Abstract
Acanthamoeba spp. are free-living amoeba found in a wide variety of natural habitats. The high percentage of the presence of Acanthamoeba in different environmental sources represents a sanitary risk for public health, especially immunocompromised patients and contact lens wearers. Acanthamoeba can cause granulomatous amoebic encephalitis, otitis, lung lesions, and skin infections in individuals with immune deficiencies. In the present study, the status of contamination of water sources in Isfahan, central Iran is analyzed through parasitological method. Totally 93 samples were utilized consisting of 59 samples of tap water and 34 samples of environmental water collected from Isfahan in May and June 2014. After filtering, cultivation was done in non-nutrient agar medium, and then the cultured media were kept at 25–30 °C. The samples were analyzed based on the morphological criteria. Acanthamoeba spp. were found in 25 (73.53 %) out of 34 environmental water samples and 17 (28.8 %) out of 59 tap water. Generally, Acanthamoeba spp. were found in 42 (45.16 %) of the samples. The results of the present study showed that the water contamination with Acanthamoeba spp. in different regions of Isfahan can be a potential infection source for at high risk people. It could be suggested that public education and precaution are quiet necessary.
Keywords: Acanthamoeba, Water source, Isfahan, Iran
Introduction
Acanthamoeba is an important genus of free-living amoebae that has been commonly found in various water sources such as drinking water, swimming pools, sea water, mineral water and thermally polluted waters. Acanthamoeba spp., as an opportunistic pathogen, are among the major colonizing pathogen for granulomatous amoebic encephalitis (GAE) and a common infectious agent in amoebic keratitis (AK) and immunocompromised hosts such as AIDS patients (Siddiqui and Khan 2012; Trabelsi et al. 2012). Acanthamoeba is a Trojan horse for microorganisms such as Escherichia coli O157, Legionella pneumophila, Coxiella burnetii, Helicobacter pylori, Chlamydophila pneumoniae, Vibrio cholerae, Listeria monocytogenes, Campylobacter jejuni, Mycobacterium leprae and Pseudomonas aeruginosa (Axelsson-Olsson et al. 2005; Greub and Raoult 2004; Winiecka-Krusnell and Linder 2001). Prevalence of Acanthamoeba is correlated with the amount of organic matter present in the water, and its frequency is high in sediments and biofilms, which constitute ecological niches where they can feed on bacteria (Loret and Greub 2010). Two developmental stages of Acanthamoeba species can be found in their life cycle including a trophozoite stage that is vegetative and active with a size of about 13–23 μm, and a resistant cyst with a diameter about 13–23 μm (Siddiqui and Khan 2012). In addition, morphological features of the trophozoite are including the presence of spiny pseudopodia, called acanthopodia, and a large central karyosome in a vesicular nucleus, and a prominent contractile vacuole in the cytoplasm with a double-walled wrinkled cyst made up of an ectocyst and an endocyst. Cysts are resistant to antibiotics, chlorination, and biocides and survive in low temperatures (Marciano-Cabral and Cabral 2003). Since the importance of AK is increasing as a factor causing eye infection worldwide (Lorenzo-Morales et al. 2015), and due to the limited number of investigations, therefore, information on the occurrence and distribution of Acanthamoeba genus in environmental and drinking water in Isfahan province remains unclear. The aim of this study was to determine the epidemiological status of Acanthamoeba spp. in Isfahan, Iran.
Materials and methods
In this cross-sectional study, tap water (domestic tap water and public tap water) and environmental water (fountains) were studied. Using the cluster sampling, Isfahan city was divided into four clusters, and water samples were collected from each cluster randomly. A total of 93 water samples including 59 samples of drinking water (domestic tap water and public tap water) and 32 samples of environmental water were taken during May to June 2014 (Table 1). One liter of each water source transferred to the Department of Parasitology, Isfahan University of Medical Sciences. Isolation of Acanthamoeba spp. was done through 0.45 μm pore-sized cellulose nitrate membranes (Sartorius Stedim Biotech GmbH 37070 Goettingen Germany) (Rezaeian et al. 2008). The filters were immediately placed on 1.5 % non-nutrient agar (NNA) medium and the plates were sealed with Parafilm®, and were incubated at 25 °C for 2 months to have opportunity for growth of the amoebae. (Rahdar et al. 2012), and they were daily evaluated for growth of Acanthamoeba spp. Although, the media were pre-prepared using Amoeba Page Saline (0.04 mM CaCl2–6H2O, 1 mM KH2PO4, 2.5 mM NaCl, 0.02 mM MgSO2. 7H2O, 0.5 mM Na2HPO4 at PH 6.9). The plates were covered with a layer of E. coli (ATCC 25922) to be nutritious environment for Acanthamoeba. The final identification of samples was performed by using Giemsa and Trichrome staining methods (Garcia and Bruckner 1997) according to Page key (Page 1988).
Table 1.
Nnmber of samples taken from each cluster from Isfahan, central Iran, 2014
| Clusters | TW-public | TW-domestic | Environmental water | Total |
|---|---|---|---|---|
| CL 1 | 14 | 5 | 11 | 30 |
| CL 2 | 4 | 10 | 6 | 20 |
| CL 3 | 8 | 5 | 6 | 19 |
| CL 4 | 9 | 4 | 11 | 24 |
| Total | 35 | 24 | 34 | 93 |
TW Tap water
Results
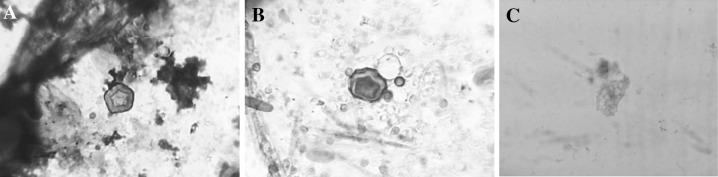
Acanthamoeba species were detected using parasitological methods based on morphological keys (Fig. 1).
Fig. 1.
a Acanthamoeba cyst X1000 (Giemsa stain), b Acanthamoeba cyst X100 (Trichrome stain), c Acanthamoeba trophozoite X100 (Trichrome stain)
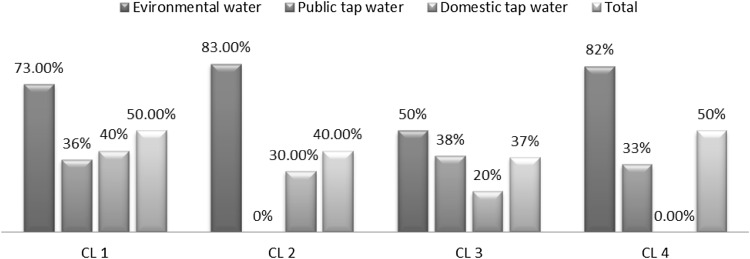
There were several Acanthamoeba spp. cysts and trophozoite in 42 samples (45.16 %) based on parasitological analyses of the amoeba growth from public tap water, domestic tap water and environmental (Table 2). The Acanthamoeba contamination rate in different water sources in different sampling area in Isfahan city is available in Fig. 2.
Table 2.
Prevalence of Acanthamoeba spp. in different water sources in Isfahan, Iran
| Water sources | Acanthamoeba spp. | Total | |
|---|---|---|---|
| Positive N (%) | Negative N (%) | ||
| Public (TW) | 11 (31.43) | 19 (67.57) | 35 |
| Domestic (TW) | 6 (25) | 18 (75) | 24 |
| Environmental | 25 (73.53) | 9 (26.47) | 34 |
| Total | 42 (45.16) | 51 (54.84) | 93 |
Fig. 2.
Acanthamoeba contamination rate of different water sources in Isfahan city divided clusters in 2014
Discussion
The results of this study showed that 42 samples out of 93 samples were contaminated by Acanthamoeba spp. In addition, the environmental water contamination rate was higher than that of drinking water. However, the contamination in public drinking water was observed higher than the residential drinking water. It should be noted that the extensive distribution network and the old public drinking water facilities may be are the factors. Given that the environmental water exposure through air and soil and other infectious sources, such as feces of birds and animals, as well as lack of water treatment and replacement and clean up too late are the factors that the contamination rate raises in the sources. Total contamination rate in cluster 1 and 2 were more than the other clusters, maybe it’s because they are old quarters with rusty distribution network. As the studied drinking water sources showed fairly high rates of contamination with the amoebae, and Acanthamoeba keratitis is increasing due to the increase in the use of contact lenses in developing countries in the recent years (Trabelsi et al. 2012) and the public education about the problem seems necessary. In addition, higher levels of public drinking water contamination with Acanthamoeba can cause corrosion in public drinking water distribution networks. The fact that public drinking water is more susceptible to be polluted by air and soil is not negligible, because the soil and air are among the major sources of Acanthamoeba (Trabelsi et al. 2012). Rahdar et al. (2012) and Yaygınlığı et al. (2012) reported 71.6 and 4.4 % of contamination rate with Achantomoeba, respectively, which are not close to the results of the present study. Similarly, Bagheri et al. (2010) in Iran were showed that Acanthamoeba spp. are present in 48 % of totals samples collected from fourteen cities of Iran (Bagheri et al. 2010). Also the contamination rate of drinking water with the amoebae in Taiwan was reported 39.5 % in 2014 (Kao et al. 2014). Furthermore, Winck et al. (2011), analysed 136 samples of tap water which showed lower contamination rates compared with ours (Winck et al. 2011). Due to the limitation in the sampling of drinking water in the public places, additional studies require with more samples. However, problems such as sampling, transportation of the samples and expensive filtration are other limitations in these kinds of studies. Due to the high rate of the contamination, in Isfahan area, water can be regarded as the potential infection source for at risk people, and using the contaminated water to wash the eyes may put them at risk of acquiring keratitis. Public education and precautions about Acanthamoeba seems quiet necessary in Isfahan.
Acknowledgments
This study is a part of MS thesis of Medical Parasitology and was financially supported by Vice-chancellor of Research and Technology, Isfahan University of Medical Sciences, Isfahan, Iran.
References
- Axelsson-Olsson D, Waldenström J, Broman T, Olsen B, Holmberg M. Protozoan Acanthamoebapolyphaga as a potential reservoir for Campylobacter jejuni. Appl Environ Microbiol. 2005;71:987–992. doi: 10.1128/AEM.71.2.987-992.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagheri H, Shafiei R, Shafiei F, Sajjadi S. Isolation of Acanthamoeba Spp. from drinking waters in several hospitals of Iran. Iran J Parasitol. 2010;5:19. [PMC free article] [PubMed] [Google Scholar]
- Garcia LS, Bruckner DA. Diagnostic medical parasitology. 4. Washington: ASM Press; 1997. [Google Scholar]
- Greub G, Raoult D. Microorganisms resistant to free-living amoebae. Clin Microbiol Rev. 2004;17:413–433. doi: 10.1128/CMR.17.2.413-433.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao P-M, et al. Seasonal distribution of potentially pathogenic Acanthamoeba species from drinking water reservoirs in Taiwan. Environ Sci Pollut Res Int. 2014;22:3766–3773. doi: 10.1007/s11356-014-3651-8. [DOI] [PubMed] [Google Scholar]
- Lorenzo-Morales J, Khan NA, Walochnik J. An update on Acanthamoeba keratitis: diagnosis, pathogenesis and treatment. Parasite. 2015;22:10. doi: 10.1051/parasite/2015010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loret J-F, Greub G. Free-living amoebae: biological by-passes in water treatment. Int J Hyg Environ Health. 2010;213:167–175. doi: 10.1016/j.ijheh.2010.03.004. [DOI] [PubMed] [Google Scholar]
- Marciano-Cabral F, Cabral G. Acanthamoeba spp. as agents of disease in humans. Clin Microbiol Rev. 2003;16:273–307. doi: 10.1128/CMR.16.2.273-307.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page FC. A new key to freshwater and soil gymnamoebae. Ambleside: Freshwater Biological Association; 1988. p. 122. [Google Scholar]
- Rahdar M, Niyyati M, Salehi M, Feghhi M, Makvandi M, Pourmehdi M, Farnia S. Isolation and genotyping of Acanthamoeba strains from environmental sources in Ahvaz City, Khuzestan Province, Southern Iran. Iran J Parasitol. 2012;7:22. [PMC free article] [PubMed] [Google Scholar]
- Rezaeian M, Niyyati M, Farnia S, Haghi AM. Isolation of Acanthamoeba spp. from different environmental sources. Iran J Parasitol. 2008;3:44–47. [Google Scholar]
- Siddiqui R, Khan NA. Biology and pathogenesis of Acanthamoeba. Parasit Vectors. 2012;5:262. doi: 10.1186/1756-3305-5-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trabelsi H, et al. Pathogenic free-living amoebae: epidemiology and clinical review. Pathol Biol (Paris) 2012;60:399–405. doi: 10.1016/j.patbio.2012.03.002. [DOI] [PubMed] [Google Scholar]
- Winck MAT, Caumo K, Rott MB. Prevalence of Acanthamoeba from tap water in Rio Grande do Sul, Brazil. Curr Microbiol. 2011;63:464–469. doi: 10.1007/s00284-011-0003-5. [DOI] [PubMed] [Google Scholar]
- Winiecka-Krusnell J, Linder E. Bacterial infections of free-living amoebae. Res Microbiol. 2001;152:613–619. doi: 10.1016/S0923-2508(01)01240-2. [DOI] [PubMed] [Google Scholar]
- Yaygınlığı ÇSK, ve Morfotiplendirmesi I. The prevalence, isolation and morphotyping of potentially pathogenic free-living amoebae from tap water and environmental water sources in Sivas Turkiye. Parazitol Derg. 2012;36:198–203. doi: 10.5152/tpd.2012.48. [DOI] [PubMed] [Google Scholar]