Abstract
Cryptosporidium spp. are recognized as one of the most important enteric pathogens causing enteritis and severe diarrhoea in calves up to 1 month of age. Although the infection may be responsible for some mortality, its impact is mainly associated with the impairment of gastrointestinal functions and lower performance of animals. A female buffalo calf of 25 days old was presented to OPD section, College of Veterinary and Animal Sciences, SVPUA&T, Meerut, with the symptoms of severe voluminous watery cholera like diarrhea with mucous and blood tinge since 4–5 days. On physical examination, calf was dehydrated, weak, and emaciated with normal temperature. Parasitological examination of the faeces by the direct smear and modified Ziehl–Neelsen staining technique revealed presence of high number of Cryptosporidium spp. oocysts. The affected female buffalo calf was treated with azithromycin and provided supportive care. Diarrohoeal symptoms were stopped from 3rd day and animal returned to normal condition by 7th day post treatment.
Keywords: Cryptosporidium spp., Modified Ziehl–Neelsen technique, Azithromycin, Buffalo calf
Introduction
Cryptosporidium spp. are cosmopolitan protozoan parasites which infect a wide range of vertebrate hosts including human beings. A varying level of prevalence of Cryptosporidium spp. in neonatal and young bovine calves, resulting high morbidity and mortality along with their ability to transmit infection to human beings through faeco-oral route, have been reported from various parts of the world including India (Fayer and Xiao 2008; Venu et al. 2012; Maurya et al. 2013). In cattle, the clinical form of the disease (Randhawa et al. 2012) and the shedding of oocysts in faeces is usually limited to calves of few months age (Bhat et al. 2012), but there are some reports of sub-clinical oocyst shedding in adult as well as in immunocompromised animals (Lorenzo et al. 1993). Cryptosporidiosis in animal is seen as a threatening source of infection for humans because of shedding of huge number of resistant oocysts, thus contaminating the surface of water (Maurya et al. 2013) and there are indications that the disease can potentially reduce the growth performance of ruminants (Ralston et al. 2003) and cause high morbidity and sometimes high mortality rates in calves (Singh et al. 2006; Fayer et al. 1997; Olson et al. 2004). The present article describes a case of cryptosporidiosis in buffalo calf and its successful management.
Materials and Methods
A 25 days old female buffalo calf was presented to OPD section, College of Veterinary and Animal Sciences, SVPUA&T, Meerut with the history of severe voluminous watery cholera like diarrhea with mucous and blood tinge since 4–5 days. On physical examination, animal was dehydrated, dull, weak and showing the symptoms of straining and diarrhea. Clinical examination revealed normal rectal temperature, heart rate and respiratory rate. The consistency of the stool was watery, pale and blood tinged along with mucous. The stool sample was collected by rectal swab in sterile vial without any preservatives and then sent to Parasitology Department. Direct faecal smears were prepared on clean grease-free microscopic glass slides with one slide for direct wet smear examination for detection of Cryptosporidium spp. oocysts and others were stained by the modified Ziehl–Neelsen (MZN) staining technique (Henriksen and Pohlenz 1981) for confirmation. Briefly, air dried smears were first fixed with methanol for 5 min, air dried and then smears were transiently fixed over a flame and kept on staining rack. The smears were flooded with concentrated carbol fuchsin and allowed to stain for 40 min. The slide were then washed under running water for 5 min, decolourised using 10 % H2SO4 for 10–15 s and then washed again in water. The smears were counter stained with 5 % malachite green for 5 min and then washed in running tap water for 5 min. Subsequently the calf was treated with azithromycin (ZADY READYMIX, Mankind Pharma) at 1500 mg/calf/day BW orally for 7 days and supportive care (fluids, electrolytes and good nutrition) was provided for speedy recovery.
Results and discussion
Coprological examination by direct wet smear under 40×, revealed oocysts which are rounded, measuring 4–7 mm in diameter with sporozoites inside (Fig. 1). It was further confirmed by MZN technique which revealed presence of high number of pinkish red colored Cryptosporidium spp. oocysts with a hollow at centre (Fig. 2). The diarrohoeal symptoms stopped from 3rd day and animal returned to normal condition by 7th day post treatment.
Fig. 1.
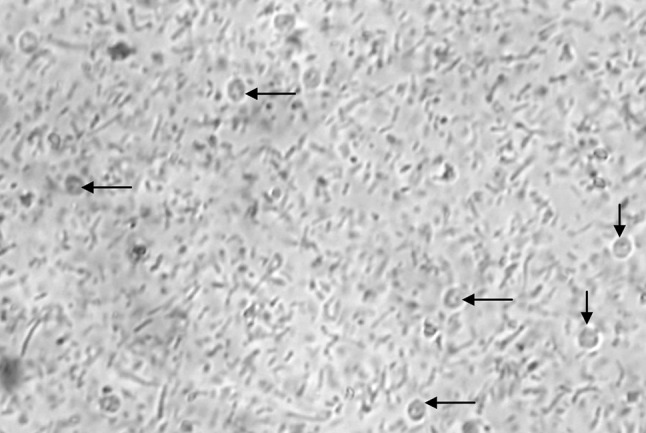
Unstained Cryptosporidium spp. sporulated oocysts (arrows) by direct smear examination (×40)
Fig. 2.
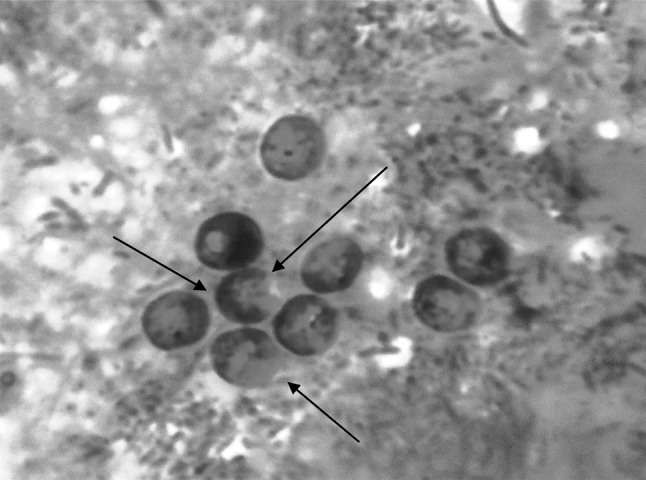
Cryptosporidium spp. oocysts (arrows) observed under high power microscope by modified Ziehl–Neelsen (MZN) staining technique (×100)
Cryptosporidiosis is common in livestock and causes significant morbidity and mortality, particularly among young animals. It is characterized by acute gastro intestinal disturbances, mucoid or haemorrhagic watery diarrohoea, fever, lethargy, anorexia and loss of condition (Navin and Juranek 1984), leading to significant economic losses in farm animals (Xiao et al. 1999). In India, the disease was reported for the first time in Uttar Pradesh (Dubey et al. 1992) and subsequently it has been reported from other parts of country. The infection rate, in India is significantly higher in bovine calves below 1 month of age than animals between 1 and 3 months (Maurya et al. 2012). We reported here a case of bovine cryptosporidiosis and its successful management in Meerut district of Western Uttar Pradesh. Our results are in line with the observations made by previous authors (Mead 2002; Elitok et al. 2005; Nasir et al. 2013) which confirmed the efficacy of azithromycin against cryptosporidiosis in bovines.
Acknowledgments
The authors are thankful to the Dean, College of Veterinary and Animal Sciences and the Vice Chancellor, Sardar Vallabhbhai Patel University of Agriculture and Technology, Meerut for providing the facilities for carrying out this work.
References
- Bhat SA, Juyal PD, Singla LD. Prevalence of cryptosporidiosis in neonatal buffalo calves in Ludhiana district of Punjab, India. Asian J Anim Vet Adv. 2012;7:512–520. doi: 10.3923/ajava.2012.512.520. [DOI] [Google Scholar]
- Dubey JP, Fayer R, Rao JR. Cryptosporidial oocysts in faeces of water buffalo and Zebu Calves in India. J Vet Parasitol. 1992;6:55–56. [Google Scholar]
- Elitok BO, Elitok M, Pulat H. Efficacy of azithromycin dihydrate in treatment of cryptosporidiosis in naturally infected dairy calves. J Vet Intern Med. 2005;19:590–593. doi: 10.1111/j.1939-1676.2005.tb02732.x. [DOI] [PubMed] [Google Scholar]
- Fayer R, Xiao L. Cryptosporidium and cryptosporidiosis. 2. Boca Raton: CRC; 2008. [Google Scholar]
- Fayer R, Speer C, Dubey J. The general biology of Cryptosporidium. In: Fayer R, editor. Cryptosporidium and cryptosporidiosis. Boca Raton: CRC Press; 1997. pp. 1–41. [Google Scholar]
- Henriksen A, Pohlenz JFL. Staining of cryptosporidia by a modified Zeihl-Neelson technique. Acta Vet Scand. 1981;22:594–596. doi: 10.1186/BF03548684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo LMJ, Ares-Mazás E, Villacorta MI. Detection of oocysts and IgG antibodies to Cryptosporidium parvum in asymptomatic adult cattle. Vet Para. 1993;47:9–15. doi: 10.1016/0304-4017(93)90171-I. [DOI] [PubMed] [Google Scholar]
- Maurya PS, Rakesh RL, Pradeep B, Kumar Saroj, Kundu K, Garg Rajat, Ram Hira, Kumar Ashok, Banerjee PS. Prevalence and risk factors associated with Cryptosporidium spp. infection in young domestic livestock in India. Trop Anim Health Prod. 2012;45:941–946. doi: 10.1007/s11250-012-0311-1. [DOI] [PubMed] [Google Scholar]
- Maurya PS, Garg R, Banerjee PS, Kumar S, Rakesh RL, Kundu K, Ram H, Raina OK. Genotyping of Cryptosporidium spp. reveals prevalence of zoonotic C. parvum subtype in bovine calves of north India. Indian J Anim Sci. 2013;83(10):1018–1023. [Google Scholar]
- Mead RJ. Cryptosporidiosis and the challenges of chemotherapy. Drug Resist Update. 2002;5:47–57. doi: 10.1016/S1368-7646(02)00011-0. [DOI] [PubMed] [Google Scholar]
- Nasir A, Avais M, Khan MS, Khan JA, Hameed S, Reichel MP. Treating Cryptosporidium parvum infection in calves. J Parasitol. 2013;99(4):715–717. doi: 10.1645/12-42.1. [DOI] [PubMed] [Google Scholar]
- Navin TR, Juranek DD. Cryptosporidiosis: clinical, epidemiologic and parasitologic review. Rev Infect Dis. 1984;6:313. doi: 10.1093/clinids/6.3.313. [DOI] [PubMed] [Google Scholar]
- Olson ME, Ralston BJ, O’handley R, Guselle NJ, Applebee AJ. What is the clinical and zoonotic significance of cryptosporidiosis in domestic animals and wildlife. In: Thompson RCA, Armson A, Ryan UM, editors. Cryptosporidium: from molecules to disease. Amsterdam: Elsevier Sci; 2004. [Google Scholar]
- Ralston BJ, Mcallister TA, Olson ME. Prevalence and infection pattern of naturally acquired giardiasis and cryptosporidiosis in range beef calves and their dams. Vet Para. 2003;114:113–122. doi: 10.1016/S0304-4017(03)00134-1. [DOI] [PubMed] [Google Scholar]
- Randhawa SS, Zahid UN, Randhawa Swaran S, Juyal PD, Singla LD, Uppal SK. Therapeutic management of cryptosporidiosis in cross bred dairy calves. IV J. 2012;89:17–19. [Google Scholar]
- Singh BB, Sharma R, Kumar H, Banga HS, Aulakh RS, Gill JPS, Sharma JK. Prevalence of Cryptosporidium parvum infection in Punjab (India) and its association with diarrhea in neonatal dairy calves. Vet Para. 2006;140:162–165. doi: 10.1016/j.vetpar.2006.03.029. [DOI] [PubMed] [Google Scholar]
- Venu R, Latha BR, Basith SA, Raj GD, Sreekumar C, Raman M. Molecular prevalence of Cryptosporidium spp. in dairy calves in southern states of India. Vet Para. 2012;188:19–24. doi: 10.1016/j.vetpar.2012.02.025. [DOI] [PubMed] [Google Scholar]
- Xiao L, Escalante L, Yang C, Sulaiman I, Escalante AA, Monsali RJ, Fayer R, Lal AA. Phylogenetic analysis of Cryptosporidium parasites based on the small subunit rRNA gene locus. Appl Environ Microbiol. 1999;65:1578–1583. doi: 10.1128/aem.65.4.1578-1583.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]


