Abstract
The identification of the cellular origins of myofibroblasts has led to the discovery of novel pathways that potentially drive myofibroblast perpetuation in disease. Here, we further investigated the role of innate immune signaling pathways in this process. In mice, renal injury-induced activation of pericytes, which are myofibroblast precursors attached to endothelial cells, led to upregulated expression of TNF receptor superfamily member 12a, also known as fibroblast growth factor-inducible 14 (Fn14), by these cells. In live rat kidney slices, administration of the Fn14 ligand, TNF-related weak inducer of apoptosis (TWEAK), promoted pericyte-dependent vasoconstriction followed by pericyte detachment from capillaries. In vitro, administration of TWEAK activated and differentiated pericytes into cytokine-producing myofibroblasts, and further activated established myofibroblasts in a manner requiring canonical and noncanonical NF-κB signaling pathways. Deficiency of Fn14 protected mouse kidneys from fibrogenesis, inflammation, and associated vascular instability after in vivo injury, and was associated with loss of NF-κB signaling. In a genetic model of spontaneous CKD, therapeutic delivery of anti-TWEAK blocking antibodies attenuated disease progression, preserved organ function, and increased survival. These results identify the TWEAK-Fn14 signaling pathway as an important factor in myofibroblast perpetuation, fibrogenesis, and chronic disease progression.
Keywords: pericyte, myofibroblast, vascular biology, Alport nephropathy, chronic, kidney disease
Fibrosis is a central problem in many forms of chronic disease affecting internal organs including the liver, lung, heart, central nervous system, and kidney. The deposition of pathologic fibrotic material between and within organ units, as well as the cellular activation required for such deposition, have been strongly implicated in the perpetuation and progression of chronic diseases. In the kidney, fibrosis manifests histologically as interstitial fibrosis, glomerulosclerosis, and arteriosclerosis.1 The numerical expansion and activation of myofibroblasts is central to fibrosis. Although a number of cell types have the potential to produce and secrete extracellular matrix (ECM) proteins, including epithelial cells, endothelial cells, and leukocytes, it is myofibroblasts that produce the pathogenic, fibrillar collagenous matrix in vivo, especially collagen types I, III, V, VII, and XI, microfibril collagens including types VI, VIII, and XII, and other matrix proteins including fibrillin, hyaluronan, and fibronectin.2–4 Recently, genetic fate-mapping studies have demonstrated that in virtually all organs, discrete populations of resident mesenchymal cells form the cellular link between initial tissue injury and excessive, pathologic matrix production by migrating from their niche and depositing pathologic matrix as (myo)fibroblasts.2,5–12 In the kidney, many of these mesenchymal cells are closely associated with capillaries, perform vascular functions, have mesenchymal stem cell characteristics, and may be referred to as pericytes or perivascular cells.1,3,9,13,14 In the mouse kidney, many of these perivascular cells lack expression of NG2 but express PDGFRβ, whereas in rat and human kidneys the expression of NG2 is more variable.15–17
The activation of pericytes results in migration from the capillary to the interstitial space. This detachment from the capillary is associated with vascular instability, which may result in pathologic angiogenesis or capillary loss.3,13,18 Studies suggest that vascular instability and pericyte activation are directly linked due the loss of survival signals, quiescence signals, and basement membrane maintenance. Failure to resolve these responses, either as a result of repetitive injury or loss of normal restorative mechanisms, appears to be central to the progression to chronic disease.
Although several secreted factors that contribute to cellular crosstalk and perpetuation of the fibrotic response have been identified, including TGFβ, VEGF, CTGF, WNT ligands, Hedgehog ligands, and PDGFs, additional factors likely play critical roles in fibrogenesis. In particular, myofibroblasts show activation of innate immune signaling pathways, suggesting that additional cytokine/receptor interactions contribute to their appearance and maintenance in a pathologic state.3,4,11,19,20
TNF receptor and TNF superfamily receptor–signaling have been identified as potentially contributing to the pathogenesis of kidney diseases, including diabetic nephropathy, IgA nephropathy, and lupus nephritis.21–25 TNF-related weak inducer of apoptosis (TWEAK) and its receptor fibroblast growth factor-inducible 14 (Fn14) are TNF superfamily (TNFSF) members and have been implicated in models of kidney injury and disease, including models featuring renal fibrosis, particularly where autoimmunity is thought to drive the pathogenesis. Such links with autoimmunity may derive from reports that TWEAK overexpression in vivo leads to the development of autoimmunity. However, the receptor and ligand are upregulated in human chronic fibrosing diseases of the kidney, such as FSGS and diabetic nephropathy, where there is no autoimmunity26,27; yet a role for this receptor and ligand directly in fibrogenesis remains undetermined.28–30 Fn14 signaling via TNF receptor-associated factors has been reported to stabilize NF-κB–inducing kinase (NIK), thereby activating the P52/RelB NF-κB complex and causing downstream activation of NF-κB target genes, which have been suggested to be important factors in human kidney disease.31 Recently, we identified Fn14 (TNFRSF12) as an upregulated receptor on activated fibroblasts precursors, known as pericytes, and their pathologic counterparts in diseased kidney, known as myofibroblasts. Transcripts for Fn14 were found to be enriched in myofibroblasts relative to other cell types in the diseased kidney.3,4,20 Fn14 was reported to contribute to microvascular permeability in cerebral ischemia, as well as pathologic angiogenesis in tumors.32,33 Furthermore, release of TWEAK by increased blood flow in the kidney may be an early feature of the development of kidney disease.34 Since such microvascular problems are recognized to drive kidney disease,14 and since pericytes are directly implicated in the fibrogenic process, we hypothesized that Fn14 may contribute to fibrogenesis by regulating pericyte functions directly. Here, we evaluate the role of Fn14 in the activation of kidney myofibroblast precursors, as well as in the persistence of established myofibroblasts, and determined its contribution to renal fibrogenesis and end organ failure.
Results
Fn14 is Expressed by Pericytes and Myofibroblasts in the Kidney
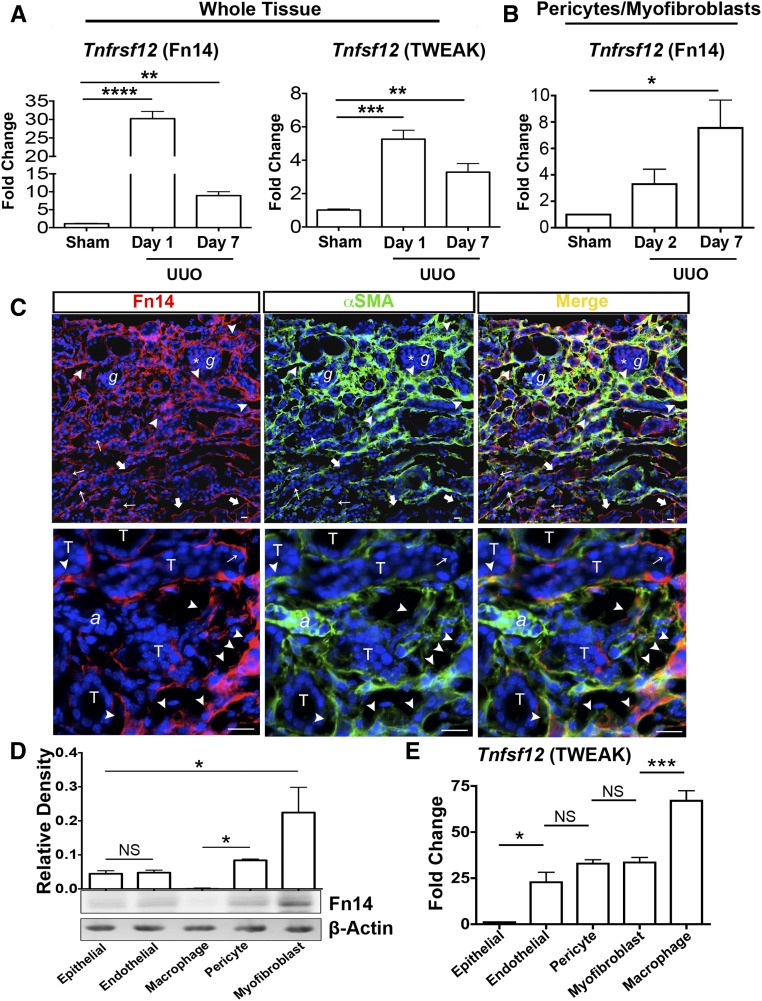
Tnfrsf12 (Fn14) and Tnfsf12 (TWEAK) were highly upregulated in the setting of kidney injury with fibrosis caused by surgical unilateral ureteral obstruction (UUO) (Figure 1, A and B). Flow cytometry-sorted pericytes from normal kidneys, and activated pericytes (which progressively differentiate into myofibroblasts) sorted from diseased kidneys were evaluated for expression of Tnfrsf12 by quantitative PCR (qPCR). Tnfrsf12 was present in healthy pericytes and expression progressively increased with disease (Figure 1B). In keeping with high expression of Fn14 by myofibroblasts, kidney tissue sections from the UUO model were stained with anti-Fn14 antibodies and costained with markers for myofibroblasts. Fn14 was detected at high levels in interstitial myofibroblasts, but was also detected in endothelial cells (Figure 1C). A minority of injured epithelial cells also expressed a low level of Fn14. Notably, only a subpopulation of myofibroblasts appeared to express Fn14 and these were in the areas of greatest myofibroblast accumulation. Fn14 staining in kidneys lacking Fn14 did not detect any specific signal, highlighting the specificity of this method for detecting protein expression (Figure 1C, Supplemental Figure 1). A similar pattern of staining was detected in biopsies from human CKD, where the majority of staining was detected in the interstitium in myofibroblasts and endothelium (Supplemental Figure 2). Fn14 expression was not detected in human glomeruli. Further validation of these observations was made in primary cultures of mouse kidney cells. Pericytes expressed Fn14, seen as a single band by Western blotting. Myofibroblasts, purified from chronically diseased and fibrotic kidneys, expressed the receptor at significantly higher levels. Epithelial and endothelial cells weakly expressed the receptor in vitro. Cultured macrophages did not express the receptor (Figure 1D). A similar pattern of expression was seen in primary cultures of human kidney cells (Supplemental Figure 2). In contrast to this pattern of expression, transcripts for Tnfsf12 were not detected in epithelial cells but were detected at high levels in cultured macrophages (Figure 1E).
Figure 1.
Fn14 is highly expressed by kidney myofibroblasts and TWEAK is highly expressed by macrophages. (A) Quantitative PCR (qPCR) results showing changes in Tnfrsf12 (Fn14) and Tnfsf12 (TWEAK) transcript levels in whole tissue from the UUO model of kidney disease in mice versus sham. (B) qPCR showing changes in Tnfrsf12 transcript levels in FACS-sorted collagen I-producing cells in the UUO model of kidney disease in mice versus sham. (C) Immunofluorescence images showing the localization of Fn14 in the UUO kidney disease model at day 10. Note a predominantly interstitial pattern of expression and predominant colocalization with a subpopulation of myofibroblasts, particularly in areas of intense interstitial cell accumulation (arrowheads), whereas some myofibroblasts do not coexpress Fn14. Weak expression in the mesangium (*) of glomeruli (g) can be seen. Expression of Fn14 on endothelium is also detected (thick arrows) and while almost all tubular epithelium (T) does not express Fn14, a minority of injured cells can be seen to express Fn14 (thin arrows) (a, arteriole). (D) Western blot showing Fn14 protein expression in primary cultures of cells. Quantitation is based on three independent experiments. (E) qPCR showing relative expression of Tnfsf12 transcripts in primary cell cultures. Bar, 25 μm; *P<0.05; **P<0.01; ***P<0.001; n=5/group unless otherwise stated.
Fn14 Receptor Activation Converts Pericytes into Inflammatory Myofibroblasts In Vitro
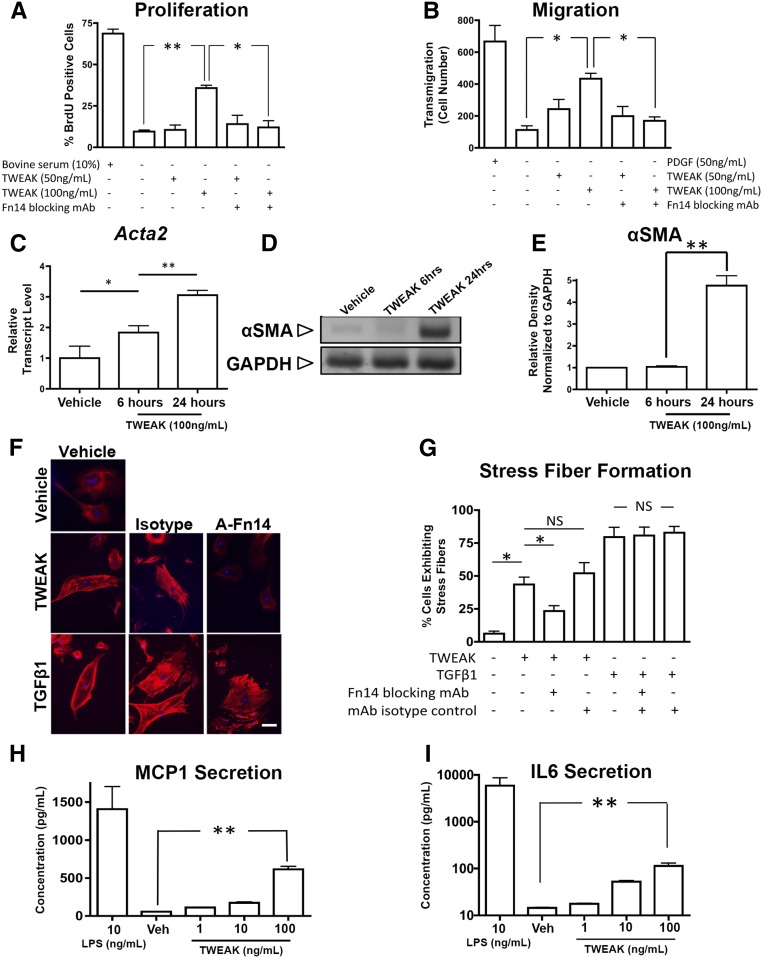
TWEAK induced dose-dependent proliferation of pericytes at higher concentrations, an effect blocked by antibodies against Fn14 (Figure 2A). An important component of myofibroblast function is the acquisition of migratory characteristics. Pericytes were interrogated for their capacity to migrate across 50 μm transwell membranes in response to the chemoattractant PDGF-BB or TWEAK (Figure 2B). At higher concentrations, TWEAK induced migration similar to PDGF. Next, we evaluated the effect of TWEAK on expression of the intermediate filament αSMA, often used as a marker of myofibroblast differentiation and contractile capacity. TWEAK upregulated Acta2 transcripts within 6 hours (Figure 2C) and increased protein synthesis within 24 hours (Figure 2, D and E). Consistent with myofibroblast differentiation, TWEAK stimulated stress fiber formation, as detected by phalloidin staining of cells in culture (Figure 2, F and G). The extent of stress fiber formation was similar to the effect of TGFβ1 stimulation, but was selectively blocked by anti-Fn14 antibodies. Therefore, TWEAK triggers myofibroblast transition in vitro. In addition, TWEAK-activated pericytes released significant amounts of the monocyte chemoattractant MCP1, as well as the inflammatory cytokine IL6 (Figure 2, H and I), though not as robustly as TLR4 stimulation by highly pure LPS.
Figure 2.
Kidney pericyte cultures are activated by TWEAK. (A, B) Graphs showing the (A) proliferative response and (B) migratory response of pericytes across a transwell barrier in response to TWEAK. (C–E) The effect of TWEAK on pericyte expression of Acta2 transcript levels and its protein, the myofibroblast marker αSMA (C) quantitative PCR, (D) Western blot, and (E) normalized densitometry of Western blots. (F) Fluorescence images and (G) quantification of stress fiber formation as detected by phalloidin-Cy3 in pericytes in response (24 hours) to TWEAK or TGFβ1. Note, anti-Fn14 (A-Fn14) blocking antibodies inhibit TWEAK-mediated stress fiber formation, but not TGFβ-mediated stress fibers. (H, I) Graphs showing release of (H) MCP1 or (I) IL6 into the supernatant by primary pericyte cultures. Note, at higher concentrations pericytes release substantial amounts of these cytokines. Bar, 25 μm; *P<0.05; **P<0.01; n=4/group.
Fn14 Activation Results in Sustained Pericyte-Mediated Vasoconstriction followed by Pericyte Detachment from Capillaries
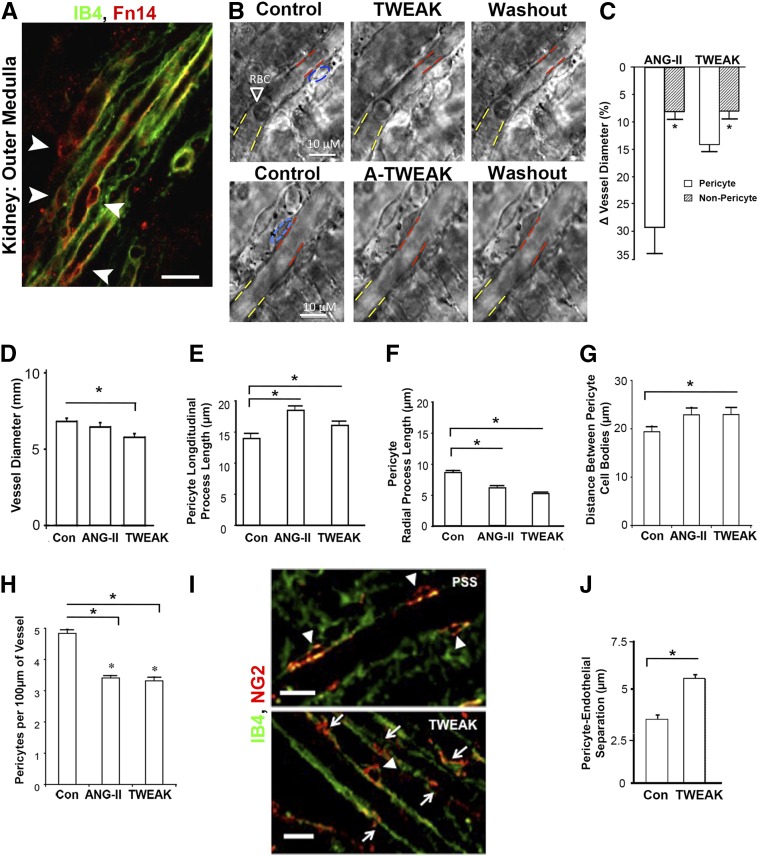
Given the pronounced effects of TWEAK on pericyte biology, we tested the functional consequences of pericyte exposure to TWEAK in situ in the medulla of the kidney. Medullary pericytes regulate blood flow into the medulla of the kidney.35 Live rat kidney slices were initially immunostained for Fn14, which was found to be expressed strongly by medullary pericytes (Figure 3A). We applied TWEAK or Angiotensin II (AngII) to the slice. Within 30 seconds, TWEAK induced pericyte contraction around the capillaries (Figure 3, B and C), which was clearly detectable and was similar to the effect of AngII. The capillary contraction was predominantly at the site of the pericyte cell body. To test whether TWEAK exerted a tonic effect on capillaries, we applied anti-TWEAK antibodies (Figure 3B), but observed no dilation of capillaries, indicating TWEAK does not tonically regulate capillary diameter in healthy conditions. Next, we determined the effect of chronic TWEAK exposure (4 hours) in the live kidney slice assay (Figure 3, D–H). TWEAK stimulated sustained contraction of the capillaries (Figure 3D) and caused a shape change to pericytes, characterized by increased pericyte longitudinal process length and reduced radial process length (Figure 3, E and F). This was associated with increased separation of pericytes and reduced pericyte number attached to capillaries (Figure 3, G and H). The combination of shape change characterized by retraction of radial processes and reduced pericyte density on the capillary suggested pericytes may be detaching in response to sustained TWEAK exposure. Such detachment could be observed over the 4-hour period, by separation of cell processes from the capillary, spreading of cell bodies away from the capillary and by quantifying the separation of pericytes from the inner endothelial surface (Figure 3, I and J). Sustained contraction followed by detachment is indicative of capillary dysfunction that may lead to abnormal salt and water homeostasis, and the subsequent detachment of pericytes is consistent with the early stages of their transition to interstitial myofibroblasts.
Figure 3.
TWEAK stimulates acute microvascular vasoconstriction mediated by pericytes in situ, followed by pericyte detachment. (A) Fluorescence micrograph showing isolectin (IB4) labeled endothelium and immunodetection of Fn14 in rat kidney slice. Note prominent expression of Fn14 by perivascular cells (arrowheads) (bar, 25 µm). (B) Phase contrast images of medullary capillaries (vasa recta) in live kidney slice showing the effect of TWEAK on capillary diameter after 270 seconds of exposure (blue: pericyte body, red: diameter at pericyte body, yellow: diameter at nonpericyte area). Note washout of TWEAK (after 6 minutes) results in relaxation. Application of anti-TWEAK antibodies has no effect on diameter. (C) Quantification of change in capillary diameter 300 seconds after application of AngII or TWEAK in pericyte or nonpericyte areas of the capillary (n=8/group). (D) Graph of capillary diameter after treatment of kidney slice with TWEAK or AngII for 4 hours. (E, F) Graphs showing the length of processes of NG2-labeled pericytes where (E) longitudinal processes increase in length but (F) radial processes retract. (G, H) Morphometric measurements of pericyte density long the vasa recta capillary 4 hours after treatment with TWEAK or AngII compared with control. (I) Fluorescent images of pericytes labeled with NG2 and capillary lumen labeled with IB4. In control settings (PSS), long pericyte processes remain longitudinal with the capillary and associated with cell bodies (shown by arrowheads), whereas after 4 hours of TWEAK, pericyte processes without associated cell bodies are seen (arrows) indicative of separation, and in other instances cell bodies show enlargement and spreading (arrowhead) (bar, 10 µm). (J) Morphometry of pericyte endothelial distance between pericyte basal membrane and capillary wall. *P<0.05; n=12/group. PSS, physiological saline solution; RBC, red blood cell.
TWEAK Enhances Myofibroblast Activation and Increases Inflammatory Cytokine Production
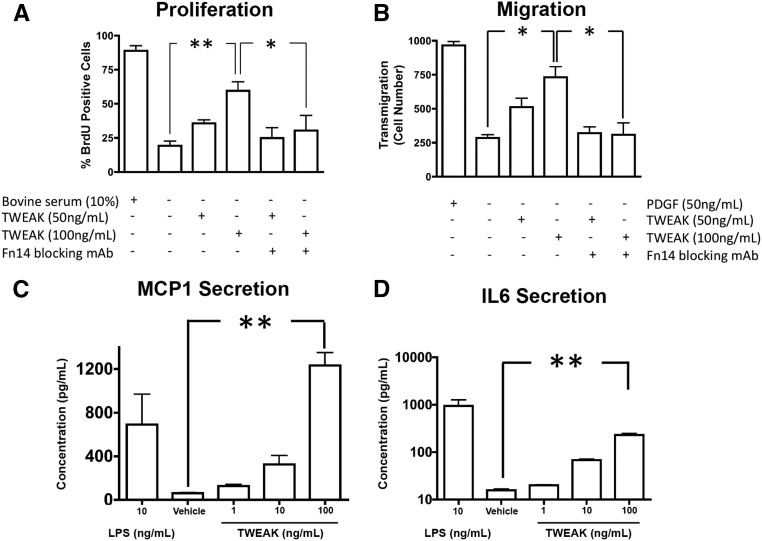
We next studied the effect of TWEAK on established myofibroblasts that are characteristic of chronic disease. Myofibroblasts, purified from kidneys 3 weeks following chronic ischemic injury when severe fibrosis has become established, exhibited enhanced proliferation, migration, and spontaneous cytokine production compared with primary pericyte cultures in identical conditions (Figures 2 and 4). TWEAK exposure enhanced proliferation and migration of myofibroblasts to a similar level as those achieved by serum or PDGF-BB, respectively (Figure 4, A and B). The TWEAK effect was blocked by anti-Fn14 blocking antibodies, confirming TWEAK selectivity for Fn14. In addition to these characteristics, TWEAK enhanced MCP-1 and IL6 release by myofibroblasts to similar levels achieved by LPS (Figure 4, C and D), indicating TWEAK was a potent proinflammatory cytokine for myofibroblasts and that established myofibroblasts had greater sensitivity to Fn14 signaling than pericytes. Similar to pericytes, however, myofibroblasts generated a repertoire of chemokines and cytokines in response to TWEAK (Supplemental Table 1).
Figure 4.
Established kidney myofibroblasts are highly sensitive to TWEAK mediated activation. (A, B) Graphs showing the proliferative response at (A) 16 hours and (B) migratory response (24 hours) across a transwell barrier of myofibroblasts in response to TWEAK. (C, D) Graphs showing release of (C) MCP1 or (D) IL6 into the supernatant by myofibroblast cultures. Note, at higher concentrations myofibroblasts release large amounts of these cytokines. *P<0.05; **P<0.01; n=4/group.
Bioinformatic Analysis Identifies TWEAK Signaling Pathways in Myofibroblasts
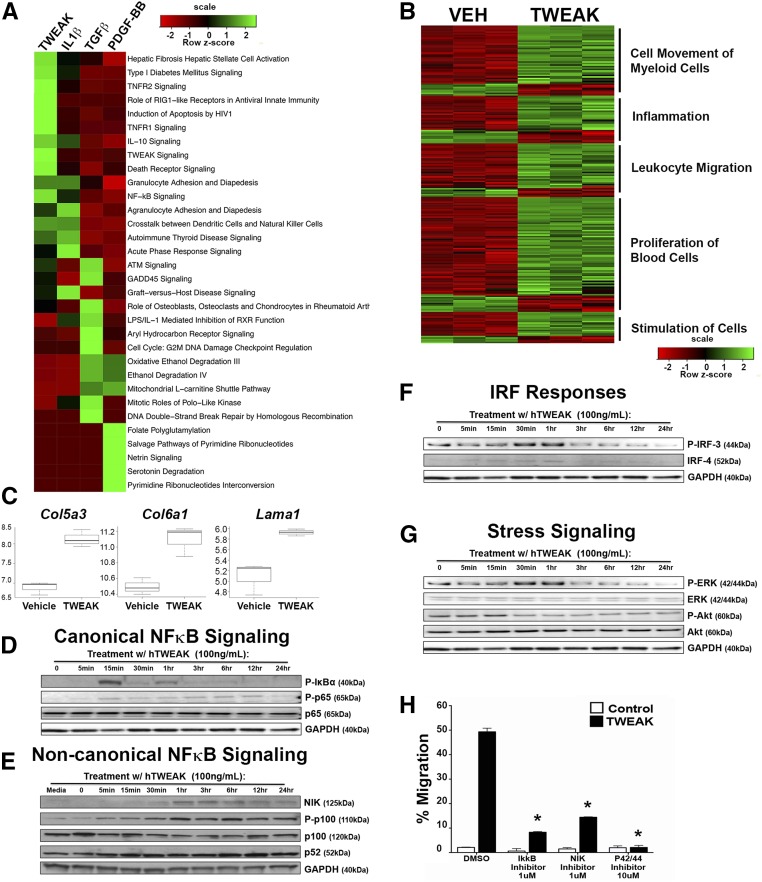
The mechanism by which TWEAK acts on myofibroblasts and the significance in driving fibrogenic responses in myofibroblasts was dissected by comparing transcriptional responses of myofibroblasts to cytokines implicated in fibrogenesis, including TWEAK, using hierarchical clustering analysis. TWEAK prominently activated pathways in myofibroblasts that promote inflammation and fibrosis (Figure 5A, Supplemental Figure 3), including the pathway of hepatic fibrosis mediated by hepatic stellate cells. Notably, TWEAK increases collagen V, collagen VI, laminin, and fibrillin matrix genes in myofibroblasts (Figure 5C, Supplemental Figure 3D, Supplemental Table 1). TWEAK activates intracellular signaling pathways including canonical and noncanonical NF-κB, JNK, AKT, and MAPK, as annotated in type 1 diabetes mellitus signaling, NF-κB signaling, and TNF receptor 2 signaling pathways (Figure 5A). Strikingly, TWEAK promotes greater enrichment of genes involved in fibrotic pathways than other known fibrogenic cytokines, including TGFβ. When the myofibroblast responsive genes were re-evaluated to enrich specifically for TWEAK-responsive genes, additional pathways IFN signaling, MIF regulation of innate immunity, and iNOS signaling were identified, collectively resulting in enhanced biologic functions in myofibroblasts including immune cell movement, cell proliferation, and cell-to-cell signaling (Figure 5B, Supplemental Figure 3A). Genes regulated by TWEAK within the cell movement module were those whereby myofibroblasts promote leukocyte recruitment, including chemokines CXCL-1,-2,-10, and -16, (also known as KC, MIP2, and IP10 respectively) and CCL-2,-7, -9, and -20 (also known as MCP1, MCP3, MIP1, and MIP3A respectively), and cell adhesion molecules (ICAM and VCAM), many of which are implicated in myeloid cell recruitment and inflammation (Figure 5B, Supplemental Figure 3, B–D, Supplemental Table 1). Altered cell movement genes included Rho/RAC family members GRK5, RND1, and ARHGEF6, troponins (Supplemental Figure 3, Supplemental Table 1), and ACTA2, (Figure 2) all of which are involved in contraction, migration, and cell signaling in response to changes in the stiffness of the ECM microenvironment. The cell proliferation module regulated by TWEAK includes cell cycle regulators, such as cyclin D1, as well as cytokines such as PDGF-B and CSF-1, whereby myofibroblasts can exert autocrine and paracrine effects on stromal cells and macrophages. The cell-to-cell signaling module includes the most highly upregulated gene, the IL33 receptor, known as IL1LR1 (ST2), as well as IL-1R1 and TNF receptor-2, which may serve to amplify innate immune signaling (Supplemental Figure 3, B–D, Supplemental Table 1). In addition, canonical and noncanonical NF-κB transcriptional regulators NFKB2 (P100), NFKBIA (IκBα), NFKBIE, and RELB are upregulated. JUNB, NRF3, and FOXS1, regulators of inflammatory signaling pathways, such as AKT, are enhanced (Figure 5A, Supplemental Figure 3A, Supplemental Table 1). Also notable is the upregulation of multiple vascular permeability regulators, including S1PR3, S1PR2, VCAM, ICAM1, ECSCR, VEGF-C, VEGFR1, and RGS5 (Supplemental Figure 3, B–D, Supplemental Table 1). Since the promotion of vascular permeability, reflecting the destabilization of vessels, is associated with pathologic fibrosis when chronically maintained, these findings further implicate TWEAK in the process of vascular instability.
Figure 5.
Transcriptional analysis of cytokine-stimulated myofibroblasts identifies TWEAK-induced fibrogenic signaling via canonical and noncanonical NF-κB, IRF, and ERK. (A) Ingenuity IPA software was used to identify pathways that are enriched in myofibroblasts treated with TWEAK, IL1β, TGFβ, or PDGF-BB compared with vehicle after 16 hours. The top ten pathways for each cytokine based on P values were pooled, and the heatmap shows z-scores for these pathways across all stimuli. (B) The heatmap shows the intensity of genes differentially expressed with TWEAK treatment at 16 hours for each of the biologic processes shown. Vehicle and TWEAK treatments were performed in triplicate. (C) Normalized gene expression for matrix proteins upregulated by TWEAK treatment (log base 2). (D) Blots showing canonical NF-κB activation by the increase in P-IκBα, P-p65 (RELA) with time in myofibroblasts in response to TWEAK. (E) Blots showing noncanonical NF-κB activation by accumulation of NIK and activation of P-p100 to generate the cleaved and active p52 subunit. (F) Blots showing the effect of TWEAK on active P-IRF-3 accumulation and IRF-4 accumulation. (G) The effect of TWEAK on active P-ERK and P-AKT signaling adaptors. (H) The effect of canonical NF-κB inhibitor (IκKβ inhibitor), noncanonical NF-κB inhibitor (NIK inhibitor), and ERK inhibitor on TWEAK stimulated myofibroblast migration. *P<0.05.
Consistent with the bioinformatic analysis, TWEAK-treated myofibroblasts phosphorylated IκBα and p65, indicative of canonical NF-κB activation (Figure 5D). The noncanonical pathway was also activated, with kinetics slower and more sustained than for other downstream signaling pathways, with evidence of NIK accumulation, increased phosphorylation of p100, and p100 cleavage to yield the p52 subunit (Figure 5E). As suggested by the bioinformatic analysis, TWEAK activates IFN regulatory factors (IRF), which may be responsible for IFN-β production and MAPK activation (Figure 5F). Also, TWEAK activates the ERK signaling pathway, whereas AKT signaling, constitutively active in myofibroblasts, is downregulated by TWEAK (Figure 5G). To evaluate the importance of these regulated pathways in myofibroblast responses, canonical NF-κB signaling was blocked with the IκKβ inhibitor, ACHP, resulting in an 80% reduction in migration. A NIK inhibitor, which blocks noncanonical pathway signaling, similarly inhibited migration, as did an inhibitor of ERK phosphorylation (Figure 5H). Overall, these results are consistent with the in vitro functional studies reported above, whereby TWEAK promotes more pathologic myofibroblast characteristics.
Fn14 Deficiency Attenuates Fibrogenesis and Vascular and Tubular Injury in the UUO Model of Kidney Fibrosis
The studies above, strongly implicate Fn14 as an important receptor in vascular reactivity, in differentiation of perivascular cells to myofibroblasts, myofibroblast persistence, and leukocyte recruitment to tissues. To test the contribution of Fn14 and TWEAK in vivo, we studied fibrogenesis in the UUO model, a robust model for the study of early fibrogenesis. Mice deficient in Fn14 (Tnfrsf12−/−) (Supplemental Figure 1) were subjected to surgical UUO, compared with wild-type littermates, and evaluated at days 5, 7, and 10.
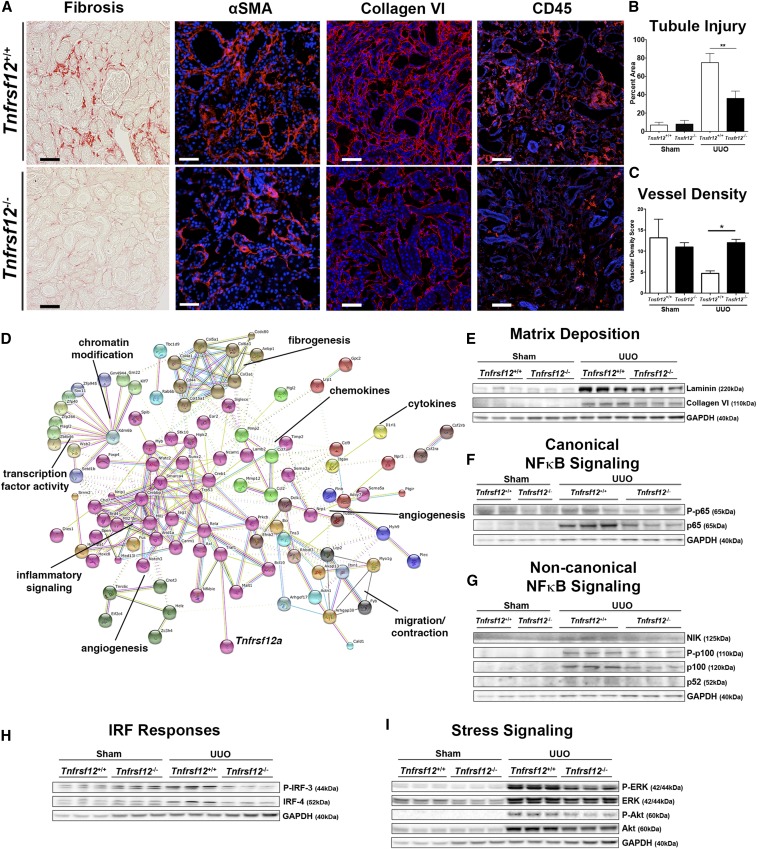
Tnfrsf12−/− mice have normal appearing and functioning kidneys. At day 10 of disease, there was substantially less fibrosis in the kidney tissue of Tnfrsf12−/− mice compared with littermate controls (Figure 6A, Supplemental Figure 4). The appearance of αSMA+ cells was markedly reduced, as was the recruitment of leukocytes (Figure 6A, Supplemental Figure 4). Consistent with reduced activation of myofibroblasts and reduced leukocyte infiltration, the tubular epithelial cells of the nephron were less injured and there was significant preservation of the microvasculature (Figure 6, B and C). Whole tissue transcriptional analysis was performed in healthy kidneys and diseased kidneys at day 10. A total of 977 probe sets were differentially expressed between Tnfrsf12−/− and littermate controls, with the majority (74%, n=720) being more highly expressed in Tnfrsf12−/− mice. Reduced expression genes were analyzed by KEGG annotated ontology pathways (Supplemental Figure 4E, Supplemental Table 2). The most prominent pathways included amoebiasis, which encapsulates cell movement, followed by pathways involving focal adhesion, ECM deposition, leukocyte transendothelial migration, chemokine signaling, and NF-κB signaling annotated in B cell signaling. In addition, enrichment for genes in Notch and VEGF signaling pathways implicated in angiogenesis was reduced through absence of Fn14. The proteins regulated by Fn14 signaling were evaluated by String protein-protein interaction analysis to identify nodes or modules (Figure 6D).36 Strikingly, eight distinct nodes emerged controlling inflammatory signaling, cytokine and chemokine generation, angiogenesis, contraction, fibrogenesis, and chromatin modification. Prominent among matrix genes regulated by Fn14 in vivo were collagen VI and collagen V, as had been identified in the myofibroblast cultures (Figure 5). In fact, many of the signaling nodes identified in the myofibroblast and pericyte cultures overlapped with the whole tissue analysis, pointing to a key role for Fn14 in regulating myofibroblast behavior in vivo. Consistent with these findings, collagen VI and laminin were deposited in response to kidney injury, and the increase in these proteins was almost completely abated by the absence of Tnfrsf12 (Figure 6, A and E, Supplemental Figure 4).
Figure 6.
Fn14 deficiency (Tnfrsf12−/−) reduces fibrogenesis in the UUO model of kidney disease. (A) Photomicrographic images showing the extent of fibrosis (day 10), αSMA+ myofibroblasts (day 7), collagen type VI (day 7), and CD45+ leukocytes (day 7) in the UUO model. (B, C) Graphs quantifying tubular injury and vascular density. (D) String analysis showing known and predicted protein-protein interactions of 200 genes most enriched as a result of loss of Fn14 in the diseased kidney. Different types of evidence denoted by the color of the connecting lines, where green is neighborhood, red is gene fusion, blue is co-occurrence, black is coexpression, purple is experiments, cyan is databases, yellow is text mining, and lilac is homology. Proteins are colored according to nodes of interaction. (E) Blots showing the effect of Fn14 deficiency on matrix protein accumulation in whole tissue. (F, G) Blots showing the effect of disease and Fn14 deficiency on activation of factors in the (F) canonical and (G) noncanonical NF-κB signaling pathways. (H) Blots showing the effect of disease and Fn14 deficiency on activation of P-IRF-3 and IRF-4. (I) Blots showing the effect of Fn14 deficiency on active P-ERK and P-AKT. *P<0.05; **P<0.01; n=7/group; bar, 50 μm.
Next, we dissected the signaling pathways downstream of Fn14 that we had identified in myofibroblasts (Figure 5) and found significant reductions in canonical NF-κB component p65 and noncanonical NF-κB pathway activation, indicated by reduced NIK and P-p100 levels in the Tnfrsf12-deficient kidneys. In addition, Tnfrsf12 deficiency led to reduced P-IRF-3 and IRF-4 signaling (Figure 6, F–I). The reduction of P65 activity correlated closely with total levels of P65, whereas the reduction in P52 activity was directly regulated by upstream accumulation of NIK, suggesting that loss of Fn14 results primarily in the reduction of noncanonical pathway activation in vivo. In addition, the ERK, MAPK, and AKT signaling pathways were also less activated when Tnfrsf12 was absent.
Anti-TWEAK Blocking Antibodies Slow Progression of Chronic Fibrotic Disease of the Kidney and Enhance Survival
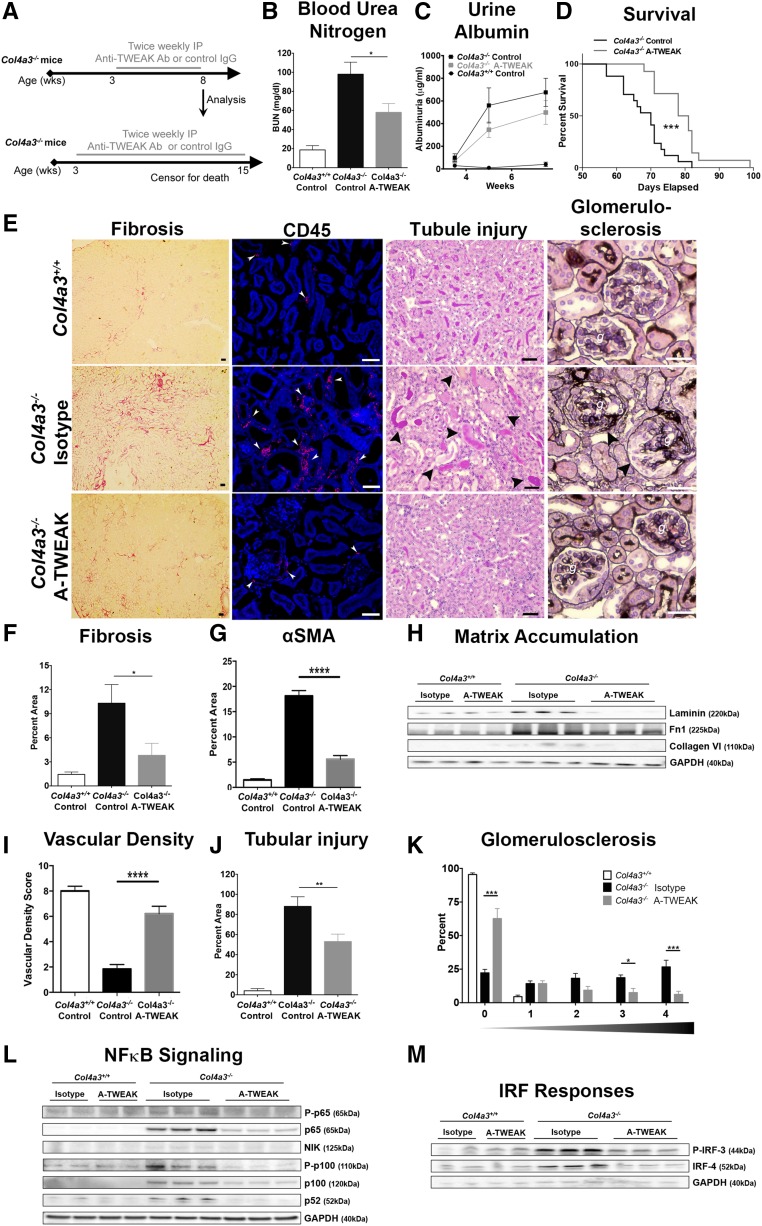
The studies above provided compelling evidence that Fn14 signaling and its downstream pathways might be important drivers of chronic disease progression in the kidney through actions on myofibroblasts, potentially mediated by the secretion of TWEAK from recruited macrophages. To evaluate the importance of this pathway in chronic disease triggered by microvascular injury, we delivered anti-TWEAK antibodies that block its interaction with Fn1437 therapeutically to mice with a mutation in the capillary basement membrane gene, Col4a3, which causes microvascular instability in the kidney, leading to chronic fibrotic kidney disease analogous to human Alport nephropathy (Figure 7).38 Col4a3−/− mice on the 129sv background develop albuminuria by 3 weeks and develop progressive glomerulosclerosis, tubulointerstitial kidney disease, and loss of organ function, followed by death from kidney failure by 60–80 days, with a median survival of 70 days. Mice received either an isotype-matched control Ig or anti-TWEAK antibody (10 mg/kg) twice weekly by intraperitoneal (i.p.) injection from 24 days onward, once albuminuria had developed (Figure 7A). In one cohort, kidneys were analyzed at 56 days to assess kidney function and pathology. Anti-TWEAK antibody treatment reduced levels of BUN, a marker of kidney function, by approximately 50%, and attenuated albumin leak, another marker of kidney function (Figure 7, B and C). A second cohort of mice (n=14/group) was treated from 24 days onward with anti-TWEAK antibody (10 mg/kg i.p. injection twice a week) to evaluate the effect on longevity. At this concentration, anti-TWEAK antibodies specifically enhanced survival, with a median improvement of 9.5 days (equivalent to a 13.5% increase in survival) (Figure 7D). Standard histologic evaluation revealed a marked reduction in interstitial fibrosis and interstitial myofibroblasts (Figure 7, E–G), as well as a substantial reduction in matrix proteins, including collagen VI and laminin (Figure 7H). Such a reduction in myofibroblasts was closely associated with a reduction in leukocytes (Figure 7E, Supplemental Figure 5), preservation of the microvasculature (Figure 7I), and a reduction in injury to the epithelium (Figure 7J). Moreover, the loss of glomerular capillaries and glomerular scarring that occurs with this disease was also attenuated by anti-TWEAK antibodies (Figure 7, I–K, Supplemental Figure 5). The major signaling pathways blocked by chronic anti-TWEAK antibody treatment were assessed. Marked reduction in both canonical and noncanonical NF-κB signaling was detected, with the most striking changes in the noncanonical pathway (Figure 7L). In addition, IRF signaling was attenuated (Figure 7M), whereas ERK and AKT activation were not reduced in this chronic disease model (Supplemental Figure 5).
Figure 7.
Anti-TWEAK antibodies block fibrogenesis and the progression of CKD in Col4a3−/− mice. (A) Schema showing the experimental approach. (B) BUN levels at 8 weeks showing the effect of anti-TWEAK in Col4a3−/− mice. (C) Time course of albuminuria in Col4a3−/− mice (D) Kaplein–Meier survival curve showing the effect of anti-TWEAK in Col4a3−/− mice. (E) Photomicrographs showing the extent of interstitial fibrosis (Sirius red stain), CD45+ leukocytes, tubular epithelial injury (periodic acid–Schiff stain), and glomerular scarring (silver stain) at 8 weeks of age in Col4a3−/− kidneys. (F–H) Quantification of Sirius red+ fibrosis, αSMA+ myofibroblasts, and matrix proteins. (I, J) Quantification of vascular density and tubular epithelial injury. (K) Glomerulosclerosis scores showing percentage of glomeruli with each of the disease scores (0=no sclerosis, 1=1%–25% sclerosis, 2=25%–50% sclerosis 3=50%–75% sclerosis, 4=75%–100% sclerosis). (L) Representative blots showing the effect of anti-TWEAK antibodies on canonical and noncanonical NF-κB signaling factors. (M) Representative blots showing the effect of anti-TWEAK antibodies on P-IRF-3 and IRF-4. *P<0.05; ** P<0.01; n=14/group; bar, 50 μm.
Discussion
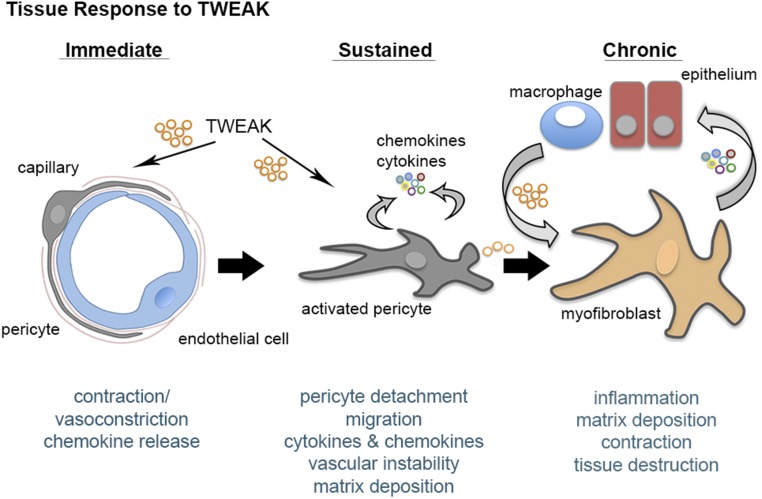
These results presented here identify a novel role for the TNF superfamily receptor signaling pathway, TWEAK-Fn14, as an important inflammatory signaling cascade that regulates pericyte and myofibroblast biology, and as a consequence, microvascular responses. In acute settings, TWEAK triggers sustained contraction of kidney capillaries, whose flow is critical to kidney function only in areas of pericyte coverage. Sustained microvascular contraction gives way to pericyte detachment, followed by differentiation to myofibroblasts. TWEAK is not only sufficient to cause pericyte differentiation to myofibroblasts, but also causes inflammatory cytokine and chemokine release by pericytes that causes leukocyte recruitment. TWEAK strongly activates established myofibroblasts, which derive from perivascular cells1,9,10,20 (Figure 8). Importantly, comparative studies showed that TWEAK also activates fibrogenic and inflammatory signaling in established myofibroblasts more strongly than other well established fibrogenic cytokines. Moreover, TWEAK stimulates myofibroblast proliferation, suggesting that in chronic disease, TNF superfamily signaling may be important in sustaining pathologic tissue responses. Although TWEAK is found in several cell types, macrophages are shown here to produce high levels; therefore, TWEAK may be one of the paracrine factors used by macrophages to promote fibrogenic responses (Figure 8). The studies also demonstrate TWEAK or its receptor Fn14 to be potential targets in the treatment of chronic fibrosing diseases, such as those initiated by mutations in the basement membrane proteins, including Alport nephropathy, thereby identifying TWEAK and Fn14 as important amplifiers of the chronic fibrogenic process. Evidence of activation of the pathway in chronic human fibrosing diseases, including FSGS and diabetic kidney disease, point to a possible role of this pathway in driving many chronic fibrosing diseases.26,27 In addition, the findings here further support the notion that the blockade of myofibroblast activation can attenuate tissue injury, as well as the deposition of ECM, and therefore improve tissue function.
Figure 8.
Tweak triggers vasoconstriction, pericyte activation and myofibroblast persistence in the kidney. Schema summarizing the effect of TWEAK on the fibroblast lineage in kidney disease.
Factors that drive fibrogenic signaling in vivo include several developmental morphogenic receptor-signaling pathways, including TGFβ, WNT, Hedgehog, PDGF, and VEGF. However, there has been relatively little attention given to innate immune inflammatory signaling pathways directly in the fibrogenic process. The findings reported here suggest TNF superfamily member TWEAK, signaling through Fn14, may be a critical fibrogenic signaling pathway. It is interesting to note that pirfenidone, a small molecule recently shown to be effective in treating human lung fibrosis, as well as kidney fibrosis,39,40 has significant impact in the generation of inflammatory cytokines, including the TNF family,41 but its precise mechanism of action is poorly understood. Unlike TNFα signaling, Fn14 is known to activate both canonical and noncanonical NF-κB signaling pathways in cells, but does not activate a cell death pathway. We show here that in established myofibroblasts, Fn14 activation does signal through both NF-κB pathways, as well as ERK, but that inhibition of the noncanonical pathway alone is sufficient to attenuate downstream effects of TWEAK in myofibroblasts, implicating noncanonical signaling as well as canonical signaling in fibrogenesis. In the disease models, it was the noncanonical pathway that was most suppressed by loss of Fn14. Fn14 activation in myofibroblasts triggers an IFN response, particularly activating IRF-3 and IRF-4, both of which have recently been implicated in the fibrogenic process in other tissues.42,43 These findings suggest that myofibroblasts may be an important, unappreciated source of IFNβ. Whereas these IFN signaling pathways have been shown to play key roles in immune cell response to infections, their role in pathologic fibrogenic responses to tissue injury has not been explored. One of the conundrums in fibrogenesis is that the process is widely considered an aberrant wound healing response. Whereas many signaling pathways that coordinately regulate tissue genesis or regeneration become activated in pathologic disease, the reason they are pathologic is unclear. One explanation for this is the requirement for the coactivation of pathways associated with innate immune responses in stromal cells as a prerequisite for pathologic responses to tissue injury.
The bioinformatic studies highlight several important aspects of Fn14 signaling in myofibroblasts that are instructive. Firstly, Fn14 directly promotes matrix synthesis, but the major collagens promoted by Fn14 are collagens V and VI, as well as laminin and fibrillin. In vivo, fibrillar collagens I and III are also reduced by absence of Fn14. Recent evidence indicates that many organs, including the kidney, generate significant levels of fibrillar collagens when healthy, yet these are not assembled into a complex matrix but are degraded. In fact, chronic disease is a state of low matrix turnover.44 It is striking, therefore, that collagens V and VI, and microfibrillar collagens are highly regulated in myofibroblasts by Fn14 signaling. Collagen VI has been implicated in liver and cardiac fibrosis, and recent human studies of CKD identify collagens V and VI as important discriminators of pathologic fibrosis.45,46 Collagen V is important in assembly of collagens I and III, and collagen VI is widely believed to be important in organizing fibrils with proteoglycans and anchoring them to basement membranes in collagens I and III47,48. One implication from these observations is that by regulating collagens V and VI, the deposition of stable crosslinked fibrillar matrix can be controlled.
In addition to an important role in matrix production and stabilization, these new studies highlight the high level of expression of Fn14 in activated pericytes and their descendants, myofibroblasts. Combined with significant endothelial expression, the receptor is therefore implicated in vascular responsiveness. In acute settings, TWEAK behaves similarly to AngII, having the capacity to vasoconstrict kidney capillaries. Therefore, TWEAK may regulate BP and sodium balance in the setting of tissue injury. Furthermore, pericyte detachment from capillaries, stimulated by TWEAK, is believed to contribute to vascular instability and eventual capillary rarefaction, thereby further promoting fibrogenesis through hypoxia-induced signals. In addition, TWEAK stimulates myofibroblasts to produce a number of factors that may adversely regulate vascular homeostasis, including S1P receptors and VEGF-C.49
The downstream signaling pathways activated by Fn14 in pericytes and myofibroblasts are identified in these studies. In addition to noncanonical and canonical NF-κB signaling, TWEAK activates ERK and IRF signaling, both of which may play roles in the fibrogenic process. NF-κB signaling is activated in a wide range of settings, particularly in response to infection. However, we increasingly recognize chronic diseases as states of inflammation, and NF-κB activation has been reported in these settings. The role of noncanonical NF-κB has been thought to be restricted to regulation by receptors such as CD40, BAFF, APRIL, LTβR, RANK, and Fn14. Several of these receptors have been implicated in fibrosis, and all these receptors have the capacity to induce canonical as well as noncanonical NF-κB signaling. But, the relative contribution of canonical versus noncanonical NF-κB in myofibroblasts from TNFSF receptors has not been determined previously. We report distinct differences between IL1β-mediated signaling in myofibroblasts, which activates P65 predominantly, compared with TWEAK. TWEAK promotes a stronger tendency toward noncanonical NF-κB signaling and stimulates chemokine production and genes associated with contraction/migration. Therefore, the noncanonical NF-κB pathway appears to be an important new signaling pathway in myofibroblasts that may contribute to their pathologic functions.
Blocking antibodies against TWEAK or Fn14 deficiency are protective in a rodent model of autoimmunity with features of lupus nephritis25; a progressive kidney disease characterized by autoimmune B cell activation, immune complex deposition, as well as leukocyte recruitment in the kidney, where immune complex deposition is thought to play a major role in the early pathogenesis. However, lupus nephritis progresses as a microvascular fibrosing kidney disease, similar to many other forms of CKD. Our studies show that anti-TWEAK antibodies not only inhibit fibrosis in an acute model of kidney injury, but also protect against the progression of a CKD triggered by a mutation in a kidney capillary basement membrane protein, Col4a3. This disease model closely replicates human Alport syndrome and has close similarities to a number of other human CKDs characterized by inflammation and fibrosis. Therefore, our studies suggest anti-TWEAK antibodies may be effective at retarding other forms of CKD, including Alport nephropathy, IgA nephropathy, and other forms of ischemic CKD, by directly targeting the fibrogenic process.
Concise Methods
Animals, Human Tissue, and Models of Kidney Disease
C57BL/6J mice were purchased from Jackson ImmunoResearch Laboratories (West Grove, PA). Col1a1-GFPTg mice on the C57BL6 background have been previously described.2 Tnfrsf12−/− (also known as Fn14−/−) mice on the C57BL6 background have been previously described and genotyping was performed as reported.50 Littermate control colonies were maintained as controls. Col4a3+/− mice were purchased from Jackson ImmunoResearch Laboratories (129-Col4a3tm1Dec/J) and are on the 129 background. Mice were bred as heterozygotes and genotyping was performed at 3 weeks as described.51 Col4a3−/− mice were weighed at 3 weeks and randomized in a sex-matched manner to vehicle or specific antibody. Anti-TWEAK antibodies or isotype-matched control Ig (10 mg/kg) were subcutaneously administered in 200 μl of saline twice weekly until week 8 or until week 12. Mice (n=12/group) were euthanized at week 8.5 for analysis of blood, urine, and tissue, or studied for survival (n=14/group). Timed urine collections were performed at weeks 4, 6, and 8. Adult (12–16 week old) mice (C57BL/6 littermate controls or Tnfrsf12−/−) were anesthetized with ketamine/xylazine (100/10 mg/kg, i.p. injection) before surgery. UUO or sham surgery was performed as previously described2 and kidneys were collected on days 0, 2, 5, 7, and 10. In other experiments, Col1a1-GFPTg male mice were anesthetized and unilateral ischemia reperfusion injury was performed by clamping the left renal artery for 30 minutes, as described.52 All animal studies were performed under protocols approved by Department of Comparative Medicine, University of Washington (Protocol 4244–01) or the Institutional Animal Care and Use Committee at Biogen (Protocol 0489–2013). Human kidney tissue was obtained from discarded kidneys during nephrectomy procedures performed at Tufts New England Medical Center under a research agreement. All patients consented for use of discarded tissue for research purposes.
Live Kidney Slice Functional Experiments
Live kidney slices prepared as described.53 Due to technical limitations, the studies were performed using rat kidneys. Pericytes on the vasa recta capillaries were identified by their previously established ‘bump-on-a-log’ morphology, and differential interference contrast images were captured through an Olympus 60× water immersion objective (0.9 NA). Real-time video images of changes in vasa recta diameter were collected every second by an attached Rolera XR CCD camera and recorded using Image Pro Software (Media Cybernetics Inc., Buckinghamshire, UK). Live kidney slices were superfused with AngII (100 nM), TWEAK (100 ng/ml), and anti-TWEAK (10 μg/ml). Time-series analysis of live kidney slice experiments was carried out using the public domain software ImageJ (National Institutes of Health, http://rsb.info.nih.gov.ij). For each experiment, vessel diameter was measured at both a pericyte site and a nonpericyte site as identified on a single vasa recta. All diameter measurements were calculated and expressed as a percentage of the corresponding baseline value for both pericyte and nonpericyte sites.
Monoclonal Antibodies
Anti-TWEAK monoclonal antibody, mP2D10, with a mouse IgG2a Fc region, neutralizes the activity of TWEAK, and was generated as previously described.37 A mouse IgG2a antibody specific for hen egg lysozyme (Biogen) was employed as the isotype-matched control Ig. Anti-Fn14 mAb, mP4A8, blocks TWEAK-induced Fn14 signaling and was generated as described.50
Disclosures
Employees of Biogen, Cambridge, MA (I.G.G., A.M.R., A.A., B.G.J., G.K., T.S.Z., S.S., L.C.B., J.S.D.) have stock in the company.
Supplementary Material
Acknowledgments
We are grateful to Nicol Hutchison (University of Washington) for assisting with experiments, to Bill Stallcup for generating anti-PDGFRβ antibodies, to the Lynne and Mike Garvey Microscopy Suite at University of Washington, to Catherine Quigley and Michelle Ols (Biogen) for assistance with experiments, and to G. Mabbutt, K. Saromi, and Carol Crawford (University of Kent) for assistance with live kidney slice experiments and confocal imaging of fixed tissue.
These studies were funded by Biogen, National Institutes of Health grants (DK087389, DK093493, DK094768, HL122895, and TR000504), an American Heart Association grant 12040023 (to J.S.D.), and a Core Fulbright Visiting Scholarship (to Nicol Hutchison).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Beyond EMT: Epithelial STAT3 as a Central Regulator of Fibrogenesis,” on pages 3502–3504.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2015111227/-/DCSupplemental.
References
- 1.Duffield JS: Cellular and molecular mechanisms in kidney fibrosis. J Clin Invest 124: 2299–2306, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lin SL, Kisseleva T, Brenner DA, Duffield JS: Pericytes and perivascular fibroblasts are the primary source of collagen-producing cells in obstructive fibrosis of the kidney. Am J Pathol 173: 1617–1627, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schrimpf C, Xin C, Campanholle G, Gill SE, Stallcup W, Lin SL, Davis GE, Gharib SA, Humphreys BD, Duffield JS: Pericyte TIMP3 and ADAMTS1 modulate vascular stability after kidney injury. J Am Soc Nephrol 23: 868–883, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grgic I, Krautzberger AM, Hofmeister A, Lalli M, DiRocco DP, Fleig SV, Liu J, Duffield JS, McMahon AP, Aronow B, Humphreys BD: Translational profiles of medullary myofibroblasts during kidney fibrosis. J Am Soc Nephrol 25: 1979–1990, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lemos DR, Babaeijandaghi F, Low M, Chang CK, Lee ST, Fiore D, Zhang RH, Natarajan A, Nedospasov SA, Rossi FM: Nilotinib reduces muscle fibrosis in chronic muscle injury by promoting TNF-mediated apoptosis of fibro/adipogenic progenitors. Nat Med 21: 786–794, 2015 [DOI] [PubMed] [Google Scholar]
- 6.Dulauroy S, Di Carlo SE, Langa F, Eberl G, Peduto L: Lineage tracing and genetic ablation of ADAM12(+) perivascular cells identify a major source of profibrotic cells during acute tissue injury. Nat Med 18: 1262–1270, 2012 [DOI] [PubMed] [Google Scholar]
- 7.Kisseleva T, Cong M, Paik Y, Scholten D, Jiang C, Benner C, Iwaisako K, Moore-Morris T, Scott B, Tsukamoto H, Evans SM, Dillmann W, Glass CK, Brenner DA: Myofibroblasts revert to an inactive phenotype during regression of liver fibrosis. Proc Natl Acad Sci U S A 109: 9448–9453, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Puche JE, Lee YA, Jiao J, Aloman C, Fiel MI, Muñoz U, Kraus T, Lee T, Yee HF Jr, Friedman SL: A novel murine model to deplete hepatic stellate cells uncovers their role in amplifying liver damage in mice. Hepatology 57: 339–350, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kramann R, Schneider RK, DiRocco DP, Machado F, Fleig S, Bondzie PA, Henderson JM, Ebert BL, Humphreys BD: Perivascular Gli1+ progenitors are key contributors to injury-induced organ fibrosis. Cell Stem Cell 16: 51–66, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Humphreys BD, Lin SL, Kobayashi A, Hudson TE, Nowlin BT, Bonventre JV, Valerius MT, McMahon AP, Duffield JS: Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am J Pathol 176: 85–97, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hung C, Linn G, Chow YH, Kobayashi A, Mittelsteadt K, Altemeier WA, Gharib SA, Schnapp LM, Duffield JS: Role of lung pericytes and resident fibroblasts in the pathogenesis of pulmonary fibrosis. Am J Respir Crit Care Med 188: 820–830, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Göritz C, Dias DO, Tomilin N, Barbacid M, Shupliakov O, Frisén J: A pericyte origin of spinal cord scar tissue. Science 333: 238–242, 2011 [DOI] [PubMed] [Google Scholar]
- 13.Kida Y, Ieronimakis N, Schrimpf C, Reyes M, Duffield JS: EphrinB2 reverse signaling protects against capillary rarefaction and fibrosis after kidney injury. J Am Soc Nephrol 24: 559–572, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin SL, Chang FC, Schrimpf C, Chen YT, Wu CF, Wu VC, Chiang WC, Kuhnert F, Kuo CJ, Chen YM, Wu KD, Tsai TJ, Duffield JS: Targeting endothelium-pericyte cross talk by inhibiting VEGF receptor signaling attenuates kidney microvascular rarefaction and fibrosis. Am J Pathol 178: 911–923, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kramann R, Fleig SV, Schneider RK, Fabian SL, DiRocco DP, Maarouf O, Wongboonsin J, Ikeda Y, Heckl D, Chang SL, Rennke HG, Waikar SS, Humphreys BD: Pharmacological GLI2 inhibition prevents myofibroblast cell-cycle progression and reduces kidney fibrosis. J Clin Invest 125: 2935–2951, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stefanska A, Eng D, Kaverina N, Pippin JW, Gross KW, Duffield JS, Shankland SJ: Cells of renin lineage express hypoxia inducible factor 2α following experimental ureteral obstruction. BMC Nephrol 17: 5, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ligresti G, Nagao RJ, Xue J, Choi YJ, Xu J, Ren S, Aburatani T, Anderson SK, MacDonald JW, Bammler TK, Schwartz SM, Muczynski KA, Duffield JS, Himmelfarb J, Zheng Y: A Novel Three-Dimensional Human Peritubular Microvascular System [published online ahead of print December 11, 2015]. J Am Soc Nephrol doi: 10.1681/ASN.2015070747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hammes HP: Pericytes and the pathogenesis of diabetic retinopathy. Horm Metab Res 37[Suppl 1]: 39–43, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Olson LE, Soriano P: PDGFRβ signaling regulates mural cell plasticity and inhibits fat development. Dev Cell 20: 815–826, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Souma T, Nezu M, Nakano D, Yamazaki S, Hirano I, Sekine H, Dan T, Takeda K, Fong GH, Nishiyama A, Ito S, Miyata T, Yamamoto M, Suzuki N: Erythropoietin Synthesis in Renal Myofibroblasts Is Restored by Activation of Hypoxia Signaling. J Am Soc Nephrol 27: 428–438, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sanchez-Nino, MD, Benito-Martin, A, Goncalves, S, Sanz, AB, Ucero, AC, Izquierdo, MC, Ramos, AM, Berzal, S, Selgas, R, Ruiz-Ortega, M, Egido, J, Ortiz, A: TNF superfamily: a growing saga of kidney injury modulators. Mediators of inflammation 2010: 182958, 2010 [DOI] [PMC free article] [PubMed]
- 22.McCarthy DD, Kujawa J, Wilson C, Papandile A, Poreci U, Porfilio EA, Ward L, Lawson MA, Macpherson AJ, McCoy KD, Pei Y, Novak L, Lee JY, Julian BA, Novak J, Ranger A, Gommerman JL, Browning JL: Mice overexpressing BAFF develop a commensal flora-dependent, IgA-associated nephropathy. J Clin Invest 121: 3991–4002, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang J, Anders RA, Wu Q, Peng D, Cho JH, Sun Y, Karaliukas R, Kang HS, Turner JR, Fu YX: Dysregulated LIGHT expression on T cells mediates intestinal inflammation and contributes to IgA nephropathy. J Clin Invest 113: 826–835, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vincent FB, Morand EF, Schneider P, Mackay F: The BAFF/APRIL system in SLE pathogenesis. Nat Rev Rheumatol 10: 365–373, 2014 [DOI] [PubMed] [Google Scholar]
- 25.Michaelson JS, Wisniacki N, Burkly LC, Putterman C: Role of TWEAK in lupus nephritis: a bench-to-bedside review. J Autoimmun 39: 130–142, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hodgin JB, Borczuk AC, Nasr SH, Markowitz GS, Nair V, Martini S, Eichinger F, Vining C, Berthier CC, Kretzler M, D’Agati VD: A molecular profile of focal segmental glomerulosclerosis from formalin-fixed, paraffin-embedded tissue. Am J Pathol 177: 1674–1686, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Woroniecka KI, Park AS, Mohtat D, Thomas DB, Pullman JM, Susztak K: Transcriptome analysis of human diabetic kidney disease. Diabetes 60: 2354–2369, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xia Y, Herlitz LC, Gindea S, Wen J, Pawar RD, Misharin A, Perlman H, Wu L, Wu P, Michaelson JS, Burkly LC, Putterman C: Deficiency of fibroblast growth factor-inducible 14 (Fn14) preserves the filtration barrier and ameliorates lupus nephritis. J Am Soc Nephrol 26: 1053–1070, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hotta K, Sho M, Yamato I, Shimada K, Harada H, Akahori T, Nakamura S, Konishi N, Yagita H, Nonomura K, Nakajima Y: Direct targeting of fibroblast growth factor-inducible 14 protein protects against renal ischemia reperfusion injury. Kidney Int 79: 179–188, 2011 [DOI] [PubMed] [Google Scholar]
- 30.Ucero AC, Benito-Martin A, Fuentes-Calvo I, Santamaria B, Blanco J, Lopez-Novoa JM, Ruiz-Ortega M, Egido J, Burkly LC, Martinez-Salgado C, Ortiz A: TNF-related weak inducer of apoptosis (TWEAK) promotes kidney fibrosis and Ras-dependent proliferation of cultured renal fibroblast. Biochim Biophys Acta 1832: 1744–1755, 2013 [DOI] [PubMed] [Google Scholar]
- 31.Sanz AB, Sanchez-Niño MD, Izquierdo MC, Jakubowski A, Justo P, Blanco-Colio LM, Ruiz-Ortega M, Selgas R, Egido J, Ortiz A: TWEAK activates the non-canonical NFkappaB pathway in murine renal tubular cells: modulation of CCL21. PLoS One 5: e8955, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yepes M: TWEAK and Fn14 in the Neurovascular Unit. Front Immunol 4: 367, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Winkles JA: The TWEAK-Fn14 cytokine-receptor axis: discovery, biology and therapeutic targeting. Nat Rev Drug Discov 7: 411–425, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yilmaz MI, Carrero JJ, Ortiz A, Martín-Ventura JL, Sonmez A, Saglam M, Yaman H, Yenicesu M, Egido J, Blanco-Colio LM: Soluble TWEAK plasma levels as a novel biomarker of endothelial function in patients with chronic kidney disease. Clin J Am Soc Nephrol 4: 1716–1723, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kennedy-Lydon TM, Crawford C, Wildman SS, Peppiatt-Wildman CM: Renal pericytes: regulators of medullary blood flow. Acta Physiol (Oxf) 207: 212–225, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Szklarczyk D, Franceschini A, Wyder S, Forslund K, Heller D, Huerta-Cepas J, Simonovic M, Roth A, Santos A, Tsafou KP, Kuhn M, Bork P, Jensen LJ, von Mering C: STRING v10: protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res 43: D447–D452, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Campbell S, Burkly LC, Gao HX, Berman JW, Su L, Browning B, Zheng T, Schiffer L, Michaelson JS, Putterman C: Proinflammatory effects of TWEAK/Fn14 interactions in glomerular mesangial cells. J Immunol 176: 1889–1898, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Hudson BG, Tryggvason K, Sundaramoorthy M, Neilson EG: Alport’s syndrome, Goodpasture’s syndrome, and type IV collagen. N Engl J Med 348: 2543–2556, 2003 [DOI] [PubMed] [Google Scholar]
- 39.King TE Jr, Bradford WZ, Castro-Bernardini S, Fagan EA, Glaspole I, Glassberg MK, Gorina E, Hopkins PM, Kardatzke D, Lancaster L, Lederer DJ, Nathan SD, Pereira CA, Sahn SA, Sussman R, Swigris JJ, Noble PW ASCEND Study Group : A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med 370: 2083–2092, 2014 [DOI] [PubMed] [Google Scholar]
- 40.Sharma K, Ix JH, Mathew AV, Cho M, Pflueger A, Dunn SR, Francos B, Sharma S, Falkner B, McGowan TA, Donohue M, Ramachandrarao S, Xu R, Fervenza FC, Kopp JB: Pirfenidone for diabetic nephropathy. J Am Soc Nephrol 22: 1144–1151, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grattendick KJ, Nakashima JM, Feng L, Giri SN, Margolin SB: Effects of three anti-TNF-alpha drugs: etanercept, infliximab and pirfenidone on release of TNF-alpha in medium and TNF-alpha associated with the cell in vitro. Int Immunopharmacol 8: 679–687, 2008 [DOI] [PubMed] [Google Scholar]
- 42.Tsushima K, Osawa T, Yanai H, Nakajima A, Takaoka A, Manabe I, Ohba Y, Imai Y, Taniguchi T, Nagai R: IRF3 regulates cardiac fibrosis but not hypertrophy in mice during angiotensin II-induced hypertension. FASEB J 25: 1531–1543, 2011 [DOI] [PubMed] [Google Scholar]
- 43.Jiang DS, Bian ZY, Zhang Y, Zhang SM, Liu Y, Zhang R, Chen Y, Yang Q, Zhang XD, Fan GC, Li H: Role of interferon regulatory factor 4 in the regulation of pathological cardiac hypertrophy. Hypertension 61: 1193–1202, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maahs DM, Siwy J, Argilés A, Cerna M, Delles C, Dominiczak AF, Gayrard N, Iphöfer A, Jänsch L, Jerums G, Medek K, Mischak H, Navis GJ, Roob JM, Rossing K, Rossing P, Rychlík I, Schiffer E, Schmieder RE, Wascher TC, Winklhofer-Roob BM, Zimmerli LU, Zürbig P, Snell-Bergeon JK: Urinary collagen fragments are significantly altered in diabetes: a link to pathophysiology. PLoS One 5: e13051, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Veidal SS, Karsdal MA, Vassiliadis E, Nawrocki A, Larsen MR, Nguyen QH, Hägglund P, Luo Y, Zheng Q, Vainer B, Leeming DJ: MMP mediated degradation of type VI collagen is highly associated with liver fibrosis--identification and validation of a novel biochemical marker assay. PLoS One 6: e24753, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Naugle JE, Olson ER, Zhang X, Mase SE, Pilati CF, Maron MB, Folkesson HG, Horne WI, Doane KJ, Meszaros JG: Type VI collagen induces cardiac myofibroblast differentiation: implications for postinfarction remodeling. Am J Physiol Heart Circ Physiol 290: H323–H330, 2006 [DOI] [PubMed] [Google Scholar]
- 47.Keene DR, Engvall E, Glanville RW: Ultrastructure of type VI collagen in human skin and cartilage suggests an anchoring function for this filamentous network. J Cell Biol 107: 1995–2006, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kielty CM, Whittaker SP, Grant ME, Shuttleworth CA: Type VI collagen microfibrils: evidence for a structural association with hyaluronan. J Cell Biol 118: 979–990, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shea BS, Brooks SF, Fontaine BA, Chun J, Luster AD, Tager AM: Prolonged exposure to sphingosine 1-phosphate receptor-1 agonists exacerbates vascular leak, fibrosis, and mortality after lung injury. Am J Respir Cell Mol Biol 43: 662–673, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Girgenrath M, Weng S, Kostek CA, Browning B, Wang M, Brown SA, Winkles JA, Michaelson JS, Allaire N, Schneider P, Scott ML, Hsu YM, Yagita H, Flavell RA, Miller JB, Burkly LC, Zheng TS: TWEAK, via its receptor Fn14, is a novel regulator of mesenchymal progenitor cells and skeletal muscle regeneration. EMBO J 25: 5826–5839, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gomez IG, MacKenna DA, Johnson BG, Kaimal V, Roach AM, Ren S, Nakagawa N, Xin C, Newitt R, Pandya S, Xia TH, Liu X, Borza DB, Grafals M, Shankland SJ, Himmelfarb J, Portilla D, Liu S, Chau BN, Duffield JS: Anti-microRNA-21 oligonucleotides prevent Alport nephropathy progression by stimulating metabolic pathways. J Clin Invest 125: 141–156, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lin SL, Li B, Rao S, Yeo EJ, Hudson TE, Nowlin BT, Pei H, Chen L, Zheng JJ, Carroll TJ, Pollard JW, McMahon AP, Lang RA, Duffield JS: Macrophage Wnt7b is critical for kidney repair and regeneration. Proc Natl Acad Sci U S A 107: 4194–4199, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Crawford C, Kennedy-Lydon T, Sprott C, Desai T, Sawbridge L, Munday J, Unwin RJ, Wildman SS, Peppiatt-Wildman CM: An intact kidney slice model to investigate vasa recta properties and function in situ. Nephron, Physiol 120: 17–31, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.