Abstract
APOL1 risk variants are associated with kidney disease in blacks, but the mechanisms of renal injury associated with APOL1 risk variants are unknown. Because APOL1 is unique to humans and some primates, we created transgenic (Tg) mice using the promoter of nephrin-encoding Nphs1 to express the APOL1 reference sequence (G0) or the G2 risk variant in podocytes, establishing Tg lines with a spectrum of APOL1 expression levels. Podocytes from Tg-G0 and Tg-G2 mice did not undergo necrosis, apoptosis, or autophagic cell death in vivo, even in lines with highly expressed transgenes. Further, Tg-G0 and Tg-G2 mice did not develop kidney pathology, proteinuria, or azotemia as of 300 days of age. However, by 200 days of age, Tg-G2 mice had significantly lower podocyte density than age-matched WT and Tg-G0 mice had, a difference that was not evident at weaning. Notably, a pregnancy-associated phenotype that encompassed eclampsia, preeclampsia, fetal/neonatal deaths, and small litter sizes occurred in some Tg-G0 mice and more severely in Tg-G2 mice. Similar to human placenta, placentas of Tg mice expressed APOL1. Overall, these results suggest podocyte depletion could predispose individuals with APOL1 risk genotypes to kidney disease in response to a second stressor, and add to other published evidence associating APOL1 expression with preeclampsia.
Keywords: genetic renal disease, podocyte, transgenic mouse, focal segmental glomerulosclerosis, chronic kidney disease
Polymorphisms in the gene for APOL1 are common in individuals of African ancestry and are associated with the excess prevalence of CKD in blacks.1,2 Although most vertebrate genomes have an APO-L locus, humans and some Old World primates have evolved novel APO-L proteins that are structurally and functionally distinct from rodent APO-L proteins.3,4 In addition, the human/primate APOL1 protein is unique in that it contains a secretory signal and circulates in the blood at approximately 0.3 mg/dl as part of HDL particles. The only known function of human/primate APOL1 is mediated by this circulating form, which provides innate immunity against the kinetoplastid pathogens Trypanosoma brucei5 and Leishmania,6 both endemic in Africa.
The two APOL1 coding variants associated with CKD, known as G1 and G2, underwent strong natural selection possibly by providing parasite-killing activity toward additional T. brucei subspecies or other pathogens.1,4,6 This innate immune function is dominant, such that individuals with one G1 or G2 allele (heterozygotes) are resistant to trypanosomiasis. However, individuals with two G1 or G2 alleles (homozygotes or compound heterozygotes, with no reference sequence APOL1 alleles, known as G0) have an increased risk for developing CKD. The biology underlying the association of APOL1 variants with CKD remains unclear; APOL1 variants may cause CKD or APOL1-G0 may protect against CKD.
Although APOL1 is an abundant plasma protein, our prior work demonstrated that circulating APOL1 levels are not associated with CKD or APOL1 genotype, although APOL1 risk genotypes are associated with CKD and CKD progression.7 Others have shown kidney allograft failure was more common with donor kidneys obtained from individuals with APOL1 kidney disease risk genotypes; in contrast, graft survival was similar in recipients with and without APOL1 risk genotypes.8–10 Together, these data strongly support the hypothesis that circulating APOL1 does not cause kidney injury. As an alternative approach, we and others examined additional sites where APOL1 may be expressed which could identify a link with renal injury. APOL1 protein was present in normal and diseased kidney tissue within several renal cell types including, podocytes, proximal tubules, and endothelial and smooth muscle cells of small arteries and arterioles.11,12 Thus, APOL1 expression in renal parenchymal cells and a novel intracellular function for APOL1 could underlie its association with CKD.
Since mice do not have an ortholog of human APOL1, we created two transgenic mouse models expressing either reference sequence APOL1-G0 or the CKD-associated variant APOL1-G2 to investigate the phenotypic impact of intracellular expression of APOL1 in podocytes. We focused on APOL1-G2 because it is a single mutation, and the genetic evidence has shown essentially equivalent odds for kidney diseases in subjects who are homozygous for G1 or G2 risk alleles or are compound heterozygotes for one copy of each.13 In addition, we limited transgene expression to podocytes since it recreates an in vivo expression pattern documented in human biopsies, and the CKDs associated with APOL1 risk variants typically result from podocyte or glomerular injury. APOL1 expression, podocyte viability, and the development of spontaneous phenotypes were examined in these transgenic mice.
Results
Transgenic Mice Creation and Kidney Expression and Phenotype
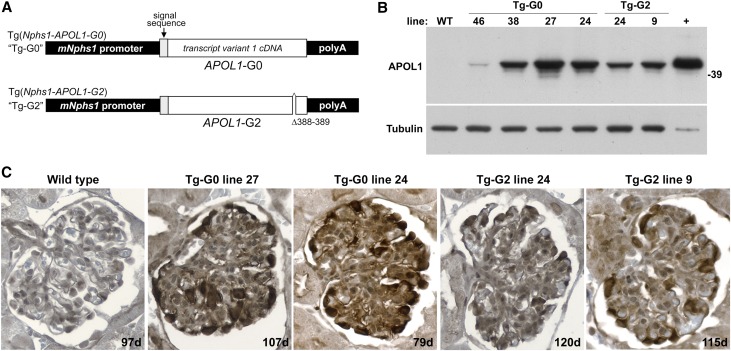
Transgenic mice were created using pronuclear microinjection of fertilized FVB/N oocytes using transgenes expressing either APOL1-G0 (Tg-G0) or APOL1-G2 (Tg-G2) under the control of the 4.2 kb murine Nephrin gene (Nphs1) promoter (Figure 1A). The transgenes were full length cDNAs of transcript variant 1, which includes the signal sequence and encodes the 398 amino acid APOL1 isoform A. Numerous founders were obtained (Supplemental Table 1) for both Tg-G0 (n=29) and Tg-G2 (n=8), and when mated with wild-type (WT) FVB/N breeders, transgenic offspring were obtained in the expected Mendelian ratios.
Figure 1.
Transgenic mouse models expressed human APOL1-G0 and APOL1-G2 in podocytes. (A) Transgenic mice expressing human APOL1-G0 (Tg-G0) and APOL1-G2 (Tg-G2) were created as described (see Concise Methods). The transgenes consisted of a full length coding cDNA (splice variant 1, protein isoform A) containing the signal peptide sequence. Expression was controlled by the 4.2 kb murine Nphs1 promoter (mNphs1) and the rabbit β-globin gene polyA tail. (B) Transgene expression was estimated by Western blotting for APOL1 protein in whole-cell extracts from isolated glomeruli from offspring of the various founders used for line development. Glomeruli from a WT mouse were used as a negative control. Whole cell extracts from Chinese hamster ovary cells transfected with a full length APOL1 cDNA expression plasmid were used as a positive (+) control. This blot was reprobed with an anti-Tubulin antibody to demonstrate loading of equivalent protein amounts in each lane. (C) Transgene expression level was corroborated by immunohistochemistry (color reaction product is brown with hematoxylin counterstain) and podocyte-restricted expression was confirmed in offspring of founders used for line development. Age of animal given on lower right in days (d).
APOL1 was consistently expressed in podocytes but the expression levels varied, likely reflecting either transgene copy number or influences from flanking DNA or chromatin structure at the insertion site. APOL1 expression levels detected by Western blotting of isolated glomeruli (Figure 1B) or by immunohistochemistry (Figure 1C) correlated, and the expression pattern in the kidney was restricted to podocyte cytoplasm by immunofluorescence (Supplemental Figure 1). No Tg-G2 founders were obtained that exhibited the highest levels of transgene expression observed in several Tg-G0 founders (Supplemental Table 1). APOL1 protein was not observed in renal tubules or endothelium using immunohistochemistry or immunofluorescence. Kidney expression levels of APOL1 did not change with successive generations of breeding (data not shown).
All of the founders that survived initial breeding were longitudinally examined for proteinuria until at least 200 days of age (mean age of 302 days, see Supplemental Table 1 for terminal ages). Littermates negative for the APOL1 transgene DNA were used as age-matched, WT controls. No founders, except Tg-G2 F9, were proteinuric at any time point (Supplemental Figure 2A). Tg-G2 F9 had a moderate level of proteinuria that was evident at weaning, but the proteinuria did not worsen with age.
Unique lines of mice were established from four Tg-G0 founders (lines 27, 24, 38, and 46) and two Tg-G2 founders (lines 24 and 9) and used for phenotyping studies. Male and females from each of the established lines were longitudinally examined for proteinuria (mean age of 270 days). None had evidence of proteinuria except for some (but not all) offspring from Tg-G2 line 9, which had a similar level of proteinuria as the founder animal (Supplemental Figure 2B). The cause of this incompletely penetrant, nonprogressive proteinuria in Tg-G2 line 9 is unclear. However, no other Tg-G2 founder (n=5) or their progeny developed proteinuria, including those with similar levels of transgene expression (n=3, see Supplemental Table 1). These data suggest that the proteinuria in Tg-G2 line 9 reflects multiple transgene insertion sites and/or insertional modification of a host gene rather than APOL1-G2–dependent kidney injury. Therefore, the mechanism of the incompletely penetrant proteinuria in the Tg-G2 line 9 was not studied further.
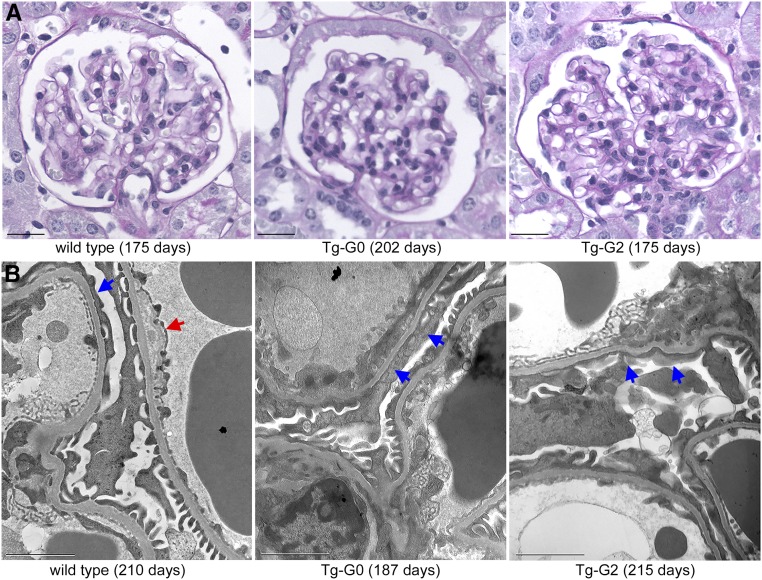
Similarly, there was no evidence of kidney histopathology (Figure 2A) in either Tg-G0 or Tg-G2 mice at 200 days of age when compared with age-matched controls. Transmission electron microscopy identified minor glomerular changes in basement membrane thickness and podocyte foot process effacement (Figure 2B), but these changes were not different compared with age-matched WT mice (Supplemental Table 2).
Figure 2.
Tg-G0 and Tg-G2 mice did not develop kidney pathology. (A) Kidney histopathology (period acid–Schiff stain) from established Tg-G0 and Tg-G2 lines, and age-matched WT mouse (ages given in parentheses). No significant differences were noted between Tg-G0, Tg-G2, and WT mice. Scale bar, 20 µm. (B) Transmission electron microscopy of glomerular capillary loops from established Tg-G0 and Tg-G2 lines, and WT FVB/N mice (ages given in parentheses). All transgenic and WT kidneys had evidence of some ultrastructural changes including basement membrane thickness anomalies (red arrow) and podocyte foot process effacement or widening (blue arrows) which were quantified in Supplemental Table 2. Scale bar, 2 µm.
Evaluation of Podocyte Cell Death In Vivo
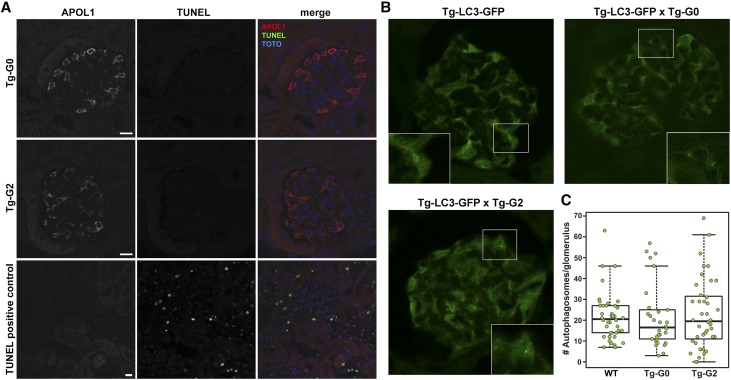
The pathologic mechanism of APOL1-mediated kidney disease is currently unknown, but recent studies using cell culture models have suggested kidney injury may be initiated by variant APOL1-dependent cytotoxicity (reviewed in Limou et al.14). To assess for evidence of cell death in vivo, fixed kidney tissue from Tg-G0 and Tg-G2 mice were probed for DNA fragmentation (common in necrotic and apoptotic cell death) using the terminal deoxynucleotidyl transferase–mediated digoxigenin-deoxyuridine nick-end labeling (TUNEL) assay. However, no TUNEL-positive cells were detected in glomeruli from Tg-G0 or Tg-G2 mouse kidneys (Figure 3A).
Figure 3.
Tg-G0 and Tg-G2 mice had no evidence of podocyte cell death. (A) Glomeruli were examined using the TUNEL assay to detect cell death processes characterized by nuclear fragmentation (necrosis and apoptosis) and were negative in multiple Tg-G0 (n=6, ages examined ranged from 35 to 211 days) and Tg-G2 (n=4, ages examined ranged from 54 to 275 days) mice. Scale bar, 10 µm. Sections were costained with APOL1 and TOTO-3 was used as a nuclear counterstain. (B) Tg-G0 and Tg-G2 mice were intercrossed with mice ubiquitously expressing GFP-tagged LC3, a marker for autophagosome formation. Cytoplasmic GFP-LC3 fluorescence in glomeruli was strong in podocytes and green fluorescence that concentrated in subcellular puncta (inset) were counted individually as autophagosomes. (C) Number of autophagosomes in glomeruli were not significantly different between dual transgenic Tg-LC3-GFP×Tg-G0 (Tg-G0, n=3 mice) and Tg-LC3-GFP×Tg-G2 (Tg-G2, n=4 mice) when compared with Tg-LC3-GFP mice (WT, n=4 mice). Box plot denotes interquartile range with a median line and 95th percentile whiskers. Each dot represents one glomerulus, ten glomeruli were scored for each mouse. All mice were age-matched females, similar results were obtained for age-matched males (not shown). No significant differences were detected between groups using generalized linear mixed models and Markov Chain Monte Carlo samples.
To evaluate cell death pathways associated with autophagy induction, in vivo autophagosome formation was examined using another transgenic mouse model ubiquitously expressing a green fluorescent protein-tagged microtubule-associated protein 1 light chain 3 (GFP-LC3). LC3 labels autophagosomal membranes and GFP-LC3 can be visualized as intensely fluorescent intracellular puncta. LC3 is highly expressed in podocytes, and these Tg-LC3-GFP mice have been used previously to quantify in vivo autophagy induction in podocytes.15,16 Tg-G0 and Tg-G2 mice were intercrossed with Tg-LC3-GFP mice and kidneys from dual transgenic mice were fixed and GFP-expressing autophagosomes in glomeruli were scored by observers blinded to genotype. Numbers of autophagosomes were not statistically different between WT, Tg-G0, and Tg-G2 transgenic mice (Figure 3, B and C) indicating that APOL1-G0 or APOL1-G2 expression failed to activate autophagy in vivo. As previously reported, no GFP-positive puncta were seen in the cortical tubules of the LC3-GFP mice.16
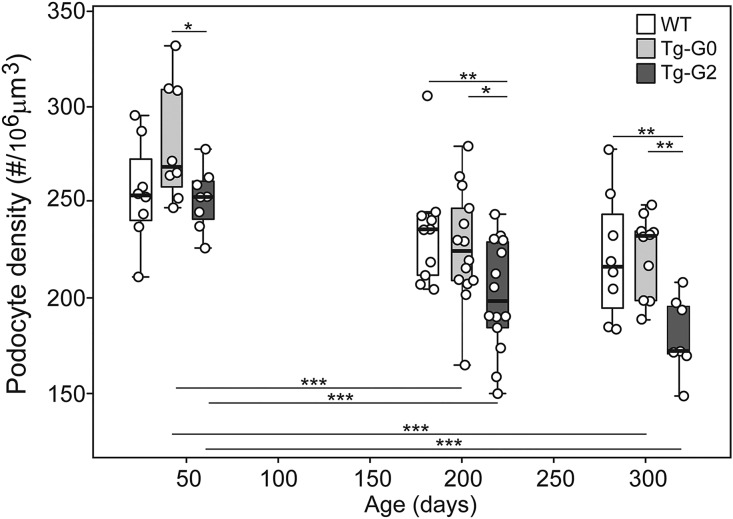
Since all of the methods used to detect cell death produce transient signals that may be missed at a given time point, we also quantified podocyte densities in WT, Tg-G0, and Tg-G2 mouse kidneys using a method developed by Roger Wiggins (University of Michigan).17 This assay can reveal if a chronic, low level of podocyte loss (either by death, detachment, or dedifferentiation) accumulates over time, resulting in a pathologically significant depletion of podocytes with age. At 40 days of age, when kidney development is complete in the mouse, there was no significant difference in glomerular tuft volume (Supplemental Figure 3A), podocyte number (Supplemental Figure 3B), or the calculated podocyte density (Figure 4) between WT (nontransgenic littermates), Tg-G0, or Tg-G2 mice. At the 200 day time point, all mice had significantly increased glomerular volume (Supplemental Figure 3A), which has been previously shown as an age-related change,18 although glomerular volume increases in Tg-G0 and Tg-G2 mice were significantly greater compared with WT mice. Unexpectedly, the Tg-G2 mice had significantly decreased podocyte number at 200 days of age (Supplemental Figure 3B). The calculated podocyte density (ratio of podocyte number and glomerular volume) was therefore significantly decreased in Tg-G2 mice compared with WT (Figure 4). No difference in podocyte density was detected between Tg-G0 and WT mice. By 300 days of age, podocyte densities in Tg-G2 continued to trend downward and remained significantly less compared with WT and Tg-G0 (Figure 4). All mice had significant increases in glomerular tuft volume between 200 and 300 days of age (Supplemental Figure 3A), but podocyte number remained significantly less in Tg-G2 mice compared with WT and Tg-G0 mice (Supplemental Figure 3B).
Figure 4.
Tg-G2 mice had significantly decreased podocyte density at 200 and 300 but not 40 days of age. At approximately 40, 200, and 300 days of age, glomerular volumes (see Supplemental Figure 3A) and podocytes counts (see Supplemental Figure 3B) were quantified and data were transformed to account for section thickness and the nuclear caliper diameter and shape coefficient (see Concise Methods). Podocyte densities were calculated from the transformed podocyte counts and glomerular volumes. Box plots show quartiles with a median line and 95th percentile whiskers. Each dot is the average of about 100 glomeruli. Statistical significance was determined by repeat measures (ANOVA). Significant differences between genotypes at each time point are shown above boxes, and significant differences within a genotype comparing the three time points are indicated below the boxes (*P<0.05; **P<0.01; ***P<0.001). Numbers in each group (mean group age) for the 40-day time point were: Wild-type (WT), n=8 (39 days); Tg-G0, n=8 (35 days); Tg-G2, n=8 (35 days); for the 200-day time point: WT, n=8 (211 days); Tg-G0, n=14 (207 days); Tg-G2, n=11 (225 days); and for the 300-day time point: WT, n=8 (315 days); Tg-G0, n=10 (317 days); Tg-G2, n=7 (315 days).
Pregnancy-Associated Phenotype
In the initial breeding of the founders, there was an unexpected occurrence of sudden death in mid- to late-term pregnant Tg-G0 and Tg-G2 founder females. Excluding founder females with very low levels of transgene expression (see Supplemental Table 1), 100% (two of two) Tg-G2 and 29% (two of seven) Tg-G0 founder females died during their first pregnancy. Pregnant females that died were either found dead with signs of seizure activity (ptyalism) or were observed having grand mal seizures and were euthanized. In addition, WT FVB/N females used as dams for founder males also exhibited seizures or sudden death while pregnant or <24 hours after delivering a viable litter, thereby linking this phenotype to the presence of transgenic fetuses or fetal-derived placental tissue. Of the surviving founders that initially reproduced successfully, pregnancy-associated deaths occurred sporadically in the breeding of both their female offspring and in dams of male offspring. However, in continued breeding, the occurrence of pregnancy-associated deaths has become rare, as would be expected for a trait that causes death before reproduction.
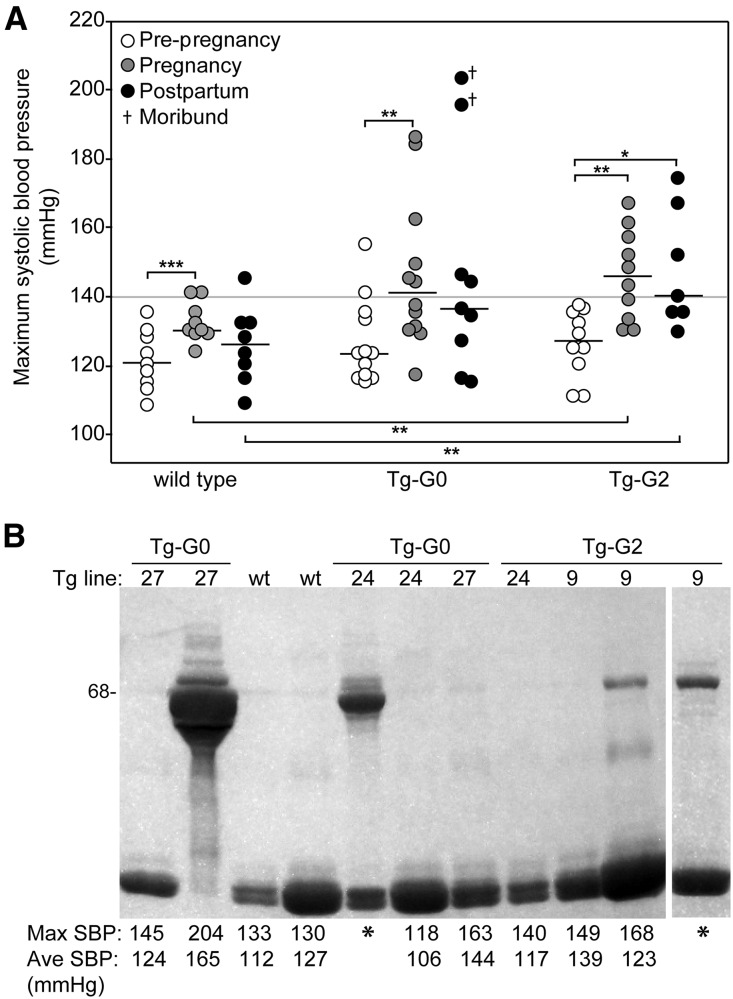
Because of the similarity of this phenotype with eclampsia, pregnant transgenic mice were evaluated for preeclampsia using WT FVB/N pregnant mice for comparison. Hallmarks of preeclampsia are high BP, proteinuria, endotheliosis, and elevated levels of soluble fms-like tyrosine kinase-1 (sFlt-1). Systolic BPs were monitored prepregnancy, during pregnancy, and postpartum in Tg-G0, Tg-G2, and WT FVB/N mice. Baseline, prepregnancy pressures were not different in transgenic mice compared with WT mice (Figure 5A). None of the WT mice had systolic BPs exceeding 145 mmHg. However, approximately 50% of the transgenic pregnant mice (five of 12 Tg-G0 and five of ten Tg-G2) had systolic BP >145 mmHg, with some >180 mmHg. Hypertension was only significantly higher in pregnant Tg-G2 mice compared with WT mice. In affected animals, the onset of hypertension occurred during the second half of pregnancy, which is temporally correlated with the formation of the definitive placenta (Supplemental Figure 4).
Figure 5.
Pregnant Tg-G0 and Tg-G2 mice were susceptible to preeclampsia. (A) Systolic BPs were monitored before mating, during pregnancy, and postpartum. Maximum systolic pressures recorded during each period are presented for WT (n=9), Tg-G0 (n=12), and Tg-G2 (n=10) mice (see Supplemental Figure 4 for longitudinal graphs of individual BP readings). Two Tg-G0 mice became moribund in the postpartum period. Median lines are shown. Paired and unpaired t tests were used to evaluate differences in BPs within genotypes and between genotypes respectively; significant differences are noted as follows: *P<0.05; **P≤0.01; ***P<0.001. (B) Example of pregnancy-associated albuminuria detected by coomassie-stained polyacrylamide gel; each animal shown was pregnant and urine was collected near term or immediate postpartum. Asterisk (*) indicates a seizing animal and BPs were not recorded; urine was collected at euthanasia. Below the gel are the maximum systolic BP readings (Max SBP) detected during pregnancy/postpartum and the average systolic BP readings (Ave SBP) during the last five days of pregnancy. Low molecular weight bands are the major urinary proteins which are common and normal in mice. WT, WT pregnant mice.
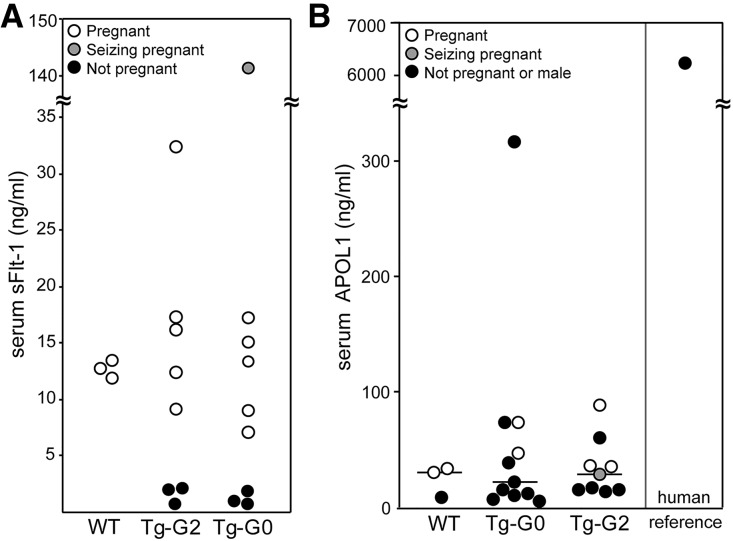
On clinical evaluation, there were no differences in serum electrolytes or liver function enzymes between WT pregnant and Tg-G0 or Tg-G2 pregnant mice, including those exhibiting seizures and hypertension (Supplemental Table 3). There were no gross pathologic changes in the brains of seizing mice (data not shown). The more severely hypertensive and seizing mice had proteinuria (Figure 5B), and seizing mice had high serum levels of sFlt-1 (Figure 6A). However, there was no APOL1 protein detected in the serum of the pregnant females, nor was APOL1 detectable in the circulation of any nonpregnant female or male transgenic mouse (Figure 6B).
Figure 6.
APOL1 was not present in the serum of Tg-G0 or Tg-G2 mice. Circulating factors in pregnant Tg-G0 and Tg-G2 mice were assayed by ELISA. (A) Quantification of sFlt-1 levels in pregnant and nonpregnant transgenic mice with pregnant WT mice for comparison. (B) Quantification of APOL1 levels in transgenic mice, including pregnant females, using a human APOL1-specific ELISA. Median lines are shown. There is no possibility of human APOL1 in the circulation of nontransgenic mice, thus, the low levels detected in WT nontransgenic mice represents nonspecific crossreactivity of mouse serum. Human serum given as a positive control and as a reference for the typical circulating level in humans.
Kidney histopathology of the seizing pregnant females had no gross pathologic changes (Supplemental Figure 5A) or evidence of fibrin-containing clots in glomeruli (data not shown), but exhibited reduced immunostaining for the endothelial marker CD31 in glomerular capillaries (Supplemental Figure 5B). This loss of CD31 in glomerular capillaries was previously shown to occur in human preeclampsia19 and suggests glomerular endothelial cell damage. APOL1 expression by RT-PCR was confirmed in transgenic placentas (Supplemental Figure 6A) and by immunofluorescence in the human placenta (Supplemental Figure 6B). APOL1 expression, however, was undetectable in transgenic brains (by immunohistochemistry, data not shown). Expression of Nephrin and the Nphs1 promoter has been previously demonstrated in some anatomic locations of the brain and spinal cord in various mouse models,20–23 although this expression pattern of endogenous protein was not substantiated in human or pig tissues.24,25
In conjunction with the occurrence of an eclampsia-like phenotype in some founder females, other founders never mated successfully or produced few viable pups (Supplemental Table 1). Of the successfully reproducing founders, the weaned litter size was lower than expected for FVB/N mice. Litters trended toward smaller sizes inversely proportional to transgene expression level in the Tg-G0 mice, and Tg-G2 litters trended smaller than Tg-G0 litters. Tg-G2 line 24 litters were significantly smaller than WT FVB/N litters (Supplemental Figure 7). This reduced litter size was caused by a combination of fetal death in utero, stillborn pups, and pups that failed to thrive and died in the immediate postnatal period (<1 week after birth). Although initial founder matings with WT FVB/N mice produced expected Mendelian ratios of transgenic pups, subsequent filial generations from some founders were unable to consistently produce transgenic offspring. In addition, records from the transgenic core facility indicated that despite implanting similar numbers of injected oocytes into an equal number of pseudo-pregnant females, there were fewer live births and weaned pups for the Tg-G2 transgene compared with Tg-G0. For Tg-G0, eight of eight implanted females delivered litters and weaned 73 pups averaging 9 pups/litter. For Tg-G2, seven of eight implanted females delivered litters but two of the seven litters died shortly after birth. Of the surviving five litters, a total of 25 pups were weaned, averaging 5 pups/litter. There was no difference in the success rate for transgene integration, as there were comparable percentages (40% versus 42%) of transgene-positive to transgene-negative weanlings from both the Tg-G0 and Tg-G2 microinjections, respectively.
Discussion
We have characterized Tg-G0 and Tg-G2 mice and made two novel observations. First, neither Tg-G0 nor Tg-G2 mice, with a range of transgene expression levels, developed kidney disease as assessed by proteinuria and creatinine levels or standard histology testing when aged to approximately 300 days. These data faithfully model the observations in humans, which show most individuals with APOL1 risk genotypes do not develop kidney diseases in the absence of an additional stressor, a “second hit.”1,2,26 Mice do not have a human APOL1 ortholog and may lack molecules necessary for APOL1-dependent kidney disease. However, podocyte densities were significantly reduced in Tg-G2 mice. These data suggest, for the first time, that APOL1-G2 diminished podocyte density and that the APOL1-dependent effector pathways needed for kidney disease are present in mice.
The podocyte depletion hypothesis is based on the pathologic descriptions by Kriz et al.27 and the careful modeling of podocyte number and density in experimental models and human disease by Wiggins et al. (reviewed in Kikuchi et al.28). In rodent models and humans, once podocyte density losses exceed 40% (in humans, <100 podocyte/106 µm3) glomerular disease becomes progressive, regardless of the inciting injury, as a result of hypertrophic cell stress.29–31 Podocyte density in the Tg-G2 mice remained above this threshold from birth through to 300 days of age, explaining the lack of overt kidney disease. Even if the rate of podocyte density change is extrapolated, Tg-G2 mice would not be at risk for overt kidney disease within the normal lifespan of FVB/N mice. In humans, normal aging is associated with loss of podocyte density, estimated at 0.9% per year in adults, resulting from both increased glomerular volume (hypertrophy) and decreased podocyte numbers.30 Careful histomorphometric analyses of kidney microanatomy in an autopsy series of blacks without kidney diseases demonstrated that APOL1 risk alleles exaggerated age-related increases in glomerular volume, which may predispose these individuals to kidney diseases.32 Since the podocyte is postmitotic, this additional glomerular tuft enlargement reduces podocyte density even in the absence of podocyte loss. The Tg-G2 mice suggest blacks with risk genotypes may have inadequate podocyte numbers for their age-related increases in glomerular volume. Thus, podocyte density decreases may be a mechanism of genetic susceptibility mediated by APOL1 risk alleles, which can result in a predisposition to glomerulosclerosis if a subsequent injury further reduces the podocyte reserve to a level that compromises glomerular function.
The mechanisms for reduced podocyte density in the Tg-G2 mice are not clear. Since glomerular volumes were equivalent in Tg-G0 and Tg-G2 mice, reduced podocyte densities in the Tg-G2 mice resulted from fewer podocytes. Despite the eclampsia/preeclampsia phenotype, intrauterine stress did not cause the podocyte depletion phenotype; podocyte densities were the same in 40-day-old WT and Tg-G2 mice. We found no evidence for necrosis, apoptosis, or autophagic cell death after analyzing multiple sections from multiple mice. Alternatively, rodent and human studies have shown podocytes can detach from the glomerulus without dying (reviewed in Oliveira Arcolino et al.33). Since WT-1 immunostaining was used to quantify podocytes, missing podocytes may be present but dedifferentiated and no longer expressing WT-1. Podocyte WT-1 staining is lost in some genetic and acquired human glomerular diseases. However, reduced podocyte number and density in the Tg-G2 mice occurred when the mice had no overt kidney disease phenotype, making dedifferentiation a less likely explanation for reduced podocyte density.
In contrast to our data in these APOL1 transgenic mice, several recent studies have linked intracellular expression of APOL1 with cytotoxicity.14 However, these studies were limited to robust in vitro34–41 or in vivo ectopic expression models4 with toxicity attributed to either apoptosis, necrosis, autophagy induction, or intracellular potassium depletion. APOL1 is expressed in podocytes in situ in healthy kidney tissue,11,12 suggesting physiologic APOL1 expression is not cytotoxic. Although our in vivo models do not use the native APOL1 promoter, we used the mouse Nphs1 promoter to generate APOL1 expression at levels consonant with other proteins constitutively expressed in the podocyte and we failed to find evidence of cell death or clinical kidney disease. Since rodents lack an APOL1 ortholog, these transgenic mice model humans homozygous for APOL1-G0 and APOL1-G2 and accurately recapitulate the observation that individuals with an APOL1 risk genotype do not develop kidney disease in the absence of a relevant stressor. Thus, these mice will provide a useful model to test stressors that may then cause kidney disease in the setting of a risk genotype.
Our second novel and unexpected finding in our transgenic mice was some pregnant Tg-G0 and Tg-G2 mice developed an eclampsia/preeclampsia phenotype, suggesting that APOL1 has a placental function. Although the pregnancy-associated phenotype occurred in both Tg-G0 and Tg-G2 founders and established lines, Tg-G2 mice were more severely affected as demonstrated by: (1) pregnancy failures, loss of litters, and reduced litter size; (2) greater percentage of founder females died of eclampsia; and (3) significant BP elevation (preeclampsia). Since these phenotypes also occurred in WT FVB/N mice carrying transgenic litters, the presence of transgenic pups or placentas must be causal. The lack of circulating APOL1 in dams and sires supports this premise. Incomplete penetrance and variable expressivity of the adverse pregnancy phenotypes may result from the variable, aggregate transgene load for each pregnancy. For example, more transgenic fetuses or higher fetal transgene expression (homozygote versus heterozygote fetuses) may determine a threshold to initiate the cascade of events leading to abnormal pregnancy outcomes. Both APOL1-G0 and APOLl-G2 could initiate the pregnancy phenotype but APOL1-G2 caused more severe disease. Further work is needed to establish the association of APOL1 variants with human pregnancy complications and the mechanisms involved.
By using the Nephrin promoter, we appear to have recreated normal human expression of APOL1 in the placenta. We observed APOL1 was abundantly expressed in human trophoblasts, which confirms genome-wide expression studies of normal human tissues that document both Nphs1 (Nephrin) and APOL1 expression in placenta, with APOL1 being one of the most abundant transcripts in the placenta.42,43 The original descriptions of the human APO-L gene cluster identified high APOL1 expression in the placenta and suggested aberrant expression in preeclampsia.44–46 Placental Nephrin expression has also been documented in other studies.47–49 The pregnancy phenotype was not caused by an artifact of transgenesis, as none of the many published transgenic mouse lines created using the Nphs1 promoter have reported adverse pregnancy outcomes.
Preeclampsia, preterm birth, and low birth weight are more prevalent in sub-Saharan Africans and women of African Ancestry (reviewed in Nakimuli et al. and Shahul et al.50,51). APOL1 levels, APOL1-derived peptides, and APOL1 autoantibodies in mothers are linked to both preeclampsia and intrauterine growth retardation.52–54 The pregnancy-associated phenotypes observed in our transgenic mice suggest an important role for Nephrin and APOL1 in placental function. Data from the Nephrotic Syndrome Study Network cohort,55 confirmed in the Chronic Kidney Disease in Children cohort,56 found preterm birth was more common in black children with glomerular disease and two APOL1 risk alleles. Importantly, this association may be determined by the genotype of the child, not the genotype of the mother. These observations are consistent with the known paternal influence on preeclampsia,57 since APOL1 allele contributions from each parent appear necessary to predispose the mother to an at-risk pregnancy.
In summary, APOL1 expression regulated by the mouse Nphs1 promoter does not cause overt kidney disease according to standard clinical phenotyping tools. Importantly, the Tg-G2 mice have reduced podocyte densities, providing a “first hit” that may predispose blacks to progressive kidney diseases after a subsequent stressor, or second hit. We also identified greater adverse pregnancy outcomes in Tg-G2 mice, which in conjunction with published human data, suggest variant APOL1s may contribute to pregnancy complications.
Concise Methods
Transgenic Mice
To generate the APOL1 transgene, a plasmid containing the APOL1 coding sequence in transcript variants 1 and 3 (isoform A, NM_00366.1.3) was purchased from GeneCopeia (Rockville, MD). In this plasmid the 6 bp in-frame deletion for the G2 variant (rs71785313) was engineered using site-directed mutagenesis (QuickChange, Stratagene, La Jolla, CA). Full length cDNAs were amplified in parallel using the primer pair: forward 5′-GGACTCAGATCTCGAGGCCGCCACCATGGAGGGAGCTGCTTTGCTG-3′ and reverse 5′- GGCGACCGGTTCTAGATCACAGTTCTTGGTCCGCCTG-3′. The forward primer was designed to engineer a Kozak sequence between the Xho1 restriction and the start codon of APOL1. The 1238 and 1232 bp amplicons for APOL1-G0 and APOL1-G2, respectively, were digested using XhoI and XbaI, gel purified, and directionally ligated into the multicloning site between the 4.2 kb murine Nphs1 promoter and the splice donor, splice acceptor, polyadenylation signal of the rabbit β-globin gene of an existing vector developed and kindly provided by Dr. Susan Quaggin.58 After sequence confirmation, the transgene cassette containing the Nphs1 promoter, Kozak sequence, APOL1 cDNA, and splice donor, splice acceptor, polyadenylation signal was excised from the expression vector using PacI, NotI digest. The resulting fragment was gel purified and submitted to the transgenic animal facility at Case Western Reserve University for pronuclear injection of fertilized, single-cell FVB/N oocytes. For genotyping, transgene DNA was detected in genomic DNA from mouse tissues using standard PCR with the following primers: forward 5′-ACAAGCCCAAGCCCACGACC-3′ and reverse 5′-AGGGGCCACATCCGTGAGCT-3′, under the following amplification conditions; 94°C for 10 minutes, followed by 28 cycles of 94°C for 30 seconds, 56°C for 30 seconds, and 72°C for 1 minute.
Transgenic mice ubiquitously expressing GFP-LC3 were obtained from RIKEN (Hirosawa, Japan) and have been used previously to assess in vivo autophagosome formation in podocytes.15,16 Tg-GFP-LC3 mice were intercrossed with Tg-G0 and Tg-G2 and maintained on a standard light cycle with ad libitum food and water. Animals were euthanized at the same time of the light cycle and kidney tissues were fixed in neutral buffered formalin overnight, frozen in optimal cutting temperature compound, and 10 µm cryosections were mounted to visualize GFP fluorescence. LC3-II–positive puncta in glomeruli were counted by two blinded observers.
BP and Clinical Evaluations
Systolic BP was measured using tail cuff plethysmography with equipment from IITC (San Diego, CA). Mice were acclimated to the procedure followed by a minimum of three prepregnancy readings to establish baseline BP the week before mating. BPs were measured through pregnancy and up to 4 days postpartum. Serum chemistries were performed at the Cleveland facilities of Marshfield Labs Veterinary Services (Waukesha, WI). Serum levels of APOL1 were quantified by ELISA with a kit from USCN Life Sciences (Cedarlane Labs, Burlington, NC) as previously described,7 using 1:10 and 1:50 dilutions of mouse serum. Serum levels of sFlt-1 were quantified by Quantikine ELISA (R&D Systems, Minneapolis, MN) following the manufacturer’s instructions using maternal serum collected near term pregnancy. All samples for ELISAs were assayed in duplicate and averaged. Urinary albumin levels were monitored by PAGE with coomassie staining.
Histopathology and TUNEL Assay
Periodic acid–Schiff staining (Sigma-Aldrich, St. Louis, MO) was performed on formalin-fixed, paraffin-embedded, 4-μm thick, tissue sections for light microscopy. Ultrastructure of glomerular basement membranes, foot processes, and endothelial fenestrae were imaged using standard transmission electron microscopy on gluteraldehyde-formaldehyde-fixed, epon-embedded sections. Histopathology and ultrastructural changes were evaluated by two renal pathologists blinded to sample identity. Glomerular changes were semiquantitatively scored using a method developed for deep phenotyping human biopsies.59
TUNEL assays were performed on formalin-fixed, paraffin-embedded, 4-μm thick, tissue sections using the in situ Cell Death Detection Kit (Fluorescein) from Roche Diagnostics (Indianapolis, IN) according to the manufacturer’s recommendations. Two sections for each mouse kidney were examined, one with and one without coimmunostaining for APOL1.
Podocyte Density
Podocyte density and glomerular volumes were quantified using a method developed by Roger Wiggins (University of Michigan), for single slides,17 using WT-1 immunostaining to identify podocyte nuclei and synaptopodin immunostaining to demarcate glomerular tuft boundaries. Four-micrometer thick sections were used and approximately 100 glomeruli were quantified for each animal with a minimum of eight animals per time point for each group. The three groups examined were WT, Tg-G0 (line 27, line 24, and line 38 animals), and Tg-G2 (line 24 animals). Podocyte nuclear caliper diameter, podocyte number, and glomerular area were scored and podocyte density and glomerular tuft volumes were calculated using the supplemental table provided in Venkatareddy et al.17
Expression Analyses
Immunohistochemistry on mouse and human tissue was performed as previously described using formalin-fixed, paraffin-embedded tissue with antigen retrieval.12 Antibodies were a rabbit polyclonal antibody to human APOL1 (18885, Sigma-Aldrich); CD31 (ab317830; Abcam, Inc., Cambridge, MA); cytokeratin-7 (M7018; DAKO, Carpinteria, CA), synaptopodin (clone G1D4; Meridian, Memphis, TN); and WT-1 (C-19; Santa Cruz Biotechnology, Santa Cruz, CA). Antibody binding was identified with either horseradish peroxidase-conjugated secondary antibodies followed by avidin-biotin amplification and detection with 3, 3′-diaminobenzidine substrate using reagents from Vector Labs (Burlingame, CA), or with fluorescent-conjugated secondary antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA) and detection with confocal fluorescent microscopy. For Western blotting, glomeruli were enriched using the sieving method and whole-cell protein extracts were prepared for analysis by denaturing PAGE and Western blotting. Immunodetection used the same rabbit polyclonal used for immunohistochemistry, diluted 1:200, and goat anti-rabbit horseradish peroxidase-labeled secondary antibody (Jackson ImmunoResearch) with chemiluminescence detection (Pierce ECL, Life Sciences, Grand Island, NY).
Placental expression of APOL1 in mouse tissues was confirmed from whole-tissue RNA extracted using a Qiagen RNeasy micro kit (Germantown, MD), reverse transcribed using AMV RT with oligo-dT primers (Roche Life Sciences), and amplified using PCR primers as previously described.12 Deidentified, discard human placental tissue was kindly provided by Perrie O’Tierney-Ginn. Immunofluorescence staining was performed as described above on formalin-fixed, paraffin-embedded 4-µm thick sections.
Statistical Analyses
Group differences in podocyte density and glomerular volumes were analyzed using ANOVA. Generalized linear mixed models and Markov Chain Monte Carlo samples were used to evaluate differences in autophagosome number, podocyte density, glomerular volume, and corrected podocyte count between groups. Paired and unpaired t tests were used to evaluate differences in BPs within groups and between groups, respectively. Differences in transgenic litter size compared with WT mice were determined by t test followed by Bonferroni correction. P values <0.05 were considered significant. Numbers in each comparison group are provided in figures or in figure legends.
Disclosures
J.R.S. is a member of the advisory boards for Eli Lilly and Company, Indianapolis, IN and Bristol-Myers Squibb, New York, NY. All other authors declare no conflict of interest.
Supplementary Material
Acknowledgments
We thank Ron Conlon and Rachel Mann of the Case Western Reserve University Transgenic Core Facility, Perrie O’Tierney-Ginn for providing human placental tissue and assistance with placental anatomy, Susan Quaggin for providing the vector containing the murine Nphs1 promoter, Roger Wiggins for assistance with quantification of podocyte density, and Jeffrey Schelling and Thomas Hostetter for critical review of the manuscript. L.A.B., J.R.S., and J.F.O. conceived and designed experiments, interpreted data, assembled figures, and wrote the manuscript. L.A.B., Z.W., L.L., S.M.M., M.K., J.R.S., and J.F.O. conducted experiments. P.E.D. performed all statistical analyses, prepared graphs for figures, and edited the final manuscript. L.B. and D.B.T. provided pathological evaluation, scored pathology phenotypes, and edited the final manuscript.
This work was supported by National Institutes of Health grant DK097836 and the Clinical and Translational Science Collaborative of Cleveland supported by the National Center for Advancing Translational Sciences grant UL1TR000439. S.M.M. was supported by training grant DK007470.
This work was presented in preliminary form at the American Society of Nephrology Renal Week, November 5–8, 2015, Philadelphia, PA.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2015111220/-/DCSupplemental.
References
- 1.Genovese G, Friedman DJ, Ross MD, Lecordier L, Uzureau P, Freedman BI, Bowden DW, Langefeld CD, Oleksyk TK, Uscinski Knob AL, Bernhardy AJ, Hicks PJ, Nelson GW, Vanhollebeke B, Winkler CA, Kopp JB, Pays E, Pollak MR: Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science 329: 841–845, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tzur S, Rosset S, Shemer R, Yudkovsky G, Selig S, Tarekegn A, Bekele E, Bradman N, Wasser WG, Behar DM, Skorecki K: Missense mutations in the APOL1 gene are highly associated with end stage kidney disease risk previously attributed to the MYH9 gene. Hum Genet 128: 345–350, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith EE, Malik HS: The apolipoprotein L family of programmed cell death and immunity genes rapidly evolved in primates at discrete sites of host-pathogen interactions. Genome Res 19: 850–858, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thomson R, Genovese G, Canon C, Kovacsics D, Higgins MK, Carrington M, Winkler CA, Kopp J, Rotimi C, Adeyemo A, Doumatey A, Ayodo G, Alper SL, Pollak MR, Friedman DJ, Raper J: Evolution of the primate trypanolytic factor APOL1. Proc Natl Acad Sci U S A 111: E2130–E2139, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vanhamme L, Paturiaux-Hanocq F, Poelvoorde P, Nolan DP, Lins L, Van Den Abbeele J, Pays A, Tebabi P, Van Xong H, Jacquet A, Moguilevsky N, Dieu M, Kane JP, De Baetselier P, Brasseur R, Pays E: Apolipoprotein L-I is the trypanosome lytic factor of human serum. Nature 422: 83–87, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Samanovic M, Molina-Portela MP, Chessler AD, Burleigh BA, Raper J: Trypanosome lytic factor, an antimicrobial high-density lipoprotein, ameliorates Leishmania infection. PLoS Pathog 5: e1000276, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bruggeman LA, O’Toole JF, Ross MD, Madhavan SM, Smurzynski M, Wu K, Bosch RJ, Gupta S, Pollak MR, Sedor JR, Kalayjian RC: Plasma apolipoprotein L1 levels do not correlate with CKD. J Am Soc Nephrol 25: 634–644, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Freedman BI, Julian BA, Pastan SO, Israni AK, Schladt D, Gautreaux MD, Hauptfeld V, Bray RA, Gebel HM, Kirk AD, Gaston RS, Rogers J, Farney AC, Orlando G, Stratta RJ, Mohan S, Ma L, Langefeld CD, Hicks PJ, Palmer ND, Adams PL, Palanisamy A, Reeves-Daniel AM, Divers J: Apolipoprotein L1 gene variants in deceased organ donors are associated with renal allograft failure. Am J Transplant 15: 1615–1622, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee BT, Kumar V, Williams TA, Abdi R, Bernhardy A, Dyer C, Conte S, Genovese G, Ross MD, Friedman DJ, Gaston R, Milford E, Pollak MR, Chandraker A: The APOL1 genotype of African American kidney transplant recipients does not impact 5-year allograft survival. Am J Transplant 12: 1924–1928, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reeves-Daniel AM, DePalma JA, Bleyer AJ, Rocco MV, Murea M, Adams PL, Langefeld CD, Bowden DW, Hicks PJ, Stratta RJ, Lin JJ, Kiger DF, Gautreaux MD, Divers J, Freedman BI: The APOL1 gene and allograft survival after kidney transplantation. Am J Transplant 11: 1025–1030, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma L, Shelness GS, Snipes JA, Murea M, Antinozzi PA, Cheng D, Saleem MA, Satchell SC, Banas B, Mathieson PW, Kretzler M, Hemal AK, Rudel LL, Petrovic S, Weckerle A, Pollak MR, Ross MD, Parks JS, Freedman BI: Localization of APOL1 protein and mRNA in the human kidney: nondiseased tissue, primary cells, and immortalized cell lines. J Am Soc Nephrol 26: 339–348, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Madhavan SM, O’Toole JF, Konieczkowski M, Ganesan S, Bruggeman LA, Sedor JR: APOL1 localization in normal kidney and nondiabetic kidney disease. J Am Soc Nephrol 22: 2119–2128, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kopp JB, Nelson GW, Sampath K, Johnson RC, Genovese G, An P, Friedman D, Briggs W, Dart R, Korbet S, Mokrzycki MH, Kimmel PL, Limou S, Ahuja TS, Berns JS, Fryc J, Simon EE, Smith MC, Trachtman H, Michel DM, Schelling JR, Vlahov D, Pollak M, Winkler CA: APOL1 genetic variants in focal segmental glomerulosclerosis and HIV-associated nephropathy. J Am Soc Nephrol 22: 2129–2137, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Limou S, Dummer PD, Nelson GW, Kopp JB, Winkler CA: APOL1 toxin, innate immunity, and kidney injury. Kidney Int 88: 28–34, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hartleben B, Gödel M, Meyer-Schwesinger C, Liu S, Ulrich T, Köbler S, Wiech T, Grahammer F, Arnold SJ, Lindenmeyer MT, Cohen CD, Pavenstädt H, Kerjaschki D, Mizushima N, Shaw AS, Walz G, Huber TB: Autophagy influences glomerular disease susceptibility and maintains podocyte homeostasis in aging mice. J Clin Invest 120: 1084–1096, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mizushima N, Yamamoto A, Matsui M, Yoshimori T, Ohsumi Y: In vivo analysis of autophagy in response to nutrient starvation using transgenic mice expressing a fluorescent autophagosome marker. Mol Biol Cell 15: 1101–1111, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Venkatareddy M, Wang S, Yang Y, Patel S, Wickman L, Nishizono R, Chowdhury M, Hodgin J, Wiggins PA, Wiggins RC: Estimating podocyte number and density using a single histologic section. J Am Soc Nephrol 25: 1118–1129, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Glassock RJ, Rule AD: The implications of anatomical and functional changes of the aging kidney: with an emphasis on the glomeruli. Kidney Int 82: 270–277, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao S, Gu X, Groome LJ, Wang Y: Decreased nephrin and GLEPP-1, but increased VEGF, Flt-1, and nitrotyrosine, expressions in kidney tissue sections from women with preeclampsia. Reprod Sci 16: 970–979, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beltcheva O, Kontusaari S, Fetissov S, Putaala H, Kilpeläinen P, Hökfelt T, Tryggvason K: Alternatively used promoters and distinct elements direct tissue-specific expression of nephrin. J Am Soc Nephrol 14: 352–358, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Moeller MJ, Kovari IA, Holzman LB: Evaluation of a new tool for exploring podocyte biology: mouse Nphs1 5′ flanking region drives LacZ expression in podocytes. J Am Soc Nephrol 11: 2306–2314, 2000 [DOI] [PubMed] [Google Scholar]
- 22.Putaala H, Sainio K, Sariola H, Tryggvason K: Primary structure of mouse and rat nephrin cDNA and structure and expression of the mouse gene. J Am Soc Nephrol 11: 991–1001, 2000 [DOI] [PubMed] [Google Scholar]
- 23.Putaala H, Soininen R, Kilpeläinen P, Wartiovaara J, Tryggvason K: The murine nephrin gene is specifically expressed in kidney, brain and pancreas: inactivation of the gene leads to massive proteinuria and neonatal death. Hum Mol Genet 10: 1–8, 2001 [DOI] [PubMed] [Google Scholar]
- 24.Kuusniemi AM, Kestilä M, Patrakka J, Lahdenkari AT, Ruotsalainen V, Holmberg C, Karikoski R, Salonen R, Tryggvason K, Jalanko H: Tissue expression of nephrin in human and pig. Pediatr Res 55: 774–781, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Palmén T, Ahola H, Palgi J, Aaltonen P, Luimula P, Wang S, Jaakkola I, Knip M, Otonkoski T, Holthöfer H: Nephrin is expressed in the pancreatic beta cells. Diabetologia 44: 1274–1280, 2001 [DOI] [PubMed] [Google Scholar]
- 26.Freedman BI, Kopp JB, Langefeld CD, Genovese G, Friedman DJ, Nelson GW, Winkler CA, Bowden DW, Pollak MR: The apolipoprotein L1 (APOL1) gene and nondiabetic nephropathy in African Americans. J Am Soc Nephrol 21: 1422–1426, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kriz W, LeHir M: Pathways to nephron loss starting from glomerular diseases-insights from animal models. Kidney Int 67: 404–419, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Kikuchi M, Wickman L, Hodgin JB, Wiggins RC: Podometrics as a Potential Clinical Tool for Glomerular Disease Management. Semin Nephrol 35: 245–255, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wharram BL, Goyal M, Wiggins JE, Sanden SK, Hussain S, Filipiak WE, Saunders TL, Dysko RC, Kohno K, Holzman LB, Wiggins RC: Podocyte depletion causes glomerulosclerosis: diphtheria toxin-induced podocyte depletion in rats expressing human diphtheria toxin receptor transgene. J Am Soc Nephrol 16: 2941–2952, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Hodgin JB, Bitzer M, Wickman L, Afshinnia F, Wang SQ, O’Connor C, Yang Y, Meadowbrooke C, Chowdhury M, Kikuchi M, Wiggins JE, Wiggins RC: Glomerular Aging and Focal Global Glomerulosclerosis: A Podometric Perspective. J Am Soc Nephrol 26: 3162–3178, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim YH, Goyal M, Kurnit D, Wharram B, Wiggins J, Holzman L, Kershaw D, Wiggins R: Podocyte depletion and glomerulosclerosis have a direct relationship in the PAN-treated rat. Kidney Int 60: 957–968, 2001 [DOI] [PubMed] [Google Scholar]
- 32.Hoy WE, Hughson MD, Kopp JB, Mott SA, Bertram JF, Winkler CA: APOL1 Risk Alleles Are Associated with Exaggerated Age-Related Changes in Glomerular Number and Volume in African-American Adults: An Autopsy Study. J Am Soc Nephrol 26: 3179–3189, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oliveira Arcolino F, Tort Piella A, Papadimitriou E, Bussolati B, Antonie DJ, Murray P, van den Heuvel L, Levtchenko E: Human Urine as a Noninvasive Source of Kidney Cells. Stem Cells Int 2015: 362562, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lan X, Jhaveri A, Cheng K, Wen H, Saleem MA, Mathieson PW, Mikulak J, Aviram S, Malhotra A, Skorecki K, Singhal PC: APOL1 risk variants enhance podocyte necrosis through compromising lysosomal membrane permeability. Am J Physiol Renal Physiol 307: F326–F336, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lan X, Wen H, Saleem MA, Mikulak J, Malhotra A, Skorecki K, Singhal PC: Vascular smooth muscle cells contribute to APOL1-induced podocyte injury in HIV milieu. Exp Mol Pathol 98: 491–501, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wan G, Zhaorigetu S, Liu Z, Kaini R, Jiang Z, Hu CA: Apolipoprotein L1, a novel Bcl-2 homology domain 3-only lipid-binding protein, induces autophagic cell death. J Biol Chem 283: 21540–21549, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheng D, Weckerle A, Yu Y, Ma L, Zhu X, Murea M, Freedman BI, Parks JS, Shelness GS: Biogenesis and cytotoxicity of APOL1 renal risk variant proteins in hepatocytes and hepatoma cells. J Lipid Res 56: 1583–1593, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heneghan JF, Vandorpe DH, Shmukler BE, Giovinnazo JA, Raper J, Friedman DJ, Pollak MR, Alper SL: BH3 domain-independent apolipoprotein L1 toxicity rescued by BCL2 prosurvival proteins. Am J Physiol Cell Physiol 309: C332–C347, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nichols B, Jog P, Lee JH, Blackler D, Wilmot M, D’Agati V, Markowitz G, Kopp JB, Alper SL, Pollak MR, Friedman DJ: Innate immunity pathways regulate the nephropathy gene Apolipoprotein L1. Kidney Int 87: 332–342, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khatua AK, Cheatham AM, Kruzel ED, Singhal PC, Skorecki K, Popik W: Exon 4-encoded sequence is a major determinant of cytotoxicity of apolipoprotein L1. Am J Physiol Cell Physiol 309: C22–C37, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Olabisi OA, Zhang JY, VerPlank L, Zahler N, DiBartolo S 3rd, Heneghan JF, Schlöndorff JS, Suh JH, Yan P, Alper SL, Friedman DJ, Pollak MR: APOL1 kidney disease risk variants cause cytotoxicity by depleting cellular potassium and inducing stress-activated protein kinases. Proc Natl Acad Sci U S A 113: 830–837, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dezso Z, Nikolsky Y, Sviridov E, Shi W, Serebriyskaya T, Dosymbekov D, Bugrim A, Rakhmatulin E, Brennan RJ, Guryanov A, Li K, Blake J, Samaha RR, Nikolskaya T: A comprehensive functional analysis of tissue specificity of human gene expression. BMC Biol 6: 49, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.She X, Rohl CA, Castle JC, Kulkarni AV, Johnson JM, Chen R: Definition, conservation and epigenetics of housekeeping and tissue-enriched genes. BMC Genomics 10: 269, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Duchateau PN, Pullinger CR, Cho MH, Eng C, Kane JP: Apolipoprotein L gene family: tissue-specific expression, splicing, promoter regions; discovery of a new gene. J Lipid Res 42: 620–630, 2001 [PubMed] [Google Scholar]
- 45.Page NM, Butlin DJ, Lomthaisong K, Lowry PJ: The human apolipoprotein L gene cluster: identification, classification, and sites of distribution. Genomics 74: 71–78, 2001 [DOI] [PubMed] [Google Scholar]
- 46.Monajemi H, Fontijn RD, Pannekoek H, Horrevoets AJ: The apolipoprotein L gene cluster has emerged recently in evolution and is expressed in human vascular tissue. Genomics 79: 539–546, 2002 [DOI] [PubMed] [Google Scholar]
- 47.Beall MH, Amidi F, Gayle DA, Wang S, Beloosesky R, Ross MG: Placental and fetal membrane Nephrin and Neph1 gene expression: response to inflammation. J Soc Gynecol Investig 12: 298–302, 2005 [DOI] [PubMed] [Google Scholar]
- 48.Li Y, Zheng R, Wang R, Lu X, Zhu C, Lin HY, Wang H, Yu X, Fu J: Involvement of nephrin in human placental trophoblast syncytialization. Reproduction 149: 339–346, 2015 [DOI] [PubMed] [Google Scholar]
- 49.Yun BH, Lee SM, Cho HY, Kim JY, Son GH, Kim YH, Park YW, Lim BJ, Kwon JY: Expression of nephrin in the human placenta and fetal membranes. Mol Med Rep 12: 5116–5120, 2015 [DOI] [PubMed] [Google Scholar]
- 50.Nakimuli A, Chazara O, Byamugisha J, Elliott AM, Kaleebu P, Mirembe F, Moffett A: Pregnancy, parturition and preeclampsia in women of African ancestry. Am J Obstet Gynecol 210: 510–520.e1, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shahul S, Tung A, Minhaj M, Nizamuddin J, Wenger J, Mahmood E, Mueller A, Shaefi S, Scavone B, Kociol RD, Talmor D, Rana S: Racial Disparities in Comorbidities, Complications, and Maternal and Fetal Outcomes in Women With Preeclampsia/eclampsia. Hypertens Pregnancy 34: 506–515, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Elliott SE, Parchim NF, Liu C, Xia Y, Kellems RE, Soffici AR, Daugherty PS: Characterization of antibody specificities associated with preeclampsia. Hypertension 63: 1086–1093, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ruis-González MD, Cañete MD, Gómez-Chaparro JL, Abril N, Cañete R, López-Barea J: Alterations of protein expression in serum of infants with intrauterine growth restriction and different gestational ages. J Proteomics 119: 169–182, 2015 [DOI] [PubMed] [Google Scholar]
- 54.Wen Q, Liu LY, Yang T, Alev C, Wu S, Stevenson DK, Sheng G, Butte AJ, Ling XB: Peptidomic Identification of Serum Peptides Diagnosing Preeclampsia. PLoS One 8: e65571, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sampson MG, Robertson CC, Martini S, Mariani LH, Lemley KV, Gillies CE, Otto EA, Kopp JB, Randolph A, Vega-Warner V, Eichinger F, Nair V, Gipson DS, Cattran DC, Johnstone DB, O'Toole JF, Bagnasco SM, Song PX, Barisoni L, Troost JP, Kretzler M, Sedor JR: Integrative Genomics Identifies Novel Associations with APOL1 Risk Genotypes in Black NEPTUNE Subjects [published online ahead of print July 6, 2015]. J Am Soc Nephrol 10.1681/ASN.2014111131 [DOI] [PMC free article] [PubMed]
- 56.Ng DK, Robertson CC, Woroniecki RP, Limou S, Gilles CE, Reidy KJ, Winkler CA, Hingorani S, Gibson KL, Hjorten R, Sethna CB, Kopp JB, Moxey-Mims M, Furth SL, Warady BA, Kretzler M, Sedor JR, Kaskel FJ, Sampson MG. APOL1-associated glomerular disease among African American children: A collaboration of the Chronic Kidney Disease in Children (CKiD) and Nephrotic Syndrome Study Network (NEPTUNE) cohorts. Nephrol Dialysis Transplant. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Katsi V, Felekos I, Siristatidis C, Kasioni S, Drakontaidis A, Farmakides G, Makris T, Aggeli C, Nihoyannopoulos P, Tousoulis D, Kallikazaros I: Preeclampsia: What Does the Father Have to Do with It? Curr Hypertens Rep 17: 60, 2015 [DOI] [PubMed] [Google Scholar]
- 58.Eremina V, Wong MA, Cui S, Schwartz L, Quaggin SE: Glomerular-specific gene excision in vivo. J Am Soc Nephrol 13: 788–793, 2002 [DOI] [PubMed] [Google Scholar]
- 59.Barisoni L, Nast CC, Jennette JC, Hodgin JB, Herzenberg AM, Lemley KV, Conway CM, Kopp JB, Kretzler M, Lienczewski C, Avila-Casado C, Bagnasco S, Sethi S, Tomaszewski J, Gasim AH, Hewitt SM: Digital pathology evaluation in the multicenter Nephrotic Syndrome Study Network (NEPTUNE). Clin J Am Soc Nephrol 8: 1449–1459, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.