Abstract
The impact of the combined effects of heat stress, increased vapor pressure deficit (VPD) and water deficit on the physiology of major crops needs to be better understood to help identifying the expected negative consequences of climate change and heat waves on global agricultural productivity. To address this issue, rice, wheat, and maize plants were grown under control temperature (CT, 25°C, VPD 1.8 kPa), and a high temperature (HT, 38°C, VPD 3.5 kPa), both under well-watered (WW) and water deficit (WD) conditions. Gas-exchange measurements showed that, in general, WD conditions affected the leaf conductance to CO2, while growth at HT had a more marked effect on the biochemistry of photosynthesis. When combined, HT and WD had an additive effect in limiting photosynthesis. The negative impacts of the imposed treatments on the processes governing leaf gas-exchange were species-dependent. Wheat presented a higher sensitivity while rice and maize showed a higher acclimation potential to increased temperature. Rubisco and PEPC kinetic constants determined in vitro at 25°C and 38°C were used to estimate Vcmax, Jmax, and Vpmax in the modeling of C3 and C4 photosynthesis. The results here obtained reiterate the need to use species-specific and temperature-specific values for Rubisco and PEPC kinetic constants for a precise parameterization of the photosynthetic response to changing environmental conditions in different crop species.
Keywords: photosynthesis, high temperature, water deficit, crops, C3, C4
Introduction
Mean global air temperatures are predicted to rise on average 0.3–0.6°C per decade over the next century, with heat waves becoming more frequent, intense and persistent (IPCC, 2013). In certain geographical regions, increased annual temperatures and heat wave frequency might be accompanied by decreased precipitation, causing decreased water availability for plants. While predicted increases in the concentration of atmospheric CO2 may be positive for plant productivity (Long et al., 2006), in some agricultural regions these beneficial effects are likely to be offset by negative impacts of increased temperature and water deficit (Gornall et al., 2010). Hence, future predicted environments will compromise agricultural productivity and food security for the increasing world population. A more detailed understanding of the capacity of major crops, which sustain most of the human caloric intake, to respond and acclimate to water deficit and high temperature is key to mitigate the negative impacts of climate change on plant productivity.
Decreased crop productivity under water deficit and high temperature tend to be primarily caused by limited photosynthetic carbon assimilation and persisting mitochondrial respiration (Atkin et al., 2006; Flexas et al., 2006a; Ainsworth and Ort, 2010). Under water deficit, stomatal conductance (gs) decreases, minimizing water loss, with parallel decreases in mesophyll conductance (gm) (Flexas et al., 2013; Théroux-Rancourt et al., 2014). As a consequence, the capacity of the leaf to transfer CO2 from the atmosphere to the sites of carboxylation in the chloroplast stroma decreases under drought conditions (Galmés et al., 2011). On the other hand, for most species, the photosynthetic machinery is robust enough to be largely unaffected by conditions of mild to moderate water deficit (Flexas et al., 2006a; Galmés et al., 2007b, 2013). Therefore, limitations in water availability decrease CO2 assimilation mainly through diffusive rather than metabolic limitations (Flexas et al., 2006b; Galmés et al., 2007b). Increased temperature often results in increased vapor pressure deficit (VPD), which may exacerbate even more the diffusional limitations (Perez-Martin et al., 2009).
Photosynthetic processes are strongly temperature dependent, and moderate increases above the thermal optimum cause decreases in photosynthetic CO2 uptake. Contrarily to water deficit, the negative impact of high temperature on the rate of net CO2 assimilation (AN) is mostly due to biochemical limitations (Scafaro et al., 2011; Carmo-Silva et al., 2012). Rubisco activase is extremely heat-sensitive and this results in deactivation of Rubisco catalytic sites at moderately high temperatures (Salvucci and Crafts-Brandner, 2004; Yamori et al., 2011). Moreover, increases in the maximum catalytic rate of carboxylation (kcatc) with temperature are offset by decreases in the affinity of Rubisco for CO2 (i.e., increases in the Michaelis–Menten constant for CO2, Kc, and decreases in the specificity factor, Sc/o) and the lower CO2/O2 ratio in solution, which increase photorespiration (Sage and Kubien, 2007). On the other hand, mitochondrial respiration appears to be relatively unaffected by water availability but increases with temperature compared to photosynthesis (Atkin and Tjoelker, 2003; Galmés et al., 2007c; Atkin and MacHerel, 2009; Rodríguez-Calcerrada et al., 2010; Silim et al., 2010). Thus, the response of mitochondrial respiration to combined heat and drought would tend to further decrease the leaf carbon balance.
The above responses correspond to general trends observed when stresses are applied over relatively short periods of 15–20 days. In nature plants face long-term exposure to water deficit and high temperature, and photosynthesis and mitochondrial respiration have been shown to acclimate to both water (Walters, 2005; Galmés et al., 2006; Flexas et al., 2009) and heat stress (Berry and Björkman, 1980; Yamori et al., 2005; Campbell et al., 2007; Sage and Kubien, 2007), although the capacity and mechanisms of plant acclimation may differ between species (Hikosaka et al., 2006; Kattge and Knorr, 2007; Dillaway and Kruger, 2011; Scafaro et al., 2011; Cheesman and Winter, 2013). In semi-arid climates like the Mediterranean, drought, and heat stress occur simultaneously and exert a combined effect on plant functioning (Mittler, 2006).
Most mechanistic models of carbon uptake and release in C3 and C4 leaves currently do not account for long-term responses to changes in the environmental conditions (e.g., von Caemmerer, 2000; Pittermann and Sage, 2001; Hu et al., 2010). Further, these models are usually parameterized with invariable values and temperature responses for the Rubisco kinetic parameters and gm among different species. In fact, the use of Rubisco kinetics and gm values experimentally measured in Nicotiana tabacum have been employed in most of the studies modeling leaf gas exchange responses to variations in the environment (Bernacchi et al., 2001, 2002, 2003; Diaz-Espejo, 2013; von Caemmerer, 2013). However, Rubisco kinetic constants and gm, as well as their dependence on temperature vary among species. These species-specific differences in Rubisco parameters explain differences in photosynthetic responses to temperature, and significantly bias modeling of C3 photosynthesis (Diaz-Espejo, 2013; Walker et al., 2013; Galmés et al., 2014; Flexas and Díaz-Espejo, 2015; von Caemmerer and Evans, 2015). Further, C4 photosynthesis modeling at variable temperatures has received little attention (Massad et al., 2007; Sage and Kubien, 2007; von Caemmerer, 2013). It is important that future approaches incorporate the temperature dependence of phosphoenolpyruvate carboxylase (PEPC) activity and the thermal response of the underlying kinetic parameters, such as the affinity of PEPC for CO2 (KP).
Rice (Oryza sativa), wheat (Triticum aestivum), and maize (Zea mays) are major commercially important crops. Together, these species account for ~85% of global cereal production and contribute the majority of the energy in food of humans eaten directly as staple foods or indirectly through consumption of livestock fed with grain (Grassini et al., 2013). The three cereals were domesticated in different climates and differ largely in their growth environments: rice and maize are cultivated in tropical hot and wet climates, whereas wheat tends to be grown in cooler temperate climates (Makino, 2011). Further, these species differ in their photosynthetic mechanism, maize is a C4 crop, and rice and wheat are C3 crops. The objectives of the present study were: (i) to analyze the patterns of response of leaf photosynthesis and respiration to long-term drought, VPD, and temperature stress in these three crops; (ii) to compare the sensitivity and acclimation capacity of leaf photosynthesis and respiration to these stresses in the three species; and (iii) to compare the effect on C3 and C4 photosynthetic models of using species-specific kinetics of Rubisco and PEPC, and their species-specific response to temperature.
Materials and methods
Plant material, growth conditions, and treatments
Plants of rice (O. sativa L. cv. Bomba), wheat (T. aestivum L. cv. Cajeme), and maize (Z. mays cv. Carella) were grown from seeds in a greenhouse in 3.5 L pots containing a 70:30 mixture (v:v) of horticultural substrate (Projar S.A, Spain) and perlite (granulometry A13, Projar S.A, Spain). After 2 weeks, seedlings were selected to uniform size and were moved to a controlled environment room. Light was provided by metal halide lamps (OSRAM, Germany) placed at specific distances from the plants to obtain a photosynthetically active photon flux density (PPFD) of 500 μmol m−2 s−1, with a photoperiod of 12 h day/12 h night. Ambient temperature and relative humidity were monitored with portable sensors Testo 175-H1 data logger (Gerilab, Spain). Relative humidity (RH) was maintained at 40–60% using humidifiers. For logistical reasons, assays were performed in two consecutive experiments with two sets of plants of identical age. For the first experiment a first set of plants of the three species was grown under control conditions (CT, 25/20°C day/night), which combined with the set RH resulted in a VPD of 1.8/1.0 kPa day/night. A second set of plants, for the second experiment, was grown at higher temperature (HT, 38/33°C, resulting in VPD 3.5/2.3 kPa day/night). Only temperature and VPD differed between the two sets of plants or experiments, while all other environmental conditions (e.g., light intensity and quality, air removal, photoperiod duration) were identical and computer-controlled.
For each set of plants, i.e., for each growing temperature and VPD treatment, 10 pots per species were grown at soil field capacity until plants presented fully expanded leaves (typically 2 weeks). Thereafter, 20 days after germination, pots of all species were randomly assigned to two irrigation treatments: five pots per species were maintained at field capacity throughout the experiment (well-watered treatment, WW) and five were maintained at 45% of field capacity (moderate water deficit treatment, WD). The level of water availability was determined gravimetrically by weighing the pots daily and maintained by compensating water losses with 50% Hoagland's solution. Plants were considered to be under water deficit when gs was decreased by 40% compared to the well-watered plants; gs was considered as a good indicator of the water deficit status, as previously demonstrated (Medrano et al., 2002).
New leaves were allowed to develop and expand under the two irrigation treatments for a minimum of 30 days. All measurements and samples were taken 40–50 days after the water treatment was initiated (i.e., 60–70 days after germination), on new leaves developed completely under the temperature and/or water treatments (Perdomo, 2015).
Gas exchange and chlorophyll a fluorescence measurements
Leaf gas exchange and chlorophyll a fluorescence measurements were performed with a portable photosynthesis system (Li-6400; Li-Cor Inc., USA) equipped with a leaf chamber fluorometer (Li-6400-40, Li-Cor Inc., USA), the latter using the multi-flash protocol (Loriaux et al., 2013). The response of net CO2 assimilation rate (AN) to varying intercellular airspace CO2 concentration (Ci) was measured on the youngest fully expanded leaf at a saturating photosynthetic active radiation (PAR) of 1500 μmol m−2 s−1 (10% blue light), a relative humidity of the incoming air between 40 and 50% and at two leaf temperatures: 25°C and 38°C. AN-Ci curves were initiated by allowing the leaf to reach steady-state AN and stomatal conductance (gs) at a CO2 concentration in the leaf chamber (Ca) of 400 μmol CO2 mol−1 air, before varying the Ca between 50 and 2000 μmol CO2 mol−1 air. Corrections for the leakage of CO2 into and out of the leaf chamber were applied to all gas-exchange data (Flexas et al., 2007).
The photochemical efficiency of photosystem II (ΦPSII) was determined according to Genty et al. (1989):
| (1) |
where Fs is the steady-state fluorescence yield and the maximum fluorescence yield obtained with a light-saturating pulse of 8000 μmol m−2 s−1.
ΦPSII was used for the calculation of the linear rate of electron transport (ETR) according to Krall and Edwards (1992):
| (2) |
where α is the leaf absorptance and β is the partitioning of absorbed quanta between photosystems I and II. β was assumed to be 0.5 (Laisk and Loreto, 1996; Tosens et al., 2012). α was measured for all species grown under each treatment inside a dark chamber using the light source from the Li-6400 and a spectroradiometer (HR2000CG-UV-NIR; Ocean Optics Inc., USA), as described by Schultz (1996). All values obtained for α were 0.86–0.87, with non-significant differences between species and species × treatment combinations.
Modeling C3 photosynthesis in wheat and rice
From combined gas-exchange and chlorophyll a fluorescence measurements, mesophyll conductance to CO2 (gm) was estimated for wheat and rice according to the variable J method (Harley et al., 1992):
| (3) |
where AN and Ci were obtained from gas exchange measurements at saturating light. The rate of non-photorespiratory CO2 evolution in the light (RL) was determined as half of the mitochondrial respiration at pre-dawn (Rdark), which was measured at a Ca of 400 μmol CO2 mol−1 air and leaf temperatures of 25°C or 38°C. The chloroplast CO2 compensation point in the absence of mitochondrial respiration (Γ*) was calculated from the in vitro measurements of Rubisco specificity factor (Sc/o) as:
| (4) |
AN-Ci curves were converted into AN-Cc curves using the values of gm:
| (5) |
Maximum velocity of Rubisco carboxylation (Vcmax) and maximum electron transport rate (Jmax) were calculated from AN-Cc curves according to Bernacchi et al. (2002), but using the Rubisco kinetic constants (the Michaelis–Menten constants for CO2 and O2 and the Sc/o) measured for each species at 25°C and 38°C. For comparative purposes, Vcmax and Jmax were also calculated for rice and wheat using the values for the Rubisco kinetics parameters and respective temperature dependencies reported for tobacco by Bernacchi et al. (2001, 2002).
Modeling C4 photosynthesis in maize
The C4 photosynthesis model described by von Caemmerer (2000) was applied to the AN-Ci curves measured for maize as detailed by Massad et al. (2007), with the modifications of Carmo-Silva et al. (2008). The maximum velocity of Rubisco carboxylation (Vcmax) and the maximum velocity of PEPC carboxylation (Vpmax), as well as the CO2 concentrations in the bundle sheath (Cs) and in the mesophyll cells (Cm) were estimated from the hyperbolic function describing the AN-Ci curves using a Ci step-size of 5 μmol mol−1, by applying the equations:
| (6) |
| (7) |
| (8) |
In these equations, the oxygen partial pressure in the bundle sheath and mesophyll cells (O), the bundle sheath conductance to CO2 (gbs), and the mesophyll conductance to CO2 (gm) were assumed to be invariable between water and temperature treatments, as in Carmo-Silva et al. (2008) and Massad et al. (2007), respectively. Constant values for these parameters were O = 210 mbar, gbs = 3 mmol m−2 s−1, and gm = 2 mol m−2 s−1 (von Caemmerer, 2000).
The model also requires values for kinetic constants of Rubisco and PEPC: the Rubisco specificity for CO2/O2 (Sc/o, from which Γ* is calculated as 0.5 O/Sc/o), the Michaelis–Menten constants of Rubisco for CO2 (Kc) and O2 (Ko), and the Michaelis–Menten constant of PEPC for CO2 (Kp). Vcmax and Vpmax values calculated using the in vitro kinetic constants of maize Rubisco and PEPC at 25°C and 38°C were compared to Vcmax and Vpmax calculated using the values at 25°C for Γ* (0.000193), Kc (65 Pa) Ko (45 kPa), and Kp (8 Pa) reported in von Caemmerer (2000). The temperature equations provided by Bernacchi et al. (2001, 2002) were used to calculate values for the Rubisco kinetic constants at 38°C, while Kp was assumed to be invariable with temperature changes.
Determination of michaelis–menten constants of rubisco and PEPC for their gaseous substrates
The Michaelis–Menten constants of Rubisco for CO2 (Kc) and O2 (Ko) were determined at 25°C and 38°C using leaf samples of rice, wheat, and maize, as previously described Galmés et al. (2015). In the present study, assays were done under either 0% O2 (100% N2) or 21% O2 (in 79% N2), and thus Ko was estimated using the equation:
| (9) |
TheMichaelis–Menten constant of PEPC for CO2 (Kp) was determined for maize at 25°C and 38°C, essentially as described by Uedan and Sugiyama (1976). PEPC was extracted from leaf samples (1.2 cm2) by grinding in a mortar with 46 mg insoluble PVPP and 2 mL of ice-cold extraction buffer containing 50 mM Bicine-NaOH (pH 8.2), 1 mM EDTA, 0.18% (w/v) PEG4000, 11 mM ε-aminocaproic acid, 2.2 mM benzamidine, 1.8 mg bovine serum albumin (BSA), 2.8% (v/v) Tween, and 1.8 mM Na2H2PO4. The homogenate was centrifuged for 4 min at 13,000 g and 4°C. Eight 7 mL septum-sealed vials containing 990 μL assay buffer [50 mM Bicine-NaOH (pH 8.2), 5 mM MgCl2, 1 mM EDTA, 1 mM DTT, 100 mM phosphoenolpyruvate (PEP), 20 mM NADH, 100 mM malic dehydrogenase (MDH), 100 mM glucose-6-phospate] and varying concentrations of NaH14CO3 (0–10 mM, 1.3 × 1010 Bq mol−1) were equilibrated with nitrogen (N2) for 30 min. Reactions were started by the addition of 10 μl leaf extract and quenched after 1 min with 10 M formic acid. Acid stable 14C was measured by liquid scintillation counting. To convert Kc, Ko, and Kp values from concentration to partial pressures, solubilities for CO2 of 0.0334 mol (L bar)−1 at 25°C and 0.0243 mol (L bar)−1 at 38°C and for O2 of 0.00126 mol (L bar)−1 at 25°C and 0.00102 mol (L bar)−1 at 38°C were used.
Rubisco specificity factor determination
Rubisco specificity for CO2/O2 (Sc/o) was measured at 25°C and 38°C for rice, wheat, and maize (n = 6–12) using purified leaf extracts obtained as in Galmés et al. (2006) and the oxygen electrode (Model DW1; Hansatech, Kings Lynn., UK) method described by Parry et al. (1989). Reaction mixtures contained (final concentrations) 100 mM Bicine-NaOH (pH 8.2), 10 mM MgCl2, 0.15 mg mL−1 carbonic anhydrase, 2 mM NaH14CO3 (18.5 kBq mol−1), activated Rubisco from purified extracts (20 μL), and 2.5 μM RuBP. The basic buffer was pre-equilibrated with CO2-free air at the temperature of measurement. RuBP oxygenation was calculated from the oxygen consumption and carboxylation from the amount of 14C incorporated into PGA when all the RuBP had been consumed. To convert the Sc/o values from concentration to partial pressures, the CO2 and O2 solubilities were used as described above for the Rubisco and PEPC kinetics.
Temperature/VPD sensitivity and acclimation
The effect of temperature/VPD on the main leaf gas exchange parameters was examined using two indexes. The temperature sensitivity index (TSI), to assess the impact of an increase in the measuring temperature on a given parameter (Y) in plants grown at 25°C (CT), was calculated as:
| (10) |
The temperature acclimation index ratio (TAI) of the same leaf gas exchange parameters measured and grown at a specific temperature (Silim et al., 2010) was calculated as:
| (11) |
Statistical analysis
The statistical significance of trait variation was tested by factorial ANOVA, with species, irrigation and temperature/VPD regimes, and the interaction between treatments, as fixed factors. Post hoc comparison between treatments was performed with Duncan test (P < 0.05) using Statistica 6.0 software package (StatStof Inc., USA). Regressions coefficients were calculated with Sigma Plot 11.0 software package.
Results
Leaf CO2 conductances and assimilation in rice, wheat, and maize grown under water deficit and elevated temperature and VPD
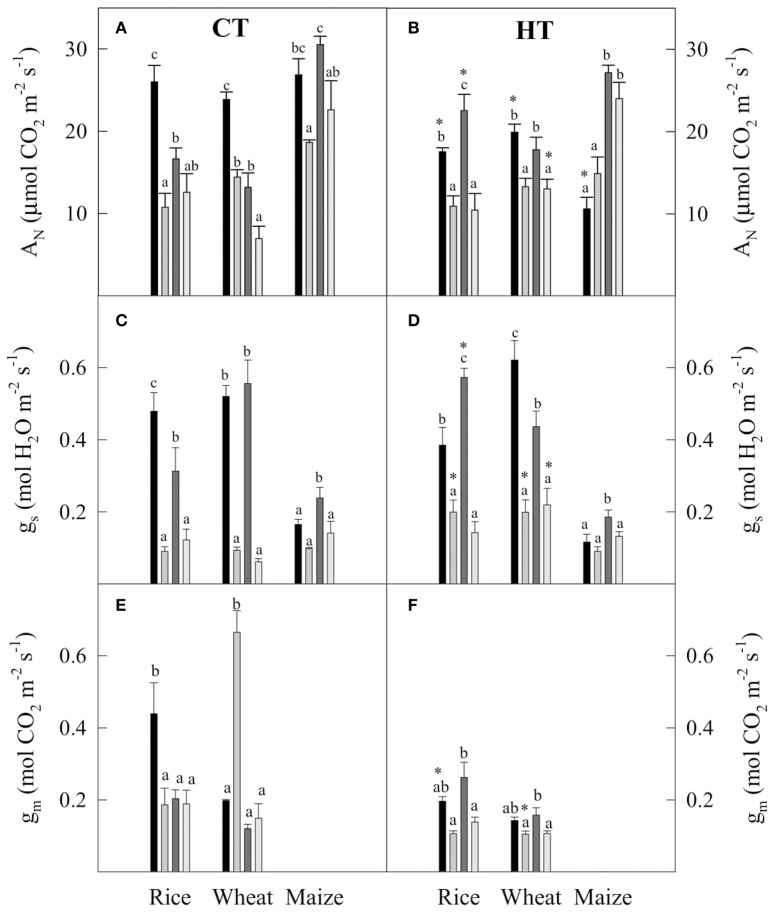
Plants of rice, wheat, and maize grown at 25°C and 1.8 kPa VPD with optimal water supply (CT-WW) had similar values of net CO2 assimilation rate (AN) at 25°C (Figure 1A). By comparison, when AN was measured at 38°C in the same plants it had similar values to that in maize at 25°C in maize, but was decreased slightly in rice and substantially in wheat. In plants grown at 38°C and 3.5 kPa VPD with optimal water supply (HT-WW), AN measured at 38°C was higher in maize than in rice or wheat, and AN measured at 25°C was largely decreased in maize, and slightly decreased in rice and wheat as compared to measurements at the higher temperature (Figure 1B).
Figure 1.
(A,B) The net CO2 assimilation rate (AN), (C,D) the stomatal conductance (gs) and (E,F) the mesophyll conductance (gm) in plants grown at CT (A,C,E) and HT (B,D,F) for rice, wheat, and maize measured at  WW-25°C,
WW-25°C,  WD-25°C,
WD-25°C,  WW-38°C, and
WW-38°C, and  WD-38°C. Values are means ± standard error (n = 3–5). Different letters denote statistically significant differences between treatments within each species and growth temperature and asterisks between the two growth temperatures within the same species, irrigation treatment, and temperature of measurement (Duncan analysis, P < 0.05).
WD-38°C. Values are means ± standard error (n = 3–5). Different letters denote statistically significant differences between treatments within each species and growth temperature and asterisks between the two growth temperatures within the same species, irrigation treatment, and temperature of measurement (Duncan analysis, P < 0.05).
Growth under conditions of water deficit (CT-WD and HT-WD) had a negative impact on AN for all plants except for maize grown at HT, mostly as a consequence of decreased stomatal conductance (gs, Figures 1C,D). Effects of water deficit and growth temperature on mesophyll conductance (gm) estimated for the C3 species showed less obvious trends (Figures 1E,F). Comparison of the results for gm, obtained with the three different methods (Figure S1), showed some scattering in the data, but significant positive correlations (P < 0.01) were obtained between the method of Harley (Harley et al., 1992; adopted in this work for subsequent comparisons and modeling) and two alternative methods (Ethier and Livingston, 2004; Yin et al., 2009). No clear pattern was observed for the 4 treatments out of 16 showing discrepancies, e.g., in some cases measurements were at 25°C and in others at 38°C. The unexpected increase in gm in wheat plants grown at 25°C under WD compared to WW conditions was confirmed by the three estimation methods (Figure S1).
Decreases in gs and gm largely explained the limitation of AN in rice and wheat plants under WD conditions (Figure 1), so that a tight correlation was observed between the total leaf conductance to CO2 (gt, calculated from integration of gs and gm) and AN (Figure S2). A similar trend was observed for gs in maize (Figure S2), supporting the conclusion that diffusive limitations to photosynthesis predominate in plants exposed to moderate water deficit conditions. Conversely, in wheat plants grown at 25°C and measured at 38°C, AN was much decreased even though gt was mostly unaffected (Figure S2).
Leaf mitochondrial dark respiration (Rdark) in rice, wheat, and maize grown under water deficit and elevated temperature and VPD
Plants of all three species grown at CT showed similar responses of mitochondrial dark respiration rate (Rdark) to the imposed treatments (Figure 2). These responses consisted of a boost of Rdark after a sudden increase in the temperature of measurement. The effects of WD on Rdark in CT plants were non-significant at the measurement temperature of 25°C in all three species, but became significant in wheat and maize measured at 38°C. In HT grown plants, the patterns of response of Rdark to the imposed treatments were radically different to those displayed by CT plants. In HT plants, Rdark became sensitive to the irrigation treatment in the C3 crops (except for wheat measured at 25°C), but not in maize. In addition, in HT plants the effects of the measuring temperature on Rdark were less evident than in CT plants, with significant changes only observed in maize and in HT-WW wheat.
Figure 2.
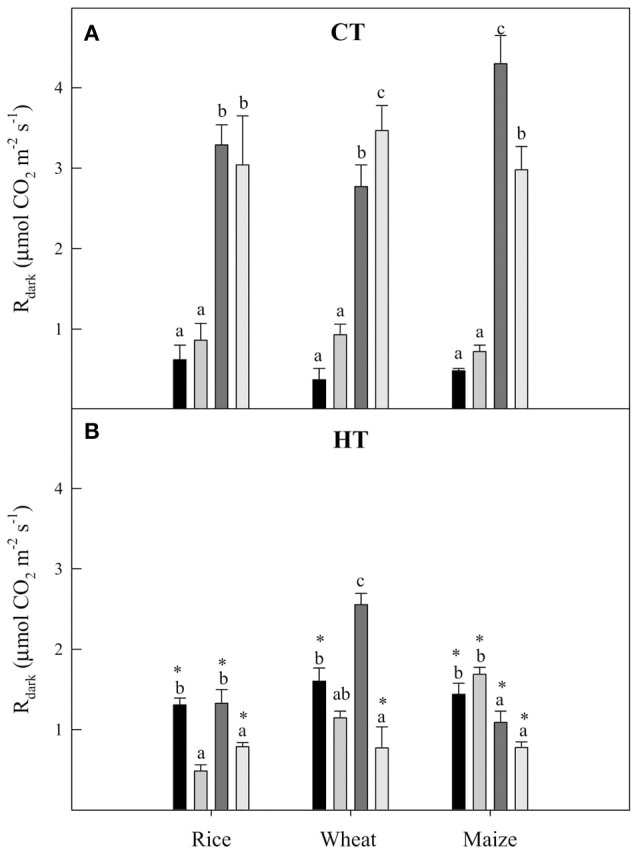
The mitochondrial respiration at pre-dawn (Rdark) in plants grown at CT (A) and HT (B) for rice, wheat, and maize measured at  WW-25°C,
WW-25°C,  WD-25°C,
WD-25°C,  WW-38°C, and
WW-38°C, and  WD-38°C. Values are means ± standard error (n = 3–5). Different letters denote statistically significant differences among treatments within each species and same growth temperature and asterisks between the two growth temperatures within the same species, irrigation treatment and temperature of measurement (Duncan analysis P < 0.05).
WD-38°C. Values are means ± standard error (n = 3–5). Different letters denote statistically significant differences among treatments within each species and same growth temperature and asterisks between the two growth temperatures within the same species, irrigation treatment and temperature of measurement (Duncan analysis P < 0.05).
Long-term effects of water deficit and high temperature and VPD stress on the photosynthetic biochemistry of the three crops
The response of photosynthesis to increasing CO2 concentration was analyzed in the three species on the basis of the CO2 concentration in the chloroplastic stroma (i.e., AN–Cc curves in rice and wheat, and AN–Cs in maize). All crops displayed the well-described response of AN to increasing Cc or Cs (Figures S3–S5).
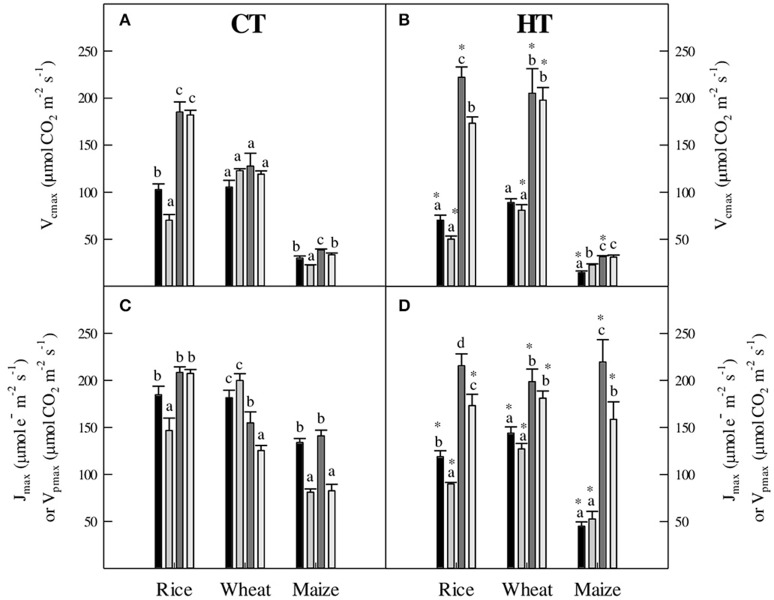
In general, for rice and wheat, the effect of temperature was more evident than that of water availability on the shape of the AN-Cc curves (Figures S3, S4). This observation suggests a higher resilience of photosynthetic biochemistry to water deficit than to high temperatures. The biochemical parameters derived from AN-Cc curves confirmed this same pattern. In CT plants, the maximum velocity of Rubisco carboxylation (Vcmax) was more responsive to the increase in measuring temperature from 25°C to 38°C in rice than in wheat (Figure 3A). By contrast, both species showed decreased Vcmax in HT plants when lowering the measuring temperature from 38°C to 25°C (Figure 3B). Significant effects of WD on Vcmax were observed in rice under CT-25°C and HT-38°C, and were absent in wheat.
Figure 3.
(A,B) The maximum velocity of Rubisco carboxylation (Vcmax), (C,D) the maximum electron transport rate (Jmax) and the maximum velocity of PEPC carboxylation (Vpmax), in plants grown at CT (A,C) and HT (B,D). Vcmax were measured for wheat, rice and maize, Jmax in wheat and rice, and Vpmax only in maize. All parameters were measured at  WW-25°C,
WW-25°C,  WD-25°C,
WD-25°C,  WW-38°C, and
WW-38°C, and  WD-38°C. Values are means ± standard error (n = 3–5). Different letters denote statistically significant differences among treatments within each species and same growth temperature and asterisks between the two growth temperatures within the same species, irrigation treatment and temperature of measurement (Duncan analysis P < 0.05).
WD-38°C. Values are means ± standard error (n = 3–5). Different letters denote statistically significant differences among treatments within each species and same growth temperature and asterisks between the two growth temperatures within the same species, irrigation treatment and temperature of measurement (Duncan analysis P < 0.05).
Compared to Vcmax, the maximum rate of electron transport (Jmax) was less affected by changes in the temperature of measurement, but similarly by changes in the irrigation treatment (Figures 3C,D). In consequence, in both rice and wheat, the ratio Jmax/Vcmax was lower when measured at 38°C compared to 25°C, irrespective of the growth temperature (data not shown). The effect of the growth temperature on Jmax/Vcmax ratio was significant when plants were measured at 38°C in the two species. By contrast, significant effects of WD were restricted to CT-25°C rice and CT-38°C wheat.
Long-term growth under WD had more evident effects on the shape of AN-Cs curves in maize compared to the effects on the AN-Cc curves in the C3 crops (Figure S5). However, these effects were restricted to the linear part of the AN-Cs curve, informative of PEPC activity. Accordingly, the maximum rate of PEPC carboxylation (Vpmax) was affected by WD under all treatments except HT-25°C (Figures 3C,D). The effect of the measuring temperature on Vpmax was dependent on the growth temperature: no effects were observed in CT-grown plants, but Vpmax decreased dramatically in HT-grown plants when the measurement temperature decreased from 38°C to 25°C. Vpmax was also highly responsive to the growth temperature, showing a high capacity for thermal acclimation (i.e., highest values when Vpmax was measured at the respective growth temperature). In maize, Vcmax was also significantly affected by the irrigation treatment in plants grown at CT, while at HT-25°C Vcmax increased under WD (Figures 3A,B). Likewise, Vcmax in maize increased with the measuring temperature at both growth temperatures irrespective of water availability (Perdomo, 2015).
Kinetic properties of rubisco and PEPC and their relevance for modeling photosynthesis of C3 and C4 plants
The gross CO2 assimilation rate (AG) was calculated from the sum of AN and half of the mitochondrial respiration in the dark (Rdark). In rice and wheat, AG increased linearly with the ratio of CO2 and O2 concentrations in the chloroplast (Cc/O) (Figure 4). For a given temperature treatment, WD plants showed a lower AG due to decreased Cc/O, in both rice and wheat. It is remarkable that rice plants measured at the same temperature but grown at different temperatures (e.g., compare CT-25°C and HT-25°C) presented different AG values for a given Cc/O, suggesting that the carboxylase/oxygenase activity of Rubisco was sensitive to the growth temperature.
Figure 4.
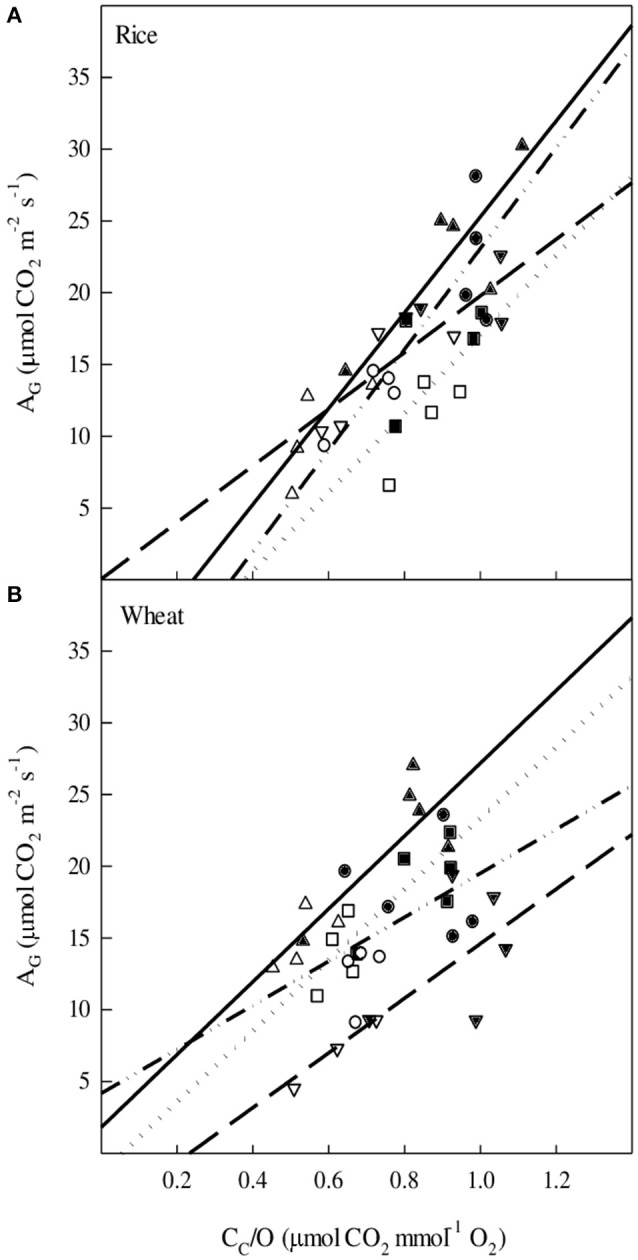
The relationship between: the gross photosynthesis rate (AG) and the relative concentrations of CO2 and O2 (Cc/O) for (A) rice and (B) wheat. Symbols, treatments and lines as follows: ▴ CT-WW-25°C, ▵ CT-WD-25°C, solid regression ( ); ▾ CT-WW-38°C, ▿ CT-WD-38°C, dashed regression (
); ▾ CT-WW-38°C, ▿ CT-WD-38°C, dashed regression ( ); ■ HT-WW-25°C, □ HT-WD-25°C, dotted regression (
); ■ HT-WW-25°C, □ HT-WD-25°C, dotted regression ( ); • HT-WW-38°C, ° HT-WD-38°C, dashed-dotted regression (
); • HT-WW-38°C, ° HT-WD-38°C, dashed-dotted regression ( ). In rice, solid regression R2 = 0.90 P < 0.001, dashed regression R2 = 0.69 P < 0.01, dotted regression R2 = 0.54 P < 0.05, and dashed-dotted regression R2 = 0.77 P < 0.001. In wheat, solid regression R2 = 0.79 P < 0.001, dashed regression R2 = 0.60 P < 0.01, and dotted regression R2 = 0.65 P < 0.01.
). In rice, solid regression R2 = 0.90 P < 0.001, dashed regression R2 = 0.69 P < 0.01, dotted regression R2 = 0.54 P < 0.05, and dashed-dotted regression R2 = 0.77 P < 0.001. In wheat, solid regression R2 = 0.79 P < 0.001, dashed regression R2 = 0.60 P < 0.01, and dotted regression R2 = 0.65 P < 0.01.
Rubisco and PEPC kinetic constants, required for photosynthesis modeling, were measured in vitro at the two temperatures of measurement to enable a more accurate modeling. As expected, all kinetic constants increased at 38°C with respect to 25°C in the three species (Table 1). Differences between the two C3 crops and maize were significant for the Michaelis–Menten constant of Rubisco for CO2 (Kc) at both temperatures and for the Michaelis–Menten constant of Rubisco for O2 (Ko) at 38°C.
Table 1.
Kinetic parameters of Rubisco and PEPC from rice, wheat, and maize measured at 25 and 38°C.
| Species | Kc (Pa) | Ko (kPa) | Γ* (Pa) | KP (Pa) | ||||
|---|---|---|---|---|---|---|---|---|
| 25°C | 38°C | 25°C | 38°C | 25°C | 38°C | 25°C | 38°C | |
| Rice | 29.1 ± 1.6a | 87.7 ± 5.2a* | 45.7 ± 4.6a | 58.3 ± 5.7a* | 4.19 ± 0.19a | 6.34 ± 0.36b* | – | – |
| Wheat | 31.6 ± 1.1a | 89.3 ± 3.6a* | 39.2 ± 3.8a | 50.3 ± 4.5a* | 3.87 ± 0.21a | 5.32 ± 0.32a* | – | – |
| Maize | 85.8 ± 7.0b | 188.3 ± 5.7b* | 39.8 ± 3.5a | 74.5 ± 7.5b* | 4.30 ± 0.30a | 6.47 ± 0.26b* | 8.0 ± 3.0 | 13.2 ± 5.0* |
Four replicates were used for the Michaelis–Menten constants of Rubisco for CO2 and O2 (Kc and Ko) and the Michaelis–Menten constant of PEPC for CO2 (KP), and five replicates for the Rubisco specificity factor (Sc/o). The chloroplast CO2 compensation point in the absence of mitochondrial respiration (Γ*) was calculated from Sc/o as explained in the Material and Methods. Different letters and asterisks denote statistically significant differences (Duncan analysis, P < 0.05) among species within the same temperature and between measurement temperatures within the same species, respectively.
The Vcmax estimated for the C3 species by applying the model of Farquhar et al. (1980), and using the values of the in vitro Rubisco kinetics specific for each species at each measurement temperature/VPD (Table 1) tended to be lower than the Vcmax estimated using the kinetic parameters from Bernacchi et al. (2001, 2002) (Table 2). However, the estimates obtained by each method for the different species under each treatment were highly related. None of the differences between Vcmax values estimated using specific kinetics and kinetics from Bernacchi et al. (2001, 2002) were significant in rice, and only in 3 cases the differences were significant in wheat (CT-WD-25°C, HT-WW-25°C and HT-WD-25°C). Indeed, the correlation between the two Vcmax estimates was high (r2 > 0.99) in both species, however the slope in wheat (0.86) was lower than in rice (0.98) and thus more distant to a 1:1 relationship (data not shown). Significant differences between values of Jmax estimated using specific and Bernacchi kinetics (Bernacchi et al., 2001, 2002) were observed for three treatments in rice (CT-WW-25°C, CT-WD-25°C, and HT-WD-25°C) and two in wheat (CT-WW-25°C and CT-WD-25°C) (Table 2). To strengthen these observations, the estimates for Vcmax and Jmax obtained using the species-specific values of Rubisco kinetics measured in the present study and those reported for tobacco in Bernacchi et al. (2001, 2002) were compared by applying the method described by Ethier and Livingston (2004); significant differences in Vcmax were observed in two cases for each species, while significant differences in Jmax were only observed in CT-WD-25°C wheat (Table S1).
Table 2.
Comparison of the maximum velocity of Rubisco carboxylation (Vcmax) and maximum rate of electron transport (Jmax) in plants grown at CT and HT, under WW and WD, and measured at 25 and 38°C, using the Rubisco kinetics parameters (Kc, Ko, and Sc/o) measured in the present study for rice and wheat (own kinetics), and using the parameters reported for tobacco by Bernacchi et al. (2001, 2002).
| Species | Growth T (°C) | Irrigation treatment | Measurement T (°C) | Own kinetics | Kinetics by Bernacchi et al. (2001, 2002) | ||
|---|---|---|---|---|---|---|---|
| Vcmax | Jmax | Vcmax | Jmax | ||||
| Rice | CT | WW | 25 | 102.7±6.1 | 184.5±9.2 | 116.0±6.0 | 112.7±22.3* |
| Rice | CT | WD | 25 | 70.1±6.2 | 146.7±13.2 | 77.9±7.2 | 82.8±4.5* |
| Rice | CT | WW | 38 | 185.1±10.8 | 208.3±5.9 | 206.2±10.8 | 197.6±6.9 |
| Rice | CT | WD | 38 | 182.2±5.0 | 207.3±4.2 | 195.8±3.1 | 198.5±0.3 |
| Rice | HT | WW | 25 | 70.2±5.4 | 118.8±6.6 | 78.0±5.8 | 108.8±5.1 |
| Rice | HT | WD | 25 | 50.2±3.3 | 90.0±1.6 | 59.2±3.8 | 79.3±2.7* |
| Rice | HT | WW | 38 | 222.1±11.0 | 215.4±12.6 | 228.3±14.4 | 198.5±11.0 |
| Rice | HT | WD | 38 | 173.2±6.8 | 173.4±11.8 | 176.5±15.2 | 161.4±5.8 |
| Wheat | CT | WW | 25 | 105.4±7.2 | 181.5±7.9 | 125.8±9.7 | 101.5±15.4* |
| Wheat | CT | WD | 25 | 122.9±2.1 | 199.8±7.1 | 144.1±3.3* | 81.9±2.5* |
| Wheat | CT | WW | 38 | 127.7±13.7 | 154.7±11.6 | 151.6±16.2 | 156.9±11.3 |
| Wheat | CT | WD | 38 | 119.2±3.3 | 125.3±5.4 | 134.5±8.9 | 119.7±5.8 |
| Wheat | HT | WW | 25 | 88.9±4.2 | 143.8±6.8 | 110.0±6.1* | 133.6±5.9 |
| Wheat | HT | WD | 25 | 80.9±5.9 | 127.2±5.7 | 104.5±6.1* | 122.8±6.6 |
| Wheat | HT | WW | 38 | 205.2±26.0 | 198.7±13.3 | 247.7±29.2 | 186.8±10.9 |
| Wheat | HT | WD | 38 | 197.8±13.5 | 181.1±7.6 | 230.3±15.9 | 174.2±8.6 |
Vcmax and Jmax were calculated on a Cc basis as estimated by the method of Harley et al. (1992). Values are means ± standard errors (n = 5). Asterisks denote statistically significant differences between the two Vcmax or Jmax values (Duncan analysis, P < 0.05) within the same treatment (growth T and irrigation treatment).
Regarding C4 modeling, the comparison was established between Vcmax and Vpmax estimates using the Rubisco and PEPC kinetics reported in the present study for maize and those reported in von Caemmerer (2000) (Table 3). von Caemmerer (2000) used the temperature dependence of Rubisco kinetic constants reported by Bernacchi et al. (2002), while Kp was assumed to be invariable with temperature. Differences in Vcmax estimates between the two approaches were non-significant under all treatments, while significant differences in Vpmax were found in CT-grown plants, irrespective of the irrigation treatment and the measuring temperature (Table 3).
Table 3.
Comparison of the maximum velocity of Rubisco carboxylation (Vcmax) and the maximum velocity of PEPC carboxylation (Vpmax) in plants grown at CT and HT, under WW and WD, and measured at 25 and 38°C, using Rubisco and PEPC kinetic parameters (Kc, Ko, Sc/o, and Kp) measured in the present study for maize, and using the parameter values reported by von Caemmerer (2000).
| Species | Growth T (°C) | Irrigation treatment | Measurement T (°C) | Own kinetics | Kinetics by von Caemmerer (2000) | ||
|---|---|---|---|---|---|---|---|
| Vcmax | Vpmax | Vcmax | Vpmax | ||||
| Maize | CT | WW | 25 | 29.9±2.3 | 133.8±4.3 | 29.7±2.2 | 112.8±3.8* |
| Maize | CT | WD | 25 | 22.5±0.3 | 81.1±3.5 | 22.3±0.3 | 69.5±2.6* |
| Maize | CT | WW | 38 | 38.7±1.0 | 141.1±5.9 | 41.5±0.9 | 117.5±6.4* |
| Maize | CT | WD | 38 | 33.6±1.7 | 82.6±6.9 | 37.5±0.8 | 62.0±4.4* |
| Maize | HT | WW | 25 | 14.4±1.8 | 44.9±4.7 | 14.1±1.8 | 38.4±4.1 |
| Maize | HT | WD | 25 | 22.7±1.2 | 52.8±8.2 | 22.2±1.2 | 46.8±7.0 |
| Maize | HT | WW | 38 | 31.7±0.9 | 219.5±23.7 | 32.6±1.1 | 182.0±15.0 |
| Maize | HT | WD | 38 | 31.1±2.2 | 158.7±18.6 | 32.2±2.2 | 141.4±10.0 |
Values are means ± standard errors (n = 5). Asterisks denote statistically significant differences between the two Vcmax or Vpmax values (Duncan analysis, P < 0.05) within the same treatment (growth T and irrigation treatment).
Sensitivity and acclimation capacity to high temperature and water deficit in rice, wheat, and maize
A temperature sensitivity index (TSI, Table 4) was calculated for the main photosynthetic parameters as the ratio between the value at CT-25°C and that at CT-38°C. The photosynthetic machinery of maize was particularly insensitive to the sudden increase in the measuring temperature in CT-grown plants, both under WW and WD. By contrast, AN, gs, and gm were affected by short-term heat stress in rice and wheat (as denoted by the asterisks), although relative sensitivity was dependent on the irrigation treatment. Irrespective of the irrigation treatment, wheat was the unique species with TSI > 1 for Jmax, and rice presented the lowest TSI for Vcmax (i.e., the largest increment due to the increase in the temperature of measurement). Rdark was the most sensitive parameter to the increase in the temperature of measurement, particularly under WW conditions.
Table 4.
Temperature sensitivity index (TSI) for the net CO2 assimilation rate (AN), stomatal conductance (gs), mesophyll conductance (gm), maximum velocity of Rubisco carboxylation (Vcmax), maximum rate of electron transport (Jmax), maximum velocity of PEPC carboxylation (Vpmax), and mitochondrial respiration at pre-dawn (Rdark).
| Parameter | WW | WD | ||||
|---|---|---|---|---|---|---|
| Rice | Wheat | Maize | Rice | Wheat | Maize | |
| AN | 1.6±0.2* | 1.7±0.2* | 0.9±0.1 | 0.8±0.2 | 2.0±0.8* | 0.8±0.2 |
| gs | 1.5±0.3 | 1.0±0.2 | 0.7±0.1 | 0.8±0.3 | 1.5±0.3* | 0.7±0.2 |
| gm | 2.5±0.9* | 1.7±0.2* | – | 1.0±0.4 | 4.4±1.3* | – |
| Vcmax | 0.6±0.1* | 0.9±0.1 | 0.8±0.1 | 0.4±0.1* | 1.0±0.1 | 0.7±0.1* |
| Jmax | 0.9±0.1 | 1.2±0.1 | – | 0.7±0.1* | 1.6±0.1* | – |
| Vpmax | – | – | 1.0±0.1 | – | – | 1.0±0.1 |
| Rdark | 0.2±0.1* | 0.2±0.1* | 0.1±0.1* | 0.3±0.1* | 0.3±0.1* | 0.2±0.1* |
TSI was calculated, under both well-watered (WW) and water deficit (WD) conditions, as the ratio between the values from plants grown and measured at 25°C and those from plants grown at CT and measured at 38°C [TSI = (CT-25°C)/(CT-38°C)]. The asterisks indicate significant differences between CT-25°C and CT-38°C. Values are means ± standard errors (n = 5).
The temperature acclimation index (TAI, Table 5) provides a tool for comparison of plants grown and measured at the same temperature (CT-25°C and HT-38°C). Under WW, wheat was the species with the lowest TAI for AN, and both rice and wheat presented TAI > 1 for Vcmax, while maize TAI for Vpmax was also >1 (Table 5). Under WD, maize was the unique species with TAI for AN significantly higher than 1, all three species presented TAI > 1 for gs and Vcmax, and maize for Vpmax. TAI for Rdark was not significantly different from 1 under WD but increased under WW conditions in all species. In wheat WW, TAI <1 for AN and TAI >1 for Rdark, suggesting a lower capacity of acclimation to increased temperature (Perdomo, 2015).
Table 5.
Temperature acclimation index (TAI) for the net CO2 assimilation rate (AN), stomatal conductance (gs), mesophyll conductance (gm), maximum velocity of Rubisco carboxylation (Vcmax), maximum rate of electron transport (Jmax), maximum velocity of PEPC carboxylation (Vpmax), and mitochondrial respiration at pre-dawn (Rdark).
| Parameter | WW | WD | ||||
|---|---|---|---|---|---|---|
| Rice | Wheat | Maize | Rice | Wheat | Maize | |
| AN | 0.9±0.1 | 0.7±0.1* | 1.0±0.1 | 1.0±0.4 | 0.9±0.1 | 1.3±0.1* |
| gs | 1.2±0.1 | 0.8±0.1 | 1.1±0.1 | 1.6±0.6 | 2.4±0.5* | 1.3±0.1* |
| gm | 0.6±0.1 | 0.8±0.1 | – | 0.8±0.5 | 0.2±0.1* | – |
| Vcmax | 2.2±0.1* | 1.9±0.2* | 1.1±0.1 | 2.6±0.3* | 1.6±0.1* | 1.4±0.1* |
| Jmax | 1.2±0.1 | 1.1±0.1 | – | 1.2±0.2 | 0.9±0.1 | – |
| Vpmax | – | – | 1.6±0.2* | – | – | 2.0±0.4* |
| Rdark | 2.1±1.0* | 5.5±1.3* | 2.3±0.3* | 0.8±0.1 | 0.8±0.2 | 1.1±0.2 |
TAI was calculated, under both well-watered (WW) and water deficit (WD) conditions, as the ratio between the values from plants grown and measured at 38°C and those from plants grown and measured at 25°C [TAI = (HT-38°C)/(CT-25°C)]. The asterisks indicate significant differences between CT-25°C and HT-38°C. Values are means ± standard errors (n = 5).
Discussion
Long-term responses to increased temperature, VPD and drought stress were compared in rice, wheat, and maize, to improve our understanding of how these three major global crops will respond to the future climate. In addition, we used the data obtained as well as the Rubisco in vitro kinetics for each species and treatments to check the validity of commonly employed “universal Rubisco constants” to parameterize photosynthesis models in different species. These two objectives are discussed independently in the next sections.
Long-term acclimation to high temperature and drought stress in three important global crops
Plants grown and measured at 25°C under well-watered conditions (CT-WW-25°C) showed similar values for AN in the three species (Figure 1). However, WD resulted in a significant decrease of AN in all three species, the strongest effect being observed in rice and the mildest in maize. In the two C3 species, these limitations were mostly due to stomatal conductance (gs), which largely decreased in both species, while the mesophyll conductance to CO2 (gm) decreased under water deficit in rice but apparently increased in wheat (Kalaji and Nalborczyk, 1991; Choluj et al., 1997). Parameters reflecting photosynthetic activity (Vcmax and Jmax) were largely unaffected by WD (Figure 3), as is often found in C3 species (Flexas et al., 2002, 2006b; Galmés et al., 2007b). In C4 maize, the drought stress-induced decrease in AN was due to combined decreases on Vcmax and Vpmax plus gs only when measured at 38°C (Figures 1, 3); this is in agreement with previous reports in C4 plants (Lal and Edwards, 1996; Carmo-Silva et al., 2010).
When WW plants grown at CT were measured at 38°C, significant decreases of photosynthesis were observed in all species but maize, although these effects were of smaller magnitude than those induced by WD except in wheat, where the two stresses resulted in responses of similar magnitude (Figure 1). These results suggest that, of the three crops, rice was the most sensitive species to drought stress and wheat was the most sensitive to increased measurement temperature, while maize was the least sensitive to both drought stress and increased measurement temperature (see also Table 4). In many studies, short-term responses are taken as evidence to predict the future photosynthetic performance of a given species under a changing climate. However, there are at least two factors that can bias these responses: (i) interactions between stresses, and (ii) acclimation in the long-term (Centritto et al., 2002; Flexas et al., 2006a; Vile et al., 2012; Cheesman and Winter, 2013). Regarding interactions, these are evidenced by measuring at 38°C plants grown at CT and subjected to water deficit (CT-WD-38°C). In rice, AN values were somewhat larger under WD when plants were measured at 38°C than at 25°C, however, the effect of WD at 38°C was not significant (Figure 1). In other words, in rice the high measurement temperature and drought stress interacted somehow to increase photosynthesis as compared to measuring drought plants at the lower temperature. In wheat, in contrast, the interaction of high measurement temperature and WD resulted in a cumulative effect of both stresses, so that photosynthesis under WD-38°C was about half of the value observed when stress treatments were applied independently (Figure 1).
Considering the comparison of WW-CT plants measured at 25°C and WD-CT plants measured at 38°C, it can no longer be considered that rice is more drought stress sensitive and wheat more temperature sensitive. Instead, both species are similarly sensitive to the combination of high measurement temperature and drought stress. This result illustrates how short-term studies observing the response to isolated stresses may fail to reproduce plant responses to the most complex, combined stress conditions that are often experienced in the field (Shah and Paulsen, 2003; Prasad et al., 2008; Vile et al., 2012; Tozzi et al., 2013). In maize, the combination of high temperature/VPD and WD resulted in photosynthesis rates only marginally lower than those displayed by WW plants measured at low temperature, as expected for a C4 species (Edwards et al., 2001; Crafts-Brandner and Salvucci, 2002; Osborne and Beerling, 2006).
Long-term acclimation responses may further confound the predictive value of short-term observations. Acclimation was evident for the three species. Values of AN were very similar between plants grown and measured at 25°C and those grown and measured at 38°C (i.e., TAI close to 1, Table 5), both under WW and WD conditions (Figures 1A,B). Only in WW wheat AN was lower in plants grown and measured at high temperature (HT-WW-38°C) than in plants grown at control temperature and measured at 25°C (CT-WW-25°C), and in WD maize AN was higher in plants grown and measured at 38°C than at 25°C, confirming the adaptation of these two species to cool and hot temperature conditions, respectively (Hikosaka et al., 2006; Nagai and Makino, 2009; Yamori et al., 2009). A similar acclimation to growth temperature—i.e., AN is kept constant—has also been observed in poplar (Silim et al., 2010). Interestingly, acclimation of photosynthesis to high temperature in the three species was achieved through different homeostatic mechanisms. For instance, in both WW and WD rice and WD wheat, the same AN at the two temperatures was achieved by increasing gs and Vcmax but decreasing gm (Figures 1, 3). Contrarily, in WW wheat, a lower AN was observed at high temperature despite increased Vcmax, which was in part attributable to large increases in respiration (Figure 2). These results indicate that changing climate results in species-dependent changes in the ratios between biochemical and diffusive parameters even in cases where net photosynthesis does not change.
In summary, the present results illustrate that the photosynthetic responses to climate conditions—e.g., drought stress and increased temperature and VPD—differ when analyzed in the short- or long-term, in a species-specific manner. Therefore, it is necessary to be cautious when deriving generalizations or predictions from short-term studies with few species subjected to isolated stress conditions. Rather, detailed long-term experiments with different species and stress interactions are urged for a better understanding of crop responses to withstanding climate change conditions.
Species-specific rubisco kinetics and their effects on accurate parameterization of C3 and C4 photosynthesis models
Photosynthesis models such as that of Farquhar et al. (1980) for C3 plants, or that of von Caemmerer (2000) for C4 plants, allow the estimation of biochemical traits such as the maximum velocity of Rubisco carboxylation (Vcmax) and the maximum rate of electron transport (Jmax) in C3 plants, and the maximum velocities of PEPC (Vpmax) and Rubisco (Vcmax) carboxylation in C4 species (von Caemmerer, 2013). While this parameterization was originally applied on a Ci basis (Berry and Björkman, 1980; Farquhar et al., 1980), it is now widely recognized that correct parameterization should take into account the CO2 concentration at the Rubisco site inside chloroplasts (Cc), for which knowledge of the mesophyll conductance to CO2 (gm) is required. For instance, a recent survey on 130 species reveals that assuming infinite gm underestimates, on average, Vcmax by as much as 75% and Jmax by 60% (Gu and Sun, 2014; Sun et al., 2014). Under severe drought stress conditions the underestimations may be even larger (Flexas et al., 2006b).
On the other hand, gm typically decreases under drought stress (Flexas et al., 2002; Galmés et al., 2007a; Gallé et al., 2009; Hu et al., 2010) and increases with temperature, at least up to a certain threshold (Silim et al., 2010; Evans and von Caemmerer, 2013; Walker et al., 2013). The response of gm to temperature has recently been shown to be strongly species-dependent (von Caemmerer and Evans, 2015), although the mechanisms for this are still unclear (Flexas and Díaz-Espejo, 2015; von Caemmerer and Evans, 2015). Therefore, to correctly parameterize photosynthesis, gm should be precisely determined for plants at each experimental condition and measurement temperature. In this study, gm decreased in rice after the increase in the measurement temperature, but this decrease was not significant in wheat. In contrast, an increase in gm with measurement temperature was reported by von Caemmerer and Evans (2015) and supported by data from Xiong et al. (2015) in rice. A recent reported showed a decline in gm as leaves aged from fully expanded to senescing (Barbour et al., 2016), supporting that the above discrepancies reflect the importance of the experimental conditions and leaf age.
Several problems with existing methods for the estimation of gm have been raised recently (Tholen et al., 2012; Gu and Sun, 2014). On one hand, the estimated gm may not reflect purely a diffusion conductance, because AN reflects a CO2 net flux that combines photosynthesis, photorespiration and mitochondrial respiration, and these three processes move CO2 along different distances and diffusion pathways (Tholen et al., 2012). On the other hand, apparent responses of gm to varying light and CO2 may be artefactual, resulting from analysis of gm dependence on variables that are explicitly included in the equations used to calculate gm (Gu and Sun, 2014). These type of errors should affect only methods that estimate different gm values at any given Ci (i.e., Harley et al., 1992 and Yin et al., 2009), but not methods that solve for a single gm estimate along a Ci gradient (i.e., Ethier and Livingston, 2004). Since, in most cases, the estimates based on these three different methods of estimation show a significant agreement (Figure S1) we may dismiss the importance of these errors in the present study, but since different values were obtained with the different methods for some treatments potential errors cannot be completely ruled out.
Hence, while recognizing that some of the values presented may represent an approximation to the true gm, we can still use these predictions to check for the effects of species-specific differences in Rubisco kinetic constants and their temperature response and acclimation on the parameterization of photosynthesis models. This is because in addition to precise knowledge on gm and its temperature dependency, a priori knowledge of Rubisco kinetic constants (Sc/o, Kc, and Ko), as well as their temperature dependencies, is required to parameterize photosynthesis models. Since these constants are unknown for Rubiscos from many species, it is becoming a common practice to use “standard” constants for any given species. The most commonly used “standard” Rubisco kinetics and temperature functions are those for tobacco as obtained by Bernacchi et al. (2002). However, it is well documented that significant differences occur among species in Rubisco kinetics (e.g., Galmés et al., 2005, 2015; Savir et al., 2010; Orr et al., 2016; Prins et al., 2016), and these differences result in significant bias in model parameterization (Walker et al., 2013). These authors also indicate that in vitro Rubisco kinetics may not accurately describe the operation of Rubisco under physiological conditions, due to degradation and/or inactivation of the enzyme during extraction or differences in the in vitro assay conditions compared to the chloroplast stroma. While the latter may be true, degradation, or activation of Rubisco during the extraction may affect quantitatively absolute parameters, such as the maximum Rubisco activity or Rubisco concentration, but should not affect Sc/o, Kc, and Ko.
Determination of in vivo kinetics for a large number of species with different functional types, as urged by Walker et al. (2013), may not be accomplished in the short term, as such experiments require the use of mutants with low Rubisco contents for each species, growth under low CO2 concentrations and the use of gas exchange measurements at different oxygen partial pressures, i.e., plant material that is yet to be created and techniques that are not readily available except in very few laboratories. In contrast, measuring in vitro kinetic constants of Rubisco is easier and less time consuming, so that a number of different species can be characterized in a reasonable time (Hermida-Carrera et al., 2016; Orr et al., 2016; Prins et al., 2016). Therefore, we propose using Γ* derived from in vitro Sc/o measured in each species at different temperatures to first estimate gm and, then, parameterize photosynthesis from AN-Cc curves using the species and temperature specific in vitro kinetics of Rubisco rather than “standard” values determined for model species.
The in vitro values for Γ* and Kc are within the range of values obtained in vivo for tobacco and Arabidopsis by Walker et al. (2013), supporting the use of in vitro values as a valid approach to estimate Rubisco constants comparable to those operating in vivo. Nevertheless, Ko was almost the double in rice and wheat regarding the tobacco and Arabidopsis also reported by Walker et al. (2013), which demonstrates the differences in the Rubisco kinetic parameters among species and point out the importance of considering the species-specific values instead of general “consensus values.”
Rubisco from C4 maize had a lower affinity for CO2 (i.e., higher Kc) than the Rubisco from C3 rice and wheat (Table 1), in agreement with previous reports (Christin et al., 2008; Carmo-Silva et al., 2010; Cousins et al., 2010; Savir et al., 2010; Whitney et al., 2011). At 25°C, differences in Γ* and Ko between species and photosynthetic mechanisms were non-significant, indicative that maize Rubisco presents a higher maximum catalytic turnover for the carboxylation reaction (kcatc) than the two C3 species, which is in agreement with recent studies (Sharwood et al., 2016a,b). The response of Rubisco kinetics to increased temperature followed trends already described in the literature, both in vivo (Brooks and Farquhar, 1985; Bernacchi et al., 2001; Walker et al., 2013) and in vitro in C3 and C4 species (Badger and Collatz, 1977; Jordan and Ogren, 1984; Galmés et al., 2005; Boyd et al., 2015), with increases in Kc, Ko, Kc/Ko, and Γ* (Table 1). The relative increase in Γ* with temperature was lower in wheat than in maize and rice. There are few reported measurements of the Michaelis–Menten constant for PEPC (Kp) in C4 species (Bauwe, 1986; Pfeffer and Peisker, 1998; Boyd et al., 2015) and limited studies on the temperature dependence of Kp (Boyd et al., 2015). Kp increased with temperature in Setaria viridis (Boyd et al., 2015) and in maize (this study) to a lesser extent than the increase of Kc. This fact, together with the higher temperature-driven increase of Vpmax as compared to that of Vcmax (Figure 3), suggests increased Rubisco limitations for C4 photosynthesis at high temperatures. That the Kp values for S. viridis are higher than those reported here for maize, corroborates the need to use species-specific kinetic constants for both Rubisco and PEPC for greater accuracy in C4 photosynthetic modeling.
Using each species Rubisco constants resulted in model parameterization estimates that in some cases differed significantly from those obtained using the “standard” constants by Bernacchi et al. (2002) in C3 plants. These differences represented on average 10–20% overestimation of Vcmax and a largely variable underestimation of Jmax (Table 2), with strongly biased Vcmax/Jmax ratios. The magnitude of these discrepancies, similar to that found by Walker et al. (2013), is remarkable, especially considering that the species compared (tobacco and Arabidopsis in Walker's study, rice and wheat here) are all herbaceous angiosperms. It is likely that even broader deviations would occur when using “standard” tobacco kinetics to parameterize more distant species, like woody angiosperms, gymnosperms, ferns or mosses. Part of this bias in the parameterization of Vcmax and Jmax is due to bias in the estimation of gm, as indicated by the significant differences obtained between the gm values estimated using the Rubisco kinetic values from the present study and those reported in Bernacchi et al. (2001, 2002). These differences were observed regardless of the gm estimation method (i.e., Harley et al., 1992; Ethier and Livingston, 2004 or Yin et al., 2009; Table S2). These results also demonstrate that species- and temperature-specific kinetic parameters for PEPC and Rubisco are required for accurate photosynthesis parameterization in C4 plants, in particular for estimating Vpmax (Table 3).
In summary, the present results confirm and extend the conclusion by Walker et al. (2013) that species-specific differences in in vivo Rubisco parameters are large enough to significantly bias modeling of C3 photosynthesis. It is further shown, for the first time, that differences in species-specific kinetics are large enough to bias modeling of C4 photosynthesis. It is thus strongly recommended that the use of “standard” Rubisco kinetics from tobacco is avoided when modeling photosynthesis in other species. As obtaining in vivo Rubisco kinetics for different species is not achievable in the short-term, we propose to use in vitro kinetics as determined by the methods explained here and elsewhere (Kane et al., 1994; Ruuska et al., 1998; Parry et al., 2007; Shay and Kubien, 2013; Perdomo, 2015; Galmés et al., 2016; Orr et al., 2016; Prins et al., 2016) as a first proxy for in vivo kinetics.
Author contributions
JAP performed the experiment, analyzed the data, and wrote the paper. ECS contributed to the design, analysis, and interpretation of the C4 gas-exchange model data. CH was involved in the acquisition of the Rubisco kinetic data. JF is an expert in plant physiology, contributed substantially to write the paper, and critically revised the work. JG obtained funding for the project and was a substantial contributor to the conception and design of the work.
Funding
This study was financially supported by the contract AGL2009-07999 (Plan Nacional, Spain) awarded to JG. JAP was the recipient of a FPI grant from the Govern de les Illes Balears. ECS was the recipient of a Rothamsted Research Career Fellowship that currently supports JAP. Rothamsted Research receives grant-aided support from the Biotechnology and Biological Sciences Research Council (BBSRC) 20:20 Wheat® Institute Strategic Programme.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Dr. A. Diaz-Espejo (IRNAS, CSIC) for help with modeling C4 photosynthesis, T. García (radioisotope service at UIB) for technical assistance with the radioactive measurements, Dr. J. Cifre for the statistical advice and Dr. A.J. Keys for his helpful comments on the manuscript. The manuscript resulted from the Ph.D. thesis of JAP “Acclimation of photosynthesis to water deficit and high temperature: physiological and biochemical aspects.”
Glossary
Abbreviations
- AG
gross CO2 assimilation rate
- AN
net CO2 assimilation rate
- Ca
atmospheric CO2 concentration
- Cc
chloroplastic CO2 concentration
- Ci
intercellular CO2 concentration
- CT
control temperature
- Fo
basal fluorescence of a dark adapted leaf
- Fs
steady-state fluorescence signal
- gbs
bundle sheath conductance to CO2
- gm
mesophyll conductance to CO2
- gs
stomatal conductance to CO2
- HT
high temperature
- Jmax
maximum photosynthetic electron transport rate
- Kc
Michaelis–Menten constant of Rubisco for CO2
- kcatc
reaction turnover rate for carboxylation activity of Rubisco
- Ko
Michaelis–Menten constant of Rubisco for O2
- KP
Michaelis–Menten constant of phosphoenolpyruvate carboxylase for HCO3
- Rdark
mitochondrial respiration at pre-dawn
- RL
non-photorespiratory CO2 evolution in the light
- Rm
mesophyll mitochondrial respiration
- PAR
photosynthetic active radiation
- PSII
photosystem II
- Sc/o
in vitro Rubisco specificity factor for CO2/O2
- TAI
temperature acclimation index
- TSI
temperature sensitivity index
- Vcmax
maximum velocity of Rubisco carboxylation activity
- Vpmax
maximum velocity of PEPC carboxylation activity
- VPD
vapor pressure deficit
- WD
water deficit
- WW
well-watered
- Γ*
photorespiratory CO2 compensation point.
Supplementary material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fpls.2016.01719/full#supplementary-material
References
- Ainsworth E. A., Ort D. R. (2010). How do we improve crop production in a warming world? Plant Physiol. 154, 526–530. 10.1104/pp.110.161349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkin O. K., MacHerel D. (2009). The crucial role of plant mitochondria in orchestrating drought tolerance. Ann. Bot. 103, 581–597. 10.1093/aob/mcn094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkin O. K., Scheurwater I., Pons T. (2006). High thermal acclimation potential of both photosynthesis and respiration in two lowland Plantago species in contrast to an alpine congeneric. Glob. Chang. Biol. 12, 500–515. 10.1111/j.1365-2486.2006.01114.x [DOI] [Google Scholar]
- Atkin O. K., Tjoelker M. G. (2003). Thermal acclimation and the dynamic response of plant respiration to temperature. Trends Plant Sci. 8, 343–351. 10.1016/S1360-1385(03)00136-5 [DOI] [PubMed] [Google Scholar]
- Badger M., Collatz G. (1977). Mechanism of ribulose-1,5-bisphosphate carboxylase and oxygenase reactions, with particular reference to the effect of temperature on kinetic parameters. Carnegie Inst. Washington Yearb. 76, 355–361. [Google Scholar]
- Barbour M. M., Evans J. R., Simonin K. A., von Caemmerer S. (2016). Online CO2 and H2O oxygen isotope fractionation allows estimation of mesophyll conductance in C4 plants, and reveals that mesophyll conductance decreases as leaves age in both C4 and C3 plants. New Phytol. 210, 875–889. 10.1111/nph.13830 [DOI] [PubMed] [Google Scholar]
- Bauwe H. (1986). An efficient method for the determination of Km values for HCO3 of phosphoenolpyruvate carboxylase. Planta 169, 356–360. 10.1007/BF00392131 [DOI] [PubMed] [Google Scholar]
- Bernacchi C. J., Pimentel C., Long S. P. (2003). In vivo temperature response functions of parameters required to model RuBP-limited photosynthesis. Plant Cell Environ. 26, 1419–1430. 10.1046/j.0016-8025.2003.01050.x [DOI] [Google Scholar]
- Bernacchi C. J., Portis A. R., Nakano H., von Caemmerer S., Long S. P. (2002). Temperature response of mesophyll conductance. implications for the determination of rubisco enzyme kinetics and for limitations to photosynthesis in vivo. Plant Physiol. 130, 1992–1998. 10.1104/pp.008250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernacchi C. J., Singsaas E. L., Pimentel C., Portis A. R., Jr., Long S. P. (2001). Improved temperature response functions for models of Rubisco-limited photosynthesis. Plant Cell Environ. 24, 253–259. 10.1111/j.1365-3040.2001.00668.x [DOI] [Google Scholar]
- Berry J., Björkman O. (1980). Photosynthetic response and adaptation to temperature in higher-plants. Physiol. Plant Mol. Biol. 31, 491–543. 10.1146/annurev.pp.31.060180.002423 [DOI] [Google Scholar]
- Boyd R. A., Gandin A., Cousins A. B. (2015). Temperature response of c4 photosynthesis: biochemical analysis of rubisco, phosphoenolpyruvate carboxylase and carbonic anhydrase in Setaria viridis. Plant Physiol. 169, 1850–1861. 10.1104/pp.15.00586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks A., Farquhar G. D. (1985). Effect of temperature on the CO2/O2 specificity of ribulose-1,5-bisphosphate carboxylase/oxygenase and the rate of respiration in the light. Planta 165, 397–406. 10.1007/BF00392238 [DOI] [PubMed] [Google Scholar]
- Campbell C., Atkinson L., Zaragoza-Castells J., Lundmark M., Atkin O. K., Hurry V. (2007). Acclimation of photosynthesis and respiration is asynchronous in response to changes in temperature regardless of plant functional group. New Phytol. 176, 375–389. 10.1111/j.1469-8137.2007.02183.x [DOI] [PubMed] [Google Scholar]
- Carmo-Silva A. E., Gore M. A., Andrade-Sanchez P., French A. N., Hunsaker D. J., Salvucci M. E. (2012). Decreased CO2 availability and inactivation of Rubisco limit photosynthesis in cotton plants under heat and drought stress in the field. Environ. Exp. Bot. 83, 1–11. 10.1016/j.envexpbot.2012.04.001 [DOI] [Google Scholar]
- Carmo-Silva A. E., Keys A. J., Andralojc P. J., Powers S. J., Arrabaça M. C., Parry M. A. J. (2010). Rubisco activities, properties, and regulation in three different C4 grasses under drought. J. Exp. Bot. 61, 2355–2366. 10.1093/jxb/erq071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmo-Silva A. E., Powers S. J., Keys A. J., Arrabaça M. C., Parry M. A. J. (2008). Photorespiration in C4 grasses remains slow under drought conditions. Plant Cell Environ. 31, 925–940. 10.1111/j.1365-3040.2008.01805.x [DOI] [PubMed] [Google Scholar]
- Centritto M., Lucas M. E., Jarvis P. G. (2002). Gas exchange, biomass, whole-plant water-use efficiency and water uptake of peach (Prunus persica) seedlings in response to elevated carbon dioxide concentration and water availability. Tree Physiol. 22, 699–706. 10.1093/treephys/22.10.699 [DOI] [PubMed] [Google Scholar]
- Cheesman A. W., Winter K. (2013). Growth response and acclimation of CO2 exchange characteristics to elevated temperatures in tropical tree seedlings. J. Exp. Bot. 64, 3817–3828. 10.1093/jxb/ert211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choluj D., Kalaji M. H., Niemyska B. (1997). Analysis of the gas exchange components in chilled tomato plants. Photosynthetica 34, 583–589. 10.1023/A:1006825915953 [DOI] [Google Scholar]
- Christin P. A., Salamin N., Muasya A. M., Roalson E. H., Russier F., Besnard G. (2008). Evolutionary switch and genetic convergence on rbcL following the evolution of C4 photosynthesis. Mol. Biol. Evol. 25, 2361–2368. 10.1093/molbev/msn178 [DOI] [PubMed] [Google Scholar]
- Cousins A. B., Ghannoum O., von Caemmerer S., Badger M. R. (2010). Simultaneous determination of Rubisco carboxylase and oxygenase kinetic parameters in Triticum aestivum and Zea mays using membrane inlet mass spectrometry. Plant Cell Environ. 33, 444–452. 10.1111/j.1365-3040.2009.02095.x [DOI] [PubMed] [Google Scholar]
- Crafts-Brandner S. J., Salvucci M. E. (2002). Sensitivity of photosynthesis in a C4 plant, maize, to heat stress. Plant Physiol. 129, 1773–1780. 10.1104/pp.002170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Espejo A. (2013). New challenges in modelling photosynthesis: temperature dependencies of Rubisco kinetics. Plant Cell Environ. 36, 2104–2107. 10.1111/pce.12192 [DOI] [PubMed] [Google Scholar]
- Dillaway D. N., Kruger E. L. (2011). Leaf respiratory acclimation to climate: comparisons among boreal and temperate tree species along a latitudinal transect. Tree Physiol. 31, 1114–1127. 10.1093/treephys/tpr097 [DOI] [PubMed] [Google Scholar]
- Edwards G., Furbank R., Hatch M., Osmond C. (2001). What does it take to be C4? Lessons from the evolution of C4 photosynthesis. Plant Physiol. 125, 46–49. 10.1104/pp.125.1.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ethier G. J., Livingston N. J. (2004). On the need to incorporate sensitivity to CO2 transfer conductance into the Farquhar-von Caemmerer-Berry leaf photosynthesis model. Plant Cell Environ. 27, 137–153. 10.1111/j.1365-3040.2004.01140.x [DOI] [Google Scholar]
- Evans J. R., von Caemmerer S. (2013). Temperature response of carbon isotope discrimination and mesophyll conductance in tobacco. Plant Cell Environ. 36, 745–756. 10.1111/j.1365-3040.2012.02591.x [DOI] [PubMed] [Google Scholar]
- Farquhar G. D., von Caemmerer S., Berry J. A. (1980). A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 149, 78–90. 10.1007/BF00386231 [DOI] [PubMed] [Google Scholar]
- Flexas J., Barón M., Bota J., Ducruet J.-M., Gallé A., Galmés J., et al. (2009). Photosynthesis limitations during water stress acclimation and recovery in the drought-adapted Vitis hybrid Richter-110 (V. berlandieri × V. rupestris). J. Exp. Bot. 60, 2361–2377. 10.1093/jxb/erp069 [DOI] [PubMed] [Google Scholar]
- Flexas J., Bota J., Escalona J., Sampol B., Medrano H. (2002). Effects of drought on photosynthesis in grapevines under field conditions: an evaluation of stomatal and mesophyll limitations. Funct. Plant Biol. 29, 461–471. 10.1071/PP01119 [DOI] [PubMed] [Google Scholar]
- Flexas J., Bota J., Galmés J., Medrano H., Ribas-Carbó M. (2006a). Keeping a positive carbon balance under adverse conditions: responses of photosynthesis and respiration to water stress. Physiol. Plant. 127, 343–352. 10.1111/j.1399-3054.2006.00621.x [DOI] [Google Scholar]
- Flexas J., Díaz-Espejo A. (2015). Interspecific differences in temperature response of mesophyll conductance: food for thought on its origin and regulation. Plant Cell Environ. 38, 625–628 10.1111/pce.12476 [DOI] [PubMed] [Google Scholar]
- Flexas J., Díaz-Espejo A., Berry J. A., Cifre J., Galmés J., Kaldenhoff R., et al. (2007). Analysis of leakage in IRGA's leaf chambers of open gas exchange systems: quantification and its effects in photosynthesis parameterization. J. Exp. Bot. 58, 1533–1543. 10.1093/jxb/erm027 [DOI] [PubMed] [Google Scholar]
- Flexas J., Niinemets Ü., Gallé A., Barbour M. M., Centritto M., Diaz-Espejo A., et al. (2013). Diffusional conductances to CO2 as a target for increasing photosynthesis and photosynthetic water-use efficiency. Photosyn. Res. 117, 45–59. 10.1007/s11120-013-9844-z [DOI] [PubMed] [Google Scholar]
- Flexas J., Ribas-Carbó M., Bota J., Galmés J., Henkle M., Martínez-Cañellas S., et al. (2006b). Decreased Rubisco activity during water stress is not induced by decreased relative water content but related to conditions of low stomatal conductance and chloroplast CO2 concentration. New Phytol. 172, 73–82. 10.1111/j.1469-8137.2006.01794.x [DOI] [PubMed] [Google Scholar]
- Gallé A., Florez-Sarasa I., Tomas M., Pou A., Medrano H., Ribas-Carbo M., et al. (2009). The role of mesophyll conductance during water stress and recovery in tobacco (Nicotiana sylvestris): acclimation or limitation? J. Exp. Bot. 60, 2379–2390. 10.1093/jxb/erp071 [DOI] [PubMed] [Google Scholar]
- Galmés J., Aranjuelo I., Medrano H., Flexas J. (2013). Variation in Rubisco content and activity under variable climatic factors. Photosyn. Res. 117, 73–90. 10.1007/s11120-013-9861-y [DOI] [PubMed] [Google Scholar]
- Galmés J., Flexas J., Keys A. J., Cifre J., Mitchell R., Madgwick P. J., et al. (2005). Rubisco specificity factor tends to be larger in plant species from drier habitats and in species with persistent leaves. Plant Cell Environ. 28, 571–579. 10.1111/j.1365-3040.2005.01300.x [DOI] [Google Scholar]
- Galmés J., Flexas J., Savé R., Medrano H. (2007a). Water relations and stomatal characteristics of Mediterranean plants with different growth forms and leaf habits: responses to water stress and recovery. Plant Soil 290, 139–155. 10.1007/s11104-006-9148-6 [DOI] [Google Scholar]
- Galmés J., Hermida-Carrera C., Laanisto L., Niinements Ü. (2016). A compendium of temperature responses of Rubisco kinetic traits: variability among and within photosynthetic groups and impacts on photosynthesis modeling. J. Exp. Bot. 67, 5067–5091. 10.1093/jxb/erw267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galmés J., Kapralov M. V., Andralojc P. J., Conesa M. À., Keys A. J., Parry M. A. J., et al. (2014). Expanding knowledge of the Rubisco kinetics variability in plant species: environmental and evolutionary trends. Plant Cell Environ. 37, 1989–2001. 10.1111/pce.12335 [DOI] [PubMed] [Google Scholar]
- Galmés J., Kapralov M. V., Copolovici L., Hermida-Carrera C., Niinemets Ü. (2015). Temperature responses of the Rubisco maximum carboxylase activity across domains of life: phylogenetic signals, trade-offs and importance for carbon gain. Photosyn. Res. 123, 183–201. 10.1007/s11120-014-0067-8 [DOI] [PubMed] [Google Scholar]
- Galmés J., Medrano H., Flexas J. (2006). Acclimation of Rubisco specificity factor to drought in tobacco: discrepancies between in vitro and in vivo estimations. J. Exp. Bot. 57, 3659–3667. 10.1093/jxb/erl113 [DOI] [PubMed] [Google Scholar]
- Galmés J., Medrano H., Flexas J. (2007b). Photosynthetic limitations in response to water stress and recovery in Mediterranean plants with different growth forms. New Phytol. 175, 81–93. 10.1111/j.1469-8137.2007.02087.x [DOI] [PubMed] [Google Scholar]
- Galmés J., Ribas-Carbó M., Medrano H., Flexas J. (2007c). Response of leaf respiration to water stress in Mediterranean species with different growth forms. J. Arid Environ. 68, 206–222. 10.1016/j.jaridenv.2006.05.005 [DOI] [Google Scholar]
- Galmés J., Ribas-Carbó M., Medrano H., Flexas J. (2011). Rubisco activity in Mediterranean species is regulated by the chloroplastic CO2 concentration under water stress. J. Exp. Bot. 62, 653–665. 10.1093/jxb/erq303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genty B., Briantais J., Baker N. (1989). The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta 990, 87–92. 10.1016/S0304-4165(89)80016-9 [DOI] [Google Scholar]
- Gornall J., Betts R., Burke E., Clark R., Camp J., Willett K., et al. (2010). Implications of climate change for agricultural productivity in the early twenty-first century. Philos. Trans. R. Soc. Lond. B Biol. Sci. 365, 2973–2989. 10.1098/rstb.2010.0158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassini P., Eskridge K. M., Cassman K. G. (2013). Distinguishing between yield advances and yield plateaus in historical crop production trends. Nat. Commun. 4, 2918. 10.1038/ncomms3918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu L., Sun Y. (2014). Artefactual responses of mesophyll conductance to CO2 and irradiance estimated with the variable J and online isotope discrimination methods. Plant Cell Environ. 37, 1231–1249. 10.1111/pce.12232 [DOI] [PubMed] [Google Scholar]
- Harley P. C., Loreto F., Di Marco G., Sharkey T. D. (1992). Theoretical considerations when estimating the mesophyll conductance to CO2 flux by analysis of the response of photosynthesis to CO2. Plant Physiol. 98, 1429–1436. 10.1104/pp.98.4.1429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermida-Carrera C., Kapralov M. V., Galmés J. (2016). Rubisco catalytic properties and temperature response in crops. Plant Physiol. 171, 2549–2561. 10.1104/pp.16.01846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikosaka K., Ishikawa K., Borjigidai A., Muller O., Onoda Y. (2006). Temperature acclimation of photosynthesis: mechanisms involved in the changes in temperature dependence of photosynthetic rate. J. Exp. Bot. 57, 291–302. 10.1093/jxb/erj049 [DOI] [PubMed] [Google Scholar]
- Hu L., Wang Z., Huang B. (2010). Diffusion limitations and metabolic factors associated with inhibition and recovery of photosynthesis from drought stress in a C3 perennial grass species. Physiol. Plant. 139, 93–106. 10.1111/j.1399-3054.2010.01350.x [DOI] [PubMed] [Google Scholar]
- IPCC (2013). Working Group I Contribution to the IPCC Fifth Assessment Report Climate Change 2013: The Physical Science Basis Summary for Policymakers.
- Jordan D. B., Ogren W. L. (1984). The CO2/O2 specificity of ribulose 1,5-bisphosphate carboxylase/oxygenase: dependence on ribulosebisphosphate concentration, pH and temperature. Planta 161, 308–313. 10.1007/BF00398720 [DOI] [PubMed] [Google Scholar]
- Kalaji H., Nalborczyk E. (1991). Gas-exchange of barley seedlings growing under salinity stress. Photosynthetica 25, 197–202. [Google Scholar]
- Kane H., Viil J., Entsch B., Paul K., Matthew K., and Andrews T. (1994). An improved method for measuring the CO2/O2 specificity of ribulosebisphosphate carboxylase-oxygenase. Aust. J. Plant Physiol. 21, 449–461. 10.1071/PP9940449 [DOI] [Google Scholar]
- Kattge J., Knorr W. (2007). Temperature acclimation in a biochemical model of photosynthesis: a reanalysis of data from 36 species. Plant Cell Environ. 30, 1176–1190. 10.1111/j.1365-3040.2007.01690.x [DOI] [PubMed] [Google Scholar]
- Krall J., Edwards G. (1992). Relationship between photosystem II activity and CO2 fixation in leaves. Physiol. Plant. 86, 180–187. 10.1111/j.1399-3054.1992.tb01328.x [DOI] [Google Scholar]
- Laisk A., Loreto F. (1996). Determining Photosynthetic Parameters from Leaf CO2 Exchange and Chlorophyll Fluorescence. Plant Physiol. 110, 903–912. 10.1104/pp.110.3.903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lal A., Edwards G. (1996). Analysis of inhibition of photosynthesis under water stress in the C4 species Amaranthus cruentus and Zea mays: electron transport, CO2 fixation and carboxylation. Funct. Plant Biol. 23, 403–412. [Google Scholar]
- Long S., Ainsworth E. A., Leakey A., Nösberger J., Ort D. (2006). Food for thought: lower-than-expected crop yield stimulation with rising CO2 concentrations. Science 312, 1918–1921. 10.1126/science.1114722 [DOI] [PubMed] [Google Scholar]
- Loriaux S. D., Avenson T. J., Welles J. M., McDermitt D. K., Eckles R. D., Riensche B., et al. (2013). Closing in on maximum yield of chlorophyll fluorescence using a single multiphase flash of sub-saturating intensity. Plant Cell Environ. 36, 1755–1770. 10.1111/pce.12115 [DOI] [PubMed] [Google Scholar]
- Makino A. (2011). Photosynthesis, grain yield, and nitrogen utilization in rice and wheat. Plant Physiol. 155, 125–129. 10.1104/pp.110.165076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massad R. S., Tuzet A., Bethenod O. (2007). The effect of temperature on C4-type leaf photosynthesis parameters. Plant Cell Environ. 30, 1191–1204. 10.1111/j.1365-3040.2007.01691.x [DOI] [PubMed] [Google Scholar]
- Medrano H., Escalona J. M., Bota J., Gulías J., Flexas J. (2002). Regulation of photosynthesis of C3 plants in response to progressive drought: stomatal conductance as a reference parameter. Ann. Bot. 89, 895–905. 10.1093/aob/mcf079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittler R. (2006). Abiotic stress, the field environment and stress combination. Trends Plant Sci. 11, 15–19. 10.1016/j.tplants.2005.11.002 [DOI] [PubMed] [Google Scholar]
- Nagai T., Makino A. (2009). Differences between rice and wheat in temperature responses of photosynthesis and plant growth. Plant Cell Physiol. 50, 744–755. 10.1093/pcp/pcp029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr D. J., Melao Alcantara A., Kapralov M. V., Andralojc P. J., Carmo-Silva A. E, Parry M. A. J. (2016). Surveying rubisco diversity and temperature response to improve crop photosynthetic efficiency. Plant Physiol. 172, 707–717. 10.1104/pp.16.00750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne C. P., Beerling D. J. (2006). Nature's green revolution: the remarkable evolutionary rise of C4 plants. Philos. Trans. R. Soc. Lond. B Biol. Sci. 361, 173–194. 10.1098/rstb.2005.1737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry M. A. J., Keys A. J., Gutteridge S. (1989). Variation in the specificity factor of C3 higher plant rubisco determined by the total consumption of ribulose-P2. J. Exp. Bot. 40, 317–320. 10.1093/jxb/40.3.317 [DOI] [Google Scholar]
- Parry M. A. J., Madgwick P. J., Carvalho J. F. C., Andralojc P. J. (2007). Prospects for increasing photosynthesis by overcoming the limitations of Rubisco. J. Agric. Sci. 145, 31–43. 10.1017/S0021859606006666 [DOI] [Google Scholar]
- Perdomo J. A. (2015). Acclimation of Photosynthesis to Water Deficit and High Temperature: Physiological and Biochemical Aspects. Ph.D. thesis, University of the Balearic Islands, Palma de Mallorca. [Google Scholar]
- Perez-Martin A., Flexas J., Ribas-Carbó M., Bota J., Tomás M., Infante J. M., et al. (2009). Interactive effects of soil water deficit and air vapour pressure deficit on mesophyll conductance to CO2 in Vitis vinifera and Olea europaea. J. Exp. Bot. 60, 2391–2405. 10.1093/jxb/erp145 [DOI] [PubMed] [Google Scholar]
- Pfeffer M., Peisker M. (1998). CO2 gas exchange and phosphoenolpyruvate carboxylase activity in leaves of Zea mays L. Photosyn. Res. 58, 281–291. 10.1023/A:1006188705423 [DOI] [Google Scholar]
- Pittermann J., Sage R. F. (2001). The response of the high altitude C4 grass Muhlenbergia montana (Nutt.) A.S. Hitchc. to long- and short-term chilling. J. Exp. Bot. 52, 829–838. 10.1093/jexbot/52.357.829 [DOI] [PubMed] [Google Scholar]
- Prasad P. V. V., Staggenborg S. A., Ristic Z. (2008). Impacts of drought and/or heat stress on physiological, developmental, growth, and yield processes of crop plants, in Response of Crops to Limited Water: Understanding and Modeling Water Stress Effects on Plant Growth Processes, Advances in Agricultural Systems Modeling, eds Ahuja L. R., Reddy V. R., Saseendran S. A., Yu Q. (Madison, WI: American Society of Agronomy; Crop Science Society of America; Soil Science Society of America; ), 301–356. [Google Scholar]
- Prins A., Orr D. J., Andralojc P. J., Reynolds M. P., Carmo-Silva A. E., Parry M. A. J. (2016). Rubisco catalytic properties of wild and domesticated relatives provide scope for improving wheat photosynthesis. J. Exp. Bot. 67, 1827–1838. 10.1093/jxb/erv574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Calcerrada J., Atkin O. K., Robson T. M., Zaragoza-Castells J., Gil L., Aranda I. (2010). Thermal acclimation of leaf dark respiration of beech seedlings experiencing summer drought in high and low light environments. Tree Physiol. 30, 214–224. 10.1093/treephys/tpp104 [DOI] [PubMed] [Google Scholar]
- Ruuska S., Andrews T., Badger M., Hudson G., Laisk A., Price G. et al. (1998). The interplay between limiting processes in C3 photosynthesis studied by rapid-response gas exchange using transgenic tobacco impaired in photosynthesis. Aust. J. Plant Physiol. 25, 859–870. 10.1071/PP98079 [DOI] [Google Scholar]
- Sage R. F., Kubien D. S. (2007). The temperature response of C3 and C4 photosynthesis. Plant Cell Environ. 30, 1086–1106. 10.1111/j.1365-3040.2007.01682.x [DOI] [PubMed] [Google Scholar]
- Salvucci M. E., Crafts-Brandner S. J. (2004). Relationship between the heat tolerance of photosynthesis and the thermal stability of Rubisco activase in plants from contrasting thermal environments. Plant Physiol. 134, 1460–1470. 10.1104/pp.103.038323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savir Y., Noor E., Milo R., Tlusty T. (2010). Cross-species analysis traces adaptation of Rubisco toward optimality in a low-dimensional landscape. Proc. Natl. Acad. Sci. U.S.A. 107, 3475–3480. 10.1073/pnas.0911663107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scafaro A. P., von Caemmerer S., Evans J. R., Atwell B. J. (2011). Temperature response of mesophyll conductance in cultivated and wild Oryza species with contrasting mesophyll cell wall thickness. Plant Cell Environ. 34, 1999–2008. 10.1111/j.1365-3040.2011.02398.x [DOI] [PubMed] [Google Scholar]
- Schultz H. (1996). Leaf absorptance of visible radiation in Vitis vinifera L.: estimates of age and shade effects with a simple field method. Sci. Hortic. 66, 93–102. 10.1016/0304-4238(96)00876-X [DOI] [Google Scholar]
- Shah N. H., Paulsen G. M. (2003). Interaction of drought and high temperature on photosynthesis and grain-filling of wheat. Plant Soil 257, 219–226. 10.1023/A:1026237816578 [DOI] [Google Scholar]
- Sharwood R. E., Ghannoum O., Whitney S. M. (2016a). Prospects for improving CO2 fixation in C3-crops through understanding C4-Rubisco biogenesis and catalytic diversity. Curr. Opin. Plant Biol. 31, 135–142. 10.1016/j.pbi.2016.04.002 [DOI] [PubMed] [Google Scholar]
- Sharwood R. E., Sonawane B. V., Ghannoum O., Whitney S. M. (2016b). Improved analysis of C4 and C3 photosynthesis via refined in vitro assays of their carbon fixation biochemistry. J. Exp. Bot. 67, 3137–3148. 10.1093/jxb/erw154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shay P. E., Kubien D. S. (2013). Field analysis of photoprotection in co-occurring cool climate C3 and C4 grasses. Physiol. Plant. 147, 316–328. 10.1111/j.1399-3054.2012.01662.x [DOI] [PubMed] [Google Scholar]
- Silim S. N., Ryan N., Kubien D. S. (2010). Temperature responses of photosynthesis and respiration in Populus balsamifera L.: acclimation versus adaptation. Photosyn. Res. 104, 19–30. 10.1007/s11120-010-9527-y [DOI] [PubMed] [Google Scholar]
- Sun Y., Gu L., Dickinson R. E., Pallardy S. G., Baker J., Cao Y., et al. (2014). Asymmetrical effects of mesophyll conductance on fundamental photosynthetic parameters and their relationships estimated from leaf gas exchange measurements. Plant Cell Environ. 37, 978–994. 10.1111/pce.12213 [DOI] [PubMed] [Google Scholar]
- Théroux-Rancourt G., Éthier G., Pepin S. (2014). Threshold response of mesophyll CO2 conductance to leaf hydraulics in highly transpiring hybrid poplar clones exposed to soil drying. J. Exp. Bot. 65, 741–753. 10.1093/jxb/ert436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tholen D., Ethier G., Genty B., Pepin S., Zhu X.-G. (2012). Variable mesophyll conductance revisited: theoretical background and experimental implications. Plant Cell Environ. 35, 2087–2103. 10.1111/j.1365-3040.2012.02538.x [DOI] [PubMed] [Google Scholar]
- Tosens T., Niinemets Ü., Vislap V., Eichelmann H., Castro-Díez P. (2012). Developmental changes in mesophyll diffusion conductance and photosynthetic capacity under different light and water availabilities in Populus tremula: how structure constrains function. Plant Cell Environ. 35, 839–856. 10.1111/j.1365-3040.2011.02457.x [DOI] [PubMed] [Google Scholar]
- Tozzi E. S., Easlon H. M., Richards J. H. (2013). Interactive effects of water, light and heat stress on photosynthesis in Fremont cottonwood. Plant Cell Environ. 36, 1423–1434. 10.1111/pce.12070 [DOI] [PubMed] [Google Scholar]
- Uedan K., Sugiyama T. (1976). Purification and characterization of phosphoenolpyruvate carboxylase from maize leaves. Plant Physiol. 57, 906–910. 10.1104/pp.57.6.906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vile D., Pervent M., Belluau M., Vasseur F., Bresson J., Muller B., et al. (2012). Arabidopsis growth under prolonged high temperature and water deficit: independent or interactive effects? Plant Cell Environ. 35, 702–718. 10.1111/j.1365-3040.2011.02445.x [DOI] [PubMed] [Google Scholar]
- von Caemmerer S. (2000). Biochemical Models of Leaf Photosynthesis. Collingwood, VIC: CSIRO. [Google Scholar]
- von Caemmerer S. (2013). Steady-state models of photosynthesis. Plant Cell Environ. 36, 1617–1630. 10.1111/pce.12098 [DOI] [PubMed] [Google Scholar]
- von Caemmerer S., Evans J. R. (2015). Temperature responses of mesophyll conductance differ greatly between species. Plant Cell Environ. 38, 629–637. 10.1111/pce.12449 [DOI] [PubMed] [Google Scholar]
- Walker B., Ariza L. S., Kaines S., Badger M. R., Cousins A. B. (2013). Temperature response of in vivo Rubisco kinetics and mesophyll conductance in Arabidopsis thaliana: comparisons to Nicotiana tabacum. Plant Cell Environ. 36, 2108–2119. 10.1111/pce.12166 [DOI] [PubMed] [Google Scholar]
- Walters R. G. (2005). Towards an understanding of photosynthetic acclimation. J. Exp. Bot. 56, 435–447. 10.1093/jxb/eri060 [DOI] [PubMed] [Google Scholar]
- Whitney S. M., Sharwood R. E., Orr D., White S. J., Alonso H., Galmés J. (2011). Isoleucine 309 acts as a C4 catalytic switch that increases ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) carboxylation rate in Flaveria. Proc. Natl. Acad. Sci. U.S.A. 108, 14688–14693. 10.1073/pnas.1109503108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong D., Liu X., Liu L., Douthe C., Li Y., Peng S., et al. (2015). Rapid responses of mesophyll conductance to changes of CO2 concentration, temperature and irradiance are affected by N supplements in rice. Plant Cell Environ. 38, 2541–2550. 10.1111/pce.12558 [DOI] [PubMed] [Google Scholar]
- Yamori W., Noguchi K., Hikosaka K., Terashima I. (2009). Cold-tolerant crop species have greater temperature homeostasis of leaf respiration and photosynthesis than cold-sensitive species. Plant Cell Physiol. 50, 203–215. 10.1093/pcp/pcn189 [DOI] [PubMed] [Google Scholar]
- Yamori W., Noguchi K., Terashima I. (2005). Temperature acclimation of photosynthesis in spinach leaves: analyses of photosynthetic components and temperature dependencies of photosynthetic partial reactions. Plant Cell Environ. 28, 536–547. 10.1111/j.1365-3040.2004.01299.x [DOI] [Google Scholar]
- Yamori W., Takahashi S., Makino A., Price G. D., Badger M. R., von Caemmerer S. (2011). The roles of ATP synthase and the cytochrome b6/f complexes in limiting chloroplast electron transport and determining photosynthetic capacity. Plant Physiol. 155, 956–962. 10.1104/pp.110.168435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin X., Struik P. C., Romero P., Harbinson J., Evers J. B., Van Der Putten P. E., et al. (2009). Using combined measurements of gas exchange and chlorophyll fluorescence to estimate parameters of a biochemical C3 photosynthesis model: a critical appraisal and a new integrated approach applied to leaves in a wheat (Triticum aestivum) canopy. Plant Cell Environ. 32, 448–464. 10.1111/j.1365-3040.2009.01934.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.