Abstract
Lipid synthesis is an important process in most organisms as well as in helminths. The present observation shows the variation of lipid and fatty acid uptake among cestode, Raillietina (Fuhrmannetta) echinobothrida; nematode, Ascaridia galli and their host, Gallus domesticus, the common country fowl. Total lipid (TL), neutral lipid (NL), glycolipid (GL), phospholipid (PL) and their fatty acid of cestode, nematode and liver and intestinal fluid of the host were analyzed by thin layer chromatography and gas liquid chromatography respectively. The result shows that liver take more TL, PL and GL except NL. Utilization of lipid from intestinal fluid when compare between the parasites, it is found that TL and PL content of cestode are higher than nematode, whereas, nematode absorbs more NL and GL than cestode. The percent of cholesterol is more in cestode than nematode. Palmitic, stearic, oleic and linoleic are the predominant fatty acids among all the samples. The present study reveals that the cestode having large surface area is more opportunistic in the resource utilization over the nematode as well as the host.
Keywords: Cestode, Nematode, Total lipid, Neutral lipid, Glycolipid, Phospholipid, TLC, GLC, Cholesterol
Introduction
Metabolic dependency of parasites on their host makes the relationship an obligatory one and study of relationships between hosts and the parasites may be considered an important subject (Bush et al. 2001). It is now explained that parasites are involved in exploitative trophic interaction with its host and cause some form of disadvantage or harm to the latter. This is termed as evolutionary ‘arm race’ by Dawkins (1986). Parasites derive their nutrient requirement directly from the stored nutrient (Cheng 1986) or from the ingested as well as partly digested food material of their hosts. Feeding relationships amongst organisms gave birth to animal associations. Competition between host and the parasite for food is probably the main selection pressure to initiate the evolution of host–parasite associations. Dependence of a parasite on the host for its food materials is of little importance unless it can utilize the food thus obtained.
Lipid and fatty acids requirements are different in different animals and are probably related to the need of the animal concerned. Parasitic stage of helminths does not use lipids normally as energy reserve (Barrett 1981). Lipids are believed to be the end product of carbohydrate metabolism in cestode. Lipids play an important role in the long-term adaptation and completion of life cycle during their endoparasitic stage (Sato et al. 2008). Cestodes have lost their capacity for de novo synthesis of lipids (Barrett 1983; Smyth and McManus 1989) and have become entirely dependent on the host. It is reported that the cestodes are able to absorb both short and long chain fatty acids through a mixture of diffusion and mediated transport (Smyth and McManus 1989). Triglycerides and all the phospholipids (PLs) were identified in Hymenolepis diminuta in its fasting host (rat) and cholesterol was the major unsaponifiable substance (Fairbairn et al. 1961). Parasitic worms display selective mechanisms for absorption of fatty acids from their environment and incorporate those absorbed fatty acids into various lipid fractions. The parenchyma of cestode is the most important tissue for the storage of lipids. Lipids also occur in the cestode tegument. It is thought that the ability to synthesize fatty acids de novo has been completely lost in parasitic helminths. Cestode body surface or tegument is highly active cellular covering and it acts as an interface between the worm and the intestinal content of the host and act as digestive–absorptive–protective surface (Dey and Misra 2009).
Studies of lipid metabolism in nematodes are important because lipids not only serve for storing and releasing energy, but also form important constituents of cell membranes. Nematodes that live under anaerobic conditions are not normally able to utilize lipids, but a slight increase in total lipids (TLs) has been reported in Ascaris during starvation (Beames et al. 1967). Smyth et al. (1996) identified 18 fatty acids from the surface and internal lipids of the filarial worm, Brugia malayi.
All nutrient molecules must be absorbed across the tegument through active transport, mediated diffusion and simple diffusion. The actual mechanism of lipid absorption has not been investigated, but it is likely to be a form of diffusion. Cestode tegument serves as a highly efficient digestive-absorptive layer that competes with the vertebrate mucosa for nutrients including heavy metals (Dalton et al. 2004). Fatty acids, monoglycerides, and sterols are absorbed at a considerably greater rate when they are in a micellar solution with bile salts (Bailey and Fairbairn 1968). Lipids of endoparasites play an important role in long-term adaptation for survival and completion of their life cycles in anaerobic condition (Sherman 1998; Sato et al. 2008). Changes in the dietary fatty acids usually result in changes in fatty acid composition of the animal concerned and a close relationship exists between fatty acid composition of that animal and the fatty acid content of its food source.
The major fatty acids of cestodes are usually C16 and C18 acids. Oleic (C18:1) is the major C18 acid, although linoleic acid (C18:2) or stearic acid (C18:0) predominate in some species. Of the fatty acids, 50–60 % is generally unsaturated in cestodes, which is similar to the situation in mammals. Cestodes are unable to synthesize long-chain fatty acids de novo from acetyl Co-A. Fatty acid synthesis in cestodes is restricted to chain lengthening of host derived fatty acids by the sequential addition of acetyl Co-A, although the mechanism for chain elongation is not known. According to Bankov et al. (1998), between nematodes and platyhelminthes, no gross difference present in lipid composition but TL content was higher in cestodes than digeneans and nematodes. Fatty acids of fungal feeding nematodes, Aphelenchus avenae and Aphelenchoides composticola were analyzed and different important fatty acids i.e. palmitic, stearic, arachidonic, oleic, linoleic, PL fatty acids, neutral lipid (NL) fatty acids were identified (Chen et al. 2001). Jadhav (2008) commented that mostly all the parasites absorb most of the food materials from host and fulfilling its need for development. A preliminary study has been made on major lipid classes and their fatty acids of Raillietina (Fuhrmannetta) echinobothrida (Mondal et al. 2009) and of Ascaridia galli (Ghosh et al. 2010). The total analysis of major lipid classes and fatty acids composition has already been done on trematode parasite, Paramphistomum cervi, rumen fluid and liver of the mammalian host, Capra hircus (Ghosh and Misra 2011). Ghosh and Misra (2014) made did further analysis by comparing the fatty acid components of the NLs and PLs of the trematode, P. cervi and liver of its mammalian host, C. hircus. Similar studies have not been performed in an avian system.
Detail analysis of major lipid classes and their fatty acids compositions of R. (F.) echinobothrida and A. galli and its avian host, Gallus domesticus have not yet studied indicating competition for lipid resources among the parasites and host. So, the present study is aimed to investigate in detail the major lipid classes and their fatty acid compositions of cestode, nematode and its host, country fowl, to understand the host–parasite interactions among them. The nematode parasite, A. galli and the cestode parasite, R. (F.) echinobothrida live in the upper and lower part of the intestine of the country fowl, G. domesticus respectively. These parasites obviously acquire lipids and fatty acids from the intestinal fluid of its host. Thus, both the host and the parasites compete for their lipids and fatty acids from a common pool i.e. intestinal fluid. Therefore, studies on the lipids and fatty acids of host tissues, both the parasites and intestinal fluid would help in understanding the host–parasite relationship as well as trophic interaction among them.
The tenet of the present study is to develop a comprehensive idea about the details of lipid classes and their fatty acids composition of the cestode, R. (F.) echinobothrida and nematode, A. galli and the liver of its host, G. domesticus in comparison to the lipids and fatty acid contents of the intestinal fluid. The hypothesis of the work is that though both the host and the two parasites take up lipid and fatty acids from a common source i.e. intestinal fluid; the parasites should absorb more fatty acids than its host as their requirement is high because parasite is unable to synthesize fatty acids de novo. Cestodes preferably absorb more fatty acids than the nematode because of the larger absorptive surface of the former.
To establish this hypothesis, the following working programme was followed:
Estimation of major lipid classes mainly TL, NL, glycolipid (GL) and PL of the liver of chicken (host) and intestinal fluid, nematode parasite A. galli and cestode parasite R. (F.) echinobothrida were done.
Fatty acids analysis of TL, NL, GL and PL of the nematode and cestode parasites, liver and intestinal fluid of its host were made.
Estimation of lipid fractions of the NL and PL of the nematode and cestode parasites, liver and intestinal fluid of its host, country fowl were done.
Lipid fractions and their fatty acid compositions of the components of the NLs such as hydrocarbon (HC), wax ester (WE), steryl ester (SE), triacylglycerols (TG) and sterol of nematode and cestode parasites and the liver and intestinal fluid of its host, country fowl were investigated.
Lipid fractions and their fatty acid composition of the components of PL such as cardiolipin (CL), phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylinositol (PI), phosphatidylserine (PS), and sphingomyelin (SPH) of the liver and intestinal fluid of host, G. domesticus and the nematode parasite, A. galli and the cestode parasite, R. (F.) echinobothrida were analyzed.
Materials and methods
The nematode parasite, A. galli and the cestode parasite, R. (F.) echinobothrida both inhabit in the upper and lower intestine of the country fowl, G. domesticus.
Raillietina (Fuhrmannetta) echinobothrida (Megnin 1880), (Stiles and Orleman 1926) is the most prevalent and pathogenic helminth parasite in birds, particularly in domestic fowl, G. domesticus (Linnaeus 1758). It requires two hosts, birds and ants, for completion of its life cycle. It is a hermaphrodite worm. Its body consists of a series of ribbon-like body segments. It is whitish in colour, highly elongated, dorsoventrally flattened, and entirely covered with a tegument. It measures about 25 cm in length and 1–1.5 cm in breadth. The parasite is responsible for ‘nodular tapeworm disease’ in poultry.
Ascaridia galli (Schrank 1788) is the largest nematode in birds. The body is semitransparent, creamy-white and cylindrical. The body is entirely covered with a thick proteinaceous cuticle. The life cycle of A. galli involves a single host. It causes ascaridiasis, a disease of poultry due to heavy infection, particularly in chicken and turkeys.
The chicken (Gallus gallus domesticus) is a domesticated fowl, a subspecies of the red jungle fowl. Chickens are omnivorous. They often scratch at the soil to search for seeds, insects etc. They may live for 5–10 years, depending on breed. Male chicken (roosters) have shiny, pointed feathers on their necks and backs which are brighter than females (hen) and have long tails. They have comb on their heads and hanging flaps of skin either side under their beaks called wattles.
Collection of samples
Ascaridia galli and R. (F.) echinobothrida were collected from the upper and lower portions of intestine of 120 country fowl from Uluberia, Howrah, West Bengal, India. The weight of the pooled samples i.e. cestode and nematode parasites, were 38.7 and 42.35 g respectively. Country fowl liver and intestinal fluid was also collected from the infected poultry and was pooled. Total 34.73 g of chicken liver and 178.33 ml of intestinal fluid from the infected hosts were taken for analysis. The parasites were kept in 0.85 % saline and the liver sample were washed in distilled water and then all the four samples were kept in the freezer at −20 °C for further analysis.
A flow chart of the entire work (Fig. 1) for lipid classes and their fatty acids of the cestode and nematode parasites and the liver and intestinal fluid of the host are presented.
Fig. 1.
The protocol of the entire work is represented through flow chart for lipid classes and their fractions of chicken liver, intestinal fluid, A. galli and R. (F.) echinobothrida. TL total lipid, HC hydrocarbon, CL cardiolipin, NL neutral lipid, WE wax ester, PE phosphatidylethanolamine, GL glycolipid, SE steryl ester, PC phosphatidylcholine, PL phospholipid, TG triacylglycerol, PI phosphatidylinositol, GLC gas liquid chromatography, ST sterol, SPH sphingomyelin, FAME fatty acid methyl ester, PS Phosphatidylserine
Extraction of total lipids (TL)
The TLs were extracted from the four samples following the method of Bligh and Dyer (1959) using methanol–chloroform (2:1 v/v), methanol–chloroform–water (2:1:0.8, v/v/v) and then again with the first solvent system. Samples were ground with the solvent, homogenized and filtered and residue was extracted with the next solvent system. The process was repeated. Finally, the three extracts were pooled, diluted with water and layer was allowed to separate in a separatory funnel. The chloroform layer at the bottom was withdrawn and dried over anhydrous sodium sulphate. The chloroform solution of lipid was evaporated under vacuum, weighed and redissolved in distilled n-hexane and kept at −20 °C for future use. BHT (butylated hydroxyl toluene) was added at a level of 100 mg/l to the solvent as antioxidant.
Fractionation of TL by column chromatography
A portion of the TLs were subjected to column chromatography using silicic acid (SRL) of 60–120 mesh size (Rouser et al. 1967). The neutral, glyco-, and PLs were eluted by the chloroform, acetone and methanol, respectively. The solvents were evaporated and fractions were kept in redistilled hexane at −20 °C. Each class of lipids was estimated by weighing in a microbalance.
Separation of NL components by TLC
TLC was performed on (20 cm × 20 cm) chromatoplates covered with silica gel G (0.5 mm thickness). The NL samples were fractionated to isolate the components by preparative TLC using petroleum ether (40–60 °C)—diethylether-acetic acid (80:20:1, v/v/v) according to Mangold (1969). The bands of TG and sterols (ST) were clearly separated but sterol esters (SEs), WEs and HCs, appeared as a single band were identified by comparison of their Rf values with those of the standards which were run on the same plates in narrow lanes, along with the samples, on the TLC plates. Bands were visualized by placing the plates into Iodine vapour chamber, were marked by a sharp needle, scrapped off the plates, by a sharp razor blade and extracted by charging them into mini glass columns and eluting out with chloroform, dried and weighed in an analytical balance, dissolved in n-hexane and kept in a freezer, till used.
Separation of HC, SE and WE by TLC
The three components, i.e. HC, SE and WE which were eluted out as a single band, were separated into individual components and were analysed according to Misra and Ghosh (1991) by TLC. The fractions containing HC, SE and WE together were spotted, as bands, on TLC plates (20 cm × 20 cm, coated with layer of Silica Gel, 0.5 mm thick). Standards were also spotted in separate on the TLC plates and were developed in a chamber containing n-hexane: diethyl ether (49:1, v/v). After development, plates were taken out of the chamber, air dried and put into Iodine vapour chamber to visualize the bands. Bands of the three components under investigation were marked with a sharp needle, scrapped off the plates and were put into mini glass columns (10 cm × 0.8 cm dia) and the components were eluted out by a mixture of solvents containing n-hexane:diethyl ether (1:1, v/v). The solvents were removed from the fractions thus obtained, in a rotary vacuum evaporator at 38 °C, weighed in a microbalance, dissolved in n-hexane: and kept in a freezer, till used.
Separation of PL components by TLC
The PL fractions obtained by column chromatography were further fractionated by TLC to obtain individual PLs. The PLs obtained by column chromatography were subjected to preparative TLC, according to Rouser et al. (1976). Thin layer plates (20 cm × 20 cm) were coated with silica gel-H (0.5 mm thick, Sigma Chemical Co., USA) and were activated at 120 °C for 90 min. After cooling to room temperature, samples were spotted on plates, as bands, along with standards in narrow lanes on the same plate and developed in chambers at 24 °C, containing solvent system of methanol: chloroform: water in ratio of 65:25:4, v/v/v. Replica plates containing standards, PC, PE, PI, PS and SPH were also developed in the same chamber and were sprayed with molybdenum blue reagent. The plates with samples were visualized by Iodine vapour in a glass chamber. Bands corresponding to standards were scrapped off the plates and extracted with chloroform:methanol (1:2, v/v). Solvents were removed under vacuum at 38 °C; the components thus obtained were then weighed in an analytical balance, dissolved in n-hexane and kept in a freezer, till used.
Preparation of silyl ether derivatives
Sterol samples were derivatised to their volatile trimethylsilylether derivatives for GLC analysis. Sterol samples (1–2 mg) and standards were taken into silylation vials and dried in a vacuum dessicator. To the samples and standard, 40 µl of TriSil-Z (Pierce Chemical Co., Rockford, IL, USA) were added in each of the vials, sealed and heated at 70 °C for 15 min. The samples were then cooled to room temperature and ready for GLC analysis (Misra et al. 1984). Identification was made by comparison of retention times with those of standards, according to Knights (1973).
Preparation of FAME
Fatty acid methyl esters (FAME) of triacylglycerol (TG), WEs, SEs, TLs, NLs, GLs, PLs and PL components i.e. CL, PC, PE, PI, SPH and PS were prepared by transmethylation according to Christie (2003).
The lipid samples (6–8 mg) were refluxed with methanolic sulphuric acid (1–2 %, v/v) for 2 h on a steam bath. After 2 h, most of the methanol was evaporated; the residual liquid was diluted with water and kept in a freezer. The cooled aqueous mixtures containing the methyl esters were then extracted thrice each with diethyl ether. The ethereal solutions were pooled and dried over anhydrous sodium sulfate. After drying, the solutions were filtered and diethyl ether was evaporated under vacuum at 38 °C. The methyl esters thus prepared were dissolved in n-hexane.
The crude methyl esters thus prepared were purified by thin layer chromatography (TLC) on silica gel G layers, using a solvent system of n-hexane: diethyl ether (90:10, v/v). Authentic standard fatty acid methyl esters were also run in a separate lane on the same TLC plate. After development bands were visualized by placing them into Iodine vapour chamber, marked with a sharp needle. Bands corresponding to standard methyl esters were marked and scrapped off the plate and extracted with diethyl ether, by placing them into mini glass columns. Ether was evaporated under vacuum, pure methyl esters were recovered, dissolved in n-hexane, sealed under a nitrogen stream and kept in freezer for GLC analysis.
Purification of FAME by TLC
Fatty acid methyl esters were purified by TLC using a solvent system of n-hexane-diethyl ether (90:10, v/v). A standard methyl ester was also run on the same plate in a separate lane. The location of methyl ester bands were done by placing the TLC plate in an iodine vapour chamber. The methyl ester bands corresponding to the standard were marked and then scrapped off the plate. Methyl esters were recovered by extracting the bands in a mini column with chloroform, the later was evaporated and the methyl esters were kept in n-hexane till analyzed by GLC.
Gas liquid chromatography (GLC)
GLC of fatty acid methyl esters were done on a Chemito 1000 instrument, equipped with flame ionization detector (FID). Quantization was done by computer using specific Clarity Lite software.
Analysis of fatty acid methyl esters (FAME)
GLC of FAME was done on a BPX-70 megabore capillary column of mt length and 0.53 mm i.d. obtained from SGE, Australia. Oven temperature was programmed from 150 to 240 °C with a rate of 8 °C/min. Initial and final temperatures were kept isothermal for 1 and 20 min, respectively. Injection port and detector temperatures were 250 and 300 °C, respectively. Nitrogen gas was used as carrier gas and its flow rate was 6.20 ml/min.
Analysis of sterol-trimethylsilyl ether derivatives
GLC of steryl-OTMS derivatives was done on a BP-1 megabore capillary column on 30mt length and 0.53 mm i.d. Injection port and detector temperatures were 300 and 350 °C, respectively. Nitrogen flow rate was 7.06 ml/min. Oven temperature was programmed from 280 to 320 °C @ 4 °C/min. Identification was made by comparison of retention times with those of standards, according to Knights (1973).
Analysis of hydrocarbons
HCs were analysed by directly injecting the samples into the GLC machine, without derivatization. Column used was a BP-1 megabore capillary column. Oven temperature was programmed from 150 to 300 °C @ 10 °C/min. Detector and injection port temperatures were at 350 and 300 °C respectively. Nitrogen gas flow rate was 8.1 ml/min. Identifications were made by comparison of retention times of the peaks with those of the standards, according to Misra and Ghosh (1991).
Identification of fatty acids was done by comparing their retention times with those of standards, chromatographed under identical operational conditions of GLC. A secondary standard of cod liver oil fatty acid methyl ester was also analysed under the same GLC conditions, as those for the sample methyl esters as suggested by Ackman and Burgher (1965).
Results
Percent composition of TL and three other lipid classes NL, GL, and PL of chicken liver, intestinal fluid and of the cestode and nematode parasites were presented in Table 1.
Table 1.
Composition of various classes of lipids obtained from chicken liver, chicken intestinal fluid, R. (F.) echinobothrida and A. galli
| Lipids | Chicken liver | Intestinal fluid | Raillietina | Ascaridia |
|---|---|---|---|---|
| Total lipids (TL)a | 4.06 | 2.40 | 3.23 | 2.33 |
| Neutral lipids (NL)b | 38.87 | 54.85 | 53.23 | 64.26 |
| Glycolipids (GL)b | 26.11 | 15.74 | 14.25 | 16.93 |
| Phospholipids (PL)b | 35.02 | 29.41 | 32.51 | 18.80 |
| (HC + WE + SE)c | 26.81 | 42.89 | 10.91 | 75.68 |
| Triacylglycerol (TG)c | 41.06 | 16.06 | 31.92 | 20.65 |
| Total Sterol (ST)c | 32.12 | 41.04 | 57.16 | 3.67 |
| Hydrocarbon (HC)d | 72.38 | 80.69 | 89.61 | 94.61 |
| Wax ester (WE)d | 20.08 | 17.89 | 8.43 | 4.42 |
| Steryl ester (SE)d | 7.53 | 1.41 | 1.96 | 0.96 |
| Cardiolipin (CL)e | 21.97 | 47.28 | 25.71 | 20.63 |
| Phosphatidylethanolamine (PE)e | 13.76 | 11.38 | 17.80 | 33.65 |
| Phosphatidylcholine (PC)e | 38.19 | 17.46 | 30.22 | 22.63 |
| Phosphatidylinositol (PI)e | 16.22 | 13.14 | 6.50 | 10.21 |
| Sphingomyelin (SPH)e | 6.06 | 4.25 | 7.72 | 5.40 |
| Phosphatidylserine (PS)e | 3.80 | 6.47 | 12.05 | 7.47 |
aExpressed as % w/w of wet tissue
bExpressed as % w/w of total lipids
cExpressed as % w/w of neutral lipids
dExpressed as % w/w of (HC + WE + SE)
eExpressed as % w/w of phospholipids
Chicken liver
The TL content of the liver was 4.06 % of the wet weight of the tissue, Percentage of lipid classes (expressed as % w/w of the TLs) of the liver were 38.87 (NL), 26.11 (GL) and 35.02 (PL). Triacylglycerol (41.06 %) occupied the major portion followed by total sterol (32.12 %) and HC + WE + SE (26.81 %). Among the HC + WE + SE, HC (72.38 %) was the major fraction. Liver showed highest content of WE and SE among intestinal fluid, cestode and nematode. The amount of PC (38.19 %) dominated over the other fractions of the PL.
Intestinal fluid of country fowl
The TL content of the intestinal fluid was 2.4 % (expressed as % w/w of wet weight of the tissue). The percentage of NL was highest (54.85 %) than PLs (29.41 %) and GLs (15.74 %) (Table 1). Amount of NL was maximum in nematode (64.26 %) followed by intestinal fluid (54.85 %), cestode (53.23 %) and chicken liver (38.87 %).
The percentage of HC + WE + SE (42.89) showed highest among the fractions of NL and then total sterol (41.04) took next position. The amount of triacylglycerol (16.06 %) was less in intestinal fluid than that of the other samples. HC constitute major portion 80.69 % than the WE and SE.
Cestode parasite
The TL content of the cestode parasite was 3.23 %. The percentages of NL, GL, and PL of the parasite were 53.23, 14.25 and 32.51 respectively (Table 1).
Maximum level of total sterol (57.16 %) was found among the fractions of NL. PC (30.22 %) and CL (25.71 %) was the major constituent among the PL fractions.
Nematode parasite
The TL content of the parasite was 2.33 %. The percentages of NL, GL and PL of the parasites were 64.26, 16.93, and 18.8 respectively (Table 1). Among the fractions of NL, HC + WE + SE were more in amount of which, HC was the major component and SE was the minor. Among the PL fractions, PE was found to be the dominant component followed by PC, CL, PI, PS and SPH respectively.
Total lipid
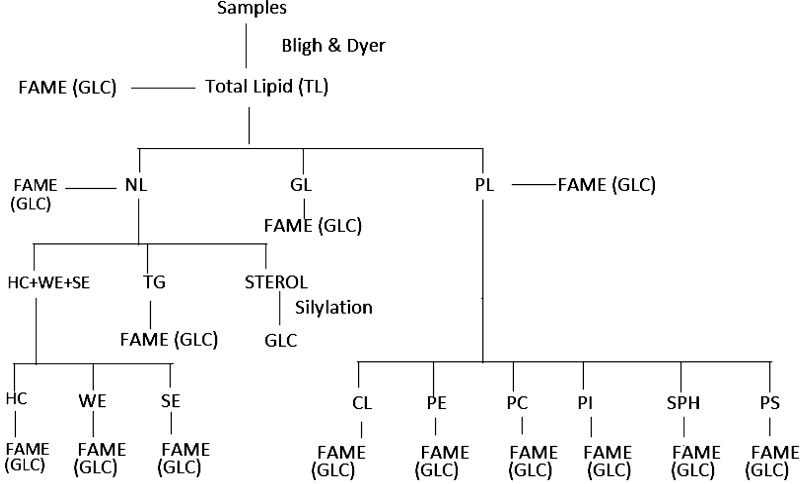
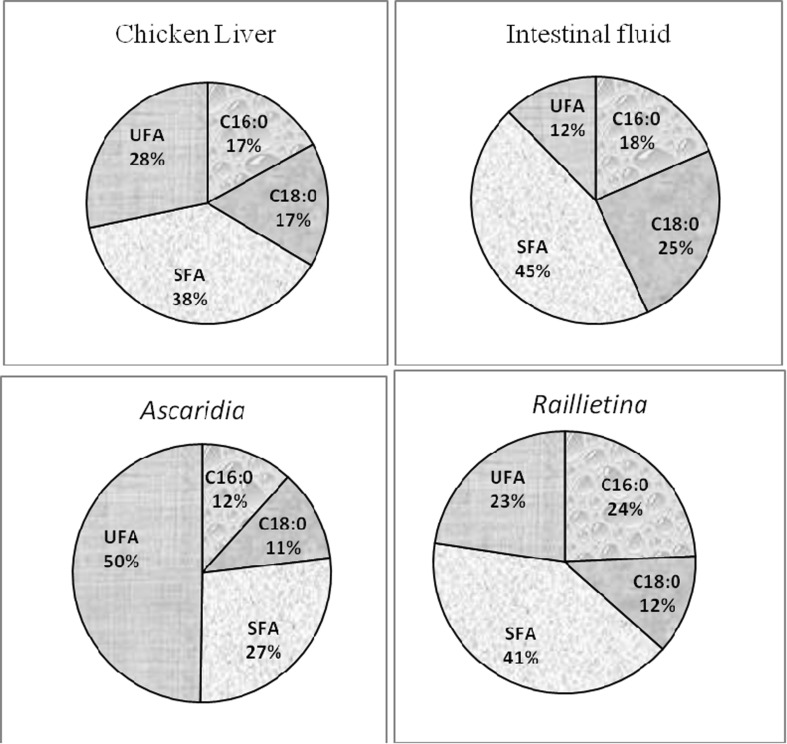
Of the 27 identified fatty acids, TL of country fowl liver, intestinal fluid, cestode and nematode parasite contained 26, 23, 23 and 21 fatty acids, respectively (Table 2). Chicken liver was the richest in saturated fatty acid and contain nearly 50.8 % saturated fatty acids (SFA), whereas, it was 42.3 % in intestinal fluid, 37.9 % in cestode and 40.5 % in nematode. Palmitic acid (C16:0), oleic acid (C18:1), and linoleic acid (C18:2) were the major components of all the four samples. Presence of higher level of stearic acid (C18:0) was also noticed in liver, intestinal fluid and nematode but it was significantly low in cestode (Fig. 2).
Table 2.
Comparison of fatty acid compositions of total lipid of chicken liver, intestinal fluid, R. (F.) echinobothrida and A. galli as determined by GLC of methylesters
| Components | Chicken liver | Intestinal fluid | Raillietina | Ascaridia |
|---|---|---|---|---|
| Saturates | ||||
| 14:0 | 0.2 | 0.5 | 1.0 | 1.0 |
| 15:0 | 0.1 | 0.2 | 0.5 | 1.9 |
| 16:0 | 27.7 | 23.5 | 27.7 | 20.2 |
| 17:0 | 0.4 | 0.3 | 0.3 | 2.8 |
| 18:0 | 14.5 | 13.6 | 5.1 | 12.4 |
| 20:0 | 0.1 | 0.3 | 1.1 | 0.8 |
| 22:0 | 7.4 | 3.7 | 1.6 | 1.0 |
| 24:0 | 0.4 | 0.2 | 0.6 | 0.4 |
| ∑ | 50.8 | 42.3 | 37.9 | 40.5 |
| Monoenes | ||||
| 16:1 | 0.6 | 0.3 | 0.7 | – |
| 17:1 | 0.1 | – | 0.1 | – |
| 18:1ω9 | 22.0 | 17.4 | 23.0 | 30.4 |
| 20:1ω9 | 0.2 | 0.4 | 0.9 | 0.6 |
| 22:1ω11 | 0.03 | 0.1 | 0.2 | 0.2 |
| 24:1 | 1.7 | 0.04 | 0.02 | – |
| ∑ | 24.63 | 18.24 | 24.92 | 31.2 |
| Dienes | ||||
| 18:2ω6 | 21.7 | 37.2 | 34.5 | 24.6 |
| 20:2 | 0.03 | – | – | – |
| ∑ | 21.73 | 37.2 | 34.5 | 24.6 |
| Polyenes | ||||
| 18:3ω6 | 0.1 | – | – | – |
| 18:3ω3 | 0.2 | 0.6 | 1.5 | 1.3 |
| 20:3ω6 | 0.4 | 0.2 | 0.4 | 0.6 |
| 20:3ω3 | 0.008 | – | – | – |
| 20:4ω6 | 0.4 | 0.3 | 0.2 | 0.1 |
| 20:4ω3 | 0.03 | 0.1 | 0.3 | 0.4 |
| 20:5ω3 | 0.006 | 0.2 | 0.1 | 0.2 |
| 21:5ω3 | 0.1 | 0.04 | 0.03 | 0.02 |
| 22:5ω6 | – | 0.02 | – | 0.01 |
| 22:5ω3 | 0.05 | 0.1 | 0.1 | 0.1 |
| 22:6ω3 | 1.6 | 0.8 | 0.3 | 0.2 |
| ∑ | 2.894 | 2.36 | 2.93 | 2.93 |
Fig. 2.
Comparison of percentage of palmitic (C16:0), stearic (C18:0), saturated fatty acid (SFA) and unsaturated fatty acid (UFA) of total lipid (TL) among chicken liver, intestinal fluid, A. galli and R. (F.) echinobothrida
Neutral lipid
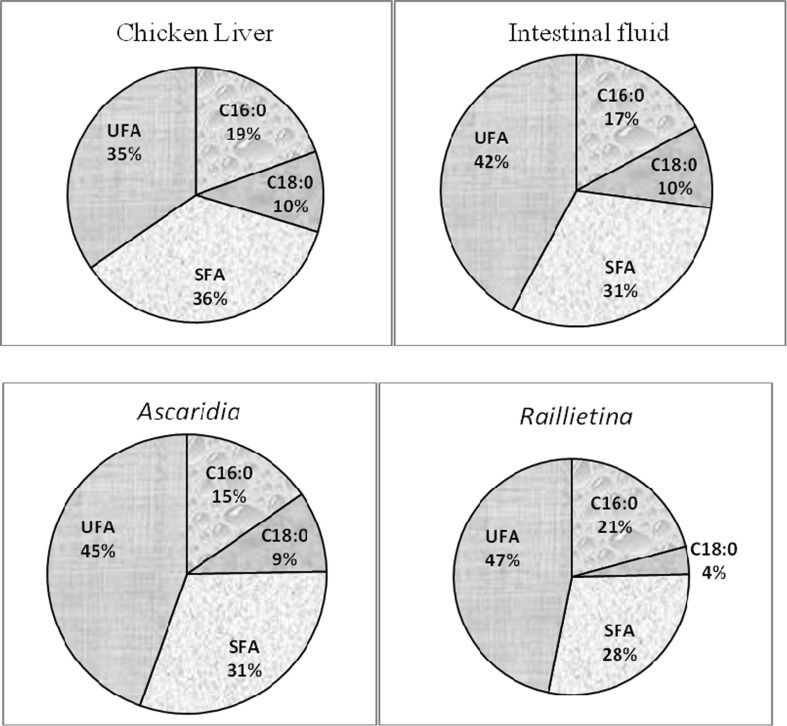
The number of identified fatty acids of NL in liver, intestinal fluid, cestode and nematode were 23, 22, 23 and 22 respectively (Table 3). The amount of total unsaturated fatty acids (UFA) was higher than total SFA in all the samples, whereas, cestode absorbed more UFA than other. Palmitic acid (C16:0) and stearic acid (C18:0) were the major SFA among all the samples, whereas, Ascaridia and Raillietina took up more palmitic acid than liver and intestinal fluid (Fig. 3). The content of MUFA was highest in liver. C14:1 only present in Ascaridia, whereas, C16:1 only found in that parasite. C17:1 was not found in intestinal fluid and nematode but a little amount of that fatty acid was observed in liver and cestode. C24:1 was absent in cestode and nematode. Oleic acid (C18:1ω9) was the predominant MUFA found in chicken liver, intestinal fluid, cestode and nematode.
Table 3.
Comparison of fatty acid compositions of neutral lipids (NL) of chicken liver, intestinal fluid, R. (F.) echinobothrida and A. galli as determined by GLC of methylesters
| Components | Chicken liver | Intestinal fluid | Raillietina | Ascaridia |
|---|---|---|---|---|
| Saturates | ||||
| 14:0 | – | 1.9 | 0.8 | 0.9 |
| 15:0 | – | 2.5 | 0.1 | 1.0 |
| 16:0 | 16.7 | 18.7 | 22.3 | 22.1 |
| 17:0 | 0.3 | 1.4 | 0.2 | 1.0 |
| 18:0 | 11.2 | 6.9 | 3.1 | 9.3 |
| 20:0 | 0.04 | 0.03 | 0.2 | 0.3 |
| 22:0 | 8.7 | 3.1 | 1.4 | 1.3 |
| 24:0 | 0.4 | 0.1 | 0.3 | 0.2 |
| ∑ | 37.34 | 34.63 | 28.4 | 36.1 |
| Monoenes | ||||
| 14:1 | – | – | – | 0.4 |
| 16:1 | 1.1 | 0.6 | 0.9 | – |
| 17:1 | 0.1 | – | 0.02 | – |
| 18:1ω9 | 31.0 | 21.2 | 26.0 | 27.5 |
| 20:1ω9 | 0.3 | 0.3 | 0.7 | 0.4 |
| 22:1ω11 | 0.04 | 0.1 | 0.1 | 0.1 |
| 24:1 | 1.2 | 0.1 | – | – |
| ∑ | 33.74 | 22.3 | 27.72 | 28.4 |
| Dienes | ||||
| 18:2ω6 | 25.9 | 40.9 | 40.2 | 31.7 |
| 20:2 | 0.02 | 0.03 | – | – |
| ∑ | 25.92 | 40.93 | 40.2 | 31.7 |
| Polyenes | ||||
| 18:3ω6 | 0.1 | – | – | – |
| 18:3ω3 | 0.3 | 1.0 | 2.3 | 1.8 |
| 20:3ω6 | 0.4 | 0.2 | 0.3 | 0.5 |
| 20:3ω3 | 0.01 | – | – | – |
| 20:4ω6 | 0.6 | 0.3 | 0.5 | 0.2 |
| 20:4ω3 | – | – | 0.03 | 0.2 |
| 20:5ω3 | 0.008 | 0.1 | 0.1 | 0.2 |
| 21:5ω3 | 0.02 | – | 0.02 | 0.6 |
| 22:5ω6 | – | 0.1 | 0.1 | 0.02 |
| 22:5ω3 | 0.1 | 0.03 | 0.1 | 0.1 |
| 22:6ω3 | 1.4 | 0.3 | 0.1 | 0.2 |
| ∑ | 2.938 | 2.03 | 3.55 | 3.82 |
Fig. 3.
Comparison of percentage of palmitic (C16:0), stearic (C18:0), saturated fatty acid (SFA) and unsaturated fatty acid (UFA) of neutral lipid (NL) among chicken liver, intestinal fluid, A. galli and R. (F.) echinobothrida
C20:2 was not present in cestode and nematode.
Among PUFA, C18:3ω6 and C20:3ω3 were only found in liver, whereas, C20:4ω3 and C22:5ω6 absent in liver. Percentage of C18:3ω3 was more in Raillietina followed by Ascaridia, intestinal fluid and then liver. Intestinal fluid lack C20:4ω3 and C21:5ω3 but found in both parasites.
Glycolipid
Twenty-seven, twenty-five, twenty-three and twenty-two fatty acids had been identified in liver, intestinal fluid, cestode and nematode parasites, respectively (Table 4). Liver had highest percentage (60.45 %) of SFA. The content of SFA was higher than UFA in liver, whereas, more UFA was found in intestinal fluid, cestode and nematode than SFA.
Table 4.
Comparison of fatty acid compositions of glycolipids (GL) of chicken liver, intestinal fluid, R. (F.) echinobothrida and A. galli as determined by GLC of methylesters
| Components | Chicken liver | Intestinal fluid | Raillietina | Ascaridia |
|---|---|---|---|---|
| Saturates | ||||
| 14:0 | 0.6 | 0.8 | 1.5 | 4.1 |
| 15:0 | 0.2 | 0.2 | 0.2 | 3.9 |
| 16:0 | 41.1 | 33.6 | 28.3 | 21.7 |
| 17:0 | 0.5 | 0.1 | 0.3 | 2.0 |
| 18:0 | 15.2 | 9.5 | 5.7 | 8.7 |
| 20:0 | 0.05 | 0.4 | 0.2 | 0.3 |
| 22:0 | 2.6 | 1.6 | 0.5 | 0.4 |
| 24:0 | 0.2 | 0.4 | 0.2 | 0.4 |
| ∑ | 60.45 | 46.6 | 36.9 | 41.5 |
| Monoenes | ||||
| 16:1 | 1.2 | – | 1.1 | – |
| 17:1 | 0.1 | 0.3 | – | – |
| 18:1ω9 | 21.5 | 15.4 | 25.8 | 32.8 |
| 20:1ω9 | 0.1 | 0.2 | 0.5 | 1.1 |
| 22:1ω11 | 0.04 | 0.1 | 0.2 | 0.8 |
| 24:1 | 0.2 | 0.1 | 0.008 | 0.01 |
| ∑ | 23.14 | 16.1 | 27.608 | 34.71 |
| Dienes | ||||
| 16:2 | – | – | – | 0.2 |
| 18:2ω6 | 15.4 | 33.4 | 33.1 | 21.4 |
| 20:2 | 0.02 | 0.02 | – | – |
| ∑ | 15.42 | 33.42 | 33.1 | 21.6 |
| Polyenes | ||||
| 18:3ω6 | 0.1 | 0.03 | – | – |
| 18:3ω3 | 0.1 | 0.7 | 1.7 | 0.6 |
| 20:3ω6 | 0.2 | 0.2 | 0.2 | 0.4 |
| 20:3ω3 | 0.003 | – | – | – |
| 20:4ω6 | 0.2 | 2.0 | 0.1 | 0.2 |
| 20:4ω3 | 0.1 | 0.1 | 0.1 | 0.2 |
| 20:5ω3 | 0.004 | 0.1 | 0.04 | – |
| 21:5ω3 | 0.1 | 0.8 | 0.03 | 0.8 |
| 22:5ω6 | 0.03 | 0.04 | 0.1 | 0.1 |
| 22:5ω3 | 0.01 | 0.04 | 0.03 | 0.02 |
| 22:6ω3 | 0.3 | 0.2 | 0.03 | 0.1 |
| ∑ | 1.147 | 4.21 | 2.33 | 2.42 |
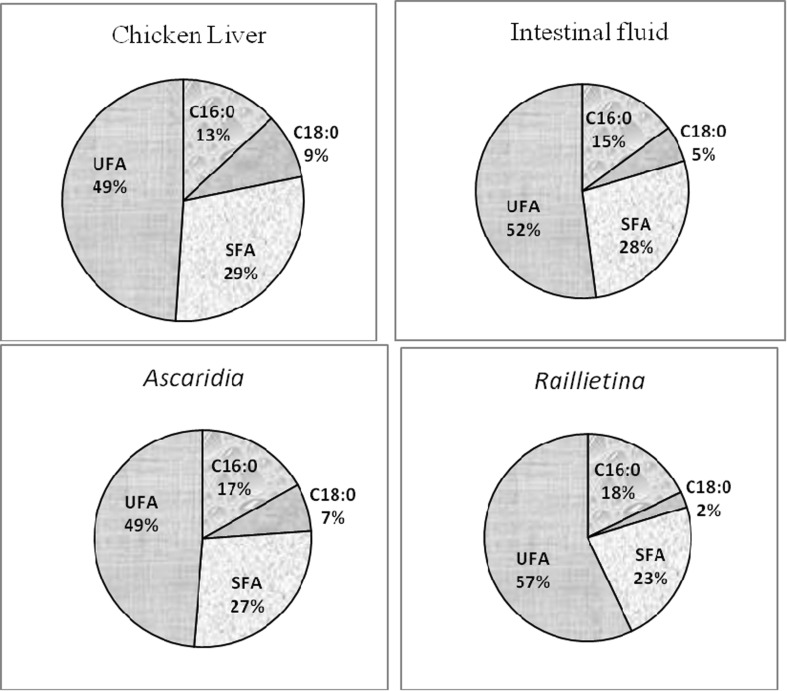
Palmitic and stearic acids were predominant SFA (Table 4). Highest amount of palmitic acid was found in liver (Fig. 4). Oleic acid was the major monounsaturated fatty acid. Linoleic (C18:2ω6) was the major DUFA found in all the four samples, whereas, the higher amount of that fatty acid was noticed in cestode than nematode and liver.
Fig. 4.
Comparison of percentage of palmitic (C16:0), stearic (C18:0), saturated fatty acid (SFA) and unsaturated fatty acid (UFA) of glycolipid (GL) among chicken liver, intestinal fluid, A. galli and R. (F.) echinobothrida
Phospholipid
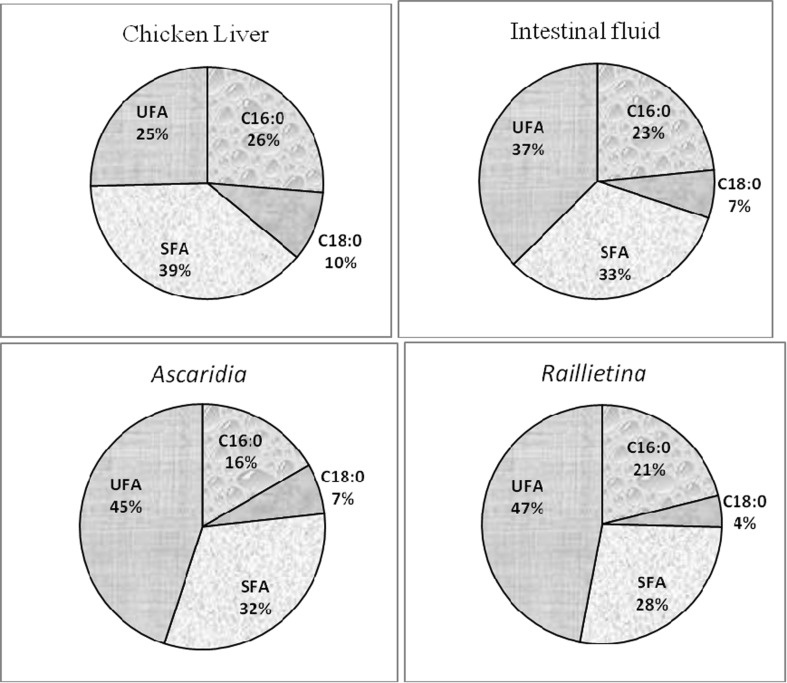
The number of identified fatty acids in the PL was same (24) in liver, intestinal fluid, cestode, while in nematode it was 25 (Table 5). It is noticed that among the UFA, A. galli exhibit highest percent of MUFA (46.82 %) and PUFA (5.54 %) than other samples. The total UFA was highest in nematode than the total SFA, whereas, in other samples i.e. chicken liver, intestinal fluid and cestode, more SFA were observed than UFA.
Table 5.
Comparison of fatty acid compositions of phospholipids (PL) of chicken liver, intestinal fluid, R. (F.) echinobothrida and A. galli as determined by GLC of methylesters
| Components | Chicken liver | Intestinal fluid | Raillietina | Ascaridia |
|---|---|---|---|---|
| Saturates | ||||
| 14:0 | – | 0.4 | 0.7 | 2.2 |
| 15:0 | – | 0.1 | 0.2 | 0.3 |
| 16:0 | 25.5 | 32.4 | 38.3 | 15.1 |
| 17:0 | 0.4 | 0.7 | 0.5 | 0.4 |
| 18:0 | 25.1 | 43.2 | 19.4 | 14.9 |
| 20:0 | 0.1 | 0.8 | 3.7 | 1.0 |
| 22:0 | 6.0 | 0.5 | 0.8 | 0.5 |
| 24:0 | 0.4 | 0.2 | 0.8 | 0.8 |
| ∑ | 57.5 | 78.3 | 64.4 | 35.2 |
| Monoenes | ||||
| 14:1 | – | – | – | 0.7 |
| 15:1 | – | – | – | 2.3 |
| 16:1 | 0.2 | 0.3 | 0.2 | 0.9 |
| 17:1 | 0.1 | 0.1 | 0.1 | 5.0 |
| 18:1ω9 | 21.0 | 6.8 | 20.8 | 34.4 |
| 20:1ω9 | 0.2 | 0.2 | 0.8 | 3.2 |
| 22:1ω11 | 0.009 | 0.4 | 0.1 | 0.3 |
| 24:1 | 2.4 | 0.005 | 0.05 | 0.02 |
| ∑ | 23.909 | 7.805 | 22.05 | 46.82 |
| Dienes | ||||
| 18:2ω6 | 16.3 | 9.7 | 11.8 | 12.2 |
| 20:2 | 0.008 | – | – | – |
| ∑ | 16.308 | 9.7 | 11.8 | 12.2 |
| Polyenes | ||||
| 18:3ω3 | 0.007 | 0.1 | 0.1 | 2.7 |
| 20:3ω6 | 0.5 | 0.1 | 0.5 | 0.6 |
| 20:3ω3 | 0.007 | – | – | – |
| 20:4ω6 | 0.2 | 3.4 | 0.1 | 0.7 |
| 20:4ω3 | 0.1 | 0.2 | 0.9 | – |
| 20:5ω3 | 0.006 | 0.01 | 0.04 | 0.6 |
| 21:5ω3 | 0.1 | 0.02 | 0.1 | 0.6 |
| 22:5ω6 | 0.008 | 0.1 | 0.03 | 0.2 |
| 22:5ω3 | 0.04 | 0.03 | 0.04 | 0.04 |
| 22:6ω3 | 1.4 | 0.2 | 0.1 | 0.1 |
| ∑ | 2.368 | 4.16 | 1.91 | 5.54 |
Among SFA, palmitic and stearic acids were predominant. Raillietina took up highest amount of C16:0 (Fig. 5). Liver was rich in C18:0 than the parasites. Among MUFA, C14:1 and C15:1 was only noticed in nematode. The maximum content of C16:1, C17:1, C18:1ω9 and C20:1ω9 were seen in Ascaridia.
Fig. 5.
Comparison of percentage of palmitic (C16:0), stearic (C18:0), saturated fatty acid (SFA) and unsaturated fatty acid (UFA) of phospholipid (PL) among chicken liver, intestinal fluid, A. galli and R. (F.) echinobothrida
Fatty acid composition of NL components
The HC profile showed that among 30 identified fatty acids, liver, intestinal fluid, cestode and nematode has 27, 28, 19 and 23 fatty acids, respectively (Table 6). Among the SFA, R. (F.) echinobothrida showed highest quantity of C14:0, C15:0, C16:0 and C17:0 and least amount of C24:0 than others. The percentage of iso fatty acids was more in cestode than others. In case of five identified anteiso fatty acids, no one was recorded from cestode parasite whereas liver, intestinal fluid and nematode contained 2, 4 and 2, respectively.
Table 6.
Comparison of compositions of hydrocarbons (HC) of chicken liver, intestinal fluid, R. (F.) echinobothrida and A. galli as determined by GLC of methylesters
| Components | Chicken liver | Intestinal fluid | Raillietina | Ascaridia |
|---|---|---|---|---|
| Saturate | ||||
| 14:0 | 1.9 | 3.7 | 5.3 | 1.7 |
| 15:0 | 3.7 | 6.1 | 7.7 | 5.3 |
| 16:0 | 4.9 | 8.4 | 11.6 | 7.2 |
| 17:0 | 7.1 | 11.4 | 11.9 | 10.2 |
| 18:0 | 14.8 | 12.4 | 13.7 | 11.9 |
| 19:0 | 7.3 | 8.9 | 6.9 | 8.9 |
| 20:0 | 8.1 | 9.3 | 8.5 | 8.7 |
| 21:0 | 8.2 | 7.4 | 4.7 | 7.7 |
| 22:0 | 9.7 | 6.4 | 4.2 | 6.6 |
| 23:0 | 6.3 | 4.1 | 2.0 | 4.8 |
| 24:0 | 4.7 | 2.7 | 0.4 | 3.6 |
| 25:0 | 2.6 | 1.4 | – | 5.4 |
| 26:0 | 1.7 | 0.7 | – | – |
| 27:0 | 0.5 | – | – | – |
| ∑ | 81.5 | 82.9 | 76.9 | 82.0 |
| Iso | ||||
| 14:iso | 0.2 | 0.5 | 0.3 | – |
| 15:iso | 1.8 | 1.5 | 4.4 | 1.3 |
| 16:iso | 1.5 | 2.1 | 2.1 | 1.6 |
| 17:iso | 2.6 | 4.1 | 5.1 | 3.8 |
| 18:iso | 2.3 | 3.4 | 3.5 | 3.5 |
| 19:iso | 2.1 | 2.8 | 4.0 | 3.2 |
| 20:iso | 2.1 | 0.2 | 2.1 | 2.5 |
| 21:iso | 1.6 | 0.2 | 1.6 | 1.2 |
| 22:iso | 2.6 | 0.2 | – | 0.2 |
| 23:iso | 0.2 | 0.2 | – | 0.1 |
| 24:iso | 0.1 | 0.5 | – | – |
| ∑ | 17.1 | 15.7 | 23.1 | 17.4 |
| Anteiso | ||||
| 20:anteiso | – | 0.4 | – | – |
| 21:anteiso | – | 0.4 | – | – |
| 22:anteiso | – | 0.4 | – | 0.4 |
| 23:anteiso | 0.6 | 0.4 | – | 0.2 |
| 24:anteiso | 0.3 | – | – | – |
| ∑ | 0.9 | 1.6 | 0 | 0.6 |
The fatty acid composition of WE was represented in Table 7. It was noticed that among identified 28 fatty acids, cestode (28) and nematode (26) had more fatty acids than that of liver (25) and intestinal fluid (25). Liver and nematode both were rich in C14:0 and C15:0 than other. Cestode also took up good content of C15:0 and C17:0. Highest amount of PUFA was found in A. galli.
Table 7.
Comparison of fatty acid compositions of wax ester (WE) of chicken liver, intestinal fluid, R. (F.) echinobothrida and A. galli as determined by GLC of methylesters
| Components | Chicken liver | Intestinal fluid | Raillietina | Ascaridia |
|---|---|---|---|---|
| Saturates | ||||
| 14:0 | 7.0 | 0.6 | 0.5 | 4.4 |
| 15:0 | 9.9 | 2.5 | 3.4 | 7.4 |
| 16:0 | 23.1 | 13.2 | 25.0 | 34.0 |
| 17:0 | 7.3 | 0.5 | 5.1 | 0.2 |
| 18:0 | 12.3 | 4.6 | 6.0 | 11.9 |
| 20:0 | 2.0 | 0.1 | 0.9 | – |
| 22:0 | 0.4 | 3.1 | 0.4 | 0.7 |
| 24:0 | 0.8 | 0.4 | 0.9 | 1.3 |
| ∑ | 62.8 | 25.0 | 42.2 | 59.9 |
| Monoenes | ||||
| 14:1 | – | – | 0.5 | 0.2 |
| 15:1 | – | – | 0.3 | 0.2 |
| 16:1 | 2.2 | 0.4 | 0.8 | 1.7 |
| 17:1 | 0.5 | 0.2 | 0.2 | 0.5 |
| 18:1ω9 | 15.7 | 26.6 | 17.6 | 5.5 |
| 20:1ω9 | 1.1 | 0.4 | 0.2 | 0.6 |
| 22:1ω11 | 0.2 | 0.4 | 0.2 | 1.4 |
| 24:1 | 0.6 | 0.1 | 0.9 | 0.6 |
| ∑ | 20.3 | 28.1 | 20.7 | 10.7 |
| Dienes | ||||
| 16:2 | – | – | 1.1 | 5.7 |
| 18:2ω6 | 6.7 | 44.8 | 23.9 | 4.3 |
| 20:2 | 0.5 | 0.1 | 0.2 | 0.5 |
| ∑ | 7.2 | 44.9 | 25.2 | 10.5 |
| Polyenes | ||||
| 18:3ω3 | 3.0 | 0.7 | 3.4 | 6.4 |
| 20:3ω6 | 1.4 | 0.3 | 2.0 | 2.7 |
| 20:4ω6 | 0.4 | 0.4 | 1.0 | 0.9 |
| 20:4ω3 | 0.5 | 0.1 | 0.4 | – |
| 20:5ω3 | 0.3 | 0.05 | 0.5 | 0.4 |
| 21:5ω3 | 0.3 | 0.1 | 0.1 | 0.2 |
| 22:5ω6 | 0.5 | 0.1 | 1.0 | 1.8 |
| 22:5ω3 | 1.2 | 0.01 | 0.9 | 1.3 |
| 22:6ω3 | 0.7 | 0.1 | 1.1 | 2.6 |
| ∑ | 8.3 | 1.86 | 10.4 | 16.3 |
Table 8 represented the fatty acid composition of SE in studied samples. Among identified 25 fatty acids, A. galli had all the 25 fatty acids, whereas R. (F.) echinobothrida, liver and intestinal fluid had 22, 17 and 21 fatty acids, respectively. It was noticed that among SFA of SE, liver showed highest percentage (52.7) than nematode 40.9 %, intestinal fluid (37 %) and cestode (16.5 %). Ascaridia absorbed all the SFA that were recorded in SE, of which, stearic and palmitic acids were predominant. Cestode took up good amount of C16:0, but stearic acid level was very low. Cestode was rich in UFA than the nematode. Oleic and linoleic fatty acids were major UFA in all the samples. C16:2 only found in nematode.
Table 8.
Comparison of fatty acid compositions of steryl ester (SE) of chicken liver, intestinal fluid, R. (F.) echinobothrida and A. galli as determined by GLC of methylesters
| Components | Chicken liver | Intestinal fluid | Raillietina | Ascaridia |
|---|---|---|---|---|
| Saturates | ||||
| 14:0 | – | 0.9 | 0.6 | 0.6 |
| 15:0 | 26.6 | 11.7 | 0.6 | 2.3 |
| 16:0 | 5.2 | 6.6 | 10.4 | 17.6 |
| 17:0 | – | – | 0.2 | 1.3 |
| 18:0 | 17.7 | 13.2 | 1.7 | 18.6 |
| 20:0 | – | 3.6 | – | 0.2 |
| 22:0 | 0.6 | 0.6 | 2.7 | 0.1 |
| 24:0 | 2.6 | 0.4 | 0.3 | 0.2 |
| ∑ | 52.7 | 37.0 | 16.5 | 40.9 |
| Monoenes | ||||
| 14:1 | – | – | 0.2 | 3.0 |
| 16:1 | 2.6 | 1.2 | 1.3 | 1.2 |
| 18:1ω9 | 9.1 | 8.4 | 34.6 | 16.7 |
| 20:1ω9 | – | 0.4 | 0.7 | 0.4 |
| 22:1ω11 | 0.9 | 1.4 | 0.3 | 0.3 |
| 24:1 | 0.8 | 0.1 | 0.1 | 0.4 |
| ∑ | 13.4 | 11.5 | 37.2 | 22.0 |
| Dienes | ||||
| 16:2 | – | – | – | 0.8 |
| 18:2ω6 | 16.1 | 47.9 | 42.9 | 32.7 |
| 20:2 | 1.2 | 0.9 | – | 0.4 |
| ∑ | 17.3 | 48.8 | 42.9 | 33.9 |
| Polyenes | ||||
| 18:3ω3 | 7.1 | 0.2 | 2.4 | 0.9 |
| 20:3ω6 | 1.6 | 0.2 | 0.4 | 0.1 |
| 20:4ω6 | 0.4 | 0.7 | 0.3 | 0.5 |
| 20:5ω3 | – | 0.1 | 0.1 | 0.05 |
| 21:5ω3 | 1.3 | – | 0.04 | 0.7 |
| 22:5ω6 | 0.8 | 0.9 | 0.004 | 0.1 |
| 22:5ω3 | – | 0.3 | 0.1 | 0.1 |
| 22:6ω3 | 4.6 | 0.02 | 0.1 | 0.9 |
| ∑ | 15.8 | 2.42 | 3.444 | 3.35 |
Comparative account of fatty acids of TG of four samples was represented in Table 9. It was recorded that among 26 fatty acids recorded, liver and nematode both have 24, whereas, intestinal fluid and cestode both have 22 fatty acids. It was also recorded that, cestode exhibit highest percentage of SFA (40.5 %) than other. Cestode was rich in palmitic acid but low in stearic acid. Highest content of stearic acid was found in liver. C14:1 and C17:1 only present in nematode. All the four samples had very good amount of oleic (C18:1w9) and linoleic (C18:2w6) acids.
Table 9.
Comparison of fatty acid compositions of triacylglycerol (TG) of chicken liver, intestinal fluid, R. (F.) echinobothrida and A. galli as determined by GLC of methylesters
| Components | Chicken liver | Intestinal fluid | Raillietina | Ascaridia |
|---|---|---|---|---|
| Saturates | ||||
| 14:0 | 3.0 | 0.6 | 1.3 | 1.5 |
| 15:0 | 3.2 | 0.2 | 0.1 | 0.1 |
| 16:0 | 19.1 | 22.6 | 36.5 | 26.2 |
| 17:0 | 2.9 | 0.3 | 0.2 | 0.3 |
| 18:0 | 8.3 | 6.3 | 1.2 | 7.6 |
| 20:0 | 0.5 | 0.3 | 0.2 | 0.05 |
| 22:0 | 3.3 | 1.5 | 0.9 | 1.2 |
| 24:0 | 0.1 | 0.1 | 0.1 | 0.02 |
| ∑ | 40.4 | 31.9 | 40.5 | 36.97 |
| Monoenes | ||||
| 14:1 | – | – | – | 0.8 |
| 16:1 | 0.8 | 0.6 | 0.6 | 0.4 |
| 17:1 | – | – | – | 0.05 |
| 18:1ω9 | 34.1 | 25.7 | 20.1 | 25.9 |
| 20:1ω9 | 0.2 | 0.4 | 0.7 | 0.3 |
| 22:1ω11 | 0.03 | 0.1 | 0.1 | 0.01 |
| 24:1 | 0.4 | – | 0.01 | 0.04 |
| ∑ | 35.53 | 26.8 | 21.51 | 27.5 |
| Dienes | ||||
| 18:2ω6 | 21.8 | 39.4 | 35.2 | 32.5 |
| ∑ | 21.8 | 39.4 | 35.2 | 32.5 |
| Polyenes | ||||
| 18:3ω6 | 0.1 | – | – | – |
| 18:3ω3 | 0.4 | 1.0 | 1.8 | 2.1 |
| 20:3ω6 | 0.5 | 0.2 | 0.3 | 0.2 |
| 20:4ω6 | 0.3 | 0.2 | 0.1 | 0.1 |
| 20:4ω3 | 0.3 | 0.1 | 0.03 | 0.02 |
| 20:5ω3 | 0.05 | 0.1 | 0.1 | 0.1 |
| 21:5ω3 | 0.2 | 0.03 | 0.2 | 0.03 |
| 22:5ω6 | 0.1 | 0.03 | – | – |
| 22:5ω3 | 0.1 | 0.05 | 0.1 | 0.02 |
| 22:6ω3 | 0.2 | 0.2 | 0.1 | 0.1 |
| ∑ | 2.25 | 1.91 | 2.73 | 2.67 |
Composition of sterols
Composition of sterols obtained from host liver, intestinal fluid, cestode and nematode were presented in Table 10. Four categories of sterols were identified and one is unidentified. Liver and intestinal fluid were highly rich in cholesterol about 96.7 and 85.9 % respectively, whereas, cestode was also high (75 %), but less than above samples and more than nematode (56.5 %). Cestode and nematode contain greater quantity of campesterol than liver and intestinal fluid nematode showed highest amount of stigmasterol (25.3 %), three times higher than cestode.
Table 10.
Comparison of compositions of sterols obtained from chicken liver, intestinal fluid, R. (F.) echinobothrida and A. galli analyzed as silyl ether derivatives by GLC
| Components | Chicken liver | Intestinal fluid | Raillietina | Ascaridia |
|---|---|---|---|---|
| Unidentified | 0.3 | 1.3 | 0.2 | – |
| Cholesterol | 96.7 | 85.9 | 75.0 | 56.5 |
| Campesterol | 0.6 | 8.0 | 17.2 | 18.2 |
| Stigmasterol | 2.1 | 4.7 | 7.1 | 25.3 |
| β-Sitosterol | 0.2 | 0.1 | 0.5 | – |
Fatty acid composition of PL components
Comparison of fatty acids composition of CL, PE, PC, PI, SPH, and PS were shown in Tables 11, 12, 13, 14, 15 and 16. From those tables, it was found that, cestode contain maximum number of fatty acids in CL, PE, SPH and PS components.
Table 11.
Comparison of fatty acid composition of cardiolipin of chicken liver, intestinal fluid, R. (F.) echinobothrida and A. galli as determined by GLC
| Components | Chicken liver | Intestinal fluid | Raillietina | Ascaridia |
|---|---|---|---|---|
| Saturates | ||||
| 14:0 | 0.2 | 10.9 | 0.4 | 6.3 |
| 15:0 | 0.2 | 12.8 | 0.9 | 7.0 |
| 16:0 | 17.0 | 26.8 | 24.0 | 18.4 |
| 17:0 | 0.7 | 10.2 | 1.2 | 5.8 |
| 18:0 | 27.6 | 9.5 | 16.2 | 15.0 |
| 20:0 | 0.04 | – | 4.2 | – |
| 22:0 | 6.5 | 0.2 | 1.2 | 0.8 |
| 24:0 | 0.6 | 0.5 | 1.1 | 0.2 |
| ∑ | 52.84 | 70.9 | 49.2 | 53.5 |
| Monoenes | ||||
| 16:1 | 0.6 | – | – | – |
| 17:1 | 0.3 | – | – | – |
| 18:1ω9 | 14.9 | 3.3 | 31.6 | 19.1 |
| 20:1ω9 | 0.2 | 2.9 | 0.6 | 1.4 |
| 22:1ω11 | 0.2 | 0.02 | 0.1 | 1.2 |
| 24:1 | 2.3 | 1.7 | 0.1 | 0.5 |
| ∑ | 18.5 | 7.92 | 32.4 | 22.2 |
| Dienes | ||||
| 18:2ω6 | 24.6 | 13.6 | 16.2 | 20.1 |
| 20:2 | 0.02 | – | – | 0.6 |
| ∑ | 24.62 | 13.6 | 16.2 | 20.61 |
| Polyenes | ||||
| 18:3ω6 | – | – | 0.02 | – |
| 18:3ω3 | 0.02 | 1.1 | 0.1 | 0.9 |
| 20:3ω6 | 0.9 | 2.2 | 0.4 | 0.1 |
| 20:4ω6 | 1.7 | 0.1 | 0.1 | 1.0 |
| 20:4ω3 | – | 1.4 | 1.1 | – |
| 20:5ω3 | – | 0.1 | 0.1 | 0.5 |
| 21:5ω3 | 0.1 | – | 0.04 | – |
| 22:5ω6 | – | 1.7 | 0.1 | 0.8 |
| 22:5ω3 | 0.1 | 0.1 | 0.05 | 0.2 |
| 22:6ω3 | 1.1 | 0.7 | 0.2 | 0.006 |
| ∑ | 3.92 | 7.4 | 2.21 | 3.506 |
Table 12.
Comparison of fatty acid composition of phosphatidylserine of chicken liver, intestinal fluid, R. (F.) echinobothrida and A. galli as determined by GLC
| Components | Chicken liver | Intestinal fluid | Raillietina | Ascaridia |
|---|---|---|---|---|
| Saturates | ||||
| 14:0 | – | 1.9 | – | – |
| 15:0 | 0.3 | 2.8 | 1.3 | – |
| 16:0 | 28.3 | 43.0 | 54.2 | 22.2 |
| 17:0 | 0.6 | 0.6 | 1.4 | 0.7 |
| 18:0 | 27.3 | 42.2 | 25.9 | 28.2 |
| 20:0 | 0.04 | 0.8 | 2.8 | – |
| 22:0 | 0.8 | 0.03 | 0.01 | 0.5 |
| 24:0 | 0.1 | 0.02 | 0.3 | 0.2 |
| ∑ | 57.44 | 91.35 | 85.91 | 51.8 |
| Monoenes | ||||
| 16:1 | – | – | 0.1 | – |
| 17:1 | 0.2 | 0.8 | 0.1 | – |
| 18:1ω9 | 6.6 | 1.4 | 7.4 | 34.0 |
| 20:1ω9 | 0.1 | 0.4 | 0.4 | 1.2 |
| 22:1ω11 | 3.7 | 0.2 | 0.1 | 0.2 |
| 24:1 | 0.2 | 0.4 | 0.1 | – |
| ∑ | 10.8 | 3.2 | 8.2 | 35.4 |
| Dienes | ||||
| 18:2ω6 | 4.2 | 2.3 | 3.9 | 9.9 |
| 20:2 | 0.1 | – | – | – |
| ∑ | 4.3 | 2.3 | 3.9 | 9.9 |
| Polyenes | ||||
| 18:3ω6 | – | 0.3 | – | – |
| 18:3ω3 | 0.1 | 0.2 | 0.1 | 1.4 |
| 20:3ω6 | – | 0.2 | 0.3 | 0.6 |
| 20:4ω6 | 26.7 | 0.4 | 0.1 | 0.3 |
| 20:4ω3 | 0.1 | 0.4 | 0.6 | – |
| 20:5ω3 | – | – | 0.003 | – |
| 21:5ω3 | 0.3 | 0.6 | 0.03 | – |
| 22:5ω6 | 0.1 | 0.2 | 0.3 | 0.5 |
| 22:5ω3 | 0.02 | – | 0.1 | – |
| 22:6ω3 | 0.1 | 0.6 | 0.1 | 0.02 |
| ∑ | 27.42 | 2.9 | 1.633 | 2.82 |
Table 13.
Comparison of fatty acid composition of phosphatidyl–ethanolamine of chicken liver, intestinal fluid, R. (F.) echinobothrida and A. galli as determined by GLC
| Components | Chicken liver | Intestinal fluid | Raillietina | Ascaridia |
|---|---|---|---|---|
| Saturates | ||||
| 14:0 | – | 1.9 | 0.5 | 0.6 |
| 15:0 | – | 2.5 | 0.6 | 0.1 |
| 16:0 | 19.3 | 17.6 | 28.7 | 16.2 |
| 17:0 | 0.6 | 2.3 | 0.7 | 1.0 |
| 18:0 | 31.1 | 21.7 | 18.9 | 19.3 |
| 20:0 | 0.1 | 1.1 | 2.9 | 0.8 |
| 22:0 | 7.5 | 1.6 | 1.3 | 0.6 |
| 24:0 | 0.6 | 0.1 | 1.1 | 0.3 |
| ∑ | 59.2 | 48.8 | 54.7 | 38.9 |
| Monoenes | ||||
| 14:1 | – | – | – | 1.2 |
| 16:1 | 0.3 | 0.9 | 0.2 | – |
| 17:1 | 0.5 | – | 0.03 | 0.6 |
| 18:1ω9 | 17.9 | 18.4 | 25.4 | 43.4 |
| 20:1ω9 | 0.2 | 0.2 | 1.1 | 2.0 |
| 22:1ω11 | 0.2 | 0.04 | 0.2 | 0.3 |
| 24:1 | 2.8 | 0.2 | 0.02 | – |
| ∑ | 21.9 | 19.74 | 26.95 | 47.5 |
| Dienes | ||||
| 18:2ω6 | 14.5 | 28.1 | 16.2 | 12.3 |
| 20:2 | 0.1 | – | 0.007 | 0.03 |
| ∑ | 14.6 | 28.1 | 16.207 | 12.33 |
| Polyenes | ||||
| 18:3ω6 | – | 0.1 | 0.006 | – |
| 18:3ω3 | – | 0.3 | 0.2 | 0.03 |
| 20:3ω6 | 0.6 | 0.8 | 0.7 | 0.6 |
| 20:4ω6 | 2.0 | 0.2 | 0.2 | 0.1 |
| 20:4ω3 | – | 0.5 | 0.8 | – |
| 20:5ω3 | – | 0.1 | 0.03 | 0.2 |
| 21:5ω3 | 0.1 | 0.03 | 0.04 | 0.2 |
| 22:5ω6 | – | 0.1 | 0.1 | – |
| 22:5ω3 | 0.1 | 0.1 | 0.05 | 0.02 |
| 22:6ω3 | 1.6 | 0.8 | 0.2 | 0.03 |
| ∑ | 4.4 | 3.03 | 2.326 | 1.18 |
Table 14.
Comparison of fatty acid composition of phosphatidylinositol of chicken liver, intestinal fluid, R. (F.) echinobothrida and A. galli as determined by GLC
| Components | Chicken liver | Intestinal fluid | Raillietina | Ascaridia |
|---|---|---|---|---|
| Saturates | ||||
| 14:0 | 0.3 | 1.6 | – | 0.6 |
| 15:0 | 0.4 | 0.3 | – | 0.2 |
| 16:0 | 41.1 | 28.0 | 39.4 | 34.3 |
| 17:0 | 0.5 | 0.8 | – | 1.1 |
| 18:0 | 18.2 | 56.1 | 30.6 | 19.8 |
| 20:0 | 0.1 | 1.2 | 3.5 | 1.0 |
| 22:0 | 0.8 | 0.03 | 0.1 | 0.2 |
| 24:0 | 0.05 | 0.2 | 0.7 | 0.2 |
| ∑ | 61.45 | 88.23 | 74.3 | 57.4 |
| Monoenes | ||||
| 14:1 | – | – | – | 0.9 |
| 15:1 | – | 0.9 | – | – |
| 16:1 | 0.2 | – | – | – |
| 17:1 | 0.1 | 1.9 | – | – |
| 18:1ω9 | 26.1 | 2.5 | 9.1 | 33.8 |
| 20:1ω9 | 0.1 | 0.4 | 0.3 | 0.9 |
| 22:1ω11 | 0.1 | 0.01 | 0.2 | – |
| 24:1 | 0.2 | 0.2 | 0.3 | – |
| ∑ | 26.8 | 5.91 | 9.9 | 35.6 |
| Dienes | ||||
| 18:2ω6 | 10.1 | 4.6 | 11.1 | 6.2 |
| 20:2 | 0.04 | – | – | 0.02 |
| ∑ | 10.14 | 4.6 | 11.1 | 6.22 |
| Polyenes | ||||
| 18:3ω6 | – | 0.3 | – | – |
| 18:3ω3 | – | 0.1 | 0.6 | 0.04 |
| 20:3ω6 | 0.2 | 0.2 | 0.6 | 0.4 |
| 20:4ω6 | 0.6 | 0.3 | 0.2 | 0.01 |
| 20:4ω3 | 0.1 | 0.2 | 0.9 | – |
| 20:5ω3 | – | – | 0.007 | 0.1 |
| 21:5ω3 | 0.1 | 0.04 | 0.007 | 0.008 |
| 22:5ω6 | – | 0.2 | 1.3 | – |
| 22:5ω3 | 0.006 | 0.007 | 0.7 | 0.004 |
| 22:6ω3 | 0.1 | 0.005 | 0.3 | 0.01 |
| ∑ | 1.106 | 1.352 | 4.614 | 0.572 |
Table 15.
Comparison of fatty acid composition of sphingomyelin of chicken liver, intestinal fluid, R. (F.) echinobothrida and A. galli as determined by GLC
| Components | Chicken liver | Intestinal fluid | Raillietina | Ascaridia |
|---|---|---|---|---|
| Saturates | ||||
| 14:0 | 0.2 | 3.4 | 1.8 | – |
| 15:0 | – | 10.6 | 6.0 | – |
| 16:0 | 31.8 | 31.5 | 20.4 | 15.5 |
| 17:0 | 0.7 | 8.0 | 4.2 | 0.6 |
| 18:0 | 26.0 | 24.9 | 34.0 | 41.3 |
| 20:0 | 1.0 | 2.3 | 4.4 | – |
| 22:0 | 1.4 | 0.04 | 0.3 | 0.9 |
| 24:0 | 0.4 | 0.5 | 0.9 | 1.5 |
| ∑ | 61.5 | 81.24 | 72.0 | 59.8 |
| Monoenes | ||||
| 14:1 | – | – | 1.0 | – |
| 16:1 | – | – | 0.4 | – |
| 17:1 | 0.3 | – | – | – |
| 18:1ω9 | 6.4 | 2.3 | 8.7 | 24.5 |
| 20:1ω9 | 0.1 | 0.04 | 0.6 | 0.9 |
| 22:1ω11 | 2.5 | 0.1 | 0.2 | – |
| 24:1 | 0.2 | 1.0 | 0.3 | – |
| ∑ | 9.5 | 3.44 | 11.2 | 25.4 |
| Dienes | ||||
| 18:2ω6 | 4.3 | 9.5 | 10.6 | 10.3 |
| 20:2 | 0.04 | – | – | – |
| ∑ | 4.34 | 9.5 | 10.6 | 10.3 |
| Polyenes | ||||
| 18:3ω3 | – | 0.8 | 0.6 | 1.9 |
| 20:3ω6 | 0.2 | 1.4 | 1.3 | 0.3 |
| 20:4ω6 | 20.4 | 0.4 | 0.3 | 1.8 |
| 20:4ω3 | 2.1 | 1.4 | 1.3 | – |
| 20:5ω3 | – | 0.03 | – | – |
| 21:5ω3 | 0.2 | 0.1 | – | – |
| 22:5ω6 | 0.03 | 0.6 | 1.5 | 0.6 |
| 22:5ω3 | 0.02 | 0.4 | 0.2 | – |
| 22:6ω3 | 0.3 | 0.1 | 0.4 | 0.02 |
| ∑ | 23.25 | 5.23 | 5.6 | 4.62 |
Table 16.
Comparison of fatty acid compositions of phosphatidylcholine of chicken liver, intestinal fluid, R. (F.) echinobothrida and A. galli as determined by GLC of methylesters
| Components | Chicken liver | Intestinal fluid | Raillietina | Ascaridia |
|---|---|---|---|---|
| Saturates | ||||
| 14:0 | 0.2 | 1.8 | 1.2 | – |
| 15:0 | 0.2 | 2.4 | 0.8 | – |
| 16:0 | 34.6 | 24.5 | 50.1 | 15.9 |
| 17:0 | 0.5 | 1.1 | 0.8 | 0.7 |
| 18:0 | 17.4 | 50.7 | 17.4 | 19.1 |
| 20:0 | 0.1 | 1.5 | 3.7 | 1.0 |
| 22:0 | 4.4 | 0.5 | 0.3 | 2.3 |
| 24:0 | 0.3 | 0.03 | 0.7 | 0.008 |
| ∑ | 57.7 | 82.53 | 75.0 | 39.008 |
| Monoenes | ||||
| 16:1 | 0.1 | – | – | – |
| 18:1ω9 | 20.8 | 5.6 | 14.8 | 42.2 |
| 20:1ω9 | 0.2 | 0.1 | 0.7 | 1.3 |
| 22:1ω11 | 0.1 | 0.04 | 0.1 | 0.3 |
| 24:1 | 1.9 | 0.2 | 0.1 | 0.1 |
| ∑ | 23.1 | 5.94 | 15.7 | 43.9 |
| Dienes | ||||
| 18:2ω6 | 16.1 | 10.0 | 7.7 | 15.7 |
| 20:2 | 0.1 | – | – | – |
| ∑ | 16.2 | 10.0 | 7.7 | 15.7 |
| Polyenes | ||||
| 18:3ω6 | – | 0.1 | 0.005 | – |
| 18:3ω3 | 0.006 | 0.04 | 0.1 | 0.02 |
| 20:3ω6 | 0.4 | 0.4 | 0.4 | 0.9 |
| 20:4ω6 | 1.2 | 0.1 | 0.1 | 0.1 |
| 20:4ω3 | – | 0.4 | 1.1 | – |
| 20:5ω3 | – | 0.01 | – | 0.2 |
| 21:5ω3 | 0.1 | 0.02 | 0.04 | 0.01 |
| 22:5ω6 | – | 0.1 | 0.03 | 0.1 |
| 22:5ω3 | 0.04 | 0.03 | 0.01 | 0.008 |
| 22:6ω3 | 1.3 | 0.2 | 0.1 | 0.1 |
| ∑ | 3.046 | 1.4 | 1.885 | 1.438 |
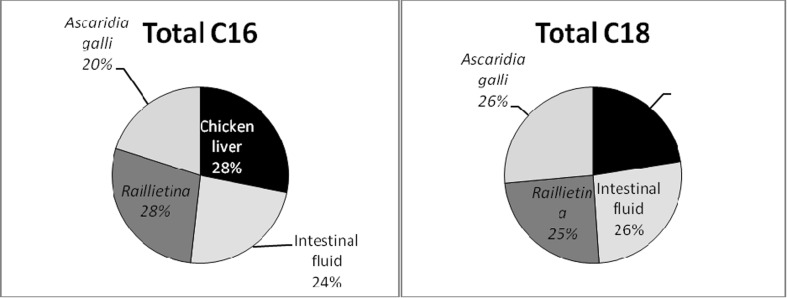
Comparable study of total C16 and total C18 of TL, of the four samples i.e. chicken liver, chicken intestinal fluid, R. (F.) echinobothrida and A. galli was shown in Fig. 6. Raillietina and liver had highest amount of total C16 of TL than Ascaridia, whereas, Ascaridia showed good percentage of total C18 (26 %) followed by Raillietina (25 %) and liver (23 %).
Fig. 6.
Comparison of total C16 and total C18 of total lipid of chicken liver, intestinal fluid, R. (F.) echinobothrida and A. galli
Comparable study of ω3, ω6 and their ratios among chicken liver, intestinal fluid, Raillietina and Ascaridia were represented in Table 17. In TL content, highest quantity of ω3 in cestode, ω6 in intestinal fluid, ratio between ω3/ω6 in liver and ratio between ω6/ω3 in intestinal fluid were presented. Similarly, in NL, nematode was highest in ω3 and the ratio of ω3/ω6, intestinal fluid in ω6 and the ratio of ω6/ω3. In case of GL, intestinal fluid, cestode and nematode had very good level of ω3 than liver, whereas, intestinal fluid and cestode also rich in ω6 and the ratios between ω3/ω6 and ω6/ω3 were high in nematode and liver, respectively. In PL content, highest quantity of ω3 in nematode, ω6 in liver, ω3/ω6 ratio in nematode and ω6/ω3 ratio in intestinal fluid were observed.
Table 17.
Comparison of ω3, ω6 and their ratios of total lipid in chicken liver, intestinal fluid, R. (F.) echinobothrida and A. galli
| Component | Chicken liver | Intestinal fluid | Raillietina | Ascaridia |
|---|---|---|---|---|
| Total lipid/ω3 | 1.99 | 1.84 | 2.33 | 2.22 |
| ω6 | 22.6 | 37.72 | 35.1 | 25.31 |
| ω3/ω6 | 0.09 | 0.049 | 0.066 | 0.088 |
| ω6/ω3 | 11.36 | 20.5 | 15.06 | 11.4 |
| Neutral lipid/ω3 | 1.84 | 1.43 | 2.65 | 3.1 |
| ω6 | 27 | 41.5 | 41.1 | 32.42 |
| ω3/ω6 | 0.068 | 0.034 | 0.064 | 0.095 |
| ω6/ω3 | 14.67 | 29.02 | 15.51 | 10.46 |
| Glycolipid/ω3 | 0.62 | 1.94 | 1.93 | 1.72 |
| ω6 | 15.93 | 35.67 | 33.5 | 22.1 |
| ω3/ω6 | 0.039 | 0.054 | 0.058 | 0.078 |
| ω6/ω3 | 25.69 | 18.39 | 17.36 | 12.85 |
| Phospholipid/ω3 | 1.66 | 0.56 | 1.28 | 4.04 |
| ω6 | 17 | 13.3 | 12.43 | 13.7 |
| ω3/ω6 | 0.098 | 0.042 | 0.103 | 0.295 |
| ω6/ω3 | 10.24 | 23.75 | 9.71 | 3.39 |
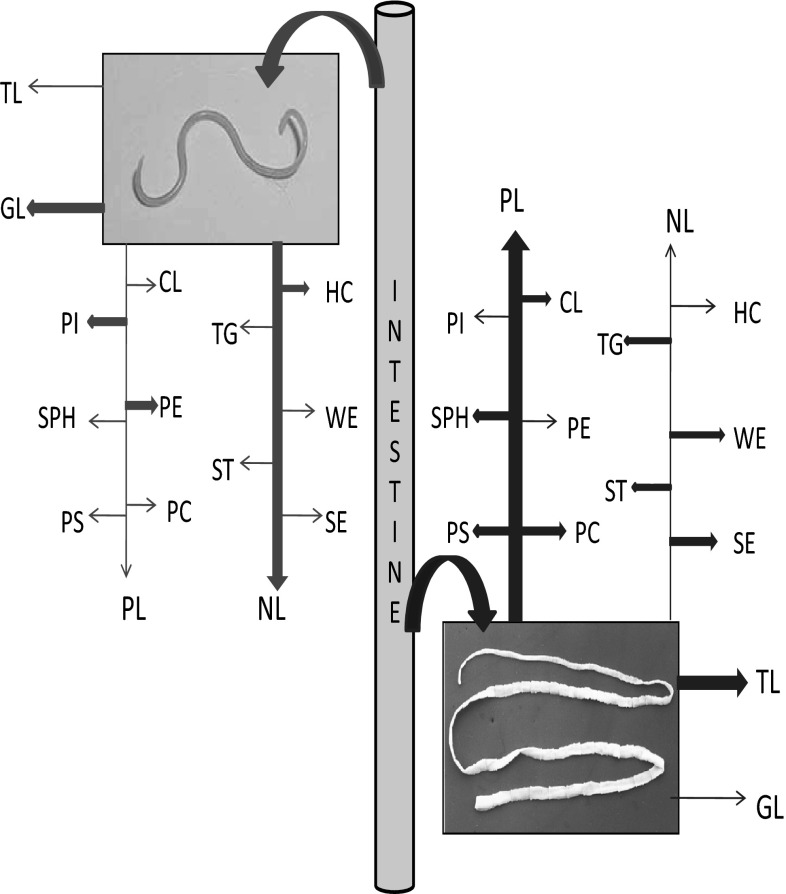
The comparison of uptake of lipid and their fractions between nematode and cestode from the intestinal fluid has been shown in Fig. 7. This figure explains competition between the parasites. This competition resulted in niche segregation between the nematode and cestode.
Fig. 7.
Comparison of the uptake of lipids and their fractions from the intestinal fluid between A. galli and R. (F.) echinobothrida. Bold arrows and light arrows denote major and minor uptake, respectively (pink arrows for Ascaridia, blue arrows for Raillietina) (color figure online)
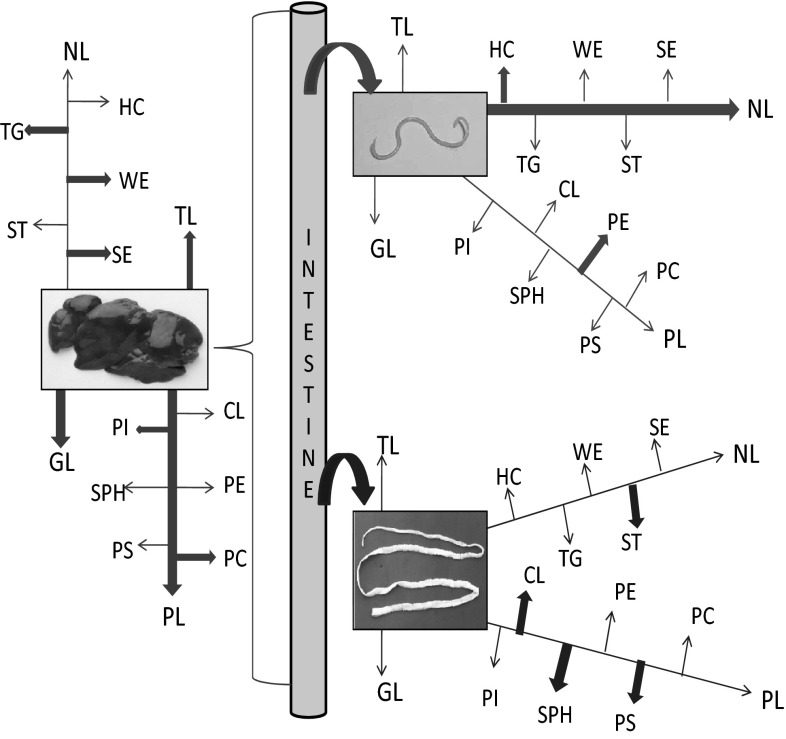
Figure 8 represented the uptake of lipids and their fractions from the intestinal fluid by the chicken liver, A. galli and R. (F.) echinobothrida. The above mentioned figure expresses the comparative account of lipid utilization among the parasites and the host depicting host–parasite interaction.
Fig. 8.
Comparison of the uptake of lipids and their fractions from the intestinal fluid by the chicken liver, A. galli and R. (F.) echinobothrida. Bold arrows and light arrows denote major and minor uptake, respectively. Curved arrows depict the uptake of lipids by nematode and cestode, large left brace denote the uptake of lipid by the liver (red arrows denote the absorption of liver, pink arrows for Ascaridia, blue arrows for Raillietina) (color figure online)
Discussion
Evolution of the host–parasite interaction mainly developed for the acquisition of nutrients from the host thus constitutes a part of the food chain. Parasites live in anaerobic condition usually needs to limit its energy budget in order to adapt to its ambient environment. This is the reason why the parasite restricts itself for de novo synthesis of fatty acids. Fatty acids are physiologically important as (1) components of PLs and GLs, (2) lipophilic modifiers of protein, (3) fuel molecules and (4) hormones and intracellular messengers. In helminths, fatty acids occur both as free fatty acids and esterified as a component of complex lipids. Ghosh and Misra (2011, 2014) have already observed that the trematode P. cervi take up almost all the lipid and fatty acids from rumen fluid of its host, C. hircus, which is required for their reproductive strategy.
Total lipid content is highest in cestode followed by nematode and trematode. In helminths, TL content varies between 10 and 30 % of the dry weight (Barrett 1981). Von Brand (1952) observed TL content in Taenia solium (16.2 %), T. saginata (11.2 %), T. hydatigena (4.9 %), Moniezia expansa (30.1 %), whereas, in H. diminuta (Fairbairn et al. 1961), H. citelli (Harrington 1965), Spirometra mansonoides (Meyer et al. 1966) and R. (F.) echinobothrida (Mondal et al. 2009), it was 20.1, 16.1, 24 and 4.05 %, respectively. Von Brand (1973) and Fairbairn (1969) also made similar observations on some nematode species i.e. Ascaris lumbricoides (8.9 %) and Nippostrongylus brasiliensis (11.9 %). However, 1.94 % of TL was found in A. galli (Ghosh et al. 2010). These variations might occur due to the availability of food materials, which is influenced by the food habit of the host, in the different microhabitat of the parasites. Key enzymes of lipid biosynthesis have been observed in Haemonchus contortus (Kapur and Sood 1987), Trichuris globulosa (Sarwal et al. 1989) and A. galli (Aggarwal et al. 1989). A. galli, being capable of synthesizing lipids into other forms, absorbs less TL than cestode. Raillietina have lost de novo synthesis of lipids and therefore, totally dependent on host for fulfilling the requirement. Beta-oxidation pathway does not take place in anaerobic condition (Ward and Fairbairn 1970). Thus, cestodes only accumulate lipids in their parenchymal cells. Alpha-oxidation of fatty acids observed in mammals and birds, but the enzyme activity of this pathway remain uncertain in helminths.
NL probably functions as storage form of fatty acids. Host, with parasitic infection, may show depleted NL content. Mayberry et al. (1972) reported the concentration of NL in the parenchyma of H. diminuta at different stages of maturation with an increase in worm age. The NL content of R. tetragona and R. echinobothrida were 74.4 and 73.1 %, respectively (Vykhrestyuk et al. 1981). NL content of A. galli is very high (64.26 %), whereas in R. (F.) echinobothrida, it is 53.23 %, but liver shows less amount (38.87 %). In an earlier study the percentage of NL in R. (F.) echinobothrida was 64.39 (Mondal et al. 2009), whereas, in A. galli it was recorded as 54.39 (Ghosh et al. 2010). Variation in NL content in parasites may be related with the feeding habit of the host. It is also noticed that in the present study each of the samples i.e. liver, nematode and cestode, itself absorb lipids in the following pattern i.e. NL > PL > GL.
A. galli contain 75.68 % of HC + WE + SE, whereas, liver and cestode had 26.81 and 10.91 %, respectively. Cestode and nematode exhibit good level of HC i.e. 89.61 and 94.61 %, respectively, than that of the host’s liver. Thus both the parasites utilize more HC than the host. Iso- and anteiso-C15:0 and C17:0 fatty acids were identified in anaerobic sulfate bacteria, D. desulfuricans, whereas, iso-C17:1 is the major and they mainly used as taxonomic markers (Boon et al. 1977). In bacteria, iso- and anteiso-fatty acids (branched chain fatty acids) are synthesized from amino acid derived precursors with branch (Kaneda 1991). The author also suggested that iso- and anteiso-fatty acids control the membrane fluidity. In the present study iso-fatty acids of HC content are highest in cestode, but lack anteiso-fatty acids, whereas, very little amount of anteiso are found in Ascaridia. Iso–anteiso methyl branched fatty acids are major components of plant surface waxes (Kunst and Samuels 2009). These fatty acids may be related with membrane fluidity of the parasites. Liver has high level of SE (7.53 %), nematode shows 0.96 %, whereas, cestode have 1.96 %. According to Ghosh and Misra (2014), SE is low in P. cervi than the host. Paratenuisentis ambiguus has specific mechanisms for the regulation of the sterol and fatty acid composition of its NLs and cholesterol as the major sterol in both P. ambiguous and its host Anguilla anguilla (Weber et al. 1994). Helminths eggs contain substantial amount of sterols (Smith et al. 1977). Vykhrestyuk and Yarygina (1982) observed that high amount of sterol accounts for the presence of higher numbers of eggs. In the present study Raillietina contain highest percentage of sterol for their large numbers of gravid proglottids containing eggs.
WE, which is composed of fatty acids and fatty alcohols, are one of the main components of plant cuticles (Jetter and Kunst 2008). Waxes play an important role as water barrier for insects, birds and animals such as sheep (Conn et al. 2003). Uropygial glands of birds secrete waxes that consist of WEs to give water proof layer to the feather (Jacob 1976). WE of Ascaris columnaris consists of large amount of palmitate and stearate, whereas A. galli contains long chain acids and alcohols (Tarr 1973). Cestode and nematode have good amount of WE as well as of triacylglycerol. It can be assumed that in the present study both R. (F.) echinobothrida and A. galli use WE somewhat for protection within the intestine of their host, G. domesticus. In the present study, both parasites show good level of TG. Mammals synthesize TG in the liver and transport to peripheral tissues as LDL. Energy storage is the main function of TG in many organisms. TG are the primary storage form of long-chain fatty acids for energy and structural purposes, and free acids can be mobilized quickly when required for transport in an appropriate form to the heart, liver and other tissues where they can be oxidized. TG functions as an energy reservoir and they are not the components of biological membrane. Therefore, Raillietina and Ascaridia might utilize TG in different organs as energy supplier because fats which consist of TG provide about six times metabolic energy of an equal weight of hydrated glycogen (Berg et al. 2002).
Cholesterol is a major constituent of cell membranes (Kurzchalia and Ward 2003) and plays an important role in cellular membrane organization, dynamics, function and sorting. It is often found distributed non-randomly in domains in membranes (Maxfield 2002). Cholesterol is also one of the vital components of cell membranes and a precursor of steroid hormones and bile salts. Cholesterol maintains the fluidity of membranes by interacting with their complex lipid components, specifically the PLs such as PC and SPH. Simons and Toomre (2000) commented that cholesterol acts by maintaining a specialized type of membrane domain, termed “lipid rafts” in a functional state. Lipid rafts are enriched in cholesterol and sphingolipids, and perform as a platform through which signal transduction events are coordinated and pathogens gain entry to infect host cells (Fredrickson and Levy 1972). Silveira et al. (1986) investigated the transfer of cholesterol and its metabolites between adult male and female worms of S. mansoni. They found that the adult male and female worms of S. mansoni are able to incorporate cholesterol and convert it into several metabolites.
According to Awharitoma et al. (1988), cholesterol, among the NL components, and PC and PE among the PL components, were the major component in four species of amphistomes Carmyerius minutes, Ceylonocotyle dicranocoelium, Cotylophoron cotylophorum, and Paramphistomum microbothrium. From the present investigation, it can be presumed that large numbers of reproductive units compelled R. (F.) echinobothrida to take large quantity of cholesterol than A. galli.
Raillietina (F.) echinobothrida and Ascaridia galli both have high percentage of campesterol i.e. 17.2 and 18.2 % respectively than intestinal fluid (8 %). Liver contain very little amount (0.6 %). Barrett et al. (1970) suggested that the intestinal parasites obtain cholesterol from their host diet or host secretion. H. diminuta cannot synthesize cholesterol, but has a specific transporter for cholesterol (Johnson and Cain 1988). The authors studied the mechanisms of cholesterol uptake by the rat tapeworm H. diminuta and suggested that the tapeworm absorbs cholesterol by a specific carrier-mediated process.
Both the parasites showed striking differences in their percent of total sterol. In the present study, cestode and nematode also have greater amount of stigmasterol than liver and intestinal fluid whereas, β-sitosterol is present only in cestode but not in nematode. Liver and intestinal fluids have very less quantity of β-sitosterol. According to Wu et al. (2006), stigmasterols are one of the major sterol in plant plasma membrane and it controls the plasma cholesterol level in vertebrate. β-sitosterol inhibits cholesterol absorption in the intestine (Matsuoka et al. 2008). Therefore, it can be assumed that stigmasterols play an important role in the life cycles of both the parasites i.e. R. (F.) echinobothrida and A. galli. β-Sitosterol may also perform an important function i.e. regulating cholesterol level in Raillietina.
The result showed that R. (F.) echinobothrida is rich (53 %) in cholesterol; P. cervi (Ghosh and Misra 2014) absorbs greater quantity of campesterol (47 %) and stigmasterol (49 %) than other, whereas A. galli takes all the three sterols in moderate amount; Raillietina and Paramphistomum take up minimum of stigmasterol and cholesterol, respectively. These sterols might be related to their greater reproductive efficiency. R. (F.) echinobothrida have large numbers of reproductive units along with greater surface area of the body than A. galli and P. cervi; that’s why Raillietina needs more cholesterol; whereas Paramphistomum which reside within rumen of herbivore goat absorb more stigmasterol than other.
GL provides energy and also serves as markers for cellular recognition. In the present study, liver has highest percent of GL (26.11 %), whereas, cestode and nematode have 14.25 and 16.93 %, respectively. Dennis et al. (1996) have shown that GLs extracted from Schistosoma mansoni adult worms can be recognized by IgG antibodies in sera from S. mansoni- and S. haematobium-infected individuals, indicating that GLs may play an active role in shaping immune responses to schistosomes. IgE antibodies reactive with GLs may play a role in immunity to schistosomes (Tielens et al. 1999). GLs along with PL are the major components of biological membranes. It may be inferred from the present findings that both the parasites utilize GLs for maintenance of their membrane function.
PL plays important roles in the transport of fat between gut and liver in mammalian digestion process; acts as building blocks of the biological cell membranes: participates in the transduction of biological signals across the membrane; involves in efficient store of energy as with triglycerides. Furlong (1991) suggested that PL play an important role in defense mechanism and act as membrane component in S. mansoni. PLs can play a role in parasite resistance to immune attack by cytotoxic antibodies and complement (Billecocq 1987). PL affects the activities of intramembrane proteins and the solubilization of xenobiotic molecules (Pallares-Trujillo et al. 2000; Kremer et al. 2001). Corroborating the above inference, it can be said that Raillietina being a cestode possess long body with large surface area which require more PL to survive against immune attack within host body and also for structuring the cell membrane.
The functions of PE, PC and PI in mammals are the participation with the membrane function (Conn et al. 2003). In S. mansoni, PC (39 %) is the major component among the PL fractions followed by PE (31 %), PI (3 %), PG (1 %), CL (1 %) and PS (1 %) of the TL (Meyer et al. 1970), whereas P. cervi exhibit highest percent of PI (31.82 %) followed by PE (27.27 %), CL (13.43 %), PC (11.98 %), SPH (10.23 %) and PS (5.27 %) of the total PL fraction (Ghosh and Misra 2014). In the present study, R. (F.) echinobothrida comprises PC (30.22 %) > CL (25.71 %) > PE (17.8 %) > PS (12.05 %) > SPH (7.72 %) > PI (6.5 %) and in A. galli, it is PE (33.65 %) > PC (22.63 %) > CL (20.63 %) > PI (10.21 %) > PS (7.47 %) > SPH (5.4 %). Among all the PL fractions, R. (F.) echinobothrida and A. galli have all the fractions i.e. CL, PE, PC, PI, SPH and PS in very good amounts. In case of, PE and PS, cestode and nematode both have greater quantity than host tissue. It can be assumed that large surface area of cestode requires more PL for their continuous membrane restructuring associated with their alteration of tegument structure along the immature, mature and gravid proglottids (Dey and Misra 2009).
CL or diphosphatidylglycerol is a unique acidic PL and involve in the activation of those enzymes concerned with oxidative phosphorylation. It has been involved in the process of apoptosis; and arachidonic and decosahexaenoic acids may be involved in this process (Chicco and Sparagna 2007). CL is considered as an important cofactor for cholesterol translocation from the outer to the inner mitochondrial membrane and is a potent stimulator of steroidogenesis (Schlame et al. 2000). Similar functions may occur in Raillietina because both CL and cholesterol are higher in amount in cestode than nematode.
Christie (2003) suggested that PE donates the ethalomine component in the biosynthesis of the glycosylphosphatidylinositol anchors for many cell surface proteins. According to Vance (2008), PE acts as a chaperone during the assembly of membrane proteins to guide the folding path for the proteins and to aid in the transition from the cytoplasmic to the membrane environment. Similar functions may be involved for these parasites.
PC has a role in signaling via the generation of diacylglycerols. PC is the biosynthetic precursor of SPH (Vance and Vance 2002). PC is a zwitterionic lipid and usually the most abundant PL in membranes of animal and plants, constituting a high proportion of the outer leaflet of the plasma membrane. It is also an integral component of the lipoproteins in plasma. It has an influence on innumerable metabolic pathways. It is also a source for choline in the synthesis of acetylcholine in cholinergic neurons. In this work, it is found that Raillietina absorb more PC than Ascaridia. Raillietina may use PC mainly for cell signalling which maintain maturation of large number of proglottids and also detachment of gravid proglottids from the body. Ascaridia also acquire PC for development of reproductive part to fulfill its requirement.
Payrastre (2004) reported that PI is an important lipid both as a key membrane constituent and as a participant in essential metabolic processes. PI is essential for more complex GLs and growth (Haites et al. 2005). It is the main source of diacylglycerols that serve as signaling molecules in membranes (Gardocki et al. 2005). Ascaridia take more PI than Raillietina. PI serves as an anchor that links a variety of proteins to the external leaflet of the plasma membrane via complex glycosyl bridges. Three glycosylphosphatidylinositol anchored surface antigens that help in protective immunity and were identified in S. mansoni (Pearce and Sher 1989). It may be assumed that A. galli can produce different surface antigens from PI than Raillietina.
SPH serves as a precursor for ceramides and other molecules, which functions as intracellular messengers. SPH play role in the regulation of cell growth, differentiation, cell–cell communication, cell–substrate interaction and intracellular signal transduction. R. (F.) echinobothrida contain more SPH than A. galli and liver. The reason may be that Raillietina require high amount of SPH for regulation of cell growth and differentiation of their repetitive segments i.e. proglottids.
PS is an essential cofactor that binds to and activates protein kinase C, which is a key enzyme in signal transduction (Vance and Steenbergen 2005). Highest percent of PS in Raillietina might be suggested that signal transduction mechanism is highly active in cestode than Ascaridia.
Saturated fatty acids (SFA)
White et al. (1964) suggested that palmitic and stearic acids are the major SFA generally found in animals lipids. Both cestode and nematode absorb good percentage of palmitic acid from WE, SE and TG than liver, whereas cestode comprises less stearic acid from these NL fractions. C16:0 and C18:0 fatty acids represented 70 % of total fatty acids in D. immitis, D. viteae and L. carinisi (Hutchinson et al. 1976); below 50 % in P. cervi (Ghosh and Misra 2011, 2014) whereas in the present study, these fatty acids constitute below 40 % in both parasites while above 50 % of palmitic acid of PC and PS are recorded in cestode. Raillietina possesses more SFA (above 70 %) in PS, PI, SPH and PC among all the PL fractions. These high amounts of palmitic acid that is recorded from the parasites might agree with Ackman (2000). Therefore, it can be said that palmitate serves as a precursor for both saturates and unsaturates in both parasites and it is quite probable that C18 fatty acids are required mainly for the chain elongation processes to form C20 and C22 acids.
Monounsaturated fatty acids (MUFA)
Among MUFA, oleic (C18:1) is predominant in all the samples. Ascaridia possess more oleic acid among all the PL fractions except CL in which Raillietina comprises major uptake. Cestode also absorbs more C18:1 from WE. Palmitoleic (C16:1) is also found which was also reported by Ghosh and Misra (2014) in WE of P. cervi. Hexadecenoic (C16:1) and octadecenoic (C18:1) were also the major fatty acids found in Oochoristica agamae (Aisien and Ogiji 1989). Palmitoleic acid (C16:1) and oleic acid (C18:1) are not essential in the diet, because, at the Δ9 position of the saturated fatty acid, tissues can introduce a double bond (Mayes and Botham 2003). They also commented that in mammals, C18:1 fatty acid can be converted to C20:1 by elongase enzyme and from C20:1, conversion of C22:1 fatty acid takes place. Barrett (1981), Brouwers et al. (1997), Ghosh and Misra (2011, 2014) and Mondal and Dey (2015) observed that C18:1 as major fatty acids in F. hepatica, S. mansoni, P. cervi and I. hypselobagri, respectively. The above observations point that C18:1 could transform into C20:1 fatty acids. In the present observation low amount of C20:1 is detected from the liver, cestode and nematode. It is also noticed that Ascaridia possess C20:1 slightly more than other. The modification of lipids in the parasite which are obtained from the host might be related with the fitness of the parasites (Tielens 1997). Thus, from the above observations, the following contention can be made that host (fowl) modify C18:1 into C20:1 from which Raillietina and Ascaridia absorb their best and nematode also could transform oleic acid into other fatty acids.
Diunsaturated fatty acids (DUFA)
Among all the samples uptake of DUFA of TL, NL and GL is highest in cestode except PL, whereas, liver possess more of that. Raillietina has approximately 25, 45 and 35 % of DUFA in WE, SE and TG, respectively. All the samples have considerable amount of dienoic fatty acids among PL fractions. Linoleic (C18:2) acid is predominant among all the samples which was also reported by Aisien and Ogiji (1989) and Ghosh and Misra (2014). F. hepatica can form C20:2 from C18:2 (Barrett 1981) and S. mansoni can elongate linoleic series of fatty acids to C22:4 (Fripp et al. 1976). In the present study eicosadienoic (C20:2) is found only in liver not in the parasites. It can be inferred from the above observations that host might transform C18:2 into C20:2 while parasites unable to do this. Hexadecaenoic (C16:2) is observed only in cestode and nematode. C16:2 might be utilized within host’s body or changed into other fatty acids, therefore, not present in the host.
Polyunsaturated fatty acids (PUFA)
PUFA are essential dietary nutrients that important for structural composition of cell membranes and act as precursor of eicosanoids, signaling molecules controlling reproduction and immunity. Barrett (1981) commented that polyenoic acids of mammals are synthesized from palmitoleic, oleic, linoleic and linolenic fatty acids by chain elongation and desaturation process. The source of linolenic acid of mammals is the plant and animals could elongate and desaturate these fatty acids into arachidonic acid and docosahexaenoic acid (Fripp et al. 1976). In the present observations all the samples contain good amount of PUFA, whereas, Ascaridia absorb more from PL and WE. C18:3ω6 and C20:3ω3 are found in host, whereas, cestode also content little amount of C18:3ω6 in CL, PE and PC which are not recorded from host. C20:4ω3 and C22:5ω6 are found mainly in cestode and nematode. Thus, host could elongate these fatty acids and parasites absorb these from the host body. Among PUFA, α-linolenic (C18:3ω3) of the TL, NL, GL and PL is predominant in R. (F.) echinobothrida (except PL) and A. galli, whereas, docosahexanoic (C22:6ω3) abundant in liver among all the lipid i.e. TL, NL, GL and PL. α-linolenic might transform into docosahexanoic acid within host. A. galli also enriched in eicosapentanoic (C20:5), arachidonic (C20:4ω6) acid in PL. Therefore, nematode could be able to elongate linolenic acid into eicosapentanoic and arachidonic acid. It can be inferred that both Raillietina and Ascaridia require arachidonic acid mainly for interaction with the tissues of host i.e. involving in host–parasite interaction. In case of WE and SE, most of the polyunSFA are predominant in all the samples. From the present study it is evident that eicosatetraenoic (C20:4ω3) is abundant in cestode among PL fractions except CL, whereas, liver has extremely greater percentage of arachidonic acid (C20:4ω6) in SPH and PS.
According to Lee et al. (2012), PUFA chain in PI has a role in some PI 3-phosphate signaling. Linoleic and linolenic acids are essential fatty acids, in that they cannot be synthesized by animals and must come from plants via the diet. They are precursors of arachidonic, eicosapentanoic and decosahexaenoic acids, which are vital components of all membrane lipids. Arachidonate, unsaturated C20 fatty acid, is the precursor of the prostaglandins, prostacyclins, thromboxanes, leukotrines and lipoxins. PUFA acts as the mediator of daf-2/insulin signaling and that daf-16 might be involved in fatty acids homeostasis under the control of PUFA (Horikawa and Sakamoto 2010). Lack of essential fatty acids in the diet of experimental animals caused atrophy of lymphoid organs and the reduction of both the T cell mediated and the independent immune response in mammals (Halas et al. 2006). Thus, it is obvious that both parasites absorb PUFA for proper functioning of cell signaling and immunity.
The ω6/ω3 ratio was significantly altered by the effect of dietary intake in the female broilers (Scaife et al. 1994). Omega-6 and ω-3 levels, especially the ω3/ω6 ratios of dietary PUFA are important for the membrane composition (Hulbert et al. 2005). Domestic animals and humans can’t synthesize ω-3 from ω-6 because they lack specific desaturase enzymes (Simopoulos 2006). It is evident from the present observation that the liver of host i.e. G. domesticus has sufficient amount of both ω3 and ω6 which they get from dietary source, whereas Ascaridia contain less ω6 than Raillietina, but both parasites contain considerable quantity of ω3. Omega-6 is high in cestode for their large body surface. The present result may suggests that ω3 and ω6 both have important role in both parasites and omega-3 might help the parasites in anti-inflammatory function against microbial environment of the GI tract.
Tapeworms probably do not derive any energy from degradation of lipids or proteins. Parasites living in anaerobic condition usually needs to limit its energy consumption in order to adapt to its environment. This is the reason why the parasite might restrict itself for de novo synthesis of fatty acids. Parasites normally do not harm host but their high nutritional requirement for growth and reproductive purpose cause nutritional depletion in host which responsible for disease condition in the host (Fekete and Kellems 2007).
From the ecological and evolutionary point of view, the host is the environment of the parasite. A host usually provides a rich and highly regulated supply of nutrients to parasites. The present study reveals three prong competitions among the host; nematode and cestode parasite. Intestinal fluid is the source for lipid fractions and their fatty acids for the host (country fowl), A. galli and R. (F.) echinobothrida. In one hand, nematode competes with the host; secondly, cestode competes with the host and thirdly, nematode and cestode compete between them (though this is not intense because their microhabitat in the gut is different). Parasites do not synthesize fatty acids de novo, thus they become opportunistic for their nutritional resource utilization. Cestode takes the maximum advantage as they absorb the nutrients throughout their body surface which is many times larger than the nematode. Cestode body has no site (organ) for lipid storage. They rely on “absorb and utilize” principle as and when required. On the other hand, nematode can reserve a little bit of lipid in their body muscle which provides energy to their muscle movement. Host (fowl) absorbs lipids and fatty acids and has the site for lipid storage. Furthermore, fowl can synthesize required fatty acids unlike those of the parasites. Host has independent capability of lipid and fatty acid uptake from the intestinal fluid as well as synthesis and storage. Thus, the host has little risk for their lipid requirement than the parasite. Cestodes require more lipids for their vitellogenesis than the nematodes because the former exhibit much higher rate of reproduction than the nematode. Cestodes have higher risk than nematode for their reproductive strategy, thus become more opportunistic than the nematode. Host absorbs much amount of TL, GL and PL than both the parasites and through their ability to synthesize fatty acids the host compensates its requirement.
There is information that chicken absorb high amount of lipid from their diet source and built a fat reserve. Poultry birds are free from parasitic infection and they store fat in many organs including skin and liver. As a result the poultry have chance to develop hepatic lipidosis i.e. accumulation of excessive fat in the liver (Aziz 2008). The fat accumulation in broiler chicken is the effect of the degree of saturation of dietary fats (Sanz et al. 2000). Gut parasitic infections eliminate the problem of liver lipidosis by up taking much of the lipid resource from the intestinal fluid. If the lipid content of two parasites is added with the host lipid content, then the amount of TL content would be 9.62 % of wet tissue instead of 4.06 % within the host body. These high amounts of lipids are normally stored in the liver and the host becomes fattier. Thus country fowl is not suffering from hepatic lipidosis because they are usually found to be infected by these parasites.
The above discussion revealed that, A. galli, having gut, reside in the upper portion of the host’s intestine and therefore, it has opportunity to digest large particles of food materials within its body and Ascaridia take highest amount of NL, HC and PE than liver and Raillietina. On the other hand, R. (F.) echinobothrida, having no gut system, live in the lower portion of the host’s intestine where they get almost digested food materials. In this microhabitat, cestodes have greater chances to absorb most of the food components. Highest content of ST, CL, PS and SPH are found in Raillietina than liver and nematode.
The concept of the present investigation is based on the host–parasite interaction from their food resource utilization. The host (fowl), nematode and cestode all are dependent on their common food resource i.e. intestinal fluid. Fowl forage in the macro-environment while gut inhabiting cestode and nematode micro-forage in their microenvironment i.e. intestinal fluid. Ultimately, the above mentioned three partners form a guild and interact among themselves to exploit the food resource present in the intestinal fluid (as depicted in Figs. 7, 8). The host takes up lipid components throughout the small intestine utilizing its large surface area. The cestode also takes up lipid fractions throughout their body surface and comes next so far TL, NL and PL, are concerned. TL and PL are more essential to cestode for maintenance of their large surface area in hostile environment. Requirement of the nematode is comparatively low because of their small body size and less reproduction rate than the cestode. The cestode resides in safer place for resource utilization. This advantage is not available to the nematodes. Cestodes with large absorptive area aggregate to a place in the intestine which is rich in nutrient and interfere with the lipid uptake of the host. Perhaps the total absorptive area of aggregated cestode population is much higher than that of the host in that particular microhabitat. Nematode is deprived of this situation, thus their population size is much smaller than the cestode.
The above discussion clearly indicates that cestode is more opportunistic in the resource utilization over the nematode. Nematode comes next to the cestode due to their limitations in absorptive area and less compatible food resource availability. Ability to synthesize and conversion of fatty acids provide some advantage to the host though their lipid uptake is hindered by the parasite. Thus, the hypothesis of the present work is tenable which proves the opportunistic behaviour of the parasites with regard to lipid resource utilization.
Acknowledgments
The authors are thankful to the Head, Department of Zoology, Vidyasagar University for necessary laboratory facilities. The authors (M.M. and K.K.M.) are also grateful to Dr. U. C. Pal, Principal, to Dr. P.P. Chakraborty, Head of the Department of Zoology, Raja N. L. Khan women’s College, Medinipur for allowing the laboratory facility of the college. In this connection, it will not be out of place to mention the support of all the respected teachers of the Department of Zoology. The authors are indebted to late Dr. A. Ghosh, President, Drug Research and Development Centre, Kolkata, India for GLC analysis of the samples and necessary help.
Compliance with ethical standards
Conflict of interest
None.
Contributor Information
Madhumita Mondal, Email: mm.mndl@gmail.com.
K. K. Misra, Email: misrakk@vsnl.com, Email: misrakk47@gmail.com
References
- Ackman RG. Fatty acids in fish and shellfish. In: Chow CK, editor. Fatty acids in foods and their health implications. New York: Marcel Dekker; 2000. pp. 153–172. [Google Scholar]
- Ackman RG, Burgher RD. Cod liver oil fatty acids as secondary reference standards in the GLC of polyunsaturated fatty acids of animal origin: analysis of a dermal oil of the Atlantic leather-back turtle. J Am Oil Chem Soc. 1965;42:38–42. doi: 10.1007/BF02558251. [DOI] [PubMed] [Google Scholar]
- Aggarwal R, Sanyal SN, Khera S. Lipid metabolism and low molecular weight solute uptake in Ascaridia galli. Acta Vet Hung. 1989;37(4):335–347. [PubMed] [Google Scholar]
- Aisien SO, Ogiji EE. Observations on the lipids of Oochoristica agamae (Cestoda) Parasitol Res. 1989;75:307–310. doi: 10.1007/BF00931815. [DOI] [PubMed] [Google Scholar]
- Awharitoma AO, Opute FI, Ali SN, Obiamiwe BA. Lipid composition of four species of amphistomes (trematode) from the rumen of cattle. Int J Parasitol. 1988;18:441–444. doi: 10.1016/0020-7519(88)90006-9. [DOI] [PubMed] [Google Scholar]
- Aziz T. Hepatic lipidosis in Turkeys. World Poult. 2008;24(2):28–29. [Google Scholar]
- Bailey HH, Fairbairn D. Lipid metabolism in helminth parasites. V. Absorption of fatty acids and monoglycerides from micellar solution by Hymenolepis diminuta (Cestoda) Comp Biochem Physiol. 1968;26:819–836. doi: 10.1016/0010-406X(68)90003-0. [DOI] [Google Scholar]
- Bankov I, Timanova A, Barrett J. Sphingomyelin synthesis in helminthes: a minireview. Folia Parasitol. 1998;45:257–260. [PubMed] [Google Scholar]
- Barrett J. Biochemistry of parasitic helminths. Baltimore: University Park Press; 1981. [Google Scholar]
- Barrett J. Lipid metabolism. In: Arme C, Pappas PW, editors. Biology of Eucestoda. London: Academic Press; 1983. pp. 391–419. [Google Scholar]
- Barrett J, Cain GD, Fairbairn D. Sterols in Ascaris lumbricoides (Nematoda), Macracan thorynchus hirudinaceus and Moniliformis dubius (Acanthocephala) and Echinostoma revolutum (Trematoda) J Parasitol. 1970;56:1004–1008. doi: 10.2307/3277525. [DOI] [PubMed] [Google Scholar]
- Beames CG, Jr, Harris BG, Hopper FA., Jr The synthesis of fatty acids from acetate by intact tissue and muscle extract of Ascaris lumbricoides suum. Comp Biochem Physiol. 1967;20:509–521. doi: 10.1016/0010-406X(67)90265-4. [DOI] [PubMed] [Google Scholar]
- Berg JM, Tymoczko JL, Stryer L, Clarke ND. Biochemistry. 5. New York: Freeman; 2002. [Google Scholar]
- Billecocq A. Protection by phospholipids of Schistosoma mansoni schistosomula against the action of cytotoxic antibodies and complement. Mol Biochem Parasitol. 1987;25(2):133–142. doi: 10.1016/0166-6851(87)90002-8. [DOI] [PubMed] [Google Scholar]
- Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Boon JJ, Leeuw JW, Hoek GJ, Vosjan JH. Significance and taxonomic value of iso and anteiso monoenoic fatty acids and branched β-hydroxyl acids in Desulfovibrio desulfuricans. J Bacteriol. 1977;129(3):1183–1191. doi: 10.1128/jb.129.3.1183-1191.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwers JF, Smeenk IM, van Golde LM, Tielens AG. The incorporation, modification and turnover of fatty acids in adult Schistosoma mansoni. Mol Biochem Parasitol. 1997;88:175–185. doi: 10.1016/S0166-6851(97)00091-1. [DOI] [PubMed] [Google Scholar]
- Bush AO, Fernandez JC, Esch GW, Seed JR. Parasitism: the diversity and ecology of animal parasites. Cambridge: Cambridge University Press; 2001. [Google Scholar]
- Chen J, Ferris H, Scow KM, Graham KJ. Fatty acid composition and dynamics of selected fungal-feeding nematodes and fungi. Comp Biochem Physiol B. 2001;130:135–144. doi: 10.1016/S1096-4959(01)00414-6. [DOI] [PubMed] [Google Scholar]
- Cheng TC. General parasitology. 2. New Yoek: Academic Press; 1986. [Google Scholar]
- Chicco AJ, Sparagna GC. Role of cardiolipin alterations in mitochondrial dysfunction and disease. Am J Physiol Cell Physiol. 2007;292:C33–C44. doi: 10.1152/ajpcell.00243.2006. [DOI] [PubMed] [Google Scholar]
- Christie WW. Lipid analysis. 3. Bridgwater: Oily Press; 2003. [Google Scholar]
- Conn EE, Stumpf PK, Bruening G, Doi RH. Outlines of biochemistry. 5. Singapore: Wiley; 2003. [Google Scholar]
- Dalton JP, Skelly P, Halton DW. Role of the tegument and gut in nutrient uptake by parasitic platyhelminthes. Can J Zool. 2004;82:211–232. doi: 10.1139/z03-213. [DOI] [Google Scholar]
- Dawkins R. The blind watchmaker. New York: W.W. Norton; 1986. [Google Scholar]
- Dennis RD, Baumeister S, Lauer G, Richter R, Geyer E. Neutral glycolipids of Schistosoma mansoni as feasible antigens in the detection of schistosomiasis. Parasitology. 1996;112:295–307. doi: 10.1017/S0031182000065811. [DOI] [PubMed] [Google Scholar]
- Dey C, Misra KK. Ultrastructural studies of internuncial processes in the tegument of a cyclophyllid cestode Raillietina (Fuhrmannetta) echinobothrida, a parasite of country fowl Gallus domesticus. Proc Zool Soc. 2009;62:29–37. doi: 10.1007/s12595-009-0004-6. [DOI] [Google Scholar]
- Fairbairn D. Lipid components and metabolism of Acanthocephala and Nematoda. In: Florkin M, Scheer BT, editors. Chemical zoology. New York: Academic Press; 1969. pp. 361–378. [Google Scholar]
- Fairbairn D, Wertheim G, Harpur RP, Schiller EL. Biochemistry of normal and irradiated strains of Hymenolepis diminuta. Exp Parasitol. 1961;11(2):248–263. doi: 10.1016/0014-4894(61)90031-5. [DOI] [PubMed] [Google Scholar]
- Fekete SG, Kellems RO. Interrelationship of feeding with immunity and parasitic infection: a review. Vet Med. 2007;52(4):131–143. [Google Scholar]
- Fredrickson DS, Levy RI. Familial hyperlipoproteinemia. In: Stanbury JB, Wyngaarden JB, Fredrickson DS, editors. In the metabolic basis of inherited disease. New York: McGraw-Hill; 1972. [Google Scholar]
- Fripp PJ, Williams G, Crawford MA. The differences between the long chain polyenoic acids of adult Schistosoma mansoni and the serum of its host. Comp Biochem Physiol. 1976;53B:505–507. doi: 10.1016/0305-0491(76)90207-8. [DOI] [PubMed] [Google Scholar]
- Furlong ST. Unique roles for lipids in Schistosoma mansoni. Parasitol Today. 1991;7:59–62. doi: 10.1016/0169-4758(91)90192-Q. [DOI] [PubMed] [Google Scholar]
- Gardocki ME, Jani N, Lopes JM. Phosphatidylinositol biosynthesis: biochemistry and regulation. Biochim Biophys Acta. 2005;1735:89–100. doi: 10.1016/j.bbalip.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Ghosh D, Misra KK. Major lipids and fatty acids in the liver and rumen fluid of the goat (Capra hircus) infected with the trematode Paramphistomum cervi. J Helminthol. 2011;85(03):246–254. doi: 10.1017/S0022149X10000532. [DOI] [PubMed] [Google Scholar]
- Ghosh D, Misra KK. Comparison of fatty acid contents of the neutral and phospholipids of the trematode Paramphistomum cervi and liver of its host, Capra hircus. J Parasit Dis. 2014;38:223–232. doi: 10.1007/s12639-012-0229-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A, Kar K, Ghosh D, Dey C, Misra KK. Major lipid classes and their fatty acids in a parasitic nematode, Ascaridia galli. J Parasit Dis. 2010;34(1):52–56. doi: 10.1007/s12639-010-0005-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haites RE, Morita YS, McConville MJ, Jacobe HB. Function of phosphatidylinositol in mycobacteria. J Biol Chem. 2005;280:10981–10987. doi: 10.1074/jbc.M413443200. [DOI] [PubMed] [Google Scholar]
- Halas V, Kovacs M, Babinszky L. Impact of nutrient supply on the immune functions in pig. Literature review. Magy Allatorv Lapja. 2006;128:535–543. [Google Scholar]
- Harrington GW. The lipid content of Hymenolepis diminuta and Hymenolepis citelli. Exp Parasitol. 1965;17:287–295. doi: 10.1016/0014-4894(65)90072-X. [DOI] [PubMed] [Google Scholar]
- Horikawa M, Sakamoto K. Polyunsaturated fatty acids are involved in regulatory mechanism of fatty acid homeostasis via daf-2/insulin signaling in Caenorhabditis elegans. Mol Cell Endocrinol. 2010;323(2):183–192. doi: 10.1016/j.mce.2010.03.004. [DOI] [PubMed] [Google Scholar]
- Hulbert AJ, Turner N, Storlein LH, Else P. Dietary fats and membrane function: implications for metabolism and disease. Biol Rev. 2005;80(1):155–169. doi: 10.1017/S1464793104006578. [DOI] [PubMed] [Google Scholar]
- Hutchinson WF, Turner AC, Grayson DP, White HB., Jr Lipid analysis of the adult dog heartworm Dirofilaria immitis. Comp Biochem Physiol. 1976;53B:495–497. doi: 10.1016/0305-0491(76)90205-4. [DOI] [PubMed] [Google Scholar]
- Jacob J. Bird waxes. In: Kolattukudy PE, editor. Chemistry and biochemistry of natural waxes. Amsterdam: Elsevier; 1976. pp. 93–146. [Google Scholar]
- Jadhav BV. Biosystematic studies of Davainea shindei n. sp. (Cestoda: Davainidae Furhmall, 1907) from Gallus gallus domesticus. Natl Acad Sci Lett. 2008;31:7–8. [Google Scholar]
- Jetter R, Kunst L. Plant surface lipid biosynthetic pathways and their utility for metabolic engineering of waxes and hydrocarbon biofuels. Plant J. 2008;54(4):670–683. doi: 10.1111/j.1365-313X.2008.03467.x. [DOI] [PubMed] [Google Scholar]
- Johnson WJ, Cain GD. Studies on the mechanism of cholesterol uptake by the rat tapeworm Hymenolepis diminuta (Cestoda) Comp Biochem Physiol B. 1988;91:59–67. doi: 10.1016/0305-0491(88)90114-9. [DOI] [PubMed] [Google Scholar]
- Kaneda T. Iso and anteiso-fatty acids in bacteria: biosynthesis, function and taxonomic significance. Microbiol Rev. 1991;55(2):288–302. doi: 10.1128/mr.55.2.288-302.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur J, Sood ML. Changes in lipids and free fatty acid fractions in adult Haemonchus contortus during incubation in vitro. Vet Parasitol. 1987;23(1–2):95–103. doi: 10.1016/0304-4017(87)90028-8. [DOI] [PubMed] [Google Scholar]
- Knights BA. Quantitative and qualitative analysis of plant sterols by gas-liquid chromatography. In: Heftman E, editor. Modern methods of steroid analysis. New York: Academic Press; 1973. pp. 103–138. [Google Scholar]
- Kremer JJ, Sklansky DJ, Murphy RM. Profile of changes in lipid bilayer structure caused by beta-amyloid peptide. Biochemistry. 2001;40:8563–8571. doi: 10.1021/bi010417x. [DOI] [PubMed] [Google Scholar]
- Kunst L, Samuels L. Plant cuticles shine: advances in wax biosynthesis and export. Curr Opin Plant Biol. 2009;12:721–727. doi: 10.1016/j.pbi.2009.09.009. [DOI] [PubMed] [Google Scholar]
- Kurzchalia TV, Ward S. Why do worms need cholesterol? Nat Cell Biol. 2003;5:684–688. doi: 10.1038/ncb0803-684. [DOI] [PubMed] [Google Scholar]
- Lee HC, Kubo T, Kono N, Kage-Nakadai E, Gengyo-Ando K, Mitani S, Inoqu T, Arai H. Depletion of mboa-7, an enzyme that incorporates polyunsaturated fatty acids into phosphatidylinositol (PI), impairs PI3-phosphate signaling in Caenorhabditis elegans. Genes Cells. 2012;17(9):748–757. doi: 10.1111/j.1365-2443.2012.01624.x. [DOI] [PubMed] [Google Scholar]
- Mangold HK (1969) In: Stahl E (ed) Thin layer chromatography. Springer, New York, p 155
- Matsuoka K, Nakazawa T, Nakamura A, Honda C, Endo K, Tsukada M. Study of thermodynamic parameters for solubilization of plant sterol and stanol in bile salt micelles. Chem Phys Lipids. 2008;154(2):87–93. doi: 10.1016/j.chemphyslip.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Maxfield FR. Plasma membrane microdomains. Curr Opin Cell Biol. 2002;14:483–487. doi: 10.1016/S0955-0674(02)00351-4. [DOI] [PubMed] [Google Scholar]
- Mayes PA, Botham KM. Metabolism of unsaturated fatty acids and eicosanoids. In: Murray RK, Granner DK, Mayes PA, Rodwell VW, editors. Harper’s illustrated biochemistry. 26. New Delhi: McGraw-Hill; 2003. [Google Scholar]
- Meyer F, Kimura S, Mueller JL. Lipid metabolism in the larval and adult forms of the tapeworm Spirometra mansonoides. J Biol Chem. 1966;241:4224–4232. [PubMed] [Google Scholar]
- Meyer F, Meyer H, Bueding E. Lipid metabolism in the parasitic and free-living flatworms, Schistosoma mansoni and Dugesia dorotocephala. Biochem Biophys Acta. 1970;210:257–266. doi: 10.1016/0005-2760(70)90170-0. [DOI] [PubMed] [Google Scholar]
- Misra S, Ghosh A. Analysis of epicuticular waxes. In: Linsken HF, Jackson JF, editors. Modern methods of plant analysis. Berlin: Springer; 1991. p. 205. [Google Scholar]
- Misra S, Ghosh A, Dutta J. Production and composition of microbial fat from Rhodotorula glutinis. J Sci Food Agric. 1984;35:59–65. doi: 10.1002/jsfa.2740350110. [DOI] [Google Scholar]
- Mondal J, Dey C. Lipid and fatty acid composition of a trematode, Isoparorchis hypselobagri Billet, 1898 (Digenea: Isoparorchiidae) infecting swim bladder of Wallago attu in the district North 24-Parganas of West Bengal. J Parasit Dis. 2015;39:67–72. doi: 10.1007/s12639-013-0283-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondal M, Mukhopadhyay D, Ghosh D, Dey C, Misra KK. Analysis of major lipid classes and their fatty acids in a cestode parasite of domestic fowl, Raillietina (Fuhrmannetta) echinobothrida. Proc Zool Soc. 2009;62(2):131–137. doi: 10.1007/s12595-009-0015-3. [DOI] [Google Scholar]
- Pallares-Trujillo J, Lopez-Soriano FJ, Argiles JM. Lipids: a key role in multidrug resistance? (review) Int J Oncol. 2000;16:783–798. doi: 10.3892/ijo.16.4.783. [DOI] [PubMed] [Google Scholar]
- Payrastre B. Phosphoinositides. In: Nicolaou A, Kokotos G, editors. Bioactive lipids. Bridgwater: Oily Press; 2004. pp. 63–84. [Google Scholar]
- Pearce EJ, Sher A. Three major surface antigens of Schistosoma mansoni are linked to the membrane by glycosylphosphatidylinositol. J Immunol. 1989;142:979–984. [PubMed] [Google Scholar]
- Rouser G, Kritchevsky G, Yamamoto A. Column Chromatographic and Associated Procedures for Separation and Determination of Phosphatides and Glycolipids. In: Marinetti GV, editor. Lipid chromatographic analysis. New York: Marcel Dekker; 1967. p. 99. [Google Scholar]
- Rouser G, Kritchevsky G, Yamamoto A (1976) Column Chromatographic and Associated Procedures for Separation and Determination of Phosphatides and Glycolipids. In: Marinetti GV (ed) Lipid chromatographic analysis, vol 2, 2nd edn. Marcel Dekker, New York, p 713–776
- Sanz M, Flores A, Lopez-Bote CJ. The metabolic use of energy from dietary fat in broilers is affected by fatty acid saturation. Br Poult Sci. 2000;41(1):61–68. doi: 10.1080/00071660086411. [DOI] [PubMed] [Google Scholar]
- Sarwal R, Sanyal SN, Khera S. Lipid metabolism in Trichuris globulosa (Nematoda) J Helminthol. 1989;63(4):287–297. doi: 10.1017/S0022149X00009160. [DOI] [PubMed] [Google Scholar]
- Sato S, Hirayama T, Hirazawa N. Lipid content and fatty acid composition of the monogenean Neobenedenia girellae and comparison between the host fish species. Parasitology. 2008;135:967–975. doi: 10.1017/S0031182008004575. [DOI] [PubMed] [Google Scholar]
- Scaife JR, Moyo J, Galbraith H, Michie W, Campbell V. Effect of different dietary supplemental fats and oils on the tissue fatty acid composition and growth of female broilers. Br Poult Sci. 1994;35(1):107–118. doi: 10.1080/00071669408417675. [DOI] [PubMed] [Google Scholar]
- Schlame M, Rua D, Greenberg ML. The biosynthesis and functional role of cardiolipin. Prog Lipid Res. 2000;39:257–288. doi: 10.1016/S0163-7827(00)00005-9. [DOI] [PubMed] [Google Scholar]
- Sherman IW. Carbohydrate metabolism of asexual stages. In: Sherman IW, editor. Malaria: parasite biology, pathogenesis and protection. Washington, DC: ASM Press; 1998. pp. 135–143. [Google Scholar]
- Silveira AM, Friche AA, Rumjanek FD. Transfer of (14C) cholesterol and its metabolites between adult male and female worms of Schistosoma mansoni. Comp Biochem Physiol B. 1986;85:851–857. doi: 10.1016/0305-0491(86)90186-0. [DOI] [PubMed] [Google Scholar]
- Simons K, Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- Simopoulos AP. Evolutionary aspects of diet, the ω-6/ω-3 ratio and genetic variation: nutritional implications for chronic diseases. Biomed Pharmacother. 2006;60:502–507. doi: 10.1016/j.biopha.2006.07.080. [DOI] [PubMed] [Google Scholar]
- Smith TM, Doughty BL, Brown JN. Fatty acid lipid class composition of Schistosoma japonicum eggs. Comp Biochem Physiol. 1977;57B:59–63. doi: 10.1016/0305-0491(77)90084-0. [DOI] [PubMed] [Google Scholar]
- Smyth JD, McManus DP. The physiology and biochemistry of cestodes. 2. Cambridge: Cambridge University Press; 1989. [Google Scholar]
- Smyth VP, Selkirk ME, Gounaris K. Identification and composition of lipid classes in surface and somatic preparations of adult Brugia malayi. Mol Biochem Parasitol. 1996;76:105–116. doi: 10.1016/S0166-6851(96)02615-1. [DOI] [PubMed] [Google Scholar]
- Tarr GE. Comparison of ascarosides from four species of ascarid and one of oxurid (Nematoda) Comp Biochem Physiol. 1973;46B:167–176. [Google Scholar]
- Tielens AGM. Biochemistry of trematodes. In: Fried B, Graczyk TK, editors. Advances in trematode biology. Boca Raton: CRC Press; 1997. pp. 309–343. [Google Scholar]
- Tielens AGM, van der Kleij D, Yazdanbakhsh M. Recognition of schistosome glycolipids by immunoglobulin E: possible role in immunity. Infect Immun. 1999;67(11):5946–5950. doi: 10.1128/iai.67.11.5946-5950.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance JE. Phosphatidylserine and phosphatidylethanolamine in mammalian cells: two metabolically related aminophospholipids. J Lipid Res. 2008;49:1377–1387. doi: 10.1194/jlr.R700020-JLR200. [DOI] [PubMed] [Google Scholar]
- Vance JE, Steenbergen R. Metabolism and functions of phosphatidylserine. Prog Lipid Res. 2005;44:207–234. doi: 10.1016/j.plipres.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Vance DE, Vance JE. Biochemistry of lipids, lipoproteins and membranes. 4. Amsterdam: Elsevier; 2002. [Google Scholar]
- Von Brand T. Chemical physiology of endoparasitic animals. New York: Academic Press; 1952. [Google Scholar]
- Von Brand T. Biochemistry of parasites. 2. New York: Academic Press; 1973. [Google Scholar]
- Vykhrestyuk NP, Yarygina GV. Preliminary studies of lipids of the trematodes Eurytrema pancreaticum, Calicophoron erschowi and the turbellarian Penecurva sibirica. Mol Biochem Parasitol. 1982;5:221–229. doi: 10.1016/0166-6851(82)90031-7. [DOI] [PubMed] [Google Scholar]
- Vykhrestyuk NP, Yarygina GV, Il’iasov IN. Lipids of Raillietina tetragona and Raillietina echinobothrida cestodes from the intestines of hens. Parazitologiia. 1981;15(6):525–532. [PubMed] [Google Scholar]
- Ward CW, Fairbairn D. Enzymes of β-oxidation and their function during development of Ascaris lumbricoides. Dev Biol. 1970;22:366–387. doi: 10.1016/0012-1606(70)90159-4. [DOI] [PubMed] [Google Scholar]
- Weber N, Vosmann K, Aitzetmuller K, Filipponi C, Taraschewski H. Sterol and fatty acid composition of neutral lipids of Paratenuisentis ambiguus and its host eel. Lipids. 1994;29(6):421–427. doi: 10.1007/BF02537311. [DOI] [PubMed] [Google Scholar]
- White A, Handler P, Smith EL. Principles of biochemistry. New York: McGraw-Hill; 1964. [Google Scholar]
- Wu R, Chen L, Yu Z, Quinn PJ. Phase diagram of stigmasterol-dipalmitoylphosphatidylcholine mixtures dispersed in excess water. Biochim Biophys Acta Biomembr. 2006;1758:764–771. doi: 10.1016/j.bbamem.2006.04.017. [DOI] [PubMed] [Google Scholar]