Abstract
The TH17 immune response has a central role in the pathogenesis of autoimmune diseases, implicating the TH17 master cytokine, IL-17A, as the critical mediator of diseases such as human and experimental crescentic GN. However, the relative importance of additional TH17 effector cytokines, including IL-17F, in immune-mediated tissue injury remains to be fully elucidated. Here, using a mouse model of acute crescentic GN (nephrotoxic nephritis), we identified CD4+ T cells and γδ T cells as the major cellular source of IL-17F in the inflamed kidney. Interventional studies using IL-17F gene–deficient mice, IL-17F–neutralizing antibodies, and adoptive transfer experiments into Rag1−/− mice demonstrated that CD4+ T cell–derived IL-17F drives renal tissue injury in acute crescentic GN. Notably, IL-17F–deficient nephritic mice had fewer renal infiltrating neutrophils than wild-type nephritic mice, and neutrophil depletion did not affect the course of GN in IL-17F–deficient mice. Moreover, in the chronic model of pristane-induced SLE, IL-17F–deficient mice developed less severe disease than wild-type mice, with respect to survival and renal injury. Finally, we show that IL-17F induced expression of the neutrophil-attracting chemokines CXCL1 and CXCL5 in kidney cells. The finding that IL-17F has a nonredundant function in the development of renal tissue injury in experimental GN might be of great importance for the development of anti–IL-17 cytokine therapies in TH17-mediated human autoimmune diseases.
Keywords: lymphocytes, cytokines, glomerulonephritis, immunology, systemic lupus erythematosus
Numerous studies have substantiated the essential role of TH17 immune responses in murine and human autoimmune diseases, and findings from animal models have led to successful targeting of the TH17/IL-17 axis in clinical practice, as, for example, in psoriasis.1,2 However, the relative importance of the TH17 master cytokine IL-17A in comparison to other TH17-associated cytokines, such as IL-17F and GM-CSF, as well as the mechanisms that they employ to induce inflammatory tissue lesions are topics of continued investigation.
The pathogenic role of the TH17/IL-17 axis in experimental crescentic GN has been clearly established, but in many studies the focus was almost exclusively on IL-17A because it is the cytokine primarily implicated in tissue injury and, ultimately, in the loss of renal function.3–6 However, many lymphocyte subsets that produce IL-17A also have the ability to produce IL-17F, another member of the IL-17 cytokine family sharing the closest homology with IL-17A.7,8 In addition, both cytokines can be secreted as homodimers or as IL-17A/F heterodimers by identical cells, which are mainly composed of adaptive and innate immune cells; that is, CD4 T cells, γδ T cells, invariant natural killer T cells, and group 3 innate lymphoid cells.9–11 In addition, both cytokines signal through the same heterodimeric receptor complex composed of IL-17RA and IL-17RC.12
So far, published data supports the concept that IL-17F has a distinct role in most experimental models of host defense against pathogens by the induction of chemokines, cytokines, and antimicrobial peptides,13,14 whereas its role in murine models of autoimmune diseases, such as in experimental autoimmune encephalitis and collagen-induced arthritis, remains unclear.15,16
Because of the uncertain role of IL-17F in autoimmune disease and the lack of analyses in immune-mediated kidney diseases, we sought to investigate the impact of IL-17F in GN. We therefore: (1) assessed the cellular expression pattern of IL-17F in the course of acute crescentic GN; (2) studied the functional role of IL-17F in two models of GN (nephrotoxic nephritis and pristane-induced lupus nephritis); and (3) elucidated its effect on resident kidney cells in vitro.
Results
CD4+ T Cells and γδ T Cells Are the Major Cellular Source of IL-17F in Experimental GN
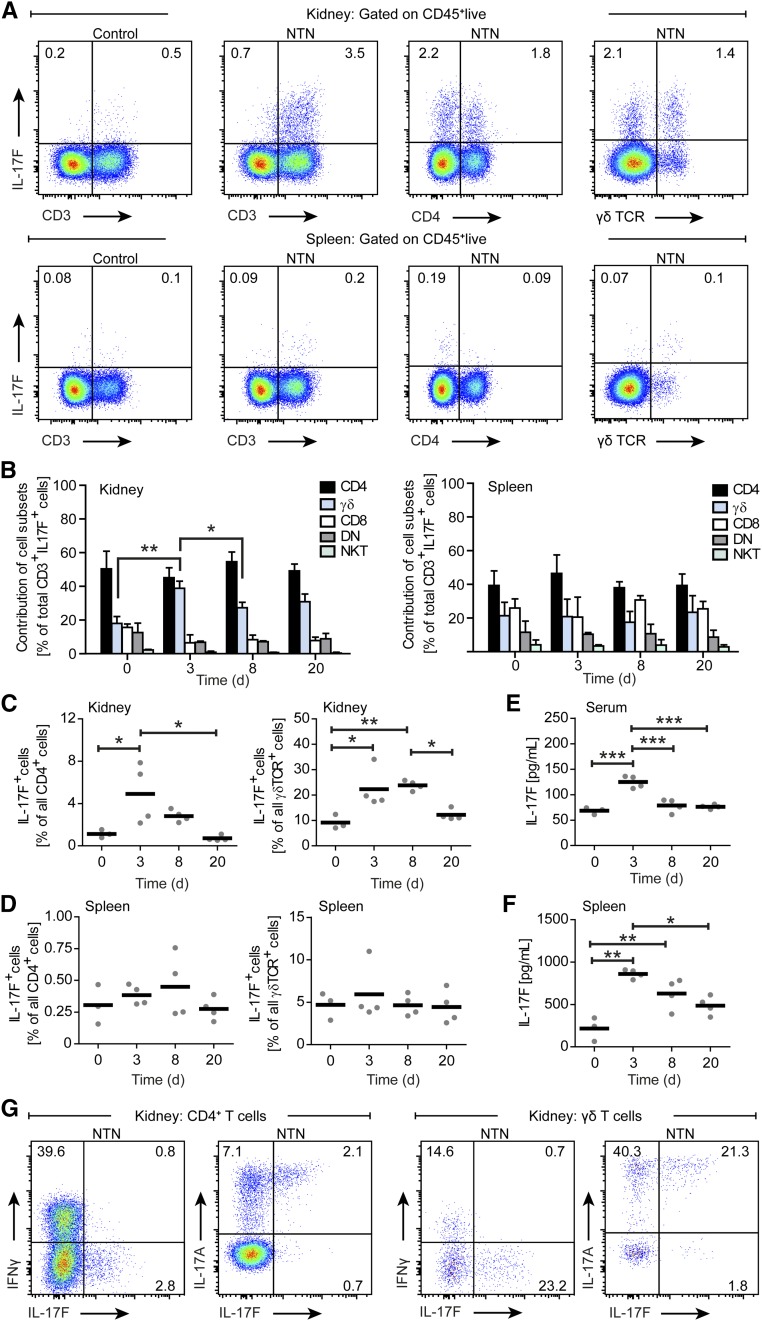
To investigate the expression pattern of IL-17F in experimental GN, we performed time kinetic analyses in nephritic mice (day 0 to day 20). Flow cytometric analyses after restimulation with PMA/ionomycin revealed robust IL-17F production by leukocytes isolated from inflamed kidneys and spleens IL-17F was almost exclusively produced by CD3+ T cells (Figure 1A). Total CD3+ T cell frequencies in the kidney increased vigorously during the course of nephrotoxic nephritis (NTN), while splenic CD3+ T cell frequencies rose shortly after NTN induction and gradually declined over the course of the disease (Supplemental Figure 1). Further analysis showed that CD4+ T cells and γδ T cells are the major cellular source of IL-17F, but its production was also observed to a lesser degree in CD3+CD8+ and CD3+CD4–CD8– (double negative) T cells, as well as in CD3+NK1.1+ natural killer T cell subsets throughout the course of disease (Figure 1B). Percentages of IL-17F–producing CD4+ T cells in the kidney among CD3+ T cells during the course of NTN remained on the same level. Three days after NTN induction, there was a strong increase in the relative contribution of γδ T cells to IL-17F production, which remained elevated throughout the period of observation. The relative contribution of CD3+ cell subsets to the splenic production of IL-17F in NTN remained stable (Figure 1B).
Figure 1.
IL-17F expression in experimental GN. (A) Representative FACS plots showing IL-17F expression after PMA/ionomycin stimulation by selected cell subsets pregated for live CD45+ cells in kidney (above) and spleen (below) on days 0 (control) and 3 of NTN. (B) Quantification of leukocyte subset contribution to renal (left) and splenic (right) IL-17F production in the course of NTN at indicated time points. (C, D) Quantification of IL-17F production from (C) renal CD4+ T cells and γδ T cells, as well as from (D) splenic CD4+ T cells and γδ T cells in the course of NTN at indicated time points. (E, F) Quantification of (E) IL-17F serum levels and (F) IL-17F in supernatants of spleen cells restimulated with sIgG at indicated time points in the course of NTN. (G) Representative FACS plots showing IFNγ, IL-17F, and IL-17A expression by CD4+ T cells and γδ T cells isolated from kidneys of nephritic wild-type mice pregated for live CD45+ cells after PMA/ionomycin stimulation on day 8 of NTN. n=3–4 mice per time point, symbols represent individual data points with the mean as horizontal line, or bar graphs with the mean±SD, *P<0.05; **P<0.01; ***P<0.001.
To further characterize renal and systemic IL-17F expression, we quantified the percentages of IL-17F+ CD4+ T cells and IL-17F+ γδ T cells and measured IL-17F levels in sera and supernatants of spleen cells restimulated with sheep IgG (sIgG). In the course of NTN, IL-17F production by renal CD4+ T cells peaked around day 3 to day 8 after NTN induction. Thereafter, IL-17F production gradually declined until day 20 (Figure 1C). Renal γδ T cells also showed markedly elevated IL-17F production 3 days after NTN induction, which persisted until day 8 and declined until day 20 (Figure 1C). Splenic CD4+ T cells and splenic γδ T cells showed no substantial differences in the IL-17F–producing capacity over time (Figure 1D). Changes in serum IL-17F concentrations of nephritic mice were only detectable at day 3 after NTN induction and returned to baseline at later time points (Figure 1E). IL-17F concentrations in supernatants of spleen cells increased 3 days after NTN induction and then declined (Figure 1F). In addition, intracellular cytokine staining of PMA/ionomycin restimulated CD4+ T cells and γδ T cells from nephritic wild-type mice at day 8 of NTN showed that the main fractions of IL-17F–producing cells were also IL-17A positive, identifying IL-17F–producing CD4+ T cells as classic TH17 cells, whereas almost no IL-17F–producing IFNγ+ TH1 cells were detectable (Figure 1G).
IL-17F Drives Renal Injury in Crescentic GN
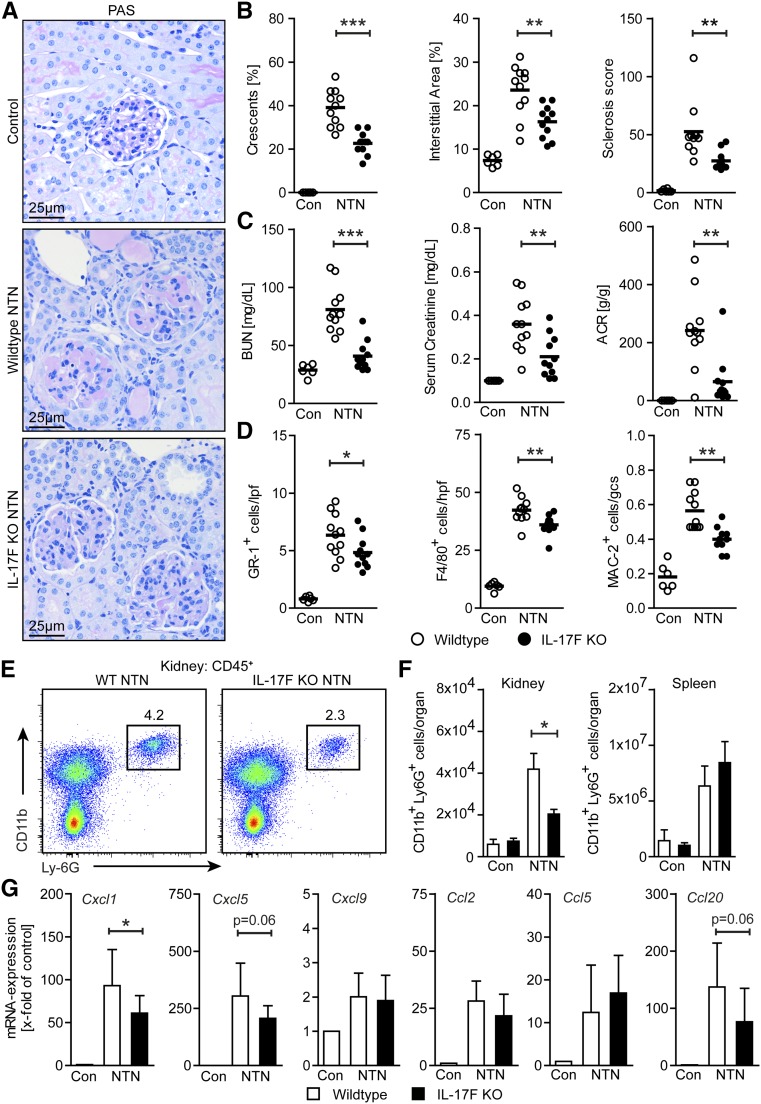
To determine whether IL-17F is of functional importance, we compared the clinical course of NTN in wild-type and IL-17F–deficient mice. To quantify renal tissue damage, periodic acid–Schiff (PAS)-stained kidney sections were evaluated for the presence of crescents, tubulointerstitial injury, and glomerular sclerosis (deposition of PAS-positive material) at day 8 of NTN (Figure 2, A and B). Quantification of all the histologic kidney structure parameters specified above showed a highly significant reduction of renal tissue damage in mice with IL-17F deficiency. Along with these structural alterations, quantification of impairment of renal function revealed a strong reduction in kidney function, as measured by BUN and serum creatinine levels, as well as a reduced albumin-to-creatinine ratio (ACR) in nephritic IL-17F–deficient mice at day 8 of NTN as compared with the nephritic wild-type group (Figure 2C). Of note, untouched wild-type and IL-17F–deficient mice did not show differences in kidney architecture and parameters of kidney function (Supplemental Figure 2, A and B).
Figure 2.
IL-17F drives renal tissue injury by mediating neutrophil infiltration. (A) Representative photographs of PAS-stained kidney sections from control, nephritic wild-type, and nephritic IL-17F–deficient mice at day 8 of NTN (original magnification ×400). (B) Quantification of glomerular crescent formation, interstitial area, and glomerular sclerosis of controls (n=6), nephritic wild-type (n=11), and nephritic IL-17F–deficient mice (n=11) 8 days after induction of nephritis. (C) BUN levels, serum creatinine, and ACR of the aforementioned groups 8 days after induction of nephritis. (D) Quantification of tubulointerstitial GR-1+ cells, F4/80+ cells, and glomerular MAC-2+ cells in the aforementioned groups 8 days after induction of nephritis. (E) Representative FACS plots showing renal neutrophils (defined as CD45+CD11b+Ly6G+ cells) in nephritic wild-type and nephritic IL-17F–deficient mice and (F) quantification of FACS analysis of renal and splenic neutrophils of wild-type controls (n=3–4), IL-17F–deficient controls (n=3–4), nephritic wild-type (n=5), and nephritic IL-17F–deficient mice (n=4) at day 8 of NTN. (G) Real-time RT-PCR analyses of renal mRNA expression of different chemokines of controls (n=6), nephritic wild-type (n=11), and nephritic IL-17F–deficient mice (n=11) 8 days after induction of nephritis. mRNA levels are expressed as x-fold of controls. Symbols represent individual data points with the mean as horizontal line, or bar graphs with the mean±SD. *P<0.05; **P<0.01; ***P<0.001.
IL-17F Promotes the Infiltration of Pathogenic Neutrophils
Next, we determined the recruitment of leukocytes to the kidneys of nephritic IL-17F–deficient and wild-type mice. Immunohistochemical staining revealed that tubulointerstitial Gr-1+ neutrophil infiltration and tubulointerstitial F4/80+, as well as glomerular Mac-2+ mononuclear phagocyte infiltration, were significantly reduced in nephritic IL-17F–deficient mice compared with their nephritic wild-type counterparts (Figure 2D). Furthermore, flow cytometric analysis of renal leukocytes confirmed a significant reduction in neutrophils of nephritic IL-17F knockout mice compared with nephritic wild-type mice, while systemic neutrophil counts in the spleen were similar in the nephritic groups (Figure 2, E and F). Untouched wild-type and IL-17F–deficient mice showed comparable neutrophil counts in the kidneys (Figure 2F, Supplemental Figure 2C). In line with that finding, mRNA analyses of whole renal cortices showed a decreased upregulation of the neutrophil-attracting chemokines CXCL1 and CXCL5 in nephritic IL-17F–deficient mice compared with their nephritic wild-type counterparts (Figure 2G). To analyze whether the less severe course of GN in IL-17F–deficient mice was a consequence of the decreased neutrophil recruitment observed, we depleted neutrophils in nephritic IL-17F–deficient mice from day 2 to day 7 after disease induction, using a Ly6G-specific monoclonal antibody (Supplemental Figure 3A). In contrast to the reported protective effect of neutrophil targeting in the NTN model,17 neutrophil depletion in nephritic IL-17F knockout mice did not affect renal tissue injury (Supplemental Figure 3, B and C). Moreover, there was no difference in BUN and serum creatinine levels, and ACR between nephritic IL-17F–deficient groups receiving or not receiving the neutrophil-depleting Ly6G-specific monoclonal antibody (Supplemental Figure 3D). Taken together, this interventional approach suggests that the ameliorated course of the disease in IL-17F–deficient mice is neutrophil dependent.
Tubulointerstitial and glomerular CD3+ T cell infiltration, tubulointerstitial FoxP3+ regulatory T cell (Treg) infiltration, as well as renal TH1 and TH17 responses, did not differ between nephritic wild-type and nephritic IL-17F–deficient mice (Supplemental Figure 4, A–C). Furthermore, nephritic wild-type and nephritic IL-17F–deficient mice had similar serum titers of anti-sheep total IgG, IgG1, IgG2a/c, IgG2b, and IgG3 antibodies (Supplemental Figure 5A). Semiquantitative scoring of glomerular sIgG, mouse IgG, and C3 deposition revealed no differences between nephritic wild-type and nephritic IL-17F–deficient mice (Supplemental Figure 5B). Moreover, cytometric bead array analyses of the cytokine production of sIgG-stimulated spleen cells showed no significant differences in TH1-, TH17-, TH2- or Treg-associated cytokine levels between the two nephritic groups (Supplemental Figure 5C). These results indicate that the absence of IL-17F did not impair the humoral or cellular systemic immune responses.
Anti–IL-17F Antibody Treatment Reduces Renal Tissue Damage in NTN
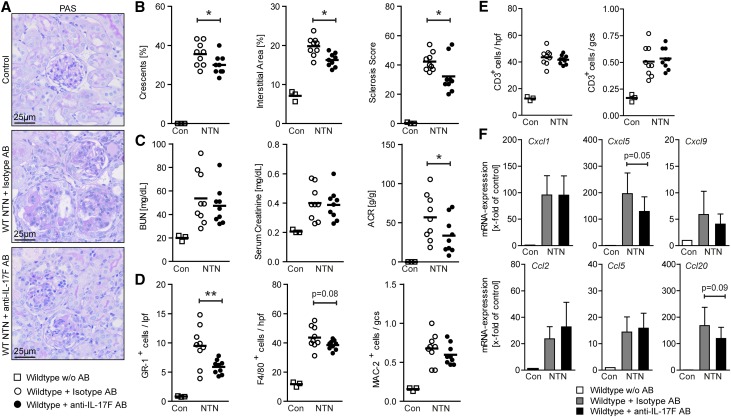
In order to investigate the effect of interventional IL-17F targeting in GN, nephritic wild-type mice were treated with either an anti–IL-17F–neutralizing antibody or an isotype mock antibody. PAS-stained kidney sections from control mice, nephritic isotype antibody-treated mice, and nephritic anti–IL-17F antibody-treated mice were assessed for glomerular and tubulointerstitial tissue injury (Figure 3A). Quantification revealed significantly reduced histologic damage scores in anti–IL-17F–treated mice compared with isotype antibody-treated mice (Figure 3B). Parameters of impairment of renal function did not differ significantly between the two treatment groups, whereas the ACR was significantly reduced in anti–IL-17F antibody-treated mice (Figure 3C).
Figure 3.
IL-17F neutralization attenuates crescentic GN. (A) Representative photographs of PAS-stained kidney sections from control mice, wild-type mice treated with isotype antibody (250 μg intraperitoneal injection on day 0 and day 4 of NTN), and wild-type mice treated with anti–IL-17F antibody (250 μg intraperitoneal injection on day 0 and day 4 of NTN), at day 8 of NTN (original magnification ×400). (B) Quantification of glomerular crescent formation, interstitial area, and glomerular sclerosis of controls (n=3), wild-type mice treated with isotype antibody (n=9), and wild-type mice treated with anti–IL-17F antibody (n=9) 8 days after induction of nephritis. (C) BUN levels, serum creatinine, and ACR of the aforementioned groups 8 days after induction of nephritis. (D) Quantification of tubulointerstitial GR-1+ cells, tubulointerstitial F4/80+ cells, and glomerular MAC-2+ cells of the aforementioned groups 8 days after induction of nephritis. (E) Quantification of tubulointerstitial CD3+ T cells and glomerular CD3+ T cells of the aforementioned groups 8 days after induction of nephritis. (F) Real-time RT-PCR analyses of renal mRNA expression of different chemokines in the aforementioned groups. mRNA levels are expressed as x-fold of controls. Symbols represent individual data points with the mean as horizontal line, or bar graphs with the mean±SD. *P<0.05; **P<0.01.
As seen in nephritic IL-17F–deficient mice, renal infiltration of tubulointerstitial Gr-1+ neutrophils was significantly reduced in mice treated with the anti–IL-17F antibody compared with mice treated with the isotype antibody. Tubulointerstitial F4/80+ and glomerular Mac-2+ mononuclear phagocytes were also reduced in anti–IL-17F–treated mice, although not to the same extent (Figure 3D), while the numbers of glomerular and tubulointerstitial CD3+ T cells were comparable between the two groups (Figure 3E). Furthermore, mRNA analyses of whole renal cortices showed a decreased upregulation of the neutrophil-attracting chemokine CXCL5 in nephritic mice treated with the anti–IL-17F antibody compared with isotype antibody–treated nephritic wild-type mice (Figure 3F).
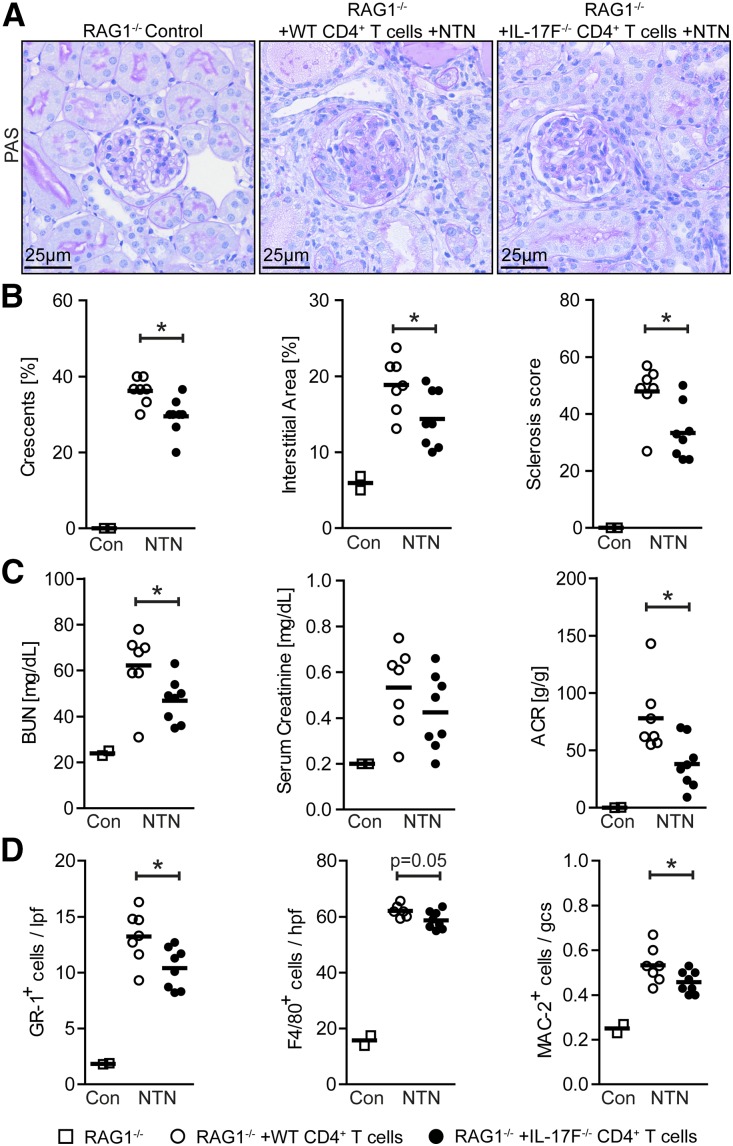
CD4+ T Cell-Derived IL-17F Significantly Contributes to Kidney Injury in Nephritic Rag1−/− Mice
To find out whether CD4+ T cell-derived IL-17F significantly influences the course of NTN, we isolated CD4+ T cells from spleens of wild-type and IL-17F–deficient mice and transferred them into Rag1–deficient mice, which lack T and B cells. Thirty-six hours after the adoptive transfer NTN was induced, PAS-stained kidney sections from Rag1−/− control mice, nephritic Rag1−/− mice reconstituted with wild-type CD4+ T cells, and nephritic Rag1−/− mice reconstituted with IL-17F−/− CD4+ T cells were assessed for glomerular and tubulointerstitial tissue injury (Figure 4A). Quantification revealed significantly reduced histologic damage scores in Rag1−/− mice reconstituted with IL-17F−/− CD4+ T cells compared with mice reconstituted with wild-type T cells (Figure 4B). Apart from serum creatinine values, parameters of impairment of renal function did also significantly differ between the two treatment groups (Figure 4C).
Figure 4.
IL-17F–producing CD4+ T cells aggravate renal tissue injury in Rag1−/− mice. (A) Representative photographs of PAS-stained kidney sections at day 8 of NTN from RAG1−/− control mice, nephritic RAG1−/− mice repopulated with 1.5×106 wild-type CD4+ T cells, and nephritic RAG1−/− mice repopulated with 1.5×106 IL-17F−/− CD4+ T cells (original magnification ×400). (B) Quantification of glomerular crescent formation, interstitial area, and glomerular sclerosis at day 8 of NTN of RAG1−/− controls (n=2), nephritic RAG1−/− mice repopulated with wild-type CD4+ T cells (n=7), and nephritic RAG1−/− mice repopulated with IL-17F−/− CD4+ T cells (n=8). (C) BUN levels, serum creatinine, and ACR of the aforementioned groups 8 days after induction of nephritis. (D) Quantification of tubulointerstitial GR-1+ cells, tubulointerstitial F4/80+ cells, and glomerular MAC-2+ cells of the aforementioned groups 8 days after induction of nephritis. Symbols represent individual data points with the mean as horizontal line. *P<0.05.
As seen in nephritic IL-17F–deficient and anti–IL-17F–treated mice, the infiltration of tubulointerstitial Gr-1+ neutrophils was significantly reduced in Rag1−/− mice reconstituted with IL-17F−/− CD4+ T cells compared with mice reconstituted with wild-type CD4+ T cells. Tubulointerstitial F4/80+ and glomerular Mac-2+ mononuclear phagocytes were also reduced in the IL-17F−/− CD4+ T cell transfer group, although not to the same extent (Figure 4D).
IL-17F Has a Nonredundant Function in the Development of Renal Tissue Injury in Experimental GN
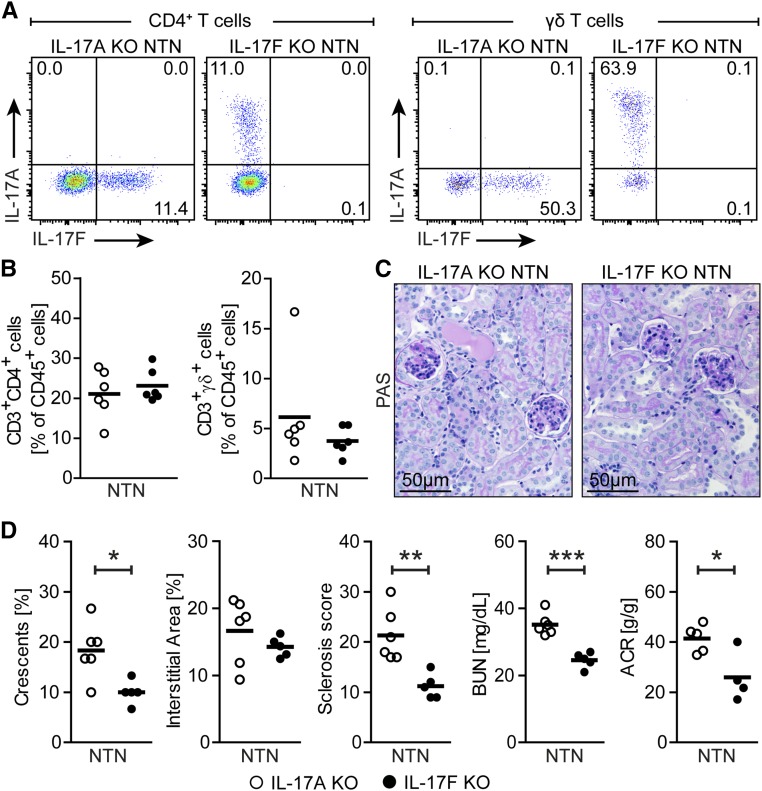
In order to evaluate whether IL-17F deficiency and IL-17A deficiency leads to a compensatory production of the respective other cytokine, we performed intracellular cytokine staining of PMA/ionomycin restimulated CD4+ T cells and γδ T cells isolated on day 8 of NTN from nephritic IL-17F–deficient and IL-17A–deficient mice, respectively. The analysis revealed that IL-17A production of CD4+ T cells and γδ T cells isolated from nephritic IL-17F–deficient mice remained stable in comparison to nephritic wild-type mice (Figures 1G and 5A). In contrast, IL-17F production of CD4+ T cells and γδ T cells isolated from nephritic IL-17A–deficient mice was strongly increased in comparison to nephritic wild-type mice (Figures 1G and 5A). Total CD4+ T cell and γδ T cell frequencies did not differ between the two knockout groups (Figure 5B).
Figure 5.
IL-17F serves a nonredundant function in crescentic GN. (A) Representative FACS plots showing IL-17A and IL-17F expression after PMA/ionomycin stimulation by CD4+ T cells and γδ T cells isolated from kidneys of nephritic IL-17A–deficient mice and nephritic IL-17F–deficient mice pregated for live CD45+ cells on day 8 of NTN. (B) Quantification of renal CD4+ T cells and γδ T cells of nephritic IL-17A–deficient mice (n=6) and nephritic IL-17F–deficient mice (n=6) at day 8 of NTN. (C) Representative photographs of PAS-stained kidney sections from nephritic IL-17A–deficient mice and nephritic IL-17F–deficient mice at day 8 of NTN (original magnification ×400). (D) Quantification of glomerular crescent formation, interstitial area, glomerular sclerosis, BUN levels, and ACR of nephritic IL-17A–deficient mice (n=6) and nephritic IL-17F–deficient mice (n=5) at day 8 of NTN. Symbols represent individual data points with the mean as horizontal line. *P<0.05; **P<0.01; ***P<0.001.
Of note, PAS-stained kidney sections showed less glomerular and tubular alterations in nephritic IL-17F–deficient mice compared with nephritic IL-17A–deficient mice (Figure 5C). Quantification of renal injury revealed significantly reduced histologic damage scores and less impairment of renal function in terms of BUN and albuminuria in IL-17F–deficient compared with IL-17A–deficient mice (Figure 5D). However, the compensatory upregulation of IL-17F in nephritic IL-17A–deficient mice did not allow a direct comparison of the relative biologic importance of IL-17F and IL-17A to be made. These experiments therefore underscore the nonredundant function of IL-17F in crescentic GN independent of IL-17A.
IL-17F Deficiency Attenuates Pristane-Induced SLE
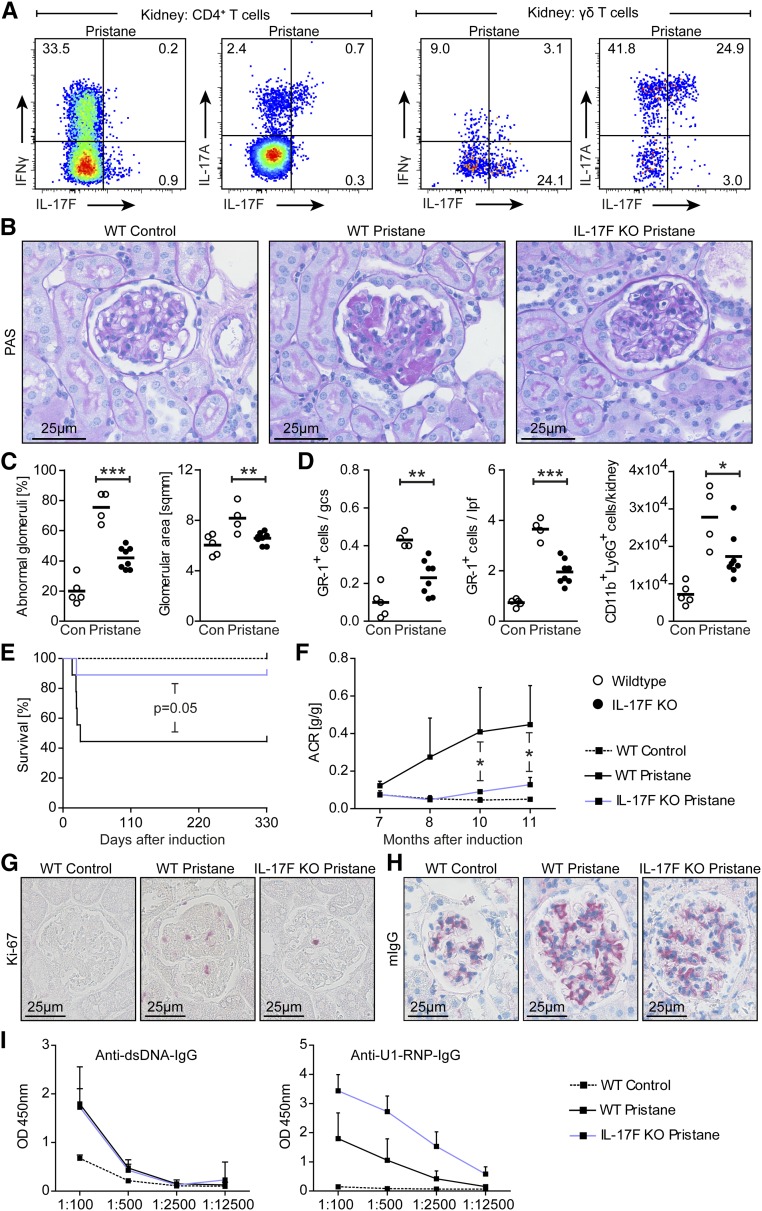
We next examined the functional role of IL-17F in a model of chronic SLE (pristane-induced SLE). Flow cytometric analyses showed increased IL-17F and IL-17A expression in renal CD4+ T cells and γδ T cells (Figure 6A), which is in line with the suggested functional role of the TH17 response in this model of renal autoimmunity.18 PAS staining of renal tissue sections 11 months after pristane injection showed that glomerular alterations, including hypercellularity, crescent formation, fibrinoid necrosis, segmental proliferation, hyalinosis, and capillary wall thickening, as well as an increase of glomerular area as a sign of immune-mediated kidney damage, were reduced in IL-17F–deficient mice (Figure 6, B and C). In accordance with our findings in the NTN model, neutrophil recruitment was decreased in lupus mice that lacked IL-17F (Figure 6D). Moreover, mortality (most likely as a consequence of reduced pulmonary vasculitis) and albuminuria were reduced in IL-17F–deficient lupus mice (Figure 6, E and F), as was glomerular cell proliferation identified by reduced Ki-67 positivity in IL-17F–deficient lupus mice (Figure 6G). The evaluation of humoral immunity determined by analyses of glomerular IgG deposition and autoantibody production did not show significant differences between wild-type and IL-17F–deficient mice (Figure 6, H and I). Taken together, these analyses highlight the unique role of IL-17F in autoimmunity, even in a chronic lupus model in which the pathology is gradually developing over months.
Figure 6.
IL-17F promotes autoimmune disease in pristane-induced lupus nephritis. (A) Representative FACS plots showing IFNγ, IL-17F, and IL-17A expression after PMA/ionomycin stimulation by CD4+ T cells and γδ T cells isolated from kidneys of wild-type and IL-17F–deficient mice pregated for live CD45+ cells 11 months after disease induction. (B) Representative photographs of PAS-stained kidney sections from control mice, wild-type lupus mice, and IL-17F–deficient lupus mice 11 months after disease induction (original magnification ×400). (C) Quantification of glomerular abnormalities and glomerular area of controls (n=5), wild-type lupus mice (n=4), and IL-17F–deficient lupus mice (n=8) 11 months after disease induction. (D) Quantification of immunohistochemically stained tubulointerstitial and glomerular GR-1+ cells, as well as quantification of FACS analysis of renal neutrophils (defined as CD45+CD11b+Ly6G+ cells) in the aforementioned groups 11 months after disease induction. (E) Kaplan–Meier plot of survival in control and lupus nephritis groups over the course of disease. (F) ACR of the groups mentioned above over the course of disease. (G, H) Representative photographs of (G) Ki-67- and (H) mouse IgG-stained kidney sections of the aforementioned groups 11 months after disease induction. (I) ELISA analyses of circulating mouse autoantibodies against dsDNA and U1-RNP from sera of control and lupus nephritis mice 11 months after disease induction. Symbols represent mean values±SD connected by lines, or individual data points with the mean as horizontal line. *P<0.05; **P<0.01; ***P<0.001.
IL-17F Induces the Expression of Neutrophil-Attracting Chemokines in Kidney Cells
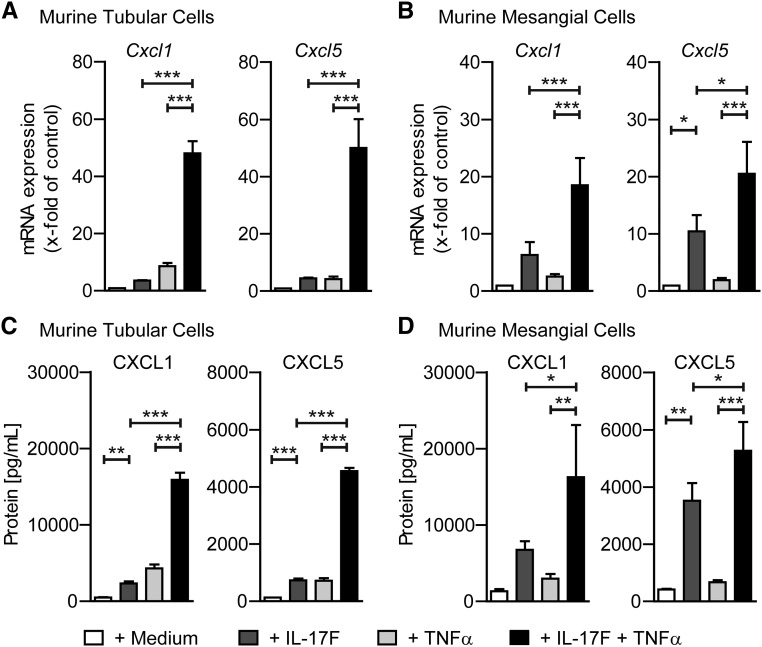
To test the effect of IL-17F on resident kidney cells, murine tubular and murine mesangial cells were cultured with IL-17F (100 ng/ml), TNFα (10 ng/ml), and both cytokines combined (100 ng/ml and 10 ng/ml, respectively). Analyses revealed mRNA upregulation of the neutrophil-attracting chemokines CXCL1 and CXCL5 in both tubular and mesangial cells, after stimulation with IL-17F, TNFα, and with both cytokines combined (Figure 7, A and B). Furthermore, CXCL1 and CXCL5 protein levels were markedly increased in the supernatants of tubular and mesangial cells after culture with either IL-17F or TNFα, and with both cytokines combined (Figure 7, C and D). Most importantly, IL-17F and TNFα showed synergistic effects on mRNA and protein levels of CXCL1 and CXCL5 that were much stronger than the effect of one cytokine alone (Figure 7, A–D). Of note, mRNA for other chemokines, such as CCL2, CCL5, and CXCL9, was not upregulated by IL-17F stimulation or the combination of IL-17F and TNFα (data not shown). This demonstrates a specific effect of IL-17F on neutrophil-attracting chemokines, which is in line with the findings observed in our in vivo experiments and eventually leads to the present hypothesis of the chain of events ultimately leading to kidney damage, as depicted in Figure 8.
Figure 7.
IL-17F induces CXCL1 and CXCL5 expression in resident kidney cells. (A, B) Real-time RT-PCR analyses of Cxcl1 and Cxcl5 mRNA expression from (A) murine tubular cells and (B) murine mesangial cells after 4 hours of in vitro culture with medium alone or with addition of IL-17F (100 ng/ml), TNFα (10 ng/ml), or the combination of IL-17F (100 ng/ml) and TNFα (10 ng/ml). (C, D) ELISA analyses of CXCL1 and CXCL5 protein levels from supernatants of cultured murine tubular and murine mesangial cells after 24 hours of incubation under the conditions mentioned above. mRNA levels are expressed as x-fold of controls (= medium alone condition). n=3–4 mice per condition, data are presented as bar graphs with the mean±SD. *P<0.05; **P<0.01; ***P<0.001.
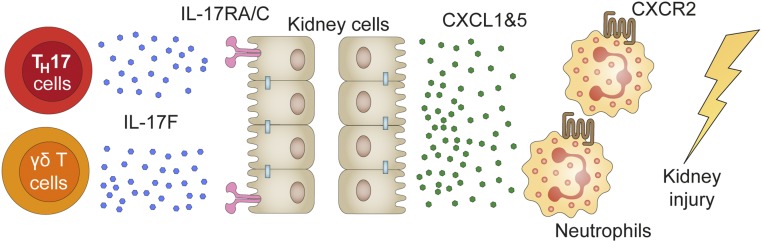
Figure 8.
IL-17F promotes tissue injury by the induction of neutrophil-attracting chemokines. Diagram depicting the present hypothesis of the chain of events leading to the recruitment of tissue-destructive neutrophils by IL-17F. CD4+ T cell- and γδ T cell-derived IL-17F induces the expression and production of chemokines CXCL1 and CXCL5, which then attract CXCR2-expressing neutrophils to the inflamed kidney, leading to the destruction of regular kidney tissue and consequent loss of renal function.
Discussion
In this study, we demonstrate the nonredundant role of IL-17F as one crucial component of disease-promoting cytokines produced by CD4+ TH17 and γδ T cells in two models of GN. This is of considerable importance because a large number of studies have established the pivotal role of the TH17 responses in the pathogenesis of autoimmune diseases. Besides, IL-23p19, IL-17RA and IL-17A have successfully been targeted for treatment of psoriasis,2,19,20 and to a lesser degree in rheumatoid arthritis and Crohn disease.21,22 However, the relative importance of TH17-associated effector cytokines, such as IL-17A, IL-17F, IL-21, IL-22, TNF-α, and GM-CSF in autoimmune diseases, as well as their suitability as a potential therapeutic target, remains to be fully elucidated.
The critical role of the TH17/IL-17 axis in GN was shown by others and us using IL-23p19, IL-17A, IL-17RA, or RORγt gene–deficient mice, each causing impaired TH17 immune responses. These knockout mice, as compared with their wild-type littermates, were less susceptible to experimental models of GN.17,18,23–26 Of note, the effect of IL-17A targeting in experimental GN seemed to be less effective than the neutralization of other TH17/IL-17 axis members (IL-23p19, IL-17RA, or RORγt), suggesting that IL-17A is not the unique effector cytokine of the TH17 response that drives kidney damage.18,23–26
Prompted by indirect evidence found for effector cytokines other than IL-17A, we detected substantial levels of IL-17F, the nearest homolog to IL-17A,7,8 in leukocytes isolated from kidneys and spleens, in the serum, and in supernatants of restimulated spleen cells of nephritic wild-type animals. Subsequent FACS analyses revealed CD4+ T cells and γδ T cells as main producers of IL-17F. Next, we investigated the functional role of this cytokine in an acute model of crescentic GN (NTN). Compared with wild-type mice, IL-17F–deficient mice developed less severe nephritis, with significantly lower albuminuria, better renal function, and a reduced frequency of glomerular crescent formation and tubulointerstitial injury. Interventional studies using IL-17F–neutralizing antibodies again revealed protection of treated mice from acute GN, though not to the extent that was seen in IL-17F–deficient mice, underlining the therapeutic potential of IL-17F blockade. The less beneficial phenotype of anti–IL-17F treatment in terms of renal function, tissue injury, and neutrophil chemoattractant expression might be due to incomplete cytokine neutralization, again indicating that thorough investigation is needed before translating findings from animal models into treatment options for human kidney diseases. Furthermore, transfer experiments in Rag1−/− mice demonstrated that CD4+ T cell-derived IL-17F significantly, if not exclusively, contributes to renal tissue injury in crescentic GN. The relative contribution of γδ T cell-derived IL-17F, as results from our time kinetic analyses are hinting, will need to be determined by further studies.
Of note, studies in the chronic model of pristane-induced SLE revealed a reduced mortality in IL-17F–deficient lupus mice and a significantly ameliorated course of lupus nephritis. Taken together, these findings highlight the pathogenic role of IL-17F in immune-mediated glomerular disease. Our study revealed one underlying mechanism: that IL-17F directly induces the renal expression of neutrophil-attracting chemokines CXCL1 and CXCL5, in vivo and in vitro, synergistically with TNFα. In line with this finding, IL-17F targeting reduced the renal infiltration of pathogenic neutrophils in GN and neutrophil depletion in nephritic IL-17F–deficient mice did not affect the course of the disease. Furthermore, reduced mortality from pristane-induced pulmonary capillaritis, in which neutrophils are the most prominent lung-infiltrating leukocyte population,27 underlines the concept that IL-17F pathologies are largely mediated by neutrophil attraction. Given the differences of renal chemokine expression in vivo compared with the results gathered in the in vitro experiments, one cannot rule out further processes that are not covered in this study, which effectively reduce neutrophil infiltration to the kidney in NTN and pristane-induced SLE.
The unexpected finding that IL-17F deficiency was even more protective in GN than the lack of IL-17A, might be due to the compensatory upregulation of IL-17F in IL-17A–deficient mice, while no upregulation of IL-17A is found in IL-17F knockout mice. Nevertheless, these results clearly underscore the nonredundant and unique role of IL-17F despite the fact that IL-17A and IL-17F mediate their biologic functions by activating the same heterodimeric IL-17RA/RC receptor complex.12 However, IL-17F binds IL-17RC with high affinity and IL-17RA with lower affinity, while IL-17A responds in the exact opposite fashion. Thus, cells with higher levels of IL-17RC expression may be more responsive to IL-17F than to IL-17A, whereas cells with higher IL-17RA expression might respond better to IL-17A stimulation.14 A detailed analysis of the molecular mechanisms, i.e., downstream targets and signal transduction pathways, employed by Il-17F, IL-17A, and their combination in kidney cells and leukocytes is still lacking and will certainly help to clarify their distinct roles in the future. Moreover, unlike IL-17RC expression, which may be limited to nonhematopoietic cells, IL-17RA seems to be more widely expressed.28 Therefore, the cell- and tissue-specific expression profiles of IL-17RA and IL-17RC, as well as distinct affinities of the respective receptor subunits, contribute to different biologic functions of IL-17F and IL-17A. This might explain the distinct phenotypes of mice deficient in either IL-17A or IL-17F in our GN models, as well as in experimental models of asthma and colitis.29
In conclusion, using gene-deficient mice, neutralizing antibodies and adoptive transfer experiments, we demonstrate for the first time that IL-17F promotes kidney injury in acute and chronic experimental GN. Our data, which challenge the paradigm of IL-17A as being the unique TH17 master cytokine, might be of direct importance for future anti–TH17/IL-17 treatment strategies in human autoimmune diseases.
Materials and Methods
Animals
IL-17F–deficient mice were obtained from B. Becher (University of Zurich, Switzerland), and IL-17A–deficient mice were provided by Y. Iwakura (University of Tokyo, Japan). RAG1−/− mice were purchased from The Jackson Laboratory (Bar Harbor, ME). All mice were on the C57BL/6J background and knockout mice underwent embryo transfer to meet the general standards of our institution. Age-matched C57BL/6J wild-type controls were also bred in our animal facility, and all animals were raised in specific pathogen-free conditions. All animal experiments were performed according to national and institutional animal care and ethical guidelines and were approved by the local committees.
Induction of Experimental GN and Functional Studies
Nephrotoxic nephritis was induced by intraperitoneal injection of nephrotoxic sheep serum (2.5 mg/gram body wt) into 8–12-week-old male mice, as previously described.30 Lupus nephritis was induced by intraperitoneal injection of 0.5 ml pristane (Sigma-Aldrich, St. Louis, MO) into 8–12-week-old male mice as previously described.18 For urine sample collection, mice were housed in metabolic cages for 5 hours. Urinary albumin excretion was determined by standard ELISA analysis (Mice-Albumin Kit; Bethyl Laboratories, Montgomery, TX), while urinary creatinine, BUN, and serum creatinine were measured using standard laboratory methods.
Real-time RT-PCR Analyses
Total RNA of the renal cortex was prepared according to standard laboratory methods. Real-time PCR was performed for 40 cycles on a StepOnePlus Real-Time PCR system (Applied Biosystems, Foster City, CA) as previously described.31 All samples were run in duplicate and normalized to 18S rRNA.
Morphologic Analyses
Glomerular injury and crescent formation, deposition of PAS-positive material, and tubulointerstitial injury were assessed as described.17,18 Paraffin-embedded sections (2 µm) were stained with an antibody directed against CD3 (A0452; Dako, Hamburg, Germany), GR-1 (Ly6 G/C, NIMP-R14; Hycult Biotech, Uden, The Netherlands), FoxP3 (FJK-16s; EMD eBiosciences, San Diego, CA), F4/80 (BM8; Dianova BMA, Augst, Switzerland), Mac-2 (M3/38; Cedarlane, Canada), Ki-67 (D3B5; Cell Signaling Technology, Danvers, MA), sIgG, or mouse IgG (both Jackson Immunoresearch Laboratories, West Grove, PA). Glomerular CD3+ and Mac-2+ cells in 30 glomerular cross-sections (magnification ×400), tubulointerstitial GR-1+ cells in 20 low-power fields (magnification ×200), and tubulointerstitial F4/80+ and CD3+ cells in 30 high-power fields (magnification ×400) per kidney were counted in a blinded manner. Glomerular mouse IgG deposition was scored from 0 to 3 in 30 glomeruli per mouse, as previously described.31 All slides were evaluated under an Axioskop light microscopy (Carl Zeiss GmbH, Jena, Germany), and photographed with an Axiocam HRc (Carl Zeiss GmbH), or by confocal microscopy with a LSM 510 meta microscope (Carl Zeiss GmbH), using the LSM software (Carl Zeiss GmbH).
Leukocyte Isolation, Stimulation, and Transfer
Previously described methods for leukocyte isolation from murine kidneys and spleens were used.32 Cell viability was assessed by trypan blue staining before stimulation, flow cytometry, and cell transfer experiments. For stimulation, after generation of a single cell suspension as mentioned above, isolated renal leukocytes and spleen cells were activated by incubation at 37°C and 5% carbon dioxide for 4.5 hours with PMA (5 ng/ml; Sigma-Aldrich) and ionomycin (1 µg/ml; EMD Millipore, Billerica, MA) in RPMI 1640 (Gibco, Grand Island, NY) with 10% FCS. After 30 minutes of incubation, Brefeldin A (10 µg/ml; Sigma-Aldrich) was added. For CD4+ T cell transfer experiments, CD4+ T cells were isolated from spleens of either wild-type or IL-17F–deficient mice using a magnetic cell separation CD4+ T cell isolation kit (Miltenyi Biotec, Bergisch Gladbach, Germany), according to manufacturer’s instruction. Viable cells were counted using trypan blue staining and 1.5×106 live cells were intravenously injected into RAG1−/− mice 36 hours before induction of NTN.
Flow Cytometry
Measurements were performed on a BD FACS LSR II, and data were analyzed with the FlowJo software (TreeStar, Inc., Ashland, OR). Cells were stained with fluorochrome-labeled antibodies against CD45, CD3, CD4, CD8, γdTCR, NK1.1, IL-17A, IL-17F, IFNγ, CD11b, and Ly6G (Biolegend, San Diego, CA; BD Biosciences, San Jose, CA; eBioscience, San Diego, CA; R&D Systems, Minneapolis, MN) as previously described.32 LIVE/DEAD staining (Near-infrared; Invitrogen, Carlsbad, CA) was used to exclude dead cells during flow cytometry and to ensure viability of the cells after the stimulation procedure.
Assessment of Humoral and Cellular Immune Responses
Mouse anti-sIgG antibody titers were measured by ELISA using sera collected 8 days after induction of nephritis, as previously described.33 To analyze the cellular immune response, spleen cells (4×106 cells/ml) were cultured in RPMI (Life Technologies, Carlsbad, CA) with 10% FCS (Gibco) and with sIgG (10 μg/ml) at 37°C for 72 hours. Cytokine concentrations in the supernatants were measured by cytometric bead array (BD Biosciences) or by ELISA, as previously described.33 Circulating anti–U1-RNP (Arotec, Wellington, New Zealand) and anti-dsDNA antibodies (precoated plates from Alpha Diagnostic International, San Antonio, TX) were analyzed by ELISA (anti-total-IgG HRP; Biozol, Eching, Germany).
Neutralization Experiments
For IL-17F neutralization experiments, either a rat antibody against mouse IL-17F (clone RN17; eBioscience) or a rat IgG1-κ isotype antibody (clone: eBRG1; eBioscience) were used, respectively. A total of 250 µg of respective antibodies were injected intraperitoneally on days 0 and 4 of NTN. For neutrophil depletion experiments, either a neutralizing rat anti-Ly6G antibody (clone 1A8; BioXCell, West Lebanon, NH) or a rat IgG2a isoptype antibody (clone 2A3; BioXCell) were used, respectively. After, 500 µg of respective antibodies were injected intraperitoneally on days 2, 4, and 6 of NTN.
Culture and Stimulation of Mouse Kidney Tubular and Mesangial Cells
Mouse kidney tubular cells34 and mouse kidney mesangial cells35 were cultured in DMEM (Life Technologies) containing 3%–10% FCS (Gibco), 100 U/ml penicillin, and 100 μg/ml streptomycin (Life Technologies) at 37°C with 5% carbon dioxide. Before stimulation, confluent cells were incubated in serum-free DMEM for 24 hours. Cells were stimulated with varying concentrations of IL-17F and TNFα (all from PeproTech, Hamburg, Germany), or with a combination of both cytokines as indicated. After 4 hours of incubation, cells were harvested and stored at –80°C for mRNA analyses. Protein levels of supernatants were determined after 24 hours of incubation by specific ELISA, according to the manufacturer's instructions (R&D Systems).
Statistical Analyses
The results are shown as the mean±SD when presented as a bar graph or as single data points with the mean in a scatter dot plot. Differences between two individual experimental groups were compared using a two-tailed t test. In the case of three or more groups, a two-way ANOVA with Bonferroni multiple comparisons test was used. For survival analysis, the Kaplan–Meier plot with a log-rank test was used. Experiments that did not yield enough independent data for statistical analysis because of the experimental setup were repeated at least three times. P<0.05 was considered to be statistically significant.
Disclosures
None.
Supplementary Material
Acknowledgments
This study was supported by a grant from the Deutsche Forschungsgemeinschaft (grant no. SFB 1192).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2015101077/-/DCSupplemental.
References
- 1.Gaffen SL, Jain R, Garg AV, Cua DJ: The IL-23-IL-17 immune axis: from mechanisms to therapeutic testing. Nat Rev Immunol 14: 585–600, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Langley RG, Elewski BE, Lebwohl M, Reich K, Griffiths CE, Papp K, Puig L, Nakagawa H, Spelman L, Sigurgeirsson B, Rivas E, Tsai TF, Wasel N, Tyring S, Salko T, Hampele I, Notter M, Karpov A, Helou S, Papavassilis C ERASURE Study Group FIXTURE Study Group : Secukinumab in plaque psoriasis--results of two phase 3 trials. N Engl J Med 371: 326–338, 2014 [DOI] [PubMed] [Google Scholar]
- 3.Kitching AR, Holdsworth SR: The emergence of TH17 cells as effectors of renal injury. J Am Soc Nephrol 22: 235–238, 2011 [DOI] [PubMed] [Google Scholar]
- 4.Turner JE, Paust HJ, Steinmetz OM, Panzer U: The Th17 immune response in renal inflammation. Kidney Int 77: 1070–1075, 2010 [DOI] [PubMed] [Google Scholar]
- 5.Couser WG: Basic and translational concepts of immune-mediated glomerular diseases. J Am Soc Nephrol 23: 381–399, 2012 [DOI] [PubMed] [Google Scholar]
- 6.Kurts C, Panzer U, Anders HJ, Rees AJ: The immune system and kidney disease: basic concepts and clinical implications. Nat Rev Immunol 13: 738–753, 2013 [DOI] [PubMed] [Google Scholar]
- 7.Hymowitz SG, Filvaroff EH, Yin JP, Lee J, Cai L, Risser P, Maruoka M, Mao W, Foster J, Kelley RF, Pan G, Gurney AL, de Vos AM, Starovasnik MA: IL-17s adopt a cystine knot fold: structure and activity of a novel cytokine, IL-17F, and implications for receptor binding. EMBO J 20: 5332–5341, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang SH, Dong C: IL-17F: regulation, signaling and function in inflammation. Cytokine 46: 7–11, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kolls JK, Lindén A: Interleukin-17 family members and inflammation. Immunity 21: 467–476, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Cua DJ, Tato CM: Innate IL-17-producing cells: the sentinels of the immune system. Nat Rev Immunol 10: 479–489, 2010 [DOI] [PubMed] [Google Scholar]
- 11.Pantelyushin S, Haak S, Ingold B, Kulig P, Heppner FL, Navarini AA, Becher B: Rorγt+ innate lymphocytes and γδ T cells initiate psoriasiform plaque formation in mice. J Clin Invest 122: 2252–2256, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Toy D, Kugler D, Wolfson M, Vanden Bos T, Gurgel J, Derry J, Tocker J, Peschon J: Cutting edge: interleukin 17 signals through a heteromeric receptor complex. J Immunol 177: 36–39, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Aujla SJ, Chan YR, Zheng M, Fei M, Askew DJ, Pociask DA, Reinhart TA, McAllister F, Edeal J, Gaus K, Husain S, Kreindler JL, Dubin PJ, Pilewski JM, Myerburg MM, Mason CA, Iwakura Y, Kolls JK: IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat Med 14: 275–281, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ishigame H, Kakuta S, Nagai T, Kadoki M, Nambu A, Komiyama Y, Fujikado N, Tanahashi Y, Akitsu A, Kotaki H, Sudo K, Nakae S, Sasakawa C, Iwakura Y: Differential roles of interleukin-17A and -17F in host defense against mucoepithelial bacterial infection and allergic responses. Immunity 30: 108–119, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Haak S, Croxford AL, Kreymborg K, Heppner FL, Pouly S, Becher B, Waisman A: IL-17A and IL-17F do not contribute vitally to autoimmune neuro-inflammation in mice. J Clin Invest 119: 61–69, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sarkar S, Justa S, Brucks M, Endres J, Fox DA, Zhou X, Alnaimat F, Whitaker B, Wheeler JC, Jones BH, Bommireddy SR: Interleukin (IL)-17A, F and AF in inflammation: a study in collagen-induced arthritis and rheumatoid arthritis. Clin Exp Immunol 177: 652–661, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Disteldorf EM, Krebs CF, Paust HJ, Turner JE, Nouailles G, Tittel A, Meyer-Schwesinger C, Stege G, Brix S, Velden J, Wiech T, Helmchen U, Steinmetz OM, Peters A, Bennstein SB, Kaffke A, Llanto C, Lira SA, Mittrücker HW, Stahl RA, Kurts C, Kaufmann SH, Panzer U: CXCL5 drives neutrophil recruitment in TH17-mediated GN. J Am Soc Nephrol 26: 55–66, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Summers SA, Odobasic D, Khouri MB, Steinmetz OM, Yang Y, Holdsworth SR, Kitching AR: Endogenous interleukin (IL)-17A promotes pristane-induced systemic autoimmunity and lupus nephritis induced by pristane. Clin Exp Immunol 176: 341–350, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mease PJ, Genovese MC, Greenwald MW, Ritchlin CT, Beaulieu AD, Deodhar A, Newmark R, Feng J, Erondu N, Nirula A: Brodalumab, an anti-IL17RA monoclonal antibody, in psoriatic arthritis. N Engl J Med 370: 2295–2306, 2014 [DOI] [PubMed] [Google Scholar]
- 20.Leonardi C, Matheson R, Zachariae C, Cameron G, Li L, Edson-Heredia E, Braun D, Banerjee S: Anti-interleukin-17 monoclonal antibody ixekizumab in chronic plaque psoriasis. N Engl J Med 366: 1190–1199, 2012 [DOI] [PubMed] [Google Scholar]
- 21.Genovese MC, Greenwald M, Cho CS, Berman A, Jin L, Cameron GS, Benichou O, Xie L, Braun D, Berclaz PY, Banerjee S: A phase II randomized study of subcutaneous ixekizumab, an anti-interleukin-17 monoclonal antibody, in rheumatoid arthritis patients who were naive to biologic agents or had an inadequate response to tumor necrosis factor inhibitors. Arthritis Rheumatol 66: 1693–1704, 2014 [DOI] [PubMed] [Google Scholar]
- 22.Sandborn WJ, Gasink C, Gao LL, Blank MA, Johanns J, Guzzo C, Sands BE, Hanauer SB, Targan S, Rutgeerts P, Ghosh S, de Villiers WJ, Panaccione R, Greenberg G, Schreiber S, Lichtiger S, Feagan BG CERTIFI Study Group : Ustekinumab induction and maintenance therapy in refractory Crohn’s disease. N Engl J Med 367: 1519–1528, 2012 [DOI] [PubMed] [Google Scholar]
- 23.Paust HJ, Turner JE, Steinmetz OM, Peters A, Heymann F, Hölscher C, Wolf G, Kurts C, Mittrücker HW, Stahl RA, Panzer U: The IL-23/Th17 axis contributes to renal injury in experimental glomerulonephritis. J Am Soc Nephrol 20: 969–979, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steinmetz OM, Summers SA, Gan PY, Semple T, Holdsworth SR, Kitching AR: The Th17-defining transcription factor RORγt promotes glomerulonephritis. J Am Soc Nephrol 22: 472–483, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tulone C, Giorgini A, Freeley S, Coughlan A, Robson MG: Transferred antigen-specific T(H)17 but not T(H)1 cells induce crescentic glomerulonephritis in mice. Am J Pathol 179: 2683–2690, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramani K, Pawaria S, Maers K, Huppler AR, Gaffen SL, Biswas PS: An essential role of interleukin-17 receptor signaling in the development of autoimmune glomerulonephritis. J Leukoc Biol 96: 463–472, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kluger MA, Melderis S, Nosko A, Goerke B, Luig M, Meyer MC, Turner JE, Meyer-Schwesinger C, Wegscheid C, Tiegs G, Stahl RA, Panzer U, Steinmetz OM: Treg17 cells are programmed by Stat3 to suppress Th17 responses in systemic lupus [published online ahead of print October 14, 2015]. Kidney Int doi: 10.1038/ki.2015.296 [DOI] [PubMed] [Google Scholar]
- 28.Gaffen SL: Structure and signalling in the IL-17 receptor family. Nat Rev Immunol 9: 556–567, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang XO, Chang SH, Park H, Nurieva R, Shah B, Acero L, Wang YH, Schluns KS, Broaddus RR, Zhu Z, Dong C: Regulation of inflammatory responses by IL-17F. J Exp Med 205: 1063–1075, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Panzer U, Steinmetz OM, Paust HJ, Meyer-Schwesinger C, Peters A, Turner JE, Zahner G, Heymann F, Kurts C, Hopfer H, Helmchen U, Haag F, Schneider A, Stahl RA: Chemokine receptor CXCR3 mediates T cell recruitment and tissue injury in nephrotoxic nephritis in mice. J Am Soc Nephrol 18: 2071–2084, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Turner JE, Paust HJ, Steinmetz OM, Peters A, Riedel JH, Erhardt A, Wegscheid C, Velden J, Fehr S, Mittrücker HW, Tiegs G, Stahl RA, Panzer U: CCR6 recruits regulatory T cells and Th17 cells to the kidney in glomerulonephritis. J Am Soc Nephrol 21: 974–985, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Riedel JH, Paust HJ, Turner JE, Tittel AP, Krebs C, Disteldorf E, Wegscheid C, Tiegs G, Velden J, Mittrücker HW, Garbi N, Stahl RA, Steinmetz OM, Kurts C, Panzer U: Immature renal dendritic cells recruit regulatory CXCR6(+) invariant natural killer T cells to attenuate crescentic GN. J Am Soc Nephrol 23: 1987–2000, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paust HJ, Ostmann A, Erhardt A, Turner JE, Velden J, Mittrücker HW, Sparwasser T, Panzer U, Tiegs G: Regulatory T cells control the Th1 immune response in murine crescentic glomerulonephritis. Kidney Int 80: 154–164, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wolf G, Mueller E, Stahl RA, Ziyadeh FN: Angiotensin II-induced hypertrophy of cultured murine proximal tubular cells is mediated by endogenous transforming growth factor-beta. J Clin Invest 92: 1366–1372, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wolf G, Haberstroh U, Neilson EG: Angiotensin II stimulates the proliferation and biosynthesis of type I collagen in cultured murine mesangial cells. Am J Pathol 140: 95–107, 1992 [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.