Abstract
Mosquitoes are blood-thirsty insects and serve as the most important vectors for spreading most notorious diseases such as malaria, yellow fever, dengue fever, and filariasis. The extensive use of synthetic mosquito repellent has resulted in resistance in mosquitoes. Therefore, the development of a reliable, eco-friendly processes for the synthesis of nano dimensional materials is an utmost important aspect of nanotechnology. In the present study, authors report absolute green synthesis of cadmium nanoparticles using marigold and rose flower petal extract. The characterization of nanomaterials was done by using UV–Vis, SEM, FTIR and fluorescent spectrophotometer analysis. Finally cadmium nanoparticles were also evaluated for their larvicidal activity of mosquito. Marigold flower petal extract shows 100 % mortality after 72 h of incubation with 10 ppm of Cd-nanoparticles. No mortality was observed in the control. Therefore, out of two flower petal mediated nanoparticles, only marigold showed better performance towards mosquito larvicidal activity than rose petal extracts. This is the first report on mosquito larvicidal activity of flower-petal mediated cadmium nanoparticles. Thus, the use of marigold petal extract to synthesize cadmium nanoparticles is a rapid, ecofriendly, and a single-step approach and the CdNps formed can be potential mosquito larvicidal agents.
Keywords: Flower extract, Marigold and rose flower, Cadmium nanoparticles, Characterization, Larvicidal activity, Mosquito
Introduction
Mosquitos are important vectors of several tropical diseases, including malaria, filariases, and numerous viral diseases, such as dengue, Japanese encephalitis and yellow fever. About 100 species of mosquitoes are vectors of human diseases among 3000 species of mosquito. Aedes is a genus of mosquitoes originally found in tropical and subtropical zones, but now found on all continents excluding Antarctica. Members of the Aedes genus are known vectors for numerous viral infections. The two most prominent species that transmit viruses are Aedes aegypti and Aedes albopictus which transmit the viruses that cause dengue fever, yellow fever, West Nile fever, chikungunya, and eastern equine encephalitis, along with many other, less notable diseases. Infections with these viruses are typically accompanied by a fever, and, in some cases, encephalitis, which can lead to death. Several insecticides namely namely, DDT, dieldrin, organophosphrous, fenithothion and propoxur were widely used in India to mitigate this dangerous problem. Persistent application of the synthetic chemical products mostly available in local markets causes undesirable consequences including production of resistant strains of mosquitoes, ecological imbalance and elimination of nontarget organism in the environment. Therefore, a demand stems out for the synthesis of bio origin mosquito repellent. Control measures are generally directed against only one or a few of the most important species and can be aimed at the adults or the larvae. Nanoparticles are now a days an emerging area of research and should be the choice to control mosquito, mainly the larval stage (Chitra et al. 2015; Mondal et al. 2014; Ramanibai and Velayutham 2015).
Nanoparticle is defined as a small object that behaves as a whole unit with respect to its transport and properties. Nanotechnology is gaining tremendous impacts in the present century due to its capability of modulating metals into their nano size. Nanoparticles have one dimension that measures 100 nm or less. The properties of many conventional materials change when formed from nanoparticles (Rajakumar and Rahuman 2011). This is typically because nanoparticles have a greater surface area per weight than larger particles which causes them to be more reactive to some other molecules (Mondal et al. 2014). As metal nanoparticles are very much prone to oxidation and agglomeration, synthesis of suitably protected nanoparticles is found to be difficult and hence different methods are being attempted. A number of approaches are available for the synthesis of heavy metal nanoparticles for example, reduction in solution (Goia and Matijevic 1998), Chemical and photochemical reactives in reverse micelles, thermal decomposition, radiation assisted, electrochemical, sonochemical, microwave assisted process (Pastoriza-Santos and Liz-Marzan 2002) and recently via green chemistry route (Song and Kim 2009). However, biological; synthesis of nanomaterials has received extensive interest due to its ecofriendly and non-hazardous in nature. But production of nanoparticles using plant materials has drawn attention of scientists because of its rapid, cost effectiveness and eco friendly nature and it provides a single step technique for the biosynthesis of nanomaterials (Medda et al. 2014; Thakkar et al. 2010). The techniques for obtaining nanoparticles using naturally occurring reagents such as vitamins, sugars, plant extracts, biodegradable polymers, and microorganisms as reductants and capping agents could be considered attractive for nanotechnology. These syntheses have led to the fabrication of limited number of inorganic nanoparticles, mainly metal nanoparticles, although several metal oxides and salts are also reported (Oluwafemi et al. 2014; Ahluwalia et al. 2014; Kalaiselvi et al. 2015; Tian et al. 2015). Among the reagents mentioned above, plant based materials seem to be the best candidates and they are suitable for large-scale ‘biosynthesis’ of nanoparticles. Plant parts such as leaf, root, latex, seed, and stem are being used for metal nanoparticle synthesis (Abdel-Aziz et al. 2014; Ajitha et al. 2015; Baghizadeh et al. 2015; Kathiravan et al. 2014). Microorganisms can also be utilized to produce nanoparticles but the rate of synthesis is slow and only limited number of sizes and shapes are amenable compared to routes involving plant based materials. Krishnamurthy et al. (2012) reported the synthesis of gold nanoparticles by using marigold flower. Marigold is a common garden plant which is rather coarse, erect, branched and grows to about 1 m high. The leaves are very deeply incised and sharply toothed flower heads are solitary, long-stalked, and thickening upward. The flowers are bright yellow, brownish-yellow or orange. This plant has been used for medicinal purposes. It has been used to treat stomach ache, parasites, diarrhea, liver illnesses, vomiting, indigestion, and toothache, among other illnesses. The other medicinal uses are it relieves chest pain and other problems associated with the chest, reduces the anxiety, expels worms from the body, purifies the blood, heals wounds, boils and skin infections caused by boils, it is used in relieving Rheumatic pains, cold, bronchitis, ulcer, disease of the eyes and uterus and also it is used as a mild laxative. Marigold had been reported to contain 5-(3-buten-1ynyl) 2, 2-bithienyl and alpha terthienyl12. Different parts of this plant including flower are used in folk medicine to cure various diseases. Leaves are used as antiseptic and in kidney troubles, muscular pain, piles and applied to boils and carbuncles. The flower is useful in fevers, epileptic fits (Ayurveda), astringent, carminative, stomachic, scabies and liver complaints and is also employed in diseases of the eyes. They are said to purify blood and flower juice is given as a remedy for bleeding piles and also used in rheumatism, colds and bronchitis.
Similarly, rose is another flower which is a woody perennial of the genus Rosa, within the family Rosaceae. There are over 100 species and thousands of cultivars. They form a group of plants that can be erect shrubs, climbing or trailing with stems that are often armed with sharp prickles. Flowers vary in size and shape and are usually large and showy, in colours ranging from white through yellows and reds. Most species are native to Asia, with smaller numbers native to Europe, North America, and northwest Africa. Species, cultivars and hybrids are all widely grown for their beauty and often are fragrant. Rose plants range in size from compact, miniature roses, to climbers that can reach seven meters in height. Different species hybridize easily, and this has been used in the development of the wide range of garden roses. Rose petals are used in making rose oil that is steam distilled by crushing. The by product of steam distillation is rose water, which is an excellent relaxing agent, soothes the nerves and adds flavor to a variety of dishes across the world. Rose essence is rich in flavanoids, tannins, antioxidants, and vitamins A, B3, C, D and E, making it beneficial in skin care. Some of the uses of rose oil, water, and essence are as follows. Rose water is an effective astringent that reduces swelling of capillaries beneath the skin. Rose petal tea is efficient in cleansing the gall bladder and liver, and it helps improve bile secretion. Rose petals are dried and crushed to make tea. Rose petals are an important ingredient in eye washes as well, as it is antiseptic in nature. Rose water benefits include nourishing the scalp and improving hair growth. It is medicinally used as an antibacterial, antiseptic, and anti-inflammatory product. There are several ways of synthesizing heavy metal nanoparticles, however, in situ formation of those materials are not well documented. This paper, aims to report a very simple, cost effective and hazard free green synthesis of spherical cadmium nanoparticles by templating marigold (Tagetes sp.) and rose (Rosa sp.) flower petals as reducing agents.
Materials and methods
Preparation of rose and marigold petal extract
Rose and marigold flowers were collected from departmental garden, Department of Environmental Science, Burdwan University, (23.2383°N, 87.8608°E) Burdwan. Flower petals were washed with distilled water separately and air dried. 5 g of dried rose and marigold petal were taken and crushed with mortar and pestle. The powdered petals then soaked in 45 ml distilled water and heated for 10–15 min. The extract was then filtered through Whatman No. 1 filter paper to remove particulate matter and to get clear solutions which were then refrigerated (4 °C) in 250 ml Erlenmeyer flasks for further experiments. In each and every steps of the experiment, sterility conditions were maintained for the effectiveness and accuracy in results without contaminations (Awwad et al. 2013). The extract was then kept in refrigerator to use within 1 week.
Preparation of CdCl2 solution
Heavy metal solution was prepared by dissolving Cadmium chloride (CdCl2) (Merck Private Limited) in double distilled water.
Synthesis of nanoparticles
According to the phyto-synthesis protocol, 88 ml of Cadmium chloride solution was added to 12 ml of the flower petal extracts. The bioreduction of metal ions was monitored at 1 h intervals by sampling aliquots of the reaction mixture. Reduction of metal ions was observed by change in their color of the reaction mixture during treatment. All experiments were carried out in triplicates. The formed metal nanoparticles was characterized further (Roopan et al. 2013).
Color of nanoparticles and its temporal variation
The flower petal extract, having characteristic color, at initial condition when solution was added to different petal extracts in different proportion and conditions it produced its initial color for different solution and after 72 h it produced ultimate characteristic color. After 8 days the solution became colorless because the particles were precipitated.
Characterization of nanoparticles
The formation of plant mediated cadmium nanoparticles was monitored by the spectral analysis. Synthesized heavy metal nanoparticles was confirmed by sampling the reaction mixture at regular intervals and the absorption maxima was scanned by UV–Vis spectra at the wavelength of 300–700 nm in Perkin Elmer Lamda Spectrophotometer. The characteristic peak indicate the production of nanoparticles (Veerakumar et al. 2014). Further, the reaction mixture was subjected to centrifugation at 60,000×g for 40 min; the resulting pellet was dissolved in deionized water and filtered through Millipore filter (0.45 µm). And the filtrate was further used for SEM, and Fourier transform infrared Spectroscopy (FTIR) study. The nanoparticles solutions were air dried in a petridish. Then the dried powder was collected and sent for the SEM study. The powder was sputter coated with gold before being mounted on aluminium stubs. The specimens were viewed and photographed using a scanning electron microscope (Hitachi, S-530) with 15 V. An aliquot of this filtrate containing cadmium nanoparticles was used for FTIR using a BRUKER (Tensor 27). Morphology of CdNPs was recorded by fluorescent spectrophotometer (SD 1000) (Mondal et al. 2014).
Collection of mosquito larvae and maintenance of mosquito culture
Larvae of A. albopictus were cultured in laboratory conditions. The eggs of A. albopictus were collected from different locations (waste tires, earthen pots, discarded jars). These were brought to the laboratory and transferred (in approximately the same aliquot numbers of eggs) to 18 cm L × 13 cm W × 4 cm D enamel trays containing 500 ml of water where they were allowed to hatch. Mosquito larvae were reared (and adult mosquitoes held). Larvae were fed 5 g ground dog biscuit and brewer’s yeast daily in a 3:1 ratio. Pupae were collected and transferred to plastic containers with 500 ml of water. The container was placed inside a screened cage (90 cm L × 90 cm H × 90 W) to retain emerging adults, for which 10 % sucrose in water solution were given. After emergence, the mosquitoes will be provided blood feeding. The adults were identified using standard identification keys following Service (1976) and Laird (1988). Glass Petri dishes lined with filter paper and containing 50 ml of water were subsequently placed inside the cage for ovipositing (Arjunan et al. 2012). After emergence of larva from the eggs fourth instar stage of A. albopictus larva were taken for mortality test.
Determination of the mosquitocidal activity by cadmium nanoparticles
Twenty-five larvae (fourth instars) was placed in 249 ml of de-chlorinated water in a 500 ml glass beaker, and 1 ml of the desired concentration of cadmium nanoparticles was added. Tests of each concentration against each instar were replicated thrice. In each case, the control comprised 25 larvae in 250 ml of distilled water (Arjunan et al. 2012). Control mortality was corrected by using Abbott’s formula (Abbott 1925), and percentage mortality was calculated as follows:
Statistical analysis
The analysis of variance was computed on statistically significant differences determined based on the appropriate F-test. The results are the mean ± SD of at least three independent replicates. The mean differences were compared utilizing Duncan’s range test. Three replicates (n = 3) were taken for all analysis from each set of experiment.
SEM study of the dead mosquito larvae
The dead larvae were collected from both the treated and control containers. Larval bodies were preserved in 70 % alcohol for further treatment for SEM study. CPD was done with the larval body and then sputter coated with gold. The coated bodies were studied under scanning electron microscope.
Results and discussion
Visual color change of CdNPs
The reduction of cadmium ions by flower extract lead to the formation of cadmium nanoparticles at room temperature. Time dependant color change was observed in cadmium nanoparticles synthesized from marigold and rose petal extract. After mixing colorless CdCl2 solution (Fig. 1) and yellow colored marigold extract straw yellow nanoparticle solution was formed (Fig. 2). Likewise, reddish rose solution when mixed with colorless CdCl2 Solution formed reddish brown solution (Fig. 3) which indicated formation of cadmium nanoparticles. Almost similar color change during formation of cadmium nanoparticles were reported by Andeani and Mohsenzadeh (2013) and Reddy et al. (2010).
Fig. 1.

Initial colour of CdCl2 solution
Fig. 2.

Cadmium solution and marigold petal extract
Fig. 3.

Cadmium nanoparticles induced by rose petal extract
UV–Vis analysis of synthesized CdNPs
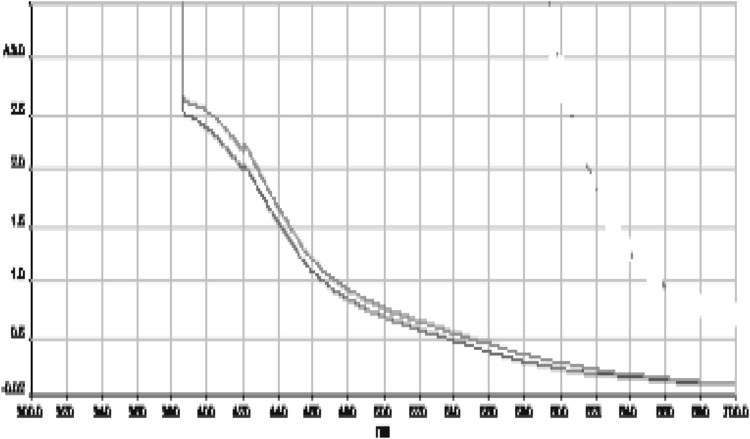
The bioreduction of cadmium ions in solution was monitored periodically by UV–Vis spectrophotometric analysis. UV–Vis spectroscopy was carried out at the wave length 300–700 nm of UV–Vis spectrophotometer (Perkin Elmer Lamda) possessing a scanning speed of 250 nm/min and a resolution of 1 nm. The UV–Vis spectrum of Cd NPs synthesized from marigold petal extract is presented in Fig. 4. From Fig. 4 it is clear that distinct peaks at 387.33 nm which is distinctly different from the peaks those are produced from pure marigold petal extract and pure cadmium salt solution. However, cadmium nanoparticles formed using rose petal extract produced a characteristic peak at 387.33 nm also (Fig. 4). Literatures also indicate that Cd NPs generally consists of a characteristic peak ranging from 300 to 400 nm.
Fig. 4.
UV–Vis spectra of Cd nanoparticles formed by rose extract (green for marigold and pink for rose) (color figure online)
Morphology of synthesized nanoparticles
The fluorescent microscope study of the nanoparticles revealed that the nano-Cd predominates absolutes spherical in shape. Most of the nanoparticles were roughly spherical in shape with smooth edges (Fig. 5).
Fig. 5.

Shape and sizes of cadmium nanoparticles
FTIR analysis of synthesized CdNPs
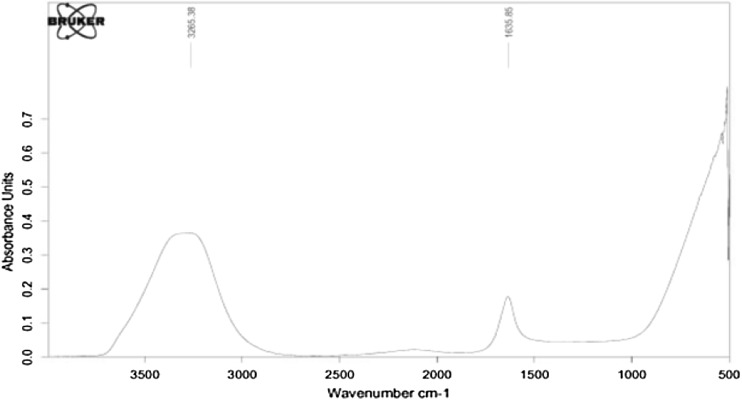
FTIR was used to recognize the possible biomolecules responsible for the reduction of the Cd ions and capping agent responsible for binding the cadmium nanoparticles. From Fig. 6 it is clear that distinct peak at 3435, 2930, 1643 and 1100 cm−1 represent the multifunctional groups indicating alcoholic –OH, amide, C–C and –OCH3 groups. However, variation of peaks may be due to some metabolites such as tannins, flavonoids, alkaloids and carotenoids which are abundant in flower extract and produce cadmium nanoparticles. Almost similar flower based gold nanoparticles was reported by Dhayananthaprabhu et al. (2013).
Fig. 6.
FTIR of Cadmium nanoparticle
Mosquito larvicidal activity
10 ppm CdCl2 salt solution showed 12.3 % mortality of A. albopictus larvae. And 72 h treatment with salt solution showed 15.1 % mortality. After 24 h of treatment with marigold petal extract induced cadmium nanoparticles (10 ppm) caused about 68.9 % mortality of A. albopictus was recorded and 100 % mortality was recorded after 72 h of incubation with same concentration of Cd-nanoparticles. However, when A. albopictus larvae were treated with rose petal extract induced Cd nanoparticles of 10 ppm concentration, 43.5 % mortality was seen after 24 h and 98.8 % after 72 h of incubation. The per cent mortality is the mean value of the replicates. All these data are presented in Tables 1 and 2. Similar mosquito larvicidal activity was reported by Shanmugasun and Balagurunathan (2013) and Balakrishnan et al. (2014) by using silver nanoparticles. Mortality of mosquito larvae was very less when treated with only CdCl2 salt (Table 3) and flower petal extract (Table 4) which indicates the efficiency of formed nanoparticles towards larvcidal activity.
Table 1.
Mean percentage Mortality of A. albopictus by cadmium nanoparticles synthesized by marigold (Tagetes erecta) petal extract
| Concentration of solution (ppm) | % mortality at day 1 | % mortality at day 3 |
|---|---|---|
| 1 | 10 ± 0.06c | 49.9 ± 0.41b |
| 5 | 45.7 ± 1.6b | 92.3 ± 0.44a |
| 10 | 68.9 ± 2.4a | 100 ± 2.88a |
Different letters (a, b and c) indicate significant differences at p < 0.01 according to the Tukey-HSD
Table 2.
Mean percentage Mortality of A. albopictus by Cadmium nanoparticles synthesized by Rose (Rosa sp.) petal extract
| Concentration of solution (ppm) | % mortality at day 1 | % mortality at day 3 |
|---|---|---|
| 1 | 9.3 ± 0.11c | 45.2 ± 0.022b |
| 5 | 35.5 ± 0.31b | 2.5 ± 0.05c |
| 10 | 43.5 ± 1.004a | 98.8 ± 0.011a |
Different letters (a, b and c) indicate significant differences at p < 0.01 according to the Tukey-HSD
Table 3.
Mean percentage mortality of A. albopictus by cadmium salt (CdCl2)
| Concentration of solution (ppm) | % mortality at day 1 | % mortality at day 3 |
|---|---|---|
| 1 | 3 ± 0.66c | 6.8 ± 1.01b |
| 5 | 7.8 ± 0.12b | 9 ± 0.7b |
| 10 | 12.3 ± 0.17a | 15.1 ± 0.4a |
Different letters (a, b and c) indicate significant differences at p < 0.01 according to the Tukey-HSD
Table 4.
Mean percentage mortality of A. albopictus by flower petal extract
| Concentration of solution | % mortality at day 1 | % mortality at day 3 |
|---|---|---|
| Rose extract | 0 | 2.1 ± 0.02a |
| Marigold extract | 0 | 1.3 ± 0.003a |
aNon-significant differences at p < 0.01 according to the Tukey-HSD
SEM study of the dead mosquito larvae
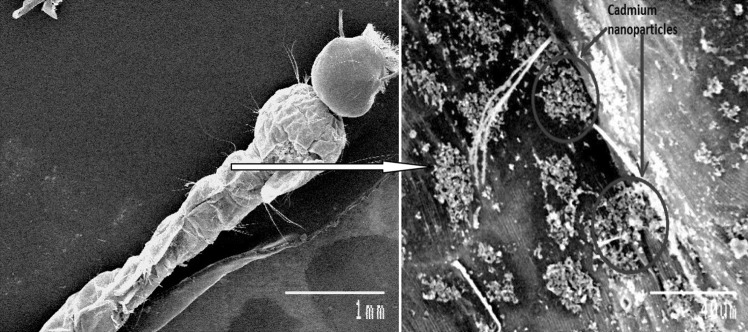
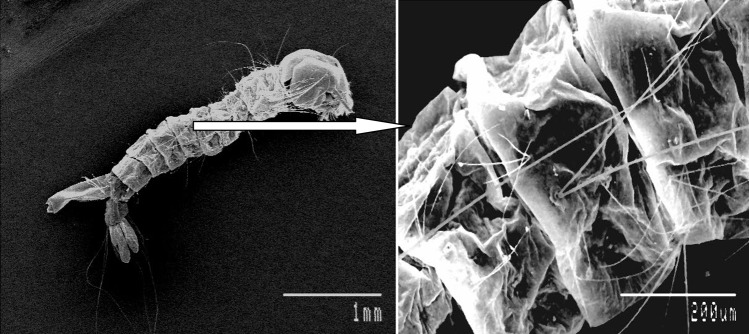
SEM study of the dead mosquito larval body clearly showed the deposition of nanoparticles on the body surface of mosquito. In Figs. 7 and 8 the SEM picture of the treated mosquito larva shows some minute particle deposition on its body surface whereas no such type of deposition can be seen in the body of control larva. This makes clear that death of mosquito larva is due to cadmium nanoparticles. But, the exact mechanism by which the nanoparticle is causing death of mosquito larva is not clearly known and need further detailed study.
Fig. 7.
Dead larva treated with Cd nanoparticles showing deposition of nanoparticles on its body surface
Fig. 8.
Control larva have no such deposition on its body
Comparative study
The larvicidal effect of aqueous leaf extracts, heavy metal nitates and synthesized nanoparticles are well documented (Mondal et al. 2014; Hajra and Mondal 2015). However, nanoinduced larvicidal effect of heavy metal is not well studied a comparative study in Table 5 is provided for better understanding the potentiality of heavy metal nanoparticles towards killing of mosquito larva.
Table 5.
Comparative study of heavy metal nanoparticles towards mosquito larvicidal activity
| Plant species | Mosquito sp. | Mortality rate | Metal nanoparticles | References |
|---|---|---|---|---|
| Mimosa pudica | Anopheles subpictus | LC50-13.90 mg/l | Ag | Marimuthu et al. (2011) |
| Culex quinquefasciatus | LC50-11.73 mg/l | |||
| Gmelina. asiatica | Anopheles stephensi | LC50-22.44 µg/ml | Ag | Muthukumaram et al. (2015) |
| LC90-40.15 µg/ml | ||||
| Aedes aegypti | LC50-25.77 µg/ml | |||
| LC90-45.98 µg/ml | ||||
| Culex quinquefasciatus | LC50-27.83 µg/ml | |||
| LC90-48.92 µg/ml | ||||
| Avicennia marina | Anopheles stephensi | LC50-4.928 mg/l | Ag | Balakrishnan et al. (2014) |
| Aedes aegypti | LC50-9.865 mg/l | |||
| Heliotropium indicum | Anopheles stephensi | LC50-18.40 µg/ml | Ag | Veerakumar et al. (2014) |
| LC90-32.45 µg/ml | ||||
| Aedes aegypti | LC50-20.10 µg/ml | |||
| LC90-35.97 µg/ml | ||||
| Culex quinquefasciatus | LC50-21.84 µg/ml | |||
| LC90-38.10 µg/ml | ||||
| Cocos nucifera | Anopheles stephensi | LC50-87.24 mg/l | Ag | Roopan et al. (2013) |
| LC90-230.90 mg/l | ||||
| Culex quinquefasciatus | LC50-49.89 mg/l | |||
| LC90-84.85 mg/l | ||||
| Cadaba indica | Anopheles stephensi | LC50-15.41 mg/l | Ag | Kalimuthu et al. (2013) |
| LC90-61.07 mg/l | ||||
| Culex quinquefasciatus | LC50-15.44 mg/l | |||
| LC90-58.37 mg/l | ||||
| Pedilanthus tithymaloides | Aedes aegypti | LC50-1.461 mg/l | Ag | Sundaravadivelan et al. (2013) |
| Culex quinquefasciatus | LC50-4.56 mg/l | |||
| LC90-13.14 mg/l | ||||
| Vinca rosea | Anopheles stephensi | LC50-16.84 mg/ml | Ag | Subarani et al. (2013) |
| LC90-68.62 mg/ml | ||||
| Culex quinquefasciatus | LC50-43.80 mg/ml | |||
| LC90-120.54 mg/ml | ||||
| Tinospora cordifolia | Anopheles subpictus | LC50-6.34 mg/l | Ag | Jayaseelan et al. (2011) |
| Culex quinquefasciatus | LC50-6.96 mg/l | |||
| Tagetes erecta | 5 ppm-45.7 % at 24 h | Cd | Present work | |
| 10 ppm-68.9 % at 24 h | ||||
| Rosa sp. | Aedes albopictus | 5 ppm-35.5 % at 24 h | ||
| 10 ppm-43.5 % at 24 h |
Conclusions
Biosynthesis of metal nanoparticles using plant derivatives is extremely studied in the last two decades. The plant metabolites induce the production of metallic nanoparticles in ecofriendly manner. In the present study, we focused on green synthesis of cadmium nanoparticles using aqueous petal extract of marigold and rose flower. The physical property of synthesized nanoparticle was characterized using relevant techniques. Further we demonstrated the possible application of CdNPs as a substitute of insecticide to shows mosquito larvicidal activity. However, present results is much better than our previous experiment with AgNPs which showed moderate mosquito larvicidal activity. With little uncovered mechanism in the current study, there is a wide scope for detailed investigation in the future for the application of CdNPs in the field of Agriculture for controlling the pathogen. The plant mediated nanoparticles have the potential to be used in various fields such as pharmaceuticals, therapeutics, sustainable and renewable energy and other commercial products.
Acknowledgments
Authors are thankful to Dr Chandan Adhikari, Department of Chemistry, Government Teachers’ Training college, Burdwan for providing lab facilities and also recording FT-IR spectra. Authors also grateful to Dr Srikanta Chatterjee, Department of Instrumentation, The University of Burdwan for recording fluorescent microscope.
Compliance with ethical standards
Conflict of interest
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- Abbott W. A method of computing the effectiveness of an insecticide. J Econ Entomol. 1925;18:265–267. doi: 10.1093/jee/18.2.265a. [DOI] [Google Scholar]
- Abdel-Aziz MS, Shaheen MS, El-Nekeety AA, Abdel-Wahhab MA. Antioxidant and antibacterial activity of silver nanoparticles biosynthesized using Chenopodium murale leaf extract. J Saudi Chem Soc. 2014;18:356–363. doi: 10.1016/j.jscs.2013.09.011. [DOI] [Google Scholar]
- Ahluwalia V, Kumar J, Sisodia R, Shakil NA, Walia S. Green synthesis of silver nanoparticles by Trichoderma harzianum and their bio-efficacy evaluation against Staphylococcus aureus and Klebsiella pneumonia. Indus Crops Products. 2014;55:202–206. doi: 10.1016/j.indcrop.2014.01.026. [DOI] [Google Scholar]
- Ajitha B, Reddy YAK, Reddy PS. Biosynthesis of silver nanoparticles using Momordica charantia leaf broth: evaluation of their innate antimicrobial and catalytic activities. J Photochem Photobiol B Biol. 2015;146:1–9. doi: 10.1016/j.jphotobiol.2015.02.017. [DOI] [PubMed] [Google Scholar]
- Andeani JK, Mohsenzadeh S. Phytosynthesis of cadmium nanoparticles from Achillea wilhelmsii flowers. J Chem. 2013 [Google Scholar]
- Arjunan NK, Murugan K, Rejeeth C, Madhiyazhagan P, Barnard DR. Green synthesis of silver nanoparticles for the control of mosquito vectors of malaria, filariasis and dengue. Vector Borne Zoonotic Dis. 2012;12(3):262–268. doi: 10.1089/vbz.2011.0661. [DOI] [PubMed] [Google Scholar]
- Awwad AM, Salem NM, Abdeen AO. Green synthesis of silver nanoparticles using carob leaf extract and its antibacterial activity. Int J Ind Chem. 2013;4:29. doi: 10.1186/2228-5547-4-29. [DOI] [Google Scholar]
- Baghizadeh A, Ranjbar S, Gupta VK, Asif M, Pourseyedi S, Karimi MJ, Mohammadinejad R. Green synthesis of silver nanoparticles using seed extract of Calendula officinalis in liquid phase. J Mole Liq. 2015;207:159–163. doi: 10.1016/j.molliq.2015.03.029. [DOI] [Google Scholar]
- Balakrishnan S, Srinivasan M, Mohanraj J. Biosynthesis silver nanoparticles from mangrove plant (Avicennia marina) extract and their potential mosquito larvicidal property. J Parasit Dis. 2014 doi: 10.1007/s12639-014-0621-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitra G, Balasubramani G, Ramkumar R, Sowmiya R, Perumal P. Mukia maderaspatana (Cucurbitaceae) extract-mediated synthesis of silver nanoparticles to control Culex quinquefasciatus and Aedes aegypti (Diptera: Culicidae) Parasitol Res. 2015;114:1407–1415. doi: 10.1007/s00436-015-4320-7. [DOI] [PubMed] [Google Scholar]
- Dhayananthaprabhu J, Narayanan RL, Thiyagarajan K. Facile synthesis of gold (Au) nanoparticles using Cassia auriculata flower extract. Advan Mater Res. 2013;678:12–16. doi: 10.4028/www.scientific.net/AMR.678.12. [DOI] [Google Scholar]
- Goia DV, Matijevic E (1998) Preparation of nanodispersed metal particles. New J Chem 22:1203–1215
- Hajra A, Mondal NK. Silver nanoparticles: an eco-friendly approach for mosquito control. Int J Sci Res Environ Sci. 2015;3(2):47–61. [Google Scholar]
- Jayaseelan C, Rahaman AA, Rajkumar G, Vishnu KA, Santhoskumar T, Marimuthu S, et al. Moonseed plant, Tinospora cordifolia Miers. Parasitol Res. 2011;109:185–194. doi: 10.1007/s00436-010-2242-y. [DOI] [PubMed] [Google Scholar]
- Kalaiselvi A, Roopan SM, Madhumitha G, Ramalingam C, Elango G. Synthesis and characterization of palladium nanoparticles using Catharanthus roseus leaf extract and its application in the photo-catalytic degradation. Spectrochem Acta A Mol Biomol Spectrosc. 2015;135:116–119. doi: 10.1016/j.saa.2014.07.010. [DOI] [PubMed] [Google Scholar]
- Kalimuthu K, Panneerselvam C, Murugan K, Hwang JS. Green synthesis of silver nanoparticles using Cabada indica lam leaf extract and its larvicidal and pupicidal activity against Anopheles stephensi and Culex quinquefasciatus. J Entomol Acarol Res. 2013;45:57–64. doi: 10.4081/jear.2013.e11. [DOI] [Google Scholar]
- Kathiravan V, Ravi S, Ashokkumar S. Synthesis of silver nanoparticles from Melia dubia leaf extract and their in vitro anticancer activity. Spectrochimica Acta A Mol Biomol Spectrosc. 2014;130:116–121. doi: 10.1016/j.saa.2014.03.107. [DOI] [PubMed] [Google Scholar]
- Krishnamurthy NB, Nagaraj B, Malakar B, Liny P, Dinesh R. Green synthesis of gold nanoparticles using Tagetes erecta l. (mari gold) flower extract and evaluation of their antimicrobial activities. Int J Pharm Bio Sci. 2012;3(1):212–221. [Google Scholar]
- Laird M. The natural history of larval mosquito habitats. New York: Academic Press; 1988. [Google Scholar]
- Marimuthu S, Rahuman AA, Rajakumar G, Santhoshkumar T, Kirthi AV, Jayaseelan C, Bagavan A, Zahir AA, Elango G, Kamaraj C. Evaluation of green synthesized silver nanoparticles against parasites. Parasitol Res. 2011;108:1541–1549. doi: 10.1007/s00436-010-2212-4. [DOI] [PubMed] [Google Scholar]
- Medda S, Hajra A, Dey U, Bose P, Mondal NK. Biosynthesis of silver nanoparticles from Aloe vera leaf extract and antifungal activity against Rhizopus sp. and Aspergillus sp. Appl Nanosci. 2014 [Google Scholar]
- Mondal NK, Choudhury A, Dey U, Mukhopadhyay P, Chatterjee S, Das K, Datta JK. Green synthesis of silver nanoparticles and its application for mosquito control. Asian Pac J Trop Dis. 2014;4(1):s204–s210. doi: 10.1016/S2222-1808(14)60440-0. [DOI] [Google Scholar]
- Muthukumaram U, Govindarajan M, Rajeswary M. Mosquito larvicidal potential of silver nanoparticles using Chomelia asiatica (Rubiaceae) against Anopheles stephensi, Aedes aegypti and Culex quinquifasciatus (Diptera: Culicidae) Parasitol Res. 2015;114(3):989–999. doi: 10.1007/s00436-014-4265-2. [DOI] [PubMed] [Google Scholar]
- Oluwafemi OS, Daramola OA, Ncapayi V. A facile green synthesis of type II water soluble CdTe/CdS core shell nanoparticles. Mater Lett. 2014;133:9–13. doi: 10.1016/j.matlet.2014.06.152. [DOI] [Google Scholar]
- Pastoriza-Santos I, Liz-Marzan LM. Synthesis of silver nanoparticles in DMF. Nano Lett. 2002;2(8):903–905. doi: 10.1021/nl025638i. [DOI] [Google Scholar]
- Rajakumar G, Rahuman AA. Larvicidal activity of synthesized silver nanoparticles using Eclipta prostrate leaf extract against filariasis and malaria vectors. Acta Trop. 2011;118:196–203. doi: 10.1016/j.actatropica.2011.03.003. [DOI] [PubMed] [Google Scholar]
- Ramanibai R, Velayutham K. Bioactive compound synthesis of Ag nanoparticles from leaves of Melia azedarach and its control for mosquito larvae. Res Vet Sci. 2015;98:82–88. doi: 10.1016/j.rvsc.2014.11.009. [DOI] [PubMed] [Google Scholar]
- Reddy S, Kumara Swamy BE, Chandra U, Sherigara BS, Jayadevappa H. Synthesis of CdO nanoparticles and their modified carbon pase electrode for determination of dopamine and ascorbic acid by using cyclic voltammetry techniques. Int J Electrochem Sci. 2010;5(1):10–17. [Google Scholar]
- Roopan SM, Rohit MadhumitaG, Rahuman AA, Kamaraj C, Bharathi A, Surendra TV. Low-cost and eco-friendly phyto-synthesis of silver nanoparticles using Cocos nucifera coir extract and its larvicidal activity. Ind Crops Prod. 2013;43:631–635. doi: 10.1016/j.indcrop.2012.08.013. [DOI] [Google Scholar]
- Service MW. Mosquito ecology: field sampling methods. USA: Springer; 1976. [Google Scholar]
- Shanmugasun T, Balagurunathan R. Mosquito larvicidal activity of silver nanoparticles synthesized using actinobacterium, Streptomyces sp. M25 against Anopheles subpictus, Culex quinquefasciatus and Aedes aegypti. J Parasit Dis. 2013 doi: 10.1007/s12639-013-0412-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JY, Kim BS. Rapid biological synthesis of silver nanoparticles using plant leaf extracts. Bioprocess Biosyst Eng. 2009;32(1):79–84. doi: 10.1007/s00449-008-0224-6. [DOI] [PubMed] [Google Scholar]
- Subarani S, Sabhanayakam S, Kamaraj C. Studies on impact of biosynthesized silver nanoparticles (AgNP) in relation to malaria and filariasis vector control against Anopheles stephensi Liston and Culex quinquefasciatus Say (Diptera: Culicidae) Parasitol Res. 2013;112:487–499. doi: 10.1007/s00436-012-3158-5. [DOI] [PubMed] [Google Scholar]
- Sundaravadivelan C, Nalini Padmanabhan M, Sivaprasath P, Kishmu L. Biosynthesized silver nanoparticles from Pedilanthus tithymaloides leaf extract with anti-developmental activity against synthesis of pediculocidal and larvicidal silver nanoparticles by leaf extract from heart leaf. Parasitol Res. 2013;112(1):303–311. doi: 10.1007/s00436-012-3138-9. [DOI] [PubMed] [Google Scholar]
- Thakkar KN, Mhatre SS, Parikh RY. Biological synthesis of metal nanoparticles. Nanomed. 2010;6(2):257–262. doi: 10.1016/j.nano.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Tian Y, Liu Y, Pang F, Wangb F, Zhangb X. Green synthesis of nanostructed Ni-reduced graphene oxide hybridsand their application for catalytic reduction of 4-nitrophenol. Colloids Surf A Physicochem Eng Asp. 2015;464:96–103. doi: 10.1016/j.colsurfa.2014.10.027. [DOI] [Google Scholar]
- Veerakumar K, Govindarajan M, Rajeswary M, Muthukumaram U. Mosquito larvicidal properties of silver nanoparticles synthesized using Heliotropium indicum (Boraginaceae) against Aedes aegypti, Anopheles stephensi and Culex quinquefasciatus (Diptera: Culicidae) Parasitol Res. 2014;113(6):2663–2673. doi: 10.1007/s00436-014-3895-8. [DOI] [PubMed] [Google Scholar]