Abstract
Toxoplasma gondii (T. gondii), an intracellular parasite, establishes a chronic infection by forming cysts preferentially in the brain. TNF-α plays an important role in controlling the infection caused by this protozoan. Thus, the blockade of TNF-α could cause reactivation of latent toxoplasmosis infection as well as increase the risk of acute toxoplasmosis. This study evaluated the effect of etanercept, a TNF-α antagonist in reactivation of latent toxoplasmosis compared to the therapeutic effect of sulfadiazine and pyrimethamine in combination on the progress of the disease. A total of 40 laboratory-bred Swiss albino mice were infected with Me49 strain of T. gondii and divided into four groups: infected control group; treated group with sulfadiazine and pyrimethamine; treated group with etanercept and treated group with both etanercept and sulfadiazine and pyrimethamine. The mean number and size of tissue cysts in brain smears of mice of each group were determined and also, serum levels of TNF-α were assessed in different study groups by an enzyme linked immunosorbent assay. The results showed that the mean TNF-α level was significantly different in the treated groups compared to that in infected control group. The highest level of TNF-α was found in the infected controls. After treatment with etanercept alone or combined with sulfadiazine and pyrimethamine, it was significantly decreased. In this study, reactivation of latent toxoplasmosis was observed by a significant increase in the mean number and sizes of Toxoplasma tissue cysts in brains of mice with established chronic toxoplasmosis after treatment with etanercept alone or combined with conventional treatment compared to both untreated chronically infected controls and infected mice treated with sulfadiazine and pyrimethamine. It was concluded that etanercept, a TNF-α antagonist may play a role in reactivation of latent toxoplasmosis. So, serological screening for toxoplasmosis might offer a valuable aid for patients treated with this drug.
Keywords: Toxoplasma gondii, Sulfadiazine, Pyrimethamine, Etanercept, TNF-α
Introduction
Toxoplasma gondii (T. gondii), the etiologic agent of toxoplasmosis, is an obligate intracellular protozoan parasite capable of infecting many warm-blooded mammals, including humans (Halonen and Weiss 2013). In most individuals is able to persist in multiple tissues where the latent stage of the parasite is mainly found in the central nervous system (El-Sayed et al. 2012). Although approximately 30 % of the world’s population have T. gondii infection and harbor cysts in the brain, overt disease symptoms such as encephalitis are only evident during immune suppression (Pusch et al. 2009). The infection in humans can be acquired by ingesting of tissue cysts in raw or undercooked infected meat; ingesting of food or water contaminated with sporulated oocysts shed in the feces of an infected cat, blood transfusion, organ transplantation and congenitally, across the placenta from the mother to the fetus (Yazar et al. 2006).
Once inside the host, T. gondii tachyzoites, a form of the parasite with high levels of metabolic activity, cross the placental or intestinal epithelium using paracellular transmigration and enter circulating cells such as macrophages and dendritic cells. These tachyzoites cross the blood–brain barrier and gain access to important sites in the brain to establish a chronic infection by forming cysts which contain hundreds to thousands of bradyzoites, a form of the parasite with low levels of metabolic activity. These resistant forms are able to escape the immune system of the host as well as most therapeutic agents (Carruthers and Suzuki 2007; Maubon et al. 2008).
In response to the infection with T. gondii, the host sets up an immune reaction, mainly of cellular type, via T lymphocytes—essentially helper T lymphocytes (Th1), characterized by pro-inflammatory cytokine production such as IL-6, interferon-gamma (IFN-γ), and tumor necrosis factor (TNF-α). IFN-γ mediated immune responses limit proliferation of tachyzoites, but the parasite establishes a chronic infection by forming cysts, primarily in the brain. These tissue cysts remain largely quiescent for the life of the host, but can reactivate and cause life-threatening diseases in immunocompromised patients (Suzuki et al. 2010).
TNF-α; a soluble inflammatory cytokine secreted by activated macrophages in response to parasitic soluble antigens. It is involved in several infections with protozoan parasites. The role of TNF during such infections greatly depends on the strain of the parasite, the state of infection, and the amount of induced TNF. It plays an important role specifically in protecting against T. gondii infection, and as a consequence, blocking this cytokine may increase risk of infectious complications (Toussirot et al. 2007).
Bio-pharmaceutical etanercept (Enbrel, trade name) is a TNF antagonist that reduces elevated TNF levels by competitively binding to both TNF-α and TNF-β and inhibiting the proinflammatory cascade (Fuchs and Hadi 2006). It was approved by the Food and Drug Administration (FDA) of the USA for its use in the treatment of autoimmune diseases; rheumatoid, juvenile rheumatoid, psoriatic arthritis, plaque psoriasis and ankylosing spondylitis that is causing an overactive immune response by inhibiting TNF-α (Braun et al. 2007; Sfikakis 2010). Despite its good results, this anti-TNF-α therapy has a number of contraindications and side effects, especially when used in combination with classical immunosuppressive agents or corticosteroids (Azevedo et al. 2010). It has been demonstrated to increase disease activity when administered to patients with opportunistic infections as toxoplasmosis and reactivation of latent tuberculosis (Sfikakis 2010). A few cases of cerebral toxoplasmosis and toxoplasmic chorioretinitis have been reported in patients who were treated with anti-TNF agents either etanercept or infliximab (Gonzalez-Vicent et al. 2003; Lassoued et al. 2007; Young and McGwire 2005). The aim of this study is to evaluate the effect of etanercept (TNF-α antagonist) in reactivation of latent toxoplasmosis compared to the therapeutic effect of sulfadiazine and pyrimethamine in combination on the progress of the disease.
Materials and methods
Parasite
Brain cysts of the Me49 non-virulent strain of T. gondii were kindly provided by Department of Zoonotic Diseases, Veterinary Research Division, National Research Center, Giza, Egypt. The parasite strain was regularly maintained by repeated inoculation of Swiss albino mouse every 8 weeks with 0.1 ml of brain homogenate of previously infected mice containing, approximately, 1 × 102 cysts/ml to establish chronic toxoplasmosis (El-Sayed and Aly 2014).
Experimental animals
Laboratory-bred male Swiss albino mice, 10 weeks-old, each weighing ~40 g, were selected from the animal house of the Research Institute of Ophthalmology, Giza, Egypt. They were housed in plastic cages with white wood chips for bedding, fed by commercial complete food mixture and tap water for drinking, and maintained under controlled conditions of lighting (12 h light/12 h dark cycle) and temperature (25 ± 2 °C). Mice were inoculated intraperitoneally with 0.1 ml of the brain cysts suspension, which containing 1 × 102 cysts/ml. Four weeks after the infection, the efficiency of the experimental Toxoplasma infection in mice was confirmed by the complement fixation test for detection of specific anti-Toxoplasma antibodies (Ondriska et al. 2003), and mice with undetected or very low concentrations of specific antibodies were excluded from the experiment. The final set of experimental animals included 40 mice.
Animals were divided into four groups of 10 mice each:
- Group I (GI)
Infected control group.
- Group II (GII)
Infected and treated with conventional treatment; a combination of sulfadiazine (Dohms Laboratories) at a dose of 200 mg/kg/day and pyrimethamine (Sigma Chemical Co., St. Louis, MO), at a dose of 12.5 mg/kg/day (Romand et al. 1993). The drugs were provided in powder form and prepared daily as liquid suspensions; after brief sonication, the homogenized suspensions were administered orally to mice via tube feeding. Six weeks post-infection; treatments were administered daily at a fixed hour for 10 days.
- Group III (GIII)
Infected and received etanercept (Enbrel, Pfizer Inc, USA). Four weeks post-infection; infected mice were injected subcutaneously by etanercept at a dose of 1 mg/kg/week for 4 weeks.
- Group IV (GIV)
T. gondii infected mice and received both etanercept as GIII and conventional treatment as GII.
At the end of the experiment (8 weeks), the blood was collected from the mouse’s orbital sinus (Hoff and Rlagt 2000). The sera were separated and stored at −20 °C until the determination of TNF-α serum levels. Then all mice were sacrificed.
The effect of the used drugs was evaluated by:
Determination of TNF-α serum level
Serum levels of TNF-α were determined by enzyme-linked immunosorbent assay (TNF-α Mouse ELISA Kit, Quantikine M, R&D systems, Minneapolis, MN, USA) according to the manufacturer’s instructions. This assay employs the quantitative sandwich enzyme immune-assay technique using one polyclonal antibody and another monoclonal antibody specific for TNF-α. Optical density (OD) values were measured at 450 nm and serum TNF-α concentration was determined from the standard curve. The sensitivity cutoff for the ELISA of this cytokine was 20 pg/ml.
-
2.
Counting the brain cysts number & measuring of their size
The brains of the sacrificed mice were grinded individually in a mortar. Then, 5 ml of normal saline were added to the grinded brain tissue of each mouse obtaining brain emulsion homogenates. The total number of cysts per mouse brain was then estimated from 25 μl four separate drops that were placed on glass slides and examined by bright-field microscope at 40× magnification (Djurković-Djaković et al. 2002). Then, the mean cysts number in each group was calculated.
After that, the slides were placed in room temperature to dry completely, and were then fixed using absolute methanol. Fix smears were stained with 20 % Giemsa solution (Merck, Darmstadt, Germany) in pH 6.8 of phosphate buffer for 20 min. Measuring brain cysts size of each group was done by computerized image analysis and accordingly the mean size in each group was calculated.
Statistical analysis
Collected data were coded and introduced to a PC using the Statistical Package for Social Science (SPSS) for windows version 11.0. Data were represented as the mean ± standard deviation (SD) (n = 10). The analysis of variance (ANOVA) procedure was used to clarify statistically significant differences between the studied groups. Values were considered statistically significant when P < 0.05.
Ethical consideration
The animal experiment was carried out according to the internationally valid guidelines of experimental animal studies and the research protocol was approved by the local ethical committee. All efforts were made to minimize the animal’s suffering.
Results
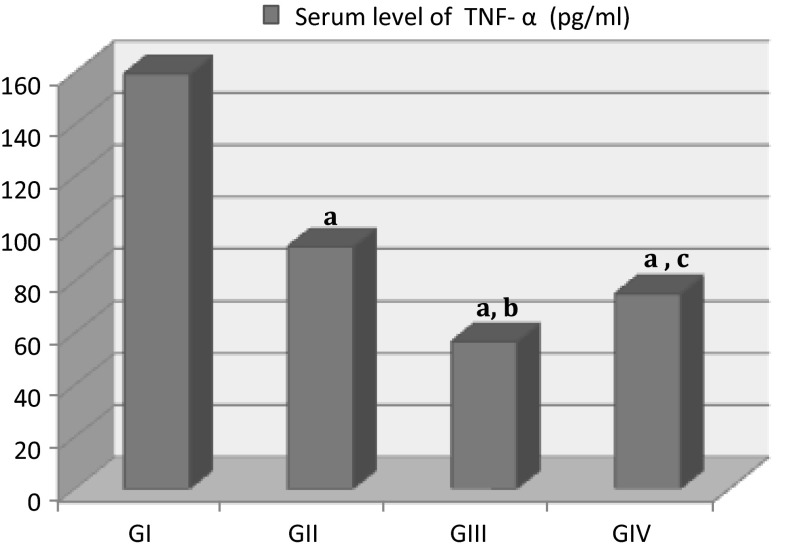
The mean serum level of TNF-α was 159.45 ± 1.65 in infected control group (GI). At the same time, a significant decrease in TNF-α levels was observed in groups treated with etanercept alone (56.65 ± 2.15) or combined with conventional treatment (75.1 ± 1.6) compared to infected control group (P = 0.03; P = 0.02, respectively). Moreover, TNF-α levels significantly decreased in the group treated with etanercept alone (P = 0.01) compared to the group treated with sulfadiazine and pyrimethamine (92.9 ± 1.6). Also, there was no significant difference between GIV and GII (P = 0.113) (Fig. 1).
Fig. 1.
Serum level of TNF-α (pg/ml) in mice with established chronic toxoplasmosis after treatment with etanercept alone (GIII) or combined with sulfadiazine and pyrimethamine (GIV) compared to untreated chronically infected control group (GI). All values were expressed as mean ± SD (n = 10). a P < 0.05 statistically significant difference in comparison to GI; b P < 0.05 statistically significant difference in comparison to GII (infected mice treated with conventional treatment). c P > 0.05 no significant difference in comparison to GII
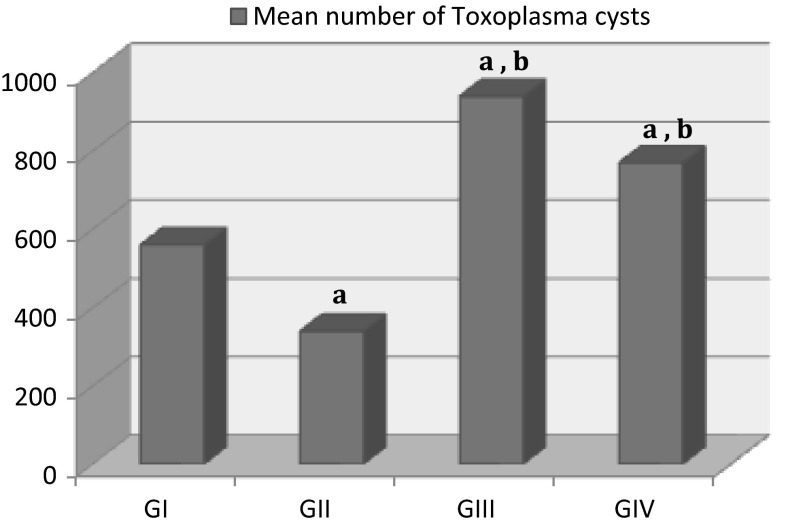
Compared to the infected control group (GI), the mean number of cysts in the brains was significantly higher in both GIII treated with etanercept alone (940 ± 84.85) and GIV treated with both etanercept and sulfadiazine and pyrimethamine (770 ± 70.71). On the other hand, there was significantly decreased in cysts number in GII (340 ± 28.28) comparing to GI (560 ± 56.57) (Fig. 2).
Fig. 2.
The mean number of Toxoplasma cysts in brains of mice with established chronic toxoplasmosis after treatment with etanercept alone (GIII) or combined with sulfadiazine and pyrimethamine (GIV) compared to untreated chronically infected controls (GI). All values were expressed as mean ± SD (n = 10). a P < 0.05 statistically significant difference in comparison to GI, b P < 0.05 statistically significant difference in comparison to GII (infected mice treated with conventional treatment)
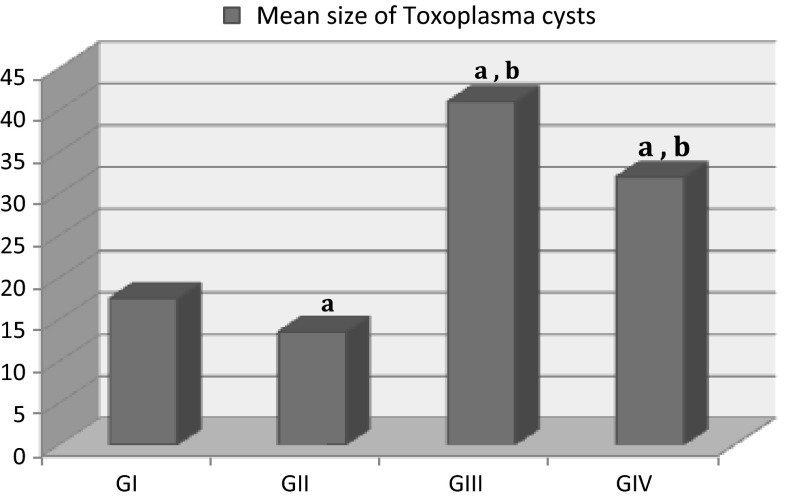
Regarding the mean size of brain cysts, it was 17.5 ± 3.54 μm in the infected mice (GI). After treatment of infected mice with sulfadiazine and pyrimethamine (GII), there was decreased in cysts size to be 13.5 ± 4.95 μm. On the other hand, it significantly increased in the infected mice that were received etanercept (GIII) to be 41.0 ± 5.66 μm. When the infected mice received both etanercept and the combination of sulfadiazine and pyrimethamine (GIV), the mean cysts size was 32.0 ± 4.24 μm (Fig. 3).
Fig. 3.
The mean size of Toxoplasma cysts in brains of mice with established chronic toxoplasmosis after treatment with etanercept alone (GIII) or combined with sulfadiazine and pyrimethamine (GIV) compared to untreated chronically infected controls (GI). All values were expressed as mean ± SD (n = 10). a P < 0.05 statistically significant difference in comparison to GI, b P < 0.05 statistically significant difference in comparison to GII (infected mice treated with conventional treatment)
Discussion
Toxoplasma gondii establishes a chronic infection by forming cysts containing bradyzoites preferentially in the brain. The cysts persist in the host tissues for years without causing any local inflammatory reaction, controlled mainly by cellular immune mechanisms (Hunter et al. 1996). However, if the balance between the host immune defenses and the parasite is disrupted, cyst rupture and renewed parasite proliferation may occur leading to clinical reactivation. Reactivation of toxoplasmosis was found to be a serious complication in patients receiving anti-TNF therapy (Ali et al. 2013).
TNF-α is a cytokine of inflammatory and immune response (Lang et al. 2007). In this in vivo experimental study, the highest level of TNF-α in serum was found in the Me49-infected mice (GI). After treatment with etanercept alone or combined with conventional treatment, it was significantly decreased. In murine toxoplasmosis, one major function of IFN-γ is the induction of TNF-α, which is produced by many cell populations; including macrophages, microglial cells, and astrocytes in the CNS for the in vivo control of T. gondii and survival of acute and chronic murine toxoplasmosis (Johnson 1992; Schlüter et al. 1997). These cytokines can subsequently activate CD8+ T cytotoxic cells to turn into major cytotoxic effector cells for lysing tachyzoite-infected cells, limiting parasite dissemination during acute infection phase (Jongert et al. 2010), as well as inhibiting cyst formation during chronic infection (Jongert et al. 2009).
In addition, TNF-α together with IL-6 can enhance proliferation and differentiation of B lymphocytes (Lang et al. 2007). It activates eosinophil cytotoxicity toward T. gondii protozoan and induces secretion of acute phase proteins via IL-6 production, resulting in inhibition of parasite replication. In addition, this cytokine plays a role in macrophage activation, differentiation and phagosome formation, and also it is critical for the clearance of intracellular pathogens (Koo et al. 2010). Indeed, anti-TNF-α antibodies aggravate toxoplasmic encephalitis in mice (Gazzinelli et al. 1993), and partially block the activity of macrophages exposed to IFN-γ. Furthermore, when these cells are exposed to a combination of TNF-α and IFN-γ at low concentrations, replication of T. gondii is inhibited to a greater extent than when IFN-γ is used alone (Chang et al. 1990).
Therefore, the reduction of TNF-α level by anti-TNF agents leads to reactivation of latent toxoplasmosis infection (Rodrigues et al. 2013) as well as increase the risk of acute toxoplasmosis. To date, only a few cases of toxoplasmosis in patients receiving anti-TNF agents have been reported. Radwan et al. (2013) reported a case of acquired ocular toxoplasmosis in a patient treated with an anti-TNF-α agent for ulcerative colitis. Young and McGwire (2005) have reported two cases of reactivation of cerebral toxoplasmosis while on treatment with infliximab. Lassoued et al. (2007) have reported two cases of ocular toxoplasmosis in patients with rheumatoid arthritis receiving anti-TNF-α agent in patients, one of the two cases was because of reactivation of previous toxoplasmosis infection, whereas the other had primary acquired ocular toxoplasmosis. In addition, Gonzalez-Vicent et al. (2003) reported a case of cerebral toxoplasmosis following etanercept treatment for idiopathic pneumonia syndrome after autologous peripheral blood progenitor cell transplantation. An interesting point to be considered is that the receiving immunosuppressive triggered the release of bradyzoites from tissue cysts which convert into tachyzoites, and proliferate in host tissue without restriction, leading to the dissemination of Toxoplasma organisms to other cells. It was found that the use of prednisolone to immunosuppress mice prior to infection with T. gondii can help propagate a greater number of the parasite from both virulent (RH, type I) and avirulent (Me49, type II) strains in mice (Puvanesuaran et al. 2012).
During the chronic stage of T. gondii infection, bradyzoites slowly replicate within the cysts and cyst sizes increase in response. In the present study, reactivation of latent toxoplasmosis was observed by a significant increase in the mean number and sizes of Toxoplasma tissue cysts in brains of mice with established chronic toxoplasmosis after treatment with etanercept alone or combined with conventional treatment compared to both untreated chronically infected controls and infected mice treated with sulfadiazine and pyrimethamine. Additionally, the patterns of cyst growth found within the brains of experimental mice were varied among the different studied groups. The tissue cysts were often spherical with well-defined cyst walls and vary in size, from small cysts (10 μm), containing 1–2 bradyzoites, to large cysts, 45 μm in diameter containing more than 50 bradyzoites. They were mostly found as individual cysts either solitary or in groups. The patterns of cysts growth found in this study are consistent with other studies which found that Toxoplasma cysts in the brain grow uniformly in size up to 2–3 months post-infection and persist in the brain for many months post-infection (Melzer et al. 2010).
The variation in sizes of Toxoplasma cysts that was observed in the current study indicated that the continual formation of new or second-generation tissue cysts in the brain occurs during the chronic infection. In vitro studies of bradyzoite differentiation and cyst formation found that intracellular bradyzoites are motile within host cells, being able to invade surrounding cells and initiate new cysts, and that the cysts can proliferate by fission indicating that bradyzoites and cysts are highly dynamic and suggesting a mechanism of parasite dissemination during the chronic infection (Dzierszinski et al. 2004). In immunocompromised individuals such as those with AIDS and organ transplants, cysts can rupture resulting in release of bradyzoites, conversion of bradyzoites into tachyzoites, and proliferation of tachyzoites, which can cause life-threatening toxoplasmic encephalitis (Israelski and Remington 1993). Even in immunocompetent host, T. gondii cysts occasionally rupture during the chronic stage of infection (Ferguson et al. 1989). In these cases, tachyzoite growth is controlled by the host’s immune response, but the parasite is most likely able to form small numbers of new cysts. Such natural rupture of cysts and the formation of new cysts are thought to result in a wide range of T. gondii cyst sizes observed in the brains of chronically infected mice.
In the present study, there was significantly decreased in the mean number and size of Toxoplasma cysts in brains of mice treated with sulfadiazine and pyrimethamine compared to untreated chronically infected controls. This is explained by the fact that, these drugs have a synergistic action by inhibiting T. gondii folic acid synthesis, which is essential for parasite survival and replication (Doliwa et al. 2013). Pyrimethamine interferes with replication of the parasite as it inhibits the enzyme dihydrofolate reductase in the folate production pathway while, sulfadiazine acts as a competitive antagonist for para-aminobenzoic acid (PABA), one of the precursors of folate production (Ng and McCluskey 2002). The observed decrease in both cysts number and size might be due to the repair of the lesions induced by treatment that reduced the number of damage cells. In fact, prompt treatment may achieve rapid resolution, minimize inflammatory damage, prevent widespread tissue destruction and decrease the chances of the parasite dissemination. Those in agreement with Soheilian et al. (2011) who observed that classic therapy (pyrimethamine, sulfadiazine) for Toxoplasma retinochoroiditis has the ability to reduce lesion size and vitreal inflammation as well as improve visual acuity.
Conclusion
It was concluded that etanercept (TNF-α antagonist) may play a role in reactivation of latent toxoplasmosis in murine models by increasing the number and size of brain tissue cysts. So, serological screening for toxoplasmosis might offer a valuable aid for patients treated with this drug.
Contributor Information
Nagwa Mostafa El-Sayed, Email: nag.elsaka@yahoo.com, Email: nagelsaka@hotmail.com.
Khadiga Ahmed Ismail, Email: khadigaahmed68@yahoo.com.
Abeer Fathy Badawy, Email: abeerfathy99@gmail.com.
Khaled Fathy Elhasanein, Email: dr_khaledfathy@hotmail.com.
References
- Ali T, Kaitha S, Mahmood S, Ftesi A, Stone J, Bronze MS. Clinical use of anti-TNF therapy and increased risk of infections. Drug Healthc Patient Saf. 2013;5:79–99. doi: 10.2147/DHPS.S28801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azevedo TVF, Pietrovski CF, de Almeida Santos M. Acute toxoplasmosis infection in a patient with ankylosing spondylitis treated with adalimumab: a case report. Reumatismo. 2010;62(4):283–285. doi: 10.4081/reumatismo.2010.283. [DOI] [PubMed] [Google Scholar]
- Braun J, McHugh N, Singh A, Wajdula JS, Sato R. Improvement in patient-reported outcomes for patients with ankylosing spondylitis treated with etanercept 50 mg once-weekly and 25 mg twice-weekly. Rheumatology (Oxford) 2007;46(6):999–1004. doi: 10.1093/rheumatology/kem069. [DOI] [PubMed] [Google Scholar]
- Carruthers VB, Suzuki Y. Effects of Toxoplasma gondii infection on the brain. Schizophr Bull. 2007;33(3):745–751. doi: 10.1093/schbul/sbm008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HR, Grau GE, Pechère JC. Role of TNF and IL-1 in infections with Toxoplasma gondii. Immunology. 1990;69(1):33–37. [PMC free article] [PubMed] [Google Scholar]
- Djurković-Djaković O, Milenković V, Nikolić A, Bobić B, Grujić J. Efficacy of atovaquone combined with clindamycin against murine infection with a cystogenic (Me49) strain of Toxoplasma gondii. J Antimicrob Chemother. 2002;50(6):981–987. doi: 10.1093/jac/dkf251. [DOI] [PubMed] [Google Scholar]
- Doliwa C, Xia D, Escotte-Binet S, Newsham EL, Sanderson SJ, Aubert D, Randle N, Wastling JM, Villena I. Identification of differentially expressed proteins in sulfadiazine resistant and sensitive strains of Toxoplasma gondii using difference-gel electrophoresis (DIGE) Int J Parasitol Drugs Drug Resist. 2013;3:35–44. doi: 10.1016/j.ijpddr.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzierszinski F, Nishi M, Ouko L, Roos DS. Dynamics of Toxoplasma gondii differentiation. Eukaryot Cell. 2004;3(4):992–1003. doi: 10.1128/EC.3.4.992-1003.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sayed NM, Aly EM. Toxoplasma gondii infection can induce retinal DNA damage: an experimental study. Int J ophthalmol. 2014;7(3):431–436. doi: 10.3980/j.issn.2222-3959.2014.03.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sayed NM, Ismail KA, Ahmed SA, Ezz-El-Din HM, Azzam HM. Possible association between Toxoplasma gondii infection and schizophrenia: Egyptian study. Infect Dis Clin Pract. 2012;20(6):394–399. [Google Scholar]
- Ferguson DJP, Hutchison WM, Pettersen E. Tissue cyst rupture in mice chronically infected with Toxoplasma gondii. Parasitol Res. 1989;75(8):599–603. doi: 10.1007/BF00930955. [DOI] [PubMed] [Google Scholar]
- Fuchs BS, Hadi S. Use of etanercept in the treatment of psoriasis and psoriatic arthritis. Rev Recent Clin Trials. 2006;1(3):259–263. doi: 10.2174/157488706778250131. [DOI] [PubMed] [Google Scholar]
- Gazzinelli RT, Eltoum I, Wynn TA, Sher A. Acute cerebral toxoplasmosis is induced by in vivo neutralization of TNF-α and correlates with the down-regulated expression of inducible nitric oxide synthase and other markers of macrophage activation. J Immunol. 1993;151(7):3672–3681. [PubMed] [Google Scholar]
- Gonzalez-Vicent M, Diaz MA, Sevilla J, Madero L. Cerebral toxoplasmosis following etanercept treatment for idiopathic pneumonia syndrome after autologous peripheral blood progenitor cell transplantation (PBPCT) Ann Hematol. 2003;82(10):649–653. doi: 10.1007/s00277-003-0705-2. [DOI] [PubMed] [Google Scholar]
- Halonen SK, Weiss LM. Toxoplasmosis. Handb Clin Neurol. 2013;114:125–145. doi: 10.1016/B978-0-444-53490-3.00008-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoff J, Rlagt LV. Methods of blood collection in the mouse. Lab Animal. 2000;29(10):47–53. [Google Scholar]
- Hunter CA, Suzuki Y, Subauste CS, Remington JS. Cells and cytokines in resistance to Toxoplasma gondii. Curr Top Microbiol Immunol. 1996;219:113–125. doi: 10.1007/978-3-642-51014-4_11. [DOI] [PubMed] [Google Scholar]
- Israelski D, Remington J. Toxoplasmosis in the non-AIDS immunocompromised host. Curr Clin Top Infect Dis. 1993;13:322–356. [PubMed] [Google Scholar]
- Johnson LL. A protective role for endogenous tumor necrosis factor in Toxoplasma gondii infection. Infect Immun. 1992;60(5):1979–1983. doi: 10.1128/iai.60.5.1979-1983.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongert E, Roberts CW, Gargano N, Forster-Waldl E, Petersen E. Vaccines against Toxoplasma gondii: challenges and opportunities. Mem Inst Oswaldo Cruz. 2009;104(2):252–266. doi: 10.1590/S0074-02762009000200019. [DOI] [PubMed] [Google Scholar]
- Jongert E, Lemiere A, Van Ginderachter J, De Craeye S, Huygen K, D’Souza S. Functional characterization of in vivo effector CD4+ and CD8+ T cell responses in acute toxoplasmosis: an interplay of IFN-gamma and cytolytic T cells. Vaccine. 2010;28(13):2556–2564. doi: 10.1016/j.vaccine.2010.01.031. [DOI] [PubMed] [Google Scholar]
- Koo S, Marty FM, Baden LR. Infectious complications associated with immunomodulating biologic agents. Infect Dis Clin N Am. 2010;24(2):285–306. doi: 10.1016/j.idc.2010.01.006. [DOI] [PubMed] [Google Scholar]
- Lang C, Gross U, Lüder CG. Subversion of innate and adaptive immune responses by Toxoplasma gondii. Parasitol Res. 2007;100(2):191–203. doi: 10.1007/s00436-006-0306-9. [DOI] [PubMed] [Google Scholar]
- Lassoued S, Zabraniecki L, Marin F, Billey T. Toxoplasmic chorioretinitis and antitumor necrosis factor treatment in rheumatoid arthritis. Semin Arthritis Rheum. 2007;36(4):262–263. doi: 10.1016/j.semarthrit.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Maubon D, Ajzenberg D, Brenier-Pinchart M-P, Dardé M-L, Pelloux H. What are the respective host and parasite contributions to toxoplasmosis? Trends Parasitol. 2008;24(7):299–303. doi: 10.1016/j.pt.2008.03.012. [DOI] [PubMed] [Google Scholar]
- Melzer TC, Cranston HJ, Weiss LM, Halonen SK. Host cell preference of Toxoplasma gondii cysts in murine brain: a confocal study. J Neuroparasitol. 2010;1:ID N10505. doi: 10.4303/jnp/N100505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng P, McCluskey PJ. Treatment of ocular toxoplasmosis. Aust Prescr. 2002;25(4):88–90. doi: 10.18773/austprescr.2002.075. [DOI] [Google Scholar]
- Ondriska F, Čatár G, Vozarová G. The significance of complement fixation test in clinical diagnosis of toxoplasmosis. Bratisl Lek Listy. 2003;104(6):89–196. [PubMed] [Google Scholar]
- Pusch L, Romeike B, Deckert M, Mawrin C. Persistent Toxoplasma bradyzoite cysts in the brain: incidental finding in an immunocompetent patient without evidence of a toxoplasmosis. Clin Neuropathol. 2009;28(3):210–212. doi: 10.5414/NPP28210. [DOI] [PubMed] [Google Scholar]
- Puvanesuaran VR, Nowroji K, Sreenivasan S, Noordin R, Balakrishnan V. Use of prednisolone to aid propagation of Toxoplasma gondii in mice. Eur Rev Med Pharmacol Sci. 2012;16(8):1028–1032. [PubMed] [Google Scholar]
- Radwan A, Baheti U, Arcinue CA, Hinkle DM. Acute unilateral Toxoplasma retinochoroiditis associated with adalimumab, a tumor necrosis factor-α antagonist. Retinal Cases Brief Rep. 2013;7(2):152–154. doi: 10.1097/ICB.0b013e3182790dbd. [DOI] [PubMed] [Google Scholar]
- Rodrigues KF, Faria e Arantes TE, Muccioli C, Neto JL, Pinheiro MM. Incidence of Toxoplasma retinochoroiditis in patients with ankylosing spondylitis after using TNF-α blockers. Parasitol Int. 2013;62(3):272–275. doi: 10.1016/j.parint.2013.02.003. [DOI] [PubMed] [Google Scholar]
- Romand S, Pudney M, Derouin F. In vitro and in vivo activities of the hydroxynaphthoquinone atovaquone alone or combined with pyrimethamine, sulfadiazine, clarithromycin, or minocycline against Toxoplasma gondii. Antimicrob Agents Chemother. 1993;37(11):2371–2378. doi: 10.1128/AAC.37.11.2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlüter D, Kaefer N, Hof H, Wiestler OD, Deckert-Schlüter M. Expression pattern and cellular origin of cytokines in the normal and Toxoplasma gondii-infected murine brain. Am J Pathol. 1997;150(3):1021–1035. [PMC free article] [PubMed] [Google Scholar]
- Sfikakis PP. The first decade of biologic TNF antagonists in clinical practice: lessons learned, unresolved issues and future directions. Curr Dir Autoimmun. 2010;11:180–210. doi: 10.1159/000289205. [DOI] [PubMed] [Google Scholar]
- Soheilian M, Ramezani A, Azimzadeh A, Sadoughi MM, Dehghan MH, Shahghadami R, Yaseri M, Peyman GA. Randomized trial of intravitreal clindamycin and dexamethasone versus pyrimethamine, sulfadiazine, and prednisolone in treatment of ocular toxoplasmosis. Ophthalmology. 2011;118(1):134–141. doi: 10.1016/j.ophtha.2010.04.020. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Wang X, Jortner BS, Payne L, Ni Y, Michie SA, Xu B, Kudo T, Perkins S. Removal of T. gondii cyst from the brain of paraffin-mediated activity CD8+ T-cells. Am J Pathol. 2010;176(4):1607–1613. doi: 10.2353/ajpath.2010.090825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toussirot É, Streit G, Wendling D. Infectious complications with anti-TNF alpha therapy in rheumatic diseases: a review. Recent Pat Inflamm Allergy Drug Discov. 2007;1(1):39–47. doi: 10.2174/187221307779815039. [DOI] [PubMed] [Google Scholar]
- Yazar S, Eser B, Yay M. Prevalence of anti-Toxoplasma gondii antibodies in Turkish blood donors. Ethiop Med J. 2006;44(3):257–261. [PubMed] [Google Scholar]
- Young JD, McGwire BS. Infliximab and reactivation of cerebral toxoplasmosis. N Engl J Med. 2005;353(14):1530–1531. doi: 10.1056/NEJMc051556. [DOI] [PubMed] [Google Scholar]