Abstract
Cutaneous leishmaniasis is one of the important skin diseases with diverse clinical manifestations. With an incidence of 0.7–1.3 million cases annually, this disease is often reported from six countries, including Iran. Accordingly, the purpose of this study was to evaluate the anti-leishmanial effect of the three plant hydroalcoholic extracts including fleawort (Plantago psyllium L.), savory (Satureja hortensis L.) and tarragon (Artemisia dracunculus L.) on Leishmania major promastigotes. The hydroalcoholic extract from each plant was extracted and its anti-leishmanial effect was evaluated in different concentrations (100–1000 µg/ml) and at various hours (24, 48 and 72 h). Savory herb inhibitory concentration 50 % (IC50) at 24, 48 and 72 h was 790.81, 398.11 and 298.42 µg/ml, respectively. In addition, tarragon herb IC50 at 24, 48 and 72 h was 962.03, 688.36 and 585.51 µg/ml, respectively. Moreover, the fleawort extract was showed the lowest effect, considering that its effect at the concentration of 1000 µg/ml was 48 % after 72 h (P > 0.05). Furthermore, the statistical analysis showed a significant difference for interaction between concentration and time regarding the tarragon and savory extracts with a P value of lower than 0.05. According to the results, the anti-leishmanial effect of the tarragon and savory extracts may make it possible to use them in the treatment of cutaneous leishmaniasis as a complementary or alternative therapy; however, further studies are necessary and should be evaluated in cell culture and in vivo conditions to confirm it.
Keywords: Leishmania major, Satureja hortensis, Artemisia dracunculus, Plantago psyllium, Extract
Introduction
Cutaneous leishmaniasis is one of the important skin diseases with diverse clinical manifestations (Salehi et al. 2014). With an incidence of 0.7–1.3 million cases annually, this disease is often reported from six countries, including Iran (WHO 2015). It seems that the incidence of this disease is increasing in the world (Karami et al. 2013; Fata et al. 2015). The annual increase of this disease, especially in endemic areas such as Iran, is thought to be due to the development of drug resistance in the disease-causing parasites (Croft et al. 2006). On the other hand, the line 1 and 2 medicines for the treatment of this disease, namely antimonial compounds and Amphotericin B, respectively, may come along with serious side effects, and furthermore, due to the relatively long period of treatment, it should be prescribed with caution in some people, and even sometimes the patient monitoring is crucial (McGwire and Satoskar 2014). Accordingly, the World Health Organization (WHO) recommended strongly the use of herbal medicine in the treatment of cutaneous leishmaniasis (Saki et al. 2015). It deserves mentioning that more than 25 % of modern medicines are of plant origin. In addition, almost 80 % of essential medical medicines such as anticancers and immunosuppressants are made of herbal components (Pan et al. 2013). Accordingly, the purpose of this study was to evaluate the anti-leishmanial effect of the three plant hydroalcoholic extracts including fleawort (Plantago psyllium L.), savory (Satureja hortensis L.) and tarragon (Artemisia dracunculus L.) on Leishmania major promastigotes.
Materials and methods
Parasite
Leishmania major promastigotes, strain MRHO/IR/75/ER, were obtained from the Medical Parasitology and Mycology department, School of Medicine, Shahid Sadoughi University of Medical Sciences, Yazd, Iran. The parasites were maintained in BALB/c mice. Amastigotes were isolated from mice spleen, and then transformed to promastigotes in Novy-Nicolle-Mac Neal (NNN) medium. Promastigotes from the third passage in NNN medium were adapted gradually to RPMI 1640 medium supplemented with 10 % fetal bovine serum (FBS), 100 µg/ml streptomycin, 100 IU/ml penicillin, at temperature of 25 °C.
Plants
Three plants native to Iran including Plantago psyllium L. (family Plantaginaceae), Satureja hortensis L. (family Lamiaceae) and Artemisia dracunculus L. (family Asteraceae) was purchased from an herbal shop and authenticated by the Herbal Medicine Research Center, School of Pharmacy, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
Extraction
Extraction was performed using maceration method. Initially, tarragon was dried at room temperature, ground and then passed through a sieve. Then 50 g of the resulting powder was poured into a sterile screw-capped glass container and mixed with 500 ml of ethyl alcohol (80 %). The extraction was carried out during 1 week. Subsequently, the extract was concentrated on a rotary evaporator under vacuum conditions at 45 °C and stored at −20 until use. Fleawort and savory were extracted according to the same procedure was used for tarragon extraction.
Experimentation
The obtained extracts were dissolved in dimethyl sulfoxide (DMSO), with a final concentration of 0.2 % DMSO in test solution. Then, 100–1000 µg of each extract was encountered with 1 × 106 exponentially growing promastigotes in separate tubes each containing RPMI medium, 10 % fetal bovine serum (FBS), 100 µg/ml streptomycin and 100 IU/ml penicillin. All of the tubes were incubated at 25 °C for 72 h. The experiment was performed in single-blind and triplicate in view of that for each concentration the average of three tubes was considered. In addition, a negative control group containing DMSO and the parasite was considered. At the 24, 48 and 72 h, 10 µl of each tube was spread on a glass slide and 10 µl of 0.4 % trypan blue was added to it and mixed gently. After 5 min, the parasites mortality rate was calculated by counting the number of live (not stained) and dead (stained) promastigotes according to the following formula:
Accordingly, at least 50 microscopic fields were examined for each sample.
Data analysis
The normal distribution of data was evaluated by Kolmogorov–Smirnov (K–S) test. Afterwards, the interaction between time and concentration was calculated by repeated-measures analysis of variance for each extract. Additionally, two-tailed t test analysis based on different concentrations was used to reveal the statistical difference between each of the extracts and the negative control group at a given hour.
Results
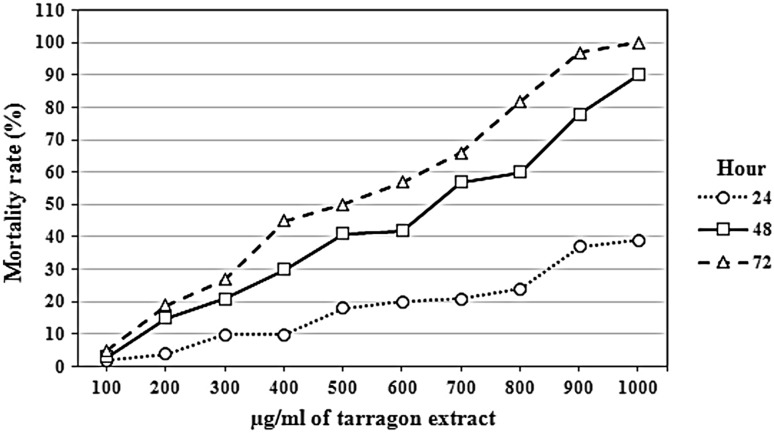
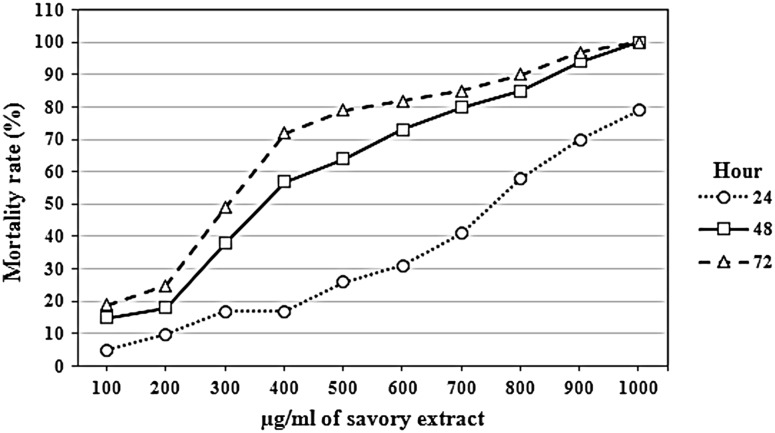
Savory IC50 at 24, 48 and 72 h was 790.81, 398.11 and 298.42 µg/ml, respectively. The effect of the savory extract in different concentrations on the parasite has been shown in Fig. 1. In addition, tarragon IC50 at 24, 48 and 72 h was 962.03, 688.36 and 585.51 µg/ml, respectively. The effect of the tarragon extract in different concentrations on the parasite has been shown in Fig. 2. Moreover, the fleawort extract was showed the lowest effect, considering that its effect at the concentration of 1000 µg/ml was 48 % after 72 h. The obtained P values from two-tailed t test analysis for savory compared to the negative control at 24, 48 and 72 h were 0.05, 0.02 and 0.02, respectively. Furthermore, for tarragon compared to the negative control it was 0.12, 0.04 and 0.02 at 24, 48 and 72 h were, respectively. The fleawort extract was showed no significant difference statistically as compared to the control group (P > 0.05). In addition, the result of repeated-measures analysis of variance for interactions between concentration and time for the fleawort extract was not significant statistically (P > 0.05) while for the tarragon and savory extracts, it was significant statistically (P < 0.05).
Fig. 1.
The effect of savory herb extract in different concentrations and at various temperatures on Leishmania major promastigotes
Fig. 2.
The effect of tarragon herb extract in different concentrations and at various temperatures on Leishmania major promastigotes
Discussion
The use of herbs in the treatment of skin diseases goes back to centuries ago. One of the important points in the use of herbal medicines is that they often have far fewer side effects than synthetic drugs (Pan et al. 2013). Until now, many studies have been carried out regarding the effect of herbal compounds on parasites. Given that cutaneous leishmaniasis is limited to the skin typically, so the use of an effective topical medicine in treating this disease is very important. In the present study, the fleawort extract showed no obvious anti-leishmanial activity, while the two others, namely the tarragon and savory extracts, showed a significant anti-leishmanial effect. Studies showed that a number of herbs have moderate to strong anti-leishmanial activity (Dutta et al. 2007; Cruz-Ede et al. 2013; Rodrigues et al. 2014; Azizi et al. 2014; Nosratabadi et al. 2015). Some authors found that a few plants can be used as an adjuvant in vaccination against Leishmania spp. (Santos et al. 1997; Kaur et al. 2014). Recent studies showed that the extracts of Hedera helix and Peganum harmala have potent anti-leishmanial effects (Khoshzaban et al. 2014; Hooshyar et al. 2014). Moreover, the anti-leishmanial activity of Juglans regia and Lawsonia inermis was shown in another study (Serakta et al. 2013). The study conducted by Peixoto et al. (2011) revealed that the hydroalcoholic extract of Miconia langsdorffii Cogn., especially C-28 methyl ester derivative, have remarkable anti-leishmanial effects. The result of one study demonstrated that α-pinene, a p-cymene-derived compound, has a notable anti-leishmanial activity (Rodrigues et al. 2015). Likewise, the result of another study showed that the plant Myrtus communis has anti-leishmanial properties in vitro, and it is likely due to its α-pinene contents (Mahmoudvand et al. 2015). There is about 5.1 % α-pinene in the tarragon essential oil (Obolskiy et al. 2011) and it seems that its anti-leishmanial effect is because of this compound. The plant savory also contains about 2.7 % of this substance (Mahboubi and Kazempour 2011) and it was capable of killing this parasite, as observed in the present study. Many studies showed that phenolic compounds have potent anti-leishmanial activity (Arias et al. 2012; Mohammadpour et al. 2012). Thymol and carvacrol as phenolic compounds are two major components of the savory essential oil (Mahboubi and Kazempour 2011) while tarragon has no phenolic compounds in its essential oil (Obolskiy et al. 2011). It seems that more effect of the savory extract on Leishmania major than the tarragon extract is because of its phenolic compounds. The anti-leishmanial effect of three herbal extracts was examined in this study, the two of which showed a considerable anti-leishmanial activity, namely savory and tarragon.
Traditionally, the two recent plants have long been used for therapeutic purposes (Obolskiy et al. 2011; Mahboubi and Kazempour 2011). According to the results, the anti-leishmanial effect of the tarragon and savory extracts may make it possible to use them in the treatment of cutaneous leishmaniasis as a complementary or alternative therapy; however, further studies are necessary and should be evaluated in cell culture and in vivo conditions to confirm it.
References
- Arias AR, Pandolfi E, Vega MC, Rolon M. Selected natural and synthetic phenolic compounds with antileishmanial activity: a five-year review. Curr Bioact Compd. 2012;8:307–333. doi: 10.2174/1573407211208040002. [DOI] [Google Scholar]
- Azizi K, Shahidi-Hakak F, Asgari Q, Hatam GR, Fakoorziba MR, Miri R, Djaefar Moemenbellah-Fard M. In vitro efficacy of ethanolic extract of Artemisia absinthium (Asteraceae) against Leishmania major L. using cell sensitivity and flow cytometry assays. J Parasit Dis. 2014 doi: 10.1007/s12639-014-0569-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croft SL, Sundar S, Fairlamb AH. Drug resistance in leishmaniasis. Clin Microbiol Rev. 2006;19:111–126. doi: 10.1128/CMR.19.1.111-126.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz-Ede M, da Silva ER, Maquiaveli-Cdo C, Alves ES, Lucon JF, Jr, dos Reis MB, de Toledo CE, Cruz FG, Vannier-Santos MA. Leishmanicidal activity of Cecropia pachystachya flavonoids: arginase inhibition and altered mitochondrial DNA arrangement. Phytochemistry. 2013;89:71–77. doi: 10.1016/j.phytochem.2013.01.014. [DOI] [PubMed] [Google Scholar]
- Dutta A, Ghoshal A, Mandal D, Mondal NB, Banerjee S, Sahu NP, Mandal C. Racemoside A, an anti-leishmanial, water–soluble, natural steroidal saponin, induces programmed cell death in Leishmania donovani. J Med Microbiol. 2007;56:1196–1204. doi: 10.1099/jmm.0.47114-0. [DOI] [PubMed] [Google Scholar]
- Fata A, Salehi Sangani G, Rafatpanah H, Mousavi Bazzaz M, Mohaghegh MA, Movahedi A. Identification of Leishmania species by kinetoplast DNA-polymerase chain reaction for the first time in Khaf district, Khorasan-e-Razavi province, Iran. Trop Parasitol. 2015;5:50–54. doi: 10.4103/2229-5070.145587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooshyar H, Talari S, Feyzi F. Therapeutic effect of Hedera helix alcoholic extract against cutaneous leishmaniasis caused by Leishmania major in Balb/c mice. Jundishapur J Microbiol. 2014;7:e9432. doi: 10.5812/jjm.9432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karami M, Doudi M, Setorki M. Assessing epidemiology of cutaneous leishmaniasis in Isfahan, Iran. J Vector Borne Dis. 2013;50:30–37. [PubMed] [Google Scholar]
- Kaur A, Kaur PK, Singh S, Singh IP. Antileishmanial compounds from Moringa oleifera Lam. Z Naturforsch C. 2014;69:110–116. doi: 10.5560/znc.2013-0159. [DOI] [PubMed] [Google Scholar]
- Khoshzaban F, Ghaffarifar F, Jamshidi-Koohsari HR. Peganum harmala aqueous and ethanol extracts effects on lesions caused by Leishmania major (MRHO/IR/75/ER) in BALB/c mice. Jundishapur J Microbiol. 2014;7:e10992. doi: 10.5812/jjm.10992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahboubi M, Kazempour N. Chemical composition and antimicrobial activity of Satureja hortensis and Trachyspermum copticum essential oil. Iran J Microbiol. 2011;3:194–200. [PMC free article] [PubMed] [Google Scholar]
- Mahmoudvand H, Ezzatkhah F, Sharififar F, Sharifi I, Dezaki ES. Antileishmanial and cytotoxic effects of essential oil and methanolic extract of Myrtus communis L. Korean J Parasitol. 2015;53:21–27. doi: 10.3347/kjp.2015.53.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGwire BS, Satoskar AR. Leishmaniasis: clinical syndromes and treatment. QJM. 2014;107:7–14. doi: 10.1093/qjmed/hct116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadpour G, Marzony ET, Farahmand M. Evaluation of the anti-Leishmaniamajor activity of Satureja bakhtiarica essential oil in vitro. Nat Prod Commun. 2012;7:133–136. [PubMed] [Google Scholar]
- Nosratabadi SJ, Sharifi I, Sharififar F, Bamorovat M, Daneshvar H, Mirzaie M. In vitro antileishmanial activity of methanolic and aqueous extracts of Eucalyptus camaldulensis against Leishmania major. J Parasit Dis. 2015;39:18–21. doi: 10.1007/s12639-013-0377-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obolskiy D, Pischel I, Feistel B, Glotov N, Heinrich M. Artemisia dracunculus L. (tarragon): a critical review of its traditional use, chemical composition, pharmacology, and safety. J Agric Food Chem. 2011;59:11367–11384. doi: 10.1021/jf202277w. [DOI] [PubMed] [Google Scholar]
- Pan SY, Zhou SF, Gao SH, Yu ZL, Zhang SF, Tang MK, Sun JN, Ma DL, Han YF, Fong WF, Ko KM. New perspectives on how to discover drugs from herbal medicines: CAM’s outstanding contribution to modern therapeutics. Evid Based Complement Altern Med. 2013;2013:627375. doi: 10.1155/2013/627375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peixoto JA, Andrade E, Silva ML, Crotti AE, Cassio Sola Veneziani R, Gimenez VM, Januário AH, Groppo M, Magalhães LG, Dos Santos FF, Albuquerque S, da Silva Filho AA, Cunha WR. Antileishmanial activity of the hydroalcoholic extract of Miconia langsdorffii, isolated compounds, and semi-synthetic derivatives. Molecules. 2011;16:1825–1833. doi: 10.3390/molecules16021825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues IA, Azevedo MM, Chaves FC, Alviano CS, Alviano DS, Vermelho AB. Arrabidaea chica hexanic extract induces mitochondrion damage and peptidase inhibition on Leishmania spp. Biomed Res Int. 2014;2014:985171. doi: 10.1155/2014/985171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues KA, Amorim LV, Dias CN, Moraes DF, Carneiro SM, Carvalho FA. Syzygium cumini (L.) Skeels essential oil and its major constituent α-pinene exhibit anti-Leishmania activity through immunomodulation in vitro. J Ethnopharmacol. 2015;160:32–40. doi: 10.1016/j.jep.2014.11.024. [DOI] [PubMed] [Google Scholar]
- Saki J, Khademvatan S, Pazyar N, Eskandari A, Tamoradi A, Nazari P. In vitro activity of Cordia myxa mucilage extract against Leishmania major and L. infantum promastigotes. Jundishapur J Microbiol. 2015;8:e19640. doi: 10.5812/jjm.19640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salehi G, Fata A, Mohaghegh MA, Mousavi-Bazzaz SM, Rafatpanah H, Movahedi A. Molecular identification of Leishmania species in Taybad district, Iran. Asian Pac J Trop Dis. 2014;4:S535–S539. doi: 10.1016/S2222-1808(14)60672-1. [DOI] [Google Scholar]
- Santos WR, Bernardo RR, Peçanha LM, Palatnik M, Parente JP, Palatnik de Sousa CB. Haemolytic activities of plant saponins and adjuvants. Effect of Periandra mediterranea saponin on the humoral response to the FML antigen of Leishmania donovani. Vaccine. 1997;15:1024–1029. doi: 10.1016/S0264-410X(96)00292-7. [DOI] [PubMed] [Google Scholar]
- Serakta M, Djerrou Z, Mansour-Djaalab H, Kahlouche-Riachi F, Hamimed S, Trifa W, Belkhiri A, Edikra N, Hamdi Pacha Y. Antileishmanial activity of some plants growing in Algeria: Juglans regia, Lawsonia inermis and Salvia officinalis. Afr J Tradit Complement Altern Med. 2013;10:427–430. doi: 10.4314/ajtcam.v10i3.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (2015) Leishmaniasis. www.who.int/mediacentre/factsheets/fs375/en. Accessed Feb 2015