Abstract
Accumulating evidence indicates that CDK2 promotes hyperproliferation and is associated to poor prognosis in multiple cancer cells. However, the physiological role of CDK2 in GBM and the biological mechanism still remains unclear. In this study, we identified that CDK2 expression was significantly enriched in GBM tumors compared with normal brain. Additionally, CDK2 was functionally required for tumor proliferation and its expression was associated to poor prognosis in GBM patients. Mechanically, CDK2 induced radio resistance in GBM cells and CDK2 knock down increased cell apoptosis when combined with radiotherapy. Therapeutically, we found that CDK2 inhibitor attenuated tumor growth both in vitro and in vivo. Collectively, CDK2 promotes proliferation, induces radio resistance in GBM, and could become a therapeutic target for GBM.
Introduction
Glioblastoma (GBM) is one of the most common malignant primary brain tumors in human adults with the median survival in the range of 14 to 18 months despite multimodality treatment comprising maximal safe resection, radiotherapy, and concomitant and adjuvant chemotherapy [1], [2]. During their clinical course, almost all patients suffer a post-treatment recurrence then become resistant to both radiotherapy and chemotherapy. Therefore, the drug resistance, high recurrence and poor prognosis of GBM promote us to identify novel biomarkers and explore novel therapeutic targets for the available clinical treatment of GBM.
Cyclin-dependent kinases (CDKs) are heterodimeric complexes composed of a catalytic kinase subunit and a regulatory cyclin subunit, and comprise a family divided into two groups based on their roles in cell cycle progression and transcriptional regulation [3], [4]. There are totally 13 CDKs in human cells and five of them, named CDK1, CDK2, CDK3, CDK4, and CDK6, respectively, are involved in the regulation of cell cycle [5]. Cyclin-dependent kinases 2 (CDK2) is one of the most essential regulators for the transition and progression in a cell-division cycle and plays a crucial role in regulating multiple events of cell division cycle including centrosome duplication, DNA synthesis, G1-S transition, and modulation of G2 progression [6], [7], [8], [9]. Accumulating evidence has been shown that CDK2 is functionally associated with hyperproliferation in multiple cancer cells and could be regarded as a potentially therapeutic target for cancer therapy [6]. Recent study indicated that CDK2 expression level was elevated in human cholangiocarcinoma tissues and apoptosis-related protein-1 dependent suppression of CDK2 induced cell cycle arrest then restrain tumor growth in cholangiocarcinoma [10]. Another study showed that inhibition of CDK2 kinase activity reduced tumor proliferation via selectively targets the CD44+/CD24−/Low stem-like cells in triple-negative breast cancer when combined with conventional chemotherapy [11]. Additionally, a newly published study demonstrated that CDK2 inhibitor exhibits anti-cancer effect in human hepatoma HepG2 and Huh7 cells and significantly inhibited tumor growth [12]. Lim et al. identified CDK2 as a direct therapeutic target of curcumin in colon cancer cells via cell cycle arrest in HCT116 cells [13]. Taken together, CDK2-dependent cell cycle regulation plays an essential role in tumor growth and might be a potential therapeutic target for cancer treatment. However, despite these findings, the physiological role of CDK2 and the biological mechanism in GBM still remains unclear.
In this study, we identified that CDK2 expression was significantly enriched in GBM tumors and was functionally required for tumor proliferation both in vitro and in vivo. Furthermore, high CDK2 expression was associated to poor prognosis in GBM patients. Mechanically, CDK2 induced radio resistance in GBM cells and CDK2 knock down increased apoptosis in GBM cells when combined with radiotherapy. Therapeutically, we found that CDK2 inhibitor attenuated tumor growth both in vitro and in vivo. Collectively, CDK2 promotes proliferation, induces radio resistance in GBM, and could be a therapeutic target for GBM.
Materials and Methods
Ethics
All the usage of experimental animals (nude mice) in this study is approved by the Scientific Ethics Committee of School of Medicine, Xi'an Jiaotong University, Xi'an, China (No. 2016-085). The collection and usage of the human tumor samples and patient information are approved by the Scientific Ethics Committee of First Affiliated Hospital of Xi'an Jiaotong University, Xi'an, China (No. 2016-18).
Reagents and Antibodies
The following reagents and primary antibodies are used in this study: DMEM-F12 (Gibco, 10,565-018), Fetal bovine serum (Gibco, 10,082-147), Accutase solution (Sigma, A6964-100), Alamar Blue (Invitrogen, DAL1100), RIPA buffer (Sigma, R0278), Phosphatase inhibitor cocktail (Sigma, P0044), Protease inhibitor cocktail (P8340), Bradford (BIORAD, 500-0006), BSA used in Bradford assay (BioLabs, B9001S), PageRuler plus prestained protein (Thermo scientific, 26,619), iScript Reverse Transcription supermix for qRT-PCR (Bio-rad, 170-8841), shCDK2 lentivirus particles (Origene, TL320291V), CDK2 Inhibitor II (Santa Cruz Biotechnology, CAS 222035-13-4), Alexa Fluor® 488 Annexin V/Dead Cell Apoptosis Kit (Thermo Fisher Scientific,V13241). Anti-CDK2 (Abcam, ab54513, Mouse, used for WB), anti-CDK2 (Abcam, ab32147, Rabbit, used for IHC), β-actin (Sigma, A5316, Mouse, used for WB).
In Vitro Cell Cultures
Glioblastoma cells are cultured in DMEM/F12 medium containing 10% FBS supplement (vol%). The culture medium is replaced every 5 to 10 days. Normal Human Astrocytes (NHA, Lonza) are used as a control sample in this study.
RNA Isolation and Quantitative Real-Time PCR
RNA is isolated by using RNeasy mini kit (QIAGEN) according to the manufacturer's instructions. RNA concentration is determined using a Nanodrop 2000 (Thermo scientific). cDNA is synthesized by using iScript reverse transcription supermix for qRT-PCR (Bio-rad) according to the manufacturer's protocol. The reverse-transcribed cDNA is analyzed by quantitative RT-PCR (qRT-PCR), and GAPDH or 18 s is used as an internal control. Each qRT-PCR includes a 10 μl reaction mixture per well that includes 2.5 μl cDNA, 0.5 μl forward primer (0.5 μM), 0.5 μl reverse primer (0.5 μM), 1.5 μl of DNase/RNase-free distilled water, and 5 μl SYBR green reagent (QIAGEN). The following cycles are performed during DNA amplification: 94 °C for 2 min, 40 cycles of 94 °C (30 s), 60 °C (30 s), and 72 °C (40 s).18S is used as an internal control. The primer sequences are showed below: CDK2-Forward: GAATCTCCAGGGAATAGGGC, CDK2-Reverse: CTGAAATCCTCCTGGGCTG, 18S-Forward: GGCCCTGTAATTGGAATGAGTC and 18S-Reverse: CCAAGATCCAACTACGAGCTT.
Western Blotting
The cell lysates are prepared in RIPA buffer containing 1% protease and 1% phosphatase inhibitor cocktail (Sigma Aldrich) on ice. The sample protein concentrations are determined by the Bradford method. Equal amounts of protein lysates (10 μg/lane) are fractionated on NuPAGE Novex 4% to 12% Bis-Tris Protein gel (Invitrogen) and transferred to a PVDF membrane (Invitrogen). Subsequently, the membranes are blocked with 5% skimmed milk for 1 h and then treated with the relevant antibody at 4 °C overnight. Protein expression is visualized with Amersham ECL Western Blot System (GE Healthcare Life Sciences). β-Actin serves as a loading control.
Flow Cytometry Analysis
Harvest the cells after the incubation period and wash in cold phosphate-buffered saline for 3 times (PBS). Re-centrifuge the washed cells (from step 2), discard the supernatant and suspend 5 × 105 cells in 100 μl 1X Annexin-binding buffer. Add 5 μl Alexa Fluor® 488 annexin V and 1 μl 100 μg/ml PI working solution thatwas prepared according to the protocol. Incubate the cells at room temperature for 15 minutes then add 400 μl 1× annexin -binding buffer, mix gently and keep the samples on ice. Analyze the stained cells by measuring the fluorescence emission at 530 nm and 575 nm with 488 nm excitation.
Immunohistochemistry Staining
For IHC, experimental mice are sacrificed and perfused with ice-cold PBS followed by 4% (wt/ vol) paraformaldehyde (PFA). Then brains are harvested and fixed in 4% (wt/vol) PFA for 24 h and then transferred into 10% formalin. Brain sections are incubated with the indicated primary antibodies overnight at 4 °C, followed by incubation with an HRP-conjugated secondary antibody for 1 h at room temperature. Signals are detected using DAB substrate kit (Vector). Nuclei are counter stained with hematoxylin or Hoechst, respectively. Samples incubate without primary antibodies are used as negative controls.
German Immunohistochemical Score
Percentage of positive cells is classified as 0 (negative), 1 (up to 10%), 2 (11% to 50%), 3 (51% to 80%), or 4 (>80% positive cells), staining intensity is classified as 0 (no staining), 1 (weak), 2 (moderate), or 3 (strong). The final immunoreactivity score is defined as the multiplication of both grading results (percentage of positive cells × staining intensity).
In Vivo Intracranial Xenograft Tumor Models
Six-week-old nude mice are used for GBM intracranial implantation. All animal experiments are carried out in Xi'an Jiaotong University. The GBM suspension (1 × 105 cells in 5 μl of PBS) transduced with non-target or shCDK2 lentivirus is injected into the brains of nude mice after anesthesia. At least six mice are used for each group. Drug treatment is done through tail vein injection and starts from 5 days after tumor cells are implanted. Mice were monitored once a day for symptoms related to tumor growth including an arched back, unsteady gait, paralysis of legs and body weight lose. Mice were euthanized by overdose of anesthesia of ketamine and xylazine after a total body weight loss of 40% or severe symptoms were observed.
Statistical Analysis
All the data are presented as mean ± SD. The number of replicates for each experiment is stated in the figure legend. Statistical differences between two groups are evaluated by two-tailed t-test. The comparison among multiple groups are performed by one-way analysis of variance (ANOVA) followed by Dunnett's posttest. The statistical significance of Kaplan–Meier survival plot is determined by log-rank analysis. Statistical analysis is performed by Prism 6 (Graphpad prism), unless mentioned otherwise in figure legend. P < .05 is considered as statistically significant.
Results
CDK2 Expression was Enriched in GBM
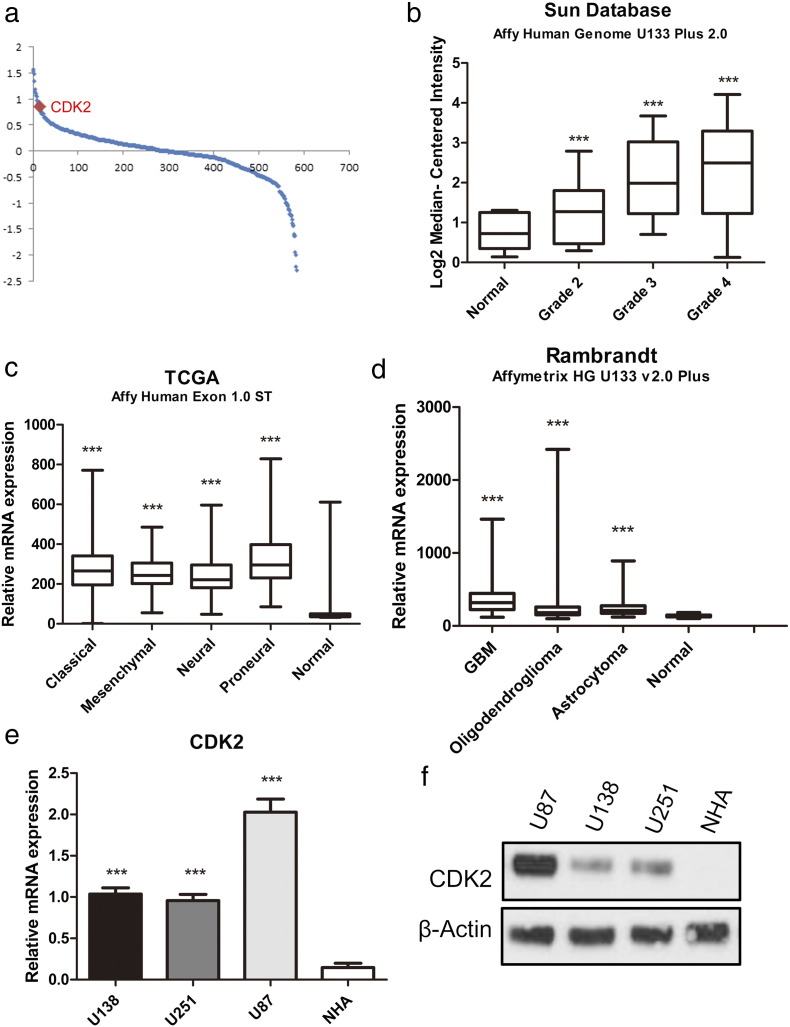
In this study, we first sought to characterize the role of CDK2 in GBM tumors. To this end, we analyzed the microarray data from TCGA database and find that CDK2 is the most up-regulated kinase-encoding gene in GBM samples compared to normal tissue (Figure 1a). Furthermore, DNA microarray data from the Sun. database (GSE 4290) database indicated that CDK2 was highly expressed in GBM when compared with normal brain and low-grade glioma [14] (Figure 1b). Additionally, we found that CDK2 expression was significantly enriched in all the four subgroups of GBM (classical, mesenchymal, neural, and proneural) compared with non-tumor tissue (Figure 1c). The data from Rembrandt database also demonstrated that CDK2 mRNA expression in GBM is significantly higher than non-tumor and other types of glioma groups (Figure 1d). To confirm this, qRT-PCR using 3 GBM cell lines and normal human astrocyte (NHA) was performed and the result exhibited the higher expression of CDK2 protein in GBM (Figure 1e). The sequences of these primers are described in methods and materials. Similar to the mRNA expression, Western blotting data demonstrated the same result (Figure 1f). Taken together, these data indicated that CDK2 was preferentially expressed in GBM.
Figure 1.
CDK2 expression was enriched in GBM.
(a) Genome-wide transcriptome microarray analysis from TCGA database showed that CDK2 is one of the most up-regulated kinases in GBM samples compared to normal tissue. (b) Analysis of Sun.'s database indicated that CDK2 expression was elevated in GBM samples. (c) Analysis of TCGA database indicated that CDK2 was highly expressed in all the 4 subgroups of GBM (classical, mesenchymal, neural and proneural) compared with non tumor tissue. (d) Analysis of Rembrandt database demonstrated that CDK2 expression in GBM is significantly higher than non tumor and other types of glioma groups. (e) qRT-PCR analysis showed CDK2 mRNA expression was elevated in 3 GBM cell lines (U138, U251, U87) compared with normal astrocytes (NHA). (f) Western blotting analysis indicated that CDK2 protein expression was enriched in 3 GBM cell lines compared with normal astrocytes.
CDK2 was Functionally Required for GBM Proliferation Both In Vitro and In Vivo
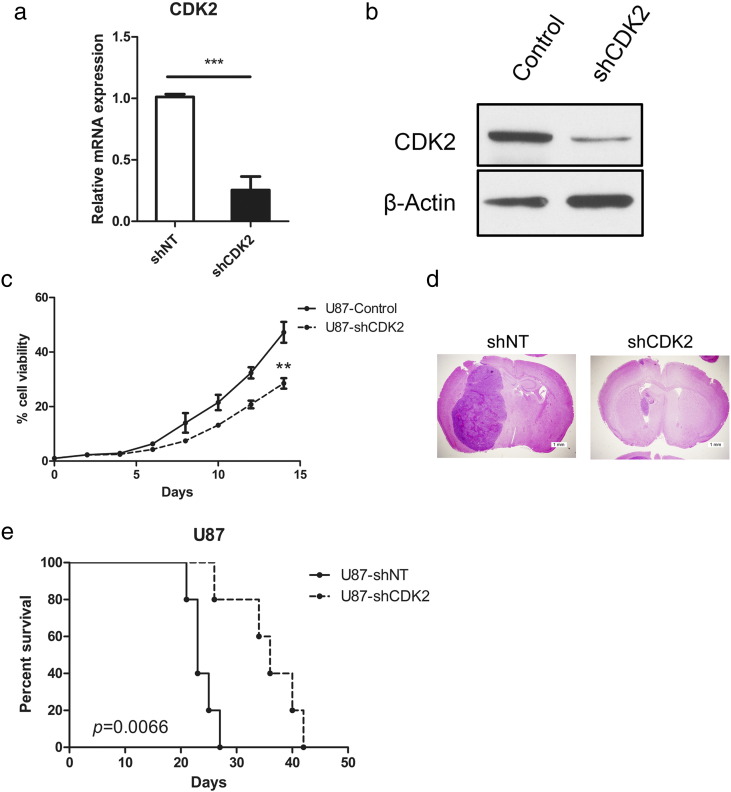
To examine the pathophysiological role of CDK2 in GBM, an in vitro GBM cell line (U87) was transduced with either lentiviral shRNA clone for CDK2 (shCDK2) or a non-targeting shRNA (shNT; negative control). qRT-PCR was performed to confirm the knockdown efficiency of the lentivirus (Figure 2a). Additionally, Western blotting exhibited that CDK2 protein expression was markedly decreased by shCDK2 (Figure 2b). In vitro cell growth kinetics of shCDEK2 transfected U87 cells was diminished proportionally to the reduction levels of CDK2 (Figure 2c). Next, we investigated the effect of CDK2 knockdown on in vivo tumor formation. To this end, we used U87 cells-derived mouse intracranial tumor models. The results showed that mice xenografted with shNT transfected U87 rapidly formed lethal hyper vascular GBM-like tumors within 30 days (median survival being 24.7 ± 2.94 days), while a longer survival could be observed in mice xenografted shCDK2 transfected U87 cells (median survival being 35.6 ± 6.23 days), highlighting a potent anti-tumorigenesis effects of CDK2 knock-down (Figure 2, d and e). All together, these findings indicated that CDK2 is required for GBM proliferation both in vitro and in vivo.
Figure 2.
CDK2 was functionally required for GBM proliferation both in vitro and in vivo.
(a) qRT-PCR analysis of U87 cells transduced with shRNA against CDK2 (shCDK2) or non-targeting control (shNT). (b) Western blot analysis of U87 cells transduced with shRNA against CDK2 (shCDK2) or non-targeting control (shNT). (c) In vitro cell growth assay showing shRNA against CDK2 (shCDK2) inhibited cell proliferation of U87 cells (P < .01, n = 6, with one-way ANOVA). (d) Representative images of H&E stained mouse brain section after the intracranial transplantation of U87 cells transduced with shRNA against CDK2 (shCDK2) or non-targeting control (shNT). (e) Kaplan–Meier analysis of nude mice harboring intracranial tumor derived from U87 cells transduced with shNT (n = 5), shCDK2 (n = 5) (P = .0066, with log-rank test).
Enhanced CDK2 Expression was Highly Expressed in GBM and was Associated to Poor Prognosis in GBM Patients
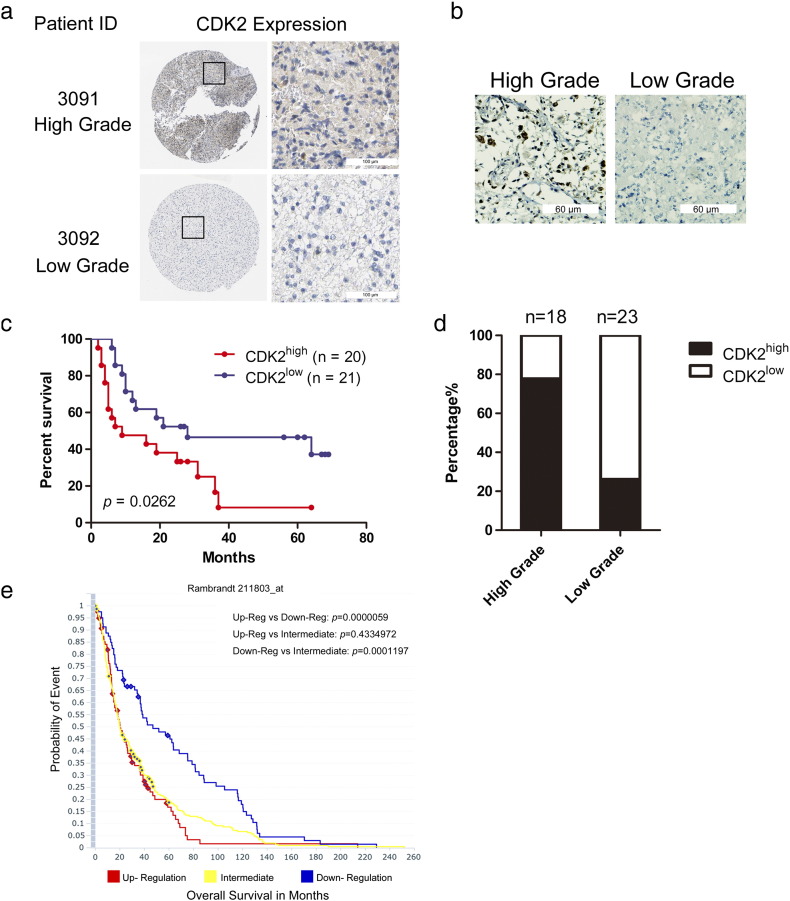
Given these experimental data, we sought to ask whether CDK2 could be a clinical target in GBM. To this end, we first checked the Human Protein Atlas (THPA) and found that CDK2 expression was enriched in compared to low grade glioma (Figure 3a). We then examined CDK2 expression in 41 glioma patient samples of varying grades, which were collected from 2006 to 2015 in First Affiliated Hospital of Xi'an Jiaotong University, by immunohistochemical and Germany immunohistochemical scores (GIS) was used to quantify the expression levels. As a result, we found CDK2 was highly expressed in high-grade glioma (14 out of 18) compared to low grade glioma samples (6 out of 23) (Figure 3b and c). Furthermore, we found that the overall survival period is significantly longer in the CDK2 low expressed group (with a median survival 28 months) than CDK2 high expressed group (with a median survival 9 months) (Figure 3d). Then we checked the data from Rembrandt database also demonstrated that GBM patients with higher CDK2 expression exhibited significantly shorter survival periods compared to those with intermediate or low expression group (Figure3e). Collectively, these data indicated that CDK2 might be a clinically relevant molecular target in GBM.
Figure 3.
Enhanced CDK2 expression was highly expressed in GBM and was associated with poor prognosis in GBM patients.
(a) CDK2 displayed a strong expression at protein level in the Human protein Atlas dataset. (b) Representative immunohistochemically image of CDK2. (c) Analysis of CDK2 in glioma samples and non-tumor brain samples showed CDK2 is highly expressed in high grade glioma samples compared to low grade glioma samples. (d) Kaplan–Meier analysis evaluating the correlation between CDK2 expression and survival of 41 glioma patients (CDK2high samples versus CDK2low samples, P = .0262, with log-rank test). (e) Analysis of the Rembrandt data indicated the inverted correlation between CDK2 mRNA expression and post-surgical survival of GBM patients.
CDK2 Regulated Radio Resistance in GBM
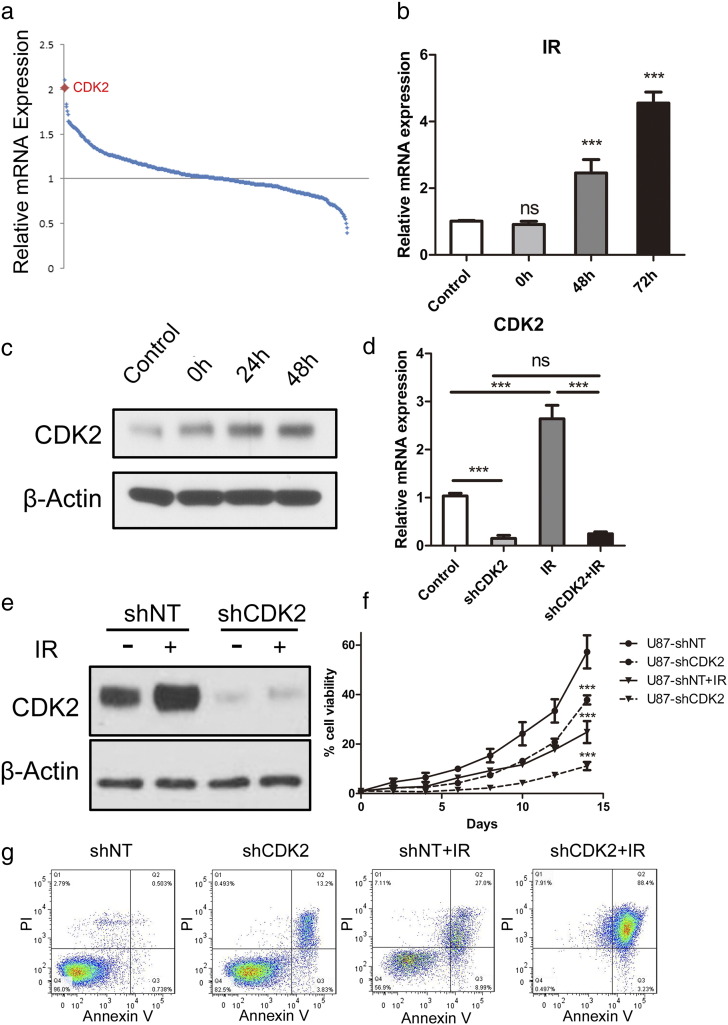
To identify the mechanism of CDK2 regulated GBM tumorigenesis and proliferation, we then assumed that CDK2 might contribute to radio-resistance in GBM, thereby leading to tumor recurrence after therapeutic failure. The DNA microarray analysis focusing on 668 kinase-encoding genes with 2 GBM cell lines demonstrated that CDK2 was the top 2 gene which were up-regulated after irradiation at 12 Gy (Figure 4a). To confirm this, we treaded U87 cells with or without radiotherapy for 12 Gy and purified mRNA or protein 72 h after treatment. qRT-PCR analysis indicated that CDK2 expression level was significantly elevated at both mRNA level (Figure 4b) and protein level after radio treatment (Figure 4c). To investigate whether CDK2 is necessary for GBM cells to maintain radio resistance, we then combined CDK2 knock down together with radiotherapy. qRT-PCR and western blotting results showed the post-radiation elevation of CDK2 was reduced by shCDK2 (Figure 4, d and e). In vitro cell proliferation was dramatically decreased by shCDK2 when combined with radio treatment (Figure 4f). At the same time, we performed flow cytometry for apoptosis with Annexin V (AV) antibody and Propidium Iodide (PI) by using shCDK2 transduced U87 cells with or without radiation treatment (12 Gy). The results indicated that proportions of cells undergoing early (AV+; PI−) and late (AV+; PI+) apoptosis were both dramatically increased after radiation, when combined with CDK2 knockdown in comparison to radiation alone (Figure 4g). Taken together, CDK2-dependent radio resistance was essential for GBM tumorigenesis and recurrence after therapeutic treatment.
Figure 4.
CDK2 regulated radio resistance in GBM.
(a) Genome-wide transcriptome microarray analysis showed that CDK2 was one of the most up-regulated kinases in GBM cells post-irradiation (12Gy) compared to naïve GBM cells. (b) qRT-PCR analysis for CDK2 mRNA expression in U87 cells post-irradiation (12Gy) compared to naïve U87 cells (P < .01, with t-test). (c) Western analysis for CDK2 protein expression in U87 cells post-irradiation (12Gy) compared to naïve U87 cells. (d) qRT-PCR analysis for CDK2 expression using U87 cells infected with shNT and shCDK2 then treated with or without radiation treatment (12 Gy). (e) Western blot analysis for CDK2 expression using U87 cells infected with shNT and shCDK2 then treated with or without radiation treatment (12 Gy). β-actin served as a control. (f) In vitro cell growth assay for U87 cells transduced with shNT or shCDK2 with or without radiation treatment (12 Gy). (g) Flow cytometry analysis for apoptosis with Annexin V antibody and Propidium Iodide using U87 cells transduced with shNT or shCDK2 with or without radiation treatment (12 Gy).
CDK2 Inhibitor Attenuated Tumor Growth by Increasing Radio Sensitivity in GBM
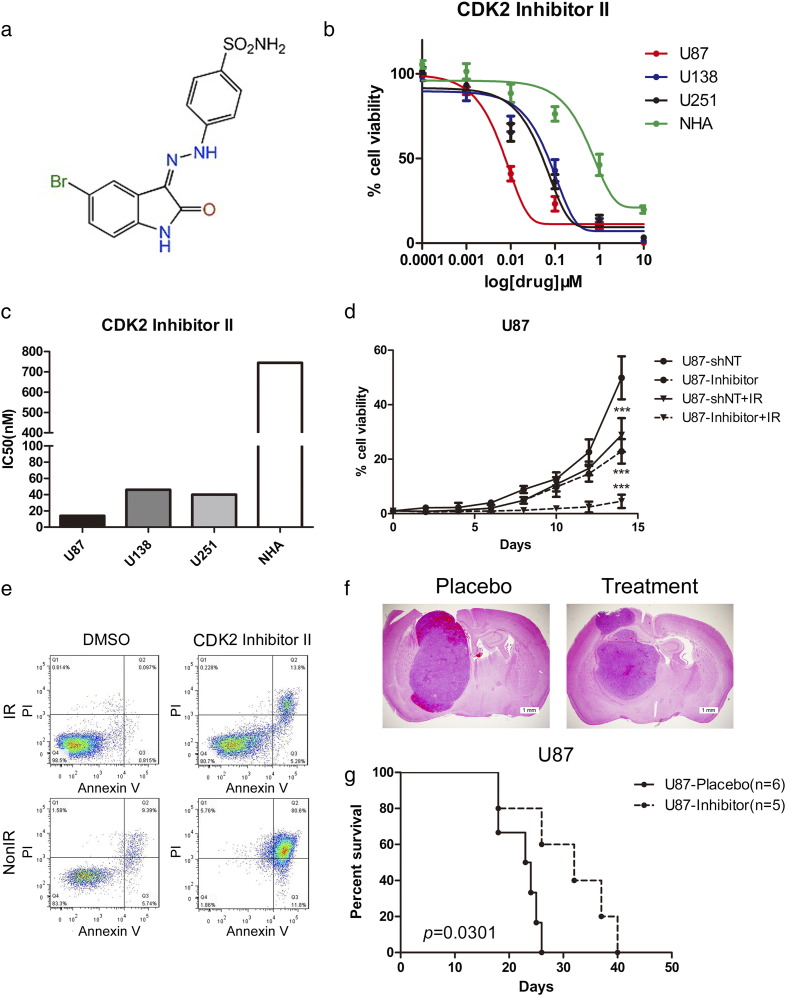
For the purpose of CDK2 targeting therapeutic development, we sought to identify whether selectively CDK2 inhibition could eliminate tumorigenesis and radio resistance. To this end, an ATP-competitive CDK2 inhibitor, CDK2 inhibitor II, was used for in vitro and in vivo experiments (Figure 5a). To characterize the efficacy of thisCDK2 inhibitor, we started from investigating the IC50s of CDK2 inhibitor II in different GBM cell lines. As expected, in vitro sensitivities of these GBM cells were correlated with their CDK2 expression levels and normal astrocytes showed more resistance to CDK2 inhibitor II (Figure 5, b and c). Furthermore, in vitro cell proliferation was significantly eliminated by CDK2 inhibitor II when combined with radiation compared with radiotherapy alone (Figure 5d). Flow cytometry results indicated that radiation treatment significantly increased the proportion of apoptotic cells when combined with C3a together (Figure 5e). To investigate the effect of CDK2 inhibitor II on in vivo tumor initiation and propagation, mouse intracranial tumor models were used and CDK2 inhibitor II treatment started from 5 days after implanted U87 cells then continued for 7 days through tail vein injection for 20 mg/kg/day. A decreased tumor growth and longer survival could be observed after CDK2 inhibitor II treatment in mice (Figure 5, f and g), indicating that CDK2 inhibition was capable of abolishing GBM growth both in vitro and in vivo and might be a promising therapeutic target for GBM therapy.
Figure 5.
CDK2 inhibitor attenuated tumor growth by increasing radio sensitivity in GBM.
(a) Chemical structure of CDK2 Inhibitor II. (b) In vitro cell viability assay for CDK2 Inhibitor II with 3 GBM cell lines (U138, U251, U87) compared with normal astrocytes (NHA). (c) IC50s of CDK2 Inhibitor II in 3 GBM cell lines (U138, U251, U87) compared with normal astrocytes (NHA). (d) In vitro cell growth assay showed that CDK2 Inhibitor II decreased cell proliferation of U87 when combined with radiation (P < .001, with ELDA analysis). (e) Flow cytometry analysis for apoptosis with Annexin V antibody and Propidium Iodide using U87 cells pretreated with CDK2 Inhibitor II with or without radiation treatment (12 Gy). (f) Representative images of H&E stained mouse brain section after the intracranial transplantation of U87 cells then followed continuously 7-day CDK2 Inhibitor II treatment or placebo by tail vein injection. (g) Kaplan–Meier analysis was performed for the comparison of survival in U87 implanted mice treat with or without CDK2 Inhibitor II (P = .0066, with log-rank test).
Discussion
Accumulating data suggests that CDKs are targets of interest for anticancer drug development as uncontrolled activation of CDKs can accelerate tumor proliferation [15]. Among all the CDKs, CDK2 was identified as a key kinase in tumorigenesis and proliferation in a range of cancer types including lung cancer, liver cancer, colon cancer and breast cancer [11], [12], [13]. In this study, we identified CDK2 expression was significantly elevated in glioma tumor especially in GBM and was functionally required for GBM cell proliferation and tumorigenesis. However, the molecular mechanism in this progress still remains unclear. Recent study identifies p53-dependent repression of CDK2 as a key mechanism during cellular senescence, essential for cell cycle exit [16], [17]. It also suggests that, clinically, CDK2 inhibition may be effective in driving pre-tumorigenic cells into a senescent state, thereby abrogating tumor progression by preventing further proliferation and possible accumulation of genetic aberrations that may otherwise lead to bypass of senescence [16], [17]. Another study demonstrates that CDK2 promotes invasion in GBM by phosphorylating Rb1, up-regulating CDC2, inactivating caldesmon and increasing MLC phosphorylation and decreases in KAP levels have the ability to activate this pathway by increasing CDK2 activation [18]. Nonetheless, whether CDK2 functions in GBM tumor growth depends on cell cycle regulation or there is still some other mechanism existing in this process still remains unclear and further studies are needed to investigate this.
Radio resistance has been proven to be the key factor of poor clinical prognosis and tumor recurrence in GBM patients. Herein, we found that CDK2 was one of the most up-regulated kinase encoding genes in GBM after radio treatment and CDK2-dependent radio resistance was essential for GBM tumorigenesis and recurrence after therapeutic treatment. However, the detailed mechanism and down-stream pathway of CDK2 in GBM are poorly identified. It has been shown that a small subpopulation of tumor cells among the whole tumor, termed glioma stem cells (GSCs), is likely responsible for tumorigenesis, treatment resistance, and recurrence [19], [20], [21], [22]. Opyrchal et al. reported that aberrant activation of cyclin E/ CDK2 oncogenic signaling is essential for the maintenance and expansion of CD44+/CD24−/Low cancer stem-like cell subpopulation in inflammatory breast cancer (IBC) and CDK2 kinase activity may represent a novel targeted therapeutic approach to treat aggressive IBC tumors [11]. Additionally, another research indicates that phosphorylation of EZH2 at T416 by CDK2 contributes to the malignancy of triple negative breast cancer [23], [24]. EZH2 is well known as one of the core functional components in polycomb repressive complexes 2 (PRC2), which functions as a lysine methyltransferase and catalyzes tri-methylation of histone H3 and EZH2 phosphorylation has been proved to be indispensable for tumorigenesis and therapy resistance in GBM [25], [26], [27]. Combined with our findings, it raises the possibility that CDK2-mediated EZH2 phosphorylation is important for GSC stemness maintainance and thus induces therapy resistance in GBM and further studies need to be done to confirm this hypothesis.
All together, our findings identified CDK2 is significantly elevated in GBM tumors and is functionally required for tumor proliferation both in vitro and in vivo. Furthermore, high CDK2 expression is associated to poor prognosis in GBM patients. Mechanically, CDK2 induces radio resistance in GBM cells and CDK2 knock down increases apoptosis in GBM cells when combined with radiotherapy. Therapeutically, we find that CDK2 inhibitor attenuates tumor growth and reduces radio resistance in GBM. In conclusion, CDK2 promotes proliferation, induces radio resistance in GBM, and could be a therapeutic target for GBM.
Compliance with Ethical Standards
Conflict of Interest: Authors declare that there is no conflict of interest.
Ethical approval: All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent: Informed consent was obtained from all individual participants included in the study.
Acknowledgements
We thank all the members in the Department of Neurosurgery, First Affiliated Hospital of Xi'an Jiaotong University.
Contributor Information
Wanfu Xie, Email: wanfu67@aliyun.com.
Maode Wang, Email: maodewang@163.com.
References
- 1.Ho VK, Reijneveld JC, Enting RH, Bienfait HP, Robe P, Baumert BG, Visser O, Dutch Society NO. Changing incidence and improved survival of gliomas. Eur J Cancer. 2014;50:2309–2318. doi: 10.1016/j.ejca.2014.05.019. [DOI] [PubMed] [Google Scholar]
- 2.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 3.Sausville EA. Complexities in the development of cyclin-dependent kinase inhibitor drugs. Trends Mol Med. 2002;8:S32–S37. doi: 10.1016/s1471-4914(02)02308-0. [DOI] [PubMed] [Google Scholar]
- 4.Shapiro GI. Cyclin-dependent kinase pathways as targets for cancer treatment. J Clin Oncol. 2006;24:1770–1783. doi: 10.1200/JCO.2005.03.7689. [DOI] [PubMed] [Google Scholar]
- 5.Idowu MA. Cyclin Dependent Kinases as Drug Targets for Cell Growth and Proliferation Disorders A Role for Systems Biology Approach in Drug Development Part II. Biotechnol Biotechnol Equip. 2011;0142:2712–2715. [Google Scholar]
- 6.Chohan TA, Qian H, Pan Y, Chen JZ. Cyclin-dependent kinase-2 as a target for cancer therapy: progress in the development of CDK2 inhibitors as anti-cancer agents. Curr Med Chem. 2015;22:237–263. doi: 10.2174/0929867321666141106113633. [DOI] [PubMed] [Google Scholar]
- 7.De Boer L, Oakes V, Beamish H, Giles N, Stevens F, Somodevilla-Torres M, Desouza C, Gabrielli B. Cyclin A/cdk2 coordinates centrosomal and nuclear mitotic events. Oncogene. 2008;27:4261–4268. doi: 10.1038/onc.2008.74. [DOI] [PubMed] [Google Scholar]
- 8.Fiset A, Xu E, Bergeron S, Marette A, Pelletier G, Siminovitch KA, Olivier M, Beauchemin N, Faure RL. Compartmentalized CDK2 is connected with SHP-1 and beta-catenin and regulates insulin internalization. Cell Signal. 2011;23:911–919. doi: 10.1016/j.cellsig.2011.01.019. [DOI] [PubMed] [Google Scholar]
- 9.Flores O, Wang Z, Knudsen KE, Burnstein KL. Nuclear targeting of cyclin-dependent kinase 2 reveals essential roles of cyclin-dependent kinase 2 localization and cyclin E in vitamin D-mediated growth inhibition. Endocrinology. 2010;151:896–908. doi: 10.1210/en.2009-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zheng J, Li Q, Wang W, Wang Y, Fu X, Wang W, Fan L, Yan W. Apoptosis-related protein-1 acts as a tumor suppressor in cholangiocarcinoma cells by inducing cell cycle arrest via downregulation of cyclin-dependent kinase subunits. Oncol Rep. 2016;35:809–816. doi: 10.3892/or.2015.4422. [DOI] [PubMed] [Google Scholar]
- 11.Opyrchal M, Salisbury JL, Iankov I, Goetz MP, McCubrey J, Gambino MW, Malatino L, Puccia G, Ingle JN, Galanis E. Inhibition of Cdk2 kinase activity selectively targets the CD44(+)/CD24(−)/Low stem-like subpopulation and restores chemosensitivity of SUM149PT triple-negative breast cancer cells. Int J Oncol. 2014;45:1193–1199. doi: 10.3892/ijo.2014.2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shi XN, Li H, Yao H, Liu X, Li L, Leung KS, Kung HF, Lu D, Wong MH, Lin MC. In Silico Identification and In Vitro and In Vivo Validation of Anti-Psychotic Drug Fluspirilene as a Potential CDK2 Inhibitor and a Candidate Anti-Cancer Drug. PLoS One. 2015;10:e0132072. doi: 10.1371/journal.pone.0132072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lim TG, Lee SY, Huang Z, Lim do Y, Chen H, Jung SK, Bode AM, Lee KW, Dong Z. Curcumin suppresses proliferation of colon cancer cells by targeting CDK2. Cancer Prev Res. 2014;7:466–474. doi: 10.1158/1940-6207.CAPR-13-0387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun L, Hui AM, Su Q, Vortmeyer A, Kotliarov Y, Pastorino S, Passaniti A, Menon J, Walling J, Bailey R. Neuronal and glioma-derived stem cell factor induces angiogenesis within the brain. Cancer Cell. 2006;9:287–300. doi: 10.1016/j.ccr.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 15.Malumbres M, Barbacid M. Cell cycle, CDKs and cancer: a changing paradigm. Nat Rev Cancer. 2009;9:153–166. doi: 10.1038/nrc2602. [DOI] [PubMed] [Google Scholar]
- 16.Linzen U, Lilischkis R, Pandithage R, Schilling B, Ullius A, Luscher-Firzlaff J, Kremmer E, Luscher B, Vervoorts J. ING5 is phosphorylated by CDK2 and controls cell proliferation independently of p53. PLoS One. 2015;10:e0123736. doi: 10.1371/journal.pone.0123736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zalzali H, Nasr B, Harajly M, Basma H, Ghamloush F, Ghayad S, Ghanem N, Evan GI, Saab R. CDK2 transcriptional repression is an essential effector in p53-dependent cellular senescence-implications for therapeutic intervention. Mol Cancer Res. 2015;13:29–40. doi: 10.1158/1541-7786.MCR-14-0163. [DOI] [PubMed] [Google Scholar]
- 18.Li H, Jiang X, Yu Y, Huang W, Xing H, Agar NY, Yang HW, Yang B, Carroll RS, Johnson MD. KAP regulates ROCK2 and Cdk2 in an RNA-activated glioblastoma invasion pathway. Oncogene. 2015;34:1432–1441. doi: 10.1038/onc.2014.49. [DOI] [PubMed] [Google Scholar]
- 19.Gu C, Banasavadi-Siddegowda YK, Joshi K, Nakamura Y, Kurt H, Gupta S, Nakano I. Tumor-specific activation of the C-JUN/MELK pathway regulates glioma stem cell growth in a p53-dependent manner. Stem Cells. 2013;31:870–881. doi: 10.1002/stem.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee Y, Kim KH, Kim DG, Cho HJ, Kim Y, Rheey J, Shin K, Seo YJ, Choi YS, Lee JI. FoxM1 Promotes Stemness and Radio-Resistance of Glioblastoma by Regulating the Master Stem Cell Regulator Sox2. PLoS One. 2015;10:e0137703. doi: 10.1371/journal.pone.0137703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee JK, Chang N, Yoon Y, Yang H, Cho H, Kim E, Shin Y, Kang W, Oh YT, Mun GI. USP1 targeting impedes GBM growth by inhibiting stem cell maintenance and radioresistance. Neuro-Oncology. 2016;18:37–47. doi: 10.1093/neuonc/nov091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nicolay NH, Lopez Perez R, Saffrich R, Huber PE. Radio-resistant mesenchymal stem cells: mechanisms of resistance and potential implications for the clinic. Oncotarget. 2015;6:19366–19380. doi: 10.18632/oncotarget.4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang CC, LaBaff A, Wei Y, Nie L, Xia W, Huo L, Yamaguchi H, Hsu YH, Hsu JL, Liu D. Phosphorylation of EZH2 at T416 by CDK2 contributes to the malignancy of triple negative breast cancers. Am J Transl Res. 2015;7:1009–1020. [PMC free article] [PubMed] [Google Scholar]
- 24.Zeng X, Chen S, Huang H. Phosphorylation of EZH2 by CDK1 and CDK2: a possible regulatory mechanism of transmission of the H3K27me3 epigenetic mark through cell divisions. Cell Cycle. 2011;10:579–583. doi: 10.4161/cc.10.4.14722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim E, Kim M, Woo DH, Shin Y, Shin J, Chang N, Oh YT, Kim H, Rheey J, Nakano I. Phosphorylation of EZH2 activates STAT3 signaling via STAT3 methylation and promotes tumorigenicity of glioblastoma stem-like cells. Cancer Cell. 2013;23:839–852. doi: 10.1016/j.ccr.2013.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim SH, Joshi K, Ezhilarasan R, Myers TR, Siu J, Gu C, Nakano-Okuno M, Taylor D, Minata M, Sulman EP. EZH2 protects glioma stem cells from radiation-induced cell death in a MELK/FOXM1-dependent manner. Stem Cell Rep. 2015;4:226–238. doi: 10.1016/j.stemcr.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Orzan F, Pellegatta S, Poliani PL, Pisati F, Caldera V, Menghi F, Kapetis D, Marras C, Schiffer D, Finocchiaro G. Enhancer of Zeste 2 (EZH2) is up-regulated in malignant gliomas and in glioma stem-like cells. Neuropathol Appl Neurobiol. 2011;37:381–394. doi: 10.1111/j.1365-2990.2010.01132.x. [DOI] [PubMed] [Google Scholar]