Abstract
Metabolic acidosis is associated with increased urinary calcium excretion and related sequelae, including nephrocalcinosis and nephrolithiasis. The increased urinary calcium excretion induced by metabolic acidosis predominantly results from increased mobilization of calcium out of bone and inhibition of calcium transport processes within the renal tubule. The mechanisms whereby acid alters the integrity and stability of bone have been examined extensively in the published literature. Here, after briefly reviewing this literature, we consider the effects of acid on calcium transport in the renal tubule and then discuss why not all gene defects that cause renal tubular acidosis are associated with hypercalciuria and nephrocalcinosis.
Keywords: renal tubular acidosis, chronic metabolic acidosis, calcium, hypercalciuria
It has been appreciated for nearly a century that metabolic acidosis is associated with increased urinary calcium excretion.1–3 Overall, this occurs via at least two mechanisms: release of calcium from bone4 and changes in calcium transport within the renal tubule.5 Given that bone is an important reservoir of buffer (with both calcium carbonate and phosphate), there is a myriad of data and a strong rationale to implicate its role in buffering acid.6,7 Dissolution of bone releases calcium, either as carbonate or phosphate. The net movement of calcium from bone into blood leads to excess calcium being excreted in urine, in an effort to stabilize systemic calcium concentrations. Metabolic acidosis increases ionized calcium in blood, by decreasing the amount bound to albumin. Furthermore, metabolic acidosis causes alterations in the renal reabsorptive capacity for calcium due to the direct inhibition of calcium transport within the nephron; this is evidenced by classic human studies where metabolic acidosis was induced and urinary calcium wasting observed, despite a fall in the filtered load of calcium.5 However, genetic diseases causing renal tubular acidosis vary with regard to increased calcium excretion and sequelae resulting from excessive urinary calcium losses, such as nephrocalcinosis and nephrolithiasis.8,9 In this review, we will first briefly summarize over 90 years of experiments examining the relationship between acidosis and urinary calcium excretion. We will then highlight selected genetic disorders of altered acid-base balance, using them to understand why a direct coupling between acidosis and renal calcium losses and their associated sequelae are not consistently observed.
Effect of Acid-Base Perturbations on Bone
Acute or chronic administration of exogenous acid increases urinary calcium excretion. Initial studies exploring the effects of acid loading on overall calcium handling concluded that intestinal calcium absorption is not affected3,4,10; however, recent work found increased solvent drag mediated calcium flux and altered expression of tight junction proteins in the duodenum of rats with chronic metabolic acidosis.11,12 Given that the vast majority of calcium in the body is stored in bone as part of the hydroxyapatite crystal, it is expected that dissolution of bone during acid loading leads to urinary calcium wasting. Extensive evidence from multiple experimental models is consistent with this.7 There is an acute physicochemical buffering of hydrogen ions (H+) followed by a more chronic effect on cells mediating bone turnover.
Acute Acidosis
Studies examining the mechanisms by which bone buffers H+ have been eloquently reviewed previously.6,7 In brief, the surface of bone is littered with negatively charged sites that bind both sodium and potassium. During acute metabolic acidosis (<3 hours) there is an exchange of both these monovalent cations with H+ thereby increasing plasma pH.13–15 There is also some rapid direct dissolution of calcium carbonate and hydroxyapatite from bone releasing calcium, which is likely part of the rapidly exchangeable pool of calcium.13,14,16 Both of these physicochemical processes occur independently of bone cell activity.
Chronic Acidosis
In contrast to the acute situation, exposure of bone to chronic metabolic acidosis i.e., for 24 hours or more, affects bone cell function. Osteoclast activity is stimulated and osteoblast activity is inhibited by lower pH.17–19 Importantly, the converse is true; metabolic alkalosis increases osteoblastic activity and inhibits osteoclastic activity, favoring bone formation.20 One mechanism whereby acidosis mediates its effect on bone involves the release of PGE2, which stimulates the expression of receptor activator of nuclear factor κ-B (RANK) ligand altering osteoclast differentiation, maturation and H+ATPase activity thereby increasing resorption.21–23
Effects of Acid-Base Perturbations on the Renal Tubule
Metabolic acidosis causes renal calcium wasting not only via the liberation of calcium from bone, but also by directly altering calcium handling within the renal tubule. This is evident as an increase in urinary calcium excretion occurs despite a decrease in filtered calcium load.5,24 Furthermore, metabolic acidosis alters renal tubular calcium handling irrespective of changes in parathyroid hormone (as patients with hypoparathyroidism demonstrate this phenomenon), and independent of tubular sodium handling.5,25,26 In order to consider how these alterations occur, we must first review tubular calcium reabsorption along the nephron.
Ionized calcium that is filtered by the glomerulus is reabsorbed via both passive paracellular and active transcellular pathways. The proximal tubule and thick ascending limb (TAL) of the Henle loop demonstrate predominantly, if not exclusively, passive paracellular transport. In the proximal tubule, water absorption is driven by transcellular sodium movement, which is largely mediated by the apical sodium hydrogen exchanger 3 (NHE3).27 Luminal water removal increases the concentration of different solutes, such as calcium, that are not reabsorbed via secondary active transport processes. Calcium then moves down its concentration gradient or with water through the tight junction.28 In the TAL, removal of water in the relatively calcium impermeable thin descending limb,29 and a lumen positive voltage, provides the driving force for paracellular calcium flux. Activity of the apically expressed sodium potassium chloride cotransporter 2 (NKCC2) in concert with potassium efflux back into the lumen via the renal outer medullary potassium channel is necessary to create the lumen positive potential. Consistent with a role in calcium reabsorption, genetic deletion or pharmacological inhibition of either NKCC2 or NHE3 causes hypercalciuria.30–34
Paracellular calcium transport requires both a driving force, and tight junction permeability. Calcium permeation of the proximal tubule and TAL is conferred by specific tight junction proteins called claudins.35 Their identity is better delineated in the TAL where claudins-16 and -19 form a cation permeable pore,36 and claudin-14, whose expression is directly induced via the calcium-sensing receptor (CaSR) signaling in the presence of increased ionized calcium levels, prevents calcium reabsorption.37 In the proximal tubule, claudin-2 may form a cation permeable pore.38 However, further work is required to clearly delineate the proteins permitting calcium permeation across this segment.
The link between transcellular sodium flux and paracellular calcium absorption under conditions of metabolic acidosis are yet to be clearly elucidated. NHE3 is essential for proximal tubular bicarbonate reabsorption.27 Consequently, metabolic acidosis increases NHE3 activity,39,40 an effect that should attenuate increased urinary calcium excretion accompanying metabolic acidosis. However, metabolic acidosis dissociates sodium reabsorption from calcium reabsorption distal to the late proximal tubular micropuncture site, causing increased urinary calcium excretion relative to sodium.25,41 The overall contribution of the TAL to changes in calcium transport under conditions of metabolic acidosis remains to be fully elucidated. Given our current understanding of TAL transport, one could envision that increased ionized calcium in blood (both mobilized from bone and due to decreased binding to albumin) would increase claudin-14 expression by activating the CaSR, thereby altering the permeability of the paracellular pore. This would increase urinary calcium excretion and could contribute to the dissociation between increased NHE3 activity and increased urinary calcium excretion observed with metabolic acidosis. However, direct evidence in support of this hypothesis has not been presented, and one must also consider the reduced sensitivity of the CaSR at acidic pH.42
Although the majority of calcium is reabsorbed by the proximal tubule and TAL, the remaining <10% is reabsorbed by the distal convoluted tubule and connecting tubule. This occurs in an active transcellular fashion and discernable changes in transport within this region have been clearly documented during acidosis.43 Apical calcium influx occurs through the transient receptor potential cation channel subfamily V member 5 (TRPV5) channel.44 The calcium binding protein calbindin-D28K buffers ionized calcium, which is extruded across the basolateral membrane via calcium dependent ATPases (plasma membrane Ca2+ ATPase) or a sodium calcium exchanger.45,46 Micropuncture studies demonstrate that calcium reabsorption from the distal nephron is inhibited by metabolic acidosis, independent of changes in parathyroid hormone (PTH), inferring a direct effect on renal transport processes.41,47,48 Consistent with this, renal Trpv5 expression is decreased by systemic acid loads and metabolic alkalosis increases expression.49 Moreover, metabolic acidosis does not further augment existing calcium wasting in the Trpv5 knockout mice.49 Together, this data suggests that systemic acid-base status, at least in part, alters urinary calcium excretion via an effect on the expression and activity of Trpv5 in the distal tubule. Importantly, Trpv5-deficient mice display reduced bone thickness due to increased osteoclast-mediated bone resorption, likely as a consequence of renal calcium wasting.50 However, Trpv5 is also expressed in the ruffled border of the osteoclast, where it partakes in vectorial calcium transport from the subosteoclastic resorption zone.51 Therefore, the effect of metabolic acidosis on Trpv5-mediated calcium reabsorption in kidney, independent of loss of Trpv5 in osteoclasts, remains to be determined.
Consideration of Type of Acidosis
Studies described thus far were limited to very controlled situations where acid-base status was manipulated by strict administration of an acid or alkali. Furthermore, the effect of respiratory perturbations on acid-base status and calcium handling reveals an important role for bicarbonate. Moreover, other than causing metabolic acidosis, additional effects of specific acids and their counter ions on calcium homeostasis and consequently urinary calcium excretion further complicate the situation. However, consideration of these effects aids our understanding of the dynamic physiologic processes occurring simultaneously.
Metabolic Versus Respiratory Acidosis
In different studies respiratory acidosis either does not increase urinary calcium excretion or increases urinary calcium excretion less so than a similar decrease in pH caused by a metabolic acidosis.52–54 As noted earlier, prostaglandin (PG) E2 promotes osteoclast maturation and H+ATPase activity via the RANK pathway thereby increasing bone resorption.21–23 PGE release is preferentially stimulated by experimental metabolic acidosis rather than respiratory acidosis.22 Moreover, elegant in vitro studies manipulating bicarbonate levels independent of pH demonstrate that calcium mobilization from bone in response to a metabolic acidosis occurs to a greater extent when bicarbonate concentration is simultaneously reduced.55,56 This may explain why respiratory acidosis is less potent than metabolic acidosis in promoting urinary calcium wasting.52–54 Consistent with this, in vitro models of acidosis with elevated bicarbonate levels can actually increase carbonate incorporation into bone.57 Consequently, plasma bicarbonate levels themselves, independent of pH, affect bone formation and dissolution. Thus, when considering the effect of acid-base status on calcium homeostasis, one must also consider bicarbonate, because it appears to have a greater effect on bone than changes in pH. We are unaware of studies directly examining renal tubular calcium handling in the presence of respiratory acidosis. However, the effects of hypercarbia on luminal bicarbonate concentration may increase distal calcium absorption, further attenuating urinary calcium excretion.
Different Exogenous Acids
Hydrochloric acid and ammonium chloride administration have been employed to model disease states such as the acidosis observed in renal failure or that induced by ingestion of a high protein diet. These models simplify complex disease processes and conclusions from them must therefore be carefully considered. In the case of uremic metabolic acidosis, it is typically accompanied by an elevated PTH and suppressed active 1,25-dihydroxyvitamin D levels. These hormones have significant effects on urinary calcium excretion by themselves. Increased PTH enhances bone resorption favoring calcium mobilization into blood. However, considerable evidence exists that the effect of acid on bone, and consequently calciuria, occurs independently of PTH.58–60 Similarly, reduced 1,25-dihydroxyvitamin D levels decrease intestinal calcium uptake, which should reduce urinary calcium excretion. However, many, but not all, studies found that metabolic acidosis increases urinary calcium excretion without altering intestinal calcium absorption,4,60,61 and argued against a direct role for 1,25-dihydroxyvitamin D in this process. Indeed 1,25-dihydroxyvitamin D generation in response to the calciuria of any metabolic acidosis is indirectly suppressed by bone resorption which elevates plasma calcium.62
The ingestion of protein has many diverse physiologic effects including the production of hormones and nonvolatile acid. A meta-analysis examining dietary interventions to increase acid producing food ingestion found a proportionate increase between urinary acid excretion and urinary calcium excretion.63 The calcium was assumed to come from bone. However, this has been called into question because another meta-analysis by the same group found that total calcium balance and markers of bone breakdown were not altered by increased dietary acid consumption.64 A high protein diet itself can increase intestinal calcium uptake, growth hormone, and insulin like growth factor 1 levels as well as lower PTH, which are factors that favor bone formation.65 Thus, translating models of ammonium chloride induced acidosis into recommendations on protein consumption to promote bone health, one needs to consider confounding factors. Similarly, diabetic ketoacidosis and lactic acidosis are typically associated with volume contraction, which reduces urinary calcium excretion by increasing proximal tubular calcium reabsorption. However, the osmotic diuresis caused by the former would attenuate proximal tubular calcium reabsorption. This and decreased insulin levels partially explain the more severe urinary calcium loss observed in diabetic ketoacidosis relative to lactic acidosis.66
Insight from Human diseases
Distal Renal Tubular Acidosis
Distal renal tubular acidosis (dRTA) is a disease characterized by a failure to acidify urine, i.e., an inappropriately elevated urine pH in the presence of a nonanion gap (or hyperchloremic) metabolic acidosis. The genetic causes of this disease are often, but not always, accompanied by hypercalciuria and nephrolithiasis and/or nephrocalcinosis (Table 1). Given the previous discussion on the effect of metabolic acidosis on bone, one would predict severe osteoporosis in these patients. However, this is rarely observed; instead, a mild reduction in bone density has been reported, which can be ameliorated by the administration of alkali.67,68 The molecular cause of dRTA has been ascribed to mutations in the H+-ATPase β 1 subunit (ATP6V1B1),69 α 4 subunit (ATP6V0A4), and the anion exchanger 1 (AE1/SLC4A1). We discuss urinary calcium excretion with respect to specific gene defects below.
Table 1.
RTA gene defects, calciuria, nephrocalcinosis and nephrolithiasis
| Gene/protein | Hypercalciuria, % | Nephrocalcinosis, % | Nephrolithiasis, % | References |
|---|---|---|---|---|
| dRTA | ||||
| ATP6V1B1/ V1-ATPase B1 subunit | 89 (25 of 28) | 100 (38 of 38) | 72 (13 of 18) | 9,69,71,117–121 |
| ATP6V0A4/ V0-ATPase A4 subunit | 78 (7 of 9) | 100 (9 of 9) | Not reported | 85,86 |
| SLC4A1/AE1, Anion Exchanger isoform 1 | 89 (8 of 9) | 66 (21 of 32) | 50 (12 of 24) | 90,92,122–124 |
| Mixed RTA | ||||
| CA2/carbonic anhydrase 2 | 0 (0 of 12) | Not Reported | Not Reported | 106–108,125,126 |
| pRTA | ||||
| SLC4A4/NBCe1, electrogenic Na+/HCO3- cotransporter | 0 (0 of 5) | 0 (0 of 6) | 0 (0 of 6) | 97,127–129 |
| Fanconi syndrome | ||||
| CTNS/cystinosin | 60 (38 of 63) | 68 (43 of 63) | 17 (11 of 63) | 130,131 |
| CLCN5/ CLC-5, Dent disease | 89 | 76 | 41 | 132 |
| ORCL1/OCRL-1, oculocerebrorenal syndrome of Lowe (but mutations can also cause Dent disease) | 88 (22 of 25) | 48 (12 of 25) | 24 (6 of 25) | 133,134 |
ATP6V1B1
Patients with mutations in ATP6V1B1 typically have dRTA accompanied by deafness, and nearly all reported cases display nephrocalcinosis.69–71 Why some younger children with mutations in ATP6V1B1 do not have hypercalciuria was recently discussed.9 Although many factors are considered, including other polymorphisms, the authors provide a strong argument for decreased intravascular volume and low sodium intake in preventing hypercalciuria. Importantly, ATP6V1B1 has been implicated in sodium absorption, and children with these mutations also have salt-losing nephropathy.72,73 The relationship between sodium ingestion and calcium excretion is well known.74 The molecular details linking the two are less clear. Given that paracellular calcium absorption is driven by sodium reabsorption from the proximal tubule and TAL,75–77 it can be argued that a low sodium diet leads to increased sodium reabsorption and consequently increased driving force for calcium across these nephron segments (Figure 1). Similarly, intravascular volume status strongly affects proximal tubule sodium, water, and consequently calcium reabsorption.78 Consistent with this, Coe et al.57 found that hypercalciuria associated with ammonium chloride-induced metabolic acidosis was prevented by salt restriction and only when subjects became salt replete did hypercalciuria ensue. It is therefore likely that the lower sodium diet ingested by infants who are either breast-fed or fed formulae masks the hypercalciuria induced by metabolic acidosis. Furthermore, when these children start to consume solids more sodium would be ingested and they consequently develop hypercalciuria. Finally, infants have a significant net positive calcium balance in order to mineralize bone, and a reduced ability to concentrate their urine79,80; both these factors would further attenuate the effect of acidosis on hypercalciuria.
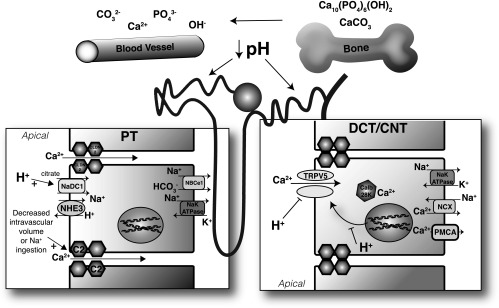
Figure 1.
Effect of metabolic acidosis on calcium homeostasis. Metabolic acidosis via a physicochemical process is acutely buffered by exchange of surface sodium (Na+) and potassium ions (K+) with protons on bone. Chronically, via osteoclast specific mechanisms, CaCO3 and Ca10(PO4)6(OH)2 are liberated. These processes are also significantly affected by serum HCO3- levels. Calcium liberated from bone is filtered by the glomerulus; however, acidosis also directly alters tubular handling. In the distal convoluted tubule (DCT) and connecting tubule (CNT), acidosis effects the expression of TRPV5, but also the luminal H+ concentration has direct effects on TRPV5 activity (H+ inhibits). Consequently, bicarbonaturia, by increasing luminal pH increases TRPV5 activity. There is debate as to whether there is significant calcium reabsorption from the collecting duct. However, α intercalated cells in this segment, when unable to secrete protons such as with dRTA, will fail to acidify the urine altering the solubility of calcium salts. In the proximal tubule, a high pH inhibits citrate reabsorption (as observed in pRTA). Further proximal sodium reabsorption, which significantly increases proximal calcium absorption, such as under conditions of hypovoemia, can effect urinary calcium excretion, but also the water reabsorption and the supersaturation of calcium (Ca2+) salts. C2, claudin-2; calb 28K, calbindin-D28K; NaDC1, Na+ dicarboxylate contransporter 1; NBCe1, sodium bicarbonate exchanger e1; NCX, sodium calcium exchanger; PMCA, plasma membrane calcium ATPase; PO4, phosphate.
Patients with incomplete dRTA due to milder mutations in ATP6V1B1 often display hypercalciuria and a tendency to form kidney stones.81,82 The etiology of nephrocalcinosis in these patients is due to intraluminal precipitation of calcium phosphate in the inner medullary collecting duct, likely resulting from an elevated intraluminal pH.83 However, it raises the intriguing question as to why some hypercalciuric disease states result in frank calcifications throughout specific parts of the kidney, i.e., nephrocalcinosis, whereas others cause discrete calcification as a stone, i.e., nephrolithiasis.
ATPV0A4
Mutations in the α 4 subunit of the H+-ATPase cause dRTA, which is sometimes associated with deafness.84,85 Nephrocalcinosis was observed in all patients for whom data are reported, although nephrolithiasis is rare. As with ATP6V1B1 mutations, a minority presented without hypercalciuria.85–87 Interestingly, the patients without hypercalciuria were aged <6 months. It is therefore likely that a low sodium intake, volume contraction, and/or a net positive calcium balance contributed to their normocalciuria, despite significant metabolic acidosis.
The Apical H+-ATPase, Volume Regulation, and Calcium Homeostasis
Patients with dRTA have long been appreciated to also have a disorder of sodium wasting.73 The molecular details of how the vacuolar H+-ATPase in the collecting duct contributes to sodium reabsorption were recently described. In combination with pendrin and Slc4A8, the H+-ATPase mediates thiazide-sensitive sodium reabsorption through the β-intercalated cell under situations of volume depletion.72,88,89 Mutations in either disease causing subunits would therefore prevent transcellular sodium reabsorption via this mechanism. Consequently, patients with mutations in the H+-ATPase would be prone to volume contraction and, as has been suggested for mutations in NCC, have increased proximal tubular sodium and consequently calcium reabsorption. Thus, if volume contracted, these patients would further attenuate hypercalciuria induced by metabolic acidosis. However, it should also be noted that volume contraction could exacerbate nephrocalcinosis and nephrolithiasis by decreasing urinary flow resulting in increased urine supersaturation of calcium, phosphate, or oxalate.
SLC4A1
Mutations in AE1 (SLC4A1) cause dRTA, which can be transmitted in either an autosomal dominant or recessive fashion. Patients present without deafness but commonly have hypercalciuria, nephrolithiasis, and nephrocalcinosis.90–93 Interestingly, not all patients with nephrolithiasis have nephrocalcinosis and vice versa.90 Hypercalciuria and its sequelae appear to be suppressed through the administration of alkali, suggesting that urinary calcium excretion is driven largely by the metabolic acidosis. As with all causes of dRTA, patients are unable to acidify their urine. Unfortunately, an alkaline pH increases the supersaturation of calcium phosphate in the tubular lumen, thereby augmenting the risk of stone formation (calcium oxalate supersaturation remains relatively independent of urinary pH). Finally, stone risk is further enhanced in dRTA patients due to reduced urinary excretion of citrate.94
Proximal Renal Tubular Acidosis
Isolated metabolic acidosis due to a defect in proximal tubular bicarbonate reabsorption is referred to as type 2 or proximal RTA (pRTA). This disease is most commonly associated with global renal dysfunction, i.e., Fanconi syndrome (see below). pRTA rarely occurs without other types of proximal tubular dysfunction. To date, only mutations in NBCe1 have been found to cause isolated pRTA in humans.8,95,96 Patients with this disorder do not have evidence of altered bone breakdown, hypercalciuria, or nephrocalcinosis; however, they typically display decreased growth, a likely consequence of their metabolic acidosis.8,95,97–99 The lack of hypercalciuria is surprising because loss of proximal bicarbonate reabsorption would result in decreased sodium and consequently calcium reabsorption,100 which might overwhelm distal tubular calcium reabsorption capacity. However, this observation could be explained by an elevated luminal pH of the distal tubule, which would increase the surface expression and activity of the apical calcium channel, TRPV5, and consequently calcium absorption.101 Finally, in contrast to dRTA and patients with metabolic acidosis, patients with pRTA have significant citrate in their urine (due to inhibition of proximal citrate reabsorption by an alkaline luminal pH). This would help prevent calcium precipitation and stone formation in patients with pRTA.102,103
Type 3 RTA and Carbonic Anhydrase 2 Deficiency
Renal tubular acidosis of a mixed nature, i.e., both proximal and distal, is called type 3. This is caused by a defect in carbonic anhydrase 2 (CA2).104,105 This enzyme is required for both acid secretion in the α intercalated cell but also for bicarbonate reabsorption from the proximal tubule. Consequently, individuals lacking CA2 activity demonstrate both bicarbonaturia and the inability to acidify their urine in the presence of metabolic acidosis.104,106,107 Given the discussion, one would predict that these patients have severe osteoporosis. However, this is not the case. They demonstrate osteopetrosis resulting from insufficient bone resorption.106,108 This occurs because CA2 is required to generate protons secreted into the subosteoclastic resorption pits by the osteoclasts, so that the mineralized matrix can be dissolved.109 Hence, even with metabolic acidosis there is impairment of calcium dissolution from bone, reinforcing the notion that bone loss during chronic metabolic acidosis is mediated by altered osteoclast activity and is not simply a physicochemical response. Consequently, CA2-deficient mice do not display hypercalciuria (R.T. Alexander, unpublished observation), and patients with CA2 deficiency are not reported to have hypercalciuria.106–108 As with pRTA, the tubular lumen of the distal convoluted tubule would contain significant bicarbonate to increase TRPV5 activity, further preventing the hypercalciuria associated with their metabolic acidosis.
Fanconi Syndrome
The Fanconi syndrome describes the presence of multiple defects in proximal tubular reabsorptive capacity including pRTA, phosphaturia, glucosuria, amino aciduria, and low molecular weight proteinuria. There are many genetic defects that can cause this disorder and discussing them all is beyond the scope of this review. However, a few points are worth considering. Twenty-five-hydroxyvitamin D circulates bound to the vitamin D binding protein. This complex is filtered at the glomerulus and is reabsorbed by the proximal tubule, providing the 1-α hydroxylase enzyme with substrate.110 Failure to endocytose this complex results in decreased conversion to active vitamin D, which reduces intestinal calcium absorption, bone turnover, and consequently affects urinary calcium excretion, as is often seen with Fanconi syndrome.
Defects in CLC5 cause Dent disease.111 This type of Fanconi syndrome is characterized by hypercalciuria and nephrocalcinosis. CLC5 is a proton-chloride exchanger expressed in endocytic vesicles in the proximal tubule, where it colocalizes with the H+ATPase.112 Importantly, defects in CLC5 can cause disorders other than Dent disease. It has been suggested that hypercalciuria in these patients is a result of increased vitamin D. This is thought to occur due to reduced 24-hydroxylation of vitamin D in the proximal tubule. Ultimately this would suppress PTH, increasing urinary calcium excretion. However, not all patients with Dent disease display increased vitamin D levels and only one of the two knockout mice strains has increased vitamin D levels.113,114 Calcium is reabsorbed in a paracellular fashion from the proximal tubule; consequently, endocytic defects caused by mutations in CLC5 could alter the permeability to and/or the molecules generating the driving force for calcium across the proximal tubule, thereby resulting in hypercalciuria. Further confusing the situation is that although uncommon, mutations in the OCRL gene can also cause hypercalciuria and nephrocalcinosis without metabolic acidosis.115,116 Understanding the mechanisms causing CLC5 and OCRL mutations to cause calciuria and nephrocalcinosis will undoubtedly provide further information on the complex interplay between metabolic acidosis and urinary calcium excretion.
Summary
It is an oversimplification to suggest that urinary calcium excretion occurs merely in response to acidosis-induced bone dissolution. Although bone is a buffer, the concentration of serum bicarbonate, i.e., type of acidosis, has a strong influence on calcium release from bone. Moreover, pH has a strong effect on tubular calcium handling, independent of PTH and vitamin D. Not only does pH regulate the expression of calcium transporting proteins in the distal nephron, the pH of the luminal fluid is likely to directly affect the activity of calcium transport mechanisms. Finally, when examining urinary calcium excretion, compensatory aspects of tubular physiology must also be considered, i.e., volume status and sodium ingestion, because these control calcium reabsorption from more proximal aspects of the nephron and supersaturation of calcium salts in luminal fluid. Much work remains to be done in order to understand why some transport defects cause hypercalciuria and nephrolithiasis as opposed to nephrocalcinosis. Our emerging understanding of the tight junction and paracellular transport may help provide these answers.
Disclosures
None.
Acknowledgments
R.T.A. is an Alberta Innovates Health Solutions Clinical Scholar and work in his laboratory is supported by grants from the Women and Children Health Research Institute, which is supported by the Stollery Children's Hospital Foundation, the Canadian Institutes of Health Research, and the Kidney Foundation of Canada. H.D. is supported by the Novo Nordisk Foundation, the Carlsberg Foundation, the Lundbeck Foundation, and the A.P. Møller Foundation.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Lamb AR, Evvard JM: The acid-base balance in animal nutrition. J Biol Chem 37: 329–342, 1919 [Google Scholar]
- 2.Williamson BJ, Freeman S: Effects of acute changes in acid-base balance on renal calcium excretion in dogs. Am J Physiol 191: 384–387, 1957 [DOI] [PubMed] [Google Scholar]
- 3.Farquharson RF, Salter WT, Tibbetts DM, Aub JC: STUDIES OF CALCIUM AND PHOSPHORUS METABOLISM: XII. The Effect of the Ingestion of Acid-producing Substances. J Clin Invest 10: 221–249, 1931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lemann J Jr, Litzow JR, Lennon EJ: The effects of chronic acid loads in normal man: further evidence for the participation of bone mineral in the defense against chronic metabolic acidosis. J Clin Invest 45: 1608–1614, 1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lemann J, Litzow JR, Lennon EJ: Studies of the mechanism by which chronic metabolic acidosis augments urinary calcium excretion in man. J Clin Invest 46: 1318–1328, 1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bushinsky DA, Frick KK: The effects of acid on bone. Curr Opin Nephrol Hypertens 9: 369–379, 2000 [DOI] [PubMed] [Google Scholar]
- 7.Krieger NS, Frick KK, Bushinsky DA: Mechanism of acid-induced bone resorption. Curr Opin Nephrol Hypertens 13: 423–436, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Igarashi T, Inatomi J, Sekine T, Cha SH, Kanai Y, Kunimi M, Tsukamoto K, Satoh H, Shimadzu M, Tozawa F, Mori T, Shiobara M, Seki G, Endou H: Mutations in SLC4A4 cause permanent isolated proximal renal tubular acidosis with ocular abnormalities. Nat Genet 23: 264–266, 1999 [DOI] [PubMed] [Google Scholar]
- 9.Tsai HY, Lin SH, Lin CC, Huang FY, Lee MD, Tsai JD: Why is hypercalciuria absent at diagnosis in some children with ATP6V1B1 mutation? Pediatr Nephrol 26: 1903–1907, 2011 [DOI] [PubMed] [Google Scholar]
- 10.Martin HE, Jones R: The effect of ammonium chloride and sodium bicarbonate on the urinary excretion of magnesium, calcium, and phosphate. Am Heart J 62: 206–210, 1961 [DOI] [PubMed] [Google Scholar]
- 11.Charoenphandhu N, Wongdee K, Tudpor K, Pandaranandaka J, Krishnamra N: Chronic metabolic acidosis upregulated claudin mRNA expression in the duodenal enterocytes of female rats. Life Sci 80: 1729–1737, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Charoenphandhu N, Tudpor K, Pulsook N, Krishnamra N: Chronic metabolic acidosis stimulated transcellular and solvent drag-induced calcium transport in the duodenum of female rats. Am J Physiol Gastrointest Liver Physiol 291: G446–G455, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Bushinsky DA, Levi-Setti R, Coe FL: Ion microprobe determination of bone surface elements: effects of reduced medium pH. Am J Physiol 250: F1090–F1097, 1986 [DOI] [PubMed] [Google Scholar]
- 14.Bushinsky DA, Wolbach W, Sessler NE, Mogilevsky R, Levi-Setti R: Physicochemical effects of acidosis on bone calcium flux and surface ion composition. J Bone Miner Res 8: 93–102, 1993 [DOI] [PubMed] [Google Scholar]
- 15.Bushinsky DA, Gavrilov K, Chabala JM, Featherstone JD, Levi-Setti R: Effect of metabolic acidosis on the potassium content of bone. J Bone Miner Res 12: 1664–1671, 1997 [DOI] [PubMed] [Google Scholar]
- 16.Pirklbauer M, Mayer G: The exchangeable calcium pool: physiology and pathophysiology in chronic kidney disease. Nephrol Dial Transplant 26: 2438–2444, 2011 [DOI] [PubMed] [Google Scholar]
- 17.Bushinsky DA: Net calcium efflux from live bone during chronic metabolic, but not respiratory, acidosis. Am J Physiol 256: F836–F842, 1989 [DOI] [PubMed] [Google Scholar]
- 18.Krieger NS, Sessler NE, Bushinsky DA: Acidosis inhibits osteoblastic and stimulates osteoclastic activity in vitro. Am J Physiol 262: F442–F448, 1992 [DOI] [PubMed] [Google Scholar]
- 19.Bushinsky DA: Stimulated osteoclastic and suppressed osteoblastic activity in metabolic but not respiratory acidosis. Am J Physiol 268: C80–C88, 1995 [DOI] [PubMed] [Google Scholar]
- 20.Bushinsky DA: Metabolic alkalosis decreases bone calcium efflux by suppressing osteoclasts and stimulating osteoblasts. Am J Physiol 271: F216–F222, 1996 [DOI] [PubMed] [Google Scholar]
- 21.Frick KK, Bushinsky DA: Metabolic acidosis stimulates RANKL RNA expression in bone through a cyclo-oxygenase-dependent mechanism. J Bone Miner Res 18: 1317–1325, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Bushinsky DA, Parker WR, Alexander KM, Krieger NS: Metabolic, but not respiratory, acidosis increases bone PGE(2) levels and calcium release. Am J Physiol Renal Physiol 281: F1058–F1066, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Nordström T, Shrode LD, Rotstein OD, Romanek R, Goto T, Heersche JN, Manolson MF, Brisseau GF, Grinstein S: Chronic extracellular acidosis induces plasmalemmal vacuolar type H+ ATPase activity in osteoclasts. J Biol Chem 272: 6354–6360, 1997 [DOI] [PubMed] [Google Scholar]
- 24.Stacy BD, Wilson BW: Acidosis and hypercalciuria: renal mechanisms affecting calcium, magnesium and sodium excretion in the sheep. J Physiol 210: 549–564, 1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sutton RA, Dirks JH: Renal handling of calcium. Fed Proc 37: 2112–2119, 1978 [PubMed] [Google Scholar]
- 26.Houillier P, Normand M, Froissart M, Blanchard A, Jungers P, Paillard M: Calciuric response to an acute acid load in healthy subjects and hypercalciuric calcium stone formers. Kidney Int 50: 987–997, 1996 [DOI] [PubMed] [Google Scholar]
- 27.Schultheis PJ, Clarke LL, Meneton P, Miller ML, Soleimani M, Gawenis LR, Riddle TM, Duffy JJ, Doetschman T, Wang T, Giebisch G, Aronson PS, Lorenz JN, Shull GE: Renal and intestinal absorptive defects in mice lacking the NHE3 Na+/H+ exchanger. Nat Genet 19: 282–285, 1998 [DOI] [PubMed] [Google Scholar]
- 28.Alexander RT, Dimke H, Cordat E: Proximal tubular NHEs: sodium, protons and calcium? Am J Physiol Renal Physiol 305: F229–F236, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rouse D, Ng RC, Suki WN: Calcium transport in the pars recta and thin descending limb of Henle of the rabbit, perfused in vitro. J Clin Invest 65: 37–42, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Simon DB, Karet FE, Hamdan JM, DiPietro A, Sanjad SA, Lifton RP: Bartter’s syndrome, hypokalaemic alkalosis with hypercalciuria, is caused by mutations in the Na-K-2Cl cotransporter NKCC2. Nat Genet 13: 183–188, 1996 [DOI] [PubMed] [Google Scholar]
- 31.Takahashi N, Chernavvsky DR, Gomez RA, Igarashi P, Gitelman HJ, Smithies O: Uncompensated polyuria in a mouse model of Bartter’s syndrome. Proc Natl Acad Sci U S A 97: 5434–5439, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pan W, Borovac J, Spicer Z, Hoenderop JG, Bindels RJ, Shull GE, Doschak MR, Cordat E, Alexander RT: The epithelial sodium/proton exchanger, NHE3, is necessary for renal and intestinal calcium (re)absorption. Am J Physiol Renal Physiol 302: F943–F956, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bomsztyk K, Calalb MB: Bicarbonate absorption stimulates active calcium absorption in the rat proximal tubule. J Clin Invest 81: 1455–1461, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suki WN, Rouse D, Ng RC, Kokko JP: Calcium transport in the thick ascending limb of Henle. Heterogeneity of function in the medullary and cortical segments. J Clin Invest 66: 1004–1009, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu AS: Claudins and the kidney. J Am Soc Nephrol 26: 11–19, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hou J, Renigunta A, Konrad M, Gomes AS, Schneeberger EE, Paul DL, Waldegger S, Goodenough DA: Claudin-16 and claudin-19 interact and form a cation-selective tight junction complex. J Clin Invest 118: 619–628, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dimke H, Desai P, Borovac J, Lau A, Pan W, Alexander RT: Activation of the Ca(2+)-sensing receptor increases renal claudin-14 expression and urinary Ca(2+) excretion. Am J Physiol Renal Physiol 304: F761–F769, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Muto S, Hata M, Taniguchi J, Tsuruoka S, Moriwaki K, Saitou M, Furuse K, Sasaki H, Fujimura A, Imai M, Kusano E, Tsukita S, Furuse M: Claudin-2-deficient mice are defective in the leaky and cation-selective paracellular permeability properties of renal proximal tubules. Proc Natl Acad Sci U S A 107: 8011–8016, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Laghmani K, Preisig PA, Moe OW, Yanagisawa M, Alpern RJ: Endothelin-1/endothelin-B receptor-mediated increases in NHE3 activity in chronic metabolic acidosis. J Clin Invest 107: 1563–1569, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu MS, Biemesderfer D, Giebisch G, Aronson PS: Role of NHE3 in mediating renal brush border Na+-H+ exchange. Adaptation to metabolic acidosis. J Biol Chem 271: 32749–32752, 1996 [DOI] [PubMed] [Google Scholar]
- 41.Sutton RA, Wong NL, Dirks JH: Effects of metabolic acidosis and alkalosis on sodium and calcium transport in the dog kidney. Kidney Int 15: 520–533, 1979 [DOI] [PubMed] [Google Scholar]
- 42.Doroszewicz J, Waldegger P, Jeck N, Seyberth H, Waldegger S: pH dependence of extracellular calcium sensing receptor activity determined by a novel technique. Kidney Int 67: 187–192, 2005 [DOI] [PubMed] [Google Scholar]
- 43.Nijenhuis T, Renkema KY, Hoenderop JG, Bindels RJ: Acid-base status determines the renal expression of Ca2+ and Mg2+ transport proteins. J Am Soc Nephrol 17: 617–626, 2006 [DOI] [PubMed] [Google Scholar]
- 44.Hoenderop JG, van Leeuwen JP, van der Eerden BC, Kersten FF, van der Kemp AW, Mérillat AM, Waarsing JH, Rossier BC, Vallon V, Hummler E, Bindels RJ: Renal Ca2+ wasting, hyperabsorption, and reduced bone thickness in mice lacking TRPV5. J Clin Invest 112: 1906–1914, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hoenderop JG, Nilius B, Bindels RJ: Calcium absorption across epithelia. Physiol Rev 85: 373–422, 2005 [DOI] [PubMed] [Google Scholar]
- 46.Alexander RT, Beggs MR, Zamani R, Marcussen N, Frische S, Dimke H: Ultrastructural and immunohistochemical localization of plasma membrane Ca2+-ATPase 4 in Ca2+-transporting epithelia. Am J Physiol Renal Physiol 309: F604–F616, 2015 [DOI] [PubMed] [Google Scholar]
- 47.Wong NL, Quamme GA, Dirks JH: Actions of parathyroid hormone are not impaired during chronic metabolic acidosis. J Lab Clin Med 105: 472–478, 1985 [PubMed] [Google Scholar]
- 48.Dubb J, Goldberg M, Agus ZS: Tubular effects of acute metabolic acidosis in the rat. J Lab Clin Med 90: 318–323, 1977 [PubMed] [Google Scholar]
- 49.Nijenhuis T, Renkema KY, Hoenderop JG, Bindels RJ: Acid-base status determines the renal expression of Ca2+ and Mg2+ transport proteins. J Am Soc Nephrol 17: 617–626, 2006 [DOI] [PubMed] [Google Scholar]
- 50.Nijenhuis T, van der Eerden BC, Hoenderop JG, Weinans H, van Leeuwen JP, Bindels RJ: Bone resorption inhibitor alendronate normalizes the reduced bone thickness of TRPV5(-/-) mice. J Bone Miner Res 23: 1815–1824, 2008 [DOI] [PubMed] [Google Scholar]
- 51.van der Eerden BC, Hoenderop JG, de Vries TJ, Schoenmaker T, Buurman CJ, Uitterlinden AG, Pols HA, Bindels RJ, van Leeuwen JP: The epithelial Ca2+ channel TRPV5 is essential for proper osteoclastic bone resorption. Proc Natl Acad Sci U S A 102: 17507–17512, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Canzanello VJ, Bodvarsson M, Kraut JA, Johns CA, Slatopolsky E, Madias NE: Effect of chronic respiratory acidosis on urinary calcium excretion in the dog. Kidney Int 38: 409–416, 1990 [DOI] [PubMed] [Google Scholar]
- 53.Lau K, Rodriguez Nichols F, Tannen RL: Renal excretion of divalent ions in response to chronic acidosis: evidence that systemic pH is not the controlling variable. J Lab Clin Med 109: 27–33, 1987 [PubMed] [Google Scholar]
- 54.Schaefer KE, Hasson M, Niemoeller H: Effect of prolonged exposure to 15 per cent CO2 on calcium and phosphorus metabolism. Proc Soc Exp Biol Med 107: 355–359, 1961 [PubMed] [Google Scholar]
- 55.Bushinsky DA: Net proton influx into bone during metabolic, but not respiratory, acidosis. Am J Physiol 254: F306–F310, 1988 [DOI] [PubMed] [Google Scholar]
- 56.Bushinsky DA, Sessler NE, Krieger NS: Greater unidirectional calcium efflux from bone during metabolic, compared with respiratory, acidosis. Am J Physiol 262: F425–F431, 1992 [DOI] [PubMed] [Google Scholar]
- 57.Bushinsky DA, Sessler NE: Critical role of bicarbonate in calcium release from bone. Am J Physiol 263: F510–F515, 1992 [DOI] [PubMed] [Google Scholar]
- 58.Coe FL, Firpo JJ Jr, Hollandsworth DL, Segil L, Canterbury JM, Reiss E: Effect of acute and chronic metabolic acidosis on serum immunoreactive parathyroid hormone in man. Kidney Int 8: 263–273, 1975 [DOI] [PubMed] [Google Scholar]
- 59.Batlle D, Itsarayoungyuen K, Hays S, Arruda JA, Kurtzman NA: Parathyroid hormone is not anticalciuric during chronic metabolic acidosis. Kidney Int 22: 264–271, 1982 [DOI] [PubMed] [Google Scholar]
- 60.Adams ND, Gray RW, Lemann J Jr: The calciuria of increased fixed acid production in humans: evidence against a role for parathyroid hormone and 1,25(OH)2-vitamin D. Calcif Tissue Int 28: 233–238, 1979 [DOI] [PubMed] [Google Scholar]
- 61.Gafter U, Kraut JA, Lee DB, Silis V, Walling MW, Kurokawa K, Haussler MR, Coburn JW: Effect of metabolic acidosis in intestinal absorption of calcium and phosphorus. Am J Physiol 239: G480–G484, 1980 [DOI] [PubMed] [Google Scholar]
- 62.Bushinsky DA, Riera GS, Favus MJ, Coe FL: Response of serum 1,25(OH)2D3 to variation of ionized calcium during chronic acidosis. Am J Physiol 249: F361–F365, 1985 [DOI] [PubMed] [Google Scholar]
- 63.Fenton TR, Eliasziw M, Lyon AW, Tough SC, Hanley DA: Meta-analysis of the quantity of calcium excretion associated with the net acid excretion of the modern diet under the acid-ash diet hypothesis. Am J Clin Nutr 88: 1159–1166, 2008 [DOI] [PubMed] [Google Scholar]
- 64.Fenton TR, Lyon AW, Eliasziw M, Tough SC, Hanley DA: Meta-analysis of the effect of the acid-ash hypothesis of osteoporosis on calcium balance. J Bone Miner Res 24: 1835–1840, 2009 [DOI] [PubMed] [Google Scholar]
- 65.Cao JJ, Nielsen FH: Acid diet (high-meat protein) effects on calcium metabolism and bone health. Curr Opin Clin Nutr Metab Care 13: 698–702, 2010 [DOI] [PubMed] [Google Scholar]
- 66.Topaloglu AK, Yildizdas D, Yilmaz HL, Mungan NO, Yuksel B, Ozer G: Bone calcium changes during diabetic ketoacidosis: a comparison with lactic acidosis due to volume depletion. Bone 37: 122–127, 2005 [DOI] [PubMed] [Google Scholar]
- 67.Domrongkitchaiporn S, Pongsakul C, Stitchantrakul W, Sirikulchayanonta V, Ongphiphadhanakul B, Radinahamed P, Karnsombut P, Kunkitti N, Ruang-raksa C, Rajatanavin R: Bone mineral density and histology in distal renal tubular acidosis. Kidney Int 59: 1086–1093, 2001 [DOI] [PubMed] [Google Scholar]
- 68.Domrongkitchaiporn S, Pongskul C, Sirikulchayanonta V, Stitchantrakul W, Leeprasert V, Ongphiphadhanakul B, Radinahamed P, Rajatanavin R: Bone histology and bone mineral density after correction of acidosis in distal renal tubular acidosis. Kidney Int 62: 2160–2166, 2002 [DOI] [PubMed] [Google Scholar]
- 69.Karet FE, Finberg KE, Nelson RD, Nayir A, Mocan H, Sanjad SA, Rodriguez-Soriano J, Santos F, Cremers CW, Di Pietro A, Hoffbrand BI, Winiarski J, Bakkaloglu A, Ozen S, Dusunsel R, Goodyer P, Hulton SA, Wu DK, Skvorak AB, Morton CC, Cunningham MJ, Jha V, Lifton RP: Mutations in the gene encoding B1 subunit of H+-ATPase cause renal tubular acidosis with sensorineural deafness. Nat Genet 21: 84–90, 1999 [DOI] [PubMed] [Google Scholar]
- 70.Ruf R, Rensing C, Topaloglu R, Guay-Woodford L, Klein C, Vollmer M, Otto E, Beekmann F, Haller M, Wiedensohler A, Leumann E, Antignac C, Rizzoni G, Filler G, Brandis M, Weber JL, Hildebrandt F: Confirmation of the ATP6B1 gene as responsible for distal renal tubular acidosis. Pediatr Nephrol 18: 105–109, 2003 [DOI] [PubMed] [Google Scholar]
- 71.Hahn H, Kang HG, Ha IS, Cheong HI, Choi Y: ATP6B1 gene mutations associated with distal renal tubular acidosis and deafness in a child. Am J Kidney Dis 41: 238–243, 2003 [DOI] [PubMed] [Google Scholar]
- 72.Gueutin V, Vallet M, Jayat M, Peti-Peterdi J, Cornière N, Leviel F, Sohet F, Wagner CA, Eladari D, Chambrey R: Renal β-intercalated cells maintain body fluid and electrolyte balance. J Clin Invest 123: 4219–4231, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sebastian A, McSherry E, Morris RC Jr: Impaired renal conservation of sodium and chloride during sustained correction of systemic acidosis in patients with type 1, classic renal tubular acidosis. J Clin Invest 58: 454–469, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kleeman CR, Bohannan J, Bernstein D, Ling S, Maxwell MH: Effect of Variations in Sodium Intake on Calcium Excretion in Normal Humans. Proc Soc Exp Biol Med 115: 29–32, 1964 [PubMed] [Google Scholar]
- 75.Ng RC, Peraino RA, Suki WN: Divalent cation transport in isolated tubules. Kidney Int 22: 492–497, 1982 [DOI] [PubMed] [Google Scholar]
- 76.Friedman PA, Figueiredo JF, Maack T, Windhager EE: Sodium-calcium interactions in the renal proximal convoluted tubule of the rabbit. Am J Physiol 240: F558–F568, 1981 [DOI] [PubMed] [Google Scholar]
- 77.Bourdeau JE, Buss SL, Vurek GG: Inhibition of calcium absorption in the cortical thick ascending limb of Henle’s loop by furosemide. J Pharmacol Exp Ther 221: 815–819, 1982 [PubMed] [Google Scholar]
- 78.Bartoli E, Romano G, Favret G: Segmental reabsorption measured by micropuncture and clearance methods during hypertonic sodium infusion in the rat. Nephrol Dial Transplant 11: 1996–2003, 1996 [DOI] [PubMed] [Google Scholar]
- 79.Edelmann CM, Barnett HL, Troupkou V: Renal concentrating mechanisms in newborn infants. Effect of dietary protein and water content, role of urea, and responsiveness to antidiuretic hormone. J Clin Invest 39: 1062–1069, 1960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fomon SJ, Owen GM, Jensen RL, Thomas LN: Calcium and phosphorus balance studies with normal full term infants fed pooled human milk or various formulas. Am J Clin Nutr 12: 346–357, 1963 [DOI] [PubMed] [Google Scholar]
- 81.Zhang J, Fuster DG, Cameron MA, Quiñones H, Griffith C, Xie XS, Moe OW: Incomplete distal renal tubular acidosis from a heterozygous mutation of the V-ATPase B1 subunit. Am J Physiol Renal Physiol 307: F1063–F1071, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dhayat NA, Schaller A, Albano G, Poindexter J, Griffith C, Pasch A, Gallati S, Vogt B, Moe OW, Fuster DG: The Vacuolar H+-ATPase B1 Subunit Polymorphism p.E161K Associates with Impaired Urinary Acidification in Recurrent Stone Formers. J Am Soc Nephrol 27: 1544–1554, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Evan AP, Lingeman J, Coe F, Shao Y, Miller N, Matlaga B, Phillips C, Sommer A, Worcester E: Renal histopathology of stone-forming patients with distal renal tubular acidosis. Kidney Int 71: 795–801, 2007 [DOI] [PubMed] [Google Scholar]
- 84.Stover EH, Borthwick KJ, Bavalia C, Eady N, Fritz DM, Rungroj N, Giersch AB, Morton CC, Axon PR, Akil I, Al-Sabban EA, Baguley DM, Bianca S, Bakkaloglu A, Bircan Z, Chauveau D, Clermont MJ, Guala A, Hulton SA, Kroes H, Li Volti G, Mir S, Mocan H, Nayir A, Ozen S, Rodriguez Soriano J, Sanjad SA, Tasic V, Taylor CM, Topaloglu R, Smith AN, Karet FE: Novel ATP6V1B1 and ATP6V0A4 mutations in autosomal recessive distal renal tubular acidosis with new evidence for hearing loss. J Med Genet 39: 796–803, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Smith AN, Skaug J, Choate KA, Nayir A, Bakkaloglu A, Ozen S, Hulton SA, Sanjad SA, Al-Sabban EA, Lifton RP, Scherer SW, Karet FE: Mutations in ATP6N1B, encoding a new kidney vacuolar proton pump 116-kD subunit, cause recessive distal renal tubular acidosis with preserved hearing. Nat Genet 26: 71–75, 2000 [DOI] [PubMed] [Google Scholar]
- 86.Karet FE, Finberg KE, Nayir A, Bakkaloglu A, Ozen S, Hulton SA, Sanjad SA, Al-Sabban EA, Medina JF, Lifton RP: Localization of a gene for autosomal recessive distal renal tubular acidosis with normal hearing (rdRTA2) to 7q33-34. Am J Hum Genet 65: 1656–1665, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gao Y, Xu Y, Li Q, Lang Y, Dong Q, Shao L: Mutation analysis and audiologic assessment in six Chinese children with primary distal renal tubular acidosis. Ren Fail 36: 1226–1232, 2014 [DOI] [PubMed] [Google Scholar]
- 88.Leviel F, Hübner CA, Houillier P, Morla L, El Moghrabi S, Brideau G, Hassan H, Parker MD, Kurth I, Kougioumtzes A, Sinning A, Pech V, Riemondy KA, Miller RL, Hummler E, Shull GE, Aronson PS, Doucet A, Wall SM, Chambrey R, Eladari D: The Na+-dependent chloride-bicarbonate exchanger SLC4A8 mediates an electroneutral Na+ reabsorption process in the renal cortical collecting ducts of mice. J Clin Invest 120: 1627–1635, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chambrey R, Kurth I, Peti-Peterdi J, Houillier P, Purkerson JM, Leviel F, Hentschke M, Zdebik AA, Schwartz GJ, Hübner CA, Eladari D: Renal intercalated cells are rather energized by a proton than a sodium pump. Proc Natl Acad Sci U S A 110: 7928–7933, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bruce LJ, Cope DL, Jones GK, Schofield AE, Burley M, Povey S, Unwin RJ, Wrong O, Tanner MJ: Familial distal renal tubular acidosis is associated with mutations in the red cell anion exchanger (Band 3, AE1) gene. J Clin Invest 100: 1693–1707, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jarolim P, Shayakul C, Prabakaran D, Jiang L, Stuart-Tilley A, Rubin HL, Simova S, Zavadil J, Herrin JT, Brouillette J, Somers MJ, Seemanova E, Brugnara C, Guay-Woodford LM, Alper SL: Autosomal dominant distal renal tubular acidosis is associated in three families with heterozygosity for the R589H mutation in the AE1 (band 3) Cl-/HCO3- exchanger. J Biol Chem 273: 6380–6388, 1998 [DOI] [PubMed] [Google Scholar]
- 92.Karet FE, Gainza FJ, Györy AZ, Unwin RJ, Wrong O, Tanner MJ, Nayir A, Alpay H, Santos F, Hulton SA, Bakkaloglu A, Ozen S, Cunningham MJ, di Pietro A, Walker WG, Lifton RP: Mutations in the chloride-bicarbonate exchanger gene AE1 cause autosomal dominant but not autosomal recessive distal renal tubular acidosis. Proc Natl Acad Sci U S A 95: 6337–6342, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rungroj N, Devonald MA, Cuthbert AW, Reimann F, Akkarapatumwong V, Yenchitsomanus PT, Bennett WM, Karet FE: A novel missense mutation in AE1 causing autosomal dominant distal renal tubular acidosis retains normal transport function but is mistargeted in polarized epithelial cells. J Biol Chem 279: 13833–13838, 2004 [DOI] [PubMed] [Google Scholar]
- 94.Zuckerman JM, Assimos DG: Hypocitraturia: pathophysiology and medical management. Rev Urol 11: 134–144, 2009 [PMC free article] [PubMed] [Google Scholar]
- 95.Horita S, Yamada H, Inatomi J, Moriyama N, Sekine T, Igarashi T, Endo Y, Dasouki M, Ekim M, Al-Gazali L, Shimadzu M, Seki G, Fujita T: Functional analysis of NBC1 mutants associated with proximal renal tubular acidosis and ocular abnormalities. J Am Soc Nephrol 16: 2270–2278, 2005 [DOI] [PubMed] [Google Scholar]
- 96.Dinour D, Chang MH, Satoh J, Smith BL, Angle N, Knecht A, Serban I, Holtzman EJ, Romero MF: A novel missense mutation in the sodium bicarbonate cotransporter (NBCe1/SLC4A4) causes proximal tubular acidosis and glaucoma through ion transport defects. J Biol Chem 279: 52238–52246, 2004 [DOI] [PubMed] [Google Scholar]
- 97.Igarashi T, Inatomi J, Sekine T, Seki G, Shimadzu M, Tozawa F, Takeshima Y, Takumi T, Takahashi T, Yoshikawa N, Nakamura H, Endou H: Novel nonsense mutation in the Na+/HCO3- cotransporter gene (SLC4A4) in a patient with permanent isolated proximal renal tubular acidosis and bilateral glaucoma. J Am Soc Nephrol 12: 713–718, 2001 [DOI] [PubMed] [Google Scholar]
- 98.Inatomi J, Horita S, Braverman N, Sekine T, Yamada H, Suzuki Y, Kawahara K, Moriyama N, Kudo A, Kawakami H, Shimadzu M, Endou H, Fujita T, Seki G, Igarashi T: Mutational and functional analysis of SLC4A4 in a patient with proximal renal tubular acidosis. Pflugers Arch 448: 438–444, 2004 [DOI] [PubMed] [Google Scholar]
- 99.Lemann J Jr, Adams ND, Wilz DR, Brenes LG: Acid and mineral balances and bone in familial proximal renal tubular acidosis. Kidney Int 58: 1267–1277, 2000 [DOI] [PubMed] [Google Scholar]
- 100.Alexander RT, Rievaj J, Dimke H: Paracellular calcium transport across renal and intestinal epithelia. Biochem Cell Biol 92: 467–480, 2014 [DOI] [PubMed] [Google Scholar]
- 101.Lambers TT, Oancea E, de Groot T, Topala CN, Hoenderop JG, Bindels RJ: Extracellular pH dynamically controls cell surface delivery of functional TRPV5 channels. Mol Cell Biol 27: 1486–1494, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Norman ME, Feldman NI, Cohn RM, Roth KS, McCurdy DK: Urinary citrate excretion in the diagnosis of distal renal tubular acidosis. J Pediatr 92: 394–400, 1978 [DOI] [PubMed] [Google Scholar]
- 103.Brennan S, Hering-Smith K, Hamm LL: Effect of pH on citrate reabsorption in the proximal convoluted tubule. Am J Physiol 255: F301–F306, 1988 [DOI] [PubMed] [Google Scholar]
- 104.Sly WS, Whyte MP, Sundaram V, Tashian RE, Hewett-Emmett D, Guibaud P, Vainsel M, Baluarte HJ, Gruskin A, Al-Mosawi M, Sakati N, Ohlsson A: Carbonic anhydrase II deficiency in 12 families with the autosomal recessive syndrome of osteopetrosis with renal tubular acidosis and cerebral calcification. N Engl J Med 313: 139–145, 1985 [DOI] [PubMed] [Google Scholar]
- 105.Sly WS, Hewett-Emmett D, Whyte MP, Yu YS, Tashian RE: Carbonic anhydrase II deficiency identified as the primary defect in the autosomal recessive syndrome of osteopetrosis with renal tubular acidosis and cerebral calcification. Proc Natl Acad Sci U S A 80: 2752–2756, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Vainsel M, Fondu P, Cadranel S, Rocmans C, Gepts W: Osteopetrosis associated with proximal and distal tubular acidosis. Acta Paediatr Scand 61: 429–434, 1972 [DOI] [PubMed] [Google Scholar]
- 107.Whyte MP, Murphy WA, Fallon MD, Sly WS, Teitelbaum SL, McAlister WH, Avioli LV: Osteopetrosis, renal tubular acidosis and basal ganglia calcification in three sisters. Am J Med 69: 64–74, 1980 [DOI] [PubMed] [Google Scholar]
- 108.Ohlsson A, Stark G, Sakati N: Marble brain disease: recessive osteopetrosis, renal tubular acidosis and cerebral calcification in three Saudi Arabian families. Dev Med Child Neurol 22: 72–84, 1980 [DOI] [PubMed] [Google Scholar]
- 109.Rousselle AV, Heymann D: Osteoclastic acidification pathways during bone resorption. Bone 30: 533–540, 2002 [DOI] [PubMed] [Google Scholar]
- 110.Nykjaer A, Dragun D, Walther D, Vorum H, Jacobsen C, Herz J, Melsen F, Christensen EI, Willnow TE: An endocytic pathway essential for renal uptake and activation of the steroid 25-(OH) vitamin D3. Cell 96: 507–515, 1999 [DOI] [PubMed] [Google Scholar]
- 111.Igarashi T, Inatomi J, Ohara T, Kuwahara T, Shimadzu M, Thakker RV: Clinical and genetic studies of CLCN5 mutations in Japanese families with Dent’s disease. Kidney Int 58: 520–527, 2000 [DOI] [PubMed] [Google Scholar]
- 112.Scheel O, Zdebik AA, Lourdel S, Jentsch TJ: Voltage-dependent electrogenic chloride/proton exchange by endosomal CLC proteins. Nature 436: 424–427, 2005 [DOI] [PubMed] [Google Scholar]
- 113.Piwon N, Günther W, Schwake M, Bösl MR, Jentsch TJ: ClC-5 Cl- -channel disruption impairs endocytosis in a mouse model for Dent’s disease. Nature 408: 369–373, 2000 [DOI] [PubMed] [Google Scholar]
- 114.Wang SS, Devuyst O, Courtoy PJ, Wang XT, Wang H, Wang Y, Thakker RV, Guggino S, Guggino WB: Mice lacking renal chloride channel, CLC-5, are a model for Dent’s disease, a nephrolithiasis disorder associated with defective receptor-mediated endocytosis. Hum Mol Genet 9: 2937–2945, 2000 [DOI] [PubMed] [Google Scholar]
- 115.Sliman GA, Winters WD, Shaw DW, Avner ED: Hypercalciuria and nephrocalcinosis in the oculocerebrorenal syndrome. J Urol 153: 1244–1246, 1995 [PubMed] [Google Scholar]
- 116.Hoopes RR Jr, Shrimpton AE, Knohl SJ, Hueber P, Hoppe B, Matyus J, Simckes A, Tasic V, Toenshoff B, Suchy SF, Nussbaum RL, Scheinman SJ: Dent Disease with mutations in OCRL1. Am J Hum Genet 76: 260–267, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gil H, Santos F, García E, Alvarez MV, Ordóñez FA, Málaga S, Coto E: Distal RTA with nerve deafness: clinical spectrum and mutational analysis in five children. Pediatr Nephrol 22: 825–828, 2007 [DOI] [PubMed] [Google Scholar]
- 118.Vargas-Poussou R, Cochat P, Le Pottier N, Roncelin I, Liutkus A, Blanchard A, Jeunemaître X: Report of a family with two different hereditary diseases leading to early nephrocalcinosis. Pediatr Nephrol 23: 149–153, 2008 [DOI] [PubMed] [Google Scholar]
- 119.Feldman M, Prikis M, Athanasiou Y, Elia A, Pierides A, Deltas CC: Molecular investigation and long-term clinical progress in Greek Cypriot families with recessive distal renal tubular acidosis and sensorineural deafness due to mutations in the ATP6V1B1 gene. Clin Genet 69: 135–144, 2006 [DOI] [PubMed] [Google Scholar]
- 120.Tasic V, Korneti P, Gucev Z, Hoppe B, Blau N, Cheong HI: Atypical presentation of distal renal tubular acidosis in two siblings. Pediatr Nephrol 23: 1177–1181, 2008 [DOI] [PubMed] [Google Scholar]
- 121.Andreucci E, Bianchi B, Carboni I, Lavoratti G, Mortilla M, Fonda C, Bigozzi M, Genuardi M, Giglio S, Pela I: Inner ear abnormalities in four patients with dRTA and SNHL: clinical and genetic heterogeneity. Pediatr Nephrol 24: 2147–2153, 2009 [DOI] [PubMed] [Google Scholar]
- 122.Weber S, Soergel M, Jeck N, Konrad M: Atypical distal renal tubular acidosis confirmed by mutation analysis. Pediatr Nephrol 15: 201–204, 2000 [DOI] [PubMed] [Google Scholar]
- 123.Buckalew VM Jr, Purvis ML, Shulman MG, Herndon CN, Rudman D: Hereditary renal tubular acidosis. Report of a 64 member kindred with variable clinical expression including idiopathic hypercalciuria. Medicine (Baltimore) 53: 229–254, 1974 [DOI] [PubMed] [Google Scholar]
- 124.Richards P, Wrong OM: Dominant inheritance in a family with familial renal tubular acidosis. Lancet 2: 998–999, 1972 [DOI] [PubMed] [Google Scholar]
- 125.Ballet JJ, Griscelli C, Coutris C, Milhaud G, Maroteaux P: Bone-marrow transplantation in osteopetrosis. Lancet 2: 1137, 1977 [DOI] [PubMed] [Google Scholar]
- 126.Ohlsson A, Cumming WA, Paul A, Sly WS: Carbonic anhydrase II deficiency syndrome: recessive osteopetrosis with renal tubular acidosis and cerebral calcification. Pediatrics 77: 371–381, 1986 [PubMed] [Google Scholar]
- 127.Igarashi T, Ishii T, Watanabe K, Hayakawa H, Horio K, Sone Y, Ohga K: Persistent isolated proximal renal tubular acidosis--a systemic disease with a distinct clinical entity. Pediatr Nephrol 8: 70–71, 1994 [DOI] [PubMed] [Google Scholar]
- 128.Winsnes A, Monn E, Stokke O, Feyling T: Congenital persistent proximal type renal tubular acidosis in two brothers. Acta Paediatr Scand 68: 861–868, 1979 [DOI] [PubMed] [Google Scholar]
- 129.Rodriguez Soriano J, Boichis H, Stark H, Edelmann CM Jr: Proximal renal tubular acidosis. A defect in bicarbonate reabsorption with normal urinary acidification. Pediatr Res 1: 81–98, 1967 [DOI] [PubMed] [Google Scholar]
- 130.Theodoropoulos DS, Shawker TH, Heinrichs C, Gahl WA: Medullary nephrocalcinosis in nephropathic cystinosis. Pediatr Nephrol 9: 412–418, 1995 [DOI] [PubMed] [Google Scholar]
- 131.Saleem MA, Milford DV, Alton H, Chapman S, Winterborn MH: Hypercalciuria and ultrasound abnormalities in children with cystinosis. Pediatr Nephrol 9: 45–47, 1995 [DOI] [PubMed] [Google Scholar]
- 132.Claverie-Martín F, Ramos-Trujillo E, García-Nieto V: Dent’s disease: clinical features and molecular basis. Pediatr Nephrol 26: 693–704, 2011 [DOI] [PubMed] [Google Scholar]
- 133.Cho HY, Lee BH, Choi HJ, Ha IS, Choi Y, Cheong HI: Renal manifestations of Dent disease and Lowe syndrome. Pediatr Nephrol 23: 243–249, 2008 [DOI] [PubMed] [Google Scholar]
- 134.Bockenhauer D, Bokenkamp A, van’t Hoff W, Levtchenko E, Kist-van Holthe JE, Tasic V, Ludwig M: Renal phenotype in Lowe Syndrome: a selective proximal tubular dysfunction. Clin J Am Soc Nephrol 3: 1430–1436, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]