Abstract
Patients treated with hemodialysis develop severely reduced functional capacity, which can be partially ameliorated by correcting anemia and through exercise training. In this study, we determined perturbations of an erythroid-stimulating agent and exercise training to examine if and where limitation to oxygen transport exists in patients on hemodialysis. Twenty-seven patients on hemodialysis completed a crossover study consisting of two exercise training phases at two hematocrit (Hct) values: 30% (anemic) and 42% (physiologic; normalized by treatment with erythroid-stimulating agent). To determine primary outcome measures of peak power and oxygen consumption (VO2) and secondary measures related to components of oxygen transport and utilization, all patients underwent numerous tests at five time points: baseline, untrained at Hct of 30%, after training at Hct of 30%, untrained at Hct of 42%, and after training at Hct of 42%. Hct normalization, exercise training, or the combination thereof significantly improved peak power and VO2 relative to values in the untrained anemic phase. Hct normalization increased peak arterial oxygen and arteriovenous oxygen difference, whereas exercise training improved cardiac output, citrate synthase activity, and peak tissue diffusing capacity. However, although the increase in arterial oxygen observed in the combination phase reached a value similar to that in healthy sedentary controls, the increase in peak arteriovenous oxygen difference did not. Muscle biopsy specimens showed markedly thickened endothelium and electron–dense interstitial deposits. In conclusion, exercise and Hct normalization had positive effects but failed to normalize exercise capacity in patients on hemodialysis. This effect may be caused by abnormalities identified within skeletal muscle.
Keywords: anemia, erythropoietin, physical activity, end stage kidney disease, citrate synthase, VO2 peak
Functional capacity and fitness are markedly reduced in patients with ESRD,1–3 which increases their risk of mortality4 and limits their ability to perform activities of daily life.5 The reduction in physical fitness observed is multifactorial; however, renal anemia contributes significantly.6 Oxygen carrying capacity is reduced by anemia, which in turn, limits an individual’s peak oxygen uptake, ability to perform exercise, and time to exhaustion, especially in ESRD.7
Oxygen uptake can be thought of as a cascade of oxygen from the lung to the blood pumped by the heart to the working tissues, where it is used to perform physical work.8 Patients with ESRD on hemodialysis can have pathologies at most of these sites. The majority of patients on hemodialysis develop a progressive anemia, because their kidneys become unable to produce sufficient erythropoietin to maintain normal hemoglobin mass.9 This anemia combined with impaired cardiovascular function limit the delivery of oxygen to the tissues. As kidney function declines, patients become even less active,10 causing further deterioration of cardiovascular function, and a downward spiral ensues, ultimately leading to severe disability.
Fortunately, the anemia of renal disease can be avoided because of the advent of recombinant erythropoietin. Anemia is typically treated with erythropoiesis-stimulating agents (ESAs), which partially normalize renal anemia, improve quality of life and cognitive function,11,12 reduce left ventricular hypertrophy,13 and modestly increase peak oxygen uptake relative to the increase in hematocrit.14 Likewise, intradialytic exercise training also increases peak oxygen uptake15,16 and has additional cardioprotective benefits.17 However, similar to treatment with ESAs, exercise training in patients with kidney disease does not restore exercise capacity to normal levels.18,19 Both interventions have been shown to produce improvements in physical fitness and peak oxygen uptake; however, they have not returned patients to the level of fitness of most sedentary people with normal renal function. It is possible in the case of raising hematocrit that, although oxygen carrying capacity of the blood is markedly improved, the other components of the oxygen cascade are unchanged and limit the potential benefit of normalizing hematocrit. Conversely, regular exercise training alone may not achieve its full benefit if oxygen transport is limited by anemia. A third scenario exists where some aspect of ESRD or dialysis20 prevents normal oxygen transport that may be unaffected by either correction of anemia or regular exercise.
Evidently, the precise mechanisms underlying the modest improvements in oxygen uptake observed after exercise training or treatment of anemia in patients on hemodialysis have not been carefully elucidated. Thus, we used a crossover-designed study to test the hypothesis that patients with ESRD on hemodialysis would have the greatest improvement in peak oxygen uptake and peak power when hematocrit was increased to within a normal physiologic range (hematocrit of 42%) and combined with intradialytic exercise training (TR42) compared with exercise training alone (TR30), normalized hematocrit alone (UN42), or anemic untrained hematocrit (UN30). Secondary aims were to elucidate the possible mechanism of exercise intolerance in ESRD.
Results
Subject Characteristics
Fifty patients with ESRD requiring hemodialysis were recruited to participate in this study. Three patients were deemed ineligible after randomization. Of the 47 remaining patients, 27 patients completed the entire study (study design is given in Figure 1). In total, 20 patients withdrew from the study, predominantly during the first and final 12-week periods (n=10 and n=7, respectively). Reasons for withdrawal included personal reasons (n=8), renal transplant (n=5), unable to achieve target hematocrit of 42% (n=2) (despite epoetin α doses >2000 U/kg per week), failure to return to hematocrit of 30% (n=1), poor hemodialysis compliance (n=3), and cancer (n=1). The characteristics of patients who were randomized and those who completed the study are presented in Table 1. The majority of patients were black (51%) or Hispanic (32%). Hypertension was the predominant cause of renal failure followed by GN and diabetes.
Figure 1.
Schematic diagram of the study protocol. Subjects were required to have a stable hematocrit (Hct) for a minimum of 4 weeks during the maintenance (increasing or decreasing) phase before any testing. Similarly, patients were required to complete ≥33 good exercise sessions (92%) before completing post-testing for each training phase. EXT, exercise training.
Table 1.
Baseline characteristics of the enrolled cohort and completed participants
| Characteristics | Randomized, n=47 | Completed, n=27 | P Value |
|---|---|---|---|
| Age, yr [range] | 44±13 [20–71] | 45±11 | 0.36 |
| Sex, n (% men) | 36 (77) | 22 (81) | 0.62 |
| Height, cm | — | 172±9 | |
| Weight, kg | — | 79±17 | |
| Body fat, % | — | 20±7 | |
| Ethnicity, n (%) | |||
| White | 6 (13) | 3 (11) | 0.83 |
| Black | 24 (51) | 15 (56) | 0.71 |
| Hispanics | 15 (32) | 8 (30) | 0.84 |
| Asian Indian | 2 (4) | 1 (4) | 0.91 |
| Cause of kidney disease, n (%) | |||
| Hypertension | 23 (49) | 16 (58) | 0.39 |
| GN | 10 (21) | 5 (19) | 0.77 |
| Diabetes | 8 (17) | 5 (19) | 0.87 |
| Other | 6 (13) | 1 (4) | 0.20 |
| Duration of hemodialysis, yr [range] | 3±3 [4 mo to 17 yr] | 3±3 | 0.99 |
| Hematocrit, % | 30.7±1.8 | 30.8±1.9 | 0.73 |
| ESA dose, U/kg per wk | 142±112 | 97±89 | 0.07 |
Values are presented as mean±SD or n (%) for categorical variables.
The dosage of ESA required to normalize hematocrit levels was approximately four times that of the baseline dose of ESA (epoetin α: 415±196 versus 97±89 U/kg per week). The higher dose of ESA significantly increased hematocrit, arterial O2 content, hemoglobin, total blood volume and red cell mass and decreased plasma volume (Table 2). Normalization of hematocrit also increased resting diastolic BP and mean arterial pressure (MAP); however, systolic BP was unchanged (Table 2).
Table 2.
Response of resting measures of metabolic and cardiovascular function to exercise training and hematocrit normalization
| Parameter | UN30 | TR30 | UN42 | TR42 | Main Effect Hct | Main Effect Training | Interaction |
|---|---|---|---|---|---|---|---|
| Hct, % | 31.4±1.7 | 30.9±3.9 | 41.5±2.1 | 42.1±2.4 | <0.0001 | 0.92 | 0.28 |
| Hemoglobin, g/dl | 10.7±0.8 | 10.5±1.3 | 14.4±0.9 | 14.6±1.0 | <0.001 | 0.99 | 0.31 |
| RBC volume, ml/kg | 17.7±2.9 | 17.6±3.3 | 24.4±3.9 | 25.3±4.9 | <0.0001 | 0.59 | 0.50 |
| Plasma volume, ml/kg | 46.3±8.5 | 46.8±8.1 | 42.3±6.2 | 43.3±8.7 | 0.016 | 0.624 | 0.87 |
| Blood volume, ml/kg | 64.0±11.2 | 64.3±10.4 | 66.6±9.7 | 68.6±13.3 | 0.113 | 0.596 | 0.69 |
| VO2, ml/kg per min | 3.0±0.6 | 2.8±0.5 | 3.1±0.6 | 3.0±0.6 | 0.179 | 0.179 | 0.65 |
| Cardiac output, L/min | 7.4±1.8 | 7.3±2.0 | 7.5±2.1 | 7.3±1.9 | 0.89 | 0.69 | 0.89 |
| Heart rate, bpm | 73±9 | 77±12 | 72±12 | 73±12 | 0.25 | 0.25 | 0.49 |
| a-vO2 difference, vol% | 3.2±0.6 | 3.2±0.7 | 3.4±0.9 | 3.4±1.0 | 0.20 | 0.99 | 0.99 |
| aO2, vol% | 14.3±1.2 | 14.2±1.9 | 18.6±1.6 | 18.8±1.9 | <0.0001 | 0.88 | 0.64 |
| Resting systolic BP, mmHg | 145±21 | 142±22 | 146±23 | 140±21 | 0.90 | 0.28 | 0.72 |
| Resting diastolic BP, mmHg | 84±13 | 83±12 | 90±15 | 87±11 | 0.045 | 0.42 | 0.686 |
| Resting MAP, mmHg | 104±12 | 103±13 | 109±15 | 104±11 | 0.23 | 0.23 | 0.42 |
Data are presented as means±SDs for a given variable at a given time point. Hct, hematocrit; RBC, red blood cell; a-vO2 difference, arteriovenous oxygen difference; aO2, arterial oxygen content.
Exercise Training Compliance
The number of completed training sessions and mean training impulse (TRIMP) scores, which provides an overall measure of training load, were similar between the anemic (TR30) and normalized hematocrit (TR42) conditions (Table 3). However, total work performed was slightly higher under the TR42 condition, meaning a greater amount of energy was expended during these exercise sessions.
Table 3.
Comparison of training compliance and load at the anemic and normalized hematocrit
| Parameter | TR30 | TR42 | P Value |
|---|---|---|---|
| Completed sessions | 38±4 | 36±2 | — |
| Mean TRIMP per session | 54±35 | 50±29 | 0.65 |
| Work performed per session, kJ | 136±55 | 152±61 | 0.32 |
| Kilojoules per TRIMP score per session | 3.0±1.5 | 3.7±1.7 | 0.12 |
Data are presented as means±SDs. kJ, kilojoules.
Effects of Hematocrit Normalization and Intradialytic Training on Cardiovascular and Metabolic Function
The effects of hematocrit normalization, exercise training, and the combination are presented in Table 4. Peak power and oxygen consumption (VO2) were both significantly improved by normalization of hematocrit and exercise training. However, the effect of the combination of the two perturbations, although incrementally greater than either intervention alone, was modest. The normalization of hematocrit increased peak arterial oxygen content and caused a widening of the arteriovenous oxygen difference, although exercise training alone tended to increase peak arteriovenous oxygen difference and arterial lactate concentration. The effects of exercise training and higher hematocrit were additive for arteriovenous oxygen difference. However, the combination did not normalize these values, which remained at only two thirds of expected values in healthy sedentary controls (Table 4). Finally, tissue diffusing capacity, a measure of oxygen transport by diffusion from the microcirculation to the mitochondria, was improved by exercise training at peak exercise by 11%.
Table 4.
Peak metabolic and cardiovascular response to exercise training and hematocrit normalization
| Parameter | UN30 | TR30 | UN42 | TR42 | Healthy Controls | Main Effect Hct | Main Effect Training | Interaction |
|---|---|---|---|---|---|---|---|---|
| Peak power, W | 101±35 | 124±45 | 110±37 | 134±48 | — | 0.238 | 0.004 | 0.95 |
| aO2, vol% | 15.7±1.1 | 15.5±2.1 | 20.3±1.3 | 20.5±1.7 | 18.6±1.6 | <0.0001 | 0.99 | 0.50 |
| VO2, ml/kg per min | 17.9±3.9 | 19.3±4.4 | 19.6±4.2 | 21.5±5.1 | 30.0±5.0 | 0.024 | 0.055 | 0.77 |
| a-vO2 difference, vol% | 7.9±1.3 | 8.3±1.4 | 8.5±1.8 | 9.0±1.8 | 15.6±2.1 | 0.036 | 0.14 | 0.87 |
| Cardiac output, L/min | 19.0±5.7 | 19.3±5.1 | 18.7±6.0 | 20.0±6.1 | 14.9±3.0 | 0.83 | 0.40 | 0.60 |
| RER | 1.22±0.12 | 1.20±0.10 | 1.15±0.10 | 1.16±0.09 | 1.26±0.08 | 0.007 | 0.80 | 0.45 |
| Heart rate, bpm | 144±24 | 145±22 | 143±22 | 144±24 | 177±8 | 0.82 | 0.82 | 0.99 |
| Stroke volume, ml | 131±40 | 135±38 | 129±35 | 135±35 | 84±17 | 0.89 | 0.48 | 0.89 |
| MAP, mmHg | 127±17 | 123±11 | 129±16 | 130±15 | 123±12 | 0.12 | 0.60 | 0.39 |
| SVR, dynes⋅s−1 per cm5 | 7.8±3.2 | 7.0±2.6 | 7.8±2.5 | 7.1±1.8 | — | 0.92 | 0.13 | 0.92 |
| Tissue diffusing capacity, ml/m per torr | 28.3±8.8 | 31.5±8.0 | 27.6±7.3 | 30.8±9.1 | — | 0.66 | 0.048 | 0.99 |
| Arterial lactate, mmol/L | 5.5±1.8 | 6.3±2.0 | 4.8±1.3 | 5.6±1.5 | 10.4±1.7 | 0.032 | 0.014 | 0.99 |
| Arterial pH | 7.33±0.06 | 7.32±0.06 | 7.29±0.03 | 7.29±0.05 | — | 0.001 | 0.615 | 0.62 |
Data are presented as means±SDs for a given variable at a given time point. For comparison, we have included a subset of historical healthy sedentary age–matched (n=20; men =75%; age =48±6 years old; Hct=41.3±3.2) control data from our laboratory (work by Carrick-Ransom and colleagues42) to highlight the disturbances to the capacity of the oxygen transport system in the patients on hemodialysis. Despite exercise training and hematocrit normalization, peak VO2 and a-vO2 difference remain severely impaired compared with those in normal sedentary individuals. Hct, hematocrit; —, no data; aO2, arterial oxygen content; a-vO2 difference, arteriovenous oxygen difference; RER, respiratory exchange ratio; SVR, systemic vascular resistance.
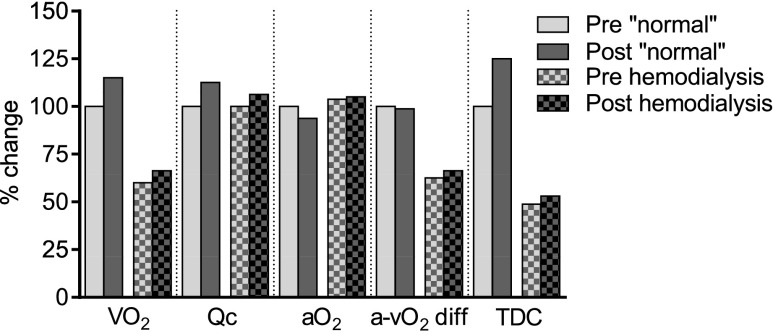
Figure 2 compares healthy normal subjects (from the work by Hartley et al.21) with the patients on hemodialysis after hematocrit normalization before and after exercise training for 12 weeks. Figure 2 highlights the blunted effects of exercise training and hematocrit normalization in patients on hemodialysis as well as the severely impaired capacity of patients at baseline. The normal training response is characterized by an increase in peak VO2 because of increased cardiac output and tissue diffusing capacity. In patients on hemodialysis, the increase in peak VO2 was one half of the observed change in the normal subjects. The exercise training had little effect on the arteriovenous oxygen difference, which remained low compared with that in the normal subjects, along with peak tissue diffusing capacity.
Figure 2.
Peak exercise response to endurance training. Comparison of maximal oxygen uptake and components in the subjects in the work by Hartley et al.21 with these data in patients on hemodialysis with a normal hematocrit. The first column in each category is normal subjects’ pretraining data, which are assigned 100%. The second column is the change from initial values in normal subjects post-training. The third column is the initial data from the patients on hemodialysis as percentages of those in the work by Hartley et al.21 The fourth column is the data from patients on hemodialysis post-training. aO2, arterial oxygen content; a-vO2, arteriovenous oxygen difference; Qc, cardiac output; TDC, tissue diffusing capacity.
Quadriceps Histology and Biochemistry
The effects of the perturbations on muscle histology and biochemistry are summarized in Table 5. Muscle histology was not significantly altered by any intervention (Table 5), although exercise training significantly increases citrate synthase activity by 43% and acyl-CoA dehydrogenase (HAD) by 16%. This indicates that exercise training increased the concentration of oxidative enzymes. However, phosphofructokinase and myoglobin were not altered by any intervention. Change in citrate synthase activity did not correlate with change in peak VO2, tissue diffusing capacity, or the work performed during exercise training.
Table 5.
Effects of intradialytic training and hematocrit normalization on skeletal muscle strength, structure, and function
| Parameter | UN30 | TR30 | UN42 | TR42 | Main Effect Hct | Main Effect Training | Interaction |
|---|---|---|---|---|---|---|---|
| Type 1 fiber, % | 33±10 | 32±9 | 31±9 | 29±9 | 0.16 | 0.40 | 0.78 |
| Area type 1, μm2 | 5366±1955 | 5226±1757 | 5171±1575 | 5140±1665 | 0.68 | 0.80 | 0.87 |
| Area type 2, μm2 | 3528±1498 | 3364±1106 | 3393±1168 | 3260±1062 | 0.61 | 0.53 | 0.95 |
| Capillary density, μm2 | 271±85 | 280±103 | 268±145 | 270±142 | 0.78 | 0.81 | 0.88 |
| Capillary-to-fiber ratio | 1.1±0.5 | 1.2±0.6 | 1.1±0.7 | 1.0±0.7 | 0.99 | 0.99 | 0.99 |
| Citrate synthase, μm/min per dry gram | 12.6±4.8 | 18.0±7.2 | 12.2±4.7 | 17.9±7.5 | 0.83 | <0.0001 | 0.90 |
| HAD, μm/min per dry gram | 25.8±8.2 | 30.0±14.7 | 22.8±7.5 | 30.6±12.9 | 0.58 | 0.0066 | 0.41 |
| Phosphofructokinase, μm/min per dry gram | 87.2±50.2 | 74.1±34.0 | 86.4±50.3 | 97.7±64.5 | 0.25 | 0.93 | 0.22 |
| Buffer capacity, μm H+ per pH per dry gram | 356±89 | 387±85 | 359±71 | 361±79 | 0.46 | 0.29 | 0.36 |
| Myoglobin, ng/wet g | 2.3±0.7 | 2.0±0.7 | 2.4±0.9 | 2.3±0.7 | 0.17 | 0.17 | 0.49 |
Data are presented as means±SDs for a given variable at a given time point. Hct, hematocrit; H+, hydrogen ions.
Adverse Events
One serious adverse event occurred during the study; a patient suffered a seizure during dialysis at the normal hematocrit level. In addition, 10 access thromboses required treatment: four occurred while patients were at the higher hematocrit, three occurred while patients were at the lower hematocrit, and three occurred while either increasing or decreasing hematocrit.
Comparison of Healthy Aged–Matched Controls with Patients on Hemodialysis: Quadriceps Electron Micrographs
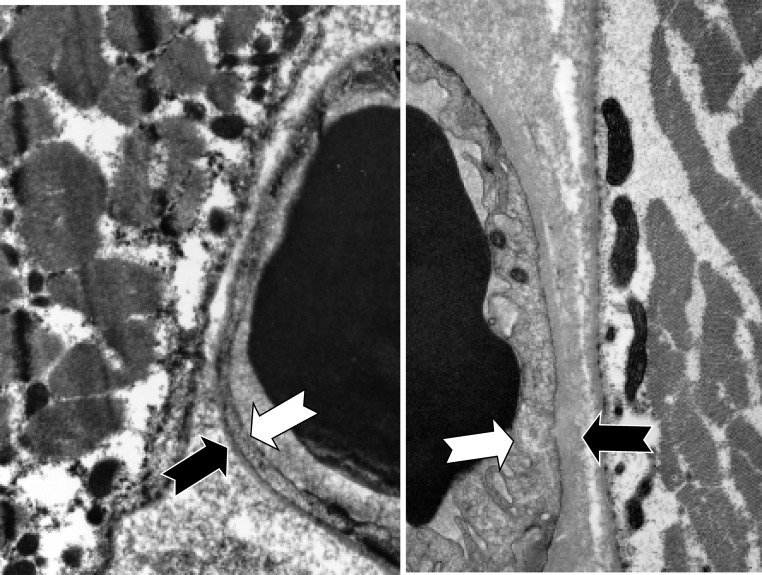
We compared a subset of patients on hemodialysis (n=18) at baseline with a group of controls with normal renal function (n=7). A typical sample from a healthy control is shown in Figure 3, left panel, and a typical hemodialysis patients is shown in Figure 3B, right panel. The sample from the hemodialysis patients is characterized by thickened capillary endothelium and capillary basement membranes, with an enlarged interstitial space that was filled with electron-dense material. Mitochondrial density was also reduced in the adjacent muscle. Both the capillary endothelium and basement membranes of the patient on hemodialysis were significantly thicker in comparison with those in the controls (Figure 3, Table 6).
Figure 3.
Comparison of representative hemodialysis patient and healthy control electron micrograph of capillary and skeletal muscle. The photo in the left panel is from an individual without renal failure, and the photo in the right panel is from a patient on hemodialysis. The large black objects on the inner edges of the two photographs are red blood cells in capillaries. The white arrows depict the inner edges of the capillary endothelium, and the black arrows depict the outer edges of the capillary basement membrane. Magnification, ×5000.
Table 6.
Comparison of capillary structure using electron micrograph
| Parameter | Patients on Hemodialysis, n=18 | Healthy Control, n=7 | P Value |
|---|---|---|---|
| Capillary endothelium, nm | 780±450 | 460±120 | 0.01 |
| Capillary basement membrane, nm | 500±270 | 220±60 | 0.002 |
| Total thickness, nm | 1130±500 | 680±190 | 0.003 |
Data are presented as means±SD.
Discussion
We sought to identify the limitations in the oxygen cascade system in hemodialysis patients by examining the response to exercise training and normalization of hematocrit on components of the Fick Equation [VO2 = cardiac output × (Ca − Cv)]. We found that both restoring hematocrit levels to within a normal range and intradialytic exercise training improve peak oxygen uptake and power, albeit by different mechanisms. The normalization of hematocrit markedly increased arterial oxygen concentration, widening the arteriovenous oxygen difference. In contrast, exercise training increased peak VO2 through improved peak tissue diffusing capacity or greater ability to diffuse oxygen from the capillary to the mitochondria. Although the combination did provide for greater peak power and VO2, the increases were far less than expected compared with changes observed in individuals not dependent on hemodialysis. A major finding was the discovery of markedly thickened capillary endothelium and electron-dense deposits in the interstitial space, which could explain the poor functional capacity found in hemodialysis patients and likely inhibits oxygen transport and removal of metabolic waste from working muscle.
Nonetheless, these improvements in functional capacity and maximal oxygen uptake are clinically relevant. The improvement in peak VO2 after training and hematocrit normalization was equivalent to an approximately 1-MET increase in fitness from the baseline hematocrit untrained state (Δ+3.6 ml/kg per minute). Several studies have shown reductions in total mortality and cardiovascular events with increasing fitness.22–24 Specifically, Blair and colleagues22,23 and Gulati et al.25 have shown that a 1-MET increase in exercise capacity is equivalent to a 12% improvement in survival in men and a 17% improvement in survival in women.
Effects of Exercise Training
In this study, intradialytic exercise training alone significantly increased peak power and VO2, citrate synthase activity and HAD, and tissue diffusing capacity. The magnitude of the increase in peak VO2 observed in our study is consistent with the results of the recent meta-analysis by Heiwe and Jacobson.26 Thus, it seems that exercise training in hemodialysis patients shows a modest but significant improvement in peak VO2 that is approximately two thirds of what is expected in healthy individuals who have received a similar dose of training and are not on hemodialysis.21
Effects of Normalization of Hematocrit
In this study, normalization of hematocrit resulted in an increase in peak VO2 and arteriovenous oxygen difference as well as increases in arterial oxygen content and red blood cell volume. In healthy subjects (nondialysis), increasing hematocrit by 20% (from 42.7% to 50.8%) increases peak VO2 by 7%.27 In contrast, increasing hematocrit by 36% (from 30.8±1.9% to 41.8±2.1%) resulted in only a 9% increase in peak VO2 in hemodialysis patients. These findings highlight the diminished response of peak VO2 to increasing arterial oxygen content in hemodialysis patients. Furthermore, despite the large increase in arterial oxygen content, the skeletal muscle was only able to extract a small amount of the additional oxygen available.
Effect of Exercise Training and Hematocrit Normalization
The effects of the combination of exercise training and hematocrit normalization significantly increased peak power, VO2, and arteriovenous oxygen difference. However, the effects were modest, and the combination did not normalize exercise capacity, despite significant improvement in the ability of the muscle to extract oxygen and increased availability of oxygen. In contrast to our findings, Painter et al.15 found no improvement in peak VO2 with exercise training and hematocrit normalization. The difference in results may be partially explained by study design; in this study, we used a crossover design, whereas Painter et al.15 randomized groups of different individuals. However, the findings from both studies do highlight the difficulty associated with improving peak VO2 in hemodialysis patients. Neither increasing arterial oxygen content nor regular exercise was able to completely overcome the apparent barrier to oxygen transport from red cell to mitochondria.
Limitations in Oxygen Transfer in Patients on Hemodialysis
Although peak arteriovenous oxygen difference did increase with both normalization of hematocrit and the combination of hematocrit and exercise training, the absolute values were still well below those of age– and sex–matched healthy controls (Figure 2, Table 4). Tissue diffusing capacity, which is the maximal ability to transfer oxygen from red blood cells in the capillary to the mitochondria,28 was also low in comparison with that observed in healthy individuals21 but did increase slightly with exercise training. This finding suggests that exercise training enhanced the transfer of oxygen from the microvasculature red cells to the mitochondria. Despite this increased ability to transfer oxygen, we did not observe any statistically significant changes in capillary density or capillary-to-fiber ratios. Our findings support previous studies19,29,30 that have shown that capillary density and capillary-to-fiber ratios are reduced in hemodialysis patients.
Thus, the results from this study and others15,31 strongly suggest that there is something unique to hemodialysis patients that limits oxygen transfer at the site of the capillary-muscle interface. Sala et al.32 conducted an elegant set of experiments, which also identified an impairment in maximal oxygen supply in young patients with ESRD. Similar to our findings, Sala et al.32 identified abnormally low oxidative diffusion conductance in the muscle but seemingly normal oxidative capacity within the muscle. A limitation of the study by Sala et al.32 was that they did not normalize hemoglobin levels; thus, their comparison group had significantly higher hemoglobin. In this study, we increased arterial oxygen content, which resulted in a modest improvement in peak VO2 but failed to restore tissue diffusing capacity to expected levels.
We identified a possibly explanation for the modest increase in VO2 typically observed after training in hemodialysis patients. In a subset of patients, we examined electron micrographs from the biopsies of skeletal muscle. The patients’ electron micrographs were characterized by markedly thickened capillary endothelium and capillary basement membranes, with an enlarged interstitial space that was filled with electron-dense material (Figure 3, Table 6). The etiology of the thickened capillary endothelium and basement membrane is not clear. However, we hypothesize two possible explanations for the accumulation of the material in the skeletal muscle: (1) chronic renal insufficiency or hemodialysis itself creating an inflammatory reaction in capillary endothelium or (2) the incomplete removal of waste products by hemodialysis, which may have resulted in the accumulation of these unidentified waste products in the skeletal muscle, limiting oxygen diffusion in the muscle. Additional studies are required to confirm these preliminary findings.
Special Considerations
This study investigated the limitations of oxygen transport in patients on hemodialysis. Patients on hemodialysis dialyze via an arteriovenous shunt created surgically by either anastomosing an artery (typically in the upper extremity) to a large superficial vein, which enlarges (“arteriolyzes”) the veins, or inserting a subcutaneous graph between artery and vein. This arteriovenous fistula was a benefit for our study by allowing technically easy access to the arterial circulation for measurements of arterial blood gases and arterial pressures during rest and peak exercise with no additional subject burden, because patients would go on to dialysis after the exercise testing. Whereas, in normal (non-hemodialysis) populations, multiple arterial cannulations carry an unwarranted risk to the subject and are rarely performed. A potential confounding explanation for the lower than expected arteriovenous oxygen difference measurements at rest is that a percentage (perhaps as much as 25%33) of resting cardiac output flows through the shunt, where there is presumably no offloading of oxygen. However, the magnitude of the shunt is likely constant at rest and peak exercise under the four conditions of our experiment (UN30, TR30, UN42, and TR42); therefore, the presence of the shunt fails to account for the less than expected modest increase in arteriovenous oxygen difference observed in this study to the perturbations of normalizing hematocrit and exercise training.
The cardiac outputs at rest and during exercise were elevated in these hemodialysis patients compared with sedentary controls. We have previously shown that, for a given VO2, cardiac output is elevated in patients dialysis.19 The increase in cardiac output is partially caused by the presence of the arteriovenous fistula, which can increase cardiac output by as much as 25%.33,34 We speculate that the combination of hemodialysis and the arteriovenous shunt over time may act to train cardiac output. In sedentary individuals, there is little training of cardiac output; despite this, the cardiac output-VO2 relationship is maintained across a wide range of fitness levels. In addition, there were also differences in the exercise testing protocols: the hemodialysis patients were tested on a bike ergometer (Monark) with a ramp protocol, whereas the sedentary control group were tested upright on a treadmill using a modified Astrand–Saltin incremental protocol. These factors may account for the higher resting and peak cardiac outputs observed in these subjects.
In conclusion, We found that both correcting the anemia of renal disease and intradialytic exercise training produced significant, clinically meaningful improvements in peak oxygen uptake and power. The improvements observed would likely translate into improvements in the ability to perform and sustain a variety of activities of daily living. However, there remains a significant limitation in the ability of the hemodialysis patients to extract oxygen from the red blood cell into the working tissue. We discovered electron microscopic evidence that suggests that the impaired ability to normalize oxygen transport and extraction may be at the site of the capillary wall and interstitial space. We hypothesize that this observation may explain the impaired ability to normalize oxygen transport in hemodialysis patients, even after markedly increasing arterial oxygen carrying capacity and despite a rigorous exercise training program, which resulted in a 43% increase in citrate synthase activity. Additional work is required to identify the electron-dense material in the interstitial space and the etiology of the thickened capillary endothelium to determine (1) whether the changes can be reversed and (2) if removal, reduction, or prevention of accumulation of the material restores the response to training and/or the functional capacity of patients with ESRD to normal.
Concise Methods
Study Participants
We recruited 50 patients undergoing maintenance hemodialysis from outpatient dialysis units in Dallas, Texas. Eligible patients were ≥18 years of age, were on hemodialysis for >3 months, and had hematocrit of 30%±3%. Patients were excluded from the study if they had orthopedic limitations to pedaling a cycle ergometer, unstable heart disease, poor dialysis compliance, or likelihood of renal transplant within 6 months. All participants provided written informed consent to a protocol approved by the Institutional Review Board of the University of Texas Southwestern Medical Center. This study followed the guidelines set forth in the Declaration of Helsinki. After enrollment, three patients were deemed ineligible for randomization (n=1 did not achieve target hematocrit and n=2 did not have ability to pedal a cycle ergometer) (Supplemental Material).
Study Design
This study had a crossover design (Figure 1). After enrollment and baseline assessment, patients were randomized to either group A or B. Group A participants’ hematocrit levels were increased to 42% (UN42), whereas group B levels were maintained at 30% (UN30). After a minimum of 12 weeks, the testing battery was repeated (with a stable hematocrit maintained for a minimum of 4 weeks). Intradialytic exercise training (36 exercise sessions) was then performed by group A with hematocrit levels at 42% (TR42) and group B with hematocrit levels at 30% (TR30). Training was then ceased; hematocrit decreased to 30% in group A and increased to 42% in group B. The testing battery was repeated again before both groups completed 12 weeks of exercise training. The final testing battery was administered on completion of the second 12 weeks of intradialytic exercise training.
Interventions
The data collection for this study was completed before the black box warning issued by the Food and Drug Administration regarding hematocrit and hemoglobin targets for patients with ESRD.
Hematocrit Management
To increase hematocrit, the patient’s maintenance dose of recombinant ESA was increased by 150%; after 2 weeks, the dose was doubled every 2 weeks until the target hematocrit (42%±3%) was obtained. The ESA dose was then titrated to maintain hematocrit within the target range. A placebo was administered to maintain or lower hematocrit to 30%±3%. Hematocrit levels were managed by one of the primary investigators (J.R.T.). This investigator (J.R.T.) did not share this knowledge of the subject’s group assignment with any patients, staff, or investigators in the study. The staff in the dialysis unit were provided with identically labeled syringes with the patients’ dosages of ESA adjusted with diluent so that the volumes were identical, and they were administered by a clinical nurse. This investigator (J.R.T.) had no involvement with study subject training or exercise testing. While participating in the study, ESA for the patient was provided complimentary by the sponsor. Staff at the dialysis units were unaware of the specific ESA dose (or placebo) delivered to the patients in the study and the patient’s hematocrit.
Exercise Training
Supervised exercise training was performed during the second hour of the dialysis session three times per week for 12 weeks. Target workloads were determined from the initial exercise test, and each patient cycled at 75% of his/her peak power output. Exercise duration and intensity were gradually increased, such that, by workout 24, participants were cycling for approximately 60 minutes at the highest tolerable workload that could be sustained. From week 9, high–intensity interval training was introduced with every third workout consisting of an interval session of either 1 minute at 200% of the pre-training peak power output or 2- to 3-minute intervals at 150%–200% of pre-training peak power. Each session was supervised by a trainer who was blinded to the subject’s hematocrit level, and heart rate (Polar) was monitored throughout the session along with cadence, which was maintained at 50 revolutions per minute.
To quantify exercise training, the absolute amount of work performed (kilojoules) in each session (kilojoules per session) was calculated along with TRIMPs of the workout (TRIMPs per session).35 The TRIMP method multiplies the duration of a training session by the average heart rate achieved during that session weighted for exercise intensity as a function of heart rate reserve. Using this method, exercise sessions of longer duration and/or greater intensity, such as interval workouts, are assigned relatively higher TRIMP values (higher heart rate and higher weighting factor) than sessions of shorter duration and/or lower intensity. TRIMP is a commonly used technique to assess and monitor exercise training load.36 The goal for each patient was to complete at least 33 good sessions (92% of all sessions) before post-training testing was performed. Post-testing was scheduled at the end of each phase when an individual subject had completed the goal of that phase (Figure 1) (either attained the target hematocrit or completion of ≥33 good training sessions). The actual time for completion of study phases was 17±10 weeks for phase 1, 15±2 weeks for phase 2, 18±10 weeks for phase 3, and 15±4 weeks for phase 4. Thus, the patients spent an average of 16 months enrolled in the study.
Measurements and Outcomes: On–Dialysis Day Assessments
Resting Metabolic and Hemodynamic Assessment
Approximately 44 hours after the last dialysis session, patients attended our laboratory. On arrival, body weight was measured before subjects were instrumented, and they laid quietly for 60 minutes in a resting metabolic suite. After 30 minutes of supine rest, resting O2 utilization was determined via the Douglas bags method. Resting heart rate, BP, and cardiac output (acetylene rebreathing) were also measured. An arterial blood gas sample was drawn and analyzed for oxyhemoglobin, pO2, pCO2, pH, and HCO3− on an ILS-1306 Blood Gas Analyzer and an ILS-482 Co-Oximeter. Arterial oxygen content was calculated from these data. Arterial lactate concentration was measured on a Yellow Springs 23L Lactate Analyzer (Yellow Springs, Yellow Springs, OH).
Blood Volume
Blood volume was measured using the Evan Blue dye dilution technique.37 Blood volume was calculated using ratios of 0.91 and 0.96 for venous to body hematocrit and plasma trapping, respectively. The volume of red cell mass was determined by subtracting plasma volume from blood volume. Values were indexed to body weight and presented as milliliters per kilogram of body mass.
Exercise Testing
Maximal exercise testing was performed on a cycle ergometer using a ramp protocol (Monark 818E). Measures of ventilatory gas exchange were made using the Douglas bag technique. Heart rate was monitored continuously by a three-lead ECG (telemetry unit; Transkinetics) throughout the test. At the end of each stage, BP (STBP-780; Colin Medical Instruments, South Plainfield, NJ), arterial blood gases (ILS-1306 Blood Gas Analyzer and ILS-482 Co-Oximeter), and cardiac output (modification of foreign gas rebreathing method38) were measured. Peak values for oxygen uptake and power were the highest values obtained during the test. Data collected from the exercise test included power output, ventilation, oxygen uptake, carbon dioxide production, cardiac output, heart rate, systolic and diastolic BPs, MAP, arterial oxygen content, arterial pH, and arterial lactate concentration. Data calculated from the collected data included respiratory quotient, stroke volume, MAP, systemic vascular resistance, arteriovenous oxygen difference, mixed venous oxygen content, and tissue diffusing capacity (peak data only). Briefly, the calculation of tissue diffusing capacity allows the assessment of oxygen transport: VO2 maximum = diffusing capacity × (mean muscle capillary pO2 − mitochondrial pO2).28,40 To calculate tissue diffusing capacity, the following variables were imputed: muscle blood flow (assumed to equal cardiac output), arterial blood gases (pO2, pCO2, pH, and O2 saturation), estimated tissue venous blood gases (on the basis of the Fick principle), hemoglobin, hemoglobin p50, and blood temperature. Muscle capillary pO2 is then calculated using a custom–designed computer program.40
Off–Dialysis Day Assessments
Quadriceps Biopsy
The day after a dialysis session, the patient would report to the laboratory for a muscle biopsy. Muscle biopsies were performed on the right vastus lateralis. All specimens from a given subject were analyzed on the same run of a given assay.
Image Analyses
The percentages of each fiber type and fiber areas were assessed in duplicate from three to four full fields at ×20 by two experienced technicians blinded to group assignment. Capillary density and the capillary-to-fiber ratio were read at ×40. Inter- and intraobserver variabilities were <10%. Fiber type is expressed as a percentage of total fibers counted. Fiber area is expressed in square micrometers. Capillary density is expressed as capillaries per square micrometer and a ratio of capillaries to fiber.
Electron Microscopy
Capillary endothelium and capillary basement membrane thickness were measured in six radial directions, taking care to avoid areas including endothelial nuclear material. Measurements were averaged and expressed in nanometers. Seven subjects (five males and two females) with normal renal function and similar age to our patients had biopsies analyzed in the same manner for comparison.
Enzyme Activities
Activities for citrate synthase, HAD, and phosphofructokinase were measured with a flurometer on muscle homogenates at pH 7.0 and room temperature. Values are expressed as micromoles of NADH per minute per gram dry weight of tissue.
Tissue Buffering Capacity
Tissue buffering capacity was measured on muscle homogenates by the method described by Nakao et al.41 Values are expressed as micromoles of H+ per pH unit per gram dry weight of tissue.
Muscle Myoglobin Concentration
Myoglobin concentrations were measured on muscle homogenates by RIA using a standardized kit. Crossreactivity/cross-sensitivity values are expressed as nanograms of myoglobin per gram wet weight of tissue.
Reference Control Group
For comparison, healthy sedentary age–matched control data are presented from previous published studies in our laboratory,42 and the effect of the normal training response is from the literature.21 Exercise testing was performed to determine the response to maximal exercise. It should be noted that the healthy sedentary cohort was predominantly tested using a treadmill in contrast to the cycle ergometer used in this study.
Statistical Analyses
There was no effect of order/carryover effect; therefore, we combined groups A and B for analysis under each condition of interest (Figure 1). An unpaired t test and a chi-squared test were used to compare baseline characteristics in randomized patients and those who completed the study. A two–way repeated measures ANOVA was used to determine the effect of normalizing hematocrit and exercise training. An unpaired t test was used for the comparison of capillary wall thickness between renal subjects and healthy subjects. Data presented as means and SDs. Commercially available statistical software was used for analysis (SAS, version 6.02; SAS Institute Inc., Cary, NC and GraphPad Prism, version 6; GraphPad Software, La Jolla, CA); α was considered significant if P≤0.05.
Disclosures
The salary of J.S.-G. was partially supported by Amgen, Inc. (Thousand Oaks, CA) during design and data acquisition phases of this study. Financial support was received from Amgen, Inc.
Supplementary Material
Acknowledgments
We thank all 50 patients who initially agreed to participate in the study, despite the burden of living with chronic renal failure, and a special thank you to the 27 patients who persevered and completed the entire 14–15 months of their personal participation in the study. The original data collection for this study was extremely complicated and involved many institutions and individuals. It would not have been possible to perform this study without the dedication, cooperation, and excellent efforts of those many individuals and institutions. We would like to acknowledge and thank the personnel involved in the design, exercise training, data acquisition, and analysis. These include personnel from Amgen, Inc. (Thousand Oaks, CA), the University of Texas Southwestern Medical Center, the Baylor/University of Texas Southwestern Human Performance Center, the Institute for Environmental and Exercise Medicine, and the six participating dialysis units. We thank and acknowledge the tireless efforts of their staff in the care of their patients and their cheerful and cooperative tolerance of our incursions and interruptions to their already busy and stressful routines. From Amgen, Inc., we would like to acknowledge and thank Cynthia Fuller, Donna Holloway, and David Goodkin for their coordinating, supervising, and advising efforts, without which the study would not have been possible. In particular, we thank Chao Wang for performing the statistical analysis on the dataset. From the University of Texas Southwestern, we acknowledge and thank the Parkland Dialysis Unit and staff and the Electron Microscopy Laboratory for their assistance, access, and use of equipment. From the Baylor/University of Texas Southwestern Human Performance Center, we acknowledge and thank Wyman Shultz for his calibration and maintenance of the exercise laboratory; J. T. Gean for his creation and maintenance of the database and the management of the data; Cyrus Oufi for assisting J.T. Gean and Wyman Shultz; William Sams, Kevin Robinzine, Chritsy Zolfogharty, and Tia Peterson for their tireless efforts coordinating, testing, and training the subjects and then, subsequently performing assays on their muscle specimens; and Lisa Baker for biochemistry. From the Institute for Exercise and Environmental Medicine, we thank Benjamin D. Levine for his support and making his facility available for much of the post study specimen analysis. We also thank Peter Wagner from the University of California, San Diego for providing us with the program to calculate tissue diffusing capacity and expert advice in performing this complex analysis.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2015091034/-/DCSupplemental.
References
- 1.Kouidi E, Albani M, Natsis K, Megalopoulos A, Gigis P, Guiba-Tziampiri O, Tourkantonis A, Deligiannis A: The effects of exercise training on muscle atrophy in haemodialysis patients. Nephrol Dial Transplant 13: 685–699, 1998 [DOI] [PubMed] [Google Scholar]
- 2.Moore GE, Brinker KR, Stray-Gundersen J, Mitchell JH: Determinants of VO2peak in patients with end-stage renal disease: On and off dialysis. Med Sci Sports Exerc 25: 18–23, 1993 [DOI] [PubMed] [Google Scholar]
- 3.Painter P, Krasnoff JB, Kuskowski M, Frassetto L, Johansen KL: Effects of modality change and transplant on peak oxygen uptake in patients with kidney failure. Am J Kidney Dis 57: 113–122, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sietsema KE, Amato A, Adler SG, Brass EP: Exercise capacity as a predictor of survival among ambulatory patients with end-stage renal disease. Kidney Int 65: 719–724, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Evans RW, Manninen DL, Garrison LP Jr., Hart LG, Blagg CR, Gutman RA, Hull AR, Lowrie EG: The quality of life of patients with end-stage renal disease. N Engl J Med 312: 553–559, 1985 [DOI] [PubMed] [Google Scholar]
- 6.Heiwe S, Jacobson SH: Exercise training for adults with chronic kidney disease. Cochrane Database Syst Rev 10: CD003236, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ossareh S, Roozbeh J, Krishnan M, Liakopoulos V, Bargman JM, Oreopoulos DG: Fatigue in chronic peritoneal dialysis patients. Int Urol Nephrol 35: 535–541, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Weibel ER, Taylor CR, Hoppeler H: The concept of symmorphosis: A testable hypothesis of structure-function relationship. Proc Natl Acad Sci U S A 88: 10357–10361, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eschbach JW, Adamson JW: Anemia of end-stage renal disease (ESRD). Kidney Int 28: 1–5, 1985 [DOI] [PubMed] [Google Scholar]
- 10.Howden EJ, Fassett RG, Isbel NM, Coombes JS: Exercise training in chronic kidney disease patients. Sports Med 42: 473–488, 2012 [DOI] [PubMed] [Google Scholar]
- 11.Canadian Erythropoietin Study Group : Association between recombinant human erythropoietin and quality of life and exercise capacity of patients receiving haemodialysis. BMJ 300: 573–578, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beusterien KM, Nissenson AR, Port FK, Kelly M, Steinwald B, Ware JE Jr.: The effects of recombinant human erythropoietin on functional health and well-being in chronic dialysis patients. J Am Soc Nephrol 7: 763–773, 1996 [DOI] [PubMed] [Google Scholar]
- 13.Macdougall IC, Lewis NP, Saunders MJ, Cochlin DL, Davies ME, Hutton RD, Fox KA, Coles GA, Williams JD: Long-term cardiorespiratory effects of amelioration of renal anaemia by erythropoietin. Lancet 335: 489–493, 1990 [DOI] [PubMed] [Google Scholar]
- 14.Johansen KL, Finkelstein FO, Revicki DA, Gitlin M, Evans C, Mayne TJ: Systematic review and meta-analysis of exercise tolerance and physical functioning in dialysis patients treated with erythropoiesis-stimulating agents. Am J Kidney Dis 55: 535–548, 2010 [DOI] [PubMed] [Google Scholar]
- 15.Painter P, Moore G, Carlson L, Paul S, Myll J, Phillips W, Haskell W: Effects of exercise training plus normalization of hematocrit on exercise capacity and health-related quality of life. Am J Kidney Dis 39: 257–265, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Painter PL, Nelson-Worel JN, Hill MM, Thornbery DR, Shelp WR, Harrington AR, Weinstein AB: Effects of exercise training during hemodialysis. Nephron 43: 87–92, 1986 [DOI] [PubMed] [Google Scholar]
- 17.Goldberg AP, Geltman EM, Hagberg JM, Gavin JR 3rd, Delmez JA, Carney RM, Naumowicz A, Oldfield MH, Harter HR: Therapeutic benefits of exercise training for hemodialysis patients. Kidney Int Suppl 16: S303–S309, 1983 [PubMed] [Google Scholar]
- 18.Howden EJ, Leano R, Petchey W, Coombes JS, Isbel NM, Marwick TH: Effects of exercise and lifestyle intervention on cardiovascular function in CKD. Clin J Am Soc Nephrol 8: 1494–1501, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moore GE, Parsons DB, Stray-Gundersen J, Painter PL, Brinker KR, Mitchell JH: Uremic myopathy limits aerobic capacity in hemodialysis patients. Am J Kidney Dis 22: 277–287, 1993 [DOI] [PubMed] [Google Scholar]
- 20.Dobre M, Meyer TW, Hostetter TH: Searching for uremic toxins. Clin J Am Soc Nephrol 8: 322–327, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hartley LH, Grimby G, Kilbom A, Nilsson NJ, Astrand I, Bjure J, Ekblom B, Saltin B: Physical training in sedentary middle-aged and older men. 3. Cardiac output and gas exchange asubmaximal and maximal exercise. Scand J Clin Lab Invest 24: 335–344, 1969 [DOI] [PubMed] [Google Scholar]
- 22.Blair SN, Kohl HW 3rd, Barlow CE, Paffenbarger RS Jr., Gibbons LW, Macera CA: Changes in physical fitness and all-cause mortality. A prospective study of healthy and unhealthy men. JAMA 273: 1093–1098, 1995 [PubMed] [Google Scholar]
- 23.Blair SN, Kohl HW 3rd, Paffenbarger RS Jr., Clark DG, Cooper KH, Gibbons LW: Physical fitness and all-cause mortality. A prospective study of healthy men and women. JAMA 262: 2395–2401, 1989 [DOI] [PubMed] [Google Scholar]
- 24.Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE: Exercise capacity and mortality among men referred for exercise testing. N Engl J Med 346: 793–801, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Gulati M, Pandey DK, Arnsdorf MF, Lauderdale DS, Thisted RA, Wicklund RH, Al-Hani AJ, Black HR: Exercise capacity and the risk of death in women: The St James Women Take Heart Project. Circulation 108: 1554–1559, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Heiwe S, Jacobson SH: Exercise training in adults with CKD: A systematic review and meta-analysis. Am J Kidney Dis 64: 383–393, 2014 [DOI] [PubMed] [Google Scholar]
- 27.Birkeland KI, Stray-Gundersen J, Hemmersbach P, Hallen J, Haug E, Bahr R: Effect of rhEPO administration on serum levels of sTfR and cycling performance. Med Sci Sports Exerc 32: 1238–1243, 2000 [DOI] [PubMed] [Google Scholar]
- 28.Wagner PD: Central and peripheral aspects of oxygen transport and adaptations with exercise. Sports Med 11: 133–142, 1991 [DOI] [PubMed] [Google Scholar]
- 29.Lewis MI, Fournier M, Wang H, Storer TW, Casaburi R, Cohen AH, Kopple JD: Metabolic and morphometric profile of muscle fibers in chronic hemodialysis patients. J Appl Physiol (1985) 112: 72–78, 2012 [DOI] [PMC free article] [PubMed]
- 30.Sakkas GK, Sargeant AJ, Mercer TH, Ball D, Koufaki P, Karatzaferi C, Naish PF: Changes in muscle morphology in dialysis patients after 6 months of aerobic exercise training. Nephrol Dial Transplant 18: 1854–1861, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Lewis MI, Fournier M, Wang H, Storer TW, Casaburi R, Kopple JD: Effect of endurance and/or strength training on muscle fiber size, oxidative capacity and capillarity in hemodialysis patients. J Appl Physiol (1985) 119: 865–871, 2015 [DOI] [PMC free article] [PubMed]
- 32.Sala E, Noyszewski EA, Campistol JM, Marrades RM, Dreha S, Torregrossa JV, Beers JS, Wagner PD, Roca J: Impaired muscle oxygen transfer in patients with chronic renal failure. Am J Physiol Regul Integr Comp Physiol 280: R1240–R1248, 2001 [DOI] [PubMed] [Google Scholar]
- 33.Dundon BK, Torpey K, Nelson AJ, Wong DT, Duncan RF, Meredith IT, Faull RJ, Worthley SG, Worthley MI: The deleterious effects of arteriovenous fistula-creation on the cardiovascular system: A longitudinal magnetic resonance imaging study. Int J Nephrol Renovasc Dis 7: 337–345, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ori Y, Korzets A, Katz M, Erman A, Weinstein T, Malachi T, Gafter U: The contribution of an arteriovenous access for hemodialysis to left ventricular hypertrophy. Am J Kidney Dis 40: 745–752, 2002 [DOI] [PubMed] [Google Scholar]
- 35.Banister EW, Morton RH, Fitz-Clarke J: Dose/response effects of exercise modeled from training: Physical and biochemical measures. Ann Physiol Anthropol 11: 345–356, 1992 [DOI] [PubMed] [Google Scholar]
- 36.Howden EJ, Perhonen M, Peshock RM, Zhang R, Arbab-Zadeh A, Adams-Huet B, Levine BD: Females have a blunted cardiovascular response to one year of intensive supervised endurance training. J Appl Physiol (1985) 119: 37–46, 2015 [DOI] [PMC free article] [PubMed]
- 37.Foldager N, Blomqvist CG: Repeated plasma volume determination with the Evans Blue dye dilution technique: The method and a computer program. Comput Biol Med 21: 35–41, 1991 [DOI] [PubMed] [Google Scholar]
- 38.Triebwasser JH, Johnson RL, Burpo RP, Campbell JC, Reardon WC, Blomqvist CG: Noninvasive determination of cardiac output by a modified acetylene rebreathing procedure utilizing mass spectrometer measurements. Aviat Space Environ Med 48: 203–209, 1977 [PubMed] [Google Scholar]
- 39.Okazaki K, Iwasaki K, Prasad A, Palmer MD, Martini ER, Fu Q, Arbab-Zadeh A, Zhang R, Levine BD: Dose-response relationship of endurance training for autonomic circulatory control in healthy seniors. J Appl Physiol (1985) 99: 1041–1049, 2005 [DOI] [PubMed] [Google Scholar]
- 40.Wagner PD: Determinants of maximal oxygen transport and utilization. Annu Rev Physiol 58: 21–50, 1996 [DOI] [PubMed] [Google Scholar]
- 41.Nakao T, Fujiwara S, Isoda K, Miyahara T: Impaired lactate production by skeletal muscle with anaerobic exercise in patients with chronic renal failure. A possible consequence of defective glycolysis in skeletal muscle. Nephron 31: 111–115, 1982 [DOI] [PubMed] [Google Scholar]
- 42.Carrick-Ranson G, Hastings JL, Bhella PS, Shibata S, Fujimoto N, Palmer D, Boyd K, Levine BD: The effect of age-related differences in body size and composition on cardiovascular determinants of VO2max. J Gerontol A Biol Sci Med Sci 68: 608–616, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.