Abstract
The gene for ADP ribosylation factor–like GTPase 13B (Arl13b) encodes a small GTPase essential for cilia biogenesis in multiple model organisms. Inactivation of arl13b in zebrafish leads to a number of phenotypes indicative of defective cilia, including cystic kidneys. In mouse, null mutation in Arl13b results in severe patterning defects in the neural tube and defective Hedgehog signaling. Human mutations of ARL13B lead to Joubert syndrome, a ciliopathy. However, patients with mutated ARL13B do not develop kidney cysts. To investigate whether Arl13b has a role in ciliogenesis in mammalian kidney and whether loss of function of Arl13b leads to cystic kidneys in mammals, we generated a mouse model with kidney–specific conditional knockout of Arl13b. Deletion of Arl13b in the distal nephron at the perinatal stage led to a cilia biogenesis defect and rapid kidney cyst formation. Additionally, we detected misregulation of multiple pathways in the cystic kidneys of this model. Moreover, valproic acid, a histone deacetylase inhibitor that we previously showed slows cyst progression in a mouse cystic kidney model with neonatal inactivation of Pkd1, inhibited the early rise of Wnt7a expression, ameliorated fibrosis, slowed cyst progression, and improved kidney function in the Arl13b mutant mouse. Finally, in rescue experiments in zebrafish, all ARL13B allele combinations identified in patients with Joubert syndrome provided residual Arl13b function, supporting the idea that the lack of cystic kidney phenotype in human patients with ARL13B mutations is explained by the hypomorphic nature of the mutations.
Keywords: polycystic kidney disease, renal fibrosis, signaling
The cilium is a cell surface organelle that is uniquely positioned to function as a cellular antenna that detects environmental signals and couples these signals to cellular responses.1–6 Ciliary defects lead to a wide range of human diseases collectively referred to as ciliopathies, including polycystic kidney diseases (PKDs). Autosomal dominant polycystic kidney disease (ADPKD) is the most common form of PKD and caused by mutations in PKD1 or PKD2 encoding Polycystin 1 and Polycystin 2, respectively.7,8 The role of cilia in PKD pathogenesis is highlighted by the facts that disruption of cilia biogenesis in mouse models almost inevitably leads to kidney cyst formation5,9 and that Polycystins are targeted to the cilium; this specific trafficking highly correlates with the in vivo function of the Polycystins. Interestingly, a recent study revealed unexpectedly that complete removal of cilia ameliorated cyst progression triggered by Polycystin inactivation, suggesting that Polycystins inhibit a novel cilia–dependent, cyst–promoting pathway.10 Although the precise relationship between Polycystins and cilia is complex and not well understood, all of these studies suggest that the cilium is a critical organelle in the pathogenesis of PKD.
The gene for ADP ribosylation factor–like GTPase 13B (Arl13b), encoding a small GTPase, was first identified as a cystic kidney gene in a large–scale genetic screen in zebrafish.4 The identified mutant scorpion shows body curvature and cystic kidney, typical of ciliary defects in zebrafish. Moreover, Arl13b protein is highly enriched within the cilium and essential for cilia biogenesis in all tissues analyzed in zebrafish.4,11 Arl13b in mouse was also found to be essential for cilia biogenesis, and it plays an important role in the patterning of the neural tube through regulation of Hedgehog signaling.12
To study the function of Arl13b in the mammalian kidney, we generated Arl13bflox/flox; Ksp-Cre mice to achieve kidney–specific conditional knockout. Deletion of Arl13b at the perinatal stage leads to rapid kidney cyst formation. We further show that the histone deacetylase (HDAC) inhibitor valproic acid (VPA) suppresses kidney disease progression in Arl13bflox/flox; Ksp-Cre mice and that Wnt7a is an early target of VPA treatment.
In humans, mutations of ARL13B have been linked to Joubert syndrome (JS), an autosomal recessive disorder characterized by a distinct cerebellar malformation.13,14 However, patients with JS do not develop cystic kidneys. Using complementation experiments in zebrafish, we complete a dataset that shows that all ALR13B pathogenic variants associated with human JS provide residual activity of Arl13b. In totality, our results support a conserved function of Arl13b in cilia biogenesis and development in vertebrate kidneys.
Results
Inactivation of Arl13b at the Perinatal Stage in the Distal Nephron Leads to Rapid Cyst Formation and Renal Failure in Mice
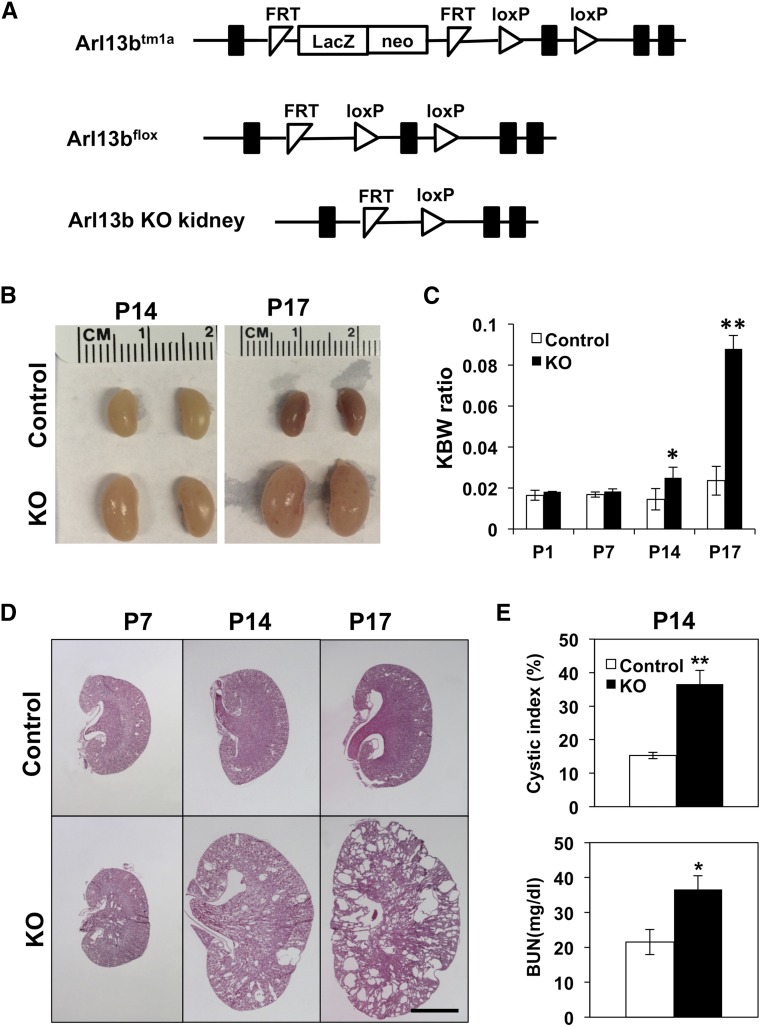
To generate a conditional allele of Arl13b, we acquired Arl13btm1a embryonic stem cell clones generated by the European Conditional Mouse Mutagenesis Program (EUCOMM). In this allele, exon 3 of Arl13b is flanked by two LoxP sites, and an FRT-LacZ-neo-FRT selection cassette is located in intron 2 (Figure 1A). Germline transmission of Arl13btm1a from injected chimeras was confirmed by long-range PCR (not shown). Arl13btm1a/+ mice were crossed with a transgenic strain carrying an actin-driven FLPe15 to generate the Arl13bflox allele. Arl13bflox/flox mice are viable with no discernible phenotypes (not shown).
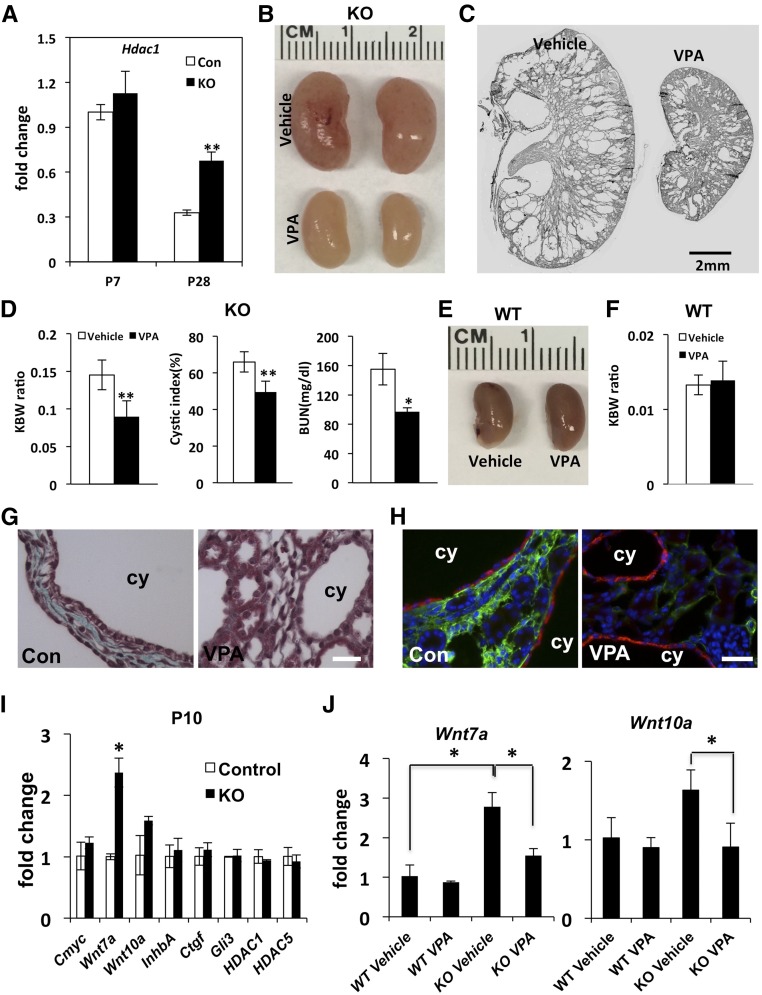
Figure 1.
Distal nephron–specific inactivation of Arl13b in Arl13bflox/flox; Ksp-Cre mice leads to rapid progression of PKD. (A) Diagram of targeting vector. Exons are showed by black boxes. FRT indicates FLPE recombinase sites, and loxP indicates Cre recombinase sites. (B) Gross morphology of kidneys from P14 and P17 Arl13bflox/flox; Ksp-Cre mice compared with control siblings. (C) Increasing KBW ratio in mutants during the neonatal stage (n=3 for each stage and each genotype). (D) Hematoxylin/eosin-stained sections from control and mutant kidneys on P7, P14, and P17. Scale bar, 2 mm. (E) Significant cyst formation quantified by cystic index and increased BUN levels in knockout mice at P14 (n=3 for each genotype). Control indicates the wild type. KO, knockout. *P<0.05; **P<0.01.
To generate kidney-specific knockout of Arl13b, we crossed Arl13bflox/+ mice with a Ksp-Cre strain.16 Ksp-Cre mediates recombination in distal segments of the nephron after E11.5.17 Arl13bflox/flox; Ksp-Cre mice were born in normal Mendelian ratios but show progressively enlarged kidneys (Figure 1B). At postnatal day 1 (P1) and P7, the size of the kidney in mutants is similar to that in the control (data not shown). From P14, the size is obviously enlarged, and by P17, the size is significantly increased (Figure 1B). Consistent with the enlargement of the kidney, the kidney-to-body weight (KBW) ratio is significantly increased in mutant mice by P14 (Figure 1C). The mutant mice die at around P60 (not shown). Histologic sections of the kidney failed to detect cysts at P1 (not shown), minimal cysts at P7, significant cysts in both the medulla and cortex region at P14, and severely cystic kidneys at P17 (Figure 1D). Quantification of cystic index verified that cyst formation is already significant in mutant mice at P14 (Figure 1E). To analyze kidney function in mutant mice, we perform BUN assay. Results show that the BUN level is significantly increased in mutant mice at P14 (Figure 1E), indicating a decline in kidney function. Combined, these results suggest a rapid progression of PKD in the mutant mouse.
Arl13b Is Essential for Cilia Biogenesis in Collecting Duct Epithelial Cells
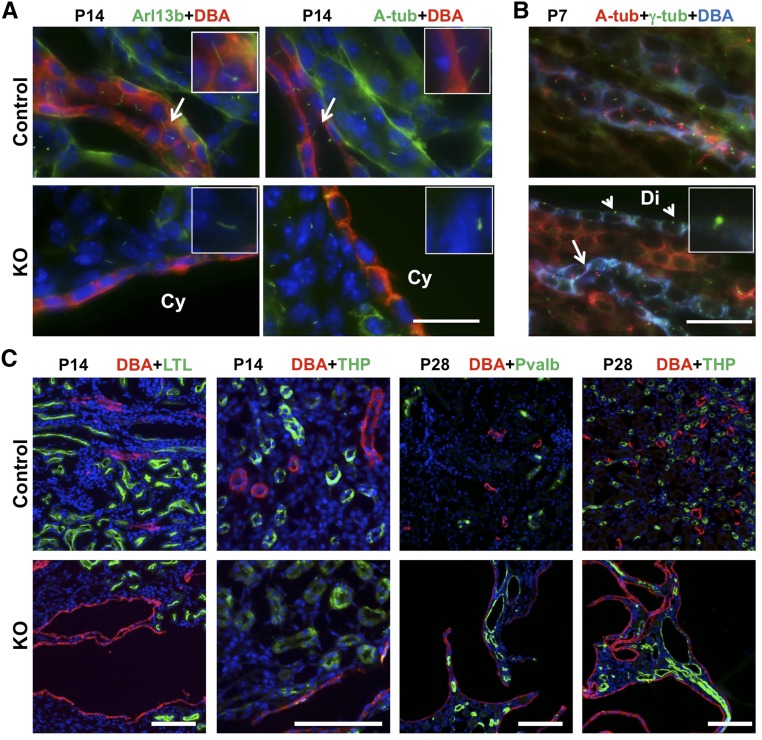
To confirm that cell type–specific knockout of Arl13b was achieved, immunostaining for Arl13b was performed and verified that Arl13b expression is absent in the collecting duct labeled by Dolichos Biflorus Agglutinin (DBA) in knockout mice at P14 (Figure 2A, left panel). We then investigated cilia biogenesis in the conditional knockout model. At P14, DBA–positive ductal cells were ciliated in control mice as shown by the cilia marker antiacetylated tubulin (Figure 2A, right panel). In contrast, in mutant mice, most DBA-positive cells were devoid of cilia (Figure 2A, right panel). To further confirm this result, we performed immunostaining using anti–γ-tubulin as a basal body marker. At P7, DBA-positive cells were frequently ciliated as shown by antiacetylated tubulin in wild–type control mice (Figure 2B). In knockout mice, some DBA-positive regions were already dilated (Figure 2B). Interestingly, in dilated regions, cilia were largely absent, whereas cilia were still present in undilated DBA–positive regions (Figure 2B), suggesting that cilia biogenesis defect in the mutant kidney correlates with kidney cyst formation.
Figure 2.
Arl13b is essential for cilia biogenesis, and its depletion leads to cyst formation in mouse kidney. (A) P14 kidney sections shown in the collecting duct are labeled by DBA (red) and Arl13b signal (green; left panel), and cilia signal labeled by antiacetylated tubulin (A-tub; green; right panel) is lacking in the mutant kidney (knockout [KO]). Nuclei are labeled with DAPI in blue. Although insets in control show cilia in the collecting duct, insets in KO show cilia outside of the collecting duct. Arrows point to cilia. Cy, cyst. (B) P7 kidney sections show basal body labeled with anti–γ-tubulin (γ-tub; green) and cilia labeled with antiacetylated tubulin (A-tub; red) in control and mutant (KO) mice. Arrow points to a cilium in DBA-positive (blue) segment. Arrowheads point to basal bodies without cilium in a dilated DBA–positive region (Di). Inset shows a basal body without cilia. (C) Cyst formation is confined to the distal nephron in Arl13bflox/flox; Ksp-Cre mice. DBA marks the collecting duct. The proximal tubule is labeled with LTL, the medullary thick ascending limb is labeled by THP, and the distal convoluted tubule is labeled with anti-Parvalbumin (Pvalb). Nuclei are labeled with DAPI in blue. Control indicates the wild type. Scale bars, 20 μm in A and B; 100 μm in C.
Cyst Formation Is Confined to the Distal Nephron in Arl13bflox/flox; Ksp-Cre Mice
To define the cellular origin of kidney cysts in this model, we labeled P14 kidney sections with the collecting duct marker DBA, the proximal tubule marker Lotus Tetragonolobus Lectin (LTL), and the thick ascending limb marker anti-Tamm–Horsfall protein (THP). At P14, cysts mainly form in the collecting duct (Figure 2C, columns 1 and 2). Consistent with the lack of Ksp-Cre expression in the proximal tubule, this segment labeled by LTL does not appear to be cystic (Figure 2C, column 1). Interestingly, the THP–positive thick ascending limb, which expresses Cre, does not appear to be cystic at this stage (Figure 2C, column 2). However, at P28, cysts are detected in the THP–positive thick ascending limb and the distal convoluted tubule labeled with anti-Parvalbumin (Figure 2C, columns 3 and 4). Combined, the overall distribution pattern of cysts is consistent with the expression pattern of Ksp-Cre.17 The difference in cyst progression in different segments may represent differential Cre expression in these regions. Alternatively, the collecting duct may be more susceptible to cyst growth.18
Arl13b Inactivation Leads to Cell Overproliferation
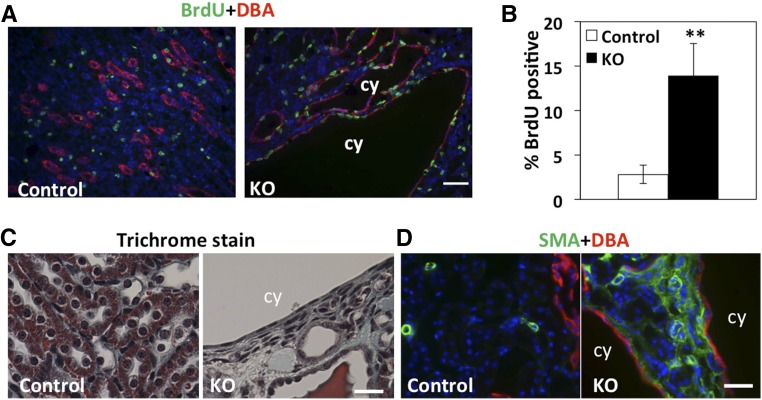
Because cell overproliferation is one of the mechanisms of cyst progression, we investigated cell proliferation in Arl13b mutant kidney compared with controls using a bromodeoxyuridine (BrdU) incorporation assay. At P14, kidney samples were collected 3 hours after intraperitoneal (ip) injection of BrdU. Immunostaining on kidney sections revealed that around 14% of DBA–positive cyst–lining cells displayed nuclear BrdU incorporation compared with around 2.8% positive nuclei in DBA-positive cells in control kidneys (Figure 3, A and B), suggesting a significant increase of cell proliferation.
Figure 3.
Increased cell proliferation and fibrosis in the kidney of Arl13bflox/flox; Ksp-Cre mice. (A) Kidney sections stained with anti-BrdU (green) and DBA (red) at P14. (B) Quantitation of BrdU-positive nuclei in DBA-positive cells (n=3 animals of each genotype). (C) Trichrome stain indicates collagen deposition (green) in mutant kidneys at P28. (D) Interstitial cells show increased smooth muscle actin (SMA; green) expression in mutant kidney compared with controls at P28. The collecting duct is labeled with DBA in red. Control indicates the wild type. cy, Cyst; KO, knockout. Scale bars, 50 μm in A; 20 μm in C and D. **P<0.01.
Arl13b Inactivation Leads to Kidney Fibrosis
Cystic kidneys are frequently fibrotic. To investigate whether Arl13b inactivation eventually leads to fibrosis in the kidney, we performed trichrome staining on kidney sections to analyze potential collagen deposition. At P21, we failed to detect any significant increase of trichrome signal in the mutant kidney (data not shown). In contrast, at P28, increase of trichrome staining was obvious in the mutant kidney (Figure 3C). We further stained kidney sections with anti-smooth muscle actin, a marker of myofibroblasts. Results showed dramatic increase in the mutant kidney at P28 (Figure 3D). Combined, these results show that cyst formation caused by Arl13b inactivation precedes overt kidney fibrosis in the later stage of the disease progression.
Multiple Signaling Pathways Are Misregulated in the Cystic Kidney of Arl13bflox/flox; Ksp-Cre Mice
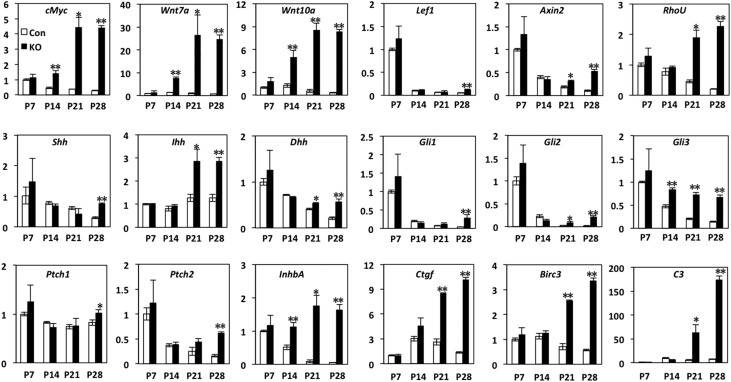
Multiple pathways have been implicated in cystic kidney progression. In the Ift140 model, targets and components of the Wnt pathway, HH pathway, and Hippo pathway are upregulated.19 To investigate potential mechanisms for cyst progression in Arl13bflox/flox; Ksp-Cre mice, we performed time course for the expression level of candidate genes through quantitative PCR (qPCR) using P7–P28 whole-kidney lysates. At P28, the expression of two Wnt ligands, Wnt7a and Wnt10a, is dramatically increased (Figure 4). The Wnt pathway targets Lef1 and Axin2 are also significantly upregulated. The expression of c-Myc, a potential target of the Wnt pathway that has been implicated in PKD,20–23 also increases dramatically. In addition, the Hedgehog pathway ligands Shh, Ihh, and Dhh are upregulated as well as HH pathway targets Gli1, Gli2, and Gli3. Similarly, the expression of Hippo pathway targets Birc3, Ctgf, and InhbA also rises. Finally, we analyzed the expression level of complement C3, an innate immune response gene that is upregulated in both autosomal recessive PKD and ADPKD models,24,25 and found that it is significantly increased as well (Figure 4).
Figure 4.
Multiple pathways are misregulated in cystic kidneys of Arl13bflox/flox; Ksp-Cre mice. qPCR analysis of genes involved in Wnt (cMyc, Wnt7a, Wnt10a, Lef1, Axin2, and RhoU), Hedgehog (Shh, Ihh, Dhh, Ptch1, Ptch2, Gli1, Gli2, and Gli3), Hippo (InhbA, Ctgf, and Birc3), and innate immunity (C3) pathways in control (Con) and knockout (KO) kidneys at P7, P14, P21, and P28. Gene expression is normalized with that of Gapdh. Unit 1 is defined as the expression level in wild-type samples at P7 (n=4 kidneys from two mice). Error bars show SDs. *P<0.05; **P<0.01.
Consistent with previous findings,19 our results show that the expression of most of these signaling pathway components steadily decreases during normal kidney development at the neonatal stage, including c-Myc, Lef1, Axin2, RhoU, and many Hedgehog pathway components (Figure 4). Within this group of genes, c-Myc is unique in that its expression increases by P14 in the mutant kidney (Figure 4). In contrast, some of the Hedgehog pathway components, including Gli1, Gli2, and Shh, show a decrease between P7 and P21 and rise at P28 in mutant kidneys (Figure 4). A second group of genes, including Wnt7a and Wnt10a, shows relatively stable or even slightly increased expression in control kidneys between P7 and P14 followed by a decrease at later time points (Figure 4). Their expression in the mutant kidney increases more dramatically at P14 and persists at P28. Complement C3 expression represents yet another pattern: although it increases from P7 to P14 and relatively stabilizes afterward in controls, it rises dramatically over the entire period in mutant kidneys (Figure 4).
Combined, these results suggest that multiple pathways are misregulated in the mutant kidney in a complex pattern. Compared with age–matched wild–type kidneys, in mutant kidneys, the expression of c-Myc, Wnt7a, Wnt10a, Gli3, and InhbA is significantly higher by P14, the expression of Axin2, RhoU, Ihh, Dhh, Gli2, Ctgf, and Birc3 is elevated by P21, and the rest become significantly increased at P28 (Figure 4).
VPA, an HDAC Inhibitor, Can Partially Suppress PKD Progression in Arl13bflox/flox; Ksp-Cre Mice
In a previous study, we found that the histone deacetylase inhibitor (HDACI) VPA slowed cyst progression and improved kidney function in a mouse ADPKD model on the basis of neonatal inactivation of Pkd1.26 We, therefore, investigated the expression level of Hdac1 in the mutant kidney. Our results show that, at P28, the expression level of Hdac1 is significantly increased, although there is no significant change at P7 (Figure 5A). We further investigated whether VPA can ameliorate disease progression in Arl13bflox/flox; Ksp-Cre mice. Results show that mutant mice treated with VPA from P10 to P25 show significantly reduced kidney size (Figure 5, B and C) and KBW ratio (Figure 5D) compared with vehicle-treated samples. Moreover, BUN level is also significantly reduced, indicating improved kidney function (Figure 5D). Furthermore, the expression of the Wnt, Hedgehog, and Hippo pathway components analyzed above is all reduced by this treatment in mutant kidneys (Supplemental Figure 1). In contrast, the effect of the same VPA treatment has minimal effect on kidney size, KBW ratio, or gene expression in wild-type controls (Figure 5, E and F, Supplemental Figure 1). Combined, these results show that VPA can slow down disease progression in Arl13bflox/flox; Ksp-Cre mice.
Figure 5.
The HDACi VPA suppresses disease progression in Arl13bflox/flox; Ksp-Cre mice. (A) qPCR on Hdac1 expression in wild-type (WT; Con) and mutant (KO) kidney. Unit 1 is defined as the expression in WT sample at P7 (n=3 mice). (B) Kidney morphology of vehicle and VPA-injected mutant (KO) mice at P25. (C) Representative hematoxylin/eosin–stained sections of kidneys from vehicle and VPA-treated mutant mice at P25. (D) KBW ratio, cystic index, and BUN in VPA and vehicle-treated mutant (KO) mice at P25 (n=3 mice). (E) Kidney morphology in vehicle and VPA–injected WT mice at P25. (F) KBW ratio in vehicle and VPA–injected WT mice at P25. (G) Trichrome staining of P25 kidney section of vehicle (Con) and VPA–treated Arl13bflox/flox; Ksp-Cre mice. (H) P25 kidney section of vehicle (Con) and VPA–treated Arl13bflox/flox; Ksp-Cre mice stained with anti-smooth muscle actin (green) and DBA (red). (I) Wnt7a expression is upregulated in mutant (KO) kidney at P10 as shown by qPCR analysis. Gene expression is normalized with that of Gapdh. Unit 1 is defined as the expression level in WT samples (n=3 kidneys from three mice). (J) qPCR analysis of Wnt7a and Wnt10a expression in mutant (KO) and WT kidney treated with vehicle or VPA. Unit 1 is defined as the expression level in vehicle–treated WT samples (n=3 kidneys from three mice). Error bars show SDs. cy, Cyst. Scale bars, 20 μm in G and H. *P<0.05; **P<0.01.
VPA Treatment Ameliorates Fibrosis and Suppresses Early Transcriptional Changes in Arl13bflox/flox; Ksp-Cre Mice
Because HDACIs have been shown to suppress fibrosis, including renal fibrosis,27–37 we investigated the effect of VPA treatment on fibrosis in Arl13bflox/flox; Ksp-Cre mice. VPA treatment from P10 to P25 significantly reduced collagen deposition in mutant mice as shown by trichrome staining (Figure 5G). Smooth muscle actin was also significantly decreased in the interstitium of VPA–treated mutant kidneys (Figure 5H).
To tease out the potential mechanism for HDACI–mediated disease inhibition, we sought to identify early transcriptional changes in the mutant kidney. qPCR using P10 kidney for genes that are misregulated at P14 revealed that a single gene, Wnt7a, was significantly upregulated at the whole–kidney lysate level in mutants (Figure 5I). The expression of Wnt10a was also increased but did not reach a statistically significant level (Figure 5I). Interestingly, VPA treatment from P7 to P10 effectively abolished the increase of Wnt7a and Wnt10a expression specifically in the mutant kidney (Figure 5J).
The Nature of ARL13B Mutations Associated with JS in Humans
Three mutant genotypes, R79Q/R79Q, W82X/R200C, and Y86C/Y86C, have been linked to JS.13,14 Although R79Q, R200C, and Y86C are single-amino substitutions, W82X is a truncation. It was reported that, although R79Q and R200C can partially rescue both the body curvature and cystic kidney phenotypes of the zebrafish scorpionhi459 mutant, Y86C was not able to rescue the body curvature phenotype, although it reduced the percentage of kidney cyst formation.13,14 To clarify the function of Y86C and formally test the function of the truncation mutation W82X, we performed zebrafish rescue experiments. As shown in our previous study, injection of mRNA encoding wild-type Arl13b fused with eGFP fully rescues the zebrafish scorpionhi459 mutant (Figure 6A). In contrast, arl13b-W82X mRNA could not rescue either body curvature or kidney cyst phenotypes (Figure 6A). Expression of Y86C reduced but did not fully eliminate the percentage of phenotypic embryos (Figure 6A). To verify that the reduction of phenotypic embryos is caused by partial recue of the mutants, we genotyped embryos with normal body axis and no kidney cyst. Results showed that some of the embryos with wild-type appearance are rescued homozygous mutants (Figure 6B), supporting the hypothesis that Y86C is, indeed, a hypomorphic mutation that retains residual activity. Thus, all ARL13B genotypes associated with JS could provide partial activity of Arl13b.
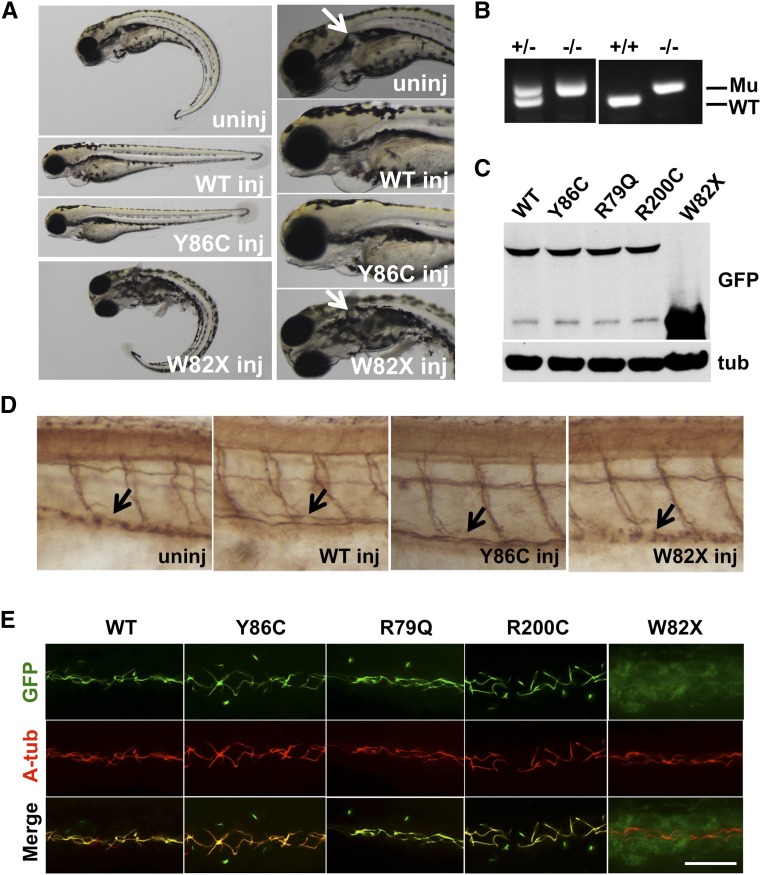
Figure 6.
Function and localization of four mutant Arl13b identified in patients with JS assayed in zebrafish. (A) Y86C-GFP could rescue the body curvature (left panel) and kidney cyst (enlarged in the right panel; white arrows) of scorpionhi459 mutants. The truncated form W82X could not rescue either phenotype. (B) PCR genotyping results. Although the wild-type (WT) band identifies the WT allele, the mutant (Mu) band identifies the mutant allele. Each lane is for a single embryo with WT morphology. (C) Western blot showing the protein levels of eGFP-tagged WT and mutant Arl13b at 12-hour postfertilization. Anti–β-tubulin (tub) is used as a loading control. (D) Side views of the trunk region of day 2 scorpionhi459 mutant embryos subjected to mRNA injection. Arrows point to cilia bundles in the kidney duct. (E) Side views of the pronephric duct in embryos injected with mRNA encoding GFP-tagged WT and mutant Arl13b. Cilia are labeled with antiacetylated tubulin in red. WT inj, Y86C inj, and W82X inj indicate injected with WT, Y86C, and W82X Arl13b-GFP mRNA, respectively. A-tub, antiacetylated tubulin; uninj, uninjected scorpionhi459 mutant. Scale bar, 10 μm.
Western blot using anti-eGFP showed that wild-type Arl13b and the three missense mutants are expressed at similar levels when equal amounts of mRNA were injected, whereas the level of W82X is much higher than the wild type (Figure 6C).
We further investigated the ability of Y86C and W82X to rescue cilia biogenesis defects in scorpionhi459 mutant using a previously established assay.11 In the wild–type zebrafish kidney duct, cilia bundle together and appear as a line between somite and yolk in whole-mount embryos stained with antiacetylated tubulin. In contrast, in mutant embryos, stunted cilia appear as spots (Figure 6D, column 1). Injection of wild-type and Y86C arl13b mRNA restored cilia bundle, whereas W82X mRNA failed to do so (Figure 6D). Combined, the ability of different versions of arl13b to rescue the morphologic defects of scorpionhi459 mutant correlates with their ability to restore ciliogenesis in this mutant.
Previous cell culture study suggests that R79Q and R200C could still traffic to cilia. We further investigated the subcellular localization pattern of all of the mutant proteins in vivo in developing zebrafish embryos by injecting mRNA into wild-type embryos. Immunostaining with anti-eGFP on injected embryos showed that all three missense mutant proteins could still traffic effectively to cilia, similar to wild-type Arl13b (Figure 6E). In contrast, W82X is diffusely localized throughout the cell (Figure 6E).
Discussion
Although originally isolated as a cystic kidney gene in zebrafish, the role of Arl13b in mammalian kidney development and homeostasis was unclear until very recently. The lack of kidney symptoms in human patients with defective Arl13b raises the possibility that there may be tissue and species variations in its function. To investigate the function of Arl13b in mammalian kidney development directly, we generated a conditional knockout mouse of Arl13b. Deletion of Arl13b in the distal nephron at the perinatal stage leads to rapid progression of cystic kidney disease, consistent with a very recent report that conditional inactivation of Arl13b via Nestin-Cre leads to kidney cyst,38 showing its essential role in developing and maintaining the elaborate three–dimensional epithelial structure of the mammalian kidney.
We previously showed that VPA ameliorated cyst progression and improved kidney function in a neonatal mouse ADPKD model based on conditional inactivation of Pkd1.26 Additionally, trichostatin A, another HDACI, was shown to suppress cyst progression in a mouse Pkd2 knockout model.39 In this study, we provide evidence that VPA is also effective against PKD progression in the Arl13bflox/flox; Ksp-Cre model. Combined, these results show that HDACIs can slow disease progression in not only ADPKD models based on inactivation of Polycystins but also, PKD models based on ciliogenesis defects.
Through chromatin remodeling, HDACs regulate transcription directly or indirectly. From the budding yeast to mammalian cells, HDACIs were reported to change the expression of 2%–10% of expressed genes, with roughly equal numbers of genes either up- or downregulated.40–45 Past studies provide some tantalizing clues for potential targets of HDACI treatment. It was shown that, in response to flow, Hdac5 is exported out of the nucleus, leading to activation of Mef2c-mediated transcription.39 Moreover, among the genes that we analyzed, c-Myc shows abnormally increased expression in Arl13b mutant kidney at P14. A recent study reported that JQ1, a small molecule inhibitor of the epigenetic regulator Brd4, reduces c-Myc expression and slows disease progression in an orthologous mouse ADPKD model.46
HDACIs have been used to suppress fibrosis, including renal fibrosis.27–37 Our results show that VPA treatment suppresses interstitial fibrosis in Arl13bflox/flox; Ksp-Cre mice. VPA treatment also inhibited the rise of Wnt7a in Arl13bflox/flox; Ksp-Cre mice at a very early stage during cystogenesis. Interestingly, Wnt7a is upregulated in renal epithelial cells in a Pkd1−/− model.47 Wnt signaling has been reported to promote renal fibrosis,48 and increasing evidence supports the notion that inflammation and fibrosis contribute to PKD progression.49–51 Combined, these results suggest that Wnt7a is an early target of HDACI treatment.
A remaining question is why patients with JS and ARL13B mutations do not develop kidney cyst. Species difference could be a factor. Alternatively, the identified mutations could be hypomorphic alleles, and the residual activity could support normal kidney development, whereas some other tissues (e.g., the cerebellum) could be more sensitive to the level of Arl13b activity. Our results in zebrafish suggest that all of the identified JS genotypes provide some level of Arl13b activity, supporting the hypomorphic allele hypothesis, which is also consistent with the observation that a complete or severe loss of function of Arl13b in either zebrafish or mouse is incompatible for survival beyond early embryonic stages.4,12
Arl13b is required for cilia biogenesis in multiple tissues and multiple organisms. Here, we show that the mammalian renal epithelial cell is no exception. Previous work suggests that Arl13b is highly enriched on cilia and that its ability to traffic to cilia is essential for the in vivo function of this protein.4,11 Combined, these results suggest that defective cilia biogenesis is a major mechanism for kidney cyst formation in Arl13b mutants.
Arl13b is an ARF family small GTPase with the GTPase domain at the N terminus followed by a C-terminal region containing a coiled coil domain. Both regions are essential for its ciliary localization and in vivo function.11 Not surprisingly, the W82X truncation, which removes more than one half of the GTPase domain and the entire C–terminal region, abolishes its function. Previous structural study on the Arl13b homolog in Chlamydomonas reinhardtii suggests that R79 and R200 residues may be involved in stabilizing the conformation of activated Arl13b.52 Interestingly, although located in the small GTPase domain and conserved from Caenorhabditis elegans to human, Y86 is not conserved in C. reinhardtii. The effective localization of Y86C to cilia suggests that its reduced function is not caused by trafficking defects. Moreover, all three missense mutants seem to be as stable as the wild-type protein. Agents stabilizing the conformation of the GTP-bound form of Arl13b could potentially increase or restore the function of these missense mutants.
Concise Methods
Animal Care Ethics
All zebrafish and mouse experiments were performed in Yale University School of Medicine in accordance with Yale University Institutional Animal Care and Use Committee guidelines.
Mouse Breeding
Arl13btm1a embryonic stem cell clones were purchased from the EUCOMM (http://www.mousephenotype.org) and then, injected into C57/BL6J blastocysts to generate chimeric mice. Chimeric animals were mated to C57/BL6J mice. Arl13btm1a was converted to Arl13bflox allele by mating with the FLPe transgenic mice. Ksp-Cre was used to delete exon 3, creating Arl13b knockout specifically in the kidney.
Genotyping of Arl13b alleles was performed using primers 5′-CTCTTTGCAGGATGCCCTTC-3′ and 5′-AAGGGTTTTTCTCTGTAGCAC-3′. Although the wild-type allele is identified by a 437-bp band, the Arl13bflox allele is identified by a 594-bp band.
Zebrafish Husbandry
Zebrafish were maintained according to standard protocols.53 Embryos were obtained through natural spawning.
Generation of Zebrafish arl13b Mutant Constructs
Zebrafish arl13b-R79Q-GFP, arl13b-Y86C-GFP, arl13b-R200C-GFP, and arl13b-W82X-GFP were generated through site-directed mutagenesis using previously described pCS2+arl13b11 as a template.
Zebrafish Embryo Microinjection and Rescue Assay
Capped mRNA was synthesized in vitro using the mMESSAGE mMACHINE Kit (Ambion) following the manufacturer’s instructions. One cell–stage zebrafish embryos from arl13b+/hi459 incross were injected with 250 pg capped mRNA. Ventral body curvature and pronephric cyst were scored at 3 days postfertilization. Genotyping PCR was performed using three primers: one viral specific (5′-CTGTTCCATCTGTTCCTGAC-3′) and two for genomic regions flanking the proviral insertion (5′-GCACTTAACGCCTCCTCTTTGTATG-3′ and 5′-CCGTGCGCACAGTCAGGG-3′). Mutant product is around 400 bp, whereas wild-type band is around 350 bp.
Immunostaining
Mouse kidneys were fixed by heart perfusion with 4% PFA, fixed in 4% PFA overnight at 4°C, embedded in OCT, and cryosectioned at 5 μm thickness.
The following primary antibodies and lectins are used: Rhodamine DBA (RL-1032; 1:100; Vector Laboratories, Burlingame, CA), Fluorescein LTL (FL-1321; 1:300; Vector Laboratories), THP (AF5175; 1:500; R&D Systems, Minneapolis, MN), anti-Parvalbumin mAb (MAB1572; 1:200; EMD Millipore), Rabbit anti-Arl13b (17711–1-AP; 1:100; Proteintech), mouse monoclonal antiacetylated tubulin (clone 6–11B-1; 1:5000; Sigma-Aldrich, St. Louis, MO), mouse α–smooth muscle actin (ab7817; 1:100; Abcam, Inc., Cambridge, MA), and anti–γ-tubulin (T3559; 1:500; Sigma-Aldrich).
For Arl13b-GFP localization in zebrafish, 24-hour postfertilization embryos were fixed in Dent's fixative (80% methanol and 20% DMSO). Goat anti–GFP antibody (600–132–215; Rockland Immunochemicals Inc., Gilbertsville, PA) was used at 1:500. Embryos were flat mounted using Fluoro-Gel (17985–1; Electron Microscopy Sciences).
For cilia biogenesis rescue experiment, zebrafish embryos from crosses between heterozygous carriers of scorpionhi459 were injected with mRNA encoding different versions of Arl13b and fixed in Dent's fixative at day 2 postfertilization. Mouse antiacetylated tubulin was used at 1:2000, and HRP goat anti–mouse IgG antibody (Pierce, Rockford, IL) was used at 1:1000. DAB reaction was used for detection of HRP signal. Brown color was visualized and photographed in bright field under a dissecting scope.
All immunofluorescence pictures were taken with a Zeiss Axioplan 2 Microscope System (Carl Zeiss GmbH, Jena, Germany) using AxioVision Rel. 4.8 software.
Protein Extraction and Western Blot
Zebrafish embryo lysate was obtained as published before.54 Briefly, embryos were deyolked, homogenized in SDS sample buffer, and boiled at 95°C for 10 minutes, and resultant lysates were cleared by centrifugation at 12,000×g for 5 minutes. Blots were incubated with primary antibodies (anti–β-tubulin; T4026; 1:5000; Sigma-Aldrich; mouse anti-GFP; clones 7.1 and 13.1; 1:1000; Roche, Basel, Switzerland) followed by HRP–conjugated secondary antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA), and signals were developed by Western Lightning Plus ECL Kit (PerkinElmer, Waltham, MA).
Proliferation Assay
Proliferation was assayed through BrdU incorporation. Kidneys were harvested 3 hours after ip injection of 100 mg/kg body wt BrdU solution (10280879001; Roche). Kidneys were fixed with 4% PFA and embedded in OCT. Cryosections were cut at 5 μm, and BrdU incorporation was detected using anti-BrdU antibody (347580; BD Biosciences, San Jose, CA). About 1300 DBA-positive cells were counted to calculate the BrdU–positive cell ratio in the collecting duct.
Cystic Index Measurement
Cystic index was measured as previously published.26 Briefly, cyst formation was quantified in sagittal sections of kidneys. Two sections were analyzed for each animal. Hematoxylin/eosin-stained sections were scanned using the scan slide module in Metamorph v.7.1 acquisition software (Universal Imaging). Cystic and noncystic areas are measured by the integrated morphometric feature in Metamorph. Cystic index is defined as cystic area to total kidney area in each section.
qPCR analysis
Mouse kidneys were harvested at P7, P10, P14, P21, and P28. Total RNA isolation and first–strand cDNA synthesis were performed as described previously.54 qPCR reactions were performed with the KAPA SYBR Fast qPCR Kit Master Mix (2×) Universal (Kapa Biosystems) using a C1000 Thermal Cycler, CFX96 Real-Time System (Bio-Rad, Hercules, CA). Previously published PCR primers19 were used for Gapdh, c-Myc, RhoU, Wnt7a, Wnt10a, Lef1, Axin2, C3, Birc3, Ctgf, InhbA, Gli1, Gli2, and Gli3, NCBI primer blast was used to design primers for the rest of the genes. They include Hdac1: 5′-CTCACCGAATCCGCATGACT-3′ and 5′-GCTTCACAGCACTTGCGAC-3′; Shh: 5′-AGACCGGCTGATGACTCAGAG-3′ and 5′-GCTCGACCCTCATAGTGTAGAGAC-3′; Ptch1: 5′-TGGCCGCATTGATCCCTATC and 5′-ACACAGGGGCTTGTGAAACA-3′; Ptch2: 5′-TGGTAATCCTCGTGGCCTCT-3′ and 5′-AACCAGCAAGCATGAGCAGA-3′; Ihh: 5′-CGGCTTCGACTGGGTGTATT-3′ and 5′-CATCACTGAAGGTGGGGGTC-3′; and Dhh: 5′-CGTACCCAACTACAACCCCG-3′ and 5′-GTGGAGTGAATCCTGTGCGT-3′. Gene expression was normalized to that of Gapdh.
VPA Treatment
VPA was purchased from Acros Organics. Daily ip injection was performed during the duration described in specific experiments. The VPA treatment group received 200 mg/kg VPA in 15% DMSO and 85% saline, whereas the vehicle control group received the same volume of 15% DMSO and 85% saline without VPA. In total, six mice (three for VPA and three for vehicle) were analyzed.
Statistical Analyses
The t test between paired samples (wild-type versus mutant or vehicle versus VPA treated) was performed using Excel (Microsoft, Redmond, WA).
Disclosures
None.
Supplementary Material
Acknowledgments
We thank members of the Brueckner laboratory, the laboratory of S.S., and the laboratory of Z.S. for helpful discussions; D. Shao and S. Mentone for histology assistance; and the Yale Mouse Metabolic Phenotyping Center (grant U24 DK059635) for BUN analysis.
This work was supported by the National Institutes of Health (NIH) grant R01 DK092808, research grant RSG-10-247-01-DDC from the American Cancer Society (to Z.S.), NIH grant 1P30DK090744 Animal Core, NIH grant R01 DK100592 (to S.S.), and research grant 191G14a (to Z.S.) from the PKD Foundation. S.J. is supported by NIH grant T32 DK07356 Investigative Training in Hepatology.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2015091004/-/DCSupplemental.
References
- 1.Hildebrandt F, Attanasio M, Otto E: Nephronophthisis: Disease mechanisms of a ciliopathy. J Am Soc Nephrol 20: 23–35, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pazour GJ, Baker SA, Deane JA, Cole DG, Dickert BL, Rosenbaum JL, Witman GB, Besharse JC: The intraflagellar transport protein, IFT88, is essential for vertebrate photoreceptor assembly and maintenance. J Cell Biol 157: 103–113, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pazour GJ, Rosenbaum JL: Intraflagellar transport and cilia-dependent diseases. Trends Cell Biol 12: 551–555, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Sun Z, Amsterdam A, Pazour GJ, Cole DG, Miller MS, Hopkins N: A genetic screen in zebrafish identifies cilia genes as a principal cause of cystic kidney. Development 131: 4085–4093, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Yoder BK, Tousson A, Millican L, Wu JH, Bugg CE Jr., Schafer JA, Balkovetz DF: Polaris, a protein disrupted in orpk mutant mice, is required for assembly of renal cilium. Am J Physiol Renal Physiol 282: F541–F552, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Yoder BK, Hou X, Guay-Woodford LM: The polycystic kidney disease proteins, polycystin-1, polycystin-2, polaris, and cystin, are co-localized in renal cilia. J Am Soc Nephrol 13: 2508–2516, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Consortium TEPKD, The European Polycystic Kidney Disease Consortium : The polycystic kidney disease 1 gene encodes a 14 kb transcript and lies within a duplicated region on chromosome 16. Cell 77: 881–894, 1994 [DOI] [PubMed] [Google Scholar]
- 8.Mochizuki T, Wu G, Hayashi T, Xenophontos SL, Veldhuisen B, Saris JJ, Reynolds DM, Cai Y, Gabow PA, Pierides A, Kimberling WJ, Breuning MH, Deltas CC, Peters DJ, Somlo S: PKD2, a gene for polycystic kidney disease that encodes an integral membrane protein. Science 272: 1339–1342, 1996 [DOI] [PubMed] [Google Scholar]
- 9.Pazour GJ, Dickert BL, Vucica Y, Seeley ES, Rosenbaum JL, Witman GB, Cole DG: Chlamydomonas IFT88 and its mouse homologue, polycystic kidney disease gene tg737, are required for assembly of cilia and flagella. J Cell Biol 151: 709–718, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma M, Tian X, Igarashi P, Pazour GJ, Somlo S: Loss of cilia suppresses cyst growth in genetic models of autosomal dominant polycystic kidney disease. Nat Genet 45: 1004–1012, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duldulao NA, Lee S, Sun Z: Cilia localization is essential for in vivo functions of the Joubert syndrome protein Arl13b/Scorpion. Development 136: 4033–4042, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caspary T, Larkins CE, Anderson KV: The graded response to Sonic Hedgehog depends on cilia architecture. Dev Cell 12: 767–778, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Cantagrel V, Silhavy JL, Bielas SL, Swistun D, Marsh SE, Bertrand JY, Audollent S, Attié-Bitach T, Holden KR, Dobyns WB, Traver D, Al-Gazali L, Ali BR, Lindner TH, Caspary T, Otto EA, Hildebrandt F, Glass IA, Logan CV, Johnson CA, Bennett C, Brancati F, Valente EM, Woods CG, Gleeson JG International Joubert Syndrome Related Disorders Study Group : Mutations in the cilia gene ARL13B lead to the classical form of Joubert syndrome. Am J Hum Genet 83: 170–179, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thomas S, Cantagrel V, Mariani L, Serre V, Lee JE, Elkhartoufi N, de Lonlay P, Desguerre I, Munnich A, Boddaert N, Lyonnet S, Vekemans M, Lisgo SN, Caspary T, Gleeson J, Attié-Bitach T: Identification of a novel ARL13B variant in a Joubert syndrome-affected patient with retinal impairment and obesity. Eur J Hum Genet 23: 621–627, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodríguez CI, Buchholz F, Galloway J, Sequerra R, Kasper J, Ayala R, Stewart AF, Dymecki SM: High-efficiency deleter mice show that FLPe is an alternative to Cre-loxP. Nat Genet 25: 139–140, 2000 [DOI] [PubMed] [Google Scholar]
- 16.Shao X, Somlo S, Igarashi P: Epithelial-specific Cre/lox recombination in the developing kidney and genitourinary tract. J Am Soc Nephrol 13: 1837–1846, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Shibazaki S, Yu Z, Nishio S, Tian X, Thomson RB, Mitobe M, Louvi A, Velazquez H, Ishibe S, Cantley LG, Igarashi P, Somlo S: Cyst formation and activation of the extracellular regulated kinase pathway after kidney specific inactivation of Pkd1. Hum Mol Genet 17: 1505–1516, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fedeles SV, Tian X, Gallagher AR, Mitobe M, Nishio S, Lee SH, Cai Y, Geng L, Crews CM, Somlo S: A genetic interaction network of five genes for human polycystic kidney and liver diseases defines polycystin-1 as the central determinant of cyst formation. Nat Genet 43: 639–647, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jonassen JA, SanAgustin J, Baker SP, Pazour GJ: Disruption of IFT complex A causes cystic kidneys without mitotic spindle misorientation. J Am Soc Nephrol 23: 641–651, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cowley BD Jr., Smardo FL Jr., Grantham JJ, Calvet JP: Elevated c-myc protooncogene expression in autosomal recessive polycystic kidney disease. Proc Natl Acad Sci U S A 84: 8394–8398, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin F, Hiesberger T, Cordes K, Sinclair AM, Goldstein LS, Somlo S, Igarashi P: Kidney-specific inactivation of the KIF3A subunit of kinesin-II inhibits renal ciliogenesis and produces polycystic kidney disease. Proc Natl Acad Sci U S A 100: 5286–5291, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Traykova-Brauch M, Schönig K, Greiner O, Miloud T, Jauch A, Bode M, Felsher DW, Glick AB, Kwiatkowski DJ, Bujard H, Horst J, von Knebel Doeberitz M, Niggli FK, Kriz W, Gröne HJ, Koesters R: An efficient and versatile system for acute and chronic modulation of renal tubular function in transgenic mice. Nat Med 14: 979–984, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trudel M, D’Agati V, Costantini F: C-myc as an inducer of polycystic kidney disease in transgenic mice. Kidney Int 39: 665–671, 1991 [DOI] [PubMed] [Google Scholar]
- 24.Mrug M, Zhou J, Woo Y, Cui X, Szalai AJ, Novak J, Churchill GA, Guay-Woodford LM: Overexpression of innate immune response genes in a model of recessive polycystic kidney disease. Kidney Int 73: 63–76, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Su Z, Wang X, Gao X, Liu Y, Pan C, Hu H, Beyer RP, Shi M, Zhou J, Zhang J, Serra AL, Wüthrich RP, Mei C: Excessive activation of the alternative complement pathway in autosomal dominant polycystic kidney disease. J Intern Med 276: 470–485, 2014 [DOI] [PubMed] [Google Scholar]
- 26.Cao Y, Semanchik N, Lee SH, Somlo S, Barbano PE, Coifman R, Sun Z: Chemical modifier screen identifies HDAC inhibitors as suppressors of PKD models. Proc Natl Acad Sci U S A 106: 21819–21824, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levine MH, Wang Z, Bhatti TR, Wang Y, Aufhauser DD, McNeal S, Liu Y, Cheraghlou S, Han R, Wang L, Hancock WW: Class-specific histone/protein deacetylase inhibition protects against renal ischemia reperfusion injury and fibrosis formation. Am J Transplant 15: 965–973, 2015 [DOI] [PMC free article] [PubMed]
- 28.Zhang X, Liu H, Hock T, Thannickal VJ, Sanders YY: Histone deacetylase inhibition downregulates collagen 3A1 in fibrotic lung fibroblasts. Int J Mol Sci 14: 19605–19617, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ota C, Yamada M, Fujino N, Motohashi H, Tando Y, Takei Y, Suzuki T, Takahashi T, Kamata S, Makiguchi T, Yamaya M, Kubo H: Histone deacetylase inhibitor restores surfactant protein-C expression in alveolar-epithelial type II cells and attenuates bleomycin-induced pulmonary fibrosis in vivo. Exp Lung Res 41: 422–434, 2015 [DOI] [PubMed] [Google Scholar]
- 30.Choi SY, Ryu Y, Kee HJ, Cho SN, Kim GR, Cho JY, Kim HS, Kim IK, Jeong MH: Tubastatin A suppresses renal fibrosis via regulation of epigenetic histone modification and Smad3-dependent fibrotic genes. Vascul Pharmacol 72: 130–140, 2015 [DOI] [PubMed] [Google Scholar]
- 31.Van Beneden K, Geers C, Pauwels M, Mannaerts I, Wissing KM, Van den Branden C, van Grunsven LA: Comparison of trichostatin A and valproic acid treatment regimens in a mouse model of kidney fibrosis. Toxicol Appl Pharmacol 271: 276–284, 2013 [DOI] [PubMed] [Google Scholar]
- 32.Liu N, He S, Ma L, Ponnusamy M, Tang J, Tolbert E, Bayliss G, Zhao TC, Yan H, Zhuang S: Blocking the class I histone deacetylase ameliorates renal fibrosis and inhibits renal fibroblast activation via modulating TGF-beta and EGFR signaling. PLoS One 8: e54001, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Van Beneden K, Mannaerts I, Pauwels M, Van den Branden C, van Grunsven LA: HDAC inhibitors in experimental liver and kidney fibrosis. Fibrogenesis Tissue Repair 6: 1, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pang M, Zhuang S: Histone deacetylase: A potential therapeutic target for fibrotic disorders. J Pharmacol Exp Ther 335: 266–272, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kinugasa F, Noto T, Matsuoka H, Urano Y, Sudo Y, Takakura S, Mutoh S: Prevention of renal interstitial fibrosis via histone deacetylase inhibition in rats with unilateral ureteral obstruction. Transpl Immunol 23: 18–23, 2010 [DOI] [PubMed] [Google Scholar]
- 36.Marumo T, Hishikawa K, Yoshikawa M, Hirahashi J, Kawachi S, Fujita T: Histone deacetylase modulates the proinflammatory and -fibrotic changes in tubulointerstitial injury. Am J Physiol Renal Physiol 298: F133–F141, 2010 [DOI] [PubMed] [Google Scholar]
- 37.Pang M, Kothapally J, Mao H, Tolbert E, Ponnusamy M, Chin YE, Zhuang S: Inhibition of histone deacetylase activity attenuates renal fibroblast activation and interstitial fibrosis in obstructive nephropathy. Am J Physiol Renal Physiol 297: F996–F1005, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seixas C, Choi SY, Polgar N, Umberger NL, East MP, Zuo X, Moreiras H, Ghossoub R, Benmerah A, Kahn RA, Fogelgren B, Caspary T, Lipschutz JH, Barral DC: Arl13b and the exocyst interact synergistically in ciliogenesis. Mol Biol Cell 27: 308–320, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xia S, Li X, Johnson T, Seidel C, Wallace DP, Li R: Polycystin-dependent fluid flow sensing targets histone deacetylase 5 to prevent the development of renal cysts. Development 137: 1075–1084, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mitsiades CS, Mitsiades NS, McMullan CJ, Poulaki V, Shringarpure R, Hideshima T, Akiyama M, Chauhan D, Munshi N, Gu X, Bailey C, Joseph M, Libermann TA, Richon VM, Marks PA, Anderson KC: Transcriptional signature of histone deacetylase inhibition in multiple myeloma: Biological and clinical implications. Proc Natl Acad Sci U S A 101: 540–545, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mariadason JM, Corner GA, Augenlicht LH: Genetic reprogramming in pathways of colonic cell maturation induced by short chain fatty acids: Comparison with trichostatin A, sulindac, and curcumin and implications for chemoprevention of colon cancer. Cancer Res 60: 4561–4572, 2000 [PubMed] [Google Scholar]
- 42.Schrump DS, Fischette MR, Nguyen DM, Zhao M, Li X, Kunst TF, Hancox A, Hong JA, Chen GA, Kruchin E, Wright JJ, Rosing DR, Sparreboom A, Figg WD, Steinberg SM: Clinical and molecular responses in lung cancer patients receiving Romidepsin. Clin Cancer Res 14: 188–198, 2008 [DOI] [PubMed] [Google Scholar]
- 43.Ekwall K: Genome-wide analysis of HDAC function. Trends Genet 21: 608–615, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Robyr D, Suka Y, Xenarios I, Kurdistani SK, Wang A, Suka N, Grunstein M: Microarray deacetylation maps determine genome-wide functions for yeast histone deacetylases. Cell 109: 437–446, 2002 [DOI] [PubMed] [Google Scholar]
- 45.Chiba T, Yokosuka O, Arai M, Tada M, Fukai K, Imazeki F, Kato M, Seki N, Saisho H: Identification of genes up-regulated by histone deacetylase inhibition with cDNA microarray and exploration of epigenetic alterations on hepatoma cells. J Hepatol 41: 436–445, 2004 [DOI] [PubMed] [Google Scholar]
- 46.Zhou X, Fan LX, Peters DJ, Trudel M, Bradner JE, Li X: Therapeutic targeting of BET bromodomain protein, Brd4, delays cyst growth in ADPKD. Hum Mol Genet 24: 3982–3993, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Qin S, Taglienti M, Cai L, Zhou J, Kreidberg JA: c-Met and NF-κB-dependent overexpression of Wnt7a and -7b and Pax2 promotes cystogenesis in polycystic kidney disease. J Am Soc Nephrol 23: 1309–1318, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.He W, Dai C, Li Y, Zeng G, Monga SP, Liu Y: Wnt/beta-catenin signaling promotes renal interstitial fibrosis. J Am Soc Nephrol 20: 765–776, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen L, Zhou X, Fan LX, Yao Y, Swenson-Fields KI, Gadjeva M, Wallace DP, Peters DJ, Yu A, Grantham JJ, Li X: Macrophage migration inhibitory factor promotes cyst growth in polycystic kidney disease. J Clin Invest 125: 2399–2412, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Karihaloo A, Koraishy F, Huen SC, Lee Y, Merrick D, Caplan MJ, Somlo S, Cantley LG: Macrophages promote cyst growth in polycystic kidney disease. J Am Soc Nephrol 22: 1809–1814, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Swenson-Fields KI, Vivian CJ, Salah SM, Peda JD, Davis BM, van Rooijen N, Wallace DP, Fields TA: Macrophages promote polycystic kidney disease progression. Kidney Int 83: 855–864, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miertzschke M, Koerner C, Spoerner M, Wittinghofer A: Structural insights into the small G-protein Arl13B and implications for Joubert syndrome. Biochem J 457: 301–311, 2014 [DOI] [PubMed] [Google Scholar]
- 53.Westerfield M: The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish (Danio rerio), Eugene, University of Oregon Press, 2000 [Google Scholar]
- 54.Zhao L, Yuan S, Cao Y, Kallakuri S, Li Y, Kishimoto N, DiBella L, Sun Z: Reptin/Ruvbl2 is a Lrrc6/Seahorse interactor essential for cilia motility. Proc Natl Acad Sci U S A 110: 12697–12702, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.