Abstract
Microvascular rarefaction distal to renal artery stenosis is linked to renal dysfunction and poor outcomes. Low–energy shockwave therapy stimulates angiogenesis, but the effect on the kidney microvasculature is unknown. We hypothesized that low–energy shockwave therapy would restore the microcirculation and alleviate renal dysfunction in renovascular disease. Normal pigs and pigs subjected to 3 weeks of renal artery stenosis were treated with six sessions of low–energy shockwave (biweekly for 3 consecutive weeks) or left untreated. We assessed BP, urinary protein, stenotic renal blood flow, GFR, microvascular structure, and oxygenation in vivo 4 weeks after completion of treatment, and then, we assessed expression of angiogenic factors and mechanotransducers (focal adhesion kinase and β1-integrin) ex vivo. A 3-week low–energy shockwave regimen attenuated renovascular hypertension, normalized stenotic kidney microvascular density and oxygenation, stabilized function, and alleviated fibrosis in pigs subjected to renal artery stenosis. These effects associated with elevated renal expression of angiogenic factors and mechanotransducers, particularly in proximal tubular cells. In additional pigs with prolonged (6 weeks) renal artery stenosis, shockwave therapy also decreased BP and improved GFR, microvascular density, and oxygenation in the stenotic kidney. This shockwave regimen did not cause detectable kidney injury in normal pigs. In conclusion, low–energy shockwave therapy improves stenotic kidney function, likely in part by mechanotransduction-mediated expression of angiogenic factors in proximal tubular cells, and it may ameliorate renovascular hypertension. Low–energy shockwave therapy may serve as a novel noninvasive intervention in the management of renovascular disease.
Keywords: renal artery stenosis, chronic kidney disease, kidney dysfunction
Atherosclerotic renal artery stenosis (ARAS) remains the leading cause of renovascular hypertension and is increasing in prevalence because of aging of the population and increased prevalence of atherosclerosis risk factors. As the disease progresses, ARAS results in gradual renal function loss1,2 and cardiovascular events.3
Restoration of vessel patency by percutaneous transluminal renal angioplasty and stenting does not often lead to improvement of renal function in ARAS compared with optimal medical therapy alone,4 likely because correction of an obstruction in the main renal artery alone cannot reverse the preexisting downstream intrarenal damage.5,6 In addition to inflammation,7,8 prolonged reduction of renal perfusion and vasoconstriction resulting from activation of the renin-angiotensin system lead to permanent changes in microvascular structure (remodeling and regression) associated with inadequate renal angiogenic signaling involving vascular endothelial growth factor (VEGF).9,10 Ischemia and oxidative stress in ARAS may also compromise the integrity of the endothelium, leading to endothelial dysfunction.5,11 In addition to glomerular podocytes, tubular epithelial cells are an important site for VEGF expression,12,13 which may fall because of tubular cell damage,14 a common histologic finding in the ARAS kidney. Furthermore, development of fibrosis restricts expansion of the microcirculation to replace lost vessels, resulting in a vicious cycle of microvascular rarefaction and consequent declines in blood and oxygen supply.15 Clearly, novel strategies developed to preserve the microvasculature could be of considerable value to slow functional decline in kidneys with ARAS.
Low–energy extracorporeal shockwave (SW) therapy, at 10% energy of the traditional SW used for lithotripsy, evokes neovascularization and improves regional blood flow and function in various ischemic tissues.16–18 The mechanical stimulus may be converted into cell signaling by upregulation of canonical mechanotransducers, like β1-integrin and its effector, focal adhesion kinase (FAK),19,20 which in turn, activate VEGF signaling and elicit angiogenesis. Experimental and clinical studies in ischemic heart disease have shown improvement in myocardial blood flow and cardiac function after SW therapy.21,22 Given that ischemic kidneys share several patterns of microvascular remodeling with ischemic hearts,23 mechanical forces that improve the myocardial microcirculation and hemodynamics may also benefit the ARAS kidney. However, whether SW can alleviate ARAS–induced ischemic kidney disease is unknown. Therefore, we hypothesized that low-energy SW would preserve the stenotic kidney microvasculature and stabilize renal function in a unilateral ARAS swine model.
Results
Animals and SW Treatments
Twenty-six pigs were randomized to ARAS (renal artery stenosis [RAS] induced after 6 weeks of an atherogenic diet) or normal controls treated or untreated with SW. Three weeks after RAS induction, low-energy SW was delivered biweekly for 3 consecutive weeks (a total of six sessions) (Supplemental Figure 1A). Serum creatinine (Scr) and mean arterial pressure (MAP) were monitored during the treatment. Four weeks after completion of SW therapy, single–kidney renal blood flow (RBF), GFR, and oxygenation (relaxivity index, R2*) were assessed in vivo. Animals were then euthanized for ex vivo studies, including microvascular remodeling as per microvascular density and renal expression of VEGF, angiopoietin-1, and hypoxia–inducible factor 1α (HIF-1α); expression of the mechanotransducers β1-integrin and its downstream effector FAK; oxidative stress by dihydroethidium (DHE); tubular injury and fibrosis; and tissue repair markers, like stromal–derived factor 1β (SDF-1β), stem cell factor (SCF), octamer–binding transcription factor 4 (Oct-4), and kidney injury molecule 1 (KIM-1). The kidney injury marker neutrophil gelatinase–associated lipocalin (NGAL) was also examined after SW to assess its safety. Moreover, the effects of SW on the stenotic kidneys were also examined in four additional pigs (prolonged ARAS and SW), in which SW treatment started after 6 weeks of RAS, and four other pigs served as controls.
SW Improved BP Control and Stabilized Renal Function in ARAS
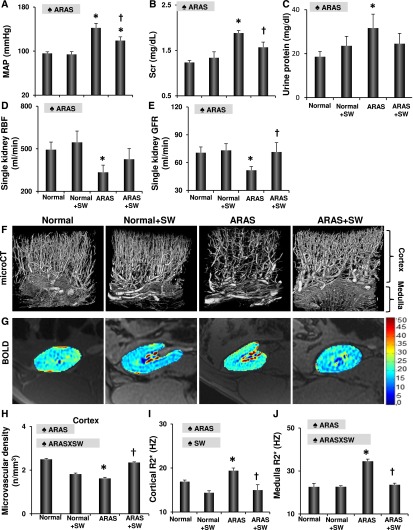
Before SW, MAP was similarly elevated in ARAS pigs compared with normal controls (Supplemental Figure 2A). After a 3-week SW regimen, MAP decreased in ARAS and SW pigs but remained unchanged in ARAS and normal pigs (Supplemental Figure 2A). Four weeks later, MAP of ARAS and SW pigs was lower than that of ARAS pigs, although it remained elevated compared with normal (Figure 1A). Plasma renin activity and norepinephrine (NE) release were both elevated in the ARAS stenotic kidney veins but not in ARAS and SW pigs (Table 1), indicating decreased activation of the renin-angiotensin system. Scr was similarly elevated in ARAS and SW pigs and ARAS pigs during the 3-week period (Supplemental Figure 2B), but by 16 weeks, it was lower in ARAS and SW pigs (Figure 1B). Urinary protein excretion of ARAS pigs was higher than normal at 16 weeks, whereas ARAS and SW pigs did not differ from normal pigs (Figure 1C). Furthermore, although ARAS decreased stenotic kidney RBF and GFR, SW improved RBF (P>0.10 versus normal) and restored GFR (Figure 1, D and E) without affecting the function of the normal kidney.
Figure 1.
SW improves stenotic kidney function and structure. (A–C) SW lowered MAP and Scr and improved urinary protein excretion in ARAS pigs 4 weeks after completion of the SW regimen. (D and E) SW improved stenotic kidney RBF and GFR in ARAS. (F–J) Representative images of microcomputed tomography (microCT) and blood oxygen level–dependent (BOLD) magnetic resonance imaging from normal, normal and SW, ARAS, and ARAS and SW pigs and quantification of microvascular density and hypoxia (R2*). SW improved microvascular density and kidney oxygenation, which were decreased in ARAS. ♠ARAS, significant effect of ARAS; ♠ARAS × SW, significant interaction of ARAS and SW (two-way ANOVA); HZ, Hertz; ♠SW, significant effect of SW. *P<0.05 versus normal; †P<0.05 versus ARAS.
Table 1.
Characteristics (mean±SEM) of normal or ARAS pigs treated or untreated with SW)
| Characteristics | Normal | ARAS | P Value for Two-Way ANOVA | ||||
|---|---|---|---|---|---|---|---|
| Untreated | SW | Untreated | SW | ARAS | SW | ARAS × SW | |
| Body weight, kg | 47.2±1.1 | 48.5±2.0 | 51.5±4.7 | 49.3±2.2 | 0.25 | 0.55 | 0.60 |
| Degree of stenosis, % | — | — | 73±6 | 76±8 | — | — | — |
| LDL, mg/dl | 51±27 | 56±21 | 200±47a | 194±41a | <0.001 | 0.96 | 0.91 |
| Total cholesterol, mg/dl | 103±34 | 107±19 | 315±61a | 301±88a | 0.004 | 0.94 | 0.89 |
| Renal vein NE, ng/ml | 0.01±0.00 | 0.01±0.00 | 0.03±0.01a | 0.02±0.00 | 0.02 | 0.28 | 0.38 |
| Renal vein PRA, pg/ml | 0.10±0.02 | 0.10±0.04 | 0.23±0.04a | 0.11±0.02 | 0.002 | 0.74 | 0.03 |
| Renal vein SDF-1β, pg/ml | 112.2±9.5 | 111.0±16.7 | 76.4±5.8a | 112.1±12.7 | 0.04 | 0.97 | 0.13 |
| Renal vein SCF-1, pg/ml | 21.5±4.5 | 21.9±5.7 | 17.5±4.4 | 54.7±18.6b | 0.21 | 0.16 | 0.01 |
—, not observed/not performed; PRA, plasma renin activity.
P<0.05 versus normal.
P<0.05 versus ARAS.
SW Promoted the Stenotic Kidney Microcirculation and Stimulated Mechanotransduction and VEGF Expression in Proximal Tubular Cells
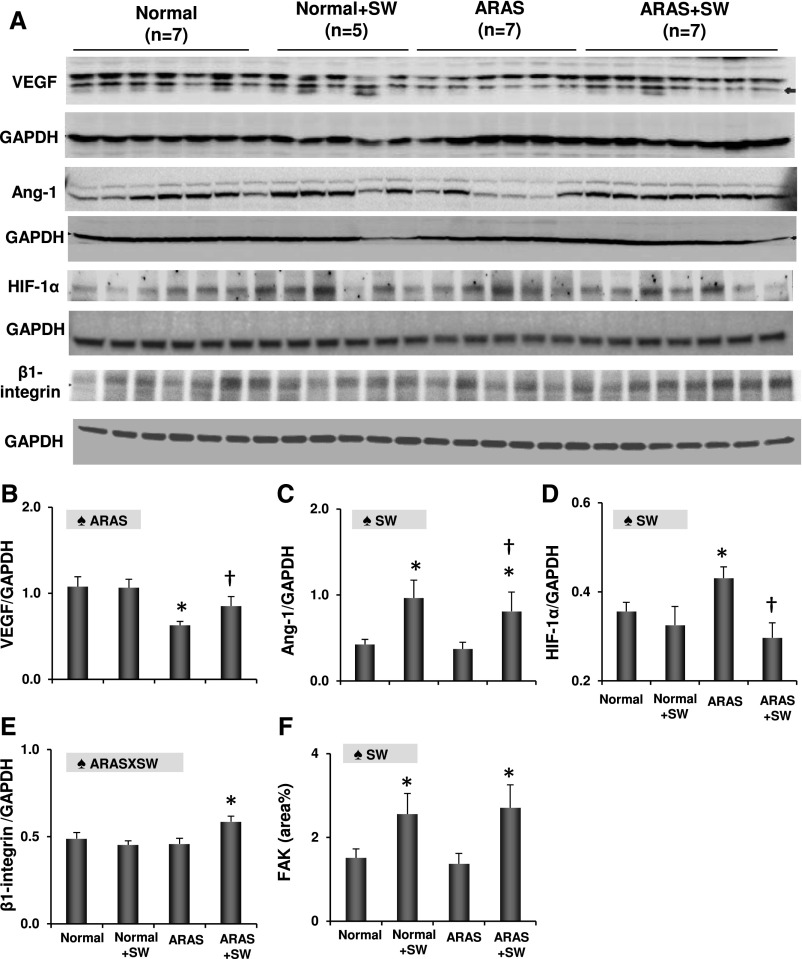
ARAS decreased the density of cortical microvessels and blunted renal oxygenation, but SW improved both (Figure 1, F–J). Similarly, SW therapy upregulated VEGF expression that was decreased by ARAS, increased angiopoietin-1, and downregulated HIF-1α (Figure 2, A–D). SW also increased angiopoietin-1 in normal kidneys. Moreover, SW improved the expression of endothelial nitric oxide synthase (eNOS), which was diminished in ARAS (Supplemental Figure 3, A and D).
Figure 2.
SW enhanced angiogenesis and mechanotransduction. (A–D) Renal expression of angiogenic VEGF, angiopoietin-1 (Ang-1), and HIF-1α. SW upregulated expression of VEGF, increased angiopoietin-1, and attenuated HIF-1α in ARAS kidneys. (E and F) SW increased both β1-integrin and focal FAK in ARAS kidneys and FAK in normal kidneys as well. ♠ARAS, significant effect of ARAS; ♠ARAS × SW, significant interaction of ARAS and SW (two-way ANOVA); GAPDH, glyceraldehyde-3-phosphate dehydrogenase; ♠SW, significant effect of SW (two-way ANOVA). *P<0.05 versus normal; †P<0.05 versus ARAS.
Expression of the mechanotransducers β1-integrin and FAK was unchanged in ARAS pigs but upregulated in ARAS and SW pigs compared with in normal pigs (Figure 2, A, E, and F), indicating stimulation of mechanotransduction signaling. SW also increased FAK expression in normal pigs. Both FAK and VEGF were localized mainly to proximal tubular cells (Supplemental Figure 4), suggesting them as a major response site in transducing SW and translating mechanical forces to angiogenic signaling.
SW Alleviated Oxidative Stress and Mediated Tissue Repair
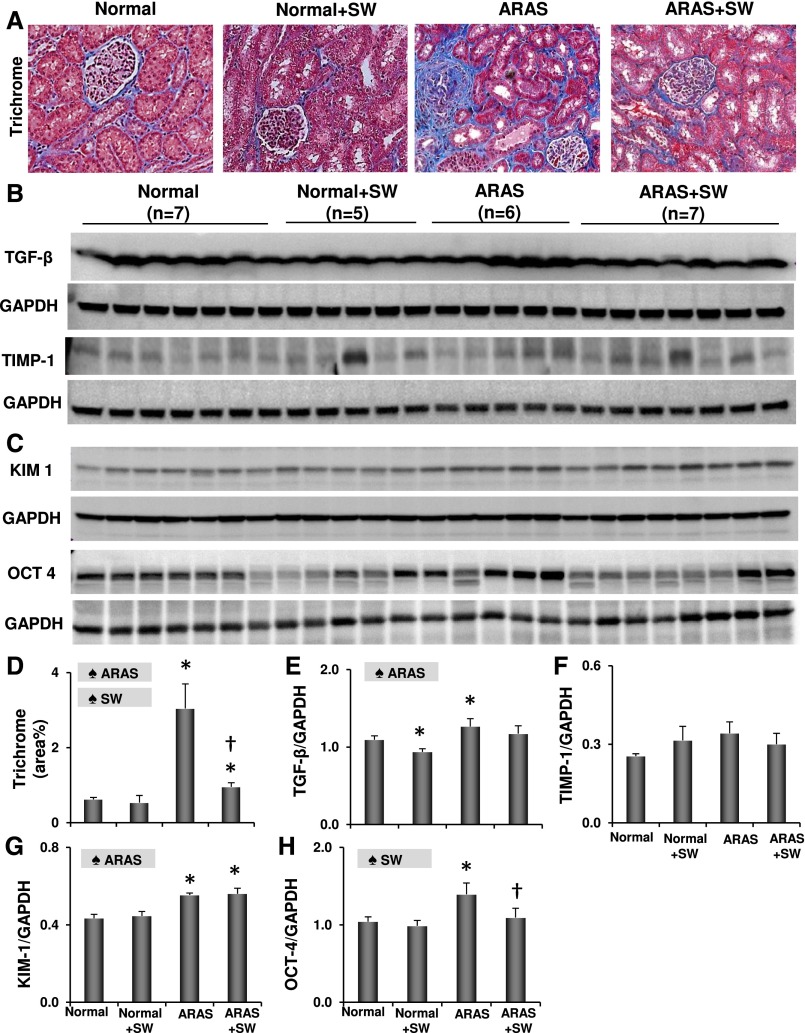
DHE staining revealed increased oxidative stress in the ARAS kidney, which was ameliorated by SW (Supplemental Figure 3, B and E) along with ARAS–induced renal fibrosis (Figure 3, A and D). SW downregulated TGF-β in normal kidneys, blunted its increase in ARAS kidneys (Figure 3, B and E), and alleviated tubular injury in ARAS (Supplemental Figure 3, C and F). SW improved renal vein and reduced levels of SDF-1β (P>0.10 versus normal) observed in ARAS, and it increased SCF in ARAS and SW pigs compared with ARAS pigs (Table 1), suggesting enhanced tissue repair potency. Moreover, kidney injury–induced regeneration markers Oct-4 and KIM-1 were both elevated in the ARAS kidney, and SW downregulated Oct-4 expression, although KIM-1 remained unchanged (Figure 3, C, G, and H).
Figure 3.
SW alleviated fibrosis and tissue injury. (A and D) Representative images and quantification of trichrome staining. (B, E, and F) Renal expression of TGF-β and tissue inhibitor of metalloproteinases 1 (TIMP-1). (C, G, and H) Renal expression of injury–induced regenerative markers KIM-1 and Oct-4. SW alleviated ARAS–induced renal fibrosis and tissue injury. ♠ARAS, significant effect of ARAS; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; ♠SW, significant effect of SW. *P<0.05 versus normal; †P<0.05 versus ARAS.
SW Did Not Induce Detectable Injury to the Kidney
In two normal animals studied immediately after a single session of SW, no gross or microscopic hematuria was observed. There was no change in urinary protein excretion or either urine or blood NGAL levels (Supplemental Table 1). Renal function, such as GFR, perfusion, and RBF, remained unaltered (Supplemental Figure 2C), and microscopic inspection of the kidney tissue showed no signs of hemorrhage or tubular injury (Supplemental Figure 2, D and E). Hence, SW did not induce measurable short–term injury to the kidney.
In the two groups treated with a 3-week SW regimen, vital signs (heart rate and BP) remained stable during each session, no hematuria or change in urinary protein excretion was observed (data not shown), and urine and plasma NGAL levels were unchanged (Supplemental Figure 2, F and G). Therefore, a 3-week SW regimen seemed to be safe for the kidney.
SW Alleviated Hyperfiltration in the Contralateral Kidneys
Both RBF and GFR were elevated in the contralateral kidneys of ARAS, indicating hyperfiltration, but not elevated in those of ARAS and SW pigs (Supplemental Figure 5, A and B). ARAS induced mild contralateral kidney fibrosis (Supplemental Figure 5, C and D), which was much lower than in the counterpart stenotic kidneys, and it remained unchanged in ARAS and SW pigs (Supplemental Figure 5, C and D).
SW Improved BP Control and Stabilized Renal Function in Prolonged ARAS
Prolonged ARAS and SW had comparable degrees of stenosis (76%±10%) and pretreatment MAP values (Supplemental Figure 6A) to ARAS (both P>0.10). MAP fell in the prolonged ARAS and SW group after treatment and became lower than ARAS (Supplemental Figure 6A). Similarly, Scr was comparably elevated in the prolonged ARAS and SW and ARAS groups at 12 weeks (Supplemental Figure 6B) compared with in the normal and SW group (Supplemental Figure 2B), and it further increased in ARAS during sham but not in prolonged ARAS and SW during SW treatment (Supplemental Figure 6B), although at 16 weeks, it remained higher than in the normal group (Supplemental Figure 6C). SW did not change stenotic RBF but significantly improved its GFR (Supplemental Figure 6, D and E). At 16 weeks, prolonged ARAS and SW RBF and GFR did not differ from either ARAS or normal (Supplemental Figure 6, F and G). In addition, SW also improved cortical microvascular density and renal oxygenation in prolonged ARAS (Supplemental Figure 7, A, B, E, and F) and alleviated fibrosis (Supplemental Figure 7, C and G), but it did not change tubular injury score (Supplemental Figure 7, D and H). These finding suggest that SW improved structure and function of the stenotic kidney in prolonged ARAS, albeit slightly less effectively than in ARAS and SW pigs.
Discussion
This study shows that low–energy SW therapy improves the poststenotic kidney oxygenation in experimental renovascular disease and preserves its function. This was associated with upregulation of mechanotransducers and angiogenic factors as well as modulation of vasoactive mediators, resulting in restoration of the renal microcirculation as well as reduced oxidative and fibrosis. No signs of renal damage were detected in SW-treated kidneys. After a more prolonged ARAS, SW also decreased BP and improved stenotic kidney GFR, albeit slightly less effectively that in ARAS and SW. We also found that the contralateral kidney of ARAS developed mild fibrosis and hyperfiltration (increased RBF and GFR) that SW improved, possibly via improvement of stenotic kidney function and fall in BP. Collectively, this study suggests a potential role for low–energy SW therapy as a safe, noninvasive, and effective treatment of the ischemic kidney distal to ARAS.
Microvascular remodeling and regression characterize the ischemic kidney, possibly secondary to protracted vasoconstriction because of activation of the renin-angiotensin system, shear stress, and increased oxidative stress.24 We have previously shown that dyslipidemia alone may increase microvascular density,25 but its coexistence with renal ischemia exacerbates microvascular loss.26,27 Decreased microvascular density, in turn, interferes with the supply and delivery of oxygen and blood, precipitating tissue hypoxia and damage. This study shows that SW can alleviate or prevent microvascular loss, which may contribute to preservation of renal function. Low-energy SW generates mechanical forces that induce localized stress on cell membranes that resembles shear stress28 and exerts biologic effects,29 after which upregulation of angiogenic factors, including VEGF and eNOS, and activation of reparative mechanisms17,18 mediate microcirculatory repair. β1-Integrin is a cell surface adhesion receptor with an extracellular domain linked to the cytoskeleton, which permits transmission of mechanical forces generated by SW by modulating the paracellular signaling pathway.30 Its effect on the vasculature is achieved via its chief downstream regulator and signaling molecule FAK,31 which in turn, stimulates VEGF32 and endothelial survival.33 In addition, SW-induced upregulation of angiopoietin-1, which promotes microvascular maturation and stability, can further facilitate FAK activation34 to enhance angiogenesis. Indeed, upregulation of angiogenic factors in the ARAS kidney by SW parallels activation of mechanotransducers, which possibly accounts for improved oxygenation (blood oxygen level–dependent R2*) and downregulated expression of HIF-1α.
Notably, VEGF expression was not only coexpressed with mechanotransducers but also, similarly and selectively localized to proximal tubules, identifying them as an important site for VEGF production12,35 after SW treatment. The specific mechanism responsible for this selective upregulation of mechanotransducers and angiogenic factors needs to be further explored. The population of proximal tubules–derived regenerating cells expressing Oct-4 and KIM-1 increases in response to hypoxia or injury,36–39 but cellular regeneration might be blunted in ARAS because of vasoconstriction and oxidative stress. SW alleviated vasoconstriction by improving expression of eNOS and reducing oxidative stress, and it may thereby facilitate regenerative function and tissue repair as suggested by normalized expression of Oct-4 in ARAS and SW.
Interestingly, activation of β1-integrin signaling was only observed in ARAS and SW pigs but not in normal and SW pigs, suggesting greater responsiveness of the ARAS kidney to SW. Because β1-integrin in tubular epithelial cells can redistribute to the apical surface during ischemic insults,40 proximal tubular cells in ARAS might become more sensitive to SW–elicited mechanical forces.
Consequent to improved renal structure and oxygenation, SW improved stenotic kidney function. Because stenotic kidney RBF was less markedly affected, the improved GFR may be partly secondary to alleviated tubular injury and improved tubular-glomeruli feedback. After a more prolonged (6-week) ARAS, SW also decreased BP, restored stenotic kidney microcirculation, and improved its GFR, albeit less effectively that in ARAS and SW; it remained not significantly different from either normal or ARAS kidneys, possibly because tubular injury was not ameliorated. Furthermore, because RBF did not increase, we cannot exclude the possibility that the improvement in GFR was partly attributable to afferent vasoconstriction, the mechanism of which would need to be explored in future studies. Overall, the efficacy of SW is likely at least partly dependent on injury duration.
This study shows BP-lowering effects of SW in ARAS animals. Diminished renin release from the treated renal veins might have been secondary to improved renal perfusion. Because NE was no longer elevated in ARAS after SW, the neurohumoral pathway might be implicated41 in its BP-lowering effect. Additional studies are needed to evaluate this link. Moreover, restored microvasculature and eNOS may not only improve blood and oxygen delivery but also, lower BP by antagonizing angiotensin II activity, increasing nitric oxide availability and expression, and alleviating oxidative stress.
The low–energy SW regimen that we applied in the kidney exhibited a good safety profile as reported in the ischemic heart.42–44 Rather than potentially imposing tissue damage, like traditional lithotripsy, a 3-week low–energy SW regimen contrarily decreased proteinuria, attenuated a rise in Scr observed in ARAS, and increased stenotic kidney GFR. This finding in our clinically relevant large animals may increase its translational potential. In addition, low-energy SW can promote healing through direct anti–inflammatory properties in acute myocardial infarction,45 carotid artery angioplasty,46 and cutaneous burn injury,47 suggesting that it may be an effective measure to boost tissue recovery. Interestingly, in an ischemia-reperfusion model, ultrasound recently suppressed renal inflammation by splenic modulation.48 In our study, SW was selectively applied to the right kidney, and the spleen was unlikely affected by SW. Nevertheless, whether spleen stimulation by SW could facilitate renal function recovery deserves additional studies.
Our study is limited by the short duration of the disease, but the similarity of renal structure and function in our swine model to human kidneys increases the translational potential of our results. The temporal patterns of SW therapy on the microcirculation and its long-term protection of renal function need to be examined in longitudinal studies. The effects of low-energy SW on glomerular cells and their production of angiogenic factors also warrant additional study. We have used the settings implemented on our machine and a previously validated regimen.16,43 The optimal doses, energy levels, and timing of SW treatment in the ischemic kidney and other kidney diseases need to be defined in additional studies.
Low–energy extracorporeal SW treatment improved the ARAS kidney microvasculature, alleviated fibrosis, stabilized renal function, and lowered BP. Low–energy SW therapy may be an effective and powerful noninvasive strategy for treatment of chronic ischemic kidney disease and renovascular hypertension. However, its efficacy is likely at least partly dependent on the duration of preexisting renal injury.
Concise Methods
Animals and SW Treatments
This study was approved by the Institutional Animal Care and Use Committee. Twenty-six domestic female pigs (50–60 kg) were studied during 16 weeks of observation. Pigs were randomized to ARAS or normal without (ARAS and normal groups, n=7 each) or with SW treatment (ARAS and SW, n=7; normal and SW, n=5). Normal pigs were fed isocaloric diets of standard chow, and ARAS pigs were fed with a high-fat diet containing 2% cholesterol (Harlan Teklad, Madison, WI).49 All animals had free access to water.
RAS was induced after 6 weeks of diet by placing a local irritant coil in the right main renal artery, leading to gradual development of unilateral RAS as previously described.50 The degrees of stenosis were determined by renal angiography 6 weeks later. Three weeks after RAS induction, low–energy SW sessions were initiated and delivered biweekly for 3 consecutive weeks (a total of six sessions). Because the kidney and heart undergo similar processes of microvascular remodeling secondary to upstream vascular obstructions, we used protocols that had been successfully applied to the myocardium.43,51 Four weeks after completion of this regimen, renal hemodynamics and oxygenation were assessed in vivo. Animals were then euthanized, and their kidneys were harvested (Supplemental Figure 1A).
Omnispec Vetspec Model (spark voltage =10–24 kV; energy density =0.09 mJ/mm2; frequency =120 pulse/min; Medispec LTD, Germantown, MD) was used to deliver SW. An Acuson SC2000 Ultrasound System (Global Siemens Healthcare, Erlangen, Germany) was used to guide SW localization on the kidney. Pigs were laid prone, the skin of the back was shaved, and ultrasound gel was applied to ensure adequate conduction of the ultrasound wave and SW (Supplemental Figure 1B). The ultrasound probe was placed at the lateral aspect of the right/stenotic kidney along its long axis for coronal visualization, and the SW applicator was located perpendicularly above the kidney to distribute energy through the kidney along its short axis (Supplemental Figure 1, B–D). Because the whole kidney is subjected to ischemia distal to the stenosis, the entire kidney was treated with SW with regions evenly selected (Supplemental Figure 1D), with 200 rapid shots applied to each treatment zone.16
In Vivo Studies
BP and Renal Function
Single-kidney function, including RBF and GFR, were assessed in both the stenotic and contralateral kidneys using multidetector computed tomography (MDCT) as described previously.52 Briefly, 160 consecutive scans were performed after a central venous injection of iopamidol (0.5 ml kg−1 2 s−1). Then, MDCT images were reconstructed and displayed with the Analyze software package (Biomedical Imaging Resource; Mayo Clinic, Rochester, MN). For data analysis, regions of interest (ROIs) were selected from tomographic images from the aorta, renal cortex, and medulla to generate time-attenuation curves in each region and obtain measures of renal function.52 BP was measured in all animals using an arterial catheter during MDCT. Urine was collected via bladder puncture to determine protein excretion, and blood was collected from the inferior vena cava for creatinine. Plasma renin activity and NE release were measured in blood collected from veins draining stenotic/SW-treated kidneys.
Renal Oxygenation
Blood oxygen level–dependent magnetic resonance imaging (Signa Echo Speed; GE Medical Systems, Milwaukee, WI) scanning was performed 2 days before MDCT to assess intrarenal oxygenation (evaluated as R2*).53,54 For data analysis, ROIs were manually traced in the cortex and medulla on the 7-millisecond echo time images that give the best anatomic details in each experimental period. For each echo time, the software automatically computed the average of magnetic resonance signals within each ROI.
Animals were euthanized 3 days after in vivo studies using a lethal intravenous dose of sodium pentobarbital (100 mg/kg; Fatal Plus; Vortech Pharmaceuticals, Fort Washington, PA).55 The kidneys were removed using a retroperitoneal incision, and they were immediately dissected and prepared in ice cold normal saline for microcomputed tomography, frozen in liquid nitrogen (and maintained at −80°C), or preserved in formalin for tissue studies.
Ex Vivo Studies
Microvasculature
After the kidney was flushed, microfil MV122 (an intravascular contrast agent) was perfused into the stenotic kidney through a cannula ligated in the renal artery. Samples were prepared and scanned at 0.5° angular increments at 18-μm resolution, and images were analyzed as previously described.24,56 The spatial density of microvessels (defined as diameters <500 μm) in the inner and outer renal cortices was examined.57
Renal expression of the angiogenic factors VEGF (1:200; Santa Cruz Biotechnology, Santa Cruz, CA), angiopoietin-1 (1:200; Santa Cruz Biotechnology), and HIF-1α (1:1000; Abcam, Inc., Cambridge, MA) was examined by Western blotting in the kidney. Immunoreactivity of eNOS (1:50; Abcam, Inc.) was measured by immunofluorescence staining and Western blotting. Expression of the mechanotransducers β1-integrin (1:1000; Cell Signaling Technology, Danvers, MA) and downstream FAK (1:50; Cell Signaling Technology) was assessed by Western blotting and immunofluorescence staining, respectively. FAK and VEGF were both costained with the proximal and distal renal tubular markers Phaseous vulgaris agglutinin and peanut agglutinin38 to localize their expression.
Oxidative Stress, Fibrosis, and Tissue Repair
DHE staining was performed to assess renal production of superoxide anion. Fibrosis was evaluated by trichrome staining. Tubular injury was scored in hematoxylin and eosin slides on a 1–5 scale (1, <10%; 2, 10%–25%; 3, 26%–50%; 4, 51%–75%; and 5, >75% injury) on the basis of tubular dilation, atrophy, cast formation, sloughing tubular endothelial cells, or thickening of basement membrane as previously described.58 Expression of TGF-β and tissue inhibitor of metalloproteinases 1 (both 1:200; Santa Cruz Biotechnology) was examined by Western blotting. Renal levels of SDF-1β (MBS735811 ELISA; MyBioSource) and SCF (MBS2020518 ELISA; MyBioSource), which mobilize endogenous repair mechanisms,59 were measured in the stenotic renal vein and inferior vena cava. Expression of regenerating renal cell markers Oct-4 and KIM-1 that are upregulated in response to hypoxia and injury36–39 were detected by Western blotting.
Effects of SW in Prolonged ARAS
Of eight additional ARAS pigs, SW was started in four pigs after 6 weeks of RAS (prolonged ARAS and SW); the four other pigs served as controls. BP and Scr were monitored during the 3-week regimen in all pigs. Stenotic RBF and GFR in prolonged ARAS and SW pigs were quantified before and after SW. Microvascular density, oxygenation levels, fibrosis, and tubular injury score were also assessed.
Safety of SW
Short-term safety of low-energy SW was assessed in two normal pigs receiving a single SW session. Heart rate was continuously monitored using an electrocardiogram monitor throughout the experiment, and BP was recorded every 15 minutes using a cuff placed on the front limb. Bladder urine and stenotic renal vein blood were collected before and 2 hours after SW to examine AKI indices, including microscopic hematuria, urine protein, and urine and plasma NGAL. Single-kidney function was assessed before and after SW by MDCT for GFR, perfusion, and RBF. Pigs were then euthanized, and the treated kidneys were immediately removed to inspect histologically for intrarenal hemorrhage and tubular injury.
The safety of the 3-week SW regimen was also tested in normal and SW pigs and ARAS and SW pigs. Electrocardiogram and BP were monitored during each SW session as described above. AKI markers were measured before, weekly during, and 4 weeks after completion of SW treatment.
Statistical Analyses
Continuous data were expressed as means±SEMs. Two-way ANOVA was used to analyze the effects of ARAS and SW as separate factors and their interactions followed by Tukey test as appropriate. One-way ANOVA was used for comparison among all groups to identify differences from prolonged ARAS and SW. Comparisons within groups were performed using repeated measures ANOVA followed by paired t test. P<0.05 was considered statistically significant.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank Medispec LTD for generously allowing the use of the shockwave machine.
This research was partly supported by National Institutes of Health grants HL121561, HL123160, DK73608, DK104273, and DK102325 and American Heart Association grant 13POST16810064.
The vendor (Medispec LTD) was not involved in data collection or analysis.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2015060704/-/DCSupplemental.
References
- 1.Safian RD, Textor SC: Renal-artery stenosis. N Engl J Med 344: 431–442, 2001 [DOI] [PubMed] [Google Scholar]
- 2.Lerman L, Textor SC: Pathophysiology of ischemic nephropathy. Urol Clin North Am 28: 793–803, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Edwards MS, Craven TE, Burke GL, Dean RH, Hansen KJ: Renovascular disease and the risk of adverse coronary events in the elderly: A prospective, population-based study. Arch Intern Med 165: 207–213, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Cooper CJ, Murphy TP, Cutlip DE, Jamerson K, Henrich W, Reid DM, Cohen DJ, Matsumoto AH, Steffes M, Jaff MR, Prince MR, Lewis EF, Tuttle KR, Shapiro JI, Rundback JH, Massaro JM, D’Agostino RB Sr., Dworkin LD; CORAL Investigators : Stenting and medical therapy for atherosclerotic renal-artery stenosis. N Engl J Med 370: 13–22, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lerman LO, Textor SC, Grande JP: Mechanisms of tissue injury in renal artery stenosis: Ischemia and beyond. Prog Cardiovasc Dis 52: 196–203, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Textor SC: Issues in renovascular disease and ischemic nephropathy: Beyond ASTRAL. Curr Opin Nephrol Hypertens 20: 139–145, 2011 [DOI] [PubMed] [Google Scholar]
- 7.Johns EJ: Inflammation: The underlying foe in renovascular hypertension? J Hypertens 27: 1964–1965, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Vaidya VS, Ford GM, Waikar SS, Wang Y, Clement MB, Ramirez V, Glaab WE, Troth SP, Sistare FD, Prozialeck WC, Edwards JR, Bobadilla NA, Mefferd SC, Bonventre JV: A rapid urine test for early detection of kidney injury. Kidney Int 76: 108–114, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eirin A, Zhu XY, Urbieta-Caceres VH, Grande JP, Lerman A, Textor SC, Lerman LO: Persistent kidney dysfunction in swine renal artery stenosis correlates with outer cortical microvascular remodeling. Am J Physiol Renal Physiol 300: F1394–F1401, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Favreau F, Zhu XY, Krier JD, Lin J, Warner L, Textor SC, Lerman LO: Revascularization of swine renal artery stenosis improves renal function but not the changes in vascular structure. Kidney Int 78: 1110–1118, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brodsky SV, Yamamoto T, Tada T, Kim B, Chen J, Kajiya F, Goligorsky MS: Endothelial dysfunction in ischemic acute renal failure: Rescue by transplanted endothelial cells. Am J Physiol Renal Physiol 282: F1140–F1149, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Schrijvers BF, Flyvbjerg A, De Vriese AS: The role of vascular endothelial growth factor (VEGF) in renal pathophysiology. Kidney Int 65: 2003–2017, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Villegas G, Lange-Sperandio B, Tufro A: Autocrine and paracrine functions of vascular endothelial growth factor (VEGF) in renal tubular epithelial cells. Kidney Int 67: 449–457, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Lindenmeyer MT, Kretzler M, Boucherot A, Berra S, Yasuda Y, Henger A, Eichinger F, Gaiser S, Schmid H, Rastaldi MP, Schrier RW, Schlöndorff D, Cohen CD: Interstitial vascular rarefaction and reduced VEGF-A expression in human diabetic nephropathy. J Am Soc Nephrol 18: 1765–1776, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Chade AR, Bentley MD, Zhu X, Rodriguez-Porcel M, Niemeyer S, Amores-Arriaga B, Napoli C, Ritman EL, Lerman A, Lerman LO: Antioxidant intervention prevents renal neovascularization in hypercholesterolemic pigs. J Am Soc Nephrol 15: 1816–1825, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Fukumoto Y, Ito A, Uwatoku T, Matoba T, Kishi T, Tanaka H, Takeshita A, Sunagawa K, Shimokawa H: Extracorporeal cardiac shock wave therapy ameliorates myocardial ischemia in patients with severe coronary artery disease. Coron Artery Dis 17: 63–70, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Wang CJ, Huang KE, Sun YC, Yang YJ, Ko JY, Weng LH, Wang FS: VEGF modulates angiogenesis and osteogenesis in shockwave-promoted fracture healing in rabbits. J Surg Res 171: 114–119, 2011 [DOI] [PubMed] [Google Scholar]
- 18.Aicher A, Heeschen C, Sasaki K, Urbich C, Zeiher AM, Dimmeler S: Low-energy shock wave for enhancing recruitment of endothelial progenitor cells: A new modality to increase efficacy of cell therapy in chronic hind limb ischemia. Circulation 114: 2823–2830, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Luu NT, Glen KE, Egginton S, Rainger GE, Nash GB: Integrin-substrate interactions underlying shear-induced inhibition of the inflammatory response of endothelial cells. Thromb Haemost 109: 298–308, 2013 [DOI] [PubMed] [Google Scholar]
- 20.Zebda N, Dubrovskyi O, Birukov KG: Focal adhesion kinase regulation of mechanotransduction and its impact on endothelial cell functions. Microvasc Res 83: 71–81, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Y, Guo T, Cai HY, Ma TK, Tao SM, Sun S, Chen MQ, Gu Y, Pang JH, Xiao JM, Yang XY, Yang C: Cardiac shock wave therapy reduces angina and improves myocardial function in patients with refractory coronary artery disease. Clin Cardiol 33: 693–699, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kikuchi Y, Ito K, Ito Y, Shiroto T, Tsuburaya R, Aizawa K, Hao K, Fukumoto Y, Takahashi J, Takeda M, Nakayama M, Yasuda S, Kuriyama S, Tsuji I, Shimokawa H: Double-blind and placebo-controlled study of the effectiveness and safety of extracorporeal cardiac shock wave therapy for severe angina pectoris. Circ J 74: 589–591, 2010 [DOI] [PubMed] [Google Scholar]
- 23.Urbieta Caceres VH, Lin J, Zhu XY, Favreau FD, Gibson ME, Crane JA, Lerman A, Lerman LO: Early experimental hypertension preserves the myocardial microvasculature but aggravates cardiac injury distal to chronic coronary artery obstruction. Am J Physiol Heart Circ Physiol 300: H693–H701, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bentley MD, Rodriguez-Porcel M, Lerman A, Sarafov MH, Romero JC, Pelaez LI, Grande JP, Ritman EL, Lerman LO: Enhanced renal cortical vascularization in experimental hypercholesterolemia. Kidney Int 61: 1056–1063, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Li ZL, Woollard JR, Ebrahimi B, Crane JA, Jordan KL, Lerman A, Wang SM, Lerman LO: Transition from obesity to metabolic syndrome is associated with altered myocardial autophagy and apoptosis. Arterioscler Thromb Vasc Biol 32: 1132–1141, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang X, Li ZL, Woollard JR, Eirin A, Ebrahimi B, Crane JA, Zhu XY, Pawar AS, Krier JD, Jordan KL, Tang H, Textor SC, Lerman A, Lerman LO: Obesity-metabolic derangement preserves hemodynamics but promotes intrarenal adiposity and macrophage infiltration in swine renovascular disease. Am J Physiol Renal Physiol 305: F265–F276, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun D, Eirin A, Ebrahimi B, Textor SC, Lerman A, Lerman LO: Early atherosclerosis aggravates renal microvascular loss and fibrosis in swine renal artery stenosis. J Am Soc Hypertens 10: 325–335, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maisonhaute E, Prado C, White PC, Compton RG: Surface acoustic cavitation understood via nanosecond electrochemistry. Part III: Shear stress in ultrasonic cleaning. Ultrason Sonochem 9: 297–303, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Sun CK, Shao PL, Wang CJ, Yip HK: Study of vascular injuries using endothelial denudation model and the therapeutic application of shock wave: A review. Am J Transl Res 3: 259–268, 2011 [PMC free article] [PubMed] [Google Scholar]
- 30.Elias BC, Mathew S, Srichai MB, Palamuttam R, Bulus N, Mernaugh G, Singh AB, Sanders CR, Harris RC, Pozzi A, Zent R: The integrin β1 subunit regulates paracellular permeability of kidney proximal tubule cells. J Biol Chem 289: 8532–8544, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eke I, Deuse Y, Hehlgans S, Gurtner K, Krause M, Baumann M, Shevchenko A, Sandfort V, Cordes N: β₁Integrin/FAK/cortactin signaling is essential for human head and neck cancer resistance to radiotherapy. J Clin Invest 122: 1529–1540, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen XL, Nam JO, Jean C, Lawson C, Walsh CT, Goka E, Lim ST, Tomar A, Tancioni I, Uryu S, Guan JL, Acevedo LM, Weis SM, Cheresh DA, Schlaepfer DD: VEGF-induced vascular permeability is mediated by FAK. Dev Cell 22: 146–157, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Braren R, Hu H, Kim YH, Beggs HE, Reichardt LF, Wang R: Endothelial FAK is essential for vascular network stability, cell survival, and lamellipodial formation. J Cell Biol 172: 151–162, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cascone I, Napione L, Maniero F, Serini G, Bussolino F: Stable interaction between alpha5beta1 integrin and Tie2 tyrosine kinase receptor regulates endothelial cell response to Ang-1. J Cell Biol 170: 993–1004, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim BS, Chen J, Weinstein T, Noiri E, Goligorsky MS: VEGF expression in hypoxia and hyperglycemia: Reciprocal effect on branching angiogenesis in epithelial-endothelial co-cultures. J Am Soc Nephrol 13: 2027–2036, 2002 [DOI] [PubMed] [Google Scholar]
- 36.Smeets B, Boor P, Dijkman H, Sharma SV, Jirak P, Mooren F, Berger K, Bornemann J, Gelman IH, Floege J, van der Vlag J, Wetzels JF, Moeller MJ: Proximal tubular cells contain a phenotypically distinct, scattered cell population involved in tubular regeneration. J Pathol 229: 645–659, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Berger K, Bangen JM, Hammerich L, Liedtke C, Floege J, Smeets B, Moeller MJ: Origin of regenerating tubular cells after acute kidney injury. Proc Natl Acad Sci U S A 111: 1533–1538, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gupta S, Verfaillie C, Chmielewski D, Kren S, Eidman K, Connaire J, Heremans Y, Lund T, Blackstad M, Jiang Y, Luttun A, Rosenberg ME: Isolation and characterization of kidney-derived stem cells. J Am Soc Nephrol 17: 3028–3040, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Bussolati B, Moggio A, Collino F, Aghemo G, D’Armento G, Grange C, Camussi G: Hypoxia modulates the undifferentiated phenotype of human renal inner medullary CD133+ progenitors through Oct4/miR-145 balance. Am J Physiol Renal Physiol 302: F116–F128, 2012 [DOI] [PubMed] [Google Scholar]
- 40.Zuk A, Bonventre JV, Brown D, Matlin KS: Polarity, integrin, and extracellular matrix dynamics in the postischemic rat kidney. Am J Physiol 275: C711–C731, 1998 [DOI] [PubMed] [Google Scholar]
- 41.Grisk O, Rettig R: Interactions between the sympathetic nervous system and the kidneys in arterial hypertension. Cardiovasc Res 61: 238–246, 2004 [DOI] [PubMed] [Google Scholar]
- 42.Nishida T, Shimokawa H, Oi K, Tatewaki H, Uwatoku T, Abe K, Matsumoto Y, Kajihara N, Eto M, Matsuda T, Yasui H, Takeshita A, Sunagawa K: Extracorporeal cardiac shock wave therapy markedly ameliorates ischemia-induced myocardial dysfunction in pigs in vivo. Circulation 110: 3055–3061, 2004 [DOI] [PubMed] [Google Scholar]
- 43.Cassar A, Prasad M, Rodriguez-Porcel M, Reeder GS, Karia D, DeMaria AN, Lerman A: Safety and efficacy of extracorporeal shock wave myocardial revascularization therapy for refractory angina pectoris. Mayo Clin Proc 89: 346–354, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Prasad M, Wan Ahmad WA, Sukmawan R, Magsombol EB, Cassar A, Vinshtok Y, Ismail MD, Mahmood Zuhdi AS, Locnen SA, Jimenez R, Callleja H, Lerman A: Extracorporeal shockwave myocardial therapy is efficacious in improving symptoms in patients with refractory angina pectoris--a multicenter study. Coron Artery Dis 26: 194–200, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Abe Y, Ito K, Hao K, Shindo T, Ogata T, Kagaya Y, Kurosawa R, Nishimiya K, Satoh K, Miyata S, Kawakami K, Shimokawa H: Extracorporeal low-energy shock-wave therapy exerts anti-inflammatory effects in a rat model of acute myocardial infarction. Circ J 78: 2915–2925, 2014 [DOI] [PubMed] [Google Scholar]
- 46.Shao PL, Chiu CC, Yuen CM, Chua S, Chang LT, Sheu JJ, Sun CK, Wu CJ, Wang CJ, Yip HK: Shock wave therapy effectively attenuates inflammation in rat carotid artery following endothelial denudation by balloon catheter. Cardiology 115: 130–144, 2010 [DOI] [PubMed] [Google Scholar]
- 47.Davis TA, Stojadinovic A, Anam K, Amare M, Naik S, Peoples GE, Tadaki D, Elster EA: Extracorporeal shock wave therapy suppresses the early proinflammatory immune response to a severe cutaneous burn injury. Int Wound J 6: 11–21, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gigliotti JC, Huang L, Ye H, Bajwa A, Chattrabhuti K, Lee S, Klibanov AL, Kalantari K, Rosin DL, Okusa MD: Ultrasound prevents renal ischemia-reperfusion injury by stimulating the splenic cholinergic anti-inflammatory pathway. J Am Soc Nephrol 24: 1451–1460, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stulak JM, Lerman A, Porcel MR, Caccitolo JA, Romero JC, Schaff HV, Napoli C, Lerman LO: Renal vascular function in hypercholesterolemia is preserved by chronic antioxidant supplementation. J Am Soc Nephrol 12: 1882–1891, 2001 [DOI] [PubMed] [Google Scholar]
- 50.Lerman LO, Schwartz RS, Grande JP, Sheedy PD, Romero JC: Noninvasive evaluation of a novel swine model of renal artery stenosis. J Am Soc Nephrol 10: 1455–1465, 1999 [DOI] [PubMed] [Google Scholar]
- 51.Zuozienė G, Laucevičius A, Leibowitz D: Extracorporeal shockwave myocardial revascularization improves clinical symptoms and left ventricular function in patients with refractory angina. Coron Artery Dis 23: 62–67, 2012 [DOI] [PubMed] [Google Scholar]
- 52.Daghini E, Primak AN, Chade AR, Krier JD, Zhu XY, Ritman EL, McCollough CH, Lerman LO: Assessment of renal hemodynamics and function in pigs with 64-section multidetector CT: Comparison with electron-beam CT. Radiology 243: 405–412, 2007 [DOI] [PubMed] [Google Scholar]
- 53.Warner L, Glockner JF, Woollard J, Textor SC, Romero JC, Lerman LO: Determinations of renal cortical and medullary oxygenation using blood oxygen level-dependent magnetic resonance imaging and selective diuretics. Invest Radiol 46: 41–47, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ebrahimi B, Gloviczki M, Woollard JR, Crane JA, Textor SC, Lerman LO: Compartmental analysis of renal BOLD MRI data: Introduction and validation. Invest Radiol 47: 175–182, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang X, Li ZL, Crane JA, Jordan KL, Pawar AS, Textor SC, Lerman A, Lerman LO: Valsartan regulates myocardial autophagy and mitochondrial turnover in experimental hypertension. Hypertension 64: 87–93, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhu XY, Rodriguez-Porcel M, Bentley MD, Chade AR, Sica V, Napoli C, Caplice N, Ritman EL, Lerman A, Lerman LO: Antioxidant intervention attenuates myocardial neovascularization in hypercholesterolemia. Circulation 109: 2109–2115, 2004 [DOI] [PubMed] [Google Scholar]
- 57.Zhang X, Eirin A, Li Z-L, Crane JA, Krier JD, Ebrahimi B, Pawar AS, Zhu X-Y, Tang H, Jordan KL, Lerman A, Textor SC, Lerman LO: Angiotensin receptor blockade has protective effects on the poststenotic porcine kidney. Kidney Int 84: 767–775, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Eirin A, Li Z, Zhang X, Krier JD, Woollard JR, Zhu XY, Tang H, Herrmann SM, Lerman A, Textor SC, Lerman LO: A mitochondrial permeability transition pore inhibitor improves renal outcomes after revascularization in experimental atherosclerotic renal artery stenosis. Hypertension 60: 1242–1249, 2012 [DOI] [PubMed] [Google Scholar]
- 59.Yamaguchi J, Kusano KF, Masuo O, Kawamoto A, Silver M, Murasawa S, Bosch-Marce M, Masuda H, Losordo DW, Isner JM, Asahara T: Stromal cell-derived factor-1 effects on ex vivo expanded endothelial progenitor cell recruitment for ischemic neovascularization. Circulation 107: 1322–1328, 2003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.