Abstract
Infection with Babesia and Theileria Causes high mortality and economical losses in livestock and has a relatively high prevalence in Iran. In Zabol, animals are susceptible to this disease because of presence of vector ticks, weather conditions and smuggle animal across the border and they cause great damages to the economy and production. However, few studies have been done for differentiation of Theileria and Babesia in sheep in this area. The purpose of the present study was to determine the prevalence of Babesiosis and Theileriosis in sheep in Zabol. A number of 80 animals were randomly selected and their blood samples were examined. The presence of Theileria and Babesia parasites in each sample was determined with PCR and microscopic examination. Of 80 blood samples obtained from sheep, 8 cases (10 %) were positive by microscopic examination where 6 samples were infected with Theileria and 2 samples with Babesia. The result of PCR method determined the prevalence of 66.25 % (53 samples) and 3.75 % (3 samples) for Theileria and Babesia, respectively. The correlation between these two methods (PCR and microscopic examination) was determined with Kappa statistical test. Based on the obtained results, it is concluded that Babesiosis has a low prevalence among the sheep of Zabol. This is the first report in which ovine Babesiosis has been studied in this region using molecular identification techniques.
Keywords: Zabol, Theileria, Babesia, PCR
Introduction
Hemoparasitic diseases caused by protozoan pathogen of the Phylum Apicomplexa are the most pathogenic parasitic infections in ruminant. Babesia and Theileria species are the important tick borne protozoa in tropical and subtropical areas and cause economic losses. Ovine Babesiosis is haemoparasitic disease of small ruminants and it reveals with clinical signs such as fever, anemia, icterus, haemoglobinuria and causes high mortality and morbidity. At first, Delpi determined the presence of B. motasi and B. ovis in Iran in 1939. B. ovis, B. motasi, and B. crassa have been identified in sheep and goats in Iran and Theileria species detected as T. lestoquardi and T. ovis (Hashemi-Fesharaki 1997; Soulsby 1982). The most common procedures used in the diagnosis of these piroplasms are microscopic examination of blood smears and Serological methods. Blood smears detection with Giemsa stain is a method which is commonly used, but because of occasional technical mistakes, this method may lead to false morphologic diagnosis. Serologic methods for diagnosis of sub-clinical infections in epidemiological studies are also highly used. These methods don’t have specificity and have cross reaction with other piroplasm species; In addition, false positive and negative results are also commonly observed. Molecular methods are sensitive, specific, and rapid for diagnosis of Babesia and Theileria and provide more accurate information for future investigation (Uilenberg 2006; Razmi et al. 2003; Schnittger et al. 2004; Altay et al. 2005).According to clinical and morphological observations, Livestock in Zabol are determined to have these disease causing great damages to the economy and production in this area. Regarding the importance of pathogenic protozoa, Babesia and Theileria, wide geographical distribution in Iran and few studies on their prevalence, especially Babesia, in livestock in Zabol, the accurate detection of these protozoa is a certain necessity to improve control measures. Due to morphological diagnostic problems, use of molecular method is considered in this study.
The purpose of the present study, therefore, is to determine the prevalence of Ovine Theileria and Babesia infection in this region using PCR method compared with common methods of microscopic examination.
Materials and methods
Sistan and Balochestan province is located in south eastern part of Iran and Zabol lies in north part of this province. The latitude and longitude GPS coordinates of Zabol (Iran) is: Lat: 31.0385, long: 61.4962. According to Koopen’s classification, Zabol is characterized by a warm dry climate with dry and warm summers and, according to Amberjeh’s classification, it has a mild desert climate (Ahmadian et al. 2009). In this study, 80 blood samples were taken randomly from sheep of different places of Zabol in spring and summer. 3 ml blood samples were collected from each sheep into the labeled EDTA tube (as preserver) and mixed gently. Simultaneously, prepared thin blood smear from their blood and transferred to the parasitology laboratory of Veterinary Faculty in Zabol. The blood smears were fixed in methanol and stained in 10 % Giemsa solution in phosphate buffered saline (PBS) pH 7.2, in order to determine of the presence of haemoprotozoal parasites. Genomic DNA was extracted from blood sample using a DNA Isolation kit (MBST, Iran) following manufacture’s recommendation and used as template for PCR. The considered nucleotide sequence was amplified using Babesia/Theileria Sense (S1) and Babesia/Theileria anti Sense (S2) derived from flanking part of hyper variable region of 18ssrRNA (Table 1) according to the method of Shayan and Rahbari (2007), in order to simultaneous differentiation between Theileria and Babesia. The PCR was performed on 50 µl reaction volumes including, 4 µl extracted DNA, 25 µl Taq DNA polymerase 2 × Master Mix (Pishgam Company, Iran), 30 pmol of each primer and sterile distilled water up to 50 ml in automated Thermocycler with the following program: 5 min incubation at 95 °C to denature double strand DNA, 38 cycles of 45 s at 95 °C, 45 s at 56° (annealing step), 45 s at 72 °C and this was followed by final extension step at 72 °C for 10 min. The sample was confirmed microscopic piroplasm used as positive control and negative control (no template) was always run simultaneously with our PCR experiments. PCR product was analyzed on 1.5 % agarose gel in 0.5X-TBE buffer and visualized using ethidium bromide and an UV illuminator (Fig. 2). The PCR products of 426–430 and 389–402 bp were produced for Theileria spp. and Babesia spp., respectively. Babesia and Theileria was determined by difference of 30 bp in the nucleotide sequence of the PCR products. For the aim of data analysis, the correlation between PCR and microscopic examination was determined with Kappa statistical test (Kappa). Moreover, the sensitivity and specificity of microscopic examination was determined in comparison to PCR method. In Bootstrap method, the 95 % interval confidence for Kappa was counted. With Binomial distribution, 95 % confidence interval for sensitivity and specificity was counted. For this purpose, the statistical program (IBM® PASW/SPSS® Statistics 18.0-2009) was used.
Table 1.
The nucleotide sequence of primers, from the hypervariable region V4 of the 18S rRNA gene of piroplasms Babesia and Theileria (Shayan and Rahbari 2007)
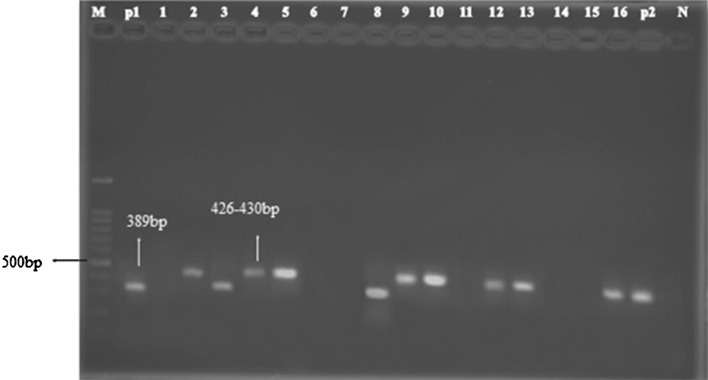
Fig. 2.
Agarose-gel electrophoresis of amplification products obtained from Theileria spp. and Babesia sp. M 100 bp ladder DNA marker; 3, 8 (Babesia); 2,4,5,9,10,12,13,16 (Theileria); P1, P2, positive control of Babesia and Theileria; N negative control
Results
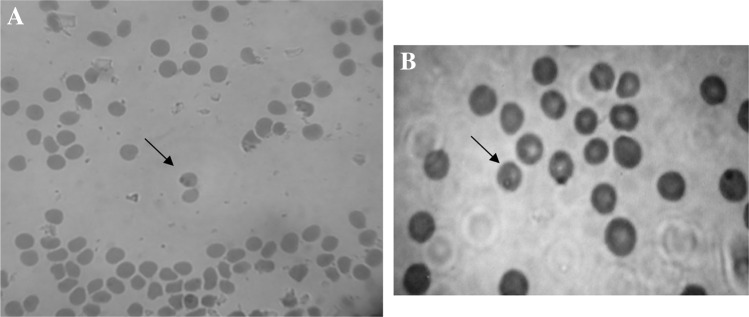
Microscopic observation on 80 stained smear of blood samples determined 6 samples were infected with Theileria (7.5 %) and 2 samples with Babesia (2.5 %) (Fig. 1); while prevalence of Theileria infection was estimated 66.25 % (53 samples) and Babesia infection 3.75 % (3 samples) by using PCR method (Fig. 2).
Fig. 1.
Blood smear from infected sheep was stained with Giemsa a Babesia, b Theileria
Comparison of microscopic examination and PCR methods
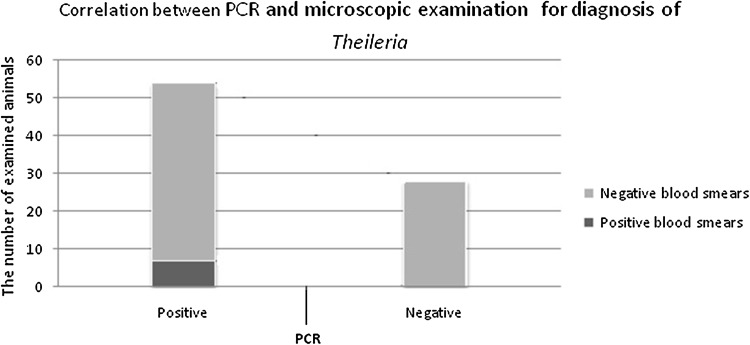
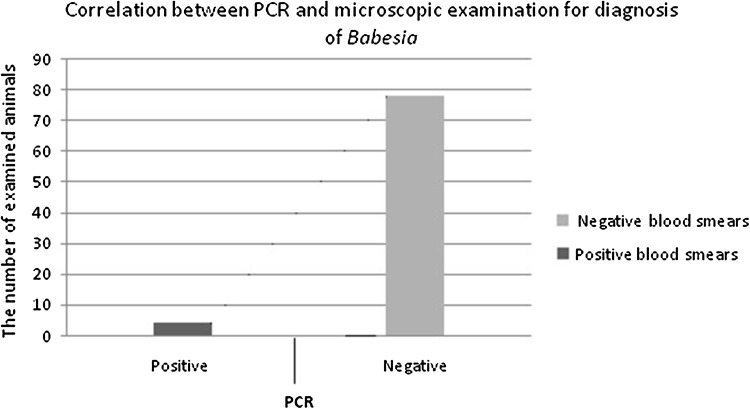
In detection of Theileria, the infections occurred in 6 samples with microscopic examination, Of 53 blood samples which were positive by PCR, whereas all of the positive samples by thin blood smears were also determined to be positive by PCR method. Theileria were not found by microscopic examination in all 27 samples were negative by PCR test. In detection of Theileria, sensitivity of microscopic examination method towards the PCR method was (11.3 %) and specificity of microscopic examination method towards PCR method was (100 %) (95 % confidence interval (CI) for sensitivity and specificity was 4.4–23.9 and 87.3–100.0 %, respectively). Results of this study revealed that microscopic examination for diagnosis of Theileria does not have enough sensitivity. Kappa test showed that there is a few correlation between the results of PCR examination and those of microscopic examination (κ = 0.079, 95 % confidence interval 0.027–0.0158) (Fig. 3). In diagnosis of Babesia infection, protozoa were observed in 2 samples with microscopic examination, Of 3 blood samples which were positive by PCR and were not found by microscopic examination in all 77 samples were negative by PCR test. Thus, sensitivity of microscopic examination method towards the PCR method was (66.7 %) and specificity of microscopic examination method towards the PCR was (100 %) (95 % confidence interval for sensitivity and specificity was 9.4–99.2 and 95.2–100.0 %, respectively). Kappa test revealed that there is an acceptable correlation between the results of PCR examination and those of microscopic examination (κ = 0.794, 95 % confidence interval 0.0388–0.100) (Fig. 4).
Fig. 3.
Comparison of PCR method and blood smears for diagnosis of Theileria
Fig. 4.
Comparison of PCR method and blood smears for diagnosis of Babesia
Discussion
Babesia and Theileria, that belongs to the phylum Apicomplexa and order Piroplasmida, are the pathogenic agents in domestic and wild animals. These organisms tend to high economical and hygienic losses worldwide, because of causing severe disease and death in domestic animals, either importance of transmission of some species to human (Ahmed et al. 2002). B. motasi, B. ovis and B. crassa have been identified as common pathogenic species in sheep and goats in Iran (Hashemi-Fesharaki 1997), On the other hand, Theileriosis caused by T. lestoquard and T. ovis. Based on clinical and morphological studies, T. ovis is widespread throughout the country (Hashemi-Fesharaki 1997; Razmi et al. 2006). There are different methods to study the prevalence of Babesia and Theileria species in small ruminants. The methods which are generally used to diagnosis of these blood protozoa are serologic methods and microscopic examination of blood smears. Microscopic method shows false negative results, because of artifact, low parasitemia and destruction of pyroplasmic shapes in erythrocyte due to hemolysis and thickness of blood smears (Dumanli et al. 2005). Moreover protozoa may have different shapes at different stages of development and in different hosts. On the other hand, different species of Piroplasm can be very similar, for instance Uilenberg believed that what is generally called B. microti, may not be Babesia, rather is Theileria, or it was better to name the horse little piroplasm, “T. equi” (Uilenberg 2006). In study of ovine theileriosis in Oman, Shayan et al. (2011) reported that they were not able to diagnose parasite with microscopic examination, but they identified T. ovis and T. lestoquardi with PCR method. Razmi et al. (2003); Schnittger et al. (2004); Altay et al. (2005) and Aktas et al. (2005) believed that for diagnosis of Theileria and Babesia species, more accurate and sensitive methods than microscopic examination of stained blood smear, like PCR method is required. However, the PCR method enables us to detect clinical, subclinical and chronic infections (Dumanli et al. 2005).In this study which conducted on sheep in Zabol, by microscopic examination 8 cases (10 %) were positive where 6 samples were infected with Theileria and 2 samples with Babesia and by PCR method, the prevalence of infection with Theileria was 66.25 % (53 samples) and Babesia 3.75 % (3 samples). The results show that the prevalence of Babesiosis was very low and mixed infection was not observed. Our results confirm the findings of other studies that molecular method is more specific and sensitive in comparison with the microscopic examination, especially for diagnosis of Theileria.
Besides, these results are the same as veterinaries’ idea about high level of Theileria infection among sheep of this area. Several studies have been done to investigate the epidemiology of Theileriosis and Babesiosis in Iran. Razmi et al. (2003) reported 26.1 % of sheep and 14.8 % of goats were infected with Babesia in Mashhad. Serological study of Babesiosis indicated a seroprevalence of 47.5 % in Khuzestan province (Hashemzadeh et al. 2006). The prevalence of Babesia and Theileria have been reported in sheep and goats in different geographic areas of Iran using PCR method as 5.58, 53 and 11.1 % for mixed infection (Dehkordi et al. 2010), in their study the prevalence of Babesiosis was lower and the result is also the same as our findings.
Seidabadi et al. (2014) reported the prevalence of 6.6 % for Babesia in North Khorasan using PCR. In addition Low prevalence of Babesia in other countries such as Turkey and Germany have also been reported (Altay et al. 2005; Theodoropoulos et al. 2006).
Zaeemi et al. (2011) and Heidarpour-Bami et al. (2010) in a study on the prevalence of Theileria species by PCR-RFLP method in west and east of Iran determined the frequencies of Theileria spp. infection in sheep in the range of 32.8–60 %, respectively. Our results are similar to the findings of Heidarpour-Bami et al. (2010).
This research actually is a primitive attempt to diagnose Babesiosis and Theileriosis prevalence and is also the first molecular report of Babesia infection among sheep in this region. Considering the limitations of current studies and technical problems in using morphology approach, it is better to use molecular method in future studies for the diagnosis of Theileria and Babesia species and their vectors so as to control the disease and reduce its side effect.
References
- Ahmadian A, Ghanbari A, Mir B, Razmjoo E. Intraction effect of drought stress and animal manure on yield componets, essential oil and its compositions on Cumin. Iran J Plant Tehran Univ. 2009;40:173–180. [Google Scholar]
- Ahmed J, Yin H, Schnittger L, Jongejan F. Ticks and tick-borne diseases in Asia with special emphasis on China. Parasitol Res. 2002;88:51–55. doi: 10.1007/s00436-001-0574-3. [DOI] [PubMed] [Google Scholar]
- Aktas M, Altay K, Dumanli N. Survey of Theileria parasites of sheep in eastern Turkey using polymerase chain reaction. Small Rumin Res. 2005;60:289–293. doi: 10.1016/j.smallrumres.2005.01.002. [DOI] [Google Scholar]
- Altay K, Dumanli N, Holman PJ, Aktas M. Detection of Theileria ovis in naturally infected sheep by nested PCR. Vet Parasitol. 2005;127:99–104. doi: 10.1016/j.vetpar.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Dehkordi ZS, Zakeri S, Nabian S, Bahonar A, Ghasemi F, Noorollahi F, Rahbari S. Molecular and biomorphometrical identification of ovine babesiosis in Iran. Iran J Parasitol. 2010;5(4):21. [PMC free article] [PubMed] [Google Scholar]
- Dumanli N, Aktas M, Cetinkaya B, Cakmak A, Koroglu E, Saki CE, et al. Prevalence and distribution of tropical theileriosis in eastern Turkey. Vet Parasitol. 2005;127:9–15. doi: 10.1016/j.vetpar.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Hashemi-Fesharaki R. Tick-borne diseases of sheep and goats and their related vectors in Iran. Parasitologia. 1997;39:115–117. [PubMed] [Google Scholar]
- Hashemzadeh F, Nabavi L, Seyfabad Shapouri MR, Rahbari S, Azizi F, et al. Development of an ELISA technique for the detection of B. ovis and serological survey of the parasite in Khuzestan province, southern Iran. Iran J Univ Shiraz. 2006;7(2):15. [Google Scholar]
- Heidarpour-Bami M, Khazraiinia P, Haddadzadeh HR, Kazemi B. Identification of Theileria species in sheep in the eastern half of Iran using nested PCR-RFLP and microscopic techniques. Iran J Vet Res. 2010;11(3):262–266. [Google Scholar]
- Razmi GR, Naghibi A, Aslani MR, Dastjerdi K, Hosseini H. An epidemiological study on Babesia infection in small ruminants in Mashhad suburb, Khorasan province. Iran Small Rumin Res. 2003;50(1):39–44. doi: 10.1016/S0921-4488(03)00107-X. [DOI] [Google Scholar]
- Razmi GR, Eshrati H, Rashtibaf M. Prevalence of Theileria spp. infection in sheep in South Khorasan province, Iran. Vet Parasitol. 2006;140:239–243. doi: 10.1016/j.vetpar.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Schnittger L, Yin H, Qi B, Gubbels MJ, Beyer D, Niemann S, Jongejan F, Ahmed JS. Simultaneously detection and differentiation of Theileria and Babesia parasite infection small ruminants by revers line blotting. Parasitol Res. 2004;92:189–196. doi: 10.1007/s00436-003-0980-9. [DOI] [PubMed] [Google Scholar]
- Seidabadi M, Razmi GhR, Naghibi A. Molecular detection of Babesia spp. in sheep and vector ticks in North Khorasan province, Iran. Iran J Vet Med. 2014;8(1):35–39. [Google Scholar]
- Shayan P, Rahbari S. Differentiation of sheep Theileria spp. and Babesia spp. by polymerase chain reaction. J Vet Res. 2007;62(3):250–260. [Google Scholar]
- Shayan P, Ebrahimzadeh E, Tageldin MH, Amininia N, Eckert B. Molecular study of sheep malignant theileriosis at Barka region in the Sultanate of Oman. Iran J Parasitol. 2011;6(1):66–72. [PMC free article] [PubMed] [Google Scholar]
- Soulsby EJL. Helminths, arthropods and protozoa of domesticated animals. 7. London: Bailliere Tindall; 1982. pp. 706–728. [Google Scholar]
- Theodoropoulos G, Gazouli M, Ikonomopoulos JA, Kantzoura V, Kominakis A. Determination of prevalence and risk factors of infection with Babesia in small ruminants from Greece by polymerase chain reaction amplification. Vet Parasitol. 2006;135:99–104. doi: 10.1016/j.vetpar.2005.07.021. [DOI] [PubMed] [Google Scholar]
- Uilenberg G. Babesia: a historical overview. Vet Parasitol. 2006;138(1–2):3–10. doi: 10.1016/j.vetpar.2006.01.035. [DOI] [PubMed] [Google Scholar]
- Zaeemi M, Haddadzadeh H, Khazraiinia P, Kazemi B, Bandehpour M. Identification of different Theileria species (Theileri lestoquardi, Theileriaovis, and Theileria annulata) in naturally infected sheep using nested PCR-RFL. Parasitol Res. 2011;108:837–843. doi: 10.1007/s00436-010-2119-0. [DOI] [PubMed] [Google Scholar]