contractile fibers at one side, adjacent to the hypodermis and has a myocyton (non- contractile cell body) of about the same width bulging into the pseudocoel. The myocyton contains the nuclei, large mitochondria, numerous cristae, ribosomes, endoplasmic reticulum, glycogen and lipids. The coelomyarian cell is more spindle shaped with the contractile portion at the distal end of a narrow U. The distal end of the U is placed against the hypodermis (Roberts and Janovy 1996).
Fig. 4.
Head, neck and body wall muscles. a The organization of somatic muscles in the adult C. elegans, dorsal oblique view. The body wall muscles are organized in four quadrants (only the dorsal quadrants are visible) with two rows of cells in each. Bar 50 µm. b Arrangement of head and neck muscles, ventral view. c Arrangement of the somatic tail muscles, ventral view. Original magnification, ×600 (Altun and Hall 2009)
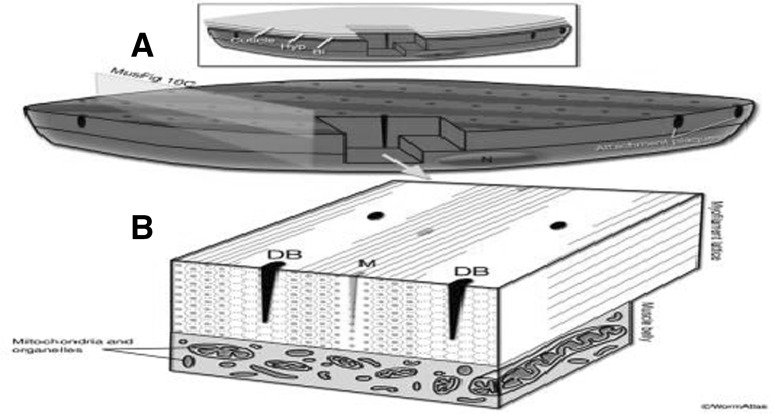
Fig. 5.
Structure of the platymyarian obliquely striated muscle. a Schematic diagram of a body wall muscle cell. Rows of dense bodies (dots) and A bands (thin lines) give the striated appearance macroscopically. Attachment plaques are localized at the end of the terminal half I bands, where the muscle cells in a quadrant contact one another. (Top inset) Layers of tissue from outside (cuticle, top) to inside (muscle, bottom). (Bl) Basal lamina between muscle and hypodermis (Hyp). b Schematic diagram of a wedge of the myofibrillar portion and the muscle belly. The nucleus and the organelles are located within the muscle belly. The myofilament lattice is arranged as a sheet of filaments on the side of the muscle cell that opposes the hypodermis. Contractile units are in register longitudinally; however, they are placed in tandem horizontally, giving rise to an alternating pattern of thick (small yellow dots and lines) and thin filaments (small black dots and lines) bands. The thin filaments are omitted from the lateral and top sides of the drawing. (DB) Dense body (Altun and Hall 2009). (Color figure online)
Hydrostatic skeleton
The assembly of cuticle, hypodermis and muscular layer composes the nematode body wall, and its main function is to act as hydrostatic-skeletal system (Harris and Crofton 1957). The somatic musculature and the rest of the body wall enclose a fluid-like cavity, the pseudocoelom or the pseudocele which is filled with fluid and heamolymph that appear as a clear, pink, almost cell free solution. The nematodes cuticle acts as anisometric skeleton in which the longitudinal somatic musculature contracts against the elastic cuticle made rigid by the hydrostatic pressure of the body fluid within the pseudocoelom (Chappell 1986). The pseudocoelom contains the coelomocytes, a unique cell type which are ovoid or with many branches. They provide the turgor-hydrostatic pressure for the animal as a whole, functions as a lubricant between tissues and provides a medium for intercellular signaling and nutrient transport. They may play a role in storage of vitamin B12, protein synthesis and secretory functions (Bolla et al.1972; Altun and Hall 2009).
Nervous system
The nervous system of roundworms is comprised of anterior nervous tissue surrounding the pharynx that forms dorsal and ventral nerve cords that go from end to end (Waggoner 2004). The nerve ring is called the circumpharyngeal commisure encircles the isthmus of the pharynx. In addition to the nerve ring, the central nervous system comprises interneurones and connected ganglia as well as the ventral nerve bundle. Anterior to the nerve ring, clusters of nuclei (papillary ganglia).The largest of these ganglia is the lateral one and includes the cell bodies for innervations of the amphids and most of neurons of the head (Chen et al. 2004). The ventral nerve trunk terminates in the pre-anal ganglion from which two branches encircles the rectum as the posterior nerve ring or rectal commisure. Other cell bodies are located along the ventral midline and in the tail (Lewbart 2011). It is noteworthy that nematodes are enriched with a variety of sensilla (small sense organs) including cephalic and caudal papillae that represent chemoreceptors and mechanoreceptors (Bogitsh et al. 2005).
Cephalic sense organs
Amphids
One curious structure that occurs in all Nemata is the amphid, a highly variable chemosensory organ that can be very obvious (Fig. 6) or very inconspicuous. Nematodes have two amphids which are specialized sensilla situated laterally on the head. These bilaterally symmetrical structures are usually of major importance for identification. Basically there are two kinds, spiral or non-spiral. Usually the non-spiral amphid is a pocket-like structure where the external opening (aperture) is in the form of a transverse slit leading to a cavity (fovea) filled with a gelatinous substance (corpus gelatum). In the spiral amphid the fovea is open and elongated, normally turning ventrally (from the nerve) although in a few cases the amphid may be dorsally wound (The Darwin Nematode Project 2006).
Fig. 6.
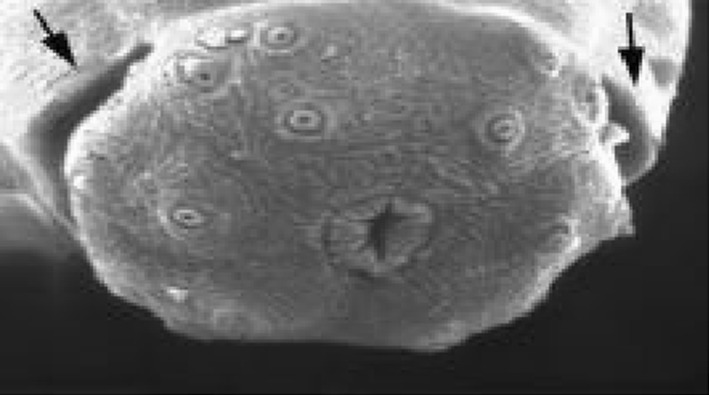
A chemosensory organ located in the anterior region of a nematode (Xiphinema americanum) (Campbell and Reece 2002)
Labial and cephalic papillae
Located around the mouth are papillae of two main types: labial papillae on the lips surrounding the mouth and cephalic papillae behind the mouth. They are mechanoreceptors innervated by papillary nerves from circumosophageal commisure (Bogitsh et al. 2005).
Caudal sense organs
Caudal papillae
observed in male nematodes and aid in copulation (Fig. 7b).
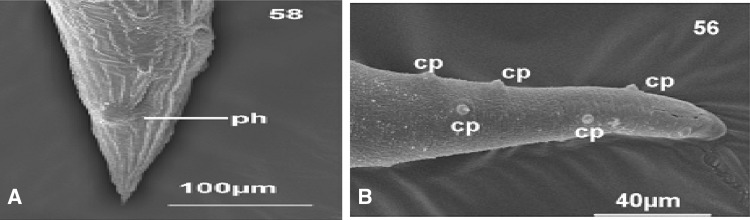
Fig. 7.
a Phasmid (ph) modifications of the cuticle in the posterior end of the body of Rhabdias sp. b Caudal papillae (cp) in the posterior end of body of males nematode parasites of amphibians (González et al. 2012)
Phasmids
paired sense organs, positioned in a bilateral manner close to the point of the tail (Fagerholm et al. 2004). While morphologically similar to amphids, phasmids bear unicellular glands opening into a depression in addition to sensory nerve endings (Fig. 7a) (Bogitsh et al. 2005).
Spicules
Males of Nematoda usually possess cuticular copulatory organs (spicules) (Fig. 8) that are inserted in the female’s vulva to attach the male to the female and to widen the vulva against the inner body pressure for sperm transfer (von Lieven and Bärmann 2005). The copulatory spicules of the nematodes Heterakis gallinarum and Nippostrongylus brasiliensis have been shown to contain nerve axons and to possess cholinesterase activity associated with these axons. The structure and position of these axons indicates that the spicule is a tactile organ which is capable of acting as a sensory probe during copulation (Lee 1973). In general, each spicule is a tubular structure consisting of a sclerotized cuticular covering and a central cytoplasmic core containing nerve tissues. Three parts can generally be distinguished: a cylindrical head, a cylindrical shaft, and a more flattened blade with two sclerotized, wing-like projections (the vela), one extending toward the dorsal and the other toward the ventral side. The vela are more conspicuous when the spicule is observed from the inner surface. The cytoplasmic core opening is usually situated on the lateral outer surface of the spicule head. There may be one or two small pores at the distal tip of each spicule (Rammah and Hirschmann 1987).
Fig. 8.
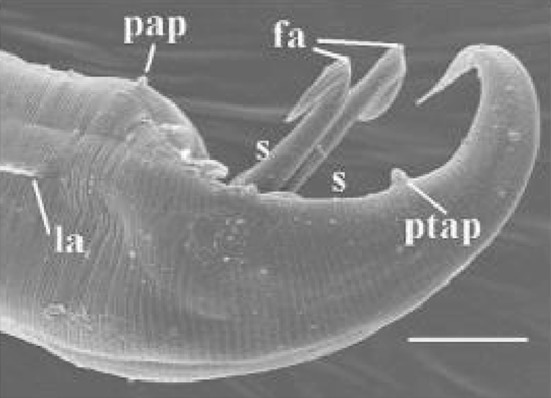
Aplectana hylambatis, male posterior end. Pap preanal papillae, ptap postanal papillae, s spicules fa fixed articulations, la lateral alae. Scale bar 40 μm (Gonzalez and Hamann 2010)
Inner body tube or digestive system
The digestive system is formed of the buccal cavity between the osophagus and the oral opening on the anterior tip of the worm ( Gutierrez 2000). It is clearly divided into stomodaeum, mesenteron and proctodeum. (Bird and Bird 1991).
The stomodaeum (foregut)
It includes the mouth and lips, buccal cavity and eosophagus. There are 6 lips (two sub-dorsal, two sub-ventral and two laterals) which surround the mouth. In some cases they may be reduced by partial fusion to 3 or by complete fusion to form a united ring around the mouth. The buccal cavity takes the form of a cylindrical or triangular tube, terminating in a valve like glottoid apparatus, which may bear the minute teeth. The cuticular lining of stoma may form teeth. The eosophagus is the largest part of stomodaeum and found between stoma and intestine. Internally, pharynx lined with cuticle and externally by membrane (basal lamella). It contains radial muscles, oesophageal glands and valves, which prevents the regurgitation of food. In some nematodes the median and posterior parts of the pharynx are swollen to form muscular bulbs (Fig. 3) (Bird and Bird 1991). Grinder is a cuticle specialization made primarily of three pairs of muscle cells rotate when the muscles contract (Avery and Thomas 1997). These interlocking “teeth” function in macerating the food, and may function as a valve to regulate one-way traffic of food into the intestine (Altun and Hall 2012). In mammalian gut parasites of the genus Trichuris (Adenophorea: Trichuridae), the stoma is lacking and the esophagus is formed of stichocytes comprising a stichosome that is glandular in function (Gardner 2001).
The intestine (midgut)
Is simple, hallow, straight non muscular tube consisting of a single layer of epithelial cells. One characteristic feature of nematode intestine is that it is anchored firmly to the oral cavity and to the anus (Fig. 9). So, when the nematode is cut the intestine will remain in place and thus, the intestine will always exist in cut sections ( Gutierrez 2000). The cytoplasm of a nematode intestinal epithelial cell has numerous structures including ribosomes, Golgi apparatus, endoplasmic reticulum and mitochondria, lipid droplets, lamellar bodies, nucleus and in some instances, microvilli covered with electron-dense amorphous material (Bird and Bird 1991).
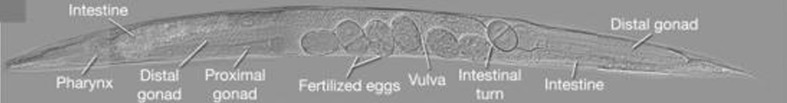
Fig. 9.
Adult intestine runs parallel to the gonad along the length of the body, ventral view. Differential interference contrast microscopy (DIC) image; magnification ×400 (Altun and Hall. 2012)
The proctodeum (hindgut)
It consists of rectum and anus in female and cloaca in male. Rectum is cuticular linings and invaginated into rectal gland. Female nematodes consist of simple tube leading to anus, whereas reproductive system opened into it and form cloaca in male contain spicules and other copulatory structure. Ultrastructurly, anus consists of slit structure on ventral side. The control of the anus opening is by a unicellular, H shaped depressor muscle (Fig. 10), which acts by raising dorsal wall of the rectum and pulling posterior lip of anus to open it (Bird and Bird 1991).
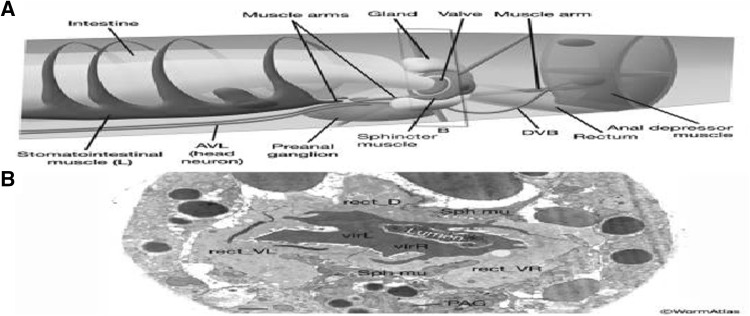
Fig. 10.
The hindgut. a Graphic rendition of the structures of the posterior alimentary canal. (Dark ovals) Muscle nuclei.b The lumen of the posterior intestine ends at the rectal valve, composed of two cells (virL and virR). The valve is surrounded by a trilobed rectal gland composed of three cells (rect_D, rect_VL, rect_VR). The gland cells connect to the lumen just posterior to the valve (not shown). The sphincter muscle (Sph mu) surrounds and pierces into the gland. TEM, transverse section; bar 1 μm (Altun and Hall 2012)
Secretory/excretory system
It is thought that the secretory-excretory(S-E) systems were named as such based simply on their morphology and before ultimate functions were detected. Strong evidence exists that most excretion occurs through the intestine (Bird and Bird 1991). Most excretory systems appear to have secretory and osmoregulatory functions. Two basic types of S-E systems exist: glandular and tubular. Most free-living nematodes (Adenophora) have the glandular type while the tubular type is commonly found in the parasitic nematodes (Secernentae) (Nelson and Albert 1983). The most common arrangement is the tubular form in which two long canals in the hypodermis connect to each other by a transverse canal near the anterior end (H shaped). Paired sub-ventral glands with granular cytoplasm thought to have a secretory function open into the transverse canal. A median, ventral duct or pore opens from the transverse canal to the outside. This is the excretory pore. Its location is species specific and serves as a taxonomic character. The second type of excretory system consists of a single ventral gland cell or renette that is present in the body cavity and connected directly to the excretory pore (Fig. 11) (Lee 2003).
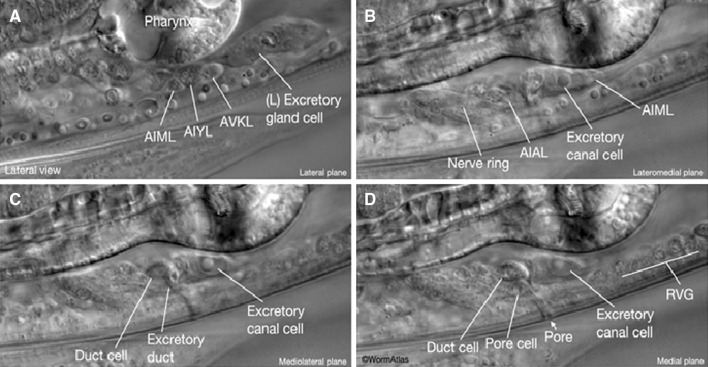
Fig. 11.
Differential interference contrast microscopy image of the excretory system in an adult C. elegans. a The excretory gland cell is located on the ventral side between the intestine and the terminal bulb of the pharynx. b The excretory cell nucleus is large and has a “fried egg” appearance with a large nucleolus. c The duct cell is anterior to the excretory cell d The excretory pore cell is ventral to the duct cell. The duct passes through the duct and pore cells and opens outside at the pore. RVG; retrovesicular ganglion (Altun and Hall 2012)
Male reproductive system
Sex organs of nematodes are simple tubular structures that are continuous with their ducts so, neither sperms nor eggs are shed into the pseudocoelom (Bird and Bird 1991). Male reproductive system is formed of testes and ducts as vas deference, seminal vesicles and ejaculatory ducts, in addition to accessory organs including spicules, copulatory bursa & genital alae (Baran 2011). In secernenteans, there is only one tests (monorchic) compared to adenophoreans which posses two testes (diorchic) (Bird and Bird 1991). The testis are tubular structures lined with epithelium and glandular tissue, sperm are produced at the end and mature as they migrate towards the shared opening of the cloaca. The proximal, upstream end of the tube is the testis which is solid, small in diameter, and enclosed by an epithelium (Fig. 12). It is filled with small, spherical primordial germ cells and has no lumen. The primordial germ cells are associated with a branching core, or rachis. Mitotic divisions of the germ cells produce spermatogonia which move downstream to undergo spermatogenesis. Several sections through the testis may be present. The next region of the male tube is the vas deferens. It is slightly larger in diameter than the testis and is also enclosed by a thin epithelium. Its interior is filled with spermatogonia and their daughters undergoing spermatogenesis. The organization of the contents of the vas deferens is looser than that of the testis and its sex cells are larger and not attached to a rachis. A lumen is present although it may not be apparent since it is filled with developing germ cells. The next region of the male duct is the seminal vesicle. The epithelial walls of this region are much thicker than those of upper regions of the reproductive tube. The ejaculatory duct, which is the downstream-most region of the male system, is too far posterior to be present in the same cross section as the testis and vas deferens (Fox 2006).
Fig. 12.
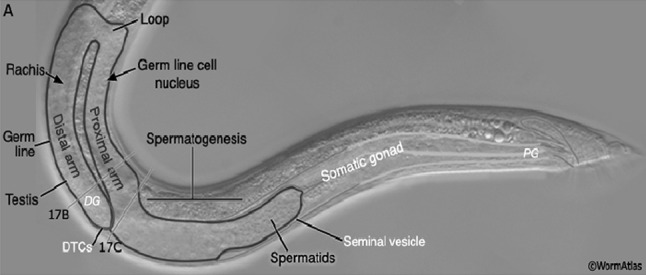
Adult male germ line organization. Nomarski DIC picture of an adult male tail region featuring the parts of the reproductive tract, lateral view. DG Distal gonad, PG proximal gonad, DTC distal tip cell, (Altun and Hall 2012)
Female reproductive system
Unlike that of the male, the female reproductive system is double so there are two ovaries, two oviducts and two uteri. There is only one vagina and gonopore but these will not be represented on the slide. The smallest sections are of the ovary. These are easily recognized because they are solid whereas the oviducts and uteri are hollow. The ovary has a central cellular core, known as the rachis, from which radiate a single layer of long narrow primordial germ cells. Their nuclei are usually easy to see. Mitotic divisions of the germ cells produce oogonia which move downstream as they undergo oogenesis. The ovary and oviduct are surrounded by thin epithelia which are often pulled away from the germinal cells leaving a white space between them. The oviduct has much the same appearance as the ovary but is a little larger in diameter and is hollow. Its epithelium is similar to that of the ovary and is thick but it has a small lumen instead of a rachis. Around the lumen is a single thick layer of oogonia (upstream) or oocytes (farther downstream).The uteri are much larger than either oviducts or ovaries. They are filled with large, shelled “eggs” in various stages of oogenesis and development and the uterine wall is thick and muscular (Fox 2006).
Implications of nematodes ultrastructural studies
The tractability and conserved genes of many nematode species have supported the significance of their ultrastructure studies which enable their use as models for diverse biological processes; including human diseases, aging, immunity, development, ecology, evolution, and host-bacterial interactions (Aboobaker and Blaxter 2000; Couillault and Ewbank 2002; Goodrich-Blair and Clarke 2007; Mitreva et al. 2009; Markaki and Tavernarakis 2010; Neher 2010; Xu and Kim 2011). Also, ultrastructural and immunolocalization of two isoenzymes, Glutathione-S-transferase (GST1) and (GST2) were carried out using immunogold electron microscopy. These isoenzymes were detected in the cuticle and the outer hypodermis of Onchocerca volvulous and were considered as secreted proteins. (Wildenburg et al. 1998). In C. elegans, detailed TEM reconstruction was necessary to understand the complexity of cell-to-cell relationships in spatial topology and intercellular junctions for communication throughout development (Nguyen et al. 1999).
In fact, diverse and disjunctive nematode ultrastructure is shown to track molecular-based phylogeny, even when classically their signals appear grossly contrasting. Consequently, studying nematodes ultrastructures has broad implications in the expression of phylogenomics, which foretells the primacy of molecular data for inferring the tree of life (Rokas et al. 2003).
These data offer an application for resolving details of multicellular complexes on a scale useful for testing hypotheses of phylogeny. As a result, precise maps of cell topography within the head regions of three distantly related nematodes are available, allowing a minimally phylogenetic approach (Ragsdale and Baldwin 2010).
Despite their sinister reputation, studying parasitic nematodes ultrastructures can also have many beneficial impacts on human interests and health. For example, entomopathogenic nematodes (EPNs), such as steinernematids and heterorhabditids, are commercially used as biological control agents for crop pests (Grewal et al. 2005).
Also, human-parasitic nematodes are being tested for therapeutic use in many autoimmune diseases (Summers et al. 2005; Kuijk and van Die 2010; Correale and Farez 2011).
In view of the phytosanitary importance of nematodes of the genus Bursaphelenchus and the limited information available on their ultrastructure, a research program on harmful phytoparasitic nematodes and their vectors has been conducted in Mediterranean forest habitats of Italy to acquire information on the morphology, biology, harmfulness and strategies for the control of indigenous species associated with the decline of host trees (Carletti et al. 2013).
Considering host-parasite interactions, the activity against filarial parasites of the antibiotics rifampicin, oxytetracycline and chloramphenicol was examined. In addition, transmission electron microscopy was used to study the effects of rifampicin and oxytetracycline on filarial tissues and on the endosymbiont bacterium, Wolbachia. Ultrastructural studies of these treated worms revealed that virtually all bacteria had been cleared from the parasite tissues. The tissues of the adult worms appeared largely intact but with a granulomatous response of host cells adhering to some specimens. However, developing uterine forms appeared to be abnormal and extensively damaged, showing an abrogation of embryogenesis (Townson et al. 2000).
Notably, methods to study the Wolbachia/filaria interaction on the ultrastructural level remained unchanged and the mechanisms for exchange of materials and for motility of endobacteria are not known. Recently, high pressure freezing/freeze substitution significantly improved the preservation of filarial tissues for electron microscopy to reveal membranes and subcellular ultrastructures that could be crucial for exchange of materials between Wolbachia and its host (Fischer et al. 2014).
In conclusion the information provided in this review might contribute towards the explication of the biology of these little known parasites. Therefore, further investigations using TEM could be directed towards correlating the nematodes ultrastructure to their functions. Moreover, prospective research in nematodes ultrastructure should focus on extending the geographical areas studied while simultaneously, expanding the examination to other possible human nematodes hosts.
References
- Aboobaker AA, Blaxter ML. Medical significance of Caenorhabditis elegans. Ann Med. 2000;32:23–30. doi: 10.3109/07853890008995906. [DOI] [PubMed] [Google Scholar]
- Aguinaldo A, Turbeville J, Linford L, Rivera M, Garey J, Raff R, Lake J. Evidence for a clade of nematodes, arthropods and other moulting animals. Nature. 1997;387:489–493. doi: 10.1038/387489a0. [DOI] [PubMed] [Google Scholar]
- Altun ZF, Hall DH (2009) Muscle system, somatic muscle. Worm Atlas. doi:10.3908/wormatlas.1.7
- Altun ZF, Hall DH (2012) Handbook of C. elegans Anatomy. Worm Atlas.http://www.wormatlas.org/hermaphrodite/hermaphroditehomepage.htm
- Avery L, Thomas JH (1997) C. elegans, Feeding and defecation In: Riddle DL, Blumenthal T, Meyer BJ (eds.). 2nd ed. Cold Spring Harbor (NY) Laboratory Press, New York [PubMed]
- Baran M. Nematodes reproductive system. Parasitology. 2011;7:163. [Google Scholar]
- Bird AF, Bird J. The structure of nematodes. San Diego: Academic press; 1991. [Google Scholar]
- Blaxter ML, Robertson WM. The cuticle. In: Perry RN, Wright DJ, editors. The Physiology and biochemistry of free-living and plant-parasitic nematodes. New York: CAB International; 1998. pp. 25–48. [Google Scholar]
- Bogitsh B, Carter C, Oeltmann T (2005) General characteristics of nematodes. In : Human parasitology 15: 308
- Bolla RI, Weinstein PP, Cain GD. Fine structure of the coelomocyte of adult Ascaris sum. J Parasitol. 1972;58:1025–1036. doi: 10.2307/3278127. [DOI] [PubMed] [Google Scholar]
- Buchsbaum R, Buchsbaum M, Pearse J, Pearse V. Animals without backbones. Chicago: University of Chicago Press; 1987. [Google Scholar]
- Burr A, Gans C. Mechanical significance of obliquely striated architecture in nematode muscle. Biol Bull. 1987;194(1):1–6. doi: 10.2307/1542507. [DOI] [PubMed] [Google Scholar]
- Campbell NC, Reece JR (2002) Sharon-taxonomy, 2010-p2.In: Nematoda Biology 6th (edn.) San Francisco: Benjamin Cummings. http://www.apsnet.org/edcenter/illglossary/Article%20Images/amphid
- Carletti B, Paoli F, Isidoro N, Roversi PF (2013) Ultrastructure of the anterior alimentary tract of Bursaphelenchus mucronatus (Nematoda Aphelenchoididae). REDIA, XCVI; 69–77
- Chappell L. Functional biology of nematodes. J Trop Ecol. 1986;2:92. doi: 10.1017/S026646740000064X. [DOI] [Google Scholar]
- Chen Z, Chen S, Dickson D. Nematology: advances and Perspectives. Basic Nerve Elem. 2004;1:221. [Google Scholar]
- Correale J, Farez M. The impact of parasite infections on the course of multiple sclerosis. J Neuroimmunol. 2011;233:6–11. doi: 10.1016/j.jneuroim.2011.01.002. [DOI] [PubMed] [Google Scholar]
- Couillault C, Ewbank J. Diverse bacteria are pathogens of Caenorhabditis elegans. Infect Immun. 2002;70:4705–4707. doi: 10.1128/IAI.70.8.4705-4707.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decraemer W, Karanastasi E, Brown D, Backeljau T. Review of the ultrastructure of the nematode body cuticle and its phylogenetic interpretation. Biol Rev. 2003;78:465–510. doi: 10.1017/S1464793102006115. [DOI] [PubMed] [Google Scholar]
- Du Four R, Guerena M, Earles R (2003) Alternative nematode control. ATTRA—National Sustainable Agriculture Information Service http://www.oisat.org/downloads/nematode
- Fagerholm H, Bruňanská M, Roepstorff A, Eriksen L. Phasmid ultrastructure of an ascaridoid nematodehysterothylacium auctum. J Parasitol. 2004;90(3):499–506. doi: 10.1645/GE-3168. [DOI] [PubMed] [Google Scholar]
- Fetterer RH, Rhoads ML. Biochemistry of the nematode cuticle: relevance to parasitic nematodes of livestock. Vet Parasitol. 1993;46(1–4):103–111. doi: 10.1016/0304-4017(93)90051-N. [DOI] [PubMed] [Google Scholar]
- Fischer K, Beatty W, Weil G, Fischer P. High pressure freezing/freeze substitution Fixation improves the ultrastructural assessment of wolbachia endosymbiont—filarial nematode host interaction. PLoS One. 2014 doi: 10.1371/journal.pone.0086383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox R. Invertebrate anatomy online. Ascaris suum Pig Roundworm: Lander University; 2006. [Google Scholar]
- Fürst von Lieven A, Bärmann VW. How can nematodes mate without spicules? Function of the male gonoduct glands in the roundworm Myolaimus. Zoology . 2005;108(3):211–216. doi: 10.1016/j.zool.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Gardner S. Worms, Nematoda. In: Levin SA, editor. Encyclopedia of biodiversity. Lincoln: Academic Press, University of Nebraska; 2001. [Google Scholar]
- Gaugler R, Bilgrami A (2004) Nematode behaviour, locomotion behavior, Role of hydrostatic skeleton, p 27
- Gibbons L. SEM guide to the morphology of nematode parasites of vertebrates. Oxu: CAB International; 1986. [Google Scholar]
- González C, Hamann M. First report of nematode parasites of Physalaemussantafecinus (Anura: Leiuperidae) from Corrientes, Argentina. Rev Mex Biodiv. 2010;81:3. [Google Scholar]
- González C, Hamann M, Salgad C (2012) Study of helminth parasites of amphibians by scanning electron microscopy, scanning electron microscopy, Dr. Viacheslav Kazmiruk (ed.), ISBN: 51
- Goodrich-Blair H, Clarke D. Mutualism and pathogenesis in Xenorhabdus and Photorhabdus: two roads to the same destination. Mol Microbiol. 2007;64:260–268. doi: 10.1111/j.1365-2958.2007.05671.x. [DOI] [PubMed] [Google Scholar]
- Grewal P, Ehlers S, Shapiro-Ilan D. Nematodes as Biocontrol agents. Wallingford: CABI Publishing; 2005. [Google Scholar]
- Gutierrez Y (2000) Nematodes, body cavity. In: Diagnostic pathology of parasitic infections with clinical correlations, 2nd (edn), p 278
- Harris JE, Crofton HD. Structure and function in the nematodes: internal pressure and cuticular structure in Ascaris. J Exp Biol. 1957;34:116–130. [Google Scholar]
- Herndon LA, Schmeissner PJ, Dudaronek JM, Brown PA, Listner KM, Sakano Y, Paupard MC, Hall DH, Driscoll M. Stochastic and genetic factors influence tissue-specific decline in ageing C. elegans. Nature. 2002;419:808–814. doi: 10.1038/nature01135. [DOI] [PubMed] [Google Scholar]
- Herndon LA, Altun ZF, Hall DH (2010) Glossary B. WormAtlas. doi:10.3908/wormatlas.6.2
- Kuijk LM, van Die I. Worms to the rescue: can worm glycans protect from autoimmune diseases? IUBMB Life. 2010;62:303–312. doi: 10.1002/iub.304. [DOI] [PubMed] [Google Scholar]
- Lee DL. Evidence for a sensory function for the copulatory spicules of nematodes. J Zool. 1973;169:281–285. doi: 10.1111/j.1469-7998.1973.tb04557.x. [DOI] [Google Scholar]
- Lee DL. The biology of nematodes, secretory-excretory system. Endokrinologie. 2003;76:112–114. [Google Scholar]
- Lewbart G (2011) Nematodes, Nervouse system In: Invertebrate Medicine, 2nd (edn). John Wiley & Sons
- Markaki M, Tavernarakis N. Modeling human diseases in Caenorhabditis elegans. Biotechnol J. 2010;5:1261–1276. doi: 10.1002/biot.201000183. [DOI] [PubMed] [Google Scholar]
- Mehlhorn H. Encyclopedia of parasitology. Great Britain: Kluwer Academic Publishers; 2008. [Google Scholar]
- Mitreva M, Smant G, Helder J. Role of horizontal gene transfer in the evolution of plant parasitism among nematodes. Methods Mol Biol. 2009;532:517–535. doi: 10.1007/978-1-60327-853-9_30. [DOI] [PubMed] [Google Scholar]
- Neher DA. Ecology of plant and free-living nematodes in natural and agricultural soil. Annu Rev Phytopathol. 2010;48:371–394. doi: 10.1146/annurev-phyto-073009-114439. [DOI] [PubMed] [Google Scholar]
- Neher DA, Powers T (2005) Nematodes. www.uvm.edu/dneher/Publications/encyclopedia
- Nelson K, Albert S. Riddle D (1983) Fine structure of the Caenorhabditis elegans secretory-excretory systemF. J Ultrastruc Res. 1983;82:156–171. doi: 10.1016/S0022-5320(83)90050-3. [DOI] [PubMed] [Google Scholar]
- Nguyen CQ, Hall DH, Yang Y, Fitch DHA. Morphogenesis of the Caenorhabditis elegans male tail tip. Dev Biol. 1999;207:86–106. doi: 10.1006/dbio.1998.9173. [DOI] [PubMed] [Google Scholar]
- Oliveira-Menezes A, Noroes J, Dreyer G, Lanfredi R. Ultrastructural analysis of wuchereria bancrofti (Nematoda: Filarioidea) body wall. Micron. 2010;41:526–531. doi: 10.1016/j.micron.2010.01.007. [DOI] [PubMed] [Google Scholar]
- Ragsdale E, Baldwin J. Resolving phylogenetic incongruence to articulate homology and phenotypic evolution: a case study from Nematoda. Proc R Soc. 2010;9277:1299–1307. doi: 10.1098/rspb.2009.2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rammah A, Hirschmann H. Morphological comparison and taxonomic utility of copulatory structures of selected nematode species. J Nematol. 1987;19(3):314–323. [PMC free article] [PubMed] [Google Scholar]
- Roberts LS, Janovy J. Foundation of parasitology. London: WMC Brown; 1996. [Google Scholar]
- Rokas A, Williams BL, King N, Carroll SB. Genome-scale approaches to resolving incongruence in molecular phylogenies. Nature. 2003;425:798–804. doi: 10.1038/nature02053. [DOI] [PubMed] [Google Scholar]
- Scheffers BR, Joppa LN, Pimm SL, Laurance WF. What we know and don’t know about Earth’s missing biodiversity. Trends Ecol Evol. 2012;27:501–510. doi: 10.1016/j.tree.2012.05.008. [DOI] [PubMed] [Google Scholar]
- Storer T, Usinger R, Stebbins R, Nybakken J. General zoology. 6. New York: McGraw-Hill Book Company; 1979. [Google Scholar]
- Summers RW, Elliott D, Urban J, Jr, Thompson R, Weinstock J. Trichuris suis therapy for active ulcerative colitis: a randomized controlled trial. Gastroenterology. 2005;128:825–832. doi: 10.1053/j.gastro.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Telford MJ, Bourlat SJ, Economou A, Papillon D, Rota-Stabelli O. The evolution of the Ecdysozoa. Philos Trans R Soc Lond B. 2008;363:1529–1537. doi: 10.1098/rstb.2007.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Darwin Nematode Project (2006) Nematode structure: General introduction to marine nematodes. Amphids. http://www.pml-nematode.org.uk
- Townson S, Hutton D, Siemienska J, Hollick L, Scanlon T, Tagboto SK, Taylor MJ. Antibiotics and Wolbachia in filarial nematodes: antifilarial activity of rifampicin, oxytetracycline and chloramphenicol against Onchocerca gutturosa, Onchocerca lienalis and Brugia pahangi. Ann Trop Med Parasitol. 2000;94(8):801–816. doi: 10.1080/00034983.2000.11813605. [DOI] [PubMed] [Google Scholar]
- Waggoner BM (2004) Study Guide Nematoda Parasitology. Fall 2012 Heth:3–10. http://www.ucmp.berkeley.edu/phyla/ecdysozoa/nematoda.html
- Wallace Lee R, Ricci C, Melone G. A cladistic analysis of pseudocoelomate (aschelminth) morphology. Invertebr Biol. 1996;115(2):104–112. doi: 10.2307/3227041. [DOI] [Google Scholar]
- Wildenburg G, Liebau E, Henkel-Duhresen K. Onchoceca volvulous : ultrastructural localization glutathione S- transferase. Exp Parasitol. 1998;88(1):34–42. doi: 10.1006/expr.1998.4189. [DOI] [PubMed] [Google Scholar]
- Wright KA, Thomson JN. The buccal capsule of C. elegans (Nematoda: Rhabditoidea): an ultrastructural study. Can J Zool. 2011;59:1952–1961. doi: 10.1139/z81-266. [DOI] [Google Scholar]
- Xu X, Kim S. The early bird catches the worm: new technologies for the Caenorhabditis elegans toolkit. Nat Rev Genet. 2011;12:793–801. doi: 10.1038/nrg3050. [DOI] [PMC free article] [PubMed] [Google Scholar]