Abstract
Because adult podocytes cannot proliferate and are therefore unable to self-renew, replacement of these cells depends on stem/progenitor cells. Although podocyte number is higher after renin-angiotensin-aldosterone system (RAAS) inhibition in glomerular diseases, the events explaining this increase are unclear. Cells of renin lineage (CoRL) have marked plasticity, including the ability to acquire a podocyte phenotype. To test the hypothesis that RAAS inhibition partially replenishes adult podocytes by increasing CoRL number, migration, and/or transdifferentiation, we administered tamoxifen to Ren1cCreERxRs-tdTomato-R CoRL reporter mice to induce permanent labeling of CoRL with red fluorescent protein variant tdTomato. We then induced experimental FSGS, typified by abrupt podocyte depletion, with a cytopathic antipodocyte antibody. RAAS inhibition by enalapril (angiotensin-converting enzyme inhibitor) or losartan (angiotensin-receptor blocker) in FSGS mice stimulated the proliferation of CoRL, increasing the reservoir of these cells in the juxtaglomerular compartment (JGC). Compared with water or hydralazine, RAAS inhibition significantly increased the migration of CoRL from the JGC to the intraglomerular compartment (IGC), with more glomeruli containing RFP+CoRL and, within these glomeruli, more RFP+CoRL. Moreover, RAAS inhibition in FSGS mice increased RFP+CoRL transdifferentiation in the IGC to phenotypes, consistent with those of podocytes (coexpression of synaptopodin and Wilms tumor protein), parietal epithelial cells (PAX 8), and mesangial cells (α8 integrin). These results show that in the context of podocyte depletion in FSGS, RAAS inhibition augments CoRL proliferation and plasticity toward three different glomerular cell lineages.
Keywords: ACE inhibitors, focal segmental glomerulosclerosis, podocyte
Podocytes are visceral glomerular epithelial cells forming a critical layer of the glomerular filtration barrier.1 Podocytes are injured in a variety of glomerular diseases, and in many instances, injury-induced apoptosis, detachment, DNA damage, and mitotic catastrophe leads to their loss.2 In contrast to other kidney epithelial cells, adult podocytes are terminally differentiated and are unable to proliferate because of cell cycle inhibition and other mechanisms.3,4 Thus, in the face of loss, the inability to self-renew leads to progressive podocyte depletion. Seminal studies have shown that podocyte depletion is a major cause of glomerulosclerosis, accompanied by reduced GFR.5–7 There is a direct correlation between the magnitude of podocyte depletion and the extent of glomerulosclerosis.8
Because of the inability of adult podocytes to proliferate, recent studies have therefore focused on stem/progenitor cells to explain how podocyte number might be partially or fully replenished under certain conditions. An emerging body of literature by our group9–12 and others13–17 supports a likely role for glomerular parietal epithelial cells (PECs) to serve as adult podocyte progenitors. Another likely adult podocyte progenitor source, and the focus of the studies herein, are cells of renin lineage (CoRL). CoRL normally reside along the afferent arterioles in the juxta-glomerular compartment (JGC).18 There is an increasing literature supporting CoRL plasticity, including their being pluripotent progenitors for podocytes,19–21 PECs,19 mesangial cells,22 vascular smooth muscle cells, and pericytes.23–25 We have shown that in states of abrupt19 and chronic20 podocyte depletion, including that of the aging kidney,21 a subset of CoRL migrate to the glomerulus, where they de novo express several proteins considered specific for podocytes, and a subpopulation also begins to acquire several ultrastructural characteristics of podocytes.
From a clinical standpoint, therapies in glomerular disease have been aimed at limiting ongoing podocyte loss. For example, inhibition of the renin-angiotensin-aldosterone system (RAAS), a mainstay therapy for glomerular diseases characterized by podocyte injury, limits podocyte apoptosis and detachment.26 More recently, studies by our group27 and others28,29 have shown that podocyte number can be increased by RAAS inhibition and that this occurs in the absence of podocyte proliferation.27,30 Similar results have been shown with corticosteroids31,32 and retinoids.11,33 Although the biologic effect of RAAS inhibition on endocrine regulation of CoRL is well documented,23,34,35 the effect of RAAS inhibition on their stemness and progenitor properties are not well understood. Moreover, it is unclear whether the higher podocyte number after RAAS inhibition in glomerular disease is due in part to their effects on CoRL.
Through use of tamoxifen inducible Ren1cCreERxRs-tdTomato-R CoRL reporter mice, the purpose of the current studies was to determine whether the higher podocyte number after RAAS inhibition in experimental FSGS was due in part to CoRL. We asked whether RAAS inhibition augments the size of the CoRL reservoir in the JGC, whether RAAS inhibition increases the migration of CoRL from the juxta- to the intraglomerular compartment, and, once the CoRL are there, whether the rate of transdifferentiation to a podocyte phenotype is increased.
Results
RAAS Inhibition Improves Outcomes in Mice with Experimental FSGS
Experimental FSGS characterized by abrupt podocyte depletion was induced in Ren1cCreERxRs-tdTomato-R mice by injecting sheep antiglomerular antibody as previously reported.19 Mice were randomized at d3, the nadir in podocyte depletion, to receive water, hydralazine, enalapril, or losartan for 25 days (Supplemental Figure 1). Sheep IgG staining confirmed the binding of injected sheep antiglomerular antibody to podocytes within glomeruli of FSGS mice and was not altered in mice receiving hydralazine, enalapril or losartan compared with control FSGS mice receiving water (Supplemental Figure 2). Therefore, RAAS inhibition did not affect the binding of the disease inducing antiglomerular antibody. Circulating white blood cells in glomeruli are not involved in the pathogenesis of this disease model.
BP was measured to ensure that any benefits from RAAS inhibition in experimental FSGS were independent of BP effects as reported previously.27 In control animals receiving water, mean BP increased by day 7 and 14 of FSGS (Supplemental Figure 3A). BP decrease significantly in all treated groups by day 7. The decrease in mean BP in FSGS mice with RAAS inhibition was similar to that in FSGS mice treated with hydralazine. These data show that hydralazine, enalapril and losartan lowered BP to a similar extent in this model.
Glomerular scarring was quantitated by glomerulosclerosis index scoring as previously published.36 The mean glomerulosclerosis score was significantly increased in all groups at day 28 compared with baseline (Supplemental Figure 3B). As expected in mice treated with enalapril or losartan, glomerulosclerosis was reduced compared with mice receiving water alone or hydralazine. Urinary albumin-to-creatinine ratio was measured at days 14 and 28 and was significantly lower in FSGS mice given enalapril or losartan compared with water- or hydralazine-treated animals (Supplemental Figure 3C).
Taken together, these data show that despite similar lowering of BP, RAAS inhibition reduced glomerulosclerosis and albuminuria in mice with experimental FSGS, consistent with previous reports.27,30 Further, renin mRNA expression in the kidney cortex showed an upregulation of renin by enalapril and losartan given to healthy or diseased animals, confirming the blockage of the negative feedback loop in the RAAS (Supplemental Figure 4, A and B).
Podocyte Number Is Higher in FSGS Mice after RAAS Inhibition
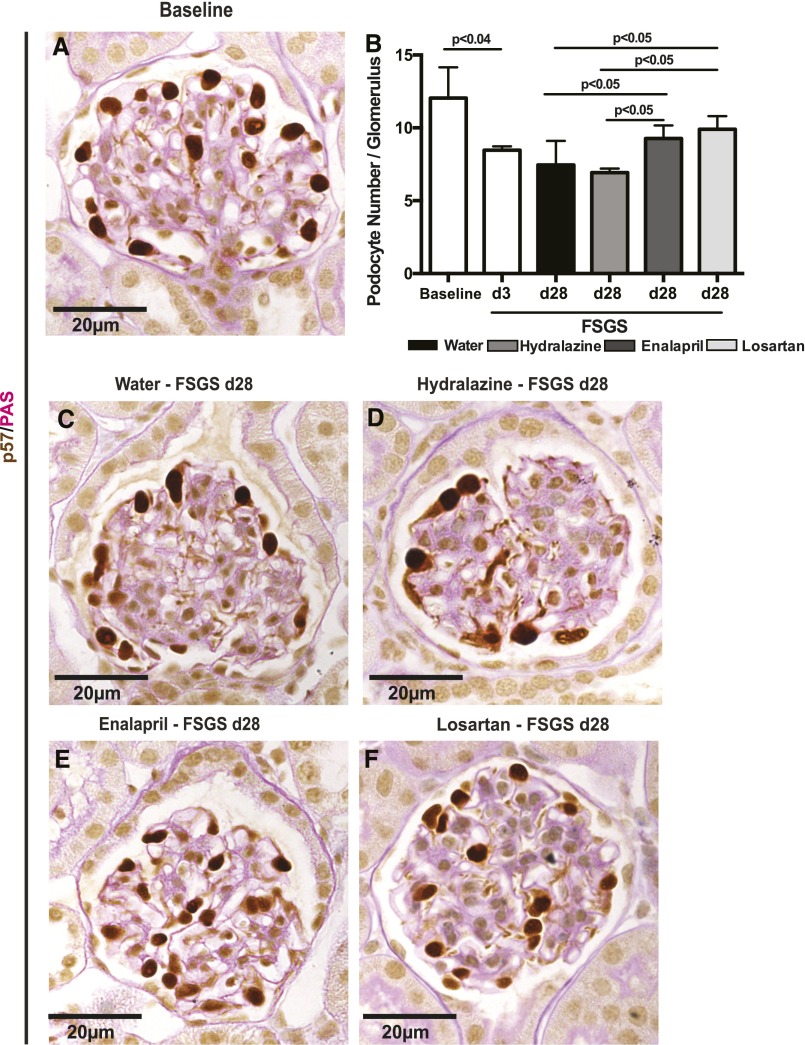
We have reported that the FSGS model used in these studies is typified by abrupt podocyte depletion.12,19,27 Total podocyte number, identified by p57 staining, was quantitated in all reporter mice at baseline, a representative group of mice at day 3 of FSGS (time point for randomization), and all four FSGS groups at day 28 of disease (Figure 1). Because glomerular volume does not change over the course of 28 days in this FSGS model (data not shown), measures of podocyte density were not needed or performed. At baseline, the average number of podocytes in the entire kidney cortex was 12.27/glomerular cross-section (Figure 1). On day 3 of FSGS, podocyte number was depleted by 40.6% from baseline to 8.43 (P<0.001 versus baseline), consistent with our previous reports.12,19,27 Mice were then blindly randomly assigned to the different treatment groups at day 3. At day 28, podocyte numbers per glomerular cross-section were 7.9 (P<0.01 versus baseline) and 6.5 (P<0.05 versus baseline) in the water and hydralazine groups respectively; 9.3 in the enalapril group (P<0.05 versus water and hydralazine groups); and 9.9 in the losartan group (P<0.5 versus water and hydralazine groups) (Figure 1). These data show that podocyte number is higher after RAAS inhibition in this FSGS model. 5-Bromo-2′-deoxyuridine staining was not detected in podocytes in any FSGS groups (data not shown) consistent with a higher podocyte number occurring in the absence of podocyte proliferation.
Figure 1.
Podocyte number is higher in FSGS mice given enalapril and losartan. p57/PAS double staining. Podocytes were identified by p57 staining (brown, nuclear); PAS was used to identify scarring. Representative pictures are shown from healthy baseline mice (A) and mice with experimental FSGS treated for 25 days with water (C), hydralazine (D), enalapril (E), or losartan (F). (B) Quantitative results show podocyte depletion at day 3, when mice were randomly assigned to treatment groups. Podocyte number remained low at day 28 in the water- and hydralazine-treated groups. Podocyte number was significantly higher in FSGS animals treated with enalapril of losartan compared with FSGS animals treated with water alone or hydralazine. Data are represented as mean±SD.
Labeled Cells of Renin Lineage Comprise a Very Small Fraction of Total Kidney Cells in Normal Mice
The overall purpose of these studies was to ultimately calculate the number of new podocytes that derive from CoRL (designated M), using the calculation N×P1×P2×P3, where N is the total CoRL population number, defined as renin-expressing cells (renin+CoRL); P1 represents the percentage labeling efficiency of CoRL; P2 represents the percentage of permanently labeled CoRL (red fluorescent protein–positive CoRL [RFP+CoRL]) that migrated from the JGC to the intraglomerular compartment (IGC); P3 represents the transdifferentiation rate of RFP+CoRL to a podocyte phenotype. The final values are shown in Table 1 (Supplemental Figure 5).
Table 1.
Formula determining rates where new podocytes derived from CoRL
| Group | N×P1 (No. RFP-Labeled Cells in JGC): CoRL Pool Size | P2 (% RFP+ in IGC): CoRL Migration | P3 (% RFP+Synpo+/ RFP+ in IGC): CoRL Transdifferentiation | M = P1×P2×P3×N (Potential No. of Traceable New RFP+ Podocytes Derived from CoRL) |
|---|---|---|---|---|
| Baseline | 442.68 | NA | NA | NA |
| FSGS+H2O | 401.49 | 5.20 | 10.04 | 2.09 |
| FSGS+hydralazine | 420.31 | 2.83 | 11.54 | 1.37 |
| FSGS+enalapril | 1282.93 | 10.70 | 17.29 | 23.74a |
| (P<0.001 versus FSGS hydralazine) | ||||
| FSGS+losartan | 1317.36 | 7.23 | 11.59 | 11.05b |
| (P=0.02 versus FSGS hydralazine) |
For details regarding formula calculation see Supplemental Figure 5. NA, not applicable.
The number of new podocytes formed by enalapril is 17-fold higher compared with hydralazine treatment.
The number of new podocytes formed by losartan is 7.92-fold higher compared with hydralazine treatment.
We began by quantitating the total number of cells in the entire kidney cortex that express renin in tamoxifen-inducible Ren1cCreERxRs-tdTomato-R CoRL reporter mice (Table 2). Figure 2 shows representative confocal images of renin staining. Because the size of the tissue sections varies occasionally from animal to animal, the total number of nuclei was measured by quantitating 4′,6-diamidino-2-phenylindole (DAPI) staining for each sample to account for this and was used to normalize all quantifications. At baseline (i.e., before disease induction), the average number (±SD) of all cell types in the cortical sections of these mice was 56,645±2941. Of these cells, 438.8±72 stained positive for renin. This value serves as N in the formula in the preceding paragraph (used in Table 1). Therefore, 0.78%±0.15% of the total kidney cortex cell population expressed renin. Renin staining was not detected in any glomeruli at baseline (Figure 2).
Table 2.
Renin+CoRL and RFP+CoRL in JGC
| Reservoir of CoRL: Data from JGC | Renin+CoRL | RFP+CoRL | ||
|---|---|---|---|---|
| Total No. of renin+CoRL in JGC per Section (Cortex) Corrected for Section Size# (Represents N in Formula) | All Kidney Cells That Are Renin+CoRL in JGC, % | Total No. # of RFP+CoRL in JGC Corrected for Section Size (Represents N×P1) | All kidney Cells That Are RFP+CoRL in JGC, % | |
| Baseline (no FSGS) | ||||
| H2O alone | 438.8±72 | 0.78±0.15 | 442.7±47.5 | 0.79±0.13 |
| Enalapril alone | 523.05±71.76 | 1.09±0.09 | 542±82.62 | 1.12±0.01a (P=0.02 versus H2O) |
| (P=0.22 versus H2O) | (P=0.02 versus H2O) | (P<0.2 versus H2O) | ||
| Losartan alone | 1439.9±18.93 | 3.09±0.49 | 1479±53.43 | 3.14±0.57b (P<0.001 versus H2O) |
| (P<0.001 versus H2O) | (P<0.001 versus H2O) | (P<0.001 versus H2O) | ||
| (P<0.001 versus enalapril) | (P<0.001 versus enalapril) | |||
| FSGS day 28 | ||||
| FSGS+H2O | 399.01±84.96 | 0.73±0.12 | 401.5±32.5 | 0.76±0.13 |
| (P=0.5 versus baseline) | (P=0.6 versus baseline) | (P=0.67 versus baseline) | (P=0.78 versus baseline) | |
| FSGS+hydralazine | 422.69±74.97 | 0.80±0.23 | 420.31±50.16 | 0.80±0.24 |
| (P=0.52 versus FSGS H2O) | (P=0.53 versus FSGS H2O) | (P=0.9 versus FSGS H2O) | (P=0.73 versus FSGS H2O) | |
| FSGS+enalapril | 1245.03±237.77 | 2.17±0.36 | 1282.93±300.66 | 2.22±0.44c |
| (P<0.001 versus FSGS hydralazine) | (P<0.001 versus FSGS hydralazine) | (P<0.001 versus FSGS hydralazine) | (P<0.001 versus FSGS Hydralazine) | |
| FSGS+losartan | 1304.07±296.52 | 2.48±0.57 | 1317.36±294.2 | 2.51±0.57d (P<0.001 versus FSGS hydralazine) |
| (P<0.001 versus FSGS hydralazine) | (P<0.001 versus FSGS hydralazine) | (P<0.001 versus FSGS hydralazine) | ||
At baseline, enalapril increased the number of RFP+CoRL by 1.23-fold over baseline.
At baseline, losartan increased the number of RFP+CoRL by three-fold over baseline.
In FSGS, enalapril increased the number of RFP+CoRL by 2.92-fold over water or hydralazine treatment.
In FSGS,-losartan increased the number of RFP+CoRL by 3.3-fold over water or hydralazine treatment.
Figure 2.
RAAS inhibition increases the number of labeled cells of renin lineage in the juxtaglomerular compartment in non-diseased mice renin/red fluorescent protein co-staining. Confocal microscopy showing co-staining for renin (green color, first column) and the RFP reporter (red color, middle column) as single panels. A merge of the two panels creates a yellow color if renin and RFP staining co-localize (right column). Nuclei stain blue with DAPI. The quantitative data is shown in panel D. (A) Non-diseased mice given water: When healthy, non diseased Ren1cCreERxRs-tdTomato-R mice were given tamoxifen, and given water alone to drink for 25 days, renin (A, i) and RFP staining (A, ii) were restricted to co-localized in the juxta-glomerular compartment (JGC) (A, iii). (A, iv – A,vi) Higher magnification images of the insets shown in the figures above. (B) Non-diseased mice given enalapril: Administering the ACE inhibitor enalapril in the drinking water for 25 days to healthy, non-diseased Ren1cCreERxRs-tdTomato-R CoRL reporter mice increased the number of cells in the JGC staining for renin (B, i) and RFP (B, ii). Renin and RFP staining merged, and was restricted to and co-localized in the JGC (B, iii). Higher magnification images of the JGC insets are shown for renin staining (B, iv), RFP staining (B, v) and merged (B, vi). (C) Non-diseased mice given losartan: non-diseased Ren1cCreERxRs-tdTomato-R CoRL reporter mice treated with ARB losartan in drinking water for 25 days increased the number of cells in the JGC staining for renin (C, i) and RFP (C, ii). The majority of cells merged. Higher magnification images of the JGC are shown for renin staining (C, iv), RFP staining (C, v) and merged (C, vi). (D) Quantitative Data: Quantification of the percentage of all cells in the kidney cortex expressing RFP showed a significant increase in healthy, non-diseased animals given either enalapril or losartan compared to water alone control mice. Original magnification is ×400. Data presented as the mean±SD. These results show that in non-diseased reporter mice, RAAS inhibition increases the number of renin stained cells, and labeled cells of renin lineage and significantly increased the percentage of cells in the total kidney cortex cell population that comprised RFP+CoRL compared to mice given water alone. Data are represented as mean ±SD.
Next we assessed the labeling efficiency of the tamoxifen-inducible system used. Temporal CoRL labeling with the tdTomato reporter was conducted in reporter mice aged 8 weeks, well beyond the period of kidney development, and all studies described herein were only initiated after a 4-week tamoxifen washout period (Supplemental Figure 1). The advantage of this inducible labeling approach is that CoRL are fate mapped after their permanent labeling with the tdTomato-red reporter following tamoxifen administration. As expected, red fluorescent protein (RFP), used to detect tdTomato-red reporter, colocalized with RFP+CoRL, and these were limited to the JGC in baseline mice (Figure 2).
Of all the renin-stained cells in the entire kidney cortex at baseline (i.e., before disease induction), 95.71%±2.91% costained for RFP in mice given tamoxifen. Thus, labeling efficiency for RFP+CoRL was 95.71% in normal mice (P1 in formula; Table 1, Supplemental Figure 5). Similar to our previous report,19 RFP staining was not detected in two negative controls: reporter mice given corn oil (vehicle for tamoxifen) and cre-negative reporter mice given tamoxifen (results not shown). No renin+CoRL, RFP+ CoRL, or renin+/RFP+ CoRL were detected in glomeruli of reporter mice at baseline (Figure 2), as described previously.19
RAAS Inhibition Increases the Number of Labeled CoRL in the JGC in Nondiseased and FSGS Mice
We next assessed the effect of RAAS inhibition on the size of the reservoir in the JGC of renin+CoRL (N in formula) and on RFP+CoRL (P1). Figure 2 and Table 2 shows that when normal (baseline) reporter mice (i.e., no disease) were given enalapril or losartan for 25 days, the number of renin+CoRL in the JGC increased by 39.74% in the enalapril group and by 29.1% in the losartan group. This translated to the renin-staining population comprising 1.09%±0.09% and 3.09%±0.49% of the total kidney cortex cell population in enalapril- and losartan-treated mice before disease induction (Table 2). Renin mRNA expression in the kidney cortex confirmed the upregulation of renin by enalapril and losartan (Supplemental Figure 4).
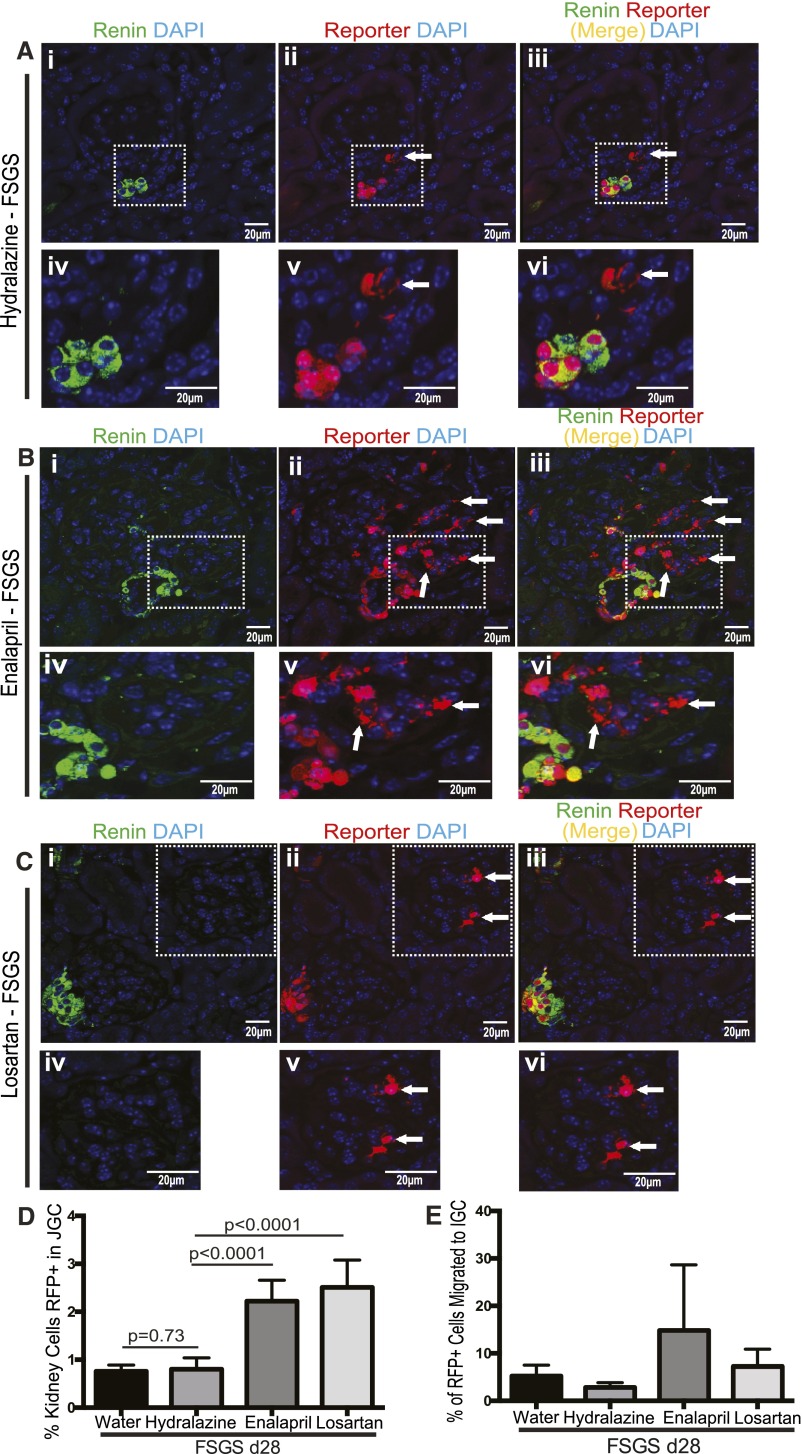
Next, we quantified renin+CoRL and RFP+CoRL in the JGC in FSGS mice at day 28 in all four treatment groups (Figure 3, Table 2). Despite a trend toward a decrease in the number of renin+CoRL in the JGC in FSGS mice given water or hydralazine, this did not reach statistical significance compared with nondiseased mice given water. Similarly, the number of RFP+CoRL in FSGS mice was not statistically significantly different in mice given water or hydralazine compared with baseline mice given water. Thus, during a 28-day period in disease, the number of renin+CoRL and RFP+CoRL did not change in the JGC in FSGS mice given water or hydralazine. When FSGS mice were given enalapril starting from day 3 of FSGS through day 28 of disease, the number of renin+CoRL and RFP+CoRL in the JGC increased 2.92-fold at day 28 of disease compared with water- and hydralazine-treated FSGS mice (Figure 3, Table 2). Similarly, the number of renin+CoRL and RFP+CoRL in the JGC increased 3.3-fold at day 28 of FSGS in the losartan-treated mice compared with water- and hydralazine-treated FSGS mice (Figure 3, Table 2). Taken together, these data show that administering enalapril or losartan to nondiseased mice or mice with FSGS increased the number of renin+CoRL (N in formula used in Table 1), as well as the number of CoRL that were permanently labeled with RFP in the JGC (i.e., RFP+CoRL = P1 in formula used in Table 1, Supplemental Figure 5).
Figure 3.
RAAS inhibition increases the number of labeled cells of renin lineage in the juxtaglomerular compartment and migration of fate-mapped cells of renin lineage in FSGS mice (A–C) Shows confocal images of co-staining for renin (green, first column) and the RFP reporter (red, middle column), and when the images were merged (right column); (D, E) shows quantitative data. Following the induction of experimental FSGS in Ren1cCreERxRs-tdTomato-R mice, diseased mice were randomized on day 3 to receive water, Hydralazine, Enalapril or Losartan in their drinking water for 25 days. Mice were analyzed at FSGS day 28. (A) Hydralazine treated FSGS mice: (A, i) In FSGS mice given Hydralazine, staining for renin was restricted to the juxta-glomerular compartment (JGC). (A, ii). RFP staining was detected in both the JGC and the intra-glomerular compartment (IGC, arrow shows example). (A, iii). RFP staining co-localized with renin staining (yellow) in the JGC, but not in the IGC. (A, iv–vi). Higher magnification images of the JGC are shown for renin staining (A, iv), RFP staining (A, v) and merged images (A, vi). (B) Enalapril treated FSGS mice: The ACE inhibitor Enalapril was added to the drinking water on day 3 FSGS for 25 days. (B, i) Renin was restricted to the JGC (B, ii). RFP staining increased in the JGC, and was readily detected in glomeruli in the IGC (arrows). (B, iii). RFP staining co-localized with RFP in the JGC. Higher magnification images of the insets for renin staining (B, iv), RFP staining (B, v) and merged images (B, vi) showing that renin localizes to the JGC, and that RFP+CoRL that have migrated to the IGC lack renin staining. (C) Losartan treated FSGS mice: Double staining for renin and RFP in FSGS mice given Losartan showed staining for renin in the JGC (C, i). RFP staining was present in the JGC and in the IGC (arrows show examples) (C, ii). RFP staining co-localized with renin in the JGC (yellow), but did not co-localize in the IGC (C, iii, arrows). Higher magnification images for renin staining (A, iv), RFP staining (A, v) and merged images (A, vi) show that RFP+CoRL in the IGC do not co-express renin. (D, E) Quantification of RFP+ cells in the juxta- (JGC) and intra- (IGC) glomerular compartment: (D) The percentage (%) of RFP+CoRL shown on the Y-axis was derived by dividing the total number of RFP stained cells in the JGC by the total number of DAPI positive cells in the kidney cortex. Enalapril and Losartan increased the % of RFP+CoRL in the JGC. (E) The percentage (%) of RFP+CoRL shown on the Y-axis was derived from the ratio of RFP+CoRL in the intra-glomerular compartment (IGC) that derived from the juxta-glomerular compartment (JGC). There was a trend in the Enalapril group to increased migration of RFP+CoRL from the JGC to the IGC in FSGS. Data presented as the mean ± standard deviation.
RAAS Inhibition Increases CoRL
Published studies show that the number of renin-expressing cells increases by the recruitment from neighboring non–renin-expressing cells (i.e., not by proliferation).34,35,37–39 However, given the marked increase in the number of RFP+CoRL, BrdU pulses were given on alternate days throughout the 25-day treatment period to accurately measure proliferation over the course of RAAS inhibition in both nondiseased and FSGS mice. Staining for BrdU (green) was not detected in RFP+CoRL in the JGC in baseline mice given water (not shown). In contrast, costaining showed that enalapril and losartan significantly increased BrdU incorporation in a subset of RFP+CoRL in the JGC (Figure 4, A and B). Similarly, when FSGS mice were given enalapril and losartan from day 3, BrdU staining was detected in both RFP+CoRL and renin+CoRL (Figure 4, C and D). In contrast, BrdU staining was absent in RFP+CoRL in FSGS mice given water or hydralazine (not shown). As stated earlier, BrdU was not detected in podocytes, and a very small number of RFP+CoRL in glomeruli of FSGS mice were BrdU positive.
Figure 4.
RAAS inhibition increases the number of labeled CoRL in the JGC and migration of fate-mapped CoRL in FSGS mice. (A–C) Confocal microscopy of co-staining of BrdU (green, left column), red fluorescent protein (RFP, red, middle column) and DAPI (blue), and merged images (yellow, right column) (A) Enalapril in non-diseased mice: (A, i) BrdU stained cell detected in the JGC; (A, ii) RFP stained cells in the JGC; (A, iii) BrdU stained cell co-localizes with RFP+ cell in the JGC (arrow head). (A, iv–vi) high power magnification of insets shown in figures above (B) Losartan in non-diseased mice: (B, i) BrdU stained cells detected in the JGC; (B,ii) RFP stained cells representing labeled cells of renin lineage; (B, iii) several RFP+ cells co-stain with BrdU (arrow heads). (B, iv–vi) high power magnification of insets shown in figures above (C) Enalapril in FSGS mice: (C, i) BrdU staining was detected in cells in the JGC (inset) and also in other cells in the tubule-interstitium; (C, ii) the number of RFP stained cells are higher in the JGC; (C, iii) BrdU co-stains in a subset RFP+CoRL in the JGC (arrow heads), but not RFP+CoRL in the IGC. (C, iv–vi) High power magnification of insets shown in figures above. (D) Losartan in FSGS mice: (D, i) BrdU staining was detected in cells in the JGC (inset); (D, ii) an increase in the number of RFP stained cells in the JGC (inset); (D, iii) BrdU co-staining in RFP+CoRL in the JGC (arrow head), but not RFP+CoRL in the IGC (Arrows). (D, iv–vi) high power magnification of insets shown in figures above.
Because RFP+CoRL were permanently labeled weeks before disease induction, BrdU administration, and RAAS inhibition, any BrdU incorporation in RFP+CoRL had to derive from proliferation of the existing population. These results therefore show for the first time that administering angiotensin-converting enzyme (ACE) inhibitors or angiotensin-receptor blockers (ARBs) to nondiseased mice and to FSGS mice increases RFP+CoRL proliferation of resident renin-expressing cells in the JGC, which contributes to their higher number.
Migration of Cells of Renin Lineage to the Glomerulus in FSGS Is Increased by RAAS Inhibition
Because RFP+CoRL were overwhelmingly detected only in the JGC before the onset of disease, we could lineage trace and fate map their migration to the IGC in disease and determine whether the rate was altered by RAAS inhibition. In baseline mice, neither renin-stained cells nor RFP+CoRL were scarcely ever detected in glomeruli (Figure 2, Table 3). At day 28 of FSGS, RFP+CoRL were detected in 4.88%±0.02% and 3.07%±0.01% of all cortical glomeruli in the water- and hydralazine-treated groups, respectively (P<0.001 versus baseline for both treatment groups).
Table 3.
RFP+CoRL that migrated to the IGC
| Data from IGC | Total No. of Glomeruli in Kidney Cortex | Total Glomeruli in Kidney Cortex That Contain RFP+CoRL, % | Total No. RFP+CoRL Detected in All Glomeruli in Kidney Cortex | Average No. RFP+CoRL per Glomerulus within Glomeruli Containing Such Cells | Labeled RFP+CoRL That Have Migrated from JGC to IGC |
|---|---|---|---|---|---|
| Baseline (no FSGS) | |||||
| H2O alone | 226.68±25.17 | 0.13±0.00 | 0.67±1.15 | 2 | 0.17±0.29 |
| FSGS day 28 | |||||
| FSGS+H2O | 214.6±26.04 | 4.88±0.024 | 21.2±8.7 | 2.04 | 5.2±2.32 |
| (P=0.54 versus H2O) | (P=0.01 versus H2O) | (P=0.01 versus H2O) | (P<0.05 versus H2O) | (P<0.01 versus H2O) | |
| FSGS+hydralazine | 196±4 | 3.07±0.01 | 17.3±6.4 | 2.89 | 2.83±0.98 |
| (P=0.28 versus FSGS H2O) | (P=0.27 versus FSGS H2O) | (P=0.53 versus FSGS H2O) | (P=0.15 versus FSGS H2O) | (P=0.15 versus FSGS H2O) | |
| FSGS+enalapril | 188.6±32.89 | 25.69±0.06a | 162.6±108.4 | 3.37b | 10.7±11.03 |
| (P=0.36 versus FSGS hydralazine) | (P=0.001 versus FSGS hydralazine) | (P<0.03 versus FSGS hydralazine) | (P=0.67 versus FSGS hydralazine) | (P=0.20 versus FSGS hydralazine) | |
| FSGS+losartan | 210.8±30.74 | 12.26±0.02c | 88±32.2 | 3.38d | 7.23±3.66 |
| (P=0.45 versus FSGS hydralazine) | (P=0.001 versus FSGS hydralazine) | (P=0.01 versus FSGS hydralazine) | (P=0.4 versus FSGS hydralazine) | (P=0.09 versus FSGS hydralazine) |
In FSGS, percentage of glomeruli with RFP+CoRL is increased 8.37-fold by enalapril compared with hydralazine treatment.
In FSGS, the number of RFP+CoRL was 1.16-fold higher in the enalapril-treated group compared with hydralazine treatment.
In FSGS, the percentage of glomeruli containing RFP+CoRL was four-fold higher in losartan group compared with hydralazine treatment.
In FSGS, within individual glomeruli containing RFP+CoRL, the number of RFP+CoRL were increased 1.17-fold by losartan compared with hydralazine treatment.
We next asked whether initiating RAAS inhibition after podocyte depletion at day 3 in experimental FSGS could augment the migration of RFP+CoRL to the glomerulus. In FSGS mice given enalapril, 25.69%±0.06% of all glomeruli contained RFP+CoRL (8.37-fold greater than hydralazine; P<0.001 versus hydralazine) (Figure 3, Table 3). In losartan-treated FSGS mice, 12.26%±0.02% of all glomeruli contained RFP+CoRL (4.0-fold greater than hydralazine; P<0.001) (Figure 3, Table 3).
These results show that of all the CoRL in the JGC in the entire kidney cortex that labeled with RFP (i.e., RFP+CoRL), 5.2%±2.32% migrated to the glomerulus in FSGS mice given water, and 2.83%±0.98% of RFP+CoRL from the JGC migrated to glomerulus in FSGS mice given hydralazine (Table 3). However, in FSGS mice, enalapril and losartan increased the rates of RFP+CoRL migration from the JGC to the IGC to 10.70%±11.03% (P=0.04 versus water) and 7.23%±3.66% (P=0.09 versus hydralazine), respectively (Table 3). Thus, RAAS inhibition significantly increased the rate of RFP+CoRL migration from the JGC to the IGC in FSGS mice. The overwhelming majority of RFP+CoRL in the IGC did not stain for renin in RAAS inhibition–treated FSGS mice (Figure 3, B and C).
We next asked whether, in addition to the higher number of glomeruli to which RFP+CoRL migrated, the number of RFP+CoRL within these glomeruli also increases. At day 28 of FSGS, those glomeruli to which RFP+CoRL had migrated contained an average of 2.04 RFP+CoRL per glomerulus in the water-treated group and 2.89 RFP+CoRL per glomerulus in the hydralazine-treated group (Table 3). However, the number increased by 1.16-fold in enalapril-treated FSGS mice (3.37 RFP+CoRL per glomerulus; P=0.67 versus water and hydralazine treatment) and by 1.17-fold in losartan-treated FSGS mice (3.38 RFP+CoRL per glomerulus; P<0.5 versus water and hydralazine treatment) (Table 3). Taken together, these results show that RFP+CoRL migrated to more glomeruli (P2 in equation) after enalapril and losartan administration to mice with FSGS, and in these glomeruli, the number of RFP+CoRL was also higher.
Transdifferentiation by CoRL to a Podocyte Phenotype Is Increased by RAAS Inhibition
Having shown a significantly higher number of RFP+CoRL in glomeruli after RAAS inhibition in FSGS, we next explored the rate of their transdifferentiation (P3 in the equation used in Table 1, Supplemental Figure 5), defined as the de novo expression of podocyte proteins, as we have previously reported.19–21 Figure 5, A and B, shows that a subset of RFP+CoRL in the IGC location coexpress the podocyte proteins synaptopodin and Wilms-tumor 1 (WT-1), respectively, and the quantitation is presented in Table 4. Transmission electron microscopy was also performed after immunoperoxidase staining for reporter (RFP) as previously described.20,21,40 RFP was detected in the CoRL surrounding the arterioles outside the glomerulus and renin granules were also observed, which served as a positive control (Figure 5C). Most podocytes lacked electron-dense (black) staining in their cell bodies and foot processes (Figure 5D). However, cells with reporter (RFP) staining contained electron-dense black material within their cell bodies and foot processes (Figure 5E), consistent with a subset of CoRL within the glomerular tuft having ultrastructural features of podocytes.
Figure 5.
CoRL express podocyte proteins and show ultrastructural features of podocytes in CoRL reporter mice with experimental FSGS after RAAS inhibition. (A) Confocal microscopy of double-staining for synaptopodin (synpo, green) and RFP (red). (A, i) in FSGS mouse at day 28, Synpo staining is restricted to podocytes; (A, ii) RFP staining detected within glomerulus (inset); (A, iii) Synpo and RFP merge (yellow, arrowhead) in the glomerulus. (A, iv–vi) Higher-power magnification of insets shown above. Note the morphology of the RFP+CoRL in the glomerulus in (A, v) that resembles characteristic podocyte features. (B) Confocal microscopy of WT-1 (green) and RFP (red) staining. (B, i) WT-1 staining is detected in the glomerulus in the nucleus in a typical podocyte distribution, and an occasional WT-1–stained cell is detected in the cytoplasm in the JGC. (B, ii) RFP staining is detected in the JGC and IGC. (B, iii) The merge shows that cells in the inset costain for nuclear WT-1 and for RFP. (B, iv–vi) High power of the insets above. Arrowhead shows example of cell in the glomerulus costaining for nuclear WT-1 and RFP. (C–E) Transmission electron micrograph of RFP-stained cells: (C) Renin granules are noted (black arrows). Arrowheads show RFP-labeled CoRL. (D) RFP does not colocalize with podocytes. (E) Subset of RFP stained cells have foot processes (arrowheads) characteristic of podocytes.
Table 4.
Transdifferentiation of RFP+CoRL to different glomerular cell types in the IGC
| Data from Intra-IGC | Podocytes | PECs | Mesangial Cells | |||
|---|---|---|---|---|---|---|
| Total No. of RFP+Synpo+ CoRL in Glomeruli in Entire Kidney Section | RFP+Synpo+/RFP+CoRL (Transdifferentiation Rate to a Podocyte Phenotype), % | Total No. of RFP+PAX8+ CoRL in Glomeruli in Entire Kidney Section | RFP+PAX8+/RFP+ CoRL (Transdifferentiation Rate to a PEC Phenotype), % | Total No. of RFP+α8I+ CoRL in Glomeruli in Entire Kidney Section | RFP+α8I+/RFP+ CoRL (Transdifferentiation Rate to a Mesangial Cell Phenotype), % | |
| Baseline (no FSGS) | ||||||
| H2O alone | 0 | 0 | 0 | 0 | 0 | 0 |
| FSGS day 28 | ||||||
| FSGS+H2O | 2.6±2.5 | 10.05±10.22 | 0 | 0 | 0 | 0 |
| (P=0.08 versus H2O) | (P>0.05 versus H2O) | |||||
| FSGS+hydralazine | 2.0±2.6 | 11.54±11.39 | 0 | 0.33±0.58 | 0 | 0 |
| (P=0.7 versus FSGS H2O) | (P=0.9 versus FSGS H2O) | (P=0.21 versus FSGS H20) | ||||
| FSGS+enalapril | 21±3.3a | 17.29±8.52 | 4.8±2.39b | 3.24±0.84 | 94.6±61.8c | 58.49±7.56 |
| (P<0.001 versus FSGS hydralazine) | (P=0.4 versus FSGS hydralazine) | (P=0.02 versus FSGS hydralazine) | (P=0.001 versus FSGS hydralazine) | (P<0.04 versus FSGS hydralazine) | (P<0.001 versus FSGS hydralazine) | |
| FSGS+losartan | 10.2±3.7d | 11.59±5.94 | 1.6±1.14 | 1.7±1.09 | 35.2±16.57 | 39.4±5.38 |
| (P=0.01 versus FSGS hydralazine) | (P=0.8 versus FSGS hydralazine) | (P=0.13 versus FSGS hydralazine) | (P=0.04 versus FSGS hydralazine) | (P=0.01 versus FSGS hydralazine) | (P<0.001 versus FSGS hydralazine) | |
In FSGS, the number of RFP+Synpo+CoRL was 10.5-fold higher in the enalapril-treated group compared with hydralazine treatment.
In FSGS, the number of RFP+PAX8+CoRL was 4.8-fold higher in the enalapril-treated group compared with hydralazine treatment.
In FSGS, the number of RFP+a8I+CoRL was 94.6-fold higher in the enalapril-treated group compared with hydralazine treatment.
In FSGS, the number of RFP+Synpo+CoRL was 5.1-fold higher in the losartan-treated group compared with hydralazine treatment.
Quantitative data in Table 4 shows the rate of transdifferentiation of RFP+CoRL in the glomerulus by measuring the percentage of RFP+CoRL that coexpress synaptopodin (P3 in the equation used in Table 1, Supplemental Figure 5). The transdifferentiation rates at day 28 were 10.05%±10.22%, 11.54%±11.39%, 17.29%±8.52%, and 11.59%±5.94% in FSGS mice given water, hydralazine, enalapril, and losartan, respectively (Table 4).
These results show that only enalapril increased the rate of CoRL transdifferentiation to a podocyte phenotype in FSGS mice.
Number of New Podocytes Derived from CoRL Is Highest after RAAS Inhibition in FSGS Mice
Table 1 shows the data used to measure the number of new podocytes (M) that derive from the pool of CoRL in the JGC under each condition tested, using the formula M=N×P1×P2×P3, where N represents the number of all CoRL in the JGC (see Table 2), P1 represents the percentage labeling efficiency (see Table 2), P2 represents the percentage of RFP+CoRL that migrated from the JGC to the IGC (see Table 3), P3 represents the transdifferentiation rate (defined as intraglomerular RFP+CoRL coexpressing synaptopodin) (see Table 4).
Under baseline conditions, M equals zero because no synpo+RFP+CoRL were detected in glomeruli. In FSGS mice treated with water or with hydralazine, M=2.09 and 1.37, respectively. In enalapril-treated FSGS mice, M=23.74, which is 17-fold higher than seen in the hydralazine group. In the losartan-treated mice, M=11.05, which was 7.92-fold higher than in the hydralazine group. Taken together, these data show that the number of podocytes derived from CoRL was significantly higher in mice with RAAS inhibition.
Labeled CoRL Coexpress PECs and Mesangial Cell Markers after RAAS Inhibition
Because we19 and others22,23 have reported that CoRL can transdifferentiate to a PEC and mesangial cell phenotype, respectively, in glomerular disease, we measured markers for PECs (PAX8) (Figure 6A) and mesangial cells (α8 integrin) (Figure 6B). The number of RFP+PAX8+ cells increased 9.8-fold and 5.2-fold in FSGS mice given enalapril and losartan, respectively, compared with FSGS mice given water and hydralazine (Table 4).
Figure 6.
CoRL express PEC and mesangial cell proteins in CoRL reporter mice with experimental FSGS after RAAS inhibition. (A) Confocal microscopy of PAX8 (green) and RFP (red) costaining. (A, i) PAX8 staining detected in classic PEC distribution along Bowman’s capsule. (A, ii) RFP-stained cell along Bowman’s capsule within inset box. (A, iii) Cell along Bowman’s capsule costaining for RFP and PAX8. (A, iv–vi) Higher-power magnification of insets above. (B) Confocal microscopy of (B, i) α8 integrin staining detected in a classic mesangial cell distribution. (B, ii) RFP-stained cells in the JGC and in the glomerulus. (B, iii) Cell costaining for α8 integrin and RFP. (B, iv–vi) Higher-power magnification of insets above.
RFP+α8integrin+ cells were not detected in the glomeruli of FSGS mice given water or hydralazine (Table 4). In contrast, RFP+α8integrin+ cells were detected in glomeruli of FSGS mice given enalapril and losartan (Table 4).
Discussion
Podocyte depletion is a critical determinant underlying the onset and magnitude of glomerulosclerosis in glomerular diseases.5,7,8 From a clinical standpoint, a goal of therapy is to raise podocyte number above the threshold at which glomerulosclerosis occurs. However, because adult podocytes are unable to proliferate,3,4 any self-renewal depends on stem/progenitor cells. Inhibiting the RAAS with ACE inhibitors and ARBs is the mainstay therapy in glomerular diseases characterized by podocyte depletion.41 In addition to their pleiotropic cellular effects on podocytes and other kidney cell types, we27 and others28,29 have recently shown that treating mice with glomerular disease by using ACE inhibitors and ARBs lead to higher podocyte numbers than control therapies, despite the absence of podocyte proliferation.30
In the current study, initiating enalapril on day 3 of FSGS when podocytes were depleted by 40.6% resulted in a higher podocyte number at day 28 compared with water or hydralazine treatments. Similar results were obtained with losartan. BrdU administration throughout the study showed that the increase in podocyte number from day 3 to day 28 was not due to podocyte proliferation. This result was consistent with other sources of cells, likely stem/progenitors that served to partially replace podocytes in disease after RAAS inhibition. The results of the current studies show that RAAS inhibition augments the formation of new podocytes derived from CoRL by increasing the size of their reservoir, their migration from the JGC to the IGC, and in addition for ACE inhibitors, an increase CoRL transdifferentiation to express podocyte proteins. In addition, the pluripotential of CoRL was augmented by RAAS inhibition when a subset of CoRL also de novo coexpressed protein markers considered unique to PECs and mesangial cells.
Renin-producing cells, also called CoRL, normally situated in the JGC along the afferent arteriole, make up <0.01% of the total kidney cell number under normal conditions.23 Several groups, including ours, have shown that adult CoRL exhibit marked plasticity under different circumstances, being capable of transdifferentiating to vascular smooth muscle cells,23 mesangial cells,22 erythropoietin-producing cells,24 pericytes,20,25 glomerular PECs,19 and podocytes.19–21 However, to date it is unclear whether CoRL plasticity can be enhanced in glomerular disease and to what extent this might contribute to replacing podocytes. Given the influence of RAAS inhibition on podocyte number, we designed the current studies in Ren1cCreERxRs-tdTomato-R reporter mice to specifically determine whether RAAS inhibition augmented the contribution of CoRL to podocyte replacement (called M) after their depletion in FSGS, quantitated by the formula M=N×P1×P2×P3, by quantitating the following variables: the reservoir of CoRL (called N in the formula), their labeling efficiency (called P1), their migration from the JGC to the IGC (called P2), and/or their transdifferentiation (called P3).
Our results show that renin+CoRL comprise 0.78%±0.15% of the entire kidney cortex cell population in Ren1cCreERxRs-tdTomato-R mice. This number constitutes the reservoir from which CoRL stem/progenitors derive (designated N in formula). When nondiseased Ren1cCreERxRs-tdTomato-R CoRL reporter mice were given tamoxifen to induce permanent labeling of this cell population, the labeling efficiency of renin+CoRL with RFP was 95.71%±2.91% (approximate labeling efficiency; P1 in formula). These labeled cells were named RFP+CoRL and represent the majority of the reservoir of cells traceable in these studies; however, it is a small population with respect to the entire kidney cortex.
The first major finding in the current study was that RAAS inhibition induces CoRL proliferation. The results from the current study shows that RAAS inhibition significantly increased the number of renin+CoRL in normal reporter mice from 0.78%±0.15% of the entire cell population in the kidney cortex to 1.09%±0.09% and 3.05%±0.49% after enalapril and losartan treatment, respectively. Previous reports have shown that RAAS inhibition increases renin+CoRL.42 RAAS inhibition also significantly increases the number of RFP+CoRL in normal mice. Thus, RAAS inhibition increases the reservoir of RFP+CoRL in normal mice from which a subset of glomerular cell stem/progenitors can arise. However, because RFP+CoRL were labeled during a finite temporal period several weeks before the study, any increase in their number had to derive from existing labeled cells. Pioneering studies by Gomez35,38,39 and Kurtz34,37 showed that to increase renin production, rather than increasing the amount of renin-production per existing cell and rather than proliferating, neighboring vascular smooth muscle cells are “recruited” to “differentiate” into renin-producing cells. This typically occurs retrograde from the JGC along preglomerular arterioles.34,35,37–39 When ACE inhibition negates the negative feedback effects of angiotensin II on renin, renin-producing cell recruitment increases significantly.23,34
Because proliferation of renin+CoRL has not been well demonstrated to date, we administered BrdU injections to normal (nondiseased) reporter mice on alternate days throughout the 25-day period of enalapril and losartan treatments. A first major finding in the current study was that RAAS inhibition significantly increased proliferation of both RFP-CoRL and RFP+CoRL, normally residing in the JGC. However, not all RFP-CoRL in the increased reservoir coexpressed BrdU, consistent with recruitment. In addition, both enalapril and losartan increased the reservoir of RFP+CoRL in the JGC in experimental FSGS compared with FSGS mice given water or hydralazine. RAAS inhibition also increased RFP+CoRL proliferation in FSGS mice. CoRL proliferation was unlikely due to BP-lowering because BPs were not significantly different in RAAS-inhibited mice compared with hydralazine-treated mice. Taken together, the data show that the reservoir (N) of CoRL from which potential progenitor might arise to replace podocytes is increased by RAAS inhibition. We also interpret these results to be consistent with a new biologic paradigm whereby RAAS inhibition increases CoRL number in the JGC by both recruitment from nonrenin cells, such as vascular cells, and by proliferation of existing CoRL. These results are similar to that reported for nonrenal endothelial progenitor cells.43,44
A second major finding was that the migration of RFP+CoRL from the JGC to the IGC was increased by RAAS inhibition after podocyte depletion. A strength of inducible cell fate mapping systems such as we have used in these studies is that a subpopulation of cells are only permanently labeled within the temporal window after tamoxifen administration. No additional labeling can occur thereafter. After a 4-week washout period, our results showed that labeled CoRL (RFP+CoRL) were restricted to the JGC; no glomeruli contained significant RFP+CoRL. Similar to what we have previously reported after abrupt podocyte depletion in FSGS,19,20 RFP+CoRL are detected in a small number of glomeruli. This is consistent with the migration of RFP+CoRL from their original location in the JGC to the new location comprising the IGC. When FSGS mice were started with RAAS inhibition at day 3, coinciding with marked podocyte depletion, the number of glomeruli containing RFP+CoRL increased 8.37-fold and 4.0-fold in the enalapril and losartan groups, respectively, compared with hydralazine treatment. These data are consistent with RAAS augmenting migration (P2 in formula above) of a labeled subpopulation of RFP+CoRL from the JGC to the IGC in FSGS. Of course we cannot measure the migration of unlabeled CoRL (RFP-CoRL), nor can we use renin as a marker because cells no longer express renin when they migrate from their original location in the JGC. Taken together, the findings show that the degree of CoRL migration is likely underreported under these conditions.
In addition to quantitating the number of glomeruli to which RFP+CoRL migrated, we also measured the number of RFP+CoRL within these glomeruli. The average number of RFP+CoRL in those glomeruli-containing labeled cells in FSGS mice given water and hydralazine was 2.04 and 2.89, respectively. Giving enalapril and losartan to FSGS mice also increased the number of RFP+CoRL within glomeruli by 1.65- and 1.9-fold, respectively. Taken together, these results show two aspects related to the migration of RFP+CoRL in disease: the number of glomeruli to which RFP+CoRL migrate is augmented substantially by RAAS inhibition, and the number of individual RFP+CoRL within these glomeruli was also higher. Finally, the quantitative data enabled us to measure the rate at which RFP+CoRL migrated from the JGC to the IGC (i.e., P2 in the formula). The results show that compared with FSGS mice given hydralazine, the rate of migration increased 17-fold after enalapril and 7.92-fold after losartan. Taken together, the data show that according to the formula M=N×P1× P2×P3 in Table 1 (Supplemental Figure 5), both N and P2 are markedly higher in FSGS mice after RAAS inhibition.
A third major finding in these studies was that transdifferentiation of intraglomerular RFP+CoRL to acquire podocyte markers was increased by enalapril. Having shown that RAAS inhibition increases the reservoir of CoRL in the JGC and the migration of RFP+CoRL from the JGC to the IGC, we next addressed the rate of transdifferentiation of RFP+CoRL (i.e., P3 in formula), defined as the rate at which RFP+CoRL in the IGC that begin to coexpress podocyte proteins. Subsets of RFP+CoRL in the IGC coexpressed synaptopodin and WT-1, and a subset exhibited typical ultrastructural features of podocytes by electron microscopy. Next, we calculated the rate of conversion of transdifferentiation of RFP+ that had migrated from the JGC to the IGC to become RFP+/synpo+ cells (i.e., P3 in formula) in FSGS mice. The results showed that P3 was 10.05% and 11.54% in water- and hydralazine-treated mice, respectively, and that losartan did not affect this process (11.59%). However, enalapril increased the rate of transdifferentiation by 1.5-fold over all other treatments. Taken together, the major mechanisms whereby RAAS augments CoRL stem/progenitors becoming podocytes is by increasing the pool of these cells, increasing migration, and to a lesser extent transdifferentiation by ACE inhibition.
A fourth major finding in the current studies was that RAAS inhibition also increased the pluripotential of RFP+CoRL toward a PEC and mesangial cell fates in FSGS. Because CoRL also have the capacity to transdifferentiate into vascular smooth muscle cells,23 mesangial cells,22 erythropoietin-producing cells,24 pericytes,20,25 and glomerular PECs,19 we performed costaining for markers of mesangial cells (α8 integrin), and PECs (PAX8) in all groups of FSGS. Within glomeruli, RFP+CoRL in the water- and hydralazine-treated groups did not coexpress mesangial markers, although occasional cells coexpressed PEC markers, as we have previously reported.19 After RAAS inhibition in FSGS mice, the number of cells coexpressing RFP+/PAX8+ along Bowman’s capsule also increased, albeit not to the extent of podocytes derived from RFP+CoRL. RFP+/α8 integrin+ cells were detected in less than 5% of all glomeruli in RAAS-treated FSGS mice, but were not detected in FSGS mice given water or hydralazine. These results show that in an experimental model of abrupt podocyte depletion followed by RAAS inhibition, a subset of RFP+CoRL that have migrated to the glomerulus no longer express renin but can coexpress markers of either podocytes, PECs, or mesangial cells. However, a large portion of intraglomerular RFP+CoRL do not coexpress any glomerular cell protein markers. It is unclear if they might with time.
Finally, when one applies the formula N×P1×P2×P3 to determine the number of new podocytes formed (M) from traceable RFP+CoRL, we learn that enalapril and losartan increases M by 17-fold and eight-fold. We acknowledge that the increase in CoRL in the glomerulus does not fully explain the higher podocyte number in RAAS-treated FSGS mice. One might therefore argue that the overall contribution by CoRL to the podocyte pool is very low, prompting the biologic question of its significance. In doing so, we need to be reminded of the seminal studies lead by Wiggins,45 who showed that incremental decreases in podocyte number had profound effects on glomerular scarring. By analogy, incremental increases in podocyte number derived from CoRL are likely to limit the progression of glomerular scarring. Romagnani14,16 has shown that PECs are stem/progenitor candidates for adult podocytes, and our data in the model used in the current studies support this notion.12 Therefore, the sum of CoRL, PECs, and perhaps other stem/progenitor cells collectively contribute to replacing podocytes, consistent with the notion that each stem/progenitor cell pool is critical. We do not expect that any stem/progenitor cell will fully restore the normal quota of podocytes. From a clinical standpoint, this is not required because the “optimal target” for podocyte restoration should be the number above which glomerular scarring occurs and/or progresses. Many studies have shown that in addition to increasing cell number, stem/progenitor cells also contribute to overall improvement by producing and secreting cytokines and growth factors that augment the function of resident and other cell types.
The mechanisms responsible for how CoRL might gain access to and populate the visceral and PEC layers of the glomerulus might include transmigration and transdifferentiation in situ. A limitation in the study is that although we show for the first time that RAAS inhibition improves podocyte number in part by augmenting CoRL as probable podocyte progenitors, specific mechanisms are not provided. Future studies will be aimed at the mechanisms of proliferation, migration, and transdifferentiation. We also do not know the duration of the beneficial effects of RAAS inhibition on CoRL.
In summary, the results of the current study show that when an ACE inhibitor or ARB is given to mice with FSGS at a disease time point when podocyte number is markedly depleted, they stimulate proliferation and increase the pool of CoRL in the JGC, and also increase their migration to the glomerulus. Once in the IGC, ACE inhibitors further augment the de novo expression of podocyte proteins by RFP+CoRL. In addition, subsets of intraglomerular RFP+CoRL also begin to coexpress markers of PECs and mesangial cells after RAAS inhibition in FSGS mice. These studies broaden the biologic benefits of RAAS inhibition in glomerular diseases typified by podocyte depletion.
Concise Methods
Transgenic Mice
We generated inducible Ren1cCreERxRs-tdTomato-R mice as described previously.19 To temporally induce permanent labeling of CoRL, Ren1cCreERxRs-tdTomato-R mice were given 100 mg/kg tamoxifen four times intraperitoneally every other day starting at 8 weeks of age (Supplemental Figure 1). A tamoxifen washout period of 4 weeks followed before initiation of experimental FSGS and/or RAAS inhibition. Mice were housed in the animal care facility of the University of Washington under specific pathogen-free conditions with food and water available ad libitum. These studies were reviewed and approved by the University of Washington Institutional Animal Care and Use Committee (2968–04).
Experimental FSGS
Experimental FSGS was induced in male Ren1cCreERxRs-tdTomato-R mice with a cytotoxic antiglomerular antibody (Supplemental Figure 1). Briefly, two doses of sheep antiglomerular antibody at 12 mg/20g body wt via intraperitoneal injection, 24 hours apart, induced abrupt podocyte depletion, accompanied by glomerulosclerosis.21,27 On day 3 of disease, when podocyte number is typically decreased by 30%–40% from baseline, mice were randomly assigned into the following treatment groups: Group 1 (n=7) received drinking water (the vehicle for hydralazine, enalapril, and losartan); group 2 (n=5) received the ACE inhibitor enalapril (75 μg/ml); group 3 (n=6) received the ARB losartan (100 μg/ml); and group 4 (n=5) received hydralazine (300 μg/ml) as a control for BP. Mice were euthanized on day 28.
In all animals described, urine was collected before euthanasia at baseline, day 14, and day 28. At euthanasia, mice were perfused with ice-cold PBS to remove excess red blood cells. Kidneys were split in half. One half was fixed overnight at 4°C in 10% neutral buffered formalin (Globe Scientific, Paramus, NJ), rinsed in 70% ethanol, processed, and embedded in paraffin. The other half was fixed for 45 minutes in 4% paraformaldehyde solution (PFA) in PBS (Affymetrix, Santa Clara, CA), washed in 30% sucrose overnight at 4°C, patted dry, rinsed briefly in optimal cutting temperature medium OCT, embedded in OCT, and frozen in a dry ice 100% ethanol bath. Sections were then cut from the paraffin or OCT blocks at a thickness of 4 μm. A piece of kidney cortex was stored in RNAlater (Ambion, Life Technologies, Grand Island, NY) until further processing of RNA (see below).
Sheep Antipodocyte Antibody Binding
To verify the binding of injected sheep antiglomerular antibody to podocytes within the glomerulus, immunofluorescence staining for sheep IgG was performed. Biotinylated anti-sheep IgG (H+L) secondary antibody (Vector Laboratories, Burlingame, CA) followed by streptavidin conjugated with AlexaFluor488 (Invitrogen, Waltham, MA) were used on cryosections as previously described.12
BrdU Labeling of Mice to Assess Proliferation
BrdU was administered to quantitate cell proliferation. All animal groups, including baseline mice, received BrdU 10 μl per g body wt (BrdU-Amersham Cell Proliferation Labeling Reagent; GE Healthcare Life Sciences, Little Chalfont, United Kingdom) for 25 days on alternate days, starting at day 3 of FSGS, until euthanasia. In the enalapril and losartan groups without FSGS, BrdU was also administered on alternate days, for 25 days total, to match the FSGS animals.
Immunostaining
All immunostaining described here was performed and analyzed on each mouse enrolled in the study (i.e., at baseline and at each time point in disease). Indirect immunoperoxidase and immunofluorescence staining were performed on 4-μm tissue sections from mouse renal biopsy specimens fixed in formalin and embedded in paraffin as described previously, with minor modifications.12,19–21,27 In brief, paraffin was removed using Histoclear (National Diagnostics, Atlanta, GA) and sections were rehydrated in a graded series of ethanol. Except where noted otherwise, antigen retrieval was performed by boiling in 10 mM citric acid buffer, pH 6.0. Nonspecific protein binding was blocked using Background Buster (Accurate Chemical & Scientific, Westbury, NY) for 20 minutes at room temperature. Endogenous biotin activity was suppressed with an Avidin/Biotin Blocking Kit (Vector Laboratories). Antibodies were diluted in 1% IgG free BSA in PBS. Initial primary antibodies were incubated overnight at 4°C. In the case of double immunostaining, subsequent primary antibodies were incubated overnight at 4°C or for 3 hours at room temperature. Secondary antibodies and streptavidin conjugates were incubated for 1 hour at room temperature. All immunofluorescence samples were mounted using Vectashield mounting medium with DAPI (Vector Laboratories).
Identifying CoRL
To identify CoRL in paraffin-embedded slides of tamoxifen-induced Ren1cCreERxRs-tdTomato-R mice, we performed immunostaining with an anti-RFP antibody (Dylite 594 conjugated RFP rabbit antibody; Rockland Immunochemicals for Research, Gilbertsville, PA). Double immunostaining with the reporter RFP and renin (biotinylated anti-renin; Innovative Research, Novi, MI) was performed to detect renin expression.
Double Staining of CoRL and Markers for Podocytes, PECs, and Mesangium
The podocyte proteins synaptopodin (Fitzgerald Industries International, Acton, MA) and WT-1 (Spring Bioscience, Pleasanton, CA) were identified by immunostaining. PECs were detected by immunostaining for PAX8 (ProteinTech Group, Inc., Rosemont, IL). Biotinylated α8 integrin (R&D Systems Inc., Minneapolis, MN) was used as a mesangial specific marker. Biotinylated anti-rabbit IgG or anti-mouse IgG followed by streptavidin conjugated with AlexaFluor488 (green signal) was applied to detect the primary antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA).
Assessment of Glomerulosclerosis and Podocyte Number
Immunostaining was performed for p57 with periodic acid-Schiff (PAS) counterstaining to measure podocyte number and assess glomerulosclerosis, as previously reported.27,36 In brief, paraffin sections were processed as described above, with antigen retrieval in 1 mM EDTA, pH 8.0. Endogenous peroxidase activity was quenched with 3% hydrogen peroxide and sections were incubated overnight at 4°C with a primary rabbit anti-p57 (1:800; Santa Cruz Biotechnology, Santa Cruz, CA), followed by rabbit-on-rodent horseradish peroxidase–polymer (Biocare Medical, Concord, CA). Visualization of immunostaining was by precipitation of diaminobenzidine (Sigma-Aldrich, St. Louis, MO). Counterstaining was performed with PAS by washing slides in fresh 0.5% periodic acid (Sigma-Aldrich) for 8 minutes, 5 minutes in ddH2O, incubating for 10 minutes at room temperature with Schiff reagent (Sigma-Aldrich), 5 minutes in fresh 0.5% sodium metabisulfate (Sigma-Aldrich), and 5–10 minutes under running warm tap water. Slides were dehydrated in ethanol and mounted with Histomount. An average of 100 glomeruli in each animal were graded for the assessment of glomerulosclerosis.
Microscopy
Immunostaining was examined on a Leica DMRB microscope and an EVOS FL Cell Imaging System using 100×, 200×, and 400× magnification. Images were collected using confocal microscopy on a Leica DMI400B.
Immunoelectron Microscopy
To better visualize the morphology of CoRL when in a glomerular location, immunoperoxidase staining was performed with a rabbit antibody to RFP (Rockland Immunochemicals for Research). The tomato red reporter was then visualized with electron-dense diaminobenzidine that was reacted with 2% OsO4, dehydrated and infiltrated with a 50/50 mixture of PolyBed (PolySciences, Inc., Warrington, PA) and propylene oxide. Ultrathin sections were prepared, mounted on grids, and examined by transmission electron microscopy, as previously described.40
Quantification of CoRL tdTomato Reporter with Renin and Markers for Podocytes, PECs, and Mesangium
The total number of cells in kidney cortex was quantified by capturing 20±5 fluorescent fields of DAPI-stained sections from each animal using a 100× magnification on an EVOS FL Cell imaging system. Counting DAPI-stained cells was done with the ImageJ 1.48d software (The National Institutes of Health, Bethesda, MD) according to The ImageJ User Guide. Briefly, ImageJ “adjust threshold” dialog was used to find the foreground areas, the watershed algorithm was used to separate touching nuclei, and the “analyze the segmented objects” tool was used to count total nuclei.
Quantification of α-RFP/renin costaining was performed on fluorescence-stained sections. The number of positively stained cells were counted per kidney cortex using a 200× magnification. Kidney cortex was divided into two compartments: intraglomerular and juxtaglomerular regions. Absolute number of RFP+, renin+, and RFP+/renin+–expressing cells were counted in each compartment. Results were expressed in two ways: as a percentage of RFP+ or renin+ cells per total cell number in each kidney cortex section and as a percentage of colocalized RFP+/renin+ cells per number of renin-expressing cells.
Quantification of RFP+ cells colocalizing with podocyte, PEC or mesangial cell markers was assessed by α-RFP/synaptopodin, α-RFP/Pax8, or α-RFP/α8 integrin fluorescence staining respectively. A double-positive cell was defined as a yellow cell, which results from an overlap between red (α-RFP) and green (synaptopodin, PAX8, or α8 integrin) signals. A mean of 198±20 glomeruli were assessed for quantification. Transdifferentiation rate of CoRL (RFP+ cells) to podocytes, PECs and mesangial cells were expressed as a percentage of colocalized cells per total number of RFP+ cells in kidney cortex.
The number of podocytes was measured by P57/PAS staining as previously described.27 An average 200±32 glomeruli were assessed for each animal for total podocyte counts.
Urine Collection, Urine Albumin Assay, and Urine Creatinine Assay
Spot urine was collected at baseline, day 14, and day 28. Urine albumin was determined using Albuwell M Elisa Assay (Exocell, Philadelphia, PA), and urine creatinine was determined using a colorimetric micro-plate assay (Cayman Chemical Company, Ann Arbor, MI).
BP Measurement
BP was measured using the CODA 6 noninvasive tail-cuff system (Kent Scientific, Torrington, CT) on conscious mice, as previously described.30,46 BP was measured before the start of disease induction (baseline reading), and during disease at days 7, 14, and 28.
Quantitative RT-PCR
Total RNA was extracted using the RNeasy Mini Kit (Qiagen, Germantown, MD). cDNA was synthesized using iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA). Quantitative RT-PCR assay was performed using an Rotor-Gene 6000 (Corbett Lifescience) and iTaq SYBR Green Supermix with ROX (Bio-Rad). The cycling conditions consisted of denaturation at 95°C for 2.5 minutes, followed by PCR cycles of 95°C for 15 seconds and amplification at 58°C for 30 seconds. Specificity of each primer pair was confirmed by verification of single PCR product with expected size in agarose gel electrophoresis. The sequences of primers are 18S forward TAGAGGGACAAGTGGCGTTC, 18S reverse CGCTGAGCCAGTCAGTGT, Ren1 forward GGAGGAAGTGTTCTCTGTCTACTACA, Ren1 reverse GCTACCTCCTAGCACCACCTC.
Statistical Analyses
Groups were compared using a two-tailed unpaired t test between two groups and ANOVA among groups of more than three. P values <0.05 represented statistically significant differences.
Disclosures
None.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health grants: 1R01DK097598-01A1, R01DK056799-10, and 5R01DK093493-04 (S.J.S.). J.L. was supported by the Deutsche Forschungsgemeinschaft (DFG, Li 2074/1-1).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2015080877/-/DCSupplemental.
References
- 1.Grahammer F, Schell C, Huber TB: The podocyte slit diaphragm--from a thin grey line to a complex signalling hub. Nat Rev Nephrol 9: 587–598, 2013 [DOI] [PubMed] [Google Scholar]
- 2.Tharaux PL, Huber TB: How many ways can a podocyte die? Semin Nephrol 32: 394–404, 2012 [DOI] [PubMed] [Google Scholar]
- 3.Pavenstädt H, Kriz W, Kretzler M: Cell biology of the glomerular podocyte. Physiol Rev 83: 253–307, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Griffin SV, Petermann AT, Durvasula RV, Shankland SJ: Podocyte proliferation and differentiation in glomerular disease: role of cell-cycle regulatory proteins. Nephrol Dial Transplant 18[Suppl 6]: vi8–vi13, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Wharram BL, Goyal M, Wiggins JE, Sanden SK, Hussain S, Filipiak WE, Saunders TL, Dysko RC, Kohno K, Holzman LB, Wiggins RC: Podocyte depletion causes glomerulosclerosis: Diphtheria toxin-induced podocyte depletion in rats expressing human diphtheria toxin receptor transgene. J Am Soc Nephrol 16: 2941–2952, 2005 [DOI] [PubMed] [Google Scholar]
- 6.D’Agati VD: Podocyte injury in focal segmental glomerulosclerosis: Lessons from animal models (a play in five acts). Kidney Int 73: 399–406, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Barisoni L: Podocyte biology in segmental sclerosis and progressive glomerular injury. Adv Chronic Kidney Dis 19: 76–83, 2012 [DOI] [PubMed] [Google Scholar]
- 8.Kim YH, Goyal M, Kurnit D, Wharram B, Wiggins J, Holzman L, Kershaw D, Wiggins R: Podocyte depletion and glomerulosclerosis have a direct relationship in the PAN-treated rat. Kidney Int 60: 957–968, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Ohse T, Vaughan MR, Kopp JB, Krofft RD, Marshall CB, Chang AM, Hudkins KL, Alpers CE, Pippin JW, Shankland SJ: De novo expression of podocyte proteins in parietal epithelial cells during experimental glomerular disease. Am J Physiol Renal Physiol 298: F702–F711, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang J, Hansen KM, Pippin JW, Chang AM, Taniguchi Y, Krofft RD, Pickering SG, Liu ZH, Abrass CK, Shankland SJ: De novo expression of podocyte proteins in parietal epithelial cells in experimental aging nephropathy. Am J Physiol Renal Physiol 302: F571–F580, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang J, Pippin JW, Vaughan MR, Krofft RD, Taniguchi Y, Romagnani P, Nelson PJ, Liu ZH, Shankland SJ: Retinoids augment the expression of podocyte proteins by glomerular parietal epithelial cells in experimental glomerular disease. Nephron, Exp Nephrol 121: e23–e37, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eng DG, Sunseri MW, Kaverina NV, Roeder SS, Pippin JW, Shankland SJ: Glomerular parietal epithelial cells contribute to adult podocyte regeneration in experimental focal segmental glomerulosclerosis. Kidney Int 88: 999–1012, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuppe C, Gröne HJ, Ostendorf T, van Kuppevelt TH, Boor P, Floege J, Smeets B, Moeller MJ: Common histological patterns in glomerular epithelial cells in secondary focal segmental glomerulosclerosis. Kidney Int 88: 990–998, 2015 [DOI] [PubMed] [Google Scholar]
- 14.Lazzeri E, Romagnani P: Podocyte biology: Differentiation of parietal epithelial cells into podocytes. Nat Rev Nephrol 11: 7–8, 2015 [DOI] [PubMed] [Google Scholar]
- 15.Ronconi E, Sagrinati C, Angelotti ML, Lazzeri E, Mazzinghi B, Ballerini L, Parente E, Becherucci F, Gacci M, Carini M, Maggi E, Serio M, Vannelli GB, Lasagni L, Romagnani S, Romagnani P: Regeneration of glomerular podocytes by human renal progenitors. J Am Soc Nephrol 20: 322–332, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sagrinati C, Netti GS, Mazzinghi B, Lazzeri E, Liotta F, Frosali F, Ronconi E, Meini C, Gacci M, Squecco R, Carini M, Gesualdo L, Francini F, Maggi E, Annunziato F, Lasagni L, Serio M, Romagnani S, Romagnani P: Isolation and characterization of multipotent progenitor cells from the Bowman’s capsule of adult human kidneys. J Am Soc Nephrol 17: 2443–2456, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Smeets B, Moeller MJ: Parietal epithelial cells and podocytes in glomerular diseases. Semin Nephrol 32: 357–367, 2012 [DOI] [PubMed] [Google Scholar]
- 18.Barajas L: Anatomy of the juxtaglomerular apparatus. Am J Physiol 237: F333–F343, 1979 [DOI] [PubMed] [Google Scholar]
- 19.Pippin JW, Sparks MA, Glenn ST, Buitrago S, Coffman TM, Duffield JS, Gross KW, Shankland SJ: Cells of renin lineage are progenitors of podocytes and parietal epithelial cells in experimental glomerular disease. Am J Pathol 183: 542–557, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pippin JW, Kaverina NV, Eng DG, Krofft RD, Glenn ST, Duffield JS, Gross KW, Shankland SJ: Cells of renin lineage are adult pluripotent progenitors in experimental glomerular disease. Am J Physiol Renal Physiol 309: F341–F358, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pippin JW, Glenn ST, Krofft RD, Rusiniak ME, Alpers CE, Hudkins K, Duffield JS, Gross KW, Shankland SJ: Cells of renin lineage take on a podocyte phenotype in aging nephropathy. Am J Physiol Renal Physiol 306: F1198–F1209, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Starke C, Betz H, Hickmann L, Lachmann P, Neubauer B, Kopp JB, Sequeira-Lopez ML, Gomez RA, Hohenstein B, Todorov VT, Hugo CP: Renin lineage cells repopulate the glomerular mesangium after injury. J Am Soc Nephrol 26: 48–54, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sequeira López ML, Pentz ES, Nomasa T, Smithies O, Gomez RA: Renin cells are precursors for multiple cell types that switch to the renin phenotype when homeostasis is threatened. Dev Cell 6: 719–728, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Kurt B, Paliege A, Willam C, Schwarzensteiner I, Schucht K, Neymeyer H, Sequeira-Lopez ML, Bachmann S, Gomez RA, Eckardt KU, Kurtz A: Deletion of von Hippel-Lindau protein converts renin-producing cells into erythropoietin-producing cells. J Am Soc Nephrol 24: 433–444, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stefanska A, Eng D, Kaverina N, Pippin JW, Gross KW, Duffield JS, Shankland SJ: Cells of renin lineage express hypoxia inducible factor 2α following experimental ureteral obstruction. BMC Nephrol 17: 5, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wennmann DO, Hsu HH, Pavenstädt H: The renin-angiotensin-aldosterone system in podocytes. Semin Nephrol 32: 377–384, 2012 [DOI] [PubMed] [Google Scholar]
- 27.Zhang J, Yanez D, Floege A, Lichtnekert J, Krofft RD, Liu ZH, Pippin JW, Shankland SJ: ACE-inhibition increases podocyte number in experimental glomerular disease independent of proliferation. J Renin Angiotensin Aldosterone Syst 16: 234–248, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Benigni A, Morigi M, Rizzo P, Gagliardini E, Rota C, Abbate M, Ghezzi S, Remuzzi A, Remuzzi G: Inhibiting angiotensin-converting enzyme promotes renal repair by limiting progenitor cell proliferation and restoring the glomerular architecture. Am J Pathol 179: 628–638, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Macconi D, Sangalli F, Bonomelli M, Conti S, Condorelli L, Gagliardini E, Remuzzi G, Remuzzi A: Podocyte repopulation contributes to regression of glomerular injury induced by ACE inhibition. Am J Pathol 174: 797–807, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guo S, Kowalewska J, Wietecha TA, Iyoda M, Wang L, Yi K, Spencer M, Banas M, Alexandrescu S, Hudkins KL, Alpers CE: Renin-angiotensin system blockade is renoprotective in immune complex-mediated glomerulonephritis. J Am Soc Nephrol 19: 1168–1176, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wada T, Pippin JW, Marshall CB, Griffin SV, Shankland SJ: Dexamethasone prevents podocyte apoptosis induced by puromycin aminonucleoside: role of p53 and Bcl-2-related family proteins. J Am Soc Nephrol 16: 2615–2625, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Wada T, Pippin JW, Nangaku M, Shankland SJ: Dexamethasone’s prosurvival benefits in podocytes require extracellular signal-regulated kinase phosphorylation. Nephron, Exp Nephrol 109: e8–e19, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Peired A, Angelotti ML, Ronconi E, la Marca G, Mazzinghi B, Sisti A, Lombardi D, Giocaliere E, Della Bona M, Villanelli F, Parente E, Ballerini L, Sagrinati C, Wanner N, Huber TB, Liapis H, Lazzeri E, Lasagni L, Romagnani P: Proteinuria impairs podocyte regeneration by sequestering retinoic acid. J Am Soc Nephrol 24: 1756–1768, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kurtz A: Renin release: Sites, mechanisms, and control. Annu Rev Physiol 73: 377–399, 2011 [DOI] [PubMed] [Google Scholar]
- 35.Kim HS, Maeda N, Oh GT, Fernandez LG, Gomez RA, Smithies O: Homeostasis in mice with genetically decreased angiotensinogen is primarily by an increased number of renin-producing cells. J Biol Chem 274: 14210–14217, 1999 [DOI] [PubMed] [Google Scholar]
- 36.Taniguchi Y, Pippin JW, Hagmann H, Krofft RD, Chang AM, Zhang J, Terada Y, Brinkkoetter P, Shankland SJ: Both cyclin I and p35 are required for maximal survival benefit of cyclin-dependent kinase 5 in kidney podocytes. Am J Physiol Renal Physiol 302: F1161–F1171, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kurt B, Karger C, Wagner C, Kurtz A: Control of renin secretion from kidneys with renin cell hyperplasia. Am J Physiol Renal Physiol 306: F327–F332, 2014 [DOI] [PubMed] [Google Scholar]
- 38.Pentz ES, Lopez ML, Kim HS, Carretero O, Smithies O, Gomez RA: Ren1d and Ren2 cooperate to preserve homeostasis: evidence from mice expressing GFP in place of Ren1d. Physiol Genomics 6: 45–55, 2001 [DOI] [PubMed] [Google Scholar]
- 39.Sequeira Lopez ML, Gomez RA: Novel mechanisms for the control of renin synthesis and release. Curr Hypertens Rep 12: 26–32, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alpers CE, Hudkins KL, Floege J, Johnson RJ: Human renal cortical interstitial cells with some features of smooth muscle cells participate in tubulointerstitial and crescentic glomerular injury. J Am Soc Nephrol 5: 201–209, 1994 [DOI] [PubMed] [Google Scholar]
- 41.Radhakrishnan J, Cattran DC: The KDIGO practice guideline on glomerulonephritis: Reading between the (guide)lines--application to the individual patient. Kidney Int 82: 840–856, 2012 [DOI] [PubMed] [Google Scholar]
- 42.Nagai Y, Nakanishi K, Akimoto T, Yamanaka N: Proliferative changes of renal arteriolar walls induced by administration of angiotensin II receptor blocker are frequent in juvenile rats. J Renin Angiotensin Aldosterone Syst 15: 440–449, 2014 [DOI] [PubMed] [Google Scholar]
- 43.Cacciatore F, Bruzzese G, Vitale DF, Liguori A, de Nigris F, Fiorito C, Infante T, Donatelli F, Minucci PB, Ignarro LJ, Napoli C: Effects of ACE inhibition on circulating endothelial progenitor cells, vascular damage, and oxidative stress in hypertensive patients. Eur J Clin Pharmacol 67: 877–883, 2011 [DOI] [PubMed] [Google Scholar]
- 44.Qian C, Schoemaker RG, van Gilst WH, Roks AJ: The role of the renin-angiotensin-aldosterone system in cardiovascular progenitor cell function. Clin Sci (Lond) 116: 301–314, 2009 [DOI] [PubMed] [Google Scholar]
- 45.Wharram BL, Goyal M, Wiggins JE, Sanden SK, Hussain S, Filipiak WE, Saunders TL, Dysko RC, Kohno K, Holzman LB, Wiggins RC: Podocyte depletion causes glomerulosclerosis: diphtheria toxin-induced podocyte depletion in rats expressing human diphtheria toxin receptor transgene. J Am Soc Nephrol 16: 2941–2952, 2005 [DOI] [PubMed] [Google Scholar]
- 46.Pichaiwong W, Hudkins KL, Wietecha T, Nguyen TQ, Tachaudomdach C, Li W, Askari B, Kobayashi T, O’Brien KD, Pippin JW, Shankland SJ, Alpers CE: Reversibility of structural and functional damage in a model of advanced diabetic nephropathy. J Am Soc Nephrol 24: 1088–1102, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.