Abstract
Belatacept is a biologic that targets CD80/86 and prevents its interaction with CD28 and its alternative ligand, cytotoxic T lymphocyte antigen 4 (CTLA-4). Clinical experience in kidney transplantation has revealed a high incidence of rejection with belatacept, especially with intensive regimens, suggesting that blocking CTLA-4 is deleterious. We performed a head to head assessment of FR104 (n=5), a selective pegylated Fab′ antibody fragment antagonist of CD28 that does not block the CTLA-4 pathway, and belatacept (n=5) in kidney allotransplantation in baboons. The biologics were supplemented with an initial 1-month treatment with low-dose tacrolimus. In cases of acute rejection, animals also received steroids. In the belatacept group, four of five recipients developed severe, steroid–resistant acute cellular rejection, whereas FR104-treated animals did not. Assessment of regulatory T cell–specific demethylated region methylation status in 1-month biopsy samples revealed a nonsignificant trend for higher regulatory T cell frequencies in FR104-treated animals. Transcriptional analysis did not reveal significant differences in Th17 cytokines but did reveal higher levels of IL-21, the main cytokine secreted by CD4 T follicular helper (Tfh) cells, in belatacept-treated animals. In vitro, FR104 controlled the proliferative response of human preexisting Tfh cells more efficiently than belatacept. In mice, selective CD28 blockade also controlled Tfh memory cell responses to KLH stimulation more efficiently than CD80/86 blockade. Our data reveal that selective CD28 blockade and belatacept exert different effects on mechanisms of renal allograft rejection, particularly at the level of Tfh cell stimulation.
Keywords: kidney transplantation, immunology, immunosuppression, lymphocytes, acute rejection
Since the successful introduction of calcineurin inhibitors (CNIs), improvements in long–term graft survival rates in kidney transplantation (KT) have been slow, a situation mainly caused by chronic allograft dysfunction. Although CNI nephrotoxicity has been implicated in chronic allograft dysfunction, recent advances show a role for chronic antibody–mediated rejection (ABMR). It is, therefore, critical to develop novel therapeutic strategies that minimize or avoid the use of CNIs that are also more effective in controlling humoral responses. This has been partially achieved by the use of belatacept (Bristol-Myers Squibb, Princeton, NJ; LEA29Y, a high-affinity variant of cytotoxic T lymphocyte Antigen 4 [CTLA-4] -Ig), a CD80–86 antagonist that prevents interaction with CD28 and its alternative ligand CTLA-4.1 Belatacept improved long–term graft function and was associated with a lower incidence of donor-specific antibody (DSA) compared with CNI.2 In a preclinical model, it prevented ABMR by inhibiting primary T follicular helper (Tfh) cell responses.3 However, belatacept is associated with a high incidence of acute rejection (AR), suggesting that blocking CTLA-4-CD80–86 interactions might be deleterious.
We have developed FR104 (Effimune), a humanized pegylated Fab′ antibody antagonist of CD28 that is devoid of agonist activity.4 In synergy with low doses of CNI, it prolongs renal allograft survival in nonhuman primates.4,5 Preserving CTLA-4 regulation while selectively antagonizing CD28 may be FR104’s main advantage as a replacement for CD80–86 antagonists. Regulation via CTLA-4 is thought to occur through both intrinsic inhibitory signaling and extrinsic actions, which are mainly through regulatory T cell (Treg) activities.6,7 Thus, selective CD28 blockade has potential advantages over belatacept, but a direct comparison in a preclinical KT model has not previously been performed.
In this study, we compared FR104 against belatacept maintenance therapy in a model of KT in primates. In contrast to belatacept-treated animals, which exhibited irreversible steroid–resistant AR, only 40% of FR104-treated animals developed AR, which was not steroid resistant. Assessment of regulatory T cell–specific demethylated region (TSDR) methylation status of 1-month kidney biopsy samples revealed a trend toward a stronger Treg signal in FR104-treated animals. Furthermore, the main cytokine secreted by Tfh, IL-21, was lower in these animals, suggesting a better response control of preexisting Tfh cells, a feature that we confirmed both in vitro in human cells and in an experimental mouse model.
Results
Belatacept and FR104 Have Comparable Pharmacodynamics in Baboons
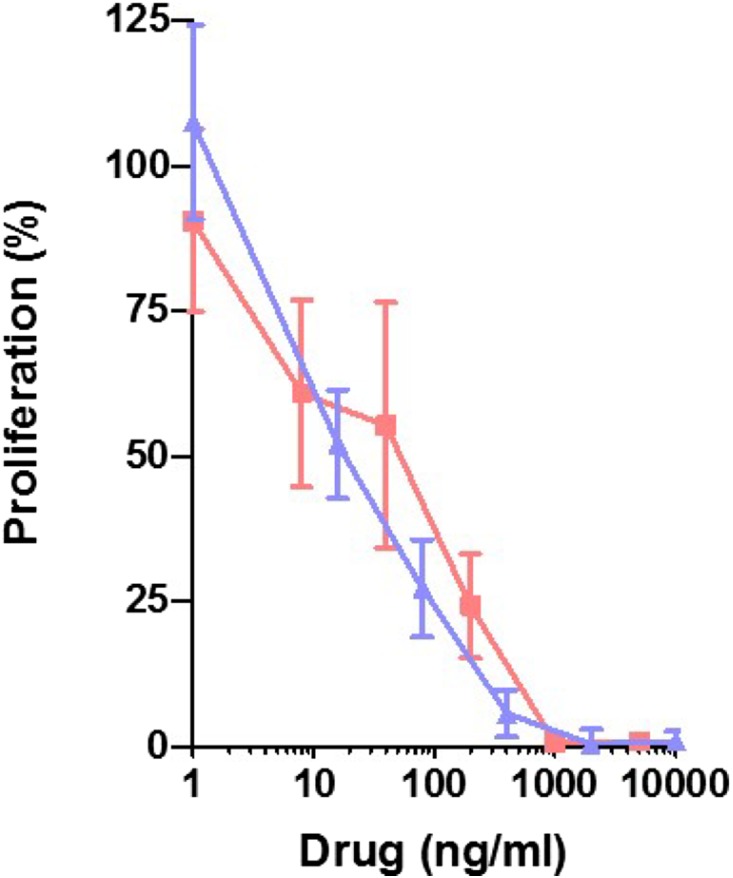
FR104 and belatacept inhibited baboon T lymphocyte proliferation similarly in a mixed lymphocyte reaction (Figure 1). In vivo, the trough concentrations measured in the two groups were not different, and they were above the ED50 of FR104 (0.3 μg/ml4; 0.07 μg/ml for belatacept) as measured by a flow cytometry assay (Supplemental Figure 1).
Figure 1.
FR104 and belatacept equally inhibit in vitro T cell proliferation. FR104 (blue triangles) and belatacept (red squares) dose response of baboon (n=4) PBMC mixed lymphocyte proliferation assays at day 5 assessed by 3H-thyminidine uptake.
FR104 Selectively Blocks CD28 Signaling and Prevents Steroid–Resistant Allograft Rejection
We designed a 1-year therapeutic protocol for KT in baboons, allowing direct comparison of selective CD28 blockade with FR104 against nonselective CD80–86 blockade with belatacept. To prevent early AR, animals also received a 1-month course of low-dose tacrolimus. In cases of biopsy-proven AR, animals received steroid boluses.
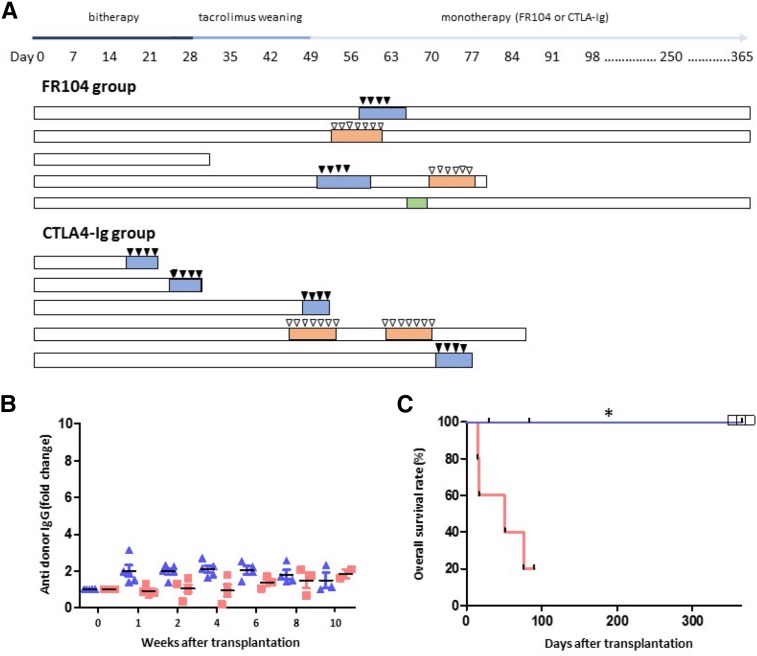
In the FR104 group (n=5), two animals developed AR (days 51 and 58), which was reversed after steroid administration. Two animals were lost because of anesthetic complications or septicemia. At 1 year post-transplantation, the three remaining FR104-treated animals exhibited stable kidney function. In the belatacept group (n=5), four recipients developed steroid-resistant AR during or after tacrolimus weaning (days 17, 28, 49, and 76). The fifth animal was lost because of pyelonephritis (Figure 2, A and C).
Figure 2.
FR104 Prevents Steroid–Resistant Allograft Rejection in contrast to belatacept therapy. (A) Timeline of immunosuppressive regimens, clinical interventions, and outcomes for FR104- and belatacept-treated groups. Highlighted rectangles represent acute renal failure episodes (blue indicates caused by rejection, red indicates caused by infection, and green indicates caused by obstruction). Black triangles represent steroid bolus, and white triangles indicate antibiotics use. (B) IgG DSAs as detected by flow cytometry after transplantation in baboons treated with FR104 (blue triangles) or belatacept (red squares). (C) Kaplan–Meier plot of overall survival for baboons treated with 1 month of low-dose tacrolimus and chronic FR104 (blue line; n=5) or 1 month of low-dose tacrolimus and chronic belatacept (red line; n=5). White squares represent animals still alive 1 year post-transplantation. Deaths by causes other than rejection have been censored, and the black marks represent recipient deaths. Survival time was evaluated by a log rank test, *P<0.05.
None of the animals exhibited DSAs (as assessed by flow cytometry) (Figure 2B). In both groups, conventional pathologic examination revealed T cell–mediated rejection (TCMR) ranging from 1A to 1B as defined by the Banff classification,8 except for one belatacept recipient (grade 2A), which exhibited an additional vascular injury without detectable C4d staining. Interestingly, 1-month protocol biopsies revealed a significant interstitial infiltrate composed primarily of CD3+ T cells in all animals, regardless of their treatment. Immunohistochemistry did not reveal differences in B lymphocyte or major T lymphocyte populations (data not shown).
FR104-Treated Recipients Show a Trend toward a Higher Treg Graft Infiltration
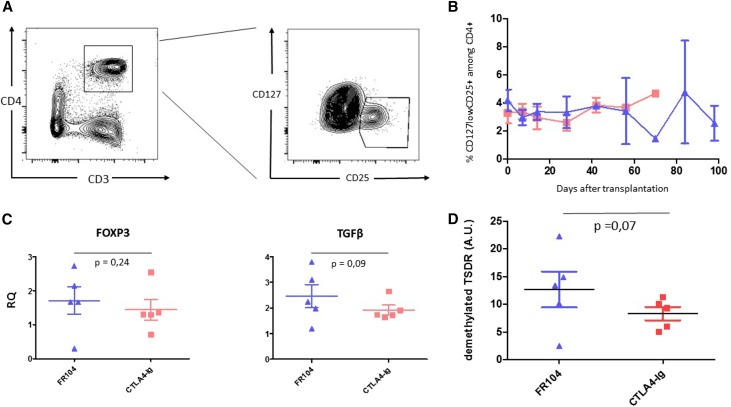
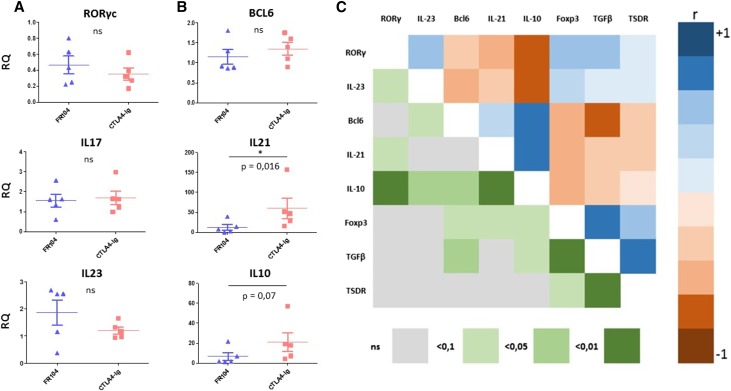
To investigate why FR104 and belatacept show differential rejection profiles, we firstly explored CTLA-4 extrinsic function through Tregs. No significant differences in the level of blood CD25hi/CD127lowCD4+ Tregs were observed (Figure 3, A and B). Similarly, no differences in Foxp3 expression as assessed by quantitative real–time PCR on 1-month protocol biopsies (or earlier in cases of rejection) were noted between the two groups (P=0.24) (Figure 3C). However, TSDR methylation analysis revealed a trend toward a stronger Treg signal in FR104-treated animals (P=0.07) (Figure 3D). In addition, we observed a statistically nonsignificant increase in TGFβ gene expression in the FR104-treated recipients (P=0.09) (Figure 3C), possibly related to the CTLA-4 extrinsic function on this inhibitory cytokine.9
Figure 3.
Assessment of TSDR methylation status reveal a nonsignificant trend for higher regulatory T cell frequencies in FR104-treated animals. (A) Flow cytometry contour plot showing gates used to assess percentage of CD4+CD25hiCD127low Treg cells. (B) Kinetics of Treg cells post-transplantation in FR104-treated recipients (blue triangles; n=5) and belatacept-treated recipients (red squares; n=5). (C) Quantitative real–time PCR measurement of Foxp3 and TGFβ gene expression in 1-month protocol biopsies or earlier biopsies in cases of rejection from animals treated with FR104 (blue triangles; n=5) or belatacept (red squares; n=5). (D) TSDR analysis in 1-month protocol biopsies or earlier biopsies in cases of rejection from animals treated with FR104 ((blue triangles; n=5) or belatacept (red squares; n=5) ; data are means±SEMs. Graph shows gene expression relative to HPRT; data are means±SEMs.
No Difference in CD28–Negative T Cells
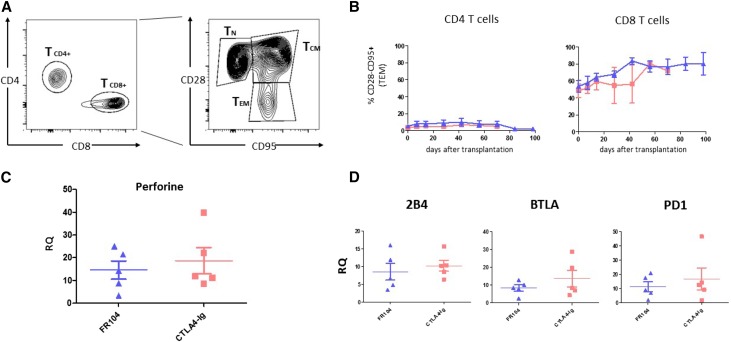
We investigated other T cell subsets suspected of being involved in belatacept-resistant rejection, in which turning off the CTLA-4 intrinsic inhibitory signaling can be deleterious. We monitored peripheral blood CD28–negative T cells, especially CD8+ cells, which are usually regarded as highly cytotoxic. No difference between the two groups was noted (Figure 4, A and B). Furthermore, assessment of cytotoxic function by measurement of perforin gene expression on 1-month biopsies showed no differences (Figure 4C). We also measured no difference in the expression of the coinhibitory receptor 2B4 gene expression, which is reportedly higher after selective CD28 blockade10 in rodents, or other PD1 and BTLA coinhibitory receptors (Figure 4D).
Figure 4.
CD28 negative T cells and the co-inhibitory receptor 2B4 are not differentially regulated between the FR104 group and the belatacept group. (A) Flow cytometry contour plot showing gates used to assess percentage of naïve T cells (TNs; CD28+CD95−), central memory T cells (TCMs; CD28+CD95+), and effector memory T cells (TEMs; CD28−CD95+) among CD4+ and CD8+. (B) Kinetics of the CD4+ and CD8+ compartments of TEMs post-transplantation in FR104-treated recipients (blue triangles; n=5) and belatacept-treated recipients (red squares; n=5). (C and D) Quantitative real–time PCR measurement of gene expression in 1-month protocol biopsies (or earlier in cases of rejection) from animals treated with FR104 (blue triangles; n=5) or belatacept (red squares; n=5). Graph shows gene expression relative to HPRT; data are means±SEMs.
Expression of IL-21 but Not Th17 Genes Is Higher in Belatacept 1-Month Biopsies Compared with FR104
Other than classic cytotoxic CD8 T cells, Th17–polarized T cells have been implicated in belatacept-resistant rejections.11,12 No difference in RORγc, IL-17, or IL-23 expression was observed on the 1-month biopsies (Figure 5A). In contrast, the main Tfh cell cytokine transcripts IL-21 (P=0.02) and to a lesser extent, IL-10 (P=0.07) were higher in belatacept recipients compared with FR104-treated animals (Figure 5B). We next analyzed the data according to both clinical status and treatment (Supplemental Figure 2). In biopsies without rejection, IL-21 gene expression was significantly higher in belatacept-treated animals (P=0.02), suggesting a differential effect between both drugs and not only the rejection process per se. Paired correlation analysis revealed three groups of positively related genes: a Th17 group (RORγc and IL-23), a Tfh group (Bcl6, IL-21, and IL-10), and a Treg group (Foxp3, TGFβ, and TSDR). Of note, there was an indication of a negative correlation between the Th17 and Tfh groups and between the latter and the Treg group (Figure 5C).
Figure 5.
The IL-21 gene expression but not the Th17 gene expression signature is increased in belatacept-treated recipients. (A and B) Quantitative PCR measurement of mRNA expression on 1-month protocol biopsies (or earlier in cases of rejection) from animals treated with FR104 (blue triangles; n=5) or belatacept (red squares; n=5). Gene expression is relative to HPRT. Data are means±SEMs. A Mann–Whitney nonparametric test was used, and P values <0.05 were considered significant. (C) Pairwise correlation analysis to determine the direction and strength of the linear relationships between genes. The upper right of the matrix is the color map of correlations. Bright colors indicate the pairs of variables closely related using the Spearman rank correlation coefficient (r), and faded colors encode for decreasing r values. The lower left of the matrix is the color map of P values.
The overall increase of Tfh-related genes in the belatacept-treated animals supports the involvement of alloreactive Tfh cells in allograft rejection in our protocol.
Graft-Infiltrating Lymphocytes Include Tfh-Like Cells
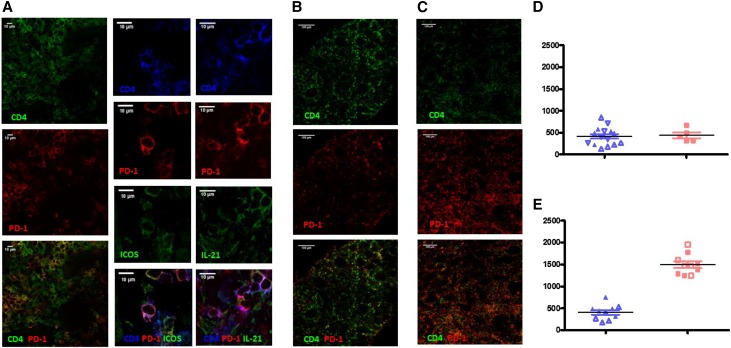
Tfh cells are normally located in the B cell zones of lymph node (LN) follicles and express high levels of CXCR5, PD1, and ICOS.13 In baboons, such as in humans and macaques,14 these CD4+CXCR5hi/PD1hi cells are restricted to the LN (Supplemental Figure 3A). By immunochemistry, we showed that only CD4+ T cells located in the B follicle (i.e., Tfh cells) were both PD1 and ICOS positive (Supplemental Figure 3B). We observed PD1–positive CD4+ T cells similar to LN Tfh cells on all graft biopsy samples. Moreover, most of these cells expressed ICOS and IL-21 (Figure 6A). Enumeration of CD4+PD1+ cells (per millimeter2) showed no difference between either group in protocol biopsies (Figure 6E). In rejection biopsies, we observed three times more CD4+PD1+ cells in belatacept-treated animals compared with FR104-treated animals (1493/mm2 versus 408/mm2; n=2) (Figure 6, B, C, and E).
Figure 6.
Tfh–like graft infiltrating lymphocytes were elevated in biopsies from belatacept-treated animals. (A) Immunostaining of a protocol biopsy showing cells with a Tfh-like phenotype (CD4+PD1+ T cells coexpressing ICOS and IL-21). (B) Representative images of CD4 and PD1 staining in rejection biopsies of FR104-treated animals. (C) Representative pictures of CD4 and PD1 staining in rejection biopsies of FR104-treated animals. (D) Number of CD4+PD1+ cells per millimeter2 in protocol biopsies in FR104- (blue; n=2) and belatacept-treated animals (red; n=1); each point represents an area (five per biopsy), and each symbol is an animal. (E) Number of CD4+PD1+ cells per millimeter2 in rejection biopsies of FR104- (blue; n=2) and belatacept-treated animals (red; n=2), and each point represents an area (five per biopsy) and each symbol is an animal.
Because the observed differences in IL-21 gene expression between the groups could be potentially explained by differing control of these infiltrating Tfh–like cells, we investigated Tfh sensitivity to both drugs in an in vitro and alternative in vivo model.
Selective CD28 Blockade Is More Effective in Controlling Proliferation of Human Primed Tfh Cells In Vitro
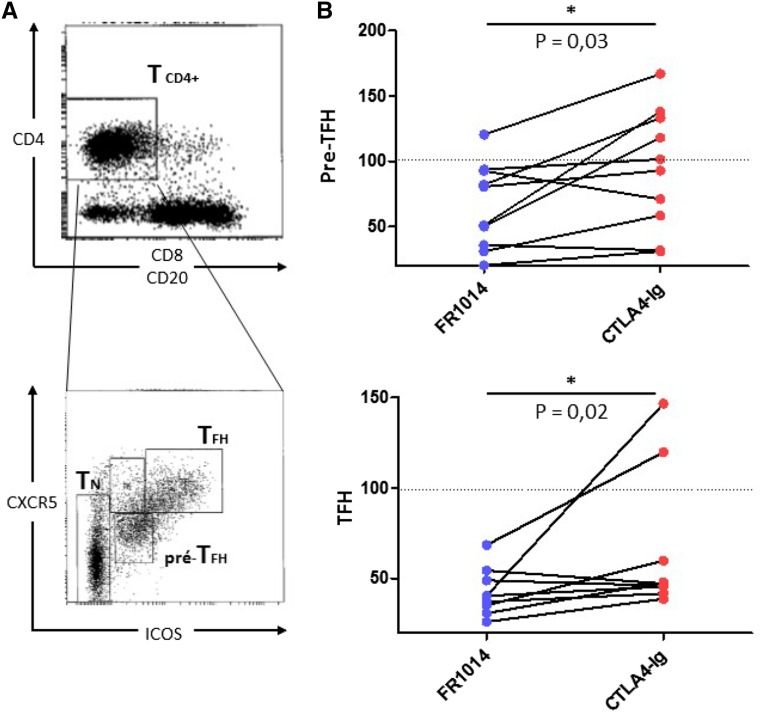
To obtain antigen–primed Tfh cells, we used human pediatric tonsils and sorted CD4+ pre–Tfh and Tfh cells expressing CXCR5 and intermediate and high levels of ICOS, respectively (Figure 7A). We tested their proliferative responses after coculture with autologous B cells in the presence of Staphylococcal enterotoxin B superantigen and either drug as previously described.15 After 8 days of culture, FR104 decreased proliferation of pre-Tfh and Tfh cells more intensively and significantly than belatacept (n=10; P=0.02 and P=0.03, respectively) (Figure 7B). Selective CD28 blockade (FR104), therefore, controlled Tfh cell responses better in vitro and might explain the differences in IL-21 gene expression observed between the two groups in vivo.
Figure 7.
Selective CD28 blockade with FR104 control in vitro Ag primed Tfh responses more effectively than belatacept. (A) Flow cytometry plot of representative of human tonsil cell suspension showing gates used to sort pre-Tfh (CD4+CXCR5+ICOS+) and Tfh (CD4+CXCR5hiICOShi). (B) Proliferative responses to autologous B cell plus Staphylococcal enterotoxin B after 8 days presented as percentages of pre-Tfh and Tfh compared with control conditions under either FR104 (blue circles) or belatacept (red circles). Each pair of connected points represents a paired experiment (n=10). The Wilcoxon matched-paired signed rank test was used. *P<0.05.
Selective CD28 Blockade Inhibits Secondary Tfh Cell Responses to KLH in Mice
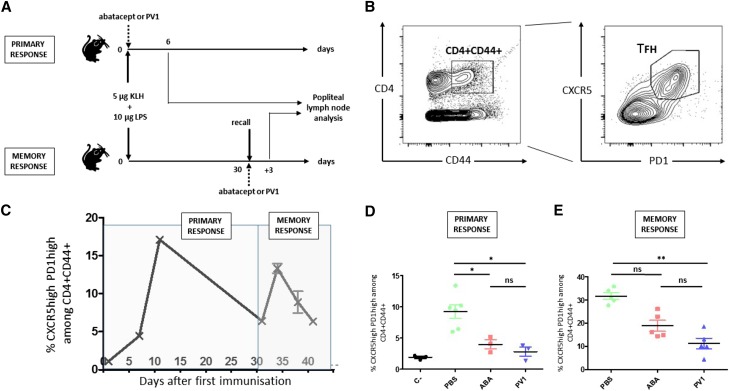
We used an in vivo experimental model of primary and secondary Tfh cell responses after KLH immunization in mice. Animals were immunized subcutaneously with KLH and LPS and treated with the Fab-PV1-Peg antibody (anti–mouse CD28 monovalent antagonist) or CTLA-4-Ig (Abatacept [Bristol-Myers Squibb]; note that belatacept does not crossreact with mouse CD80–86). Treatment and immunization were performed on day 0 to study primary responses or 1 month after the first immunization to study recall responses (Figure 8A). Tfh cell responses and levels were evaluated in popliteal draining LNs (Figure 8B). A preliminary control time course experiment revealed that, in the memory setting, the Tfh cell responses occurred earlier after the second immunization (maximum on day 3 versus on day 10 during primary response) (Figure 8C). As expected, treatments with Fab-PV1-Peg and CTLA-4-Ig were equally effective at controlling primary Tfh cell responses (n=5) (Figure 8D). In contrast, only Fab-PV1-Peg could significantly control the secondary Tfh cell response (10.7% versus 31.6%; n=5; P<0.05) (Figure 8E).
Figure 8.
Selective CD28 blockade controls Tfh recall responses to KLH immunization in mice more effectively than CTLA-4-Ig. (A) Mice were immunized with KLH and LPS and treated with either CTLA-4-Ig or the Fab-PV1-Peg antibody, an anti-CD28 antagonist effective in mice, either on day 0 to study primary responses or 1 month after a first immunization to study memory responses. (B) Postimmunization draining popliteal LNs were collected, and levels of Tfh cells (CXCR5+PD1+) among the CD4+ or CD4+CD44+ population were assessed by flow cytometry as represented in the flow cytometry contour plot. (C) Time course under control condition (n=3). Percentage of Tfh cells among CD4+CD44+ (D) on day 6 postimmunization or (E) 3 days after reimmunization according to conditions indicated on the x axis (n=5 per group). Data are means±SEMs. One-way ANOVA and Kruskal–Wallis tests were used. *P<0.05; **P<0.01.
Discussion
Our in vivo data from a preclinical model of KT show, for the first time, a differential effect between CD28 blockade and CD80–86 blockade in preventing AR, supporting previous data reported in rodent models.10
We observed severe irreversible AR in belatacept-treated recipients in contrast to FR104-treated recipients. Rejections were strictly cell mediated, with no DSAs or complement involvement. This is compatible with previous data showing that costimulation blockade with belatacept is efficient at inhibiting primary Tfh cell and antibody responses.3,16 In addition, after CNI discontinuation, FR104 monotherapy prevented AR for at least 1 year.
Evaluation of TSDR methylation status revealed a strong trend for elevated Tregs in FR104-treated animals in contrast to Foxp3 expression, which was not discriminant. Foxp3 can be transiently upregulated in activated T cells, whereas TSDR demethylation ensures stable Foxp3 expression.17,18 This lack of specificity could explain the discrepancy observed in our data. CD28 signaling is important for the generation and homeostasis of natural/thymic Tregs,19 whereas CTLA-4 is crucial for their suppressive function,20 explaining why belatacept negatively affects Tregs in different transplantation models21,22 and Foxp3 levels in patient biopsies.23 Upregulation of Treg graft infiltrates has been reported after selective CD28 blockade in KT.5,24 Although these data fail to clearly confirm these observations, preserving the CTLA-4 pathway could indirectly promote induction of peripheral Tregs (e.g., through IDO induction in APC25,26). Although the differences observed in IL-21 transcript levels provide an additional indirect explanation, a direct mechanism could also be involved. For example, in vivo costimulation through CD28 reduces the peripheral induction of Foxp3 in CD4+ T cells,27 whereas CTLA-4 promotes Foxp3 induction and Treg accumulation.28
One explanation for the occurrence of belatacept-resistant rejection could be the crossreactivity of some antigen–educated T cells that have lost the CD28 costimulation requirement for alloantigen activation, a phenomenon known as heterologous immunity.29,30 In addition, blocking the CTLA-4 pathway in these cells could be deleterious because of suppression of intrinsic inhibitory signaling. Recent advances in understanding the mechanism of action of checkpoint inhibitors show that blocking CTLA-4 could reactivate preexisting mature tumor–specific T cells.31–33 Increasing evidence also suggests that the cells involved in resistance to costimulation blockade are mature, are highly differentiated, and possess effector capacities, such as CD28–negative T cells.34–36 We did not observe any significant difference in CD28–negative T cell frequencies. Beyond the maturation stage, both the threshold of CD28 requirement and the intrinsic regulation by the CTLA-4 pathway can differ between various T cell subsets. For example, Th17–polarized T cells have been suspected of being the main culprit for belatacept-resistant rejection, because clinical association has been reported,12 and Th17 cells might be particularly sensitive to regulation by CTLA-4.11,37 We did not observe any difference in Th17 gene expression. In contrast, expression of IL-21 and IL-10, the main cytokines secreted by Tfh cells, was higher in belatacept recipients. IL-21 and IL-10 can also be produced by Th17 cells and Tregs, respectively. However, paired correlation analysis supported a positive relationship between IL-21, IL-10, and Bcl6, the Tfh cell master regulator, and did not support a positive relationship with Th17- and Treg-related genes, suggesting a Tfh gene signature. Moreover, among graft-infiltrating lymphocytes in belatacept rejection biopsies, Tfh-like cells (CD4+PD1+; also expressing ICOS and IL-21) were the predominant cell type.
Antigen–primed Tfh cells lose the requirement for CD28 costimulation for their activation.38 At this stage, CTLA-4 becomes instrumental in the control of their function in a cell-intrinsic manner.39 In addition, Tfh-like cells have been described in graft infiltrates of ABMR and TCMR biopsies.40,41 Recently, Venner et al.,42 using expression microarrays to characterize the changes most specific to TCMR compared with other diseases, including ABMR in human kidney graft biopsies, revealed that IL-21R is among the 30 transcripts most associated with TCMR. In this context, preexisting Tfh cells that crossreact with alloantigen (via heterologous immunity) may not be providing help for B cell humoral responses but rather, may be driving TCMR through IL-21 secretion itself or other effector mechanisms. This aligns with our preclinical data showing that FR104 and belatacept are equally efficient at controlling primary DSA responses controlled by Tfh cells. In our case, preexisting Tfh cells could be differentially regulated between the two groups, explaining the difference in IL-21 gene expression. The responses of the in vitro cocultured primed human Tfh cells and the Tfh responses to KLH immunization in mice support this assumption, because it was associated with various modalities of costimulation blockade. Indeed, although primary Tfh responses are equally inhibited by either selective CD28 blockade or CD80–86 blocking, only CD28 blockade reduces Tfh recall responses significantly.
In a mouse model of islet transplantation, mIL-21R-Fc combined with CTLA-4-Ig resulted in transplant tolerance in 100% of mice versus 55% for CTLA-4-Ig monotherapy alone.43 In this model, it was shown that IL-21 acted as an antitolerogenic cytokine by preventing Treg generation and inhibiting Treg function. It is possible that, in our study, IL-21 blocked both induction of Tregs and their function in the belatacept-treated group, resulting in severe AR. The observed trend toward a negative correlation between Tfh- and Treg-related genes supports this hypothesis.
The steroid resistance observed in our study seems to be quite different from results from the BENEFIT Study clinical trial,2 taking into account that we did not use an anti–CD25 induction therapy. However, AR episodes observed in the belatacept group in the BENEFIT Study were quite severe, with one third of biopsies scored as type 2B and another one third being steroid resistant versus 12% and 0%, respectively, in the control group. Clinical manifestations caused by Treg dysfunction observed in some autoimmune diseases are known to be insensitive to steroids.44,45 Using graft biopsies, Matignon et al.46 performed a molecular analysis of the T cell immune response of patients with acute TCMR. IL-21 but not IL-17, RORγt, or T-bet was significantly higher in the patients who did not experience a successful reversal of acute TCMR after anti-rejection therapy, suggesting a potential role for IL-21 in steroid resistance.
Our study shows that selective CD28 blockade in combination with suboptimal immunosuppression differently controlled AR compared with CD80–86 blockade in a preclinical model of KT in nonhuman primates. Indeed, in contrast to FR104-treated animals, belatacept therapy was associated with irreversible steroid–resistant rejection episodes. Although larger trials are required to confirm these encouraging preliminary results, CD28-selective agents could combine the advantages of belatacept (absence of nephrotoxicity and optimal control of humoral responses) while preventing belatacept-resistant rejection, suggesting that they could represent the next generation of costimulation blocking agents.
Concise Methods
Mixed Lymphocyte Reactions
PBMCs were isolated from baboon whole blood by density centrifugation over Ficoll-Paque (Eurobio). Freshly isolated PBMCs were incubated with irradiated allogeneic PBMCs (105 cells per well of each cell type) for 5 days at 37°C and 5% CO2 in complete medium (RPMI 1640 [Gibco, Carlsbad, CA], 5% human or pooled baboon serum baboon serum is from our laboratory, 2 mM l-glutamine [Sigma-Aldrich], 100 U/ml penicillin [Sigma-Aldrich, St. Louis, MO], 0.1 mg/ml streptomycin [Sigma-Aldrich], 1% nonessential amino acids [Sigma-Aldrich], 1 mM sodium pyruvate [Sigma-Aldrich], and 5 mM Hepes [Sigma-Aldrich]). Cells were pulsed with 1 mCi 3H-thymidine during the final 8 hours of culture, recovered, and counted in a scintillation counter.
Animals
Baboons (Papio anubis; 8–13 kg) were obtained from the Centre National de la Recherche Scientifique Primatology Center. Animals were housed in our laboratory’s large animal facility, and all experiments were performed in accordance with our institutional ethical guidelines. The donor-recipient combinations were chosen according to blood group compatibility and MHC mismatching (performed by DRB MHC class 2 locus typing).
Renal Transplantation in Baboons
Renal allotransplantation was performed on binephrectomized recipients as previously described5; 24-hour diuresis and BUN were monitored daily. Graftectomies were performed when plasma creatinine levels rose to >500 μmol/L. Protocol biopsies were performed 1 month post-transplantation, and surgical biopsies were performed before euthanasia for histologic examination. One biopsy fragment was collected for routine hematoxylin and eosin histology (scored blinded by a pathologist), a second fragment was snap frozen in liquid nitrogen for mRNA extraction, and a third fragment was snap frozen in Tissue-Tek (Sakura Finetek, Inc., Torrance, CA) for immunohistochemical staining. All recipients received steroids (Solumedrol; Pfizer) intravenously (iv) at 1 mg/kg per day (days 0–7) and tacrolimus (Prograf; Astellas) at 0.05 mg/kg per day (intramuscularly; adjusted to maintain trough levels between 5 and 10 ng/ml; considered as low in baboons) from day 0 to day 30. Drug weaning was then performed, with doses reduced by one half each week for 3 weeks and then stopped. In addition, they received either FR104 or belatacept iv at 10 mg/kg on days 0, 7, and 14 and then, every 2 weeks. In cases of biopsy-proven rejection, animals received steroids (iv at 5, 4, 3, 2, and 1 mg/kg on days 1–5, respectively); then, a weaning was performed intramuscularly over 3 weeks.
Phenotyping Analyses
Fluorescent mAbs against human CD3 (SP34–2), CD4 (L200), CD8 (RPA-T8), CD25 (MA251), CD28 (28.6), CD95 (DX2), and CD127 (hIL-7R-M21) were purchased from BD Biosciences (San Jose, CA). Polyclonal anti–human IgG was purchased from DAKO. Samples were acquired on a BD FACSCANTO Flow Cytometer (BD Biosciences) and analyzed with FlowJo software (FlowJo LLC).
Immunohistochemistry
Frozen sections (7 μm) were prepared from surgical or protocol renal biopsies. Slides were air dried at room temperature for 1 hour before acetone fixation for 10 minutes at room temperature. Sections were saturated with PBS containing 10% baboon serum, 2% normal goat serum, and 4% BSA. Sections were incubated overnight with primary antibodies at 4°C followed by fluorescent secondary antibodies. Primary antibodies used were monoclonal mouse IgG1 anti–human CD4 (clone 4B12; DAKO), monoclonal mouse IgG2a anti–human CD20cy (clone L26; DAKO), goat polyclonal anti–human PD1 (R&D Systems, Minneapolis, MN), monoclonal Armenian hamster IgG anti–human ICOS (clone C398.4A; BioLegend, San Diego, CA), and rabbit polyclonal anti–human IL-21 (AbD Serotec). The slides were costained with DAPI (Life Technologies, Carlsbad, CA) and mounted using Prolong Gold Antifade Reagent, and images were recorded using a Nikon A1 RSi Confocal Microscope (60× NA, 1.40 oil; Nikon, Tokyo, Japan). Image processing was carried out using FIJI software.
mRNA Analyses
mRNA was extracted from snap–frozen renal biopsies with Trizol Reagent (Life Technologies) according to the manufacturer’s instructions. The quality and quantity of mRNA were controlled by infrared spectrometry (NanoDrop; Thermo Fisher Scientific, Vernon Hills, IL). mRNA was reverse transcribed using the OmniscriptRT Kit (Qiagen, Germantown, MD), and real–time quantitative PCR was performed as previously described5 on a ViiA 7 Real-Time PCR System (Life Technologies). Amplification was performed for Foxp3 (Hs00203958_m1; Applied Biosystems, Foster City, CA), TGFβ (Hs00171257_m1), RORγc (Hs01076112_m1), IL-17F (Hs00369400_m1), IL-23 (Hs00372324_m1), Bcl6 (Rh01115889_m1), IL-21 (Rh02879198_m1), IL-10 (Hs00174086_m1), 2B4 (Rh02871839_m1), BTLA (Rh02889477_m1), PD1 (Rh03418231_m1), and perforine (Hs00169473_m1).
TSDR Analyses
Genomic DNA was isolated from frozen tissue. After RNA extraction from Trizol Buffer, 300 μl TNES-6U back extraction buffer was added (10 mM Tris-HCl, pH 7.5, 125 mM NaCl, 10 mM EDTA, pH 8, 1% SDS, 6 U urea) to the organic phase. After shaking, the mixture was incubated at room temperature for 10 minutes. Samples were then centrifuged at 18,000×g for 15 minutes at 4°C. The upper phase was removed, an equal volume of isopropanolol was added, and the samples were incubated for 2 hours at −80°C. Samples were centrifuged (18,000×g for 15 minutes at 4°C), the supernatant was removed, and the pellets were washed three times with 70% ethanol (incubated 3 minutes and then centrifuged at 18,000×g for 5 minutes). DNA samples were redissolved in TE buffer (10 mM Tris and 0.1 mM EDTA, pH 8). DNA quality and quantity were measured by infrared spectrometry (NanoDrop). Bisulfite treatment of genomic DNA was performed using the EpiTect Fast DNA Bisulfite Kit (Qiagen) according to the manufacturer’s protocol. We used an adapted protocol described by Wieczorek et al.47 on P. anubis as described.16 Real-time PCR was performed on a final reaction volume of 20 μl using Roche LightCycler 480 Probes Master (Roche Diagnostics, Indianapolis, IN) containing 15 pmol each methylation– and nonmethylation–specific forward and reverse primers for TSDR, 5 pmol hydrolysis probe, 200 ng λ-DNA (New England Biolabs, Ipswich, MA), and 60 ng bisulfite–treated genomic DNA template. Each sample was analyzed in duplicate using a LightCycler 480 System (Roche Diagnostics). Cycle conditions consisted of a 95°C preheating step for 10 minutes and 50 cycles of 95°C for 15 seconds followed by 1 minute at 61°C. The proportion of unmethylated DNA was computed as the ratio of unmethylated TSDR to methylated TSDR.
Cell Isolation
Tonsils samples were obtained from young patients (3–10 years old) undergoing tonsillectomy. Single cells were collected after mechanical disruption. B cells were first positively selected with anti–CD19 MACS Microbeads (Miltenyi Biotec, San Diego, CA). For the isolation of Tfh populations, the CD19-negative fraction was stained with mAbs against human CD4 (L200), CD8 (RPA-T8) from BD Biosciences, CD20 (2H7), ICOS (C398.4A) from BioLegend, and CXCR5 (MU5UBEE) from eBioscience (San Diego, CA). Pre-Tfh (CXCR5+ICOS+) and Tfh (CXCR5hiICOShi) cells were sorted using an FACSAria (BD Biosciences) according to the expression of ICOS and CXCR5 within the CD4+CD20−CD8− cell population.
Cell Culture
Sorted Tfh populations were cocultured with B cells (2×104 cells per well each) in RPMI medium 1640 (Gibco) supplemented with 1% l-glutamine (Sigma-Aldrich), 1% penicillin/streptomycin (Sigma-Aldrich), 1% sodium pyruvate (Sigma-Aldrich), 1% nonessential amino acids (Sigma-Aldrich), 50 μM β-mercaptoethanol (Sigma-Aldrich), 50 μg/ml gentamycin (Gibco), and 10% heat-inactivated FCS (Eurobio) in the presence of Staphylococcal enterotoxin B (1 μg/ml; Sigma-Aldrich) in U–bottomed 96–well plates. In the blocking experiment, the following reagents were added to the cocultures: FR104 (10 μg/ml) and belatacept (10 μg/ml). After 8 days of culture, cells were stained with anti-human CD3 (SP34–2; BD Biosciences), and the number of T cells per well was assessed using CountBright Absolute Counter Beads (Life Technologies).
Protein Vaccination and Treatment
C57BL/6 mice were immunized in the left hind footpad with 5 μg KLH (Stellar) and 10 μg LPS (Sigma-Aldrich) in PBS buffer. Mice were treated intraperitoneally on days 0, 3, and 6 with 10 mg/kg either Abatacept or the antagonist anti–CD28 Fab monovalent fragment Fab-PV1 produced by Effimune from the hamster anti–mouse IgG clone PV1 and subsequently conjugated to polyethylene glycol moieties to prolong its half-life in vivo (Laboratoire Celares). The same protocol was used for primary and secondary challenge (30 days after the initial priming).
Flow Cytometry
Mice were euthanized 6 days after initial priming, 3 days after secondary challenge, or on various days after immunization as indicated on the time course. Popliteal draining LNs were harvested for analysis. After mechanical disruption, cells were pelleted and resuspended in PBS. Cells were labeled with fluorescent mAbs against murine CD4 (RM4–5), CD44 (IM7), CXCR5 (2G8), and PD1 (J43) from BD Biosciences. Samples were acquired on a BD FACSCANTO Flow Cytometer (BD Biosciences) and analyzed with FlowJo software (FlowJo LLC).
Statistical Analyses
Graft survival times were plotted using Kaplan–Meier representation, and survival time between different groups was evaluated with a log rank test. Continuous variables were expressed as the means±SEMs and compared with the Mann–Whitney nonparametric test. Paired observations were analyzed using a Wilcoxon matched paired signed-rank test. Multiple comparisons were analyzed by one-way ANOVA and Kruskal–Wallis test. Pairwise correlation analysis was performed using the Spearman rank correlation coefficient.
Differences were considered significant if the P value was <0.05. All statistical analyses were performed with GraphPad Prism (GraphPad Software, La Jolla, CA).
Study Approval
Animal studies were approved by the French National Ethical Committee (CEEA-2010–17).
All donors were informed of the final use of their biologic sample and signed an informed consent.
Disclosures
N.P., S.P., C.M., and B.V. are employees of Effimune, a company developing CD28 antagonists (Effimune, Nantes, France).
Supplementary Material
Acknowledgments
The authors thank Olivier Malard and Julie Boyer from the Otorhinolaryngology Department of the Centre Hospitalier Universitaire of Nantes.
This work was supported by funds from IHU-Cesti (Investissement d'Avenir ANR-10-IBHU-005, région Pays de la Loire et Nantes Métropole).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2015070774/-/DCSupplemental.
References
- 1.Vincenti F, Larsen C, Durrbach A, Wekerle T, Nashan B, Blancho G, Lang P, Grinyo J, Halloran PF, Solez K, Hagerty D, Levy E, Zhou W, Natarajan K, Charpentier B; Belatacept Study Group : Costimulation blockade with belatacept in renal transplantation. N Engl J Med 353: 770–781, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Vincenti F, Charpentier B, Vanrenterghem Y, Rostaing L, Bresnahan B, Darji P, Massari P, Mondragon-Ramirez GA, Agarwal M, Di Russo G, Lin C-S, Garg P, Larsen CP: A phase III study of belatacept-based immunosuppression regimens versus cyclosporine in renal transplant recipients (BENEFIT study). Am J Transplant 10: 535–546, 2010 [DOI] [PubMed] [Google Scholar]
- 3.Kim EJ, Kwun J, Gibby AC, Hong JJ, Farris AB 3rd, Iwakoshi NN, Villinger F, Kirk AD, Knechtle SJ: Costimulation blockade alters germinal center responses and prevents antibody-mediated rejection. Am J Transplant 14: 59–69, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poirier N, Mary C, Dilek N, Hervouet J, Minault D, Blancho G, Vanhove B: Preclinical efficacy and immunological safety of FR104, an antagonist anti-CD28 monovalent Fab’ antibody. Am J Transplant 12: 2630–2640, 2012 [DOI] [PubMed] [Google Scholar]
- 5.Poirier N, Azimzadeh AM, Zhang T, Dilek N, Mary C, Nguyen B, Tillou X, Wu G, Reneaudin K, Hervouet J, Martinet B, Coulon F, Allain-Launay E, Karam G, Soulillou JP, Pierson RN 3rd, Blancho G, Vanhove B: Inducing CTLA-4-dependent immune regulation by selective CD28 blockade promotes regulatory T cells in organ transplantation. Sci Transpl Med 2: 17ra10, 2010 [DOI] [PMC free article] [PubMed]
- 6.Walker LSK, Sansom DM: The emerging role of CTLA4 as a cell-extrinsic regulator of T cell responses. Nat Rev Immunol 11: 852–863, 2011 [DOI] [PubMed] [Google Scholar]
- 7.Krummey SM, Ford ML: Braking bad: Novel mechanisms of CTLA-4 inhibition of T cell responses. Am J Transplant 14: 2685–2690, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haas M, Sis B, Racusen LC, Solez K, Glotz D, Colvin RB, Castro MCR, David DSR, David-Neto E, Bagnasco SM, Cendales LC, Cornell LD, Demetris AJ, Drachenberg CB, Farver CF, Farris AB 3rd, Gibson IW, Kraus E, Liapis H, Loupy A, Nickeleit V, Randhawa P, Rodriguez ER, Rush D, Smith RN, Tan CD, Wallace WD, Mengel M; Banff meeting report writing committee : Banff 2013 meeting report: Inclusion of c4d-negative antibody-mediated rejection and antibody-associated arterial lesions. Am J Transplant 14: 272–283, 2014 [DOI] [PubMed] [Google Scholar]
- 9.Chen W, Jin W, Wahl SM: Engagement of cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) induces transforming growth factor beta (TGF-beta) production by murine CD4(+) T cells. J Exp Med 188: 1849–1857, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu D, Krummey SM, Badell IR, Wagener M, Schneeweis LA, Stetsko DK, Suchard SJ, Nadler SG, Ford ML: 2B4 (CD244) induced by selective CD28 blockade functionally regulates allograft-specific CD8+ T cell responses. J Exp Med 211: 297–311, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krummey SM, Floyd TL, Liu D, Wagener ME, Song M, Ford ML: Candida-elicited murine Th17 cells express high Ctla-4 compared with Th1 cells and are resistant to costimulation blockade. J Immunol 192: 2495–2504, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krummey SM, Cheeseman JA, Conger JA, Jang PS, Mehta AK, Kirk AD, Larsen CP, Ford ML: High CTLA-4 expression on Th17 cells results in increased sensitivity to CTLA-4 coinhibition and resistance to belatacept. Am J Transplant 14: 607–614, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma CS, Deenick EK, Batten M, Tangye SG: The origins, function, and regulation of T follicular helper cells. J Exp Med 209: 1241–1253, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu H, Wang X, Lackner AA, Veazey RS: PD-1(HIGH) follicular CD4 T helper cell subsets residing in lymph node germinal centers correlate with B cell maturation and IgG production in rhesus macaques. Front Immunol 5: 85, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bentebibel S-E, Schmitt N, Banchereau J, Ueno H: Human tonsil B-cell lymphoma 6 (BCL6)-expressing CD4+ T-cell subset specialized for B-cell help outside germinal centers. Proc Natl Acad Sci U S A 108: E488–E497, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poirier N, Dilek N, Mary C, Ville S, Coulon F, Branchereau J, Tillou X, Charpy V, Pengam S, Nerriere-Daguin V, Hervouet J, Minault D, Le Bas-Bernardet S, Renaudin K, Vanhove B, Blancho G: FR104, an antagonist anti-CD28 monovalent fab’ antibody, prevents alloimmunization and allows calcineurin inhibitor minimization in nonhuman primate renal allograft. Am J Transplant 15: 88–100, 2015 [DOI] [PubMed] [Google Scholar]
- 17.Baron U, Floess S, Wieczorek G, Baumann K, Grützkau A, Dong J, Thiel A, Boeld TJ, Hoffmann P, Edinger M, Türbachova I, Hamann A, Olek S, Huehn J: DNA demethylation in the human FOXP3 locus discriminates regulatory T cells from activated FOXP3(+) conventional T cells. Eur J Immunol 37: 2378–2389, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Floess S, Freyer J, Siewert C, Baron U, Olek S, Polansky J, Schlawe K, Chang HD, Bopp T, Schmitt E, Klein-Hessling S, Serfling E, Hamann A, Huehn J: Epigenetic control of the foxp3 locus in regulatory T cells. PLoS Biol 5: e38, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang R, Huynh A, Whitcher G, Chang J, Maltzman JS, Turka LA: An obligate cell-intrinsic function for CD28 in Tregs. J Clin Invest 123: 580–593, 2013 [DOI] [PMC free article] [PubMed]
- 20.Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z, Nomura T, Sakaguchi S: CTLA-4 control over Foxp3+ regulatory T cell function. Science 322: 271–275, 2008 [DOI] [PubMed] [Google Scholar]
- 21.Riella LV, Liu T, Yang J, Chock S, Shimizu T, Mfarrej B, Batal I, Xiao X, Sayegh MH, Chandraker A: Deleterious effect of CTLA4-Ig on a Treg-dependent transplant model. Am J Transplant 12: 846–855, 2012 [DOI] [PubMed] [Google Scholar]
- 22.Charbonnier L-M, Vokaer B, Lemaître PH, Field KA, Leo O, Le Moine A: CTLA4-Ig restores rejection of MHC class-II mismatched allografts by disabling IL-2-expanded regulatory T cells. Am J Transplant 12: 2313–2321, 2012 [DOI] [PubMed] [Google Scholar]
- 23.Grimbert P, Audard V, Diet C, Matignon M, Plonquet A, Mansour H, Desvaux D, Durrbach A, Cohen JL, Lang P: T-cell phenotype in protocol renal biopsy from transplant recipients treated with belatacept-mediated co-stimulatory blockade. Nephrol Dial Transplant 26: 1087–1093, 2011 [DOI] [PubMed] [Google Scholar]
- 24.Zhang T, Fresnay S, Welty E, Sangrampurkar N, Rybak E, Zhou H, Cheng X-F, Feng Q, Avon C, Laaris A, Whitters M, Nagelin AM, O’Hara RM Jr., Azimzadeh AM: Selective CD28 blockade attenuates acute and chronic rejection of murine cardiac allografts in a CTLA-4-dependent manner. Am J Transplant 11: 1599–1609, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grohmann U, Orabona C, Fallarino F, Vacca C, Calcinaro F, Falorni A, Candeloro P, Belladonna ML, Bianchi R, Fioretti MC, Puccetti P: CTLA-4-Ig regulates tryptophan catabolism in vivo. Nat Immunol 3: 1097–1101, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Chen W, Liang X, Peterson AJ, Munn DH, Blazar BR: The indoleamine 2,3-dioxygenase pathway is essential for human plasmacytoid dendritic cell-induced adaptive T regulatory cell generation. J Immunol 181: 5396–5404, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Semple K, Nguyen A, Yu Y, Wang H, Anasetti C, Yu X-Z: Strong CD28 costimulation suppresses induction of regulatory T cells from naive precursors through Lck signaling. Blood 117: 3096–3103, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barnes MJ, Griseri T, Johnson AMF, Young W, Powrie F, Izcue A: CTLA-4 promotes Foxp3 induction and regulatory T cell accumulation in the intestinal lamina propria. Mucosal Immunol 6: 324–334, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adams AB, Williams MA, Jones TR, Shirasugi N, Durham MM, Kaech SM, Wherry EJ, Onami T, Lanier JG, Kokko KE, Pearson TC, Ahmed R, Larsen CP: Heterologous immunity provides a potent barrier to transplantation tolerance. J Clin Invest 111: 1887–1895, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Selin LK, Brehm MA: Frontiers in nephrology: Heterologous immunity, T cell cross-reactivity, and alloreactivity. J Am Soc Nephrol 18: 2268–2277, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Cha E, Klinger M, Hou Y, Cummings C, Ribas A, Faham M, Fong L: Improved survival with T cell clonotype stability after anti–CTLA-4 treatment in cancer patients. Sci Transpl Med 6: 238ra70, 2014 [DOI] [PMC free article] [PubMed]
- 32.Gubin MM, Zhang X, Schuster H, Caron E, Ward JP, Noguchi T, Ivanova Y, Hundal J, Arthur CD, Krebber W-J, Mulder GE, Toebes M, Vesely MD, Lam SSK, Korman AJ, Allison JP, Freeman GJ, Sharpe AH, Pearce EL, Schumacher TN, Aebersold R, Rammensee H-G, Melief CJM, Mardis ER, Gillanders WE, Artyomov MN, Schreiber RD: Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens. Nature 515: 577–581, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Twyman-Saint Victor C, Rech AJ, Maity A, Rengan R, Pauken KE, Stelekati E, Benci JL, Xu B, Dada H, Odorizzi PM, Herati RS, Mansfield KD, Patsch D, Amaravadi RK, Schuchter LM, Ishwaran H, Mick R, Pryma DA, Xu X, Feldman MD, Gangadhar TC, Hahn SM, Wherry EJ, Vonderheide RH, Minn AJ: Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature 520: 373–377, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weaver TA, Charafeddine AH, Agarwal A, Turner AP, Russell M, Leopardi FV, Kampen RL, Stempora L, Song M, Larsen CP, Kirk AD: Alefacept promotes co-stimulation blockade based allograft survival in nonhuman primates. Nat Med 15: 746–749, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Badell IR, Russell MC, Thompson PW, Turner AP, Weaver TA, Robertson JM, Avila JG, Cano JA, Johnson BE, Song M, Leopardi FV, Swygert S, Strobert EA, Ford ML, Kirk AD, Larsen CP: LFA-1-specific therapy prolongs allograft survival in rhesus macaques. J Clin Invest 120: 4520–4531, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu H, Perez SD, Cheeseman J, Mehta AK, Kirk AD: The allo- and viral-specific immunosuppressive effect of belatacept, but not tacrolimus, attenuates with progressive T cell maturation. Am J Transplant 14: 319–332, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ying H, Yang L, Qiao G, Li Z, Zhang L, Yin F, Xie D, Zhang J: Cutting edge: CTLA-4--B7 interaction suppresses Th17 cell differentiation. J Immunol 185: 1375–1378, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Linterman MA, Rigby RJ, Wong R, Silva D, Withers D, Anderson G, Verma NK, Brink R, Hutloff A, Goodnow CC, Vinuesa CG: Roquin differentiates the specialized functions of duplicated T cell costimulatory receptor genes CD28 and ICOS. Immunity 30: 228–241, 2009 [DOI] [PubMed] [Google Scholar]
- 39.Sage PT, Paterson AM, Lovitch SB, Sharpe AH: The coinhibitory receptor CTLA-4 controls B cell responses by modulating T follicular helper, T follicular regulatory, and T regulatory cells. Immunity 41: 1026–1039, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liarski VM, Kaverina N, Chang A, Brandt D, Yanez D, Talasnik L, Carlesso G, Herbst R, Utset TO, Labno C, Peng Y, Jiang Y, Giger ML, Clark MR: Cell distance mapping identifies functional T follicular helper cells in inflamed human renal tissue. Sci Transpl Med 6: 230ra46, 2014 [DOI] [PMC free article] [PubMed]
- 41.de Graav GN, Dieterich M, Hesselink DA, Boer K, Clahsen-van Groningen MC, Kraaijeveld R, Litjens NHR, Bouamar R, Vanderlocht J, Tilanus M, Houba I, Boonstra A, Roelen DL, Claas FHJ, Betjes MGH, Weimar W, Baan CC: Follicular T helper cells and humoral reactivity in kidney transplant patients. Clin Exp Immunol 180: 329–340, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Venner JM, Famulski KS, Badr D, Hidalgo LG, Chang J, Halloran PF: Molecular landscape of T cell-mediated rejection in human kidney transplants: Prominence of CTLA4 and PD ligands. Am J Transplant 14: 2565–2576, 2014 [DOI] [PubMed] [Google Scholar]
- 43.Petrelli A, Carvello M, Vergani A, Lee KM, Tezza S, Du M, Kleffel S, Chengwen L, Mfarrej BG, Hwu P, Secchi A, Leonard WJ, Young D, Sayegh MH, Markmann JF, Zajac AJ, Fiorina P: IL-21 is an antitolerogenic cytokine of the late-phase alloimmune response. Diabetes 60: 3223–3234, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kotsianidis I, Nakou E, Bouchliou I, Tzouvelekis A, Spanoudakis E, Steiropoulos P, Sotiriou I, Aidinis V, Margaritis D, Tsatalas C, Bouros D: Global impairment of CD4+CD25+FOXP3+ regulatory T cells in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 179: 1121–1130, 2009 [DOI] [PubMed] [Google Scholar]
- 45.Barzaghi F, Passerini L, Bacchetta R: Immune dysregulation, polyendocrinopathy, enteropathy, x-linked syndrome: A paradigm of immunodeficiency with autoimmunity. Front Immunol 3: 211, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Matignon M, Aissat A, Canoui-Poitrine F, Grondin C, Pilon C, Desvaux D, Saadoun D, Barathon Q, Garrido M, Audard V, Rémy P, Lang P, Cohen J, Grimbert P: Th-17 alloimmune responses in renal allograft biopsies from recipients of kidney transplants using extended criteria donors during acute T cell-mediated rejection. Am J Transplant 15: 2718–2725, 2015 [DOI] [PubMed]
- 47.Wieczorek G, Asemissen A, Model F, Turbachova I, Floess S, Liebenberg V, Baron U, Stauch D, Kotsch K, Pratschke J, Hamann A, Loddenkemper C, Stein H, Volk HD, Hoffmüller U, Grützkau A, Mustea A, Huehn J, Scheibenbogen C, Olek S: Quantitative DNA methylation analysis of FOXP3 as a new method for counting regulatory T cells in peripheral blood and solid tissue. Cancer Res 69: 599–608, 2009 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.